Abstract
Understanding how neuromodulators regulate behavior requires investigating their effects on functional neural systems, but also their underlying cellular mechanisms. Utilizing extensively characterized lamprey motor circuits, and the unique access to reticulospinal presynaptic terminals in the intact spinal cord that initiate these behaviours, we have investigated effects of presynaptic G protein-coupled receptors on locomotion from the systems level, to the molecular control of vesicle fusion. 5-HT inhibits neurotransmitter release via a Gβγ interaction with the SNARE complex that promotes kiss-and-run vesicle fusion. In the lamprey spinal cord we demonstrate that while presynaptic 5-HT receptors inhibit evoked neurotransmitter release from reticulospinal command neurons, their activation does not abolish locomotion, but rather modulates locomotor rhythms. Liberation of presynaptic Gβγ causes substantial inhibition of AMPA receptor-mediated synaptic responses, but leaves NMDA receptor-mediated components of neurotransmission largely intact. Because Gβγ binding to the SNARE complex is displaced by Ca2+-synaptotagmin binding, 5-HT-mediated inhibition displays Ca2+ sensitivity. We show that as Ca2+ accumulates presynaptically during physiological bouts of activity, 5-HT/Gβγ-mediated presynaptic inhibition is relieved leading to a frequency-dependent increase in synaptic concentrations of glutamate. This frequency dependent phenomenon mirrors a shift in the vesicle fusion mode and a recovery of AMPA receptor-mediated EPSCs from inhibition without a modification of NMDA receptor EPSCs. We conclude that activation of presynaptic 5-HT GPCRs state-dependently alters vesicle fusion properties to shift the weight of NMDA vs AMPA receptor-mediated responses at excitatory synapses. We have therefore identified a novel mechanism in which modification of vesicle fusion modes may profoundly alter locomotor behaviour.
Keywords: fictive locomotion, kiss-and-run, G protein-coupled receptors, serotonin, presynaptic, Gβγ
Introduction
Vertebrate locomotion mediated by spinal central pattern generators (CPGs) is initiated by excitation of brainstem reticulospinal nuclei (supplementary figure 1) (Shik et al 1969; Grillner and Shik 1973; Dubuc et al 2007). 5-HT modulates, and may excite, spinal CPGs after traumatic or experimental loss of this output (Brustein and Rossignol 1999). However, 5-HT also potently inhibits this descending excitation (Buchanan and Grillner 1991) by acting at presynaptic 5HT1B receptors both on reticulospinal axon terminals and on intraspinal excitatory interneurons (EINs) (Parker and Grillner 1999; Schwartz et al 2005). Postsynaptically, serotonin potentiates spike frequencies by inhibiting late after-hyperpolarizations and generating plateau potentials (Hounsgaard and Kiehn 1989; Wallen et al 1989; Zhong et al 2006). 5-HT projections also evoke locomotion (Liu and Jordan 2005; Jordan et al 2008), by plateau potential activation (Perrier and Delgado-Lezama 2005) and 5-HT receptors contribute to sustained, ventral root bursting (Harris-Warrick and Cohen 1985; Hounsgaard and Kiehn 1989; Parker and Grillner 1999; Kozlov et al 2001; Schwartz et al 2005; Biro et al 2006),
Vertebrate serotonergic projections form diffuse paracrine terminals that innervate extra-synaptically (Schotland et al 1995) and 5-HT prolongs bursts evoked by spinal application of glutamatergic agonists (Harris-Warrick and Cohen 1985; Christenson et al 1989). However, this artificial CPG activation bypasses reticulospinal neurons, which drive locomotion in intact organisms - a synaptic connection strongly depressed by 5-HT (Buchanan and Grillner 1991; Parker and Grillner 1999). Thus, it remains undetermined whether 5-HT can modify physiologically relevant brainstem initiated locomotion given its powerful presynaptic inhibitory effects.
At lamprey reticulospinal axons, 5-HT does not modify release probability, but liberates presynaptic Gβγ, to compete with Ca2+-dependent synaptotagmin binding to SNARE complexes (Blackmer et al 2001; Blackmer et al 2005; Gerachshenko et al 2005). This reduces synaptic cleft glutamate concentrations (Schwartz et al 2007), by causing kiss-and-run fusion (Photowala et al 2006). Thus, at high [Ca2+], preferential synaptotagmin binding to SNARE complexes occludes 5-HT receptor-mediated inhibition (Yoon et al 2007). Lower synaptic glutamate concentrations cause AMPA receptor EPSCs to be more profoundly inhibited than NMDA receptor EPSCs (Schwartz et al 2007). While kiss-and-run fusion is seen at synapses (Zhang et al 2009) and endocrine cells (Elhamdani et al 2006), behavioral roles for this phenomenon remain unexplored. At synapses it has been proposed that kiss-and-run fusion minimizes metabolic losses required for lipid and protein resorting, but also that fusion modes influence neurotransmitter secretion (Choi et al 2003). It has been proposed that kiss-and-run fusion is Ca2+-dependent, and is modified by high frequency stimulation (Harata et al 2006; Zhang et al 2009).
We demonstrate the outcome of presynaptic 5-HT1B/1D receptor activation on neurotransmission during physiological activity and behavior – brainstem-evoked locomotion. Both the target of 5-HT liberated Gβγ at the SNARE complex and the effects of Gβγ on vesicle fusion are necessary to maintain the modulatory role of 5-HT in the spinal cord. If, in contrast, 5-HT merely inhibited neurotransmitter release, no descending excitation would remain, no locomotion would occur, and other spinal modulatory effects of 5-HT would be moot in behaving vertebrates.
Materials and Methods
Experiments were performed on the isolated spinal cords or spinal cords and brainstems of larval lampreys (Petromyzon marinus). The animals were anaesthetized with tricaine methanesulfonate (MS-222; 100 mg/l; Sigma Chemical, St. Louis, MO), sacrificed by destruction of thalamus and basal ganglia, and dissected in a cold saline solution (Ringer) of the following composition (in mM): 100 NaCl, 2.1 KCl, 2.6 CaCl2, 1.8 MgCl2, 4 glucose, 5 HEPES, adjusted to a pH of 7.60. Procedures conformed to institutional guidelines (University of Illinois at Chicago, Animal Care Committee). Significance was determined using Student T tests of means. Errors are expressed as standard error of the mean.
Electrophysiology
Somata and axons of reticulospinal neurons were recorded with conventional sharp microelectrodes containing 1 to 3 M KCl. For those experiments in which EGTA was injected into the presynaptic terminal, the electrode contained 1 M KCl, 100 mM EGTA, 5 mM HEPES, pH adjusted to 7.2 with KOH. Electrode impedances ranged from 20 to 50 MΩ. EGTA was applied to the interior of the recorded axon by pressure ejection from the microelectrode. Postsynaptic neurons were recorded within the spinal ventral horn. These neurons are principally interneurons of the spinal CPG because most reticulospinal output is directed to these interneurons. Reticulospinal projections to motoneurons are principally polysynaptic (Zelenin et al 2001). Patch electrodes for whole cell postsynaptic recordings contained in mM: Cesium methane sulfonate 102.5, NaCl 1, MgCl2 1, EGTA 5, HEPES 5, pH adjusted to 7.2 with CsOH. Ventral root recordings were made with cut and polished glass micropipettes with tip diameters of 30–100 μm and filled with Ringer’s solution. These were placed over the ventral roots and recorded using conventional ac amplifiers. Locomotion was evoked by placing a pipette (tip diameter approximately 1μm) into the MLR and pressure injecting glutamate (1mM in pipette, volume per injection was approximately 100nL).
To determine the sum of ventral root output during the MLR-evoked burst of fictive locomotion, the absolute value of ventral root output from left and right ventral root pairs was summed. These data were then integrated to represent the cumulative work done. Area under noise was subtracted by fitting a straight line to the initial slope of the integral curve (prior to application of glutamate to the brainstem) and subtracting that line from the total integral. The responses shown in figure 2 are measures of the cumulative ventral root total output.
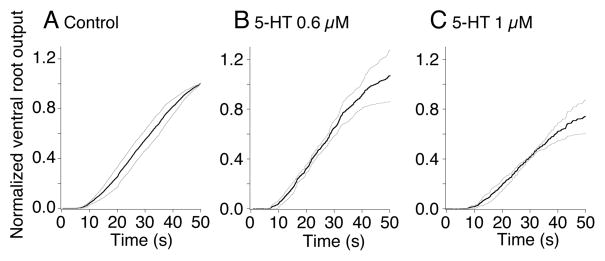
Figure 2. 5-HT does not significantly alter ventral root output power.
A. Summed normalized absolute ventral root activity is plotted against time during the MLR evoked fictive locomotion bout. Activity was measured in 3 sequential locomotor bouts activated at 5 min intervals from 5 preparations (s.e.m. shown in gray).
B. The effect of 5-HT (0.6μM) total output power remained unchanged at 107±20% of control)
C. The effect of 5-HT (1μM). Output power remained at 75±14% of control).
Data was normalized to the peak output power at the termination of the bout recorded under control conditions.
Imaging
Confocal imaging was performed using a modified Biorad MRC 600 confocal microscope using a 488 nm argon ion laser for excitation. Intensity was controlled by an AOTF on the laser fiber optic launch. The photomultiplier outputs were amplified with low noise current amplifiers (Stanford Instruments, CA) and digitized to 12 bits with a National Instruments board and custom software written under Matlab (Mathworks). The scan head mirrors were driven though the MRC 600 scan head amplifiers with the same custom software. This software is available on our website (http://alford.bios.uic.edu).
Application of FM dye
FM1-43 (5 μM) was applied as a stream from a small pipette placed over the surface of the spinal cord. Constant flow was ensured by a syringe pump. 1000 stimuli were applied to a microelectrode recorded axon during the dye application while the presence of the dye in the tissue surrounding the axons was confirmed by imaging. Dye application was subsequently terminated. During this staining protocol postsynaptic activity was blocked with glutamate receptor antagonists CNQX and AP5 (5 and 100 μM, respectively). Excess dye was removed with Advasep 7 (Kay et al, 1999; 1mM, 1 min, Cydex Inc, Lenexa, KS) to reveal areas of stimulus-dependent staining.
Labeling of reticulospinal axons with Ca2+-sensitive dye
Reticulospinal axons were filled with Ca2+-sensitive dye by pressure injection. Ca2+-sensitive dye, Fluo 5F™. (5mM in microelectrode; Molecular Probes, Inc, Eugene, OR). Line scan imaging was performed by tracking the confocal scan mirrors along the axon plasma membrane at 500 Hz.
Calibration of the Ca2+ sensitive dyes
The dye sensitivity to Ca2+ was determined using the same optical path (confocal microscope) that was used to measure Ca2+ transients in the tissue. Dyes (5 μM) were prepared in blends of two Ca2+ buffer standards (10 mM K2EGTA 100 mM KCl and 30 mM MOPS) and (10 mM CaEGTA 100 mM KCl and 30 mM MOPS) both at pH 7.2 (Molecular Probes) to make an 11 point standard curve. The dye buffer mix was placed between cover slips cooled from below with a liquid cooling system to 10°C and imaged from above with the 100X water immersion lens over the upper cover slip. It is important to note that both the need to calibrate the dyes outside the cell and that high temporal and spatial resolution cannot be followed during action potentials means that peak Ca2+ measurements cannot represent the absolute Ca2+ concentration necessary to evoke neurotransmitter release or to modify it. However, our calibrations are sufficient to ensure that the measured intensity values do not represent saturation of the dye at the time of measurement.
Results
5-HT and MLR-induced locomotion
5-HT profoundly inhibits excitatory neurotransmission both from descending reticulospinal neurons (Buchanan and Grillner 1991) and intraspinal excitatory interneurons (EINs; (Parker and Grillner 1999); for their role in the circuitry of the CPG see supplementary fig. 1). This presynaptic inhibition appears incompatible with the modulatory effect of 5-HT on locomotion during brainstem-initiated locomotion, because it would prevent descending command activation of the spinal motor system. Consequently, we determined whether locomotion initiated by brainstem stimulation could be activated when 5-HT was superfused over the spinal cord.
Locomotion was initiated in a brain-spinal cord preparation by glutamate microinjected into the mesencephalic locomotor region (MLR). Experimental effects of spinal 5-HT receptor activation were determined by selectively applying 5-HT and 5-HT1B/1D receptor agonists to the spinal cord isolated from the brain using a split-bath (Fig. 1A), with a barrier between the brain and the spinal cord placed at approximately spinal segments 2 to 5. Microinjection of L-glutamate (1mM, ~100nL) into the MLR (Fig. 1A) reproducibly induced fictive locomotor activity, monitored using a pair of extracellular electrodes placed over contralaterally opposed pairs of ventral roots, as well as by intracellular recordings from reticulospinal neurons in the middle rhombencephalic reticular nucleus (MRRN). Following the transient glutamatergic stimulation of the MLR, repetitive bursting of reticulospinal neurons coincided with fictive locomotion recorded as alternating activity in the ventral roots, in which reticulospinal neurons fire in phase with activity recorded from ventral roots of the ipsilateral rostral spinal cord (Fig 1B) (Kasicki et al 1989; Sirota et al 2000; Dubuc et al 2007).
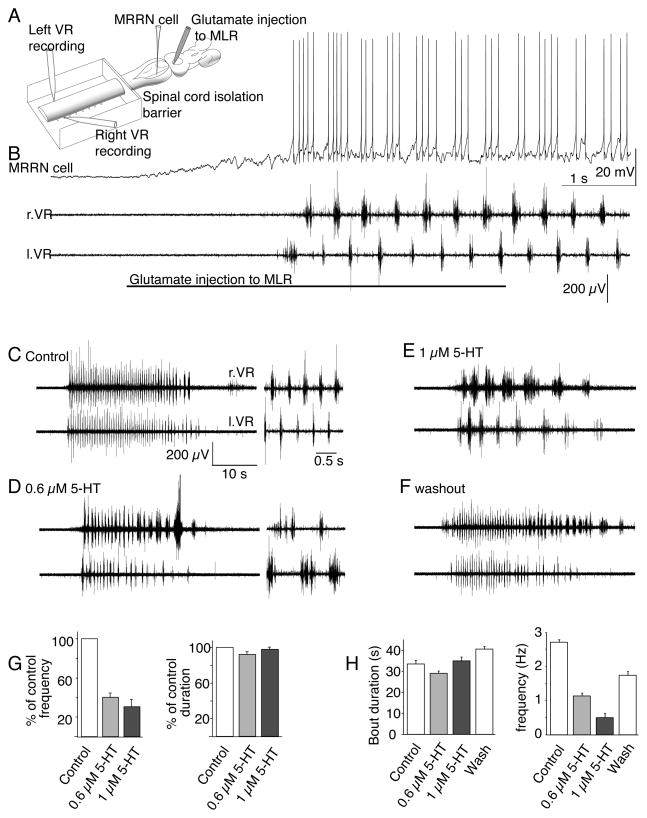
Figure 1. 5-HT reduces the cycle frequency of brainstem-evoked locomotion.
A. Recording arrangement to evaluate effects of spinal agonist/antagonist application on brainstem evoked fictive locomotion. The spinal cord was pharmacologically isolated from the brainstem with a barrier to superfusate flow at the 2nd to 5th spinal segment. Glutamate (1mM) was microinjected into the MLR from a pipette (1–2μm diameter tip). MRRN neurons were recorded intracellularly and fictive locomotion with suction electrodes over left and right pairs of spinal ventral roots (l.VR, r.VR).
B. Five second injection of L-glutamate into the MLR evokes depolarization and rhythmic firing of the MRRN neuron and alternating ventral root activity.
C. Similar control fictive locomotion to (B) recorded in a pair of ventral roots and activated by 5s glutamate microinjection to the MLR. The entire duration of the fictive swimming bout is shown along with activity from the bout in extended timebase to show the frequency of alternating activity (inset right).
D. 5-HT (0.6μM) superfused over the spinal cord reduced fictive locomotion cycle frequencies with no modification of the bout duration. Inset: timebase-expanded activity.
E. 1μM 5-HT further reduced cycle frequencies but had no effect on total bout duration.
F. Wash of 5-HT from the spinal compartment allowed recovery of the cycle frequency.
G. Mean effects of 5-HT on fictive locomotor bout duration and cycle frequency on all preparations tested. Data were normalized to control values prior to 5-HT application. 5-HT reduced cycle frequencies but left bout durations unchanged.
H. Mean effects over 3 sequential glutamate injections from which examples were taken (C–F) of 0.6 and 1μM 5-HT on total bout duration (start to end of fictive swim) and cycle frequency compared to control and wash of 5-HT.
5-HT, applied selectively to the spinal cord at doses that inhibit reticulospinal EPSCs by 50 to 80% (0.6 to1μM; (Schwartz et al 2007), dose-dependently reduced ventral root cycle frequency during brainstem-evoked fictive locomotion (Fig. 1C-F) precisely as has been reported for fictive locomotion evoked by exogenous amino acids superfused over the isolated spinal cord (Harris-Warrick and Cohen 1985; Wallen et al 1989). At 0.6μM 5-HT, cycle frequency was significantly reduced (Fig. 1D) to 40.1±4.5% of the control frequency (Fig 1G; n=5 preparations; p<0.01) and at 1μM 5-HT (Fig 1E) to 30.5±9.8% of control (n=4 preparations; Fig. 1G; p<0.01). Total duration of each locomotor bout was not significantly altered (Fig 1C–F; 92±3% of control at 0.6μM 5-HT and 98±3% of control at 1μM; Fig 1G). After wash of 5-HT from the spinal compartment, locomotor activity recovered close to control cycle frequencies while locomotor bout durations remained stable (Fig. 1C–F, 1G). Thus, the presynaptic action of 5-HT is more complex than a powerful inhibition of descending excitatory drive to the spinal CPG because such an effect would be expected to abolish MLR-evoked fictive locomotion. Moreover, it is unlikely that 5-HT only acts at a subset of reticulospinal neurons as we have recorded from hundreds of reticulospinal/spinal neuron pairs but never found a response not inhibited by 5-HT.
The effect of 5-HT on locomotion was reproducible as were bouts of evoked locomotion. Fictive locomotor bouts were of consistent duration and cycle frequency when initiated in sequential glutamate microinjections to the MLR, at 5 minute intervals in both control and during application of 5-HT (Fig 1H; mean control cycle frequency over 3 sequential glutamate microinjections in the example preparation shown in Figure 1 was 2.7±0.1Hz, mean control burst duration was 33.5±1.7s). In control experiments, with no drug in the spinal compartment, the mean cycle frequency from all 7 preparations was 1.5±0.20Hz and mean bout duration following each glutamate injection was 35.9±2.5s. Thus, 5-HT at 0.6μM and 1.0μM left bout duration unchanged, but reduced the cycle frequency (Fig 1H).
Although 5-HT did not prevent fictive locomotion, it is possible that it reduced locomotor cycle frequencies simply by reducing CPG activity following a loss of excitatory output from the reticulospinal system. If descending excitation were reduced the total output of the spinal CPG would also be reduced. This CPG output may be calculated as a sum of the combination of the cycle frequency, the number of ventral root spikes, and the amplitude of spikes as larger higher threshold neurons contribute more to the signal than smaller lower threshold neurons. To quantify whether 5-HT did alter total ventral root output power during bouts of locomotion, we rectified then summed left and right ventral root activity and calculated the integral of this activity for the duration of the MLR-evoked locomotor bout. This summed activity represents an integration of frequency and event amplitude, thus larger more readily recorded spikes will contribute more than smaller units. Three sequential evoked bouts in each of control and in 5-HT were recorded in each preparation. Output power calculated in this way was then normalized to the maximum at the end of control locomotor bouts in each preparation (Fig. 2A). At 0.6μM, 5-HT left the mean normalized output power unchanged throughout the bout (107±20% of control; Fig. 2B). Even at 1μM 5-HT, which reduces EPSCs to 20% of control (Takahashi et al 2001), output power remained at 75±14% of control (Fig. 2C; n=5). Thus, although 5-HT substantially reduced locomotor frequency, it had little effect on total ventral root output evoked by MLR stimulation. Instead, the same total output was contained within longer bursts on each side of the spinal cord.
The effect of 5-HT is mimicked by selective activation of 5-HT1B/1D receptors
There is substantial uncertainty over the physiological target of 5-HT that leads to modification of locomotion. Fictive locomotion in the isolated lamprey spinal cord can be evoked by application of exogenous glutamate agonists or uptake inhibitors (typically N-methyl-D-apartate, NMDA, or D-glutamate; (Brodin et al 1985). In the isolated spinal cord, fictive locomotion evoked by application of exogenous amino acid agonists is slowed by presynaptic 5-HT1B/1D receptors (Schwartz et al 2005) similar to the effect of 5-HT itself (Harris-Warrick and Cohen 1985). To assay directly if inhibition of synaptic transmission by presynaptic 5-HT receptors is sufficient to alter locomotion, we repeated brainstem-evoked locomotion experiments described above but substituted 5-HT with L694-247, a selective 5-HT1B/1D agonist. 5-HT1B and 1D receptors are not well resolved pharmacologically and share very similar sequences in mammals (Domenech et al 1997), however similar to mammals, in lamprey L694-247 acts selectively at presynaptic loci to inhibit EPSCs without postsynaptically altering after-hyperpolarizations, membrane potential, input impedance or spike frequencies of spinal neurons (Schwartz et al 2005). Locomotor activity was again initiated by glutamate microinjection into the MLR, and ventral root activity recorded from contralateral pairs of ventral roots. Selective activation of 5-HT1B/1D receptors in the pharmacologically isolated spinal compartment with L694-247 mimicked the effect of 5-HT; reducing the cycle frequency while leaving the duration of the locomotor bout unchanged (cycle frequency reduced to 42.6±13.5% of control at 0.1μM; p=<0.05 and 27.4±4.9% at 0.2μM, p<0.05, n=4; Fig. 3A, B, D; bout duration was 94.1±3.0 and 117.0±9.0% of control at 0.1 and 0.2μM L694-247 respectively; Fig 3E). Thus, activation of presynaptic 5-HT1B/1D receptors reproduces the effect of 5-HT (Harris-Warrick and Cohen 1985) on both exogenous amino acid (Schwartz et al 2005) and brainstem-evoked locomotion (Fig. 3B).
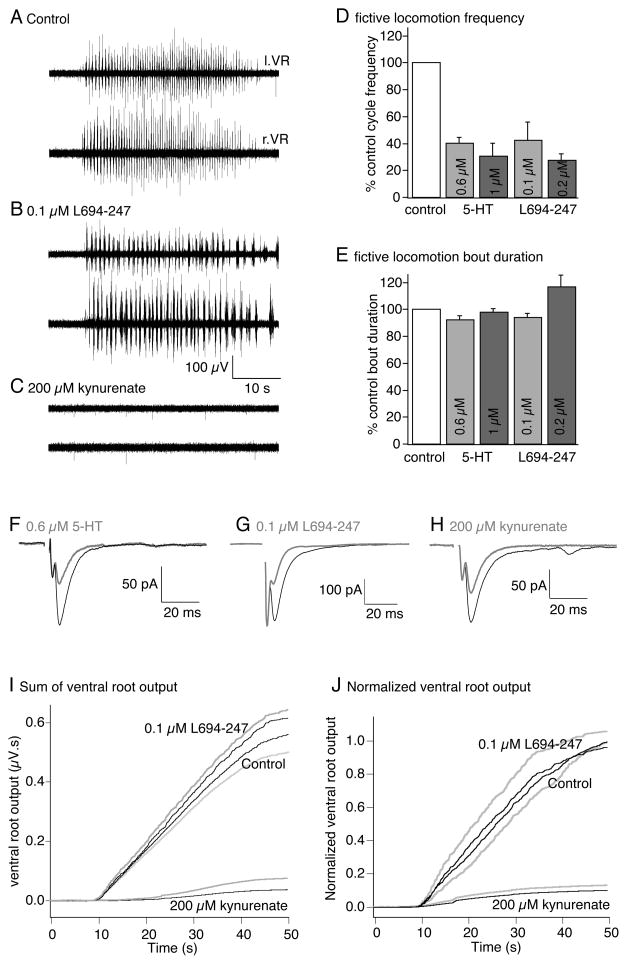
Figure 3. 5-HT1B/1D receptors reduce brainstem-activated CPG cycle frequency.
A. Fictive locomotion was activated by glutamate microinjection to the MLR as for figure 1 and recorded from pairs of ventral roots in the spinal cord.
B. Application of the 5-HT1B/1D receptor agonist L694-247 (0.1μM) to the spinal cord compartment reduced the locomotor cycle frequency but did not alter the total bout duration.
C. Application of kynurenate (200μM) to the spinal cord compartment abolished ventral root activity from the spinal cord in response to glutamate microinjection to the MLR.
D. Data normalized from all preparations to show mean effects of 5-HT (from figure 1) and L694-247 on spinal fictive locomotion cycle frequency. Both reduced the frequency dose-dependently.
E. In contrast neither 5-HT nor L694-247 modified the total bout duration following glutamate microinjection to the MLR.
F–H. A comparison of the effects of 5-HT (0.6μM), L694-247 (0.1μM) and kynurenate (200μM) on synaptic transmission (gray compared to black controls) mediated between paired reticulospinal axons and postsynaptic ventral horn neurons stimulated every 15s. These agonists at these doses gave rather similar inhibitory effects on EPSCs.
I. Summed absolute ventral root activity (μV.s) is plotted against time during the MLR evoked fictive locomotion bout. Activity was measured in 3 sequential locomotor bouts activated at 5 min intervals in control, in L694-247 (0.1μM) and kynurenate (200μM) for the preparation shown in A–C (black - mean of 3 bouts, gray – s.e.m. for L694-247 above the mean trace and for control below the mean trace, for kynurenate s.em. – gray trace above the mean trace in black).
J. Normalized summed ventral root activity from all preparations (n=5) in which L694-247 (0.1μM) was applied (black shows mean of all preparations, s.e.m. in gray, similarly to I).
Reducing synaptic strength does not mimic 5-HT-mediated decreases of locomotor cycle frequency
As for experiments in which 5-HT was applied, it is possible that 5-HT1B/1D receptors slow fictive locomotion simply by reducing synaptic drive to the CPG. If 5-HT1B/1D receptors acted by reducing neurotransmitter release probability, then a uniform averaged loss of postsynaptic response would be the outcome over the many synapses responsible for activation of fictive locomotion in the spinal cord. We wished to mimic a hypothetical reduction in excitatory drive to contrast such an effect with that of 5-HT1B/1D receptor activation. However, it is not possible to selectively reduce release probability without altering synaptic transmission within the spinal motor circuitry. Nevertheless, it is possible to uniformly reduce postsynaptic glutamate receptor activation by applying a non-selective ionotropic glutamate receptor antagonist. For these experiments, we investigated the effect of merely halving spinal EPSP amplitudes using the glutamate receptor antagonist kynurenate to mimic the effects of a simple reduction in excitatory neurotransmission - what would happen if glutamate release probability at spinal synapses was uniformly reduced rather than the more complex effects of 5-HT1B/1D receptors that we have previously demonstrated (Photowala et al 2006; Schwartz et al 2007). If 5-HT1B/1D receptors merely inhibit both descending reticulospinal and intraspinal glutamatergic synaptic excitation, kynurenate would postsynaptically mimic the distribution of 5-HT’s presynaptic inhibitory effects. The extent of 5-HT1B/1D receptor-mediated inhibition of individual evoked EPSCs using 0.1μM L694-247 is similar to that of 200μM kynurenate (Fig 3F–H). However, in contrast to the effect of L694-247, kynurenate abolished MLR-evoked fictive locomotion (200μM; n=4/4 preparations; Fig. 3C). Thus, reducing synaptic transmission by just 50% has a dramatically and qualitatively different effect than activation of 5-HT1B/1D receptors. Indeed, it is also apparent that unlike the activation of presynaptic 5-HT receptors, reducing reticulospinal output by lowering MLR activation correspondingly reduces electromyographic activity in semi-intact preparations of lamprey (Sirota et al 2000).
The effect of presynaptic 5-HT receptor activation is paradoxical in that 5-HT inhibits output from the brainstem but its effect on locomotion does not resemble either reducing brainstem drive to the CPG or generally inhibiting spinal excitatory activity. It has been suggested (Biro et al 2006) and contradicted (Parker and Grillner 1999) that 5-HT can reduce spinal inhibitory synaptic transmission. In fact, 5-HT may actually enhance spinal inhibitory synaptic transmission (Parker and Grillner 1999). However, these effects were observed at 10 to 30-fold higher 5-HT concentrations than in this study. For comparison, during paired recordings between reticulospinal axons and postsynaptic neurons 5-HT (0.6μM) or L694 247 (0.1μM) inhibited EPSCs by 55±7% (n=22) and 63±16% (n=3; Fig 2F,G) - neither prevented fictive locomotion. Kynurenate, in contrast, (200μM) reduced EPSCs by 52±5% (n=4; fig 2H), yet prevented fictive locomotion. We conclude that the effect of 5-HT on excitatory synaptic transmission is different than a simple reduction in Pr.
As for the effect of 5-HT we quantified whether selective activation of presynaptic 5-HT1B/1D receptors reduced total ventral root output power during locomotion. We again rectified then summed left and right ventral root activity and calculated the integral of this activity for the duration of the MLR-evoked locomotor bout. Three sequential evoked bouts in each of control, in L694-247 (0.1μM) and in Kynurenate (200μM) for the same preparations were averaged for this calculation. The results for the example in figure 3A–C is shown (Fig 3I). 5-HT1B/1D receptor activation had no effect on total ventral root output power during the locomotor bout. Data was again normalized to the maximum response in control to calculate mean effect of kynurenate and L694-247 in all preparations (Fig 3J; mean ventral root power in 0.1μM L694-247 was 96±10% of control; not significantly altered; n=5 preparations). We compared this quantification to the effect of kynurenate. In contrast to 5-HT1B/1D receptor activation, kynurenate (200μM) completely eliminated patterned ventral root activity. Accordingly, ventral root output power was almost abolished by 200μM kynurenate (Fig. 3C,I,J; output reduced to just 10±4% of control; p<0.01; n=5 preparations). Thus, the effect of 5-HT1B/1D receptors on spinal locomotor output was quantitatively and qualitatively different than either a simple reduction of descending drive to the spinal cord (Sirota et al 2000) as would be seen after a reduction in release probability at glutamatergic synapses.
In the isolated spinal cord during fictive locomotion activated by exogenously applied glutamatergic agonists, 5-HT1B/1D receptors modify motor activity by inhibiting output from EINs (Parker and Grillner 1999; Schwartz et al 2005). However, remaining fictive locomotion might result from properties of glutamate receptors activated directly by the exogenous agonists, rather than by synaptic glutamate release. Indeed, block of AMPA receptors does not prevent NMDA evoked fictive locomotion (Alford and Grillner 1990). Determining the effect of 5-HT receptor agonists applied to the spinal cord during brainstem-activated fictive locomotion averts this caveat. Thus, given that ventral root output power is unaffected, the effects of 5-HT and L694-247 are paradoxical if we consider presynaptic 5-HT GPCRs to act simply as presynaptic inhibitors. If activation of presynaptic 5-HT1B/1D receptors profoundly inhibits descending excitation of spinal CPGs, how does fictive locomotor output power remain largely unaltered? To answer this we have investigated the properties of presynaptic inhibition by 5-HT receptors during physiological patterns of activity necessary to sustain fictive locomotion.
Reticulospinal synaptic transmission and Ca2+ accumulation during repetitive stimulation
Previous studies characterizing reticulospinal synaptic transmission and the mechanism of 5-HT-mediated inhibition probed synaptic responses to single stimuli. In contrast, during MLR induced locomotion, reticulospinal neurons fire bursts of action potentials (up to 50 Hz (Brocard and Dubuc 2003; Fig 1) in phase with the ipsilateral and rostral spinal cord. These repetitive trains of action potentials may cause presynaptic residual Ca2+ accumulation. 5-HT1B/1D receptors inhibit release via Gβγ (Schwartz et al 2005) which competes with synaptotagmin (the Ca2+ sensor for exocytosis; Blackmer et al 2001; Gerachshenko et al 2005; Yoon et al 2007) for binding to the SNARE complex. Thus, raised [Ca2+] prevents 5-HT-mediated inhibition by allowing synaptotagmin to out-compete Gβγ for SNARE complex binding (Yoon et al 2007). We wished to determine how this competition might modify physiologically relevant trains of evoked synaptic events. Therefore, we investigated stimulus train-evoked Ca2+ accumulation in presynaptic terminals of reticulospinal axons. Axons were recorded with microelectrodes and injected through this microelectrode with the low affinity Ca2+ indicator Fluo-5F. Action potential trains were evoked in reticulospinal axons (10 shocks, 50Hz) and the Ca2+ transient recorded by confocal line-scanning microscopy with the x-scan aligned along the axon plasma membrane (Takahashi et al 2001; Fig 4A) to allow 500 Hz resolution of presynaptic Ca2+ entry.
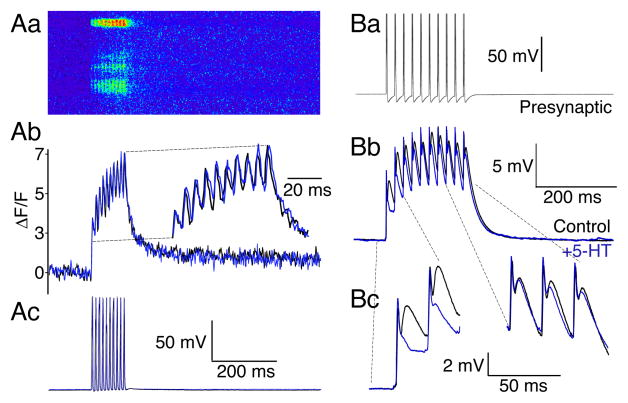
Figure 4. Presynaptic residual Ca2+ at presynaptic terminals coincides with a train-dependent loss of 5-HT mediated presynaptic inhibition.
A. Presynaptic Ca2+ transients were recorded by confocal line-scanning along the axonal plasma membrane at 500 Hz. (Aa). Line-scan recording of Ca2+ dye fluorescence in axon (3 active zones). Axon labeled with low affinity Ca2+ dye Fluo 5F from a recording microelectrode. 10 action potentials, 50Hz, evoked summating Ca2+ transients. (Ab). Ca2+ transients displayed as ΔF/F from uppermost active zone (control, black; 5-HT 1μM, blue). The timebase of the traces is expanded in the inset to clearly demonstrate the absence of any effect of 5-HT. (Ac). Presynaptic action potential train that caused the Ca2+ transients (control, black; 5-HT 1μM, blue) evoked by stimulation through the recording electrode. Panels (Aa-c) are aligned and shown with the same timebase.
B. Current clamp recording of train stimulation. (Ba), 10 presynaptic action potentials, 50 Hz evoked through the presynaptic recording electrode. (Bb). average of 10 sequential postsynaptic traces in control (black) and in 5-HT (0.6μM; gray). Note the effect of 5-HT is limited to the start of the train. (Bc). Expanded recording of the first two and last three EPSPs in the train to emphasize the loss of inhibitory effect of 5-HT at the end of the train. The initial absolute voltage of the last three traces was adjusted to that of control to emphasize the later responses in the train were not significantly inhibited by 5-HT.
The peak Ca2+ transient was estimated at 1.2μM at the first presynaptic action potential and 2.0μM at the last. Although this underestimates peak Ca2+ concentrations at the active zone (see methods) these responses were well within the linear response of the dye (kd=3.7μM at 10°C) and, thus, the lack of effect of 5-HT is not due to dye saturation. The mean increase in Ca2+ transient amplitude between the first and last stimulus was 1.8±0.1 fold (n=6), and was unaffected by 5-HT (Fig 4Ab; 0.3 to 3μM). This rise in presynaptic Ca2+ during the stimulus is sufficient to prevent 5-HT mediated presynaptic inhibition based on our previously published results (Yoon et al 2007). Indeed, experimentally induced presynaptic Ca2+ transients 1.4-fold over control prevent 5-HT-mediated inhibition (Yoon et al 2007).
To determine the effect of 5-HT on synaptic responses during stimulus trains, paired recordings were made between reticulospinal axons and their ventral horn neuron targets. Pre- and postsynaptic axons and cells were recorded in current clamp. During presynaptic action potential trains (10 at 50Hz), chemical EPSPs early in the train are inhibited by 5-HT (0.6μM; first EPSP in train reduced to 52±6% of control; p<0.01; Fig 4B), but the EPSPs recover to control at the termination of the train (Fig 4B; n=3).
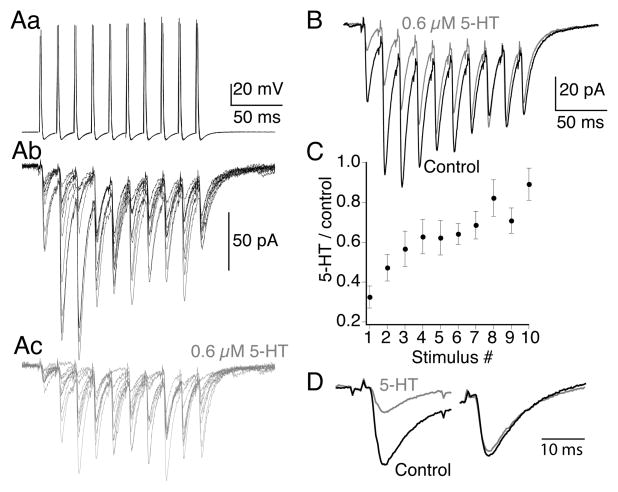
These current clamp experiments indicate a physiologically relevant relaxation of 5-HT-mediated inhibition that parallels summation of Ca2+ during the stimulus train. To quantify train-dependent effects of 5-HT on AMPA receptor EPSCs, paired recordings were made between reticulospinal axons and whole cell voltage clamped ventral horn neurons at −70mV to reveal AMPA receptor-mediated EPSCs. The presynaptic axon was repetitively stimulated (10 stimuli, 50Hz) in a series of trains (Fig 5A, shows 10 sequential responses to trains in control and in 5-HT) to reveal the quantal variance in the postsynaptic response. 5-HT (0.3μM to 1μM) markedly depressed AMPA receptor-mediated EPSCs at the start of the train, however, this inhibition was lost over the later stimuli. To reveal the extent of inhibition and its loss at the end of the train, responses to 20 trains in control and after application of 5-HT were averaged in each of the recorded pairs (Fig 5B). The mean EPSC at the start of the train was consistently inhibited, those at the end were not (Fig 5C, D; 1st EPSC inhibited to 33±6% of control in 0.6μM 5-HT; tenth response in the train was 88±9% of control; n=8) similar to current clamp recordings.
Figure 5. 5-HT-mediated inhibition of AMPA receptor-mediated EPSCs is lost during train stimulation of the presynaptic axon.
A. Paired recordings demonstrate train evoked AMPA receptor-mediated EPSCs (10 action potentials; 50Hz in each train). (Aa) presynaptic action potentials. (Ab) 10 sequential evoked EPSC trains in control and in 5-HT (Ac; 600nM; gray).
B. Means of the 10 traces shown in (Ab) and (Ac). Control, black and 5-HT, gray.
C. Ratio of EPSC amplitudes in 5-HT to control for the means of each of the 10 sequential responses in the train (n=9 preparations and pairs). 5-HT markedly inhibited the EPSCs early in the train but the response recovered toward the end.
D. The first and last traces are expanded and overlaid to emphasize the loss of inhibition at the end of the train.
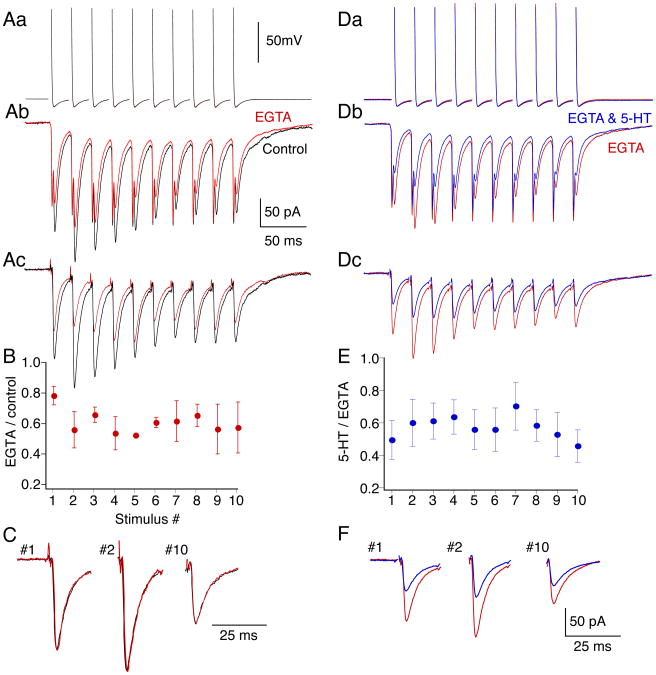
Enhanced presynaptic Ca2+ transients in high extracellular Ca2+ prevent 5-HT1B/1D receptor-mediated presynaptic inhibition (Yoon et al 2007). We now show similarly, that when presynaptic Ca2+ concentrations are raised during physiologically relevant stimulus trains (Fig. 4), 5-HT-mediated presynaptic inhibition is lost. However, during repetitive stimulation Ca2+ may represent only one of a number of effects mediating short-term plasticity. Thus, to determine whether Ca2+ summation at the active zone can account for the loss of 5-HT-mediated inhibition during the stimulus train, we prevented frequency-dependent residual Ca2+ accumulation. Reticulospinal axons were recorded from and microinjected with the slow binding Ca2+ buffer, EGTA, to mitigate train evoked presynaptic Ca2+ summation. EGTA does not prevent individual evoked EPSCs because it does not bind Ca2+ sufficiently rapidly to prevent its immediate binding to synaptotagmin (Adler et al 1991). However, EGTA will reduce the slower accumulation of residual Ca2+ at the active zone during repetitive stimulation. We injected EGTA into the presynaptic axons (electrode contained 100mM EGTA, 5mM HEPES, 1M KCl, pH 7.2; n=5). All chemical EPSCs in the train are inhibited by this injection (Fig 6A), however the first response is significantly less inhibited than the immediately subsequent responses (mean reduction of first response to 78±6% of control; mean of all later responses was to 58±2% of control, p<0.05; Fig. 6B). Note that the immediate short-term enhancement of the postsynaptic response over the beginning of the train coincides with a rise in presynaptic residual Ca2+ (Fig. 4). The later depression of the response is common at this synapse, although the underlying mechanism remains unclear. Importantly, for these experiments, the presynaptic injection did not block EPSCs, nor did it alter the kinetic profile of the individual responses during the stimulus train (Fig. 6C, traces after EGTA scaled to control peak amplitude for clarity).
Figure 6. Train-dependent loss of 5-HT-mediated inhibition is prevented by presynaptic injection of EGTA.
A. Paired recording between presynaptic reticulospinal axon and postsynaptic neuron in which EGTA (100mM) was included in the presynaptic electrode. (Aa). Presynaptic action potentials before (black) and after (red) pressure injection of EGTA into the axon. EGTA left action potentials unaffected throughout the train. (Ab). Postsynaptic EPSCs comprising electrical and chemical components evoked by presynaptic action potentials. Traces are averages of ten responses before and after injection of EGTA into the presynaptic axon. (Ac). Chemical EPSCs following subtraction of the electrical components which were obtained after blocking AMPA-mediated responses with CNQX
B. Effect of EGTA injection as proportional inhibition for each of the ten EPSCs in the train. Data from 4 paired recordings and normalized to control amplitudes of each EPSC. The first response was inhibited to a lesser extent than later responses in the train.
C. As representative examples the first, second and last EPSC in the train were compared before and after EGTA injection. The responses after EGTA were scaled to the peak amplitudes of the control responses. The kinetics of the responses (both rise and decay) were unaltered.
D. Paired recordings from cell in (A) red traces are the same as those in A after presynaptic EGTA injection. (Da). presynaptic action potentials. (Db). Evoked EPSCs in EGTA and 5-HT (600nM; blue). (Dc). Traces after subtraction of electrical components.
E. Ratio of EPSC amplitudes in 5-HT to control (n=5 preparations and pairs). 5-HT inhibited EPSCs throughout the train after presynaptic EGTA injection.
F. The first, second and last traces are expanded to emphasize that inhibition is sustained throughout the train.
After presynaptic EGTA injection, 5-HT (0.6μM) inhibited EPSCs uniformly throughout the presynaptic action potential train (Fig 6D; first response reduced to 49±12% of control; not significantly different from results without EGTA; last response reduced to 43±10% of control; not significantly different than effect of 5-HT on first response; Fig 6E,F). Thus the inhibitory effect of 5-HT on late responses during trains of presynaptic action potentials was significantly enhanced after presynaptic EGTA injection (p<0.05). We conclude that summation of presynaptic residual Ca2+ during stimulus trains mediates loss of 5-HT-mediated inhibition. This effect is consistent with a Ca2+-synaptotagmin competition with Gβγ at the SNARE complex.
High stimulus frequency enhances FM1-43 destaining in 5-HT
The results above reveal that during physiological stimulation paradigms, 5-HT acts as an activity-dependent modulator of neurotransmission, not simply an inhibitor. In addition, competition between Gβγ and synaptotagmin at the SNARE complex (Yoon et al 2007) means that Ca2+ accumulation during stimulus trains (Figs 4,5) will reduce 5-HT effects under high frequency activity seen during the activation of locomotion (Figs 1–3).
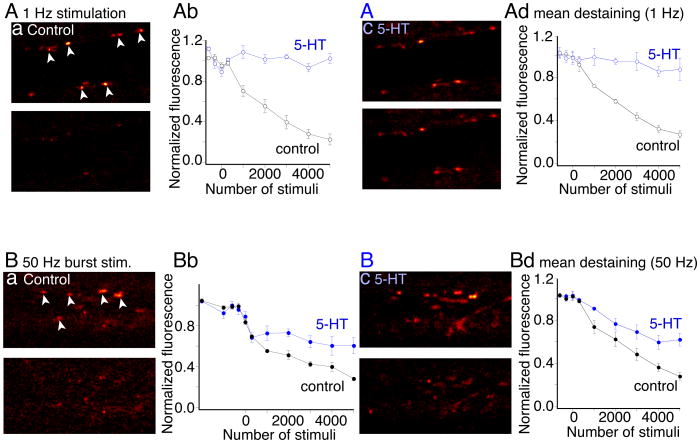
5-HT has been shown to modify vesicle fusion. A dose of 5-HT (0.6μM) that partially inhibits EPSCs, completely blocks FM1-43 destaining from the vesicles during stimulation at 1Hz (Photowala et al 2006) – a condition in which residual Ca2+ does not accumulate. We, therefore, wished to determine whether the stimulus train-dependent recovery of 5-HT inhibited AMPA receptor-mediated EPSCs is mirrored by a similar train-dependent recovery of FM1-43 destaining in 5-HT. Synaptic vesicles in single giant axons were labeled with FM1-43. Axons were stimulated (1000 stimuli, 1Hz) through an intracellular microelectrode in the presence of FM1-43 (5μM). After stimulation, FM1-43 perfusion was stopped and excess dye removed with Advasep-7 (1mM) (Kay et al 1999). Vesicles were then destained by further stimulation at 1Hz (Fig. 7A). After 5000 stimuli the mean presynaptic vesicle cluster fluorescence intensity was reduced to 27±3% of the prestimulus fluorescence (n=15 active zones, 3 preparations; p<0.01; Fig 7Ad for data in all preparations, similar to earlier results (Photowala et al 2006)). The same axons were restained (Fig 7Ac) and then 5-HT was added to the superfusate. As previously reported 5-HT (0.6 μM) abolished destaining during 1Hz stimulation (Photowala et al 2006) (Fig 7Ab,c; fluorescence after 5000 stimuli was now 87±10% of prestimulus fluorescence).
Figure 7. Effect of train stimulation on FM1-43 destaining of synaptic vesicle clusters.
Aa. FM1-43 labeled presynaptic terminals, before (top) and after (bottom) destaining at 1Hz in control. (Ab). black trace shows control destaining vs stimulus number of the terminals arrowed. The same presynaptic axon and terminals were restained with FM1-43 (Ac) then superfused with 5-HT (600nM). Stimulation (1Hz) subsequently failed to destain (Ab; blue). (Ad). Mean of data plotted as for Ab from all 5 preparations.
Ba. Presynaptic terminals (examples arrowed) labeled as (A), but destained with action potentials in trains (10 at 50 Hz; 10s intervals) with the same total number of stimuli as in A. In control destaining was indistinguishable from 1Hz destaining (Bb black). Axons and presynaptic terminals were restained (Bc) and reimaged. In 5-HT (600nM) partial destaining was observed (blue). (Bd). Mean of data plotted from all 4 preparations.
During bursts of 10 stimuli at 50Hz, 5-HT-mediated presynaptic inhibition recorded electrophysiologically was lost toward the end of the stimulus train as Ca2+ accumulated presynaptically (Figs. 5, 6). We, therefore, determined whether 5-HT-mediated trapping of FM1-43 in synaptic vesicle clusters was also prevented by high frequency stimulation. Thus, in a further 4 preparations, axons were similarly stained with FM1-43 but destained with a series of action potential trains (10 at 50Hz). Presentation of trains at 10s intervals ensured that the total number of stimuli over time was identical to 1Hz stimulation (Fig 7A). Control destaining was similar to destaining during continuous 1Hz stimulation (Fig 7Ba,b; staining reduced to 28±3% of prestimulus fluorescence; 20 active zones in 4 preparations, black trace). The terminals were restained, 5-HT (0.6μM) applied and the axon again stimulated with a series of trains. FM1-43 destaining with 50Hz trains in 5-HT was significantly greater than following continuous stimulation at 1Hz in 5-HT, although less than control destaining (after 5000 stimuli staining was reduced to 62±6% of prestimulus fluorescence; Fig 7Bd; n=25 active zones in 5 preparations; p<0.05, blue trace). This intermediate destaining rate at 50Hz in 5-HT suggests that trains of stimuli enhance destaining of FM1-43 in 5-HT. A loss of FM1-43 trapping also implies that the kiss-and-run fusion mode caused by 5-HT (Photowala et al 2006) appears to be lost during the stimulus train. Moreover, this effect is consistent with the frequency-dependent recovery of synaptic transmission inhibited by 5-HT.
High stimulus frequency reverses 5-HT-dependent reductions in cleft glutamate concentration
Consistent with its effect on vesicle fusion, 5-HT causes reductions in evoked cleft glutamate concentration following single synaptic stimuli (Schwartz et al 2007). If train stimulation enhances FM1-43 destaining from fusing vesicles in 5-HT (Photowala et al 2006) by causing full synaptic vesicle fusion at the end of the train even in 5-HT, then we may hypothesize that in 5-HT cleft glutamate concentrations rise as presynaptic residual Ca2+ summates during the stimulus train (Photowala et al 2006; Schwartz et al 2007). This can be determined by measuring efficacy of block of glutamate receptor-mediated EPSCs by low affinity antagonists (Clements et al 1992) in which a portion of the EPSC is due to non-equilibrium antagonist unbinding and its competitive replacement by synaptic glutamate. The degree to which the antagonist is replaced depends on glutamate concentration. For EPSCs evoked by a single stimulus, both low affinity AMPA and NMDA receptor antagonists revealed a supralinear inhibition of synaptic events in 5-HT, indicating a reduction in synaptic cleft glutamate (Schwartz et al 2007).
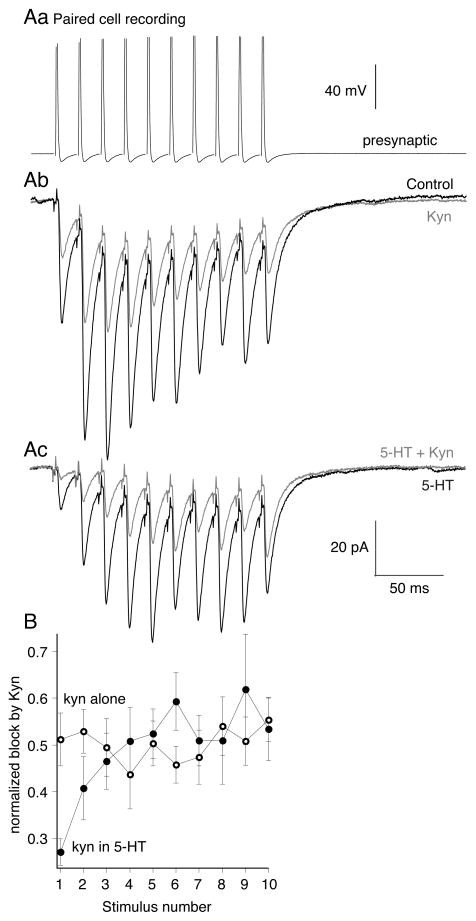
Using paired cell recordings, we probed glutamate cleft concentrations with kynurenate during presynaptic action potential trains (10 at 50Hz, Fig 8Aa). The effect of kynurenate at a half maximal dose (200μM; Fig 8Ab, red) was uniform on each sequential EPSC (n=5; kynurenate reduced the first response to 51±6% of control, the last to 55±5% of control, Fig 8B, red). Thus, in control, peak glutamate concentrations are unaffected by repetitive stimulation at 50Hz. This is consistent with our earlier finding that changes in release probability do not alter synaptic cleft glutamate concentrations (Schwartz et al 2007). 5-HT (Fig 8Ac, gray) preferentially inhibited the early EPSCs in the train. We then probed this response in 5-HT with kynurenate (200μM). Early responses in the train in 5-HT were inhibited by kynurenate to a much greater extent (to 27±3% of the 1st response in 5-HT, Fig 8B, blue) than the control effect of kynurenate alone. This result confirms that 5-HT lowers synaptic glutamate concentrations for the first stimulus in the train (Schwartz et al 2007). However, as the train progressed, the kynurenate block became indistinguishable from its effect against control responses as if no 5-HT were present (Fig. 8Ac, 8B, blue traces; EPSC amplitude reduced to 53±7% of last response in 5-HT alone, not significantly different than effect of kynurenate with no 5-HT present). We conclude that during stimulus trains in 5-HT, cleft glutamate concentrations recover to control levels as 5-HT mediated inhibition is overcome by rising presynaptic [Ca2+] and synaptic vesicle fusion is more complete. Thus, 5-HT mediated presynaptic inhibition is relaxed during repetitive stimulation characteristic of reticulospinal neuron activity that evokes locomotion.
Figure 8. 5-HT lowers evoked cleft glutamate concentrations at the start of train stimuli but not at the end.
A. To probe glutamate concentration, paired recordings were made. Presynaptic action potentials (Aa) (10 at 50Hz) evoked EPSCs (Ab) in control (black). Kynurenate (200μM; gray) reduced EPSC amplitudes throughout the train. (Ac) 5-HT (600nM) reduced EPSC amplitudes at the start of the train but not the end (compare black trace in Ab to black trace in Ac). In 5-HT kynurenate (200μM; gray) was more effective at inhibiting EPSCs at the start of the train than the end.
B. Graphs comparing the efficacy of kynurenate at blocking EPSCs throughout the stimulus train. In 5-HT (filled circles; n=9) kynurenate was more effective at inhibiting the early EPSCs in 5-HT compared to control (open circles; n=3).
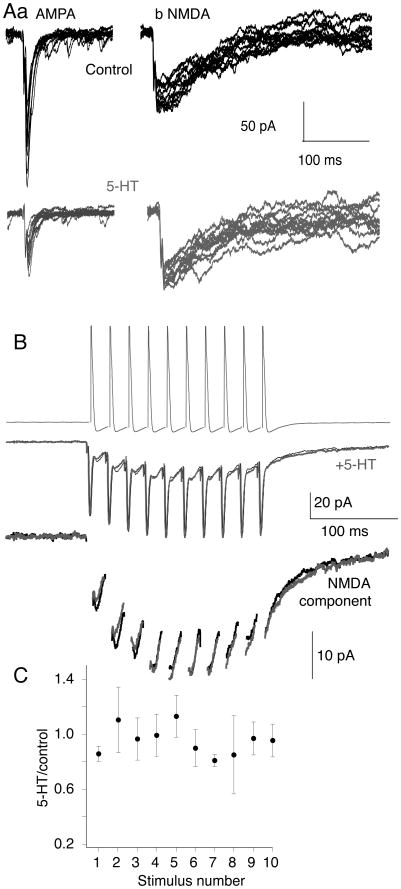
Differential efficacy of 5-HT on AMPA and NMDA receptor mediated responses
5-HT lowers evoked synaptic cleft glutamate concentrations (Schwartz et al 2007), which favors synaptic activation of NMDA over AMPA receptors (Choi et al 2003). In lamprey, presynaptic 5-HT receptors inhibit AMPA receptor-mediated EPSCs more effectively than NMDA EPSCs at the same synapses (Schwartz et al 2007). To test whether synaptic NMDA receptor activation is similarly resistant to inhibition by 5-HT during stimulus trains, we made paired recordings between reticulospinal axons – the output compartment of the MRRN neurons recorded in figure 1 – and their postsynaptic ventral horn target neurons that comprise the locomotor CPG (see supplementary fig 1). We voltage clamped the postsynaptic cell and stimulated the reticulospinal axon with a burst of action potentials (10 shocks, 50Hz; Fig 9B) in Mg2+ free superfusate to enhance NMDA receptor-mediated EPSCs at −70 mV. CNQX or NBQX (5μM) was added to reveal the slow summating NMDA receptor response under the electrical component (Fig 9B). 5-HT (0.6μM) had no effect on the NMDA component thoughout these bursts (Fig 9B,C; n=4). We conclude that 5-HT at doses that substantially inhibit AMPA receptor-mediated EPSCs and modify locomotor activity (Fig. 9A), failed to inhibit NMDA EPSCs whether evoked by single stimuli (Schwartz et al 2007) or during physiologically relevant activity trains.
Figure 9. 5-HT differentially modifies the amplitude of NMDA and AMPA-mediated synaptic responses.
A. Whole cell postsynaptic voltage clamp recording of control EPSCs, evoked by 12 sequential stimulations of a paired presynaptic reticulospinal axon. (Aa). Responses in control to show AMPA receptor mediated EPSCs (black, top). Bottom trace (gray) – after application of 5-HT (0.6μM). (Ab). Responses recorded in Mg2+ free Ringer and CNQX (5μM) to reveal the NMDA component. Bottom traces (gray) after addition of 5-HT (0.6μM). 5-HT had no effect on the NMDA EPSC.
B. Paired NMDA receptor EPSCs recorded in Mg2+ free Ringer and CNQX (5 μM) evoked by trains of sequential presynaptic action potentials (50Hz, 10 stimuli) in control (black) and in (gray) 5-HT (600nM). The EPSCs are shown with an expanded y-axis in the lower traces after removal of the electrical component for clarity.
C. Mean amplitudes of the evoked synaptic responses in the train. Each point is the mean (± s.e.m.) of each sequential EPSC recorded as the peak amplitude after the electrical component (n=3 preparations). 5-HT had no effect on the amplitude of the NMDA receptor-mediated EPSCs throughout the stimulus train.
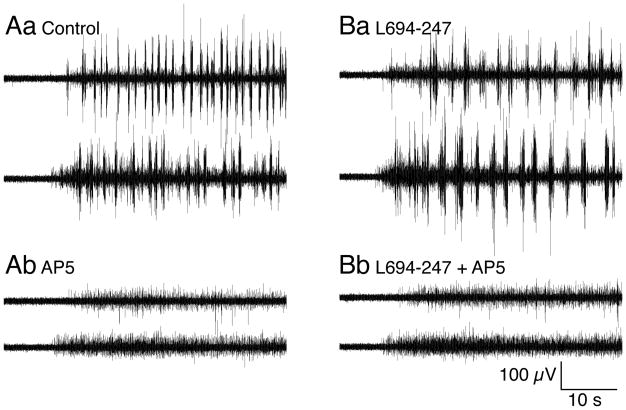
Thus, during repetitive stimulation, as during single synaptic responses, 5-HT receptor activation favors postsynaptic activation of NMDA receptors over AMPA. Spinal application of NMDA activates locomotion (Brodin et al 1985) and evokes bistable properties of spinal neurons (Sigvardt et al 1985) that initiates slower locomotion than that evoked by AMPA receptors (Brodin et al 1985; Traven et al 1993). Therefore, we determined whether NMDA receptor block could prevent MLR-activated locomotion, before and during spinal activation of 5-HT1B/1D receptors. Locomotor activity was initiated as above (Fig. 10). After obtaining reliable bouts of locomotion, the NMDA receptor antagonist D-AP5 (50μM) was applied to the spinal compartment. This prevented locomotion (Fig 10A; n=4/4 preparations). Locomotor activity was recovered on D-AP5 wash. Presynaptic 5-HT1B/1D receptor activation with L694-247 (0.1μM) in the spinal compartment slowed locomotor activity (Fig 10B), and D-AP5 (50μM) again prevented MLR-evoked locomotion.
Figure 10. NMDA receptor activation is necessary for brainstem evoked fictive locomotion.
Aa. Left and right ventral root recordings after microinjection of L-glutamate (1mM) into the MLR.
Ab. Application of the NMDA receptor antagonist D-AP5 (50μM) to the pharmacologically isolated spinal cord abolished MLR evoked fictive locomotion.
Ba. As for Figure 2, application of the 5HT1B/1D receptor agonist L694-247 (0.1μM) slowed the ventral root bursting frequency.
Bb. D-AP5 (50μM) applied in addition to L694-247 abolished fictive locomotion.
NMDA receptor activity is, therefore, necessary for brainstem-activated locomotion, but is not as strongly inhibited by presynaptic 5-HT receptors as AMPA receptor-mediated responses because the dose response of 5-HT against NMDA EPSCs is right-shifted compared to AMPA EPSCs (Schwartz et al 2007). Both in vitro and computer modeling studies indicate that selective sparing of NMDA receptor-mediated responses favors low frequency fictive locomotion (Brodin et al 1985; Traven et al 1993). Because presynaptic 5-HT receptor-meditated inhibition spares the NMDA receptor-mediated synaptic response, the relative contribution of NMDA receptors to excitation will be greater in 5-HT than in control locomotion.
Discussion
Neurotransmission is ubiquitously controlled by presynaptic GPCRs that utilize Gβγ to inhibit release by membrane-delimited mechanisms. Gβγ may modify presynaptic Ca2+ channels (Tedford and Zamponi 2006) to reduce release probability. This causes a reduction of probability of any active zone releasing transmitter, but no qualitative modulation in transmitter released at those active zones. In contrast, we have previously demonstrated that GPCRs utilize Gβγ to directly target the release apparatus to alter fusion mode independently of release probability (Photowala et al 2006). Thus, in 5-HT, vesicle fusion is dominated by kiss-and-run, trapping FM1-43 in fusing vesicles and reducing unitary quantal amplitudes (Photowala et al 2006). This reduces cleft glutamate concentrations which can still activate NMDA receptors efficiently, but not AMPA receptors (Schwartz et al 2007). We demonstrate that 5-HT1B/1D receptor-mediated reductions in synaptic cleft glutamate are modulated during stimulus trains that mimic brainstem-initiated motor behavior possibly linking frequency-dependent changes in fusion mode (Harata et al 2006; Zhang et al 2009) to Ca2+-dependent competition of synaptotagmin at the SNARE complex with Gβγ (Yoon et al 2007).
5-HT profoundly modifies vertebrate locomotion. 5-HT1B/1D receptors – presynaptic and inhibitory in vertebrates – reduce locomotion frequency (Schwartz et al 2005). 5-HT is released diffusely from paracrine terminals (Van Dongen et al 1985; Schotland et al 1995) to act pre and postsynaptically (Harris-Warrick and Cohen 1985; Buchanan and Grillner 1991; Parker and Grillner 1999). However, selective activation of presynaptic 5-HT1B/1D receptors (Schwartz et al 2005) mimics system-wide effects of 5-HT. Paradoxically, this receptor markedly inhibits output from the very neurons that convey descending brainstem commands to initiate and maintain locomotion. Yet, even at a dose (1μM) that inhibits single EPSCs by 83%, brainstem-evoked locomotion is sustained. Indeed, the resultant slower locomotion is not simply a reduction indescending drive that would be seen following a reduction in Pr, because the sum of locomotor drive in each MLR induced bout of locomotion is unaffected by 5-HT1B/1D receptors. This lack of effect of 5-HT on integrated ventral root output, which is quantitatively similar to EMG activity in lamprey (Wallén and Williams 1984), contrasts with manipulations used to alter total brainstem command output, in which total locomotor output power scales linearly with MLR stimulation used to evoke locomotion (Sirota et al 2000).
To highlight this contrast between a generalized reduction in excitation and the effect of 5-HT, we demonstrate that even partial antagonism of spinal EPSCs completely blocks brainstem evoked locomotion. Thus, we conclude that 5-HT acts in a more complex manner. It is possible that presynaptic 5-HT receptors actively select for particular outputs to recruit alternate neuron pools which might underlie different motor synergies, a phenomenon recently reported for interneurons (McLean et al 2008). However, this is unlikely because 5-HT inhibits every reticulospinal axon and EIN synapse that we have investigated with various techniques (Blackmer et al 2001; Takahashi et al 2001; Gerachshenko et al 2005; Schwartz et al 2005; Schwartz et al 2007); (Photowala et al 2006).
Reticulospinal neurons (Buchanan et al 1987) and intraspinal EINs (Buchanan and Grillner 1987) contribute to locomotor activity. Both are glutamatergic and output from both is inhibited by 5-HT (Schwartz et al 2005), with a similar profile of inhibition. During stimulus trains early responses are inhibited, whereas recovery occurs late in the train (Parker and Grillner 1999). Also in both synapses, 5-HT spares NMDA receptor-mediated responses compared to those mediated by AMPA receptors (Schwartz et al 2005; Schwartz et al 2007). While the balance of excitation provided by the two groups of neurons is unknown, both contribute significantly to locomotion. Moreover, it is clear that reticulospinal neuron activity is absolutely required (Dubuc et al 2007). Taking these characteristics into account, it becomes apparent that the complex features of 5-HT-mediated presynaptic inhibition are necessary to explain the effects of 5-HT1B receptors on locomotion. Experimentally determined results (Brodin and Grillner 1986), and results obtained with computer modeling (Traven et al 1993), indicate that selective spinal NMDA receptor activation favors lower locomotor frequencies and longer burst durations. Furthermore, computer models of the CPG indicate that excitatory responses that start depressed but then augment during bursts, like reticulospinal and EIN responses in 5-HT, favor lower frequency output (Kozlov et al 2001). It is also striking that this effect is synergistic with the postsynaptic effects of 5-HT, which slow locomotion by acting at entirely different loci (Hounsgaard and Kiehn 1989; Wallen et al 1989; Zhong et al 2006; Hammar et al 2007). Clearly, while the presynaptic action of 5-HT is in one sense simple – Gβγ released in the presynaptic terminal directly targets the SNARE complex – it leads to complex synaptic and systemic outcomes.
We propose that the effect of 5-HT1B/1D receptor activation on locomotion results directly from the presynaptic cellular mechanism of action of this receptor. Presynaptic 5HT1B/1D receptors utilize Gβγ, that competes with Ca2+-dependent synaptotagmin binding to SNARE complexes (Gerachshenko et al 2005; Yoon et al 2007). This evokes kiss-and-run vesicle fusion, reduces quantal amplitudes and lowers synaptic cleft glutamate concentrations (Photowala et al 2006; Schwartz et al 2007). This outcome allows a selective inhibition of postsynaptic AMPA receptor-mediated EPSCs but, comparatively, spares postsynaptic NMDA receptor activation (Schwartz et al 2007). The target for Gβγ liberated by the 5-HT receptor at primed vesicles provides a further layer of complexity to 5-HT1B/1D receptor-mediated inhibition. Gβγ competes with Ca2+-synaptotagmin binding to the SNARE complex, such that at raised presynaptic Ca2+ concentrations, Ca2+-synaptotagmin binding is more efficient and Gβγ-mediated inhibition is lost (Yoon et al 2007). In this study we found that this Ca2+-dependent loss of presynaptic inhibition confers a frequency-dependence on 5-HT mediated inhibition of glutamate release during physiologically relevant trains of activity. Thus, AMPA receptor-mediated EPSCs are inhibited at the start of stimulus trains but recover as Ca2+ concentrations summate in the presynaptic terminal during train stimulation to concentrations that we have demonstrated prevent Gβγ-mediated presynaptic inhibition by allowing Ca2+-synaptotagmin to compete with Gβγ for binding to the SNARE complex (Yoon et al 2007). Indeed, slow chelation of presynaptic Ca2+ abolished this frequency dependence of inhibition of AMPA receptor mediated EPSCs.
Thus, we now demonstrate during stimulus trains that at the start of the stimulus a lower glutamate concentration released into the synaptic cleft following presynaptic 5-HT receptor activation can support activation of postsynaptic NMDA receptors even when substantial inhibition of AMPA receptors occurs (Schwartz et al 2007). Moreover, as the stimulus train progresses NMDA receptor responses are retained while AMPA receptor-mediated responses recover. We have now demonstrated that this mechanism, by which vesicle fusion mode is dynamically modulated during physiological relevant trains, qualitatively alters quantal neurotransmitter release and also accounts for the behavioral outcome of 5-HT in the spinal motor system.
We have provided a possible explanation as to how a presynaptic 5-HT1B/1D receptor located on locomotor command neurons can inhibit release without preventing locomotion. However, 5-HT1B/1D receptors, which are located predominantly presynaptically, are linked to depression, aggression, memory impairment, attention deficit disorder and defects in motor and sensory processes. Thus, can this mechanism have wider implications? Kiss-and-run fusion, as a mechanism involved in vesicle recycling, is well accepted during large dense core vesicle fusion events (Elhamdani et al 2006). However, its presence and role at synapses has been more contentious. Under resting conditions some authors have claimed extensive occurrence of this form of fusion (Zhang et al 2009), others that it is rare but present (He et al 2006), while others see no evidence (Balaji and Ryan 2007). Regardless of the resting proportion of full to kiss-and-run fusion events, it has become clear that high frequency stimulus trains may modify synaptic vesicle fusion in hippocampal neurons, from a state in which kiss-and-run occurs, to one favoring complete fusion (Harata et al 2006), or an extended period of kiss-and-run vesicle fusion pore opening (Zhang et al 2007; Zhang et al 2009).
It has been proposed that kiss-and-run fusion partially accounts for synapses in which NMDA receptor activation occurs, but AMPA responses are silenced (Choi et al 2003). Modeling of AMPA receptor activation during kiss-and-run fusion implies that AMPA receptor responses can be lost because of rapid receptor desensitization during a slower initial pulse of glutamate (He et al 2006). We have previously concluded that this effect occurs in lamprey in 5-HT (Photowala et al 2006; Schwartz et al 2007). We now conclude that this causes a train position-dependence of vesicle fusion properties that not only applies to how quickly vesicles endocytose and retain FM dyes or Qdots (Harata et al 2006; Zhang et al 2007; Zhang et al 2009), but also extends to millisecond aspects of the onset of fusion, to effect efficient AMPAR activation. We propose that signal transduction mechanisms in the presynaptic terminal - Gβγ-can cause a Ca2+ (Yoon et al 2007) and therefore state-dependent modification of fusion properties and neurotransmission. It is noteworthy that a protein with a similar WD-40 structure to Gβγ exists at presynaptic terminals; the R-SNARE containing protein tomosyn. Its yeast homolog, Sro7, like Gβγ binds to the SNARE region of the SNAP-25 homolog, sec9 (Hattendorf et al 2007).
Schematic describing the basic circuitry of the lamprey spinal locomotor CPG and its excitatory pathways.
Locomotion is initiated and maintained by MLR-evoked excitation of the reticulospinal system in the mesencephalon (Dubuc et al 2007) which in turn excite left and right spinal hemi-spinal cords. The reticulospinal neurons provide excitation principally to interneurons of the spinal CPG within the ventral horn to activate locomotion(Zelenin et al 2001) although they also excite motoneurons to a lesser extent. Locomotion continues provided that the reticulospinal neurons continue to fire action potentials repetitively and to excite the spinal ventral horn interneurons. We have demonstrated that the output of these neurons is inhibited by a direct effect of Gβγ released by presynaptic 5-HT1B/1D receptor activation, on the SNARE complex. Further excitation in the spinal cord necessary for appropriate phase coupling (Williams 1992) is provided by excitatory interneurons (Buchanan and Grillner 1987) which as a class excite all their ipsilateral interneuron partners as well as motoneurons. The synaptic output of these interneurons is also inhibited by 5-HT1B/1D receptors (Parker and Grillner 1999; Schwartz et al 2005) although the presynaptic molecular targets have not yet been resolved.
Supplementary Material
Acknowledgments
We thank Heidi Hamm, Edaeni Hamid and Rejean Dubuc for their helpful discussion of the experiments and critical reading of this manuscript. This work was supported by a grant from the National Institutes and Neurological Disorders and Stroke (R01NS052699) to SA.
References
- Adler EM, Augustine GJ, Duffy SN, Charlton MP. Alien intracellular calcium chelators attenuate neurotransmitter release at the squid giant synapse. J Neurosci. 1991;11:1496–1507. doi: 10.1523/JNEUROSCI.11-06-01496.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alford S, Grillner S. CNQX and DNQX block non-NMDA synaptic transmission but not NMDA-evoked locomotion in lamprey spinal cord. Brain Res. 1990;506:297–302. doi: 10.1016/0006-8993(90)91266-j. [DOI] [PubMed] [Google Scholar]
- Balaji J, Ryan TA. Single-vesicle imaging reveals that synaptic vesicle exocytosis and endocytosis are coupled by a single stochastic mode. Proc Natl Acad Sci U S A. 2007;104:20576–20581. doi: 10.1073/pnas.0707574105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biro Z, Hill RH, Grillner S. 5-HT Modulation of identified segmental premotor interneurons in the lamprey spinal cord. J Neurophysiol. 2006;96:931–935. doi: 10.1152/jn.00309.2006. [DOI] [PubMed] [Google Scholar]
- Blackmer T, Larsen EC, Takahashi M, Martin TF, Alford S, Hamm HE. G protein betagamma subunit-mediated presynaptic inhibition: regulation of exocytotic fusion downstream of Ca2+ entry. Science. 2001;292:293–297. doi: 10.1126/science.1058803. [DOI] [PubMed] [Google Scholar]
- Blackmer T, Larsen EC, Bartleson C, Kowalchyk JA, Yoon EJ, Preininger AM, Alford S, Hamm HE, Martin TF. G protein betagamma directly regulates SNARE protein fusion machinery for secretory granule exocytosis. Nat Neurosci. 2005;8:421–425. doi: 10.1038/nn1423. [DOI] [PubMed] [Google Scholar]
- Brocard F, Dubuc R. Differential contribution of reticulospinal cells to the control of locomotion induced by the mesencephalic locomotor region. J Neurophysiol. 2003;90:1714–1727. doi: 10.1152/jn.00202.2003. [DOI] [PubMed] [Google Scholar]
- Brodin L, Grillner S. Effects of magnesium on fictive locomotion induced by activation of N-methyl-D-aspartate (NMDA) receptors in the lamprey spinal cord in vitro. Brain Res. 1986;380:244–252. doi: 10.1016/0006-8993(86)90219-2. [DOI] [PubMed] [Google Scholar]
- Brodin L, Grillner S, Rovainen CM. N-Methyl-D-aspartate (NMDA), kainate and quisqualate receptors and the generation of fictive locomotion in the lamprey spinal cord. Brain Res. 1985;325:302–306. doi: 10.1016/0006-8993(85)90328-2. [DOI] [PubMed] [Google Scholar]
- Brustein E, Rossignol S. Recovery of locomotion after ventral and ventrolateral spinal lesions in the cat. II. Effects of noradrenergic and serotoninergic drugs. J Neurophysiol. 1999;81:1513–1530. doi: 10.1152/jn.1999.81.4.1513. [DOI] [PubMed] [Google Scholar]
- Buchanan JT, Grillner S. Newly identified ‘glutamate interneurons’ and their role in locomotion in the lamprey spinal cord. Science. 1987;236:312–314. doi: 10.1126/science.3563512. [DOI] [PubMed] [Google Scholar]
- Buchanan JT, Grillner S. 5-Hydroxytryptamine depresses reticulospinal excitatory postsynaptic potentials in motoneurons of the lamprey. Neurosci Lett. 1991;122:71–44. doi: 10.1016/0304-3940(91)90196-z. [DOI] [PubMed] [Google Scholar]
- Buchanan JT, Brodin L, Dale N, Grillner S. Reticulospinal neurones activate excitatory amino acid receptors. Brain Res. 1987;408:321–325. doi: 10.1016/0006-8993(87)90397-0. [DOI] [PubMed] [Google Scholar]
- Choi S, Klingauf J, Tsien RW. Fusion pore modulation as a presynaptic mechanism contributing to expression of long-term potentiation. Philos Trans R Soc Lond B Biol Sci. 2003;358:695–705. doi: 10.1098/rstb.2002.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christenson J, Franck J, Grillner S. Increase in endogenous 5-hydroxytryptamine levels modulates the central network underlying locomotion in the lamprey spinal cord. Neurosci Lett. 1989;100:188–192. doi: 10.1016/0304-3940(89)90682-4. [DOI] [PubMed] [Google Scholar]
- Clements JD, Lester RA, Tong G, Jahr CE, Westbrook GL. The time course of glutamate in the synaptic cleft. Science. 1992;258:1498–1501. doi: 10.1126/science.1359647. [DOI] [PubMed] [Google Scholar]
- Domenech T, Beleta J, Palacios JM. Characterization of human serotonin 1D and 1B receptors using [3H]-GR-125743, a novel radiolabelled serotonin 5HT1D/1B receptor antagonist. Naunyn Schmiedebergs Arch Pharmacol. 1997;356:328–334. doi: 10.1007/pl00005058. [DOI] [PubMed] [Google Scholar]
- Dubuc R, Brocard F, Antri M, Fénelon K, Gariepy JF, Smetana R, Ménard A, LeRay D, Viana Di Prisco G, Pearlstein E, Sirota MG, Derjean D, St-Pierre M, Zielinski B, Auclair F, Veilleux D. Initiation of locomotion in lampreys. Brain Res Rev. 2007 doi: 10.1016/j.brainresrev.2007.07.016. [DOI] [PubMed] [Google Scholar]
- Elhamdani A, Azizi F, Artalejo CR. Double patch clamp reveals that transient fusion (kiss-and-run) is a major mechanism of secretion in calf adrenal chromaffin cells: high calcium shifts the mechanism from kiss-and-run to complete fusion. J Neurosci. 2006;26:3030–3036. doi: 10.1523/JNEUROSCI.5275-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerachshenko T, Blackmer T, Yoon EJ, Bartleson C, Hamm HE, Alford S. Gbetagamma acts at the C terminus of SNAP-25 to mediate presynaptic inhibition. Nat Neurosci. 2005;8:597–605. doi: 10.1038/nn1439. [DOI] [PubMed] [Google Scholar]
- Grillner S, Shik ML. On the descending control of the lumbosacral spinal cord from the “mesencephalic locomotor region”. Acta Physiol Scand. 1973;87:320–333. doi: 10.1111/j.1748-1716.1973.tb05396.x. [DOI] [PubMed] [Google Scholar]
- Hammar I, Stecina K, Jankowska E. Differential modulation by monoamine membrane receptor agonists of reticulospinal input to lamina VIII feline spinal commissural interneurons. Eur J Neurosci. 2007;26:1205–1212. doi: 10.1111/j.1460-9568.2007.05764.x. [DOI] [PubMed] [Google Scholar]
- Harata NC, Choi S, Pyle JL, Aravanis AM, Tsien RW. Frequency-dependent kinetics and prevalence of kiss-and-run and reuse at hippocampal synapses studied with novel quenching methods. Neuron. 2006;49:243–256. doi: 10.1016/j.neuron.2005.12.018. [DOI] [PubMed] [Google Scholar]
- Harris-Warrick RM, Cohen AH. Serotonin modulates the central pattern generator for locomotion in the isolated lamprey spinal cord. J Exp Biol. 1985;116:27–46. doi: 10.1242/jeb.116.1.27. [DOI] [PubMed] [Google Scholar]
- Hattendorf DA, Andreeva A, Gangar A, Brennwald PJ, Weis WI. Structure of the yeast polarity protein Sro7 reveals a SNARE regulatory mechanism. Nature. 2007;446:567–571. doi: 10.1038/nature05635. [DOI] [PubMed] [Google Scholar]
- He L, Wu XS, Mohan R, Wu LG. Two modes of fusion pore opening revealed by cell-attached recordings at a synapse. Nature. 2006;444:102–105. doi: 10.1038/nature05250. [DOI] [PubMed] [Google Scholar]
- Hounsgaard J, Kiehn O. Serotonin-induced bistability of turtle motoneurones caused by a nifedipine-sensitive calcium plateau potential. J Physiol. 1989;414:265–282. doi: 10.1113/jphysiol.1989.sp017687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan LM, Liu J, Hedlund PB, Akay T, Pearson KG. Descending command systems for the initiation of locomotion in mammals. Brain Res Rev. 2008;57:183–191. doi: 10.1016/j.brainresrev.2007.07.019. [DOI] [PubMed] [Google Scholar]
- Kasicki S, Grillner S, Ohta Y, Dubuc R, Brodin L. Phasic modulation of reticulospinal neurones during fictive locomotion and other types of spinal motor activity in lamprey. Brain Res. 1989;484:203–216. doi: 10.1016/0006-8993(89)90363-6. [DOI] [PubMed] [Google Scholar]
- Kay AR, Alfonso A, Alford S, Cline HT, Holgado AM, Sakmann B, Snitsarev VA, Stricker TP, Takahashi M, Wu LG. Imaging synaptic activity in intact brain and slices with FM1–43 in C. elegans, lamprey, and rat. Neuron. 1999;24:809–817. doi: 10.1016/s0896-6273(00)81029-6. [DOI] [PubMed] [Google Scholar]
- Kozlov A, Kotaleski JH, Aurell E, Grillner S, Lansner A. Modeling of substance P and 5-HT induced synaptic plasticity in the lamprey spinal CPG: consequences for network pattern generation. J Comput Neurosci. 2001;11:183–200. doi: 10.1023/a:1012806018730. [DOI] [PubMed] [Google Scholar]
- Liu J, Jordan LM. Stimulation of the parapyramidal region of the neonatal rat brain stem produces locomotor-like activity involving spinal 5-HT7 and 5-HT2A receptors. J Neurophysiol. 2005;94:1392–1404. doi: 10.1152/jn.00136.2005. [DOI] [PubMed] [Google Scholar]
- McLean DL, Masino MA, Koh IY, Lindquist WB, Fetcho JR. Continuous shifts in the active set of spinal interneurons during changes in locomotor speed. Nat Neurosci. 2008;11:1419–1429. doi: 10.1038/nn.2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker D, Grillner S. Activity-dependent metaplasticity of inhibitory and excitatory synaptic transmission in the lamprey spinal cord locomotor network. J Neurosci. 1999;19:1647–1656. doi: 10.1523/JNEUROSCI.19-05-01647.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrier JF, Delgado-Lezama R. Synaptic release of serotonin induced by stimulation of the raphe nucleus promotes plateau potentials in spinal motoneurons of the adult turtle. J Neurosci. 2005;25:7993–7999. doi: 10.1523/JNEUROSCI.1957-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Photowala H, Blackmer T, Schwartz E, Hamm HE, Alford S. G protein betagamma-subunits activated by serotonin mediate presynaptic inhibition by regulating vesicle fusion properties. Proc Natl Acad Sci U S A. 2006;103:4281–4286. doi: 10.1073/pnas.0600509103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schotland J, Shupliakov O, Wikström M, Brodin L, Srinivasan M, You ZB, Herrera-Marschitz M, Zhang W, Hökfelt T, Grillner S. Control of lamprey locomotor neurons by colocalized monoamine transmitters. Nature. 1995;374:266–268. doi: 10.1038/374266a0. [DOI] [PubMed] [Google Scholar]
- Schwartz EJ, Gerachshenko T, Alford S. 5-HT prolongs ventral root bursting via presynaptic inhibition of synaptic activity during fictive locomotion in lamprey. J Neurophysiol. 2005;93:980–988. doi: 10.1152/jn.00669.2004. [DOI] [PubMed] [Google Scholar]
- Schwartz EJ, Blackmer T, Gerachshenko T, Alford S. Presynaptic G-protein-coupled receptors regulate synaptic cleft glutamate via transient vesicle fusion. J Neurosci. 2007;27:5857–5868. doi: 10.1523/JNEUROSCI.1160-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shik ML, Severin FV, Orlovsky GN. Control of walking and running by means of electrical stimulation of the mesencephalon. Electroencephalogr Clin Neurophysiol. 1969;26:549. [PubMed] [Google Scholar]
- Sigvardt KA, Grillner S, Wallen P, Van Dongen PA. Activation of NMDA receptors elicits fictive locomotion and bistable membrane properties in the lamprey spinal cord. Brain Res. 1985;336:390–395. doi: 10.1016/0006-8993(85)90676-6. [DOI] [PubMed] [Google Scholar]
- Sirota MG, Di Prisco GV, Dubuc R. Stimulation of the mesencephalic locomotor region elicits controlled swimming in semi-intact lampreys. Eur J Neurosci. 2000;12:4081–4092. doi: 10.1046/j.1460-9568.2000.00301.x. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Freed R, Blackmer T, Alford S. Calcium influx-independent depression of transmitter release by 5-HT at lamprey spinal cord synapses. J Physiol. 2001;532:323–336. doi: 10.1111/j.1469-7793.2001.0323f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedford HW, Zamponi GW. Direct G protein modulation of Cav2 calcium channels. Pharmacol Rev. 2006;58:837–862. doi: 10.1124/pr.58.4.11. [DOI] [PubMed] [Google Scholar]
- Traven HG, Brodin L, Lansner A, Ekeberg O, Wallén P, Grillner S. Computer simulations of NMDA and non-NMDA receptor-mediated synaptic drive: sensory and supraspinal modulation of neurons and small networks. J Neurophysiol. 1993;70:695–709. doi: 10.1152/jn.1993.70.2.695. [DOI] [PubMed] [Google Scholar]
- Van Dongen PA, Hökfelt T, Grillner S, Verhofstad AA, Steinbusch HW, Cuello AC, Terenius L. Immunohistochemical demonstration of some putative neurotransmitters in the lamprey spinal cord and spinal ganglia: 5-hydroxytryptamine-, tachykinin-, and neuropeptide-Y-immunoreactive neurons and fibers. J Comp Neurol. 1985;234:501–522. doi: 10.1002/cne.902340408. [DOI] [PubMed] [Google Scholar]
- Wallén P, Williams TL. Fictive locomotion in the lamprey spinal cord in vitro compared with swimming in the intact and spinal animal. J Physiol. 1984;347:225–239. doi: 10.1113/jphysiol.1984.sp015063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallén P, Christenson J, Brodin L, Hill R, Lansner A, Grillner S. Mechanisms underlying the serotonergic modulation of the spinal circuitry for locomotion in lamprey. Prog Brain Res. 1989;80:321–327. doi: 10.1016/s0079-6123(08)62227-x. discussion 315–329. [DOI] [PubMed] [Google Scholar]
- Williams TL. Phase coupling by synaptic spread in chains of coupled neuronal oscillators. Science. 1992;258:662–665. doi: 10.1126/science.1411575. [DOI] [PubMed] [Google Scholar]
- Yoon EJ, Gerachshenko T, Spiegelberg BD, Alford S, Hamm HE. Gβγ regulates exocytosis by interfering with Ca2+-dependent binding of synaptotagmin to the SNARE complex. Molecular Pharmacology. 2007;72:1210–1219. doi: 10.1124/mol.107.039446. [DOI] [PubMed] [Google Scholar]
- Zelenin PV, Grillner S, Orlovsky GN, Deliagina TG. Heterogeneity of the population of command neurons in the lamprey. J Neurosci. 2001;21:7793–7803. doi: 10.1523/JNEUROSCI.21-19-07793.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Cao YQ, Tsien RW. Quantum dots provide an optical signal specific to full collapse fusion of synaptic vesicles. Proc Natl Acad Sci U S A. 2007;104:17843–17848. doi: 10.1073/pnas.0706906104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Li Y, Tsien RW. The Dynamic Control of Kiss-And-Run and Vesicular Reuse Probed with Single Nanoparticles. Science. 2009 doi: 10.1126/science.1167373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong G, Diaz-Rios M, Harris-Warrick RM. Serotonin modulates the properties of ascending commissural interneurons in the neonatal mouse spinal cord. J Neurophysiol. 2006;95:1545–1555. doi: 10.1152/jn.01103.2005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.