Abstract
Adipose-derived stem cells (ASCs) express a nonimmunogenic profile as shown by in vitro studies that demonstrate a lack of T cell proliferation to allogeneic ASCs as well as ASC-mediated suppression of mixed lymphocyte reactions. To determine whether these observations would translate in vivo, immune monitoring studies were carried out in conjunction with a rat spinal fusion study. ASCs derived from Fischer or ACI strain rats were loaded onto scaffolds and implanted in Fischer recipients that had undergone the following treatments: (1) No treatment; (2) Scaffold only; (3) Syngeneic ASCs+Scaffold; or (4) Allogeneic ASCs+Scaffold. Half of each group was sacrificed at 4 weeks postimplantation, and the remaining animals were sacrificed at 8 weeks. As determined in a separate study, allogeneic and syngeneic ASCs were equally efficacious in accelerating spinal fusion compared to No treatment and Scaffold only control groups. To determine whether donor ASCs induced an immune response in recipient rats, lymph nodes were harvested for T cell proliferation studies and serum was collected to assess antibody responses. Although T cell priming was not detected to donor alloantigens in recipients at either time point, significant antibody responses were detected to ACI ASCs in animals implanted with syngeneic or allogeneic ASCs. Antibodies were of the IgG isotype, noncytotoxic in the presence of complement, and reactive to fetal bovine serum. These results support the use of allogeneic ASCs for spinal fusion.
Introduction
Adipose-derived stem cells (ASCs) are multipotent adult stem cells derived from fat that have the ability to differentiate in vitro along a variety of lineage pathways, including bone, cartilage, adipose, neuronal-like, and muscle (reviewed in Ref.1). These cells exhibit a high expansion rate in vitro, and after several passages the population exhibits a low immunogenic profile as shown by low expression of MHC class II molecules and costimulatory molecules.2–4 When utilized as stimulator cells in a one-way mixed lymphocyte reaction (MLR), they do not stimulate a proliferative response from allogeneic T cells.2–6 Further, these cells actively inhibit MLR responses, indicating that they are immunosuppressive.2–6 These results suggest that ASCs have potential therapeutic use as allogeneic products for tissue engineering if they can maintain a nonimmunogenic and/or immunosuppressive profile after implantation in vivo.
To our knowledge, no studies have been published on the immunogenicity of allogeneic ASCs in vivo although a recent study showed that a xenogeneic graft of human ASCs placed in corneas of immunocompetent rabbits did not elicit an inflammatory response and persisted for at least 12 weeks.7 Immunosuppression by ASCs has been demonstrated in vivo by their ability to control graft-versus-host disease (GVHD) in a mouse model8 and in humans.9 In the current study, ASCs derived from ACI and Fischer strain rats were transplanted into immunocompetent Fischer strain recipients as part of a spinal fusion study. Analysis of spinal fusion, reported elsewhere,10 demonstrated that allogeneic ASCs accelerated spinal fusion equally to syngeneic ASCs, and both cell types resulted in a superior fusion product than was obtained with Scaffold only or No treatment groups. Further, at 4 weeks after surgery, inflammatory cell infiltrate was significantly lower in the fusion mass in both ASC cohorts versus scaffold alone. These results support the use of allogeneic ASCs for posterior lumbar fusion and suggest that an immune response was not initiated against these cells. In the study reported here, cellular and humoral immune responses to the implanted cells were evaluated in recipient rats to test the hypothesis that allogeneic ASCs would not be immunogenic in vivo.
Materials and Methods
Preparation of rat ASCs
Fischer and ACI strain rats were purchased from Harlan (Indianapolis, IN) under an approved IACUC protocol issued by the University of Maryland, Baltimore County, to Cognate BioServices. Inguinal fat pads were removed from a group of 10 animals and minced in a Petri dish in phosphate buffered saline (PBS; HyClone, Logan, UT) to approximately 1-mm pieces. The volume of tissue was measured with a pipette, and an equal amount of digestion medium containing 0.1% Collagenase Type I (Worthington Biochemical, Lakewood, NJ)+1% BSA (Sigma-Aldrich, St. Louis, MO) in PBS was added to the dish. The tissue was digested for 60 min at 37°C with gentle agitation. The contents of the dish were transferred to a centrifuge tube and centrifuged at 300 g for 5 min at room temperature. The fatty top layer and the supernatant were aspirated, and the stromal vascular fraction cell pellet was resuspended in the original tissue volume in complete stromal culture medium consisting of α modified Eagle's medium (α-MEM; Gibco, Grand Island, NY) supplemented with 10% screened fetal bovine serum (HyClone) and penicillin/streptomycin (Gibco).
The cells were plated at 0.1 mL tissue volume harvested/cm2 in T185 flasks. Flasks were incubated at 37°C in a humidified atmosphere containing 5% CO2. After 2 days, the medium containing nonadherent cells was aspirated and replaced with fresh medium. Medium replacement occurred every 3–4 days thereafter until adherent stromal cells became confluent (7–14 days). Adherent P0 cells were recovered from the plastic using prewarmed 0.25% trypsin (Gibco) for 5 min at 37°C. Fresh medium was added to inactivate trypsin, and the cells were washed and replated at 1.08×104/cm2. Generally, cells were passaged every week as they became confluent. By passage 4–5, ASCs were harvested and cryopreserved in FBS containing 10% DMSO (Edwards Life Sciences, Irvine, CA). Most of the frozen vials of Fischer and ACI rat ASCs were shipped to Pennington Biomedical Research Center using LN2 dry shippers (CRYO-SHIP; Custom Biogenic Systems, Burnsville, MN), where they were stored before subsequent implantation into rats at Louisiana State University. The remaining vials were placed in cryostorage on site for flow characterization studies, MLR assays, and antibody binding assays.
Characterization of rat ASCs by flow cytometry
Flow cytometry was performed as described previously.11 Briefly, approximately 5×105 cells/tube were washed once in flow wash buffer (PBS containing 0.5% BSA and 0.1% sodium azide), resuspended in 100 μL blocking buffer (wash buffer with 25 μg/mL mouse IgG), and incubated for 10 min on ice. Fluorescence-labeled monoclonal antibodies (mAbs) were added at the amount specified by the vendor. Appropriate isotype controls were added to control tubes. Antibodies directed against the following antigens (catalog #) were purchased from BioLegend (San Diego, CA) unless otherwise indicated: CD45-PE (#202207), CD80-PE (#200205), CD86-PE (#200307), CD90-AlexaFluor647 (#202507), RT1A-PE (BD Pharmingen #559993), and RT1B-PE (BD Pharmingen #554929). All tubes were incubated on ice and protected from light for 30 min. The cells were washed once in wash buffer and fixed in 200 μL of 1% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA). A minimum of 10,000 events were acquired per antibody set on a Becton Dickinson FACSCaliber flow cytometer using CELLQuest acquisition software (Becton Dickinson, San Diego, CA). Data analysis was performed using Flow Jo analysis software (Tree Star, Ashland, OR).
Characterization of rat ASCs by the immunogenicity assay
One-way MLR was used to characterize the immunogenicity of ACI strain rat ASCs. Fischer rat cervical and mesenteric lymph nodes were pooled and processed into a single-cell suspension by mechanical dissociation against a cell strainer. These lymph node cells (LNCs) were used as responder cells in the MLR. Spleen cells from ACI or Fischer rats were similarly processed and used as positive and negative control stimulator cells, respectively; ASCs derived from ACI strain rats were used as the test cells. Stimulator cells were irradiated with 5000 rad γ radiation delivered by a Cesium 137 source (Isomedix, Inc., Gammator B, Parsippany, NJ) before plating. Cultures were performed in triplicate in 96-well flat-bottom microtiter plates with low evaporation lids (Corning, Corning, NY) in Iscove's modified Dulbecco's medium plus 10% FBS (HyClone) supplemented with nonessential amino acids, sodium pyruvate, 5.5×10−5 M 2-mercaptoethanol, and antibiotics/antimycotics (medium and supplemental reagents from Gibco). Responder cells were plated at 5×105 cells/well, and stimulator cells were plated at various numbers as indicated in the figures. Cultures were incubated at 37°C in a humidified atmosphere of 5% CO2 and pulsed on day 6 with 3H-thymidine (1 μCi/well; Amersham Biosciences, Piscataway, NJ). The cells were harvested approximately 18 h later using a 96-well plate harvester (Skatron Micro96 Cell Harvester; Molecular Devices, Sunnyvale, NY), and the incorporated 3H-thymidine was determined by scintillation counting (Microbeta Trilux Scintillation and Luminescence Counter; Wallac, Gaithersburg, MD). Data are expressed as counts per minute (CPM). Test populations of cells that induced a T cell proliferative response that was significantly greater than the response to autologous Fischer spleen cells were considered immunogenic.
Characterization of rat ASCs by the suppression assay
The one-way MLR assay described previously was used to evaluate suppression by ACI strain rat ASCs. Responder cells were plated at 5×105 cells/well, and stimulator spleen cells were plated at 2×105 cells/well. ACI strain rat ASCs were titrated into the MLR cultures at numbers ranging from 0 (MLR control response) to 25,000 cells/well (confluence). T cell proliferation was determined 7 days later as previously described. Suppression was calculated by the following formula: Percent Suppression = (1 − [(Test Cell+MLR CPM)/MLR CPM])×100.
Rat spinal fusion study
This study was performed at the School of Veterinary Medicine, Louisiana State University in accordance with institutional and National Institutes of Health regulations governing the treatment of vertebrate animals. Procedures were initiated after approval by the University Animal Care Committee. Briefly, Fischer and ACI rat strain ASCs (passage 4–5) were produced at Cognate BioServices as previously described, cryopreserved in liquid nitrogen, and shipped to Pennington Biomedical Research Center. Immediately before surgery, ASCs were transferred to Louisiana State University, thawed, washed, and resuspended in complete stromal culture medium (α-MEM containing 10% FBS plus antibiotic/antimycotic). ASCs were loaded onto 7×5×2 mm β-tricalcium phosphate (80%)/type I bovine collagen blocks (20%) (Vitoss; Orthovita, Malvern, PA) by capillary action at a concentration of 7500 cells/μL to give a cell density of 3.75×106 cells/scaffold (53,571 cells/mm3). The scaffolds were 88–92% porous with pore diameters ranging from 1 to 1000 μm; further details of the scaffold can be found elsewhere.12 Cell-loaded scaffolds were cultured at 37°C in a humidified atmosphere of 5% CO2 for 48 h to allow attachment of ASCs to the scaffold. A total of 56 male Fischer rats (10 weeks old) weighing 210+2.08 g (mean ± standard error of mean) were used in the study. Animals were randomly assigned to four different treatment cohorts after bilateral decortication of the L4 and L5 transverse processes (n = 14/cohort): (1) No treatment; (2) Scaffold only; (3) Syngeneic ASCs+Scaffold; or (4) Allogeneic ASCs+Scaffold. The surgical sites were lavaged with physiological saline, and scaffolds (with or without cells) were placed on the dorsal aspect of the L4 and L5 transverse processes on each side of the spine and adjacent to the vertebral bodies. Half of each cohort was sacrificed 4 or 8 weeks after surgery to evaluate spinal fusion and to harvest tissues and blood for immunology assays. Additional details relating to the experimental design of this study as well as the spinal fusion results have been published in a separate article.10
Evaluation of recipient rat sensitization to allogeneic ASCs: T cell responses to ACI alloantigens
Fischer rat T cell activation to ACI strain alloantigens was evaluated by the one-way MLR assay. At the time of sacrifice, spleens and lymph nodes (cervical, axillary, brachial, inguinal, popliteal, and lumbar) from each rat were aseptically removed, placed in vials containing PBS, and shipped on cold packs to Cognate BioServices for processing and evaluation. Tissues were processed within 24 h of harvest. Lymph nodes from each rat were pooled and processed into a single-cell suspension. These LNCs were used as responder cells in the MLR. Spleen cells from untreated ACI or Fischer rats were similarly processed and used as stimulator cells. Stimulator cells were irradiated with 5000 rad γ radiation before plating. Cultures were performed in triplicate in 96-well flat-bottom microtiter plates in the complete Iscove's modified Dulbecco's medium described previously. Responder cells were plated at 3×105 cells/well, and spleen cell stimulators were plated at 2×105 cells/well. Control cultures consisting of responder cells cultured in medium alone or with 5 μg/mL Concanavalin A (Con A; Sigma, St. Louis, MO) were also included in the assay. Replicate culture plates were prepared for harvesting on days 3 and 7 of culture. Cultures were incubated at 37°C in a humidified atmosphere of 5% CO2 and pulsed on days 2 or 6 with 3H-thymidine as described previously. The cells were harvested approximately 18 h later, and the incorporated 3H-thymidine was determined by scintillation counting. Results are reported as ΔCPM that reflects the degree of T cell proliferation to ACI alloantigens. These numbers were derived by subtraction of the syngeneic response (CPM due to syngeneic Fischer spleen cells) from the allogeneic response (CPM due to allogeneic ACI spleen cells). Mean ΔCPM responses were compared between control and treatment groups at the 4 and 8 week time points.
Evaluation of recipient rat sensitization to allogeneic ASCs: antibody responses
Titering/isotyping studies
Serum antibody binding to ACI strain ASCs was evaluated by flow cytometry. Blood was obtained from each rat by cardiac puncture at sacrifice and allowed to clot for serum processing. Serum was placed in vials and shipped on cold packs to Cognate BioServices for evaluation. To evaluate antibody binding to ASCs, cells were washed in flow wash buffer (PBS containing 0.5% BSA and 0.1% sodium azide) and resuspended at 3×106 cells/mL. Aliquots of 80 μL of cells (2.4×105 cells) were distributed into 5 mL round-bottom tubes, and 20 μL of undiluted or diluted rat serum was added to each tube to give multiple dilutions of serum for evaluation of antibody titer. Tubes were incubated on ice for 30 min, washed twice with wash buffer, and resuspended in 100 μL of FACS buffer containing 10 μg/mL of RPE-conjugated goat antirat IgG/IgM secondary antibody (SouthernBiotech, Birmingham, AL). For isotyping studies, FITC-conjugated goat secondary antibodies to rat IgG or IgM were used (SouthernBiotech). The suspensions were incubated in the dark on ice for 30 min, washed, and fixed in 200 μL of 1% paraformaldehyde for a minimum of 15 min before acquisition on the flow cytometer. A minimum of 10,000 events was acquired for flow cytometry analysis. Mean fluorescence intensities (MFI) were determined for each serum using CellQuest software.
Cytotoxicity studies
To determine cytotoxicity of sera in the presence of complement, 20 μL undiluted serum was added to 80 μL ACI ASCs (2.4×106 cells) in PBS containing 0.5% BSA. Tubes were incubated on ice for 45 min, washed once with PBS-0.5% BSA, and Low-Tox® guinea pig complement (Cedarlane Laboratories, Ontario, Canada) was added at a final 1:20 dilution. After incubation at 37°C for 45 min, cell viability was determined by adding propidium iodide solution (Fluka BioChemika; Sigma) to the remaining cells (1 μL/tube). The percentage of dead cells that stained positive for propidium iodide was determined using a Becton Dickinson FACSCaliber flow cytometer. A positive control tube was included in the assay that utilized mouse anti-CD90 monoclonal antibody (1 μg/tube, BD Pharmingen) that bound to greater than 90% of the ASCs. A negative control tube contained cells plus complement.
FBS ELISA
To detect antibodies to FBS, FBS was adsorbed on 96-well Nunc MaxiSorp ELISA plates (Nalge Nunc International, Rochester, NY) at 10 μg/mL in carbonate buffer (pH 9.6) at 4°C for 24 h. The plates were washed five times with PBS containing 0.05% Tween 20 (PBS-T). Rat sera were serially diluted twofold down the plate in duplicate wells starting from 1:160 and ending at 1:20,480. After 3 h incubation at room temperature, the plates were washed with PBS-T, and a secondary HRP-conjugated goat antirat IgG antibody (SouthernBiotech) was added at a 1:4000 dilution. After an additional incubation for 3 h at room temperature, the plates were washed and TMB substrate (Kirkegaard and Perry, Rockville, MD) was added. Plates were read on a VERSAmax microplate reader (Molecular Devices, Sunnyvale, CA) approximately 20 min later at a wavelength of 450 nm.
Statistical analysis
The two-tailed Student's t-test was used to determine statistical significance between group means. Significant results (p < 0.05) are denoted on figures by an asterisk.
Results
Immunologic properties of rat ASCs—in vitro studies
Phenotype of ACI rat ASCs
ACI strain rat ASCs (P5), used as allogeneic source cells in this study, were phenotyped for immunologically relevant cell surface markers by flow cytometry. The results (Fig. 1A) indicate that the ASCs were positive for MHC class I major histocompatibility molecules and the stromal marker CD90; these cells were weakly positive for the costimulatory molecule CD80. Rat ASCs did not express MHC class II markers, the CD45 hematopoietic lineage marker, or the CD86 costimulatory molecule.
Fig. 1.
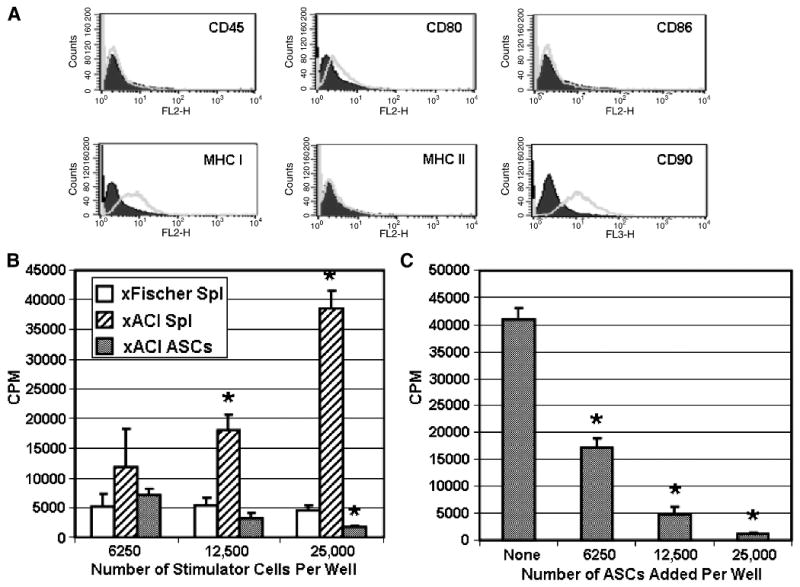
Immunologic characteristics of ACI rat strain ASCs. Passage 5 ASCs were evaluated by flow cytometry (A) for cell surface markers indicated on each histogram. The immunogenicity experiment in (B) shows the T cell proliferative response of Fischer strain LNCs (5×105 cells/well) to increasing numbers of syngeneic Fischer spleen cells and allogeneic ACI spleen cells and ASCs. Results are shown as the mean ± standard deviation of triplicate culture wells. Significant differences from the background response to syngeneic Fischer spleen cells are denoted with an asterisk (p < 0.05). The suppression experiment in (C) shows the effect of adding increasing numbers of ASCs to one-way MLR cultures consisting of Fischer responder cells (5×105 cells/well) and ACI spleen cells (2×105 cells/well). Results are shown as the mean ± standard deviation of triplicate culture wells. Significant differences from the MLR response (no ASCs added) are denoted with an asterisk (p < 0.05).
Immunogenicity
The one-way MLR assay was used to determine the immunogenicity of ACI rat ASCs in the context of the Fischer immune system. Fischer strain LNCs, obtained by pooling cervical and mesenteric lymph nodes from one rat, were used as the responder population. The LNCs were cultured with three different doses of irradiated stimulator cells: ACI strain ASCs, ACI spleen cells (positive control), or Fischer spleen cells (background response). The highest dose of ASCs (25,000 cells/well) corresponds to the number of cells required to produce a confluent monolayer in a microtiter well. As shown in Figure 1B, the Fischer T cell response to any dose of ACI ASCs was not significantly higher than the response to syngeneic spleen cells, indicating that the ASCs were not immunogenic. The assay was working properly as shown by the vigorous response of T cells to the ACI spleen cells at the two highest doses.
Suppression
ACI rat ASCs were added at various numbers to MLR cultures to determine suppression. As shown in Figure 1C, ASCs suppressed the MLR response by 58%, 88%, and 97% at doses of 6250 ASCs/well (80:1 responder:suppressor ratio), 12,500 ASCs/well (40:1 ratio), and 25,000 ASCs/well (20:1 ratio), respectively. These results demonstrate that ACI rat ASCs were highly suppressive for allogeneic responses mediated by Fischer strain T cells.
Immune responses to ACI rat ASCs in vivo
The phenotypic, immunogenicity, and suppression data generated by in vitro studies predicted that allogeneic rat ASCs would not induce an immune response in vivo. To test this hypothesis, we examined the cellular and humoral immune responses of immunocompetent Fischer rats that received allogeneic ACI ASCs in a spinal fusion model. Five to seven rats per group were analyzed for cellular studies, and seven to eight rats per group were analyzed for humoral studies.
Cellular immune responses
Recipient Fischer rat LNCs from each of the four groups in the spinal fusion model were cultured in medium, with the T cell mitogen Con A, with syngeneic spleen cells, or with allogeneic spleen cells. Responses are shown at 4 and 8 weeks after treatment, and the proliferative responses were assessed at 3 and 7 days to determine the kinetics of the response. The response to Con A was the exception with evaluation at 3 days only since this is when the peak response is known to occur.
The mean proliferative responses of LNCs to allogeneic ACI strain rat spleen cells are shown in Figure 2A. By two different criteria, the data indicate that implantation of ACI strain ASCs into Fischer rats did not sensitize the rats to respond to ACI alloantigens. First, the kinetics of the MLR response demonstrate a primary response to ACI alloantigens, not the rapid secondary response that would be expected to occur if the animals were well primed (the MLR response at 3 days was always lower than the response at 7 days). Second, the magnitude of the response to ACI spleen cells was not significantly different between any of the four groups at 4 weeks or at 8 weeks (p > 0.05). Of interest, however, was the relatively diminished allogeneic response by the group treated with ACI ASCs at 8 weeks compared to the other three groups (35–70% lower). This may be due to the statistically significant decline in the response of the ACI ASC–treated group to ACI spleen cells between 4 and 8 weeks (p ≤ 0.05, Fig. 2B). Whereas individual rat responses were variable at 4 weeks, the responses were clustered at background control levels by 8 weeks.
Fig. 2.
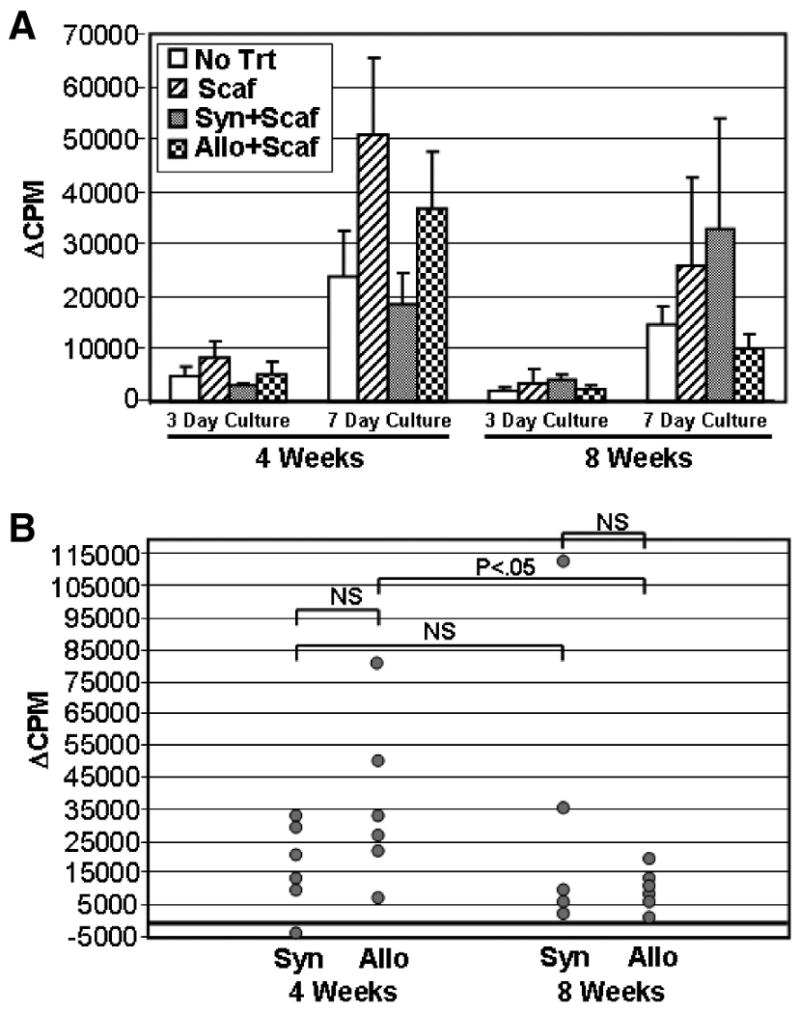
Group and individual rat T cell proliferative responses to ACI spleen cells. Lymph node cells (LNCs) were removed from recipient rats at 4 or 8 weeks after implantation with no carrier or cells (No Trt), scaffold only (Scaf), syngeneic Fischer ASCs plus scaffold (Syn+Scaf), or allogeneic ACI ASCs plus scaffold (Allo+Scaf). LNCs (3×105 cells/well) were stimulated with ACI or Fischer spleen cells (2×105 cells/well), and cultures were harvested at 3 or 7 days as indicated. Data are reported as ΔCPM that reflect that background proliferative responses to Fischer spleen cells have been subtracted from the proliferative responses to ACI spleen cells. The mean group response ± standard error of the mean is shown in (A). Individual rat responses from the Syn+Scaf and Allo+Scaf groups are shown in (B). All responses are from cultures harvested at 7 days. The significance of differences between groups are shown by brackets. NS, not significant.
The response to Con A was not significantly different (p > 0.05) between the four groups at 4 weeks or at 8 weeks (Fig. 3). We conclude that implantation of scaffold, with or without syngeneic or allogeneic ASCs, did not affect the broad T cell response to this mitogen. Although the magnitude of the Con A response declined in most of the groups between 4 and 8 weeks, the difference was not statistically significant (p > 0.05).
Fig. 3.
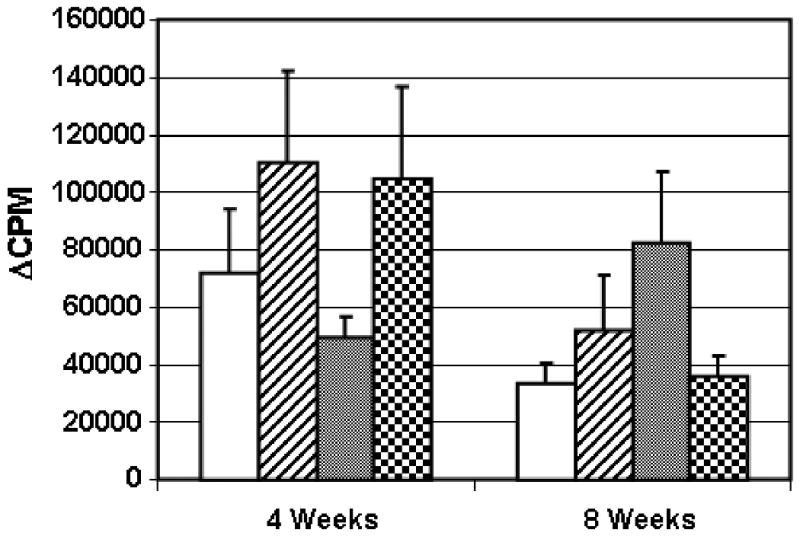
Group responses to stimulation with Con A. Groups corresponding to each bar in the graph are described in Figure 2A and in the Figure 2 legend. LNCs (3×105 cells/well) obtained from individual rats at 4 or 8 weeks were stimulated with Con A and harvested at 3 days. The response of the cells cultured in medium has been subtracted from the Con A–stimulated response (ΔCPM). The mean group response ± standard error of the mean is shown. There were no significant differences between treatment groups at either time point.
The background proliferative responses of LNCs derived from scaffold-implanted animals (with or without ASCs) were significantly elevated when compared to untreated rats when cells were cultured in medium (Fig. 4A) or with syngeneic spleen cells (Fig. 4B). This response was observed in 7 day cultures, but not in 3 day cultures, suggesting that it developed in culture. A likely explanation for this effect is that it is a response to FBS because the scaffold (with or without ASCs) was incubated in medium containing FBS before implantation. Thus, LNCs obtained from rats sensitized to FBS produced a quantitatively higher response when restimulated in culture medium containing FBS than their untreated controls. There was not a significant difference within any of the groups between LNCs harvested at 4 versus 8 weeks after treatment (p > 0.05).
Fig. 4.
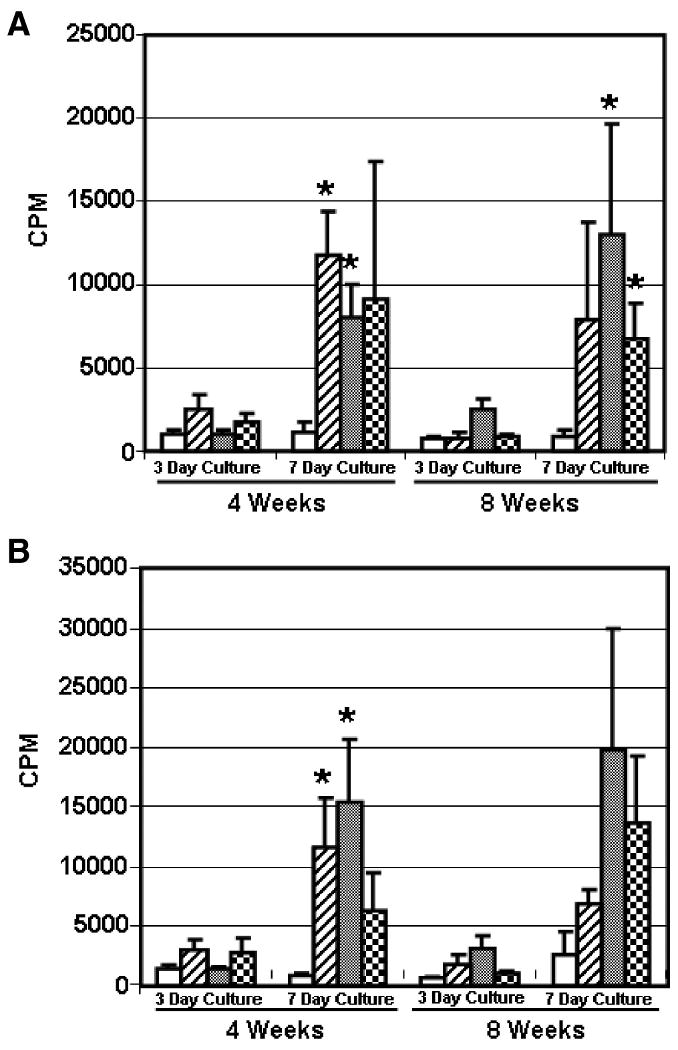
Group responses of recipient Fischer rat LNCs to control stimuli. Groups are described in Figure 2 and the Figure 2 legend. LNCs (3×105 cells/well) obtained from individual rats at 4 or 8 weeks were cultured in medium alone (A) or with syngeneic Fischer strain spleen cells (2×105 cells/well, B). The cultures were harvested at 3 or 7 days as indicated. Proliferative responses are designated as CPM because background responses were not subtracted. The mean group response ± standard error of the mean is shown. Significant differences from the No Trt background response are denoted with an asterisk (p < 0.05).
Humoral immune responses
Serum antibody binding to ACI strain ASCs was determined at 4 and 8 weeks by flow cytometry. The average mean fluorescence intensity (MFI) of serum antibody binding to ACI ASCs for each of the four groups in the spinal fusion model is shown in Figure 5A. The results demonstrate that significant antibody responses were induced in the groups of rats treated with syngeneic or allogeneic ASCs, with the highest response in the allogeneic group. The MFI for the allogeneic group was approximately threefold greater than the MFI for the syngeneic group at 4 weeks and twofold greater at 8 weeks.
Fig. 5.
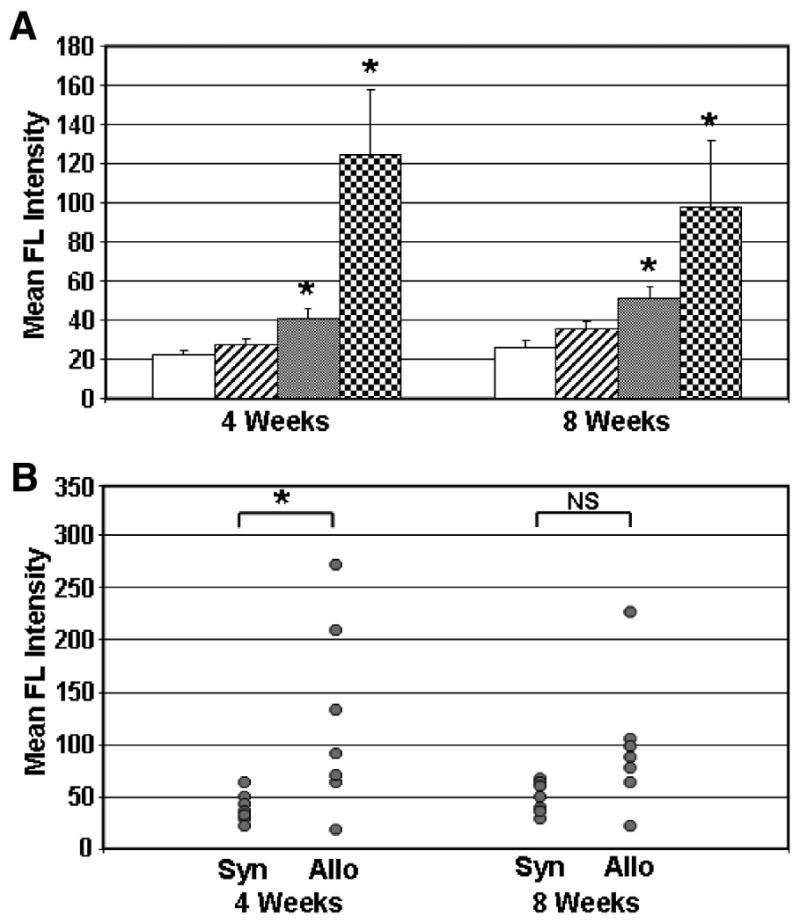
Serum antibody binding to ACI strain rat ASCs. Mean group serum antibody responses of recipient Fischer rats at 4 and 8 weeks postimplantation are shown in (A). Groups are described in Figure 2. The mean group MFI response ± standard error of the mean is shown. Significant differences from the No Trt background response are denoted with an asterisk (p < 0.05). Individual rat serum antibody responses to ACI strain rat ASCs are shown in (B). Antibody responses from individual rats are shown for the Syn+Scaf and Allo+Scaf groups at 4 and 8 weeks postimplantation. The significance of differences between groups is shown by brackets. NS, not significant.
Individual responses of rats in the syngeneic and allogeneic ASC-treated groups are shown in Figure 5B. Although the difference between these groups is significant at 4 weeks (p < 0.05), it is not significant at 8 weeks due to increased MFI in the syngeneic group and lower MFI in the allogeneic group. At each of the 4 and 8 week time points, four out of seven rats (57%) treated with allogeneic ASCs produced a significant antibody response with an MFI that was greater than or equal to the MFI of the syngeneic group antibody response plus 2 standard deviations (approximate 95% confidence interval by the empirical rule).
The antibody response to ACI ASCs in the Allogeneic ASCs+Scaffold group was characterized for isotype, cytotoxicity, and reactivity to FBS proteins. After treating ACI ASCs with sera from Allogeneic ASCs+Scaffold group or control No treatment rats, FITC-conjugated isotype-specific secondary antibodies were added to distinguish IgM from IgG antibodies bound to the cells. As shown in Figure 6A, no secondary antibody binding to IgM on ACI ASCs was detected above control levels at a 1:5 serum dilution, whereas secondary antibody to IgG detected serum antibodies in the ASC-treated rats even at a 1:500 dilution. There was not a significant difference between antibody titers at 4 and 8 weeks. When complement was added to mixtures of serum and ASCs, no cytotoxicity against the ASCs was observed (Fig. 6B). Anti-CD90 mAb, which binds to more than 90% of the ASCs and served as a positive control for the assay, reduced viability significantly in the presence of complement, indicating that the assay system was working appropriately. Finally, we tested sera for reactivity to FBS proteins by ELISA. As shown in Figure 6C, antibody activity to FBS was consistently higher in sera obtained from rats that were treated with scaffold alone or with cells plus scaffold in comparison to untreated rats. The antibody titer to FBS appears to be similar in all three groups of rats because the dilution curves overlap over a wide range of serum dilutions.
Fig. 6.
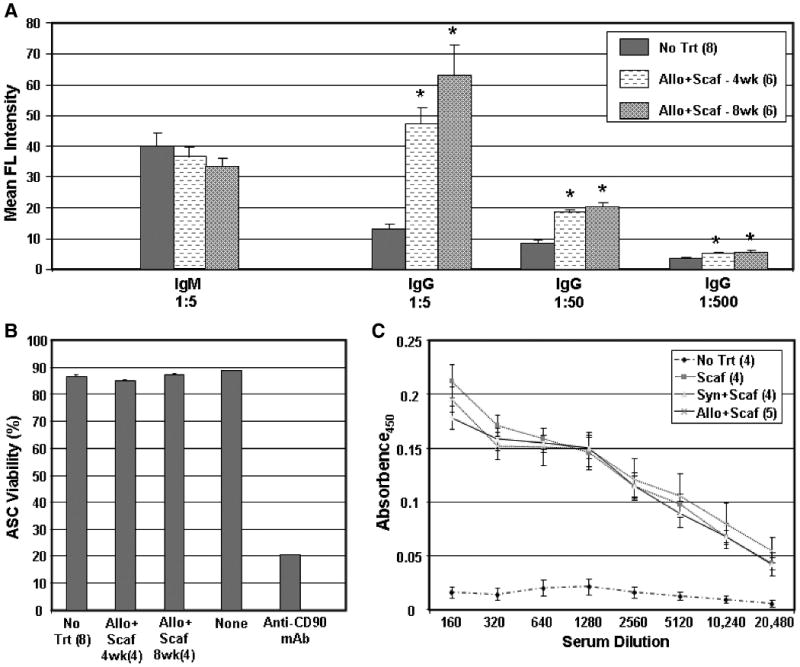
Characteristics of the humoral response. The isotype and titer of the antibody response to ACI ASCs at 4 and 8 weeks is shown in (A) for No Trt and Allo+Scaf–treated rats. Rat sera were diluted as indicated, allowed to bind to ASCs and labeled with FITC-conjugated goat anti-IgM or IgG secondary antibodies before evaluation by flow cytometry. Significant differences from No Trt controls are denoted by an asterisk (p < 0.05). Sera from the same groups of rats were evaluated for cytotoxicity against ASC target cells (B). After allowing sera to bind ASCs, guinea pig complement was added, and cell viability was determined by propidium iodide staining and flow cytometry. As controls, ASCs were incubated with complement only (“None”) or with anti-CD90 mAb (positive control). The specificity of rat sera for FBS is shown in (C). Rat sera from all four groups in the spinal fusion study were serially diluted and evaluated for binding to FBS by ELISA. In all panels, the number of rat sera tested per group is shown in parentheses, and group means are shown ± standard error.
Discussion
The current immunology study is complementary to a study that was performed to determine the relative efficacy of syngeneic and allogeneic ASCs to promote spinal fusion. The results of this complementary study, published elsewhere,10 showed that callus formation was more mature in the ASC-treated animals than in animals treated with scaffold alone. Both syngeneic and allogeneic ASCs accelerated spinal fusion, and inflammatory cell infiltration in the fusion mass was significantly lower in ASC-treated animals than scaffold alone. Hence, it was surmised that a deleterious immune response was not initiated against the allogeneic ASCs that interfered with acceleration of spinal fusion by these cells. The purpose of the current study was to determine whether we could detect either a cellular or humoral response against the allogeneic ASCs implanted in the recipient rats.
Results from the current study demonstrated that ASCs derived from rats were nonimmunogenic and immunosuppressive in vitro. Further, LNCs obtained from animals treated with allogeneic ASCs were no more responsive to donor alloantigen restimulation in vitro than LNCs obtained from untreated control animals, scaffold-treated animals, or animals treated with syngeneic ASCs. Although these results suggested that allogeneic ASCs were nonimmunogenic in vivo, antibodies were detected in the peripheral blood of host animals to donor ASCs, indicating that the ASCs elicited a humoral immune response. Characterization of the response showed that the antibodies were IgG (not IgM) and noncytotoxic in the presence of complement, and they expressed specificity for FBS antigens.
ASCs were produced from inguinal fat derived from two different strains of rats for these studies. In phenotypic studies of immunologically relevant cell surface markers, rat ASCs showed expression of MHC class I antigens and low expression of CD80 costimulatory molecules, similar to what has been previously reported for human ASCs;11 however, this differs from what has been reported for bone marrow–derived mesenchymal stem cells (BMSCs) that do not express CD80.13,14 In studies of immune function assessed by in vitro assays, rat ASCs demonstrated a nonimmunogenic and immunosuppressive profile for alloreactive immune responses, similar to reports on adult-derived human ASCs2–6 and BMSCs,13–16 as well as MSC-like cells derived from fetal tissues such as amnion,17 liver,18 and placenta.19 BMSCs from a variety of nonhuman species have been demonstrated to be nonimmunogenic and/or suppressive in vitro, including mice,20 rats,21 rabbits,22 pigs,23,24 and baboons.25 Of particular interest to a universal cell approach for enhancement of spinal fusion, BMSCs have been shown to retain their nonimmunogenic and/or suppressive properties in vitro after differentiation down the osteogenic lineage.3,13,22,23
Based on our in vitro studies showing a nonimmunogenic and suppressive profile for rat ASCs, transplanted ASCs would not be expected to induce an immune response after transplantation to an immunocompetent allogeneic host. As part of a study comparing the efficacy of syngeneic and allogeneic ASCs in a rat spinal fusion model, we evaluated recipients for cellular immunity to donor alloantigens at 4 and 8 weeks after cell transplantation. By determining two parameters of the restimulation response, magnitude and temporal kinetics, we concluded that lymph node T cells from Fischer rats transplanted with allogeneic ACI strain ASCs were not primed to ACI alloantigens in vivo. Although this type of analysis has not been previously reported for ASCs, similar results have been reported for BMSCs in a rat stroke model26 and in baboons.27 Other studies involving BMSCs have shown conflicting results. A sixfold increase in MLR activity against donor alloantigens was observed in peripheral blood T cells in swine that received intracardiac transplantation of allogeneic BMSCs.24 In mice, administration of allogeneic BMSCs has been shown to activate T cells to reject matched bone marrow.28 In another model, BMSCs transduced with erythropoietin induced their own demise by activating host T cells and natural killer cells.29 Finally, there are conflicting results as to whether BMSCs maintain their immunomodulatory properties in vivo. BMSCs have been reported to suppress GVHD in humans30 and allograft rejection in baboons25 and rats,31 but others have reported no suppressive effect of these cells on GVHD20,32 or allograft rejection.21 Although few studies have been performed with ASCs, they have been shown to exhibit suppression in a mouse model of GVHD8 and in human GVHD.9
Immune tolerance or a hyporesponsive state was not observed in the current study, but it is interesting to note that the T cell response to donor spleen cells at 8 weeks was uniformly and significantly lower than the response at 4 weeks, showing a trend with time of decreasing responsiveness to donor alloantigens. Because this response was not significantly lower than the baseline response in untreated rats, we cannot conclude that the T cells were tolerant or hyporesponsive due to suppression, clonal deletion, or other means. In a previous baboon study, intravenous administration of BMSCs resulted in T cell hyporesponsiveness that was observed at 8 weeks (but not at 4 weeks) after injection.27 The ability of BMSCs to induce tolerance has been reported in vitro33 as well as in vivo,34–37 but opposing results have been reported as well.13,28,29 Further study is needed to determine whether BMSCs and ASCs can induce unresponsiveness in vivo and, if so, the mechanism of induction.
A significant IgG antibody response, with a titer of approximately 1:500, was produced to ACI strain ASCs by recipient Fischer rats in our study. Although a portion of the antibody activity could be attributed to binding FBS proteins, there was a significant difference between syngeneic and allogeneic groups, which would suggest that a significant proportion of the antibody response was directed against ACI alloantigens. We were unable to demonstrate any cytotoxicity of these antibodies against ACI ASC target cells in the presence of complement. Antibody responses to allogeneic BMSCs have been demonstrated previously in pigs24 and baboons.27 BMSCs injected subcutaneously or into infarcted myocardium in pigs induced antibodies that were cytotoxic in the presence of complement. In the baboon study, recipient animals produced antibodies in response to a single intravenous injection of allogeneic BMSCs or after an intramuscular boost. Survival of BMSCs was more closely associated with T cell hyporesponsiveness than alloantibody titer in this model. The current results contrast with a previous study in a rat stroke model where no antibodies were produced to allogeneic BMSCs that were injected intravenously, suggesting an effect by cell type (BMSC vs. ASC) and/or route of administration in rats.26
Evidence of immune sensitization to FBS antigens appeared at both the cellular and humoral levels in all three groups of animals that were exposed to FBS on scaffold alone (Scaffold only) or scaffold plus cells (Syngeneic ASCs+Scaffold group and Allogeneic ASCs+Scaffold group). At the cellular level, T cells from all three groups produced high background proliferative responses in culture medium containing FBS that developed with time; that is, they were low or not present at a 3-day harvest but were demonstrable at 7 days. No elevated response was seen in the No treatment group. At the humoral level, anti-FBS antibodies were detected in the sera of rats implanted with scaffold or scaffold plus cells, but not in the control No treatment group. Immune responses to FBS carried over with BMSCs have been reported by other investigators38 and our experience appears to corroborate their results. Further, the scaffold used in this study has been reported to induce an inflammatory response.12 We cannot rule out the possibility that the response was due to the scaffold itself or that the scaffold exacerbated the response to FBS. In future studies, it would be prudent to avoid or reduce the use of FBS in expanding stem cells for transplantation as well as utilize noninflammatory scaffolds with reduced/nonexposure to FBS to minimize the immunogenicity of allogeneic ASCs and BMSCs in vivo. The inclusion of a Scaffold only group, not preincubated in FBS, would also sort out the effect of the scaffold in the induction of T cell responses.
The results from the current immunology study, showing the absence of a cellular T cell response coupled with a relatively low-titered, noncytotoxic humoral response, support the in vivo results presented in a companion paper that indicated equal efficacy of syngeneic and allogeneic ASCs to accelerate spinal fusion.10 The sum of these results supports the conclusion that the immune system does not negatively affect the ability of allogeneic ASCs to mediate tissue generation in vivo. Further, our data support the concept that ASCs have potential therapeutic use as allogeneic products to replace or restore damaged tissues.
Acknowledgments
This work was supported in part by funding from the Pennington Biomedical Research Foundation (J.M.G.), the NIAMS (R41AR052542) (M.J.L., K.R.M., and J.M.G.), and the CNRU Center Grant # 1P30 DK072476 titled “Nutritional Programming: Environmental and Molecular Interactions” sponsored by NIDDK (J.M.G.).
Footnotes
Disclosure Statement: K.R.M. and J.N.B. are employed by Cognate BioServices, Inc., which is owned by Toucan Capital. Toucan has licensed intellectual property to develop ASCs for tissue regeneration.
J.M.G. is a paid consultant for Toucan Capital.
No competing financial interests exist for M.J.L., N.D.S., and P.A.A.
References
- 1.Schaffler A, Buchler C. Concise review: adipose tissue-derived stromal cells—basic and clinical implications for novel cell-based therapies. Stem Cells. 2007;25:818. doi: 10.1634/stemcells.2006-0589. [DOI] [PubMed] [Google Scholar]
- 2.McIntosh K, Zvonic S, Garrett S, Mitchell JB, Floyd ZE, Hammill L, Kloster A, Di Halvorsen Y, Ting JP, Storms RW, Goh B, Kilroy G, Wu X, Gimble JM. The immunogenicity of human adipose derived cells: temporal changes in vitro. Stem Cells. 2006;24:1246. doi: 10.1634/stemcells.2005-0235. [DOI] [PubMed] [Google Scholar]
- 3.Niemeyer P, Kornacker M, Mehlorn A, Seckinger A, Vohrer J, Schmal H, Kasten P, Eckstein V, Südkamp NP, Krause U. Comparison of immunological properties of bone marrow stromal cells and adipose tissue-derived stem cells before and after osteogenic differentiation in vitro. Tissue Eng. 2007;13:111. doi: 10.1089/ten.2006.0114. [DOI] [PubMed] [Google Scholar]
- 4.Hoogduijn MJ, Crop MJ, Peeters AM, Van Osch GJ, Balk AH, Ijzermans JN, Weimar W, Baan CC. Human heart, spleen, and perirenal fat-derived mesenchymal stem cells have immunomodulatory capacities. Stem Cells Dev. 2007;16:597. doi: 10.1089/scd.2006.0110. [DOI] [PubMed] [Google Scholar]
- 5.Puissant B, Barreau C, Bourin P, Clavel C, Corre J, Bousquet C, Taureau C, Cousin B, Abbal M, Laharrague P, Penicaud L, Casteilla L, Blancher A. Immunomodulatory effect of human adipose tissue-derived adult stem cells: comparison with bone marrow mesenchymal stem cells. Br J Haematol. 2005;129:118. doi: 10.1111/j.1365-2141.2005.05409.x. [DOI] [PubMed] [Google Scholar]
- 6.Cui L, Yin S, Liu W, Li N, Zhang W, Cao Y. Expanded adipose-derived stem cells suppress mixed lymphocyte reaction by secretion of prostaglandin E2. Tissue Eng. 2007;13:1185. doi: 10.1089/ten.2006.0315. [DOI] [PubMed] [Google Scholar]
- 7.Arnalich-Montiel F, Pastor S, Blazquez-Martinez A, Fernandez-Delgado J, Nistal M, Alio JL, De Miguel MP. Adipose-derived stem cells are a source for cell therapy of the corneal stroma. Stem Cells. 2008;26:570. doi: 10.1634/stemcells.2007-0653. [DOI] [PubMed] [Google Scholar]
- 8.Yañez R, Lamana ML, García-Castro J, Colmenero I, Ramírez M, Bueren JA. Adipose tissue-derived mesenchymal stem cells have in vivo immunosuppressive properties applicable for the control of the graft-versus-host disease. Stem Cells. 2006;24:2582. doi: 10.1634/stemcells.2006-0228. [DOI] [PubMed] [Google Scholar]
- 9.Fang B, Song Y, Liao L, Zhang Y, Zhao RC. Favorable response to human adipose tissue-derived mesenchymal stem cells in steroid-refractory acute graft-versus-host disease. Transplant Proc. 2007;39:3358. doi: 10.1016/j.transproceed.2007.08.103. [DOI] [PubMed] [Google Scholar]
- 10.Lopez MJ, McIntosh KR, Spencer ND, Borneman JN, Horswell R, Anderson P, Yu G, Gaschen L, Gimble JM. Acceleration of spinal fusion using syngeneic and allogeneic adult adipose derived stem cells in a rat model. J Orthop Res. 2009;27:366. doi: 10.1002/jor.20735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitchell JB, McIntosh K, Zvonic S, Garrett S, Floyd ZE, Kloster A, Di Halvorsen Y, Storms RW, Goh B, Kilroy G, Wu X, Gimble JM. The immunophenotype of human adipose derived cells: temporal changes in stromal- and stem cell-associated markers. Stem Cells. 2006;24:376. doi: 10.1634/stemcells.2005-0234. [DOI] [PubMed] [Google Scholar]
- 12.Hing KA, Wilson LF, Buckland T. Comparative performance of three ceramic bone graft substitutes. Spine J. 2007;7:475. doi: 10.1016/j.spinee.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 13.Klyushnenkova E, Mosca JD, Zernetkina V, Majumdar MK, Beggs KJ, Simonetti DW, Deans RJ, McIntosh KR. T cell responses to allogeneic human mesenchymal stem cells: immunogenicity, tolerance, and suppression. J Biomed Sci. 2005;12:47. doi: 10.1007/s11373-004-8183-7. [DOI] [PubMed] [Google Scholar]
- 14.Tse WT, Pendleton JD, Bever WM, Egalka MC, Guinan EC. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation. 2003;75:389. doi: 10.1097/01.TP.0000045055.63901.A9. [DOI] [PubMed] [Google Scholar]
- 15.Le Blanc K, Ringdén O. Immunomodulation by mesenchymal stem cells and clinical experience. J Intern Med. 2007;262:509. doi: 10.1111/j.1365-2796.2007.01844.x. [DOI] [PubMed] [Google Scholar]
- 16.Xu G, Zhang L, Ren G, Yuan Z, Zhang Y, Zhao RC, Shi Y. Immunosuppressive properties of cloned bone marrow mesenchymal stem cells. Cell Res. 2007;17:240. doi: 10.1038/cr.2007.4. [DOI] [PubMed] [Google Scholar]
- 17.Wolbank S, Peterbauer A, Fahrner M, Hennerbichler S, van Griensven M, Stadler G, Redl H, Gabriel C. Dose-dependent immunomodulatory effect of human stem cells from amniotic membrane: a comparison with human mesenchymal stem cells from adipose tissue. Tissue Eng. 2007;13:1173. doi: 10.1089/ten.2006.0313. [DOI] [PubMed] [Google Scholar]
- 18.Gőtherstrőm C, Ringdén O, Tammik C, Zetterberg E, Westgren M, Le Blanc K. Immunologic properties of human fetal mesenchymal stem cells. Am J Obstet Gynecol. 2004;190:239. doi: 10.1016/j.ajog.2003.07.022. [DOI] [PubMed] [Google Scholar]
- 19.Chang CJ, Yen ML, Chen YC, Chien CC, Huang HI, Bai CH, Yen BL. Placenta-derived multipotent cells exhibit immunosuppressive properties that are enhanced in the presence of interferon-gamma. Stem Cells. 2006;24:2466. doi: 10.1634/stemcells.2006-0071. [DOI] [PubMed] [Google Scholar]
- 20.Sudres M, Norol F, Trenado A, Grégoire S, Charlotte F, Levacher B, Lataillade JJ, Bourin P, Holy X, Vernant JP, Klatzmann D, Cohen JL. Bone marrow mesenchymal stem cells suppress lymphocyte proliferation in vitro but fail to prevent graft-versus-host disease in mice. J Immunol. 2006;176:7761. doi: 10.4049/jimmunol.176.12.7761. [DOI] [PubMed] [Google Scholar]
- 21.Inoue S, Popp FC, Koehl GE, Piso P, Schlitt HJ, Geissler EK, Dahlke MH. Immunomodulatory effects of mesenchymal stem cells in a rat organ transplant model. Transplantation. 2006;81:1589. doi: 10.1097/01.tp.0000209919.90630.7b. [DOI] [PubMed] [Google Scholar]
- 22.Liu H, Kemeny DM, Heng BC, Liu H, Kemeny DM, Heng BC, Ouyang HW, Melendez AJ, Cao T. The immunogenicity and immunomodulatory function of osteogenic cells differentiated from mesenchymal stem cells. J Immunol. 2006;176:2864. doi: 10.4049/jimmunol.176.5.2864. [DOI] [PubMed] [Google Scholar]
- 23.Wang L, Lu XF, Lu YR, Liu J, Gao K, Zeng YZ, Li SF, Li YP, Cheng JQ, Tan WD, Wan L. Immunogenicity and immune modulation of osteogenic differentiated mesenchymal stem cells from Banna minipig inbred line. Transplant Proc. 2006;38:2267. doi: 10.1016/j.transproceed.2006.06.048. [DOI] [PubMed] [Google Scholar]
- 24.Poncelet AJ, Vercruysse J, Saliez A, Gianello P. Although pig allogeneic mesenchymal stem cells are not immunogenic in vitro, intracardiac injection elicits an immune response in vivo. Transplantation. 2007;83:783. doi: 10.1097/01.tp.0000258649.23081.a3. [DOI] [PubMed] [Google Scholar]
- 25.Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh KR, Patil S, Hardy W, Devine S, Ucker D, Deans R, Moseley A, Hoffman R. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30:42. doi: 10.1016/s0301-472x(01)00769-x. [DOI] [PubMed] [Google Scholar]
- 26.Li Y, McIntosh K, Chen J, Zhang C, Gao Q, Borneman J, Raginski K, Mitchell J, Shen L, Zhang J, Lu D, Chopp M. Allogeneic bone marrow stromal cells promote glial-axonal remodeling without immunologic sensitization after stroke in rats. Exp Neurol. 2006;198:313. doi: 10.1016/j.expneurol.2005.11.029. [DOI] [PubMed] [Google Scholar]
- 27.Beggs KJ, Lyubimov A, Borneman JN, Bartholomew A, Moseley A, Dodds R, Archambault MP, Smith AK, McIntosh KR. Immunologic consequences of multiple, high-dose administration of allogeneic mesenchymal stem cells to baboons. Cell Transplant. 2006;15:711. doi: 10.3727/000000006783981503. [DOI] [PubMed] [Google Scholar]
- 28.Nauta AJ, Westerhuis G, Kruisselbrink AW, Lurvink EG, Willemze R, Fibbe WE. Donor-derived mesenchymal stem cells are immunogenic in an allogeneic host and stimulate donor graft rejection in a nonmyeloblative setting. Blood. 2006;108:2114. doi: 10.1182/blood-2005-11-011650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eliopoulos N, Stagg J, Lejeune L, Pommey S, Galipeau J. Allogeneic marrow stromal cells are immune rejected by MHC class I- and class II-mismatched recipient mice. Blood. 2005;106:4057. doi: 10.1182/blood-2005-03-1004. [DOI] [PubMed] [Google Scholar]
- 30.Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, Lanino E, Sundberg B, Bernardo ME, Remberger M, Dini G, Egeler RM, Bacigalupo A, Fibbe W, Ringdén O. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371:1579. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- 31.Chabannes D, Hill M, Merieau E, Rossignol J, Brion R, Soulillou JP, Anegon I, Cuturi MC. A role for heme oxygenase-1 in the immunosuppressive effect of adult rat and human mesenchymal stem cells. Blood. 2007;110:3691. doi: 10.1182/blood-2007-02-075481. [DOI] [PubMed] [Google Scholar]
- 32.Min CK, Kim BG, Park G, Cho B, Oh IH. IL-10-transduced bone marrow mesenchymal stem cells can attenuate the severity of acute graft-versus-host disease after experimental allogeneic stem cell transplantation. Bone Marrow Transplant. 2007;39:637. doi: 10.1038/sj.bmt.1705644. [DOI] [PubMed] [Google Scholar]
- 33.Beyth S, Borovsky Z, Mevorach D, Liebergall M, Gazit Z, Aslan H, Galun E, Rachmilewitz J. Human mesenchymal stem cells alter antigen-presenting cell maturation and induce T-cell unresponsiveness. Blood. 2005;105:2214. doi: 10.1182/blood-2004-07-2921. [DOI] [PubMed] [Google Scholar]
- 34.Zappia E, Casazza S, Pedemonte E, Benvenuto F, Bonanni I, Gerdoni E, Giunti D, Ceravolo A, Cazzanti F, Frassoni F, Mancardi G, Uccelli A. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T cell anergy. Blood. 2005;106:1755. doi: 10.1182/blood-2005-04-1496. [DOI] [PubMed] [Google Scholar]
- 35.Gerdoni E, Gallo B, Casazza S, Musio S, Bonanni I, Pedemonte E, Mantegazza R, Frassoni F, Mancardi G, Pedotti R, Uccelli A. Mesenchymal stem cells effectively modulate pathogenic immune response in experimental autoimmune encephalomyelitis. Transplantation. 2007;83:783. doi: 10.1002/ana.21076. [DOI] [PubMed] [Google Scholar]
- 36.Augello A, Tasso R, Negrini SM, Cancedda R, Pennesi G. Cell therapy using allogeneic bone marrow mesenchymal stem cells prevents tissue damage in collagen-induced arthritis. Arthritis Rheum. 2007;56:1175. doi: 10.1002/art.22511. [DOI] [PubMed] [Google Scholar]
- 37.Itakura S, Asari S, Rawson J, Ito T, Todorov I, Liu CP, Sasaki N, Kandeel F, Mullen Y. Mesenchymal stem cells facilitate the induction of mixed hematopoietic chimerism and islet allograft tolerance without GVHD in the rat. Am J Transplant. 2007;7:336. doi: 10.1111/j.1600-6143.2006.01643.x. [DOI] [PubMed] [Google Scholar]
- 38.Spees JL, Gregory CA, Singh H, Tucker HA, Peister A, Lynch PJ, Hsu SC, Smith J, Prockop DJ. Internalized antigens must be removed to prepare hypoimmunogenic mesenchymal stem cells for cell and gene therapy. Mol Ther. 2004;9:747. doi: 10.1016/j.ymthe.2004.02.012. [DOI] [PubMed] [Google Scholar]


