Abstract
Aberrant activation of the immune system has been implicated in an increasingly large number of disease states and can influence cognition, mood, and memory. There is a long and controversial history of reports of immune activation associated with schizophrenia. In this study, we measured mitogen-stimulated cytokine levels serially in 100 medication-stabilized continuously ill subjects with schizophrenia and compared and contrasted them with mitogen-stimulated cytokine levels from 51 normal volunteers. The subjects with schizophrenia had consistently higher mitogen-stimulated IL-2 levels and lower IL-6 levels than the normal volunteers. These effects could not be explained by medications, smoking, or other clinical variables. We conclude that continuously symptomatic medication-stabilized subjects with schizophrenia have a mitogen-stimulated cytokine expression pattern that is suggestive of ongoing immune activation.
Keywords: schizophrenia, cytokine production, TH-1 immune activation, longitudinal analysis, medication effects
INTRODUCTION
There is a resurgence of interest in the role that the immune system might play in the pathogenesis of schizophrenia (Potvin et al., 2008). Some of the impetus for this renewed revival is reports that immune activation occurs in disorders ranging from peptic ulcer disease to cardiovascular and neurological disorders. A second stimulus of this renewed curiosity are preclinical findings demonstrating that cytokines mediate metabolic pathways for dopamine, serotonin, norepinephrine, and N-methyl-D aspartate (NMDA) (Dunn, 2006; Müller and Schwarz, 2006a; Müller and Schwarz, 2007). Müller and colleagues postulate that an imbalance between T-helper-cell type 1 (TH-1) and T-helper-cell type 2 (TH-2) activation leads to the production of kynurenic acid (KYN-A), an endogenous NMDA receptor antagonist, and quinolinic acid, an endogenous NMDA receptor agonist which in high doses is a neurotoxin (Müller and Schwarz, 2006a; Müller et al., 2004; Stone and Behan, 2007). They conclude that acutely ill patients with schizophrenia have TH-2 immune activation that resolves with treatment (Müller and Schwarz, 2006b). In general, studies of mitogen-stimulated cytokine levels from acutely ill individuals with schizophrenia report decreased interluken-2 (IL-2) production which could be interpreted as being consistent with the aforementioned hypothesis (Arolt et al., 2000; Bessler et al., 1995; Cazzullo et al., 2002; Cazzullo et al., 2001; Ganguli et al., 1995; Ganguli et al., 1989; Kim et al., 2001; Kim et al., 2004; Potvin et al., 2008; Rothermundt et al., 2000; Villemain et al., 1987).
Our group consistently has reported that patients with schizophrenia have increased serum levels of the soluble IL-2 receptor (sIL-2r). We have postulated that this is a subgroup effect and have demonstrated that this finding is present in medication naive, acutely ill, and chronically ill individuals, as well as individuals with schizophrenia from different ethnic groups (Rapaport et al., 1997; Rapaport and Lohr, 1994; Rapaport et al., 1994; Rapaport et al., 1993; Rapaport et al., 1991b; Rapaport et al., 1989). Increased serum sIL-2r levels are more commonly observed with immune activation (Rubin et al., 1990). In an attempt to extend our work and clarify findings from other groups, we have characterized a large cohort of continuously ill, medically stable patients with schizophrenia. We were interested in determining whether repeated sampling would find a consistent pattern of cytokine production from mitogen-stimulated leukocyte cultures. We postulated that this group of continuously ill subjects with schizophrenia would have an increase in mitogen-stimulated cytokine production favoring a TH-1 immune response. We further postulated that these findings would be stable over 6 weeks.
METHODS
Subjects
The study was approved by the Institutional Review Boards of the Veterans Affairs Medical Center, San Diego, and the University of California at San Diego. All subjects signed written informed consent to participate in the study. Subjects consisted of 100 patients who met the DSM-IV (American Psychiatric Association, 1994) diagnostic criteria for schizophrenia (male/female = 72/28; mean age = 41.1 ± 8.2 years) and 51 healthy control subjects (male/female = 41/10; mean age = 34.5 ± 11.4 years). Schizophrenic subjects were outpatients without any other current Axis I diagnosis receiving stable doses of medications. Healthy control subjects were recruited by advertisement concurrent with subjects with schizophrenia. Control subjects were non-smokers, medication-free, had never taken psychotropic medications nor had a mental disorder. A Structured Clinical Interview (DSM-IV) was performed by a trained clinician on all subjects (First, 1997). Symptom severity of subjects with schizophrenia was evaluated using the Positive and Negative Symptoms Scale (PANSS) (Kay et al., 1987). All subjects had a complete medical history and physical examination including a complete blood count, chemistry panel, urine toxicology screen and urinalysis. Subjects with acute medical or immunological disorders or positive urine toxicology screens were excluded.
Blood was drawn between 9:00 AM and 12:00 PM. Control subjects had blood drawn at one time point while subjects with schizophrenia had blood samples collected at baseline and again 6 weeks later (41 ± 1 days). Pharmacotherapy was held constant over the span of the study
In vitro cytokine production analysis
Immediately after collection, peripheral blood mononuclear cells (PBMCs) were isolated from heparinized peripheral blood using commercially available Ficoll-Paque density gradients (Pharmacia, Bridgewater, NJ). The number of viable cells was determined by a trypan blue exclusion assay. The PBMCs were resuspended in RPMI-1640 media at a concentration of 106 cells/ml, containing 1unit/ml penicillin, 1ug/ml streptomycin sulfate, 2 mM glutamate, 10 mM HEPES buffer, and 10% heat-inactivated fetal bovine serum. Cells were cultured with 10 ug/ml phytohemagglutinin (PHA) (Sigma-Aldrich, St. Louis, MO) at 37°C and 5% CO2. The cell-free supernatant was collected 48 hours later and frozen in aliquots at −80°C. Supernatant from PHA-free cell cultures served as a negative control.
Cytokine concentrations were determined using a sandwich ELISA using antibody (IL-2, IL-6, IL-10, IFN-γ) and standards purchased from Endogen (Rockford, IL). Standard curve concentrations were 35–1500 pg/ml for IL-2 assays, 10–2000 pg/ml for IL-6, 15–600 pg/ml for IL-10, and 25–1000 pg/ml for IFN-γ. The sensitivity of all assays is < 5 pg/ml and the intra- and inter-assay coefficients of variation are < 10%. Determinations of cytokine levels were performed in a masked manner, in duplicate with all subject’s samples assayed at the same time.
Statistical analysis
Demographic characteristics for schizophrenic subjects and normal volunteers were analyzed by chi-square tests for discrete variables or t-tests for continuous variables. Continuous data not normally distributed as assessed by a Kolmogrov-Smirnov test were log transformed prior to analysis. Mixed model regressions were used to compare cytokine concentrations between groups (patients with schizophrenia or control subjects) and visit number, while controlling for the demographic covariates of age, gender, and race, with the random effects of each subject over time and an unstructured covariance. Where a difference existed between schizophrenic and control subjects, separate mixed effect modeling was conducted within the group of subjects with schizophrenia for each cytokine with additional fixed predictor variables of PANSS total scores, duration of illness, sub-type of schizophrenia, types and dosages of pharmacotherapy, presence of comorbidities, comorbid medications, BMI, and smoking status. For all analysis, significant differences were declared if p < .05. Data are presented as means ± standard deviations (SD) or percent frequency where appropriate. All analysis was performed using SAS statistical software (version 9.1, SAS Institutes, Cary, NC).
RESULTS
Table 1 presents the demographic characteristics of the subjects. While there was no difference in gender, subjects with schizophrenia were significantly older (t = 3.72, 78 df, p < .01) and had a different racial distribution, with a greater proportion of subjects being of Caucasian background (χ2 = 10.1, 4 df, p = .04). However, neither age nor race were significant predictors in any subsequent analysis.
Table 1.
Demographic data as means ± SD or percentages.
| Control (n = 51) |
Schizophrenia (n = 100) |
|
|---|---|---|
| Age, years | 34.5 ± 11.4 | 41.1 ± 8.2* |
| Male | 80% | 72% |
| Ethnicity | ||
| Caucasian | 43% | 71%* |
| African-American | 22% | 11% |
| Hispanic | 14% | 12% |
| Asian | 12% | 4% |
| Other | 10% | 2% |
Significantly different from controls (p < .05).
Subjects with schizophrenia met criteria for the following subtypes: paranoid type (n = 53), disorganized type (n = 5), catatonic type (n = 4), undifferentiated type (n = 6), residual type (n = 3), or NOS (n = 29). The average age of onset was 21.3 ± 0.7 years and the mean duration of illness was 19.8 ± 1.0 years. Neuroleptic medication was not standardized and pharmacologic treatment was individualized. Subjects with schizophrenia were treated with typical antipsychotic medications (n = 31), atypical medications (n = 40) or a combination therapy (n = 29) with an average daily medication dosage of 1057 ± 942 mg of chlorpromazine equivalence units. Some of the subjects with schizophrenia were also receiving anticholinergic medications (n = 66), antidepressant medications (n = 24), mood stabilizers (n = 27), or benzodiazepines (n = 10). The subjects with schizophrenia had medical comorbidities of hypertension (n = 32), asthma (n = 14), chronic heartburn (n = 8), hypothyroidism (n = 5), hyperlipidemia (n = 5), or diabetes (n = 1). While all control subjects were non-smokers, 72% of the subjects with schizophrenia were current smokers. Over the 6 weeks of the study, subjects with schizophrenia had a statistical, but not clinically significant, decrease in total PANSS scores with a score of 82.1 ± 18.2 at visit 1 and 78.1 ± 17.6 at visit 2 (t = 2.64, 98 df, p = .01).
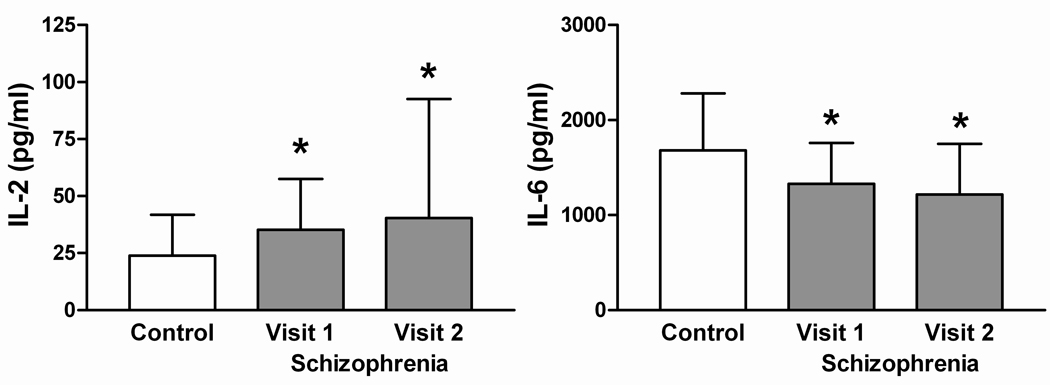
As demonstrated in table 2, subjects with schizophrenia had significantly higher mitogen-stimulated IL-2 levels (F = 23.5, 1,149 df, p < .01), lower IL-6 levels (F = 17.2, 1,149 df, p < .01), but no difference in IL-10 (F = 1.9, 1,149 df, p = .17) or IFN-γ (F = 1.0, 1,149 df, p = .31) levels when compared with normal volunteers. Over the 6 weeks of the study, cytokine production levels remained stable in subjects with schizophrenia for IL-2 (F = 0.2, 1,149 df, p = .67), IL-10 (F = 1.1, 1,149 df, p = .29) and IFN-γ (F = 2.1, 1,149 df, p = .15) while IL-6 production decreased slightly (F = 5.9, 1,149 df, p = .02; figure 1).
Table 2.
Group means ± SD of measured cytokine levels.
| Controls | Subjects with Schizophrenia | ||||||
|---|---|---|---|---|---|---|---|
| All | Smokers | Non-Smokers | |||||
| (n = 51) | (n = 100) | (n = 69) | (n = 31) | ||||
| Visit 1 | Visit 1 | Visit 2 | Visit 1 | Visit 2 | Visit 1 | Visit 2 | |
| IL-2 | 23.8 ± 17.9 | 35.3 ± 22.3* | 40.3 ± 52.2* | 33.7 ± 18.1* | 42.2 ± 61.5* | 38.7 ± 29.7* | 36.1 ± 19.3* |
| Il-6 | 1680.8 ± 600.5 | 1327.7 ± 431.8* | 1216.5 ± 532.8* | 1299.9 ± 441.8* | 1173.4 ± 555.7* | 1389.6 ± 408.6* | 1312.5 ± 472.4* |
| IL-10 | 388.6 ± 288.2 | 309.3 ± 240.2 | 312.2 ± 295.6 | 286.2 ± 247.1 | 279.0 ± 280.9 | 360.9 ± 219.3 | 386.0 ± 318.3 |
| IFN-g | 59.4 ± 106.4 | 77.3 ± 163.8 | 46.9 ± 91.1 | 76.5 ± 163.4 | 55.2 ± 106.0 | 78.9 ± 167.3 | 28.5 ± 37.4 |
Significantly different from controls (p < .05).
Figure 1.
In vitro cytokine levels. Means ± SD. *Significantly different from controls (p < .05).
Additional analysis was then performed to discern biological confounds that might explain the differences in IL-2 and IL-6 noted between controls and subjects with schizophrenia. Mixed model regressions were performed for IL-2 and IL-6 levels with PANSS-total scores, duration and sub-type of schizophrenia illness, types and dosages of pharmacotherapy, presence of comorbidities, BMI, and smoking status as fixed predictor variables in the model. In vitro IL-2 levels were found to be significantly higher in those subjects with schizophrenia treated for hyperlipidemia (F = 10.1, 1,61 df, p < .01) and significantly lower in those subjects treated for hypothyroidism (F = 5.6, 1,61 df, p = .02) compared to subjects with schizophrenia not taking these types of medications. In vitro IL-6 levels were found to be inversely correlated with PANSS total scores (F = 5.7, 1,61 df, p = .02); a 1 unit increase in total PANSS scores corresponds to a 5.4 unit decrease in IL-6 production levels. However, anticholinergic medication usage was associated with a significant increase in IL-6 levels. Subjects with schizophrenia taking anticholinergic medications had significantly higher IL-6 levels (n = 65; visit 1: 1419 ± 382; visit 2: 1302 ± 470 pg/ml) than those not taking anticholinergic agents (n = 35; visit 1: 1150 ± 471; visit 2: 1050 ± 611 pg/ml; F = 8.8, 1,61 df, p = .01). Aside from these findings, there was no significant effect of the other variables investigated.
As a confirmatory analysis of the lack of significance regarding the effects of smoking status on these cytokine measures, secondary analysis was performed to examine the differences between controls and non-smoking schizophrenics (Table 2). Results from mixed model regressions found no difference between groups in IL-10 (F = 0.3, 1,80 df, p = .59) or IFN-γ levels (F = 0.7, 1,80 df, p = .42) while controls had significantly lower mitogen-stimulated IL-2 levels than non-smoking subjects with schizophrenia of IL-2 (F = 21.4, 1,80 df, p < .01) and significantly higher mitogen-stimulated IL-6 levels than non-smoking subjects with schizophrenia (F = 5.7, 1,80 df, p = .02). Control subjects also had no difference in levels of IL-10 (F = 3.7, 1,118 df, p = .06) or IFN-γ (F = 0.9, 1,118 df, p = .35) than subjects with schizophrenia that smoked, but had lower mitogen-stimulated IL-2 levels than smoking subjects with schizophrenia of IL-2 (F = 17.0, 1,118 df, p < .01) and significantly higher mitogen-stimulated IL-6 levels (F = 16.0, 1,118 df, p < .01).
DISCUSSION
This study is the largest investigation of serial mitogen-stimulated cytokine levels ever performed in a well characterized cohort of continuously ill outpatients with schizophrenia. We observed increased mitogen-stimulated IL-2 production and decreased mitogen-stimulated IL-6 production but did not find consistent, statistically significant alterations in mitogen-stimulated IL-10 or IFN-γ levels. It is interesting to note that the general pattern of mitogen-stimulated leukocyte cytokine production is more consistent with TH-1-mediated response since IL-6 and IL-10 trend downward and IL-2 and IFN-γ in general, trend upward. Our second important finding was that mitogen stimulated cytokine production was relatively constant across the two time points in this sample. This suggests that immune dysfunction might represent a biomarker that could define a subset of patients with schizophrenia. Our preliminary analysis of the relationship between PANSS scores and cytokine levels suggested an inverse relationship between severity of illness and IL-6 production.
Although speculative, our findings may explain a previous paper we published demonstrating that augmentation with celecoxib, a cyclooxygenase-2 (Cox-2) inhibitor, was ineffective in continuously ill medication-stabilized patients with schizophrenia (Rapaport et al., 2005). Since a major function of selective COX-2 inhibitors is to decrease levels of prostaglandin-E2 (PGE2), and PGE2 has been demonstrated to be responsible for increasing IL-1 and Il-6 levels, this lack of responsiveness to COX-2 inhibitor augmentation is not surprising (Müller and Schwarz, 2008; Stolina et al., 2000).
Because of concerns about the role that medications, medical comorbidities, subtype of schizophrenia, smoking, age, and sex might have on our results, we performed mixed effect longitudinal analyses which take into account these factors. Our findings could not be explained by any of these potential confounding factors. The literature studying the effects of antipsychotic medication on immune parameters in psychiatric patients and normal volunteers is limited and inconclusive. Most studies investigate the effects of initiating antipsychotic medications in acutely ill, hospitalized patients. The majority of these small studies measure serum cytokine and/or cytokine receptor levels at admission and then periodically for up to three months. Comparing and contrasting the acute effects of haloperidol and clozapine can serve as an illustration of the complexity of the extant literature. Acute in vivo exposure to haloperidol does not effect serum cytokine and cytokine receptors, while acute exposure to clozapine therapy increases serum levels of TNF-α, the TNF-α receptor and sIL-2r (Haack et al., 1999; Maes et al., 2000; Maes et al., 1997; Monteleone et al., 1997; Müller et al., 1997; Pollmacher et al., 1997; Pollmacher et al., 1996; Rapaport et al., 1991a). This example reminds us that the acute effects of antipsychotic medication may be dramatically different. Thus, the conclusions of the recently published meta-analysis by Potvin and colleagues (2008) suggesting that serum sIL-2r elevations and decreased mitogen-stimulated IL-2 production may be medication mediated, need to be tempered by considerations about potential individual differences in the effect of antipsychotic medications on immune function as well as potential differences between the acute versus chronic effects of medications. Another factor that confounds our understanding of the literature is the inability to discern the differences between the acute effects of medication on immune function versus the impact that acute treatment has on mitigating effects of an acute exacerbation of psychosis and the subsequent stress of hospitalization has on immune function.
As discussed above, our data indicate that the class of antipsychotic medication, use of combinations of typical and atypical antipsychotic medications, anticonvulsant medication augmentation, and antidepressant medication augmentation do not account for alterations in mitogen-stimulated cytokine levels. However, secondary analyses did reveal that schizophrenic subjects who received anticholinergic medication had higher mitogen-stimulated IL-6 production than schizophrenic subjects who were not receiving anticholinergic medication. (Both groups had lower supernatant IL-6 levels than the normal control sample.) This intriguing preliminary finding is consistent with newly emerging animal studies. Preclinical work investigating pancreatitis and endotoxin-induced inflammation and shock suggest that nicotinic cholinergic activity has anti-inflammatory effects that are reversed by anticholinergic medication. In fact, pretreatment with anticholinergic compounds enhance IL-6 production in rodent models of inflammation (Giebelen et al., 2007; van Westerloo et al., 2006; Matsunaga et al., 2001). Since all medications were held constant during the course of this study, a change in anticholinergic medication usage does not explain the 100 pg/ml difference in IL-6 levels between the two sampling time points.
The association between statin use for hyperlipidemia and increased mitogen-stimulated IL-2 production is intriguing for two reasons. First, since we did not find a relationship between BMI and IL-2 production, this finding is unlikely to be an artifact of a lipocyte-mediated proinflammatory cytokine production (Wisse, 2004). Secondly, statins are known to decrease proinflammatory cytokine production and so an increase in mitogen-stimulated IL-2 production may be thought to be a counter-intuitive result (Cheng et al., 2008; Nissen et al., 2005). As is the case with our finding that thyroid replacement therapy is associated with lower mitogen-stimulated IL-2 production, these secondary analyses must be replicated in prospective data sets.
The reasons why our findings differ from other reports in the literature fall into two broad categories: differences in study population and differences in assay methodology. We deliberately chose to study continuously ill individuals who are stable but minimally responsive to current pharmacotherapy. An advantage of studying this group is that our findings are not confounded by the stress of an acute psychotic exacerbation (Arolt et al., 2000; Hornberg et al., 1995; Maes et al., 1994; Rothermundt et al., 2000; Villemain et al., 1987). Some investigators have postulated that acutely ill subjects with schizophrenia have such profound activation of the immune system that it leads to an “exhaustion” epiphenomenon….i.e., since the leukocytes are already activated, they respond less robustly to mitogen challenge than leukocytes from normal volunteers subjected to mitogen challenge (Arolt et al., 2000; Hornberg et al., 1995; Maes et al., 1994; Rothermundt et al., 2000; Villemain et al., 1987). Since we were interested in collecting a representative “real world” sample, our subjects were medically stable, but treated with a wide array of psychotropic medications. This is distinctly different than most published studies that control for medication. Another difference between our study and most of the other ones is that our subjects were outpatients rather than inpatients. Thus, this sample is different from most studies in the published literature and one we believe is more likely to include subjects with chronic immune activation.
There are two important differences in assay methodology between our study and some of the other published works in the literature. We investigated mitogen-stimulated cytokine production employing purified leukocytes rather than a whole blood assay (Arolt et al., 2000; Bessler et al., 1995; Cazzullo et al., 2002; De Groote et al., 1992; Ganguli et al., 1995; Kim et al., 1998; O'Donnell et al., 1996; Rothermundt et al., 1998; Wilke et al., 1996). A technical advantage to this approach is that one has greater precision across assays since absolute leukocyte numbers are actually counted rather than estimated from CBC results, as is the standard practice with whole blood assays. A second methodological difference between our study and many previous studies is that we chose to employ a single mitogen - PHA - rather than a combination of mitogens (Arolt et al., 2000; Hornberg et al., 1995; Maes et al., 1997; Rothermundt et al., 2000; Wilke et al., 1996). There are advantages to both approaches, but evaluating a single mitogen is simpler and enhances precision since one does not need to assure lot consistency of two mitogens. Furthermore, interpreting data about the interactions between two mitogens on leukocyte function is more complex than is frequently appreciated in the psychiatry literature.
We acknowledge that investigating complex syndromes like schizophrenia by measuring peripheral markers of immune function may not be the most exciting approach, however, data clearly demonstrate that activation of the peripheral immune system has profound effects on cognition, appetite, sleep, mood, anxiety, and psychosis (Dunn, 2006; McAfoose and Baune, 2009; Müller and Schwarz, 2006a; Myint et al., 2009). Cytokine interactions in the brain effect neurotransmitters and neurohormones involved in learning, memory and emotions (Dunn, 2006; McAfoose and Baune, 2009; Myint et al., 2009). Müller and colleagues have synthesized disparate literature and developed a cogent hypothesis that proposes immune activation stimulates the kynurenine pathway and shunts tryptophan away from production of serotonin and toward the production of kynurenine that can be metabolized to KYN-A by astrocytes or quinolinic acid by microglia (Müller and Schwarz, 2006a; Müller et al., 2004). As discussed previously, KYN-A is a naturally occurring NMDA-receptor antagonist and alpha-7 nicotinic receptor antagonist while quinolinic acid is a potent NMDA-receptor agonist. They postulate that certain forms of schizophrenia are the result of immune activation that favors a TH-2 response and increases the catabolism of kynurenine by typtophan 2,3-dioxygenase to KYN-A (Müller and Schwarz, 2006a). Although our findings do not directly support this postulate, our work is consistent with observations that immune activation may be associated both with treatment-resistance and a more chronic course of illness (Maes et al., 2000; Narayan et al., 2008; Zhang et al., 2005).
In conclusion, data from 100 well-characterized, chronically ill patients with schizophrenia who were serially sampled, suggest there is an increase in mitogen-stimulated IL-2 production and a concomitant decrease in mitogen-stimulated IL-6 production. These data require further exploration, particularly in light of newer findings suggesting that some patients with schizophrenia may have an immune mediated component to their illness.
ACKNOWLEDGEMENTS
This study was supported by funding through the National Alliance for Research on Schizophrenia and Depression, the National Institutes of Mental Health (R29-MH049746), and the Polier Endowed Chair in Schizophrenia and Related Disorders at Cedars-Sinai Medical Center.
Footnotes
STATEMENT OF INTEREST
Dr. Rapaport is a consultant to and has received honoraria for presentations from Cyberonics, Forrest Labs, Roche, Pfizer, Sanofi Synthelabo, Solvay, Sumitomo, Wyeth, GlaxoSmithKline, Janssen Pharmaceutica, Neurocrine Biosciences, Eli Lilly, and Novartis; has received research support from Astra Zeneca, Pfizer, Janssen Pharmaceutica, GlaxoSmithKine, Forrest Labs, Eli Lilly, Abbott Laboratories, Corcept Therapeutics, Cyberonics, Novartis, Pharmacia Upjohn, Sanofi Synthelabo, Solvay, Wyeth, and UCB Pharma; and is a stockholder in Forrest Labs. Ms. Bresee has no biomedical financial interests or potential conflicts of interest.
CITATIONS
- American Psychiatric Association. The diagnostic and statistical manual of mental disorders, DSM-IV. Washington, D.C: 1994. [Google Scholar]
- Arolt V, Rothermundt M, Wandinger KP, Kirchner H. Decreased in vitro production of interferon-gamma and interleukin-2 in whole blood of patients with schizophrenia during treatment. Mol Psychiatry. 2000;5:150–158. doi: 10.1038/sj.mp.4000650. [DOI] [PubMed] [Google Scholar]
- Bessler H, Levental Z, Karp L, Modai I, Djaldetti M, Weizman A. Cytokine production in drug-free and neuroleptic-treated schizophrenic patients. Biol Psychiatry. 1995;38:297–302. doi: 10.1016/0006-3223(94)00299-I. [DOI] [PubMed] [Google Scholar]
- Cazzullo CL, Sacchetti E, Galluzzo A, Panariello A, Adorni A, Pegoraro M, et al. Cytokine profiles in schizophrenic patients treated with risperidone: a 3-month follow-up study. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:33–39. doi: 10.1016/s0278-5846(01)00221-4. [DOI] [PubMed] [Google Scholar]
- Cazzullo CL, Sacchetti E, Galluzzo A, Panariello A, Colombo F, Zagliani A, et al. Cytokine profiles in drug-naive schizophrenic patients. Schizophr Res. 2001;47:293–298. doi: 10.1016/s0920-9964(00)00046-3. [DOI] [PubMed] [Google Scholar]
- Cheng SM, Lai JH, Yang SP, Tsao TP, Ho LJ, Liou JT, et al. Modulation of human T cells signaling transduction by lovastatin. Int J Cardiol. 2008 doi: 10.1016/j.ijcard.2008.10.044. [DOI] [PubMed] [Google Scholar]
- De Groote D, Zangerle PF, Gevaert Y, Fassotte MF, Beguin Y, Noizat-Pirenne F, et al. Direct stimulation of cytokines (IL-1 beta, TNF-alpha, IL-6, IL-2, IFN-gamma and GM-CSF) in whole blood. I. Comparison with isolated PBMC stimulation. Cytokine. 1992;4:239–248. doi: 10.1016/1043-4666(92)90062-v. [DOI] [PubMed] [Google Scholar]
- Dunn AJ. Effects of cytokines and infections on brain neurochemistry. Clin Neurosci Res. 2006;6:52–68. doi: 10.1016/j.cnr.2006.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB. Structured clinical interview for DSM-IV axis I disorders: SCID-I. Washington, DC: American Psychiatric Press; 1997. [Google Scholar]
- Ganguli R, Brar JS, Chengappa KR, DeLeo M, Yang ZW, Shurin G, et al. Mitogen-stimulated interleukin-2 production in never-medicated, first-episode schizophrenic patients. The influence of age at onset and negative symptoms. Arch Gen Psychiatry. 1995;52:668–672. doi: 10.1001/archpsyc.1995.03950200058014. [DOI] [PubMed] [Google Scholar]
- Ganguli R, Rabin BS, Belle SH. Decreased interleukin-2 production in schizophrenic patients. Biol Psychiatry. 1989;26:427–430. doi: 10.1016/0006-3223(89)90061-9. [DOI] [PubMed] [Google Scholar]
- Giebelen IA, van Westerloo DJ, LaRosa GJ, de Vos AF, van der Poll T. Local stimulation of alpha7 cholinergic receptors inhibits LPS-induced TNF-alpha release in the mouse lung. Shock. 2007;28:700–703. doi: 10.1097/shk.0b013e318054dd89. [DOI] [PubMed] [Google Scholar]
- Haack M, Hinze-Selch D, Fenzel T, Kraus T, Kuhn M, Schuld A, et al. Plasma levels of cytokines and soluble cytokine receptors in psychiatric patients upon hospital admission: effects of confounding factors and diagnosis. J Psychiatr Res. 1999;33:407–418. doi: 10.1016/s0022-3956(99)00021-7. [DOI] [PubMed] [Google Scholar]
- Hornberg M, Arolt V, Wilke I, Kruse A, Kirchner H. Production of interferons and lymphokines in leukocyte cultures of patients with schizophrenia. Schizophr Res. 1995;15:237–242. doi: 10.1016/0920-9964(94)00046-b. [DOI] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Kim DJ, Kim W, Yoon SJ, Go HJ, Choi BM, Jun TY, et al. Effect of risperidone on serum cytokines. Int J Neurosci. 2001;111:11–19. doi: 10.3109/00207450108986549. [DOI] [PubMed] [Google Scholar]
- Kim YK, Lee MS, Suh KY. Decreased interleukin-2 production in Korean schizophrenic patients. Biol Psychiatry. 1998;43:701–704. doi: 10.1016/s0006-3223(97)00357-0. [DOI] [PubMed] [Google Scholar]
- Kim YK, Myint AM, Lee BH, Han CS, Lee HJ, Kim DJ, et al. Th1, Th2 and Th3 cytokine alteration in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:1129–1134. doi: 10.1016/j.pnpbp.2004.05.047. [DOI] [PubMed] [Google Scholar]
- Maes M, Bocchio Chiavetto L, Bignotti S, Battisa Tura G, Pioli R, Boin F, et al. Effects of atypical antipsychotics on the inflammatory response system in schizophrenic patients resistant to treatment with typical neuroleptics. Eur Neuropsychopharmacol. 2000;10:119–124. doi: 10.1016/s0924-977x(99)00062-0. [DOI] [PubMed] [Google Scholar]
- Maes M, Bosmans E, Kenis G, De Jong R, Smith RS, Meltzer HY. In vivo immunomodulatory effects of clozapine in schizophrenia. Schizophr Res. 1997;26:221–225. doi: 10.1016/s0920-9964(97)00057-1. [DOI] [PubMed] [Google Scholar]
- Maes M, Meltzer HY, Bosmans E. Immune-inflammatory markers in schizophrenia: comparison to normal controls and effects of clozapine. Acta Psychiatr Scand. 1994;89:346–351. doi: 10.1111/j.1600-0447.1994.tb01527.x. [DOI] [PubMed] [Google Scholar]
- Matsunaga K, Klein TW, Friedman H, Yamamoto Y. Involvement of nicotinic acetylcholine receptors in suppression of antimicrobial activity and cytokine responses of alveolar macrophages to Legionella pneumophila infection by nicotine. J Immunol. 2001;167:6518–6524. doi: 10.4049/jimmunol.167.11.6518. [DOI] [PubMed] [Google Scholar]
- McAfoose J, Baune BT. Evidence for a cytokine model of cognitive function. Neurosci Biobehav Rev. 2009;33:355–366. doi: 10.1016/j.neubiorev.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Monteleone P, Fabrazzo M, Tortorella A, Maj M. Plasma levels of interleukin-6 and tumor necrosis factor alpha in chronic schizophrenia: effects of clozapine treatment. Psychiatry Res. 1997;71:11–17. doi: 10.1016/s0165-1781(97)00036-x. [DOI] [PubMed] [Google Scholar]
- Müller N, Empl M, Riedel M, Schwarz M, Ackenheil M. Neuroleptic treatment increases soluble IL-2 receptors and decreases soluble IL-6 receptors in schizophrenia. Eur Arch Psychiatry Clin Neurosci. 1997;247:308–313. doi: 10.1007/BF02922260. [DOI] [PubMed] [Google Scholar]
- Müller N, Schwarz M. Schizophrenia as an inflammation-mediated dysbalance of glutamatergic neurotransmission. Neurotox Res. 2006a;10:131–148. doi: 10.1007/BF03033242. [DOI] [PubMed] [Google Scholar]
- Müller N, Schwarz MJ. Neuroimmune-endocrine crosstalk in schizophrenia and mood disorders. Expert Rev Neurother. 2006b;6:1017–1038. doi: 10.1586/14737175.6.7.1017. [DOI] [PubMed] [Google Scholar]
- Müller N, Schwarz MJ. The immunological basis of glutamatergic disturbance in schizophrenia: towards an integrated view. J Neural Transm Suppl. 2007:269–280. doi: 10.1007/978-3-211-73574-9_33. [DOI] [PubMed] [Google Scholar]
- Müller N, Schwarz MJ. COX-2 inhibition in schizophrenia and major depression. Curr Pharm Des. 2008;14:1452–1465. doi: 10.2174/138161208784480243. [DOI] [PubMed] [Google Scholar]
- Müller N, Ulmschneider M, Scheppach C, Schwarz MJ, Ackenheil M, Moller HJ, et al. COX-2 inhibition as a treatment approach in schizophrenia: immunological considerations and clinical effects of celecoxib add-on therapy. Eur Arch Psychiatry Clin Neurosci. 2004;254:14–22. doi: 10.1007/s00406-004-0478-1. [DOI] [PubMed] [Google Scholar]
- Myint AM, Schwarz MJ, Steinbusch HW, Leonard BE. Neuropsychiatric disorders related to interferon and interleukins treatment. Metab Brain Dis. 2009 doi: 10.1007/s11011-008-9114-5. [DOI] [PubMed] [Google Scholar]
- Narayan S, Tang B, Head SR, Gilmartin TJ, Sutcliffe JG, Dean B, et al. Molecular profiles of schizophrenia in the CNS at different stages of illness. Brain Res. 2008 doi: 10.1016/j.brainres.2008.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissen SE, Tuzcu EM, Schoenhagen P, Crowe T, Sasiela WJ, Tsai J, et al. Statin therapy, LDL cholesterol, C-reactive protein, and coronary artery disease. N Engl J Med. 2005;352:29–38. doi: 10.1056/NEJMoa042000. [DOI] [PubMed] [Google Scholar]
- O'Donnell MC, Catts SV, Ward PB, Ward PB, Liebert B, Lloyd A, Wakefield D, et al. Increased production of interleukin-2 (IL-2) but not soluble interleukin-2 receptors (sIL-2R) in unmedicated patients with schizophrenia and schizophreniform disorder. Psychiatry Res. 1996;65:171–178. doi: 10.1016/s0165-1781(96)02824-7. [DOI] [PubMed] [Google Scholar]
- Pollmacher T, Hinze-Selch D, Fenzel T, Kraus T, Schuld A, Mullington J. Plasma levels of cytokines and soluble cytokine receptors during treatment with haloperidol. Am J Psychiatry. 1997;154:1763–1765. doi: 10.1176/ajp.154.12.1763. [DOI] [PubMed] [Google Scholar]
- Pollmacher T, Hinze-Selch D, Mullington J. Effects of clozapine on plasma cytokine and soluble cytokine receptor levels. J Clin Psychopharmacol. 1996;16:403–409. doi: 10.1097/00004714-199610000-00011. [DOI] [PubMed] [Google Scholar]
- Potvin S, Stip E, Sepehry AA, Gendron A, Bah R, Kouassi E. Inflammatory cytokine alterations in schizophrenia: a systematic quantitative review. Biol Psychiatry. 2008;63:801–808. doi: 10.1016/j.biopsych.2007.09.024. [DOI] [PubMed] [Google Scholar]
- Rapaport MH, Caligiuri MP, Lohr JB. An association between increased serum-soluble interleukin-2 receptors and a disturbance in muscle force in schizophrenic patients. Prog Neuropsychopharmacol Biol Psychiatry. 1997;21:817–827. doi: 10.1016/s0278-5846(97)00082-1. [DOI] [PubMed] [Google Scholar]
- Rapaport MH, Delrahim KK, Bresee CJ, Maddux RE, Ahmadpour O, Dolnak D. Celecoxib augmentation of continuously ill patients with schizophrenia. Biol Psychiatry. 2005;57:1594–1596. doi: 10.1016/j.biopsych.2005.02.024. [DOI] [PubMed] [Google Scholar]
- Rapaport MH, Doran AR, Nelson DL, McAllister C, Magliozzi JR, Paul SM. Haloperidol and soluble interleukin-2 receptors. Biol Psychiatry. 1991a;30:1063–1064. doi: 10.1016/0006-3223(91)90127-8. [DOI] [PubMed] [Google Scholar]
- Rapaport MH, Lohr JB. Serum-soluble interleukin-2 receptors in neuroleptic-naive schizophrenic subjects and in medicated schizophrenic subjects with and without tardive dyskinesia. Acta Psychiatr Scand. 1994;90:311–315. doi: 10.1111/j.1600-0447.1994.tb01599.x. [DOI] [PubMed] [Google Scholar]
- Rapaport MH, McAllister CG, Kim YS, Han JH, Pickar D, Nelson DL, et al. Increased serum soluble interleukin-2 receptors in Caucasian and Korean schizophrenic patients. Biol Psychiatry. 1994;35:767–771. doi: 10.1016/0006-3223(94)91137-1. [DOI] [PubMed] [Google Scholar]
- Rapaport MH, McAllister CG, Kirch DG, Pickar D. The effects of typical and atypical neuroleptics on mitogen-induced T lymphocyte responsiveness. Biol Psychiatry. 1991b;29:715–717. doi: 10.1016/0006-3223(91)90147-e. [DOI] [PubMed] [Google Scholar]
- Rapaport MH, McAllister CG, Pickar D, Nelson DL, Paul SM. Elevated levels of soluble interleukin 2 receptors in schizophrenia. Arch Gen Psychiatry. 1989;46:291–292. doi: 10.1001/archpsyc.1989.01810030097017. [DOI] [PubMed] [Google Scholar]
- Rapaport MH, Torrey EF, McAllister CG, Nelson DL, Pickar D, Paul SM. Increased serum soluble interleukin-2 receptors in schizophrenic monozygotic twins. Eur Arch Psychiatry Clin Neurosci. 1993;243:7–10. doi: 10.1007/BF02191517. [DOI] [PubMed] [Google Scholar]
- Rothermundt M, Arolt V, Leadbeater J, Peters M, Rudolf S, Kirchner H. Cytokine production in unmedicated and treated schizophrenic patients. Neuroreport. 2000;11:3385–3388. doi: 10.1097/00001756-200010200-00024. [DOI] [PubMed] [Google Scholar]
- Rothermundt M, Arolt V, Weitzsch C, Eckhoff D, Kirchner H. Immunological dysfunction in schizophrenia: a systematic approach. Neuropsychobiology. 1998;37:186–193. doi: 10.1159/000026501. [DOI] [PubMed] [Google Scholar]
- Rubin RT, Poland RE, Lesser IM. Neuroendocrine aspects of primary endogenous depression. X: Serum growth hormone measures in patients and matched control subjects. Biol Psychiatry. 1990;27:1065–1082. doi: 10.1016/0006-3223(90)90044-3. [DOI] [PubMed] [Google Scholar]
- Stolina M, Sharma S, Lin Y, Dohadwala M, Gardner B, Luo J, et al. Specific inhibition of cyclooxygenase 2 restores antitumor reactivity by altering the balance of IL-10 and IL-12 synthesis. J Immunol. 2000;164:361–370. doi: 10.4049/jimmunol.164.1.361. [DOI] [PubMed] [Google Scholar]
- Stone TW, Behan WM. Interleukin-1beta but not tumor necrosis factor-alpha potentiates neuronal damage by quinolinic acid: protection by an adenosine A2A receptor antagonist. J Neurosci Res. 2007;85:1077–1085. doi: 10.1002/jnr.21212. [DOI] [PubMed] [Google Scholar]
- van Westerloo DJ, Giebelen IA, Florquin S, Bruno MJ, Larosa GJ, Ulloa L, et al. The vagus nerve and nicotinic receptors modulate experimental pancreatitis severity in mice. Gastroenterology. 2006;130:1822–1830. doi: 10.1053/j.gastro.2006.02.022. [DOI] [PubMed] [Google Scholar]
- Villemain F, Chatenoud L, Guillibert E, Pelicier Y, Bach JF. Decreased production of interleukin-2 in schizophrenia. Ann N Y Acad Sci. 1987;496:669–675. doi: 10.1111/j.1749-6632.1987.tb35828.x. [DOI] [PubMed] [Google Scholar]
- Wilke I, Arolt V, Rothermundt M, Weitzsch C, Hornberg M, Kirchner H. Investigations of cytokine production in whole blood cultures of paranoid and residual schizophrenic patients. Eur Arch Psychiatry Clin Neurosci. 1996;246:279–284. doi: 10.1007/BF02190280. [DOI] [PubMed] [Google Scholar]
- Wisse BE. The inflammatory syndrome: the role of adipose tissue cytokines in metabolic disorders linked to obesity. J Am Soc Nephrol. 2004;15:2792–2800. doi: 10.1097/01.ASN.0000141966.69934.21. [DOI] [PubMed] [Google Scholar]
- Zhang XY, Zhou DF, Cao LY, Wu GY, Shen YC. Cortisol and cytokines in chronic and treatment-resistant patients with schizophrenia: association with psychopathology and response to antipsychotics. Neuropsychopharmacology. 2005;30:1532–1538. doi: 10.1038/sj.npp.1300756. [DOI] [PubMed] [Google Scholar]