Abstract
To human health, arsenic exhibits the property of a double-edged sword. Arsenic compounds such as As2O3 is effective for the treatment of relapsed/refractory acute promyelocytic leukemia, whereas chronic exposure to environmental arsenic is associated with the development of a variety of common cancers. Because As2O3 is capable of inhibiting tubulin polymerization and inducing mitotic arrest, we examined whether there existed any functional interaction between As2O3 and paclitaxel, a well-known microtubule poison. Flow cytometry and fluorescence microscopy revealed that although As2O3 alone caused a moderate level of mitotic arrest, it greatly attenuated paclitaxel-induced mitotic arrest in cells with p53 deficiency. Western blot analysis showed that As2O3 significantly blocked phosphorylation of BubR1, Cdc20, and Cdc27 in cells treated with paclitaxel, suggesting that arsenic compromised the activation of the spindle checkpoint. Our further studies revealed that the attenuation of paclitaxel-induced mitotic arrest by As2O3 resulted primarily from sluggish cell cycle progression at S phase but not enhanced mitotic exit. The clinical efficacy of taxol is associated with its ability to induce mitotic arrest and subsequent mitotic catastrophe. Our observations that As2O3 has a negative impact on the cell cycle checkpoint activation by taxol should have significant clinical implications.
Keywords: Arsenic, Paclitaxel, Mitosis, Checkpoint, Cell Cycle
INTRODUCTION
Arsenic compounds have been used for medicinal purposes for thousand years (Halicka et al. 2002;Huang 2003). Recently, arsenic trioxide (As2O3) has been approved by FDA for the treatment of acute promyelocytic leukemia (APL). It is especially effective for treating leukemia resistant to all-trans retinoic acid (Shen et al. 1997b;Soignet et al. 1998;Zhang et al. 2001). Extensive in vitro and in vivo studies show that in APL cells arsenic trioxide induces partial differentiation at low doses (0.1–0.5 µM) and apoptosis at high doses (1.0–2.0 µM) (Shen et al. 1997a;Kitamura et al. 1997;Chen et al. 1996). Arsenic compounds also induces apoptosis in a variety of solid tumor cells as well as leukemic cells other than APL (Zhu et al. 1999;Rousselot et al. 1999;Siu, Chan, and Fung 2002;Shen et al. 2000;Uslu et al. 2000). In fact, As2O3 exhibits promising therapeutic properties in inhibiting tumor growth in an orthotopic prostate cancer model (Maeda et al. 2001). During the past a few years, clinical trials have been initiated to test the efficacy of arsenic compounds in the treatment of solid tumors as well as lymphoid malignancies (Murgo 2001).
The mechanism by which arsenic compounds including As2O3 and sodium arsenite [As(III)] induce apoptosis appears to be rather complicated. As(III) can activate c-Jun NH2-terminal kinases (Huang et al. 2001), perturb mitochondrial transmembrane potential, and activate caspase 3 (Cai et al. 2000;Larochette et al. 1999). As(III) is capable of producing reactive oxygen species, eliciting DNA damage responses, and slowing down cell cycle progression (Kligerman and Tennant 2007). Notably, As(III) can induce mitotic arrest and mitotic catastrophe in a variety of cells including leukemia cells and cells derived from solid tumors (Cai et al. 2003;Halicka et al. 2002;Park et al. 2001;Uslu et al. 2000), strongly suggesting its ability to target a common signaling pathway(s) in these cells. Supporting this notion, it has been shown that As(III) directly interferes with the function of tubulins (Li and Broome 1999a;Ling et al. 2002b;Ramirez et al. 1997), thus potentially affecting the integrity of microtubules and mitotic spindles. Indeed, As(III) inhibits GTP-induced formation of microtubules in vitro by acting as a non-competitive inhibitor (Li and Broome 1999a). It has been hypothesized that As(III) is capable of cross-linking two vicinal cysteine residues (Cys-12 and Cys-213), which inactivates the GTP binding site (Li and Broome 1999a).
Taxol is one of the most effective anti-tumor drugs used in the clinic. It has been approved for the treatment of a variety of human malignancies including breast, ovarian, and non-small cell lung cancers (Jordan and Wilson 2004). Taxol stabilizes microtubules by binding to the β-subunit of tubulin, thus preventing the dynamic instability of mitotic spindles and leading to mitotic arrest. Prolonged mitotic arrest often results in mitotic catastrophe (Castedo et al. 2004). Because of their common properties in induction of mitotic arrest and apoptosis of cancer cells, As2O3 and taxol have been used in combination in clinical trials for treating stage III osteosarcoma and Ewing sarcoma (Guo et al. 2006;Halicka et al. 2002). Intriguingly, a previous study suggests that when they are used in combination in a cell culture system, As(III) and paclitaxel behave antagonistically (Carre et al. 2002a).
Given the clinical importance of As2O3 and taxol, we carefully examined the effect of these compounds on cell cycle progression as well as spindle checkpoint activation. We demonstrated that As2O3 suppressed mitotic arrest induced by paclitaxel and interfered with paclitaxel-induced activation of BubR1, suggesting antagonism between As2O3 and taxol. The compromised mitotic arrest resulted from sluggish cell cycle progression rather than accelerated mitotic exit. We also observed that the antagonistic effect on mitotic arrest and cell death was exacerbated by p53 deficiency.
MATERIALS AND METHODS
Cell culture and treatment
HeLa cells were obtained from the American Type Culture Collection (ATCC, Manassas, VA). Cells were cultured in dishes or on Lab-Tek II chamber slides (Fisher Scientific) in Dulbesso’s modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS) with 5% CO2 at 37°C. HCT116 cell lines (p53+/+ and p53−/−) generously provided by Dr. Bert Vogelstein (Johns Hopkins, Baltimore, MD) were grown in McCoy's 5a Medium Modified supplemented with 10% fetal bovine serum at 37°C under 5% CO2 atmosphere.
As2O3 (Sigma Chemical Co., St. Luis, MO) was dissolved in 1.0 mol/l NaOH and diluted in growth medium without FBS to make a stock solution at a concentration of 50 mM. Stock solutions of paclitaxel and nocodazole (Sigma Chemical Co., St. Luis, MO) were prepared in DMSO. The maximal final concentration of DMSO used in cell culture never exceeded 0.1%. To follow cell cycle progression, HeLa cells were pulsed with 0.1 mM (final concentration) bromodeoxyuridine (BrdU, Sigma) in the presence of various drugs overnight. To synchronize cells at mitosis, HeLa cells were cultured in medium containing 2 mM thymidine for 18 h and then released into fresh medium with no thymidine for 9 hrs; these cells were again cultured in medium with 2 mM thymidine for18 hrs before releasing into medium containing 50 ng/ml nocodazole for 9 hrs. Mitotic cells were collected by shake-off.
Fluorescence microscopy
Cells of various treatments were fixed in 4% paraformaldehyde in PBS for 5 minutes on ice. Following fixation, the cells were permeabilized at room temperature for 10 minutes. Following blocking with 2% BSA, the cells were incubated with anti-human phosphorylated histone H3 (p-histone H3) monoclonal antibody (PharMingen) in PBS containing 2% bovine serum albumin for 1 h at room temperature. The cells were then washed and stained with the Alexa Fluor 488-labeled secondary antibody (Invitrogen) at room temperature for 1 h in the dark. Cells were finally stained with 4',6-diamidino-2-phenylindole (1 µg/ml, Fluka). Fluorescence microscopy was performed on a Nikon microscope, and images were captured using a digital camera (Optronics) using Optronics MagFire and Image-Pro Plus software.
Flow cytometry analysis
Cells were first fixed in 75% ethanol. Cells were then incubated with an anti-BrdU-FITC antibody (Becton Dickinson) in a staining solution [PBS with 0.5% Tween 20 (v/v), 1% BSA (w/v), 0.5 mg/ml RNAse] at 4°C overnight. DNA was subsequently stained with propidium iodide (PI). Cell cycle distributions of various treatments were analyzed on a Beckman Coulter® Epics XL-MCL™ Flow Cytometer. Cell cycle distributions were analyzed using Muticycle software (Phoenix Flow System).
Western blot
Cells were harvested and lysed in a lysis buffer as described (Ouyang et al. 1997). Cell lysates were centrifuged at 12,000 × g for 15 min at 4°C and supernatants were collected. An approximately equal amount of proteins was subjected to SDS-PAGE followed by electro-transferring to PVDF membranes. The protein blots were probed with antibodies to BubR1, securin (Novocastra), Cdc27, cyclin B, and β-actin (Santa Cruz). Specific signals were detected using horseradish peroxidaseconjugated goat-anti-rabbit (or anti-mouse) secondary antibodies (Sigma) and enhanced chemiluminescence reagents (Amersham Pharmacia Biotech).
Cell viability
Cell viability was assayed by the 3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay method. HCT116 cells (5×103 cells/well) were seeded in a 96-well plate in sextuplicates. Arsenic trioxide was added to cell culture at indicated concentrations for 24 h. MTT (15 µl) was supplied to each well. After incubation at 37 °C for additional 4 h, the medium was removed. MTT formazan precipitates were dissolved in 100 µl of an SDS/dimethylformamide solution for at least 1 h at 37 °C. Optical density of dissolved samples was measured at 570 nm using a plate reader.
RESULTS
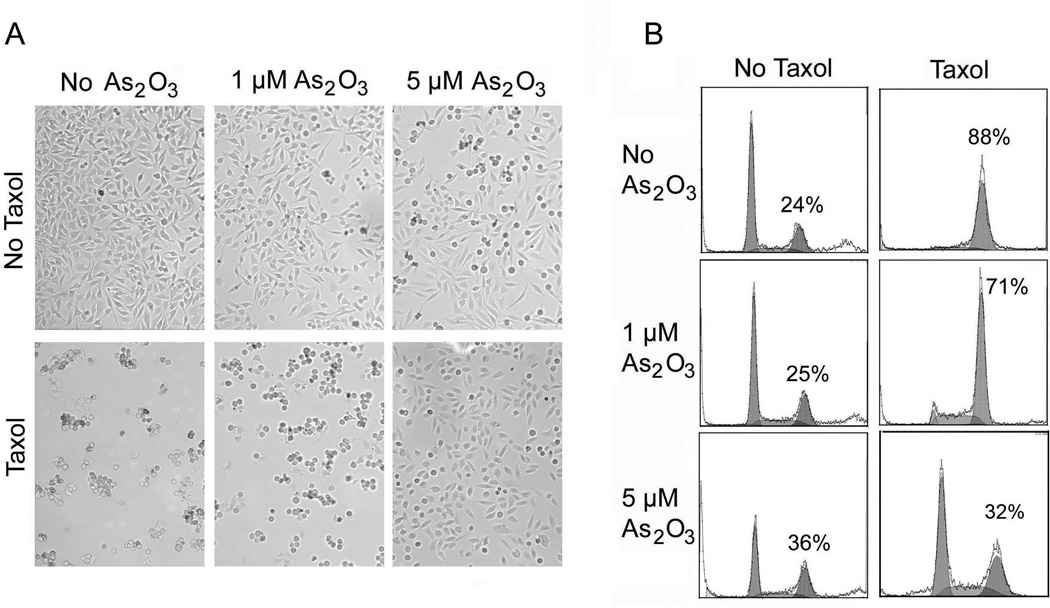
Taxol is capable of inducing mitotic arrest because of its ability to disrupt microtubule dynamics. Given that both taxol and arsenic trioxide [As(III)] are widely used in the clinic for cancer treatment, we examined whether these two compounds had a synergistic effect on causing mitotic arrest as well as mitotic catastrophe. HeLa cells treated with or without palcitaxel and/or As(III) overnight were first examined for their morphologies. After overnight treatment with paclitaxel, almost all the treated cells changed their appearance. They detached themselves from the bottom of the culture plate and became rounded-up (Fig. 1A), consistent with the established role of taxol in the induction of mitotic arrest. Cells treated with As(III) at a high concentration (5 µM) also induced the rounded-up phenotype in a small but significant fraction of cells (Fig. 1A) compared with that of the untreated control cells. Interestingly, paclitaxel-induced detachment of HeLa cells was partially blocked by co-treatment with As(III); this effect was more pronounced when a high concentration of As(III) was included in the taxol-treated culture (Fig. 1A).
Figure 1. As2O3 blocks paclitaxel-induced cell rounding and G2/M arrest.
(A) HeLa cells were treated with 10 nM paclitaxel (taxol) and/or arsenic trioxide [As2O3] at indicated concentrations for 24 h. The images were captured under a light microscope. The rounded-up cells and adherent cells are morphologically distinguishable. (B) Flow cytometry analysis of cells treated with 10 nM paclitaxel and/or As2O3 at indicated concentrations for 16h. The percentage of G2/M cells was indicated.
The fewer rounded-up cells after co-treatment with paclitaxel and As(III) suggest that arsenic may attenuate the effect of paclitaxel-induced cell cycle arrest. To test this possibility, we stained HeLa cells that had been treated with paclitaxel and/or As(III) with propidium iodide. The stained cells were then analyzed by flow cytometry to determine their cell cycle distributions. We observed that paclitaxel alone arrested a majority of cells at the G2/M stage. Paclitaxel-induced mitotic arrest was partially suppressed when HeLa cells were co-treated with As(III), and the inhibition of paclitaxel-induced G2/M arrest by As(III) was dose-dependent (Fig. 1B). Whereas about 71% of cells were in the G2/M phase when paclitaxel-treated HeLa cells were supplemented with 1 µM of As(III), about 32% of paclitaxel-treated cells were in G2/M when they were supplemented with 5 µM of As(III) (Fig. 1B).
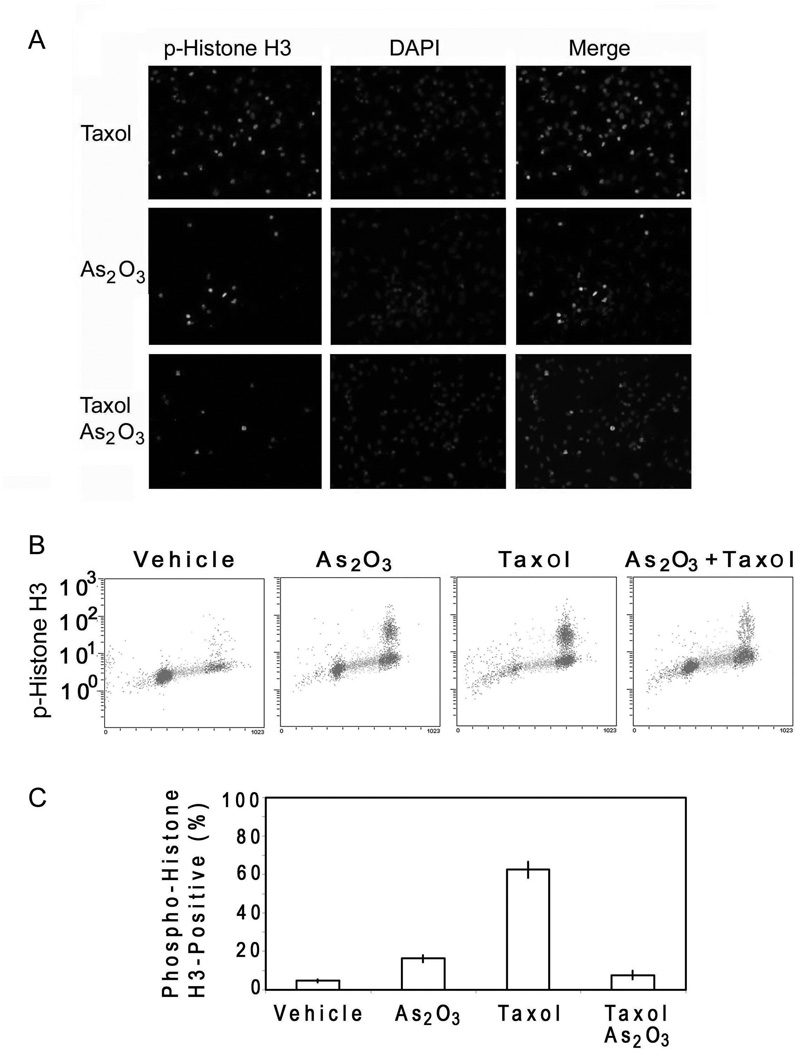
The straight analysis of cell cycle distribution by flow cytometry as shown in Fig. 1B could not differentiate G2 cells from mitotic ones. Thus, we analyzed HeLa cells treated with paclitaxel and/or As(III) for the presence of phospho-histone H3 (p-Histone H3), a specific mitotic marker. Fluorescent microscopy and flow cytometry revealed that paclitaxel-treated cells were highly positive for p-Histone H3 (Fig. 2Ato 2C). Treatment with As(III) also induced an increased number, albeit much smaller than that of paclitaxel alone, of p-Histone H3-positive cells (Fig. 2A to 2C). However, when HeLa cells were treated with paclitaxel in the presence of As(III), the p-Histone H3-positive cell population was significantly reduced compared with that of paclitaxel treated cells (Fig. 2A to 2C). These observations thus indicate that As(III) suppressed paclitaxel-induced mitotic arrest.
Figure 2. As2O3 inhibits paclitaxel-induced mitotic arrest.
(A) HeLa cells treated with or without 10 nM paclitaxel and/or 5µM As(III) for 16h were stained with the antibody to phorphorylated histone H3 (p-histone H3, green). DNA was stained with DAPI (blue). Representative cell images were shown. (B) HeLa cells treated with or without 10 nM paclitaxel and/or 5 µM As(III) for 16h were fixed and labeled with the antibody to p-histone H3 for flow cytometry analysis. (C) Flow cytometry data as shown in B were quantified from three independent experiments.
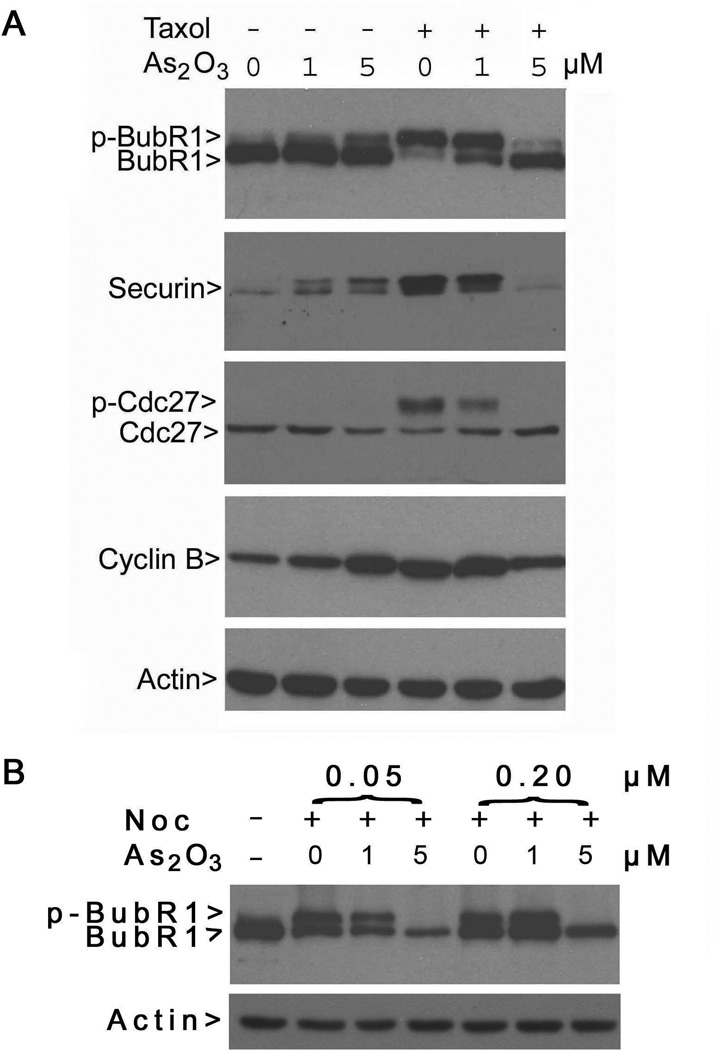
We then analyzed the spindle checkpoint status in cells exposed to paclitaxel and/or As(III). Consistent with its known effect in induction of mitotic arrest, paclitaxel treatment alone caused the activation of the spindle checkpoint manifested as the accumulation of phospho-BubR1 (p-BubR1) and phospho-Cdc27 (p-Cdc27) (Fig. 3A). Paclitaxel also significantly increased cyclin B and securin levels (Fig. 3A). On the other hand, co-treatment of HeLa cells with paclitaxel and As(III) significantly compromised the induction of p-BubR1, p-Cdc27, securin, and cyclin B, indicating a weakened mitotic arrest and spindle checkpoint activation. At a high concentration (5 µM), As(III) completely suppressed paclitaxel-induced phosphorylation of BubR1 and Cdc27. Interestingly, treatment with As(III) alone also weakly induced the accumulation of phospho-BubR1 and securin, thus consistent with its ability to induce mitotic arrest.
Figure 3. As2O3 compromises the spindle checkpoint activation.
(A) HeLa cells were treated with or without paclitaxel and/or As(III) as indicated for 24 h. Equal amounts of protein lysates prepared from the treated cells were blotted for BubR1, Cdc27, securin, cyclin B, and β-actin. The arrows p-BubR1 and p-Cdc27 denote phosphorylated BubR1 and phosphorylated Cdc27, respectively. (B) HeLa cells were treated with or without nocodazole (Noc, 0.05 and 0.2 µM) and/or As(III) for 24 h. Equal amounts of protein lysates prepared from the treated cells were blotted for BubR1 and β-actin.
As nocodazole activates the spindle checkpoint by destabilizing microtubules, we treated HeLa cells with both As(III) and nocodazole (Noc) overnight. Western blot analysis revealed that As(III) at a higher concentration (5 µM) was capable of suppressing nocodazole-induced activation of BubR1 (Fig. 3B). Combined, the above-described studies indicate that arsenic compromises mitotic arrest and the spindle checkpoint activation induced by microtubule poisons.
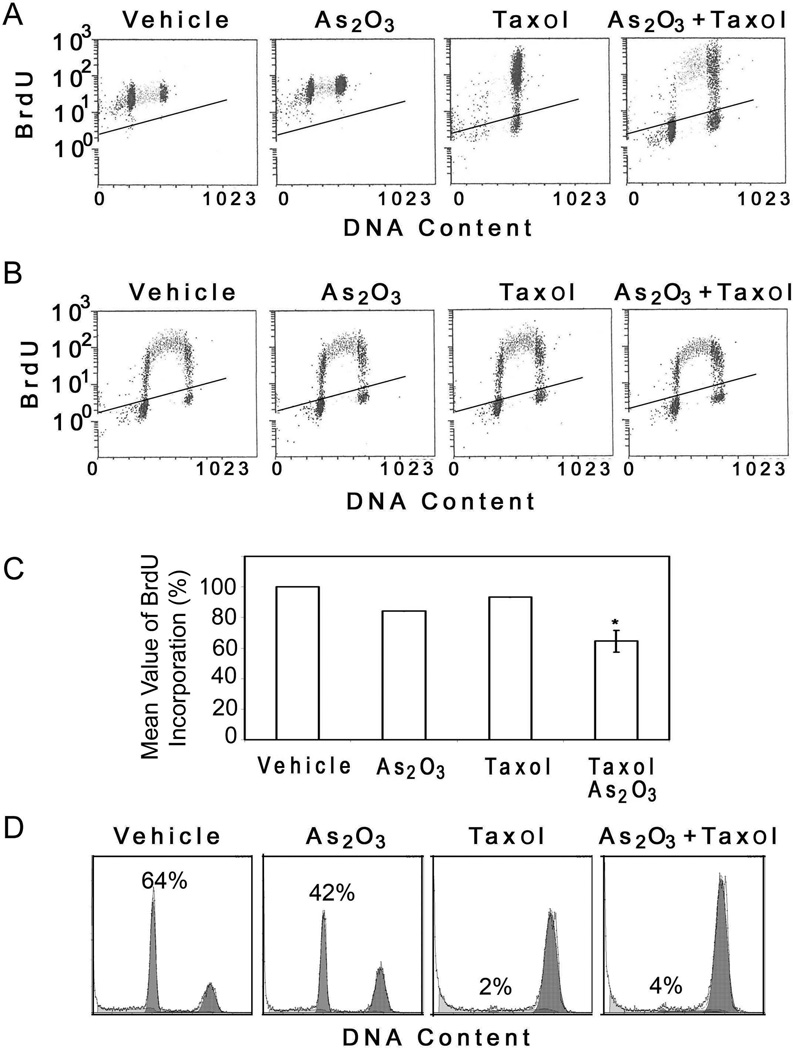
One simple and straightforward interpretation of above results is that As(III) either impeded mitotic entry or accelerated mitotic exit when cells were treated with paclitaxel. To differentiate between these two possibilities, we pulsed cells overnight with BrdU in the presence of As(III) and/or paclitaxel followed by examining a BrdU-positive population using flow cytometry. This experimental approach would allow both the use of asynchronous cell populations and the determination of the alterations in cell cycle progression specific to cells treated with As(III) and/or paclitaxel. As expected, paclitaxel treatment blocked almost all cells (either BrdU-positive or negative) in mitosis (Fig. 4A). Co-treatment with As(III) and paclitaxel greatly enriched mitotic cells with no increase in BrdU-positive G1 population; on the other hand, a significant fraction of S-phase cells remained (Fig. 4A), suggesting that As(III) treatment did not promote mitotic exit and rather slowed down cell cycle progression. To further confirm that As(III) delayed the cell cycle progression, we pulsed HeLa cells for 45 minutes with BrdU in the presence of As(III) and/or paclitaxel. Flow cytometry analysis revealed that compared with that of vehicle-treated control, the magnitude of BrdU incorporation was significantly reduced in cells treated with both As(III) and paclitaxel (Fig. 4B and 4C).
Figure 4. As2O3 delays cell cycle progression.
(A) HeLa cells were pulsed with bromo-deoxyuridine (BrdU) in the presence of vehicle, 5 µM As2O3, and/or 10 nM paclitaxel for 16h. The cells were then collected and stained with a FITC-conjugated antibody to BrdU and propidium iodide (PI) followed by flow cytometry analysis. (B) HeLa cells were pulsed with BrdU for 45 minutes in the presence of vehicle, 5 µM As2O3, and/or 10 nM paclitaxel. After that, cells were collected and stained with the FITC-conjugated BrdU antibody and PI followed by flow cytometery analysis. (C) The data presented in B were quantified from three independent experiments. * denotes the difference between the treatment and the controls (vehicle, As2O3, or paclitaxel alone) is statistically significant (p<0.01). (D) Hela cells synchronized at M phase by double-thymidine block followed by release into nocodazole-containing medium were incubated in the presence of As(III) and/or paclitaxel for 90 min. Cell cycle distributions were analyzed by flow cytometry.
To directly study the effect of As2O3 on mitotic exit, HeLa cells were synchronized at the G1/S junction by double thymidine treatment and then released into the medium containing nocodazole, which arrested cells at mitosis. Subsequently, cells were cultured in a fresh, nocodazole-free medium containing As(III) and/or paclitaxel for 90 min. Flow cytometry analysis revealed that whereas a majority of control cells (64% G1 cells) had exited from mitosis As(III) treatment significantly attenuated mitotic exit (42% G1 cells) (Fig. 4D). As expected, paclitaxel completely blocked mitotic exit with few G1 cells; co-treatment with As(III) did not significantly accelerate mitotic exit (Fig. 4D). These results thus indicate that As(III) slows down cell cycle progression at all stages and that suppression of paclitaxel-induced mitotic arrest by As(III) is not due to enhanced mitotic exit.
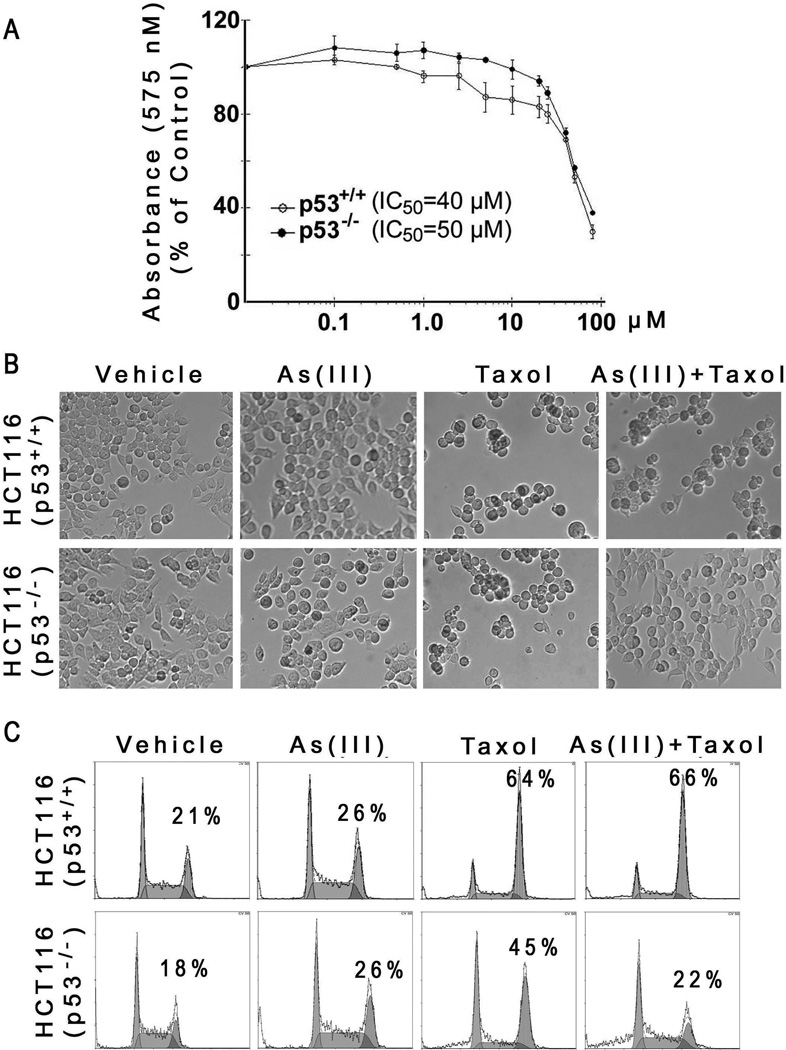
Extensive studies in the past have indicated that the p53 status significantly affects the response of cells to arsenic in vitro (Taylor et al. 2006;McNeely et al. 2006). As HeLa cells do not have functional p53, we asked whether As(III)-mediated suppression of paclitaxel-induced mitotic arrest and checkpoint activation was p53-dependent. To this end, we selected a pair of isogenic cell lines [HCT116 (p53+/+) and HCT116 (p53−/−)] for our studies. We first measured the survival rate of paired HCT116 cells treated with various concentrations of As(III) for 24 h. MTT assays revealed that HCT116 (p53+/+) cells did not exhibit significant cytotoxicity in the presence of As(III) with IC50 about 40 µM (Fig. 5A). Interestingly, isogenic HCT116 (p53−/−) cells were slightly more resistant to As(III) treatment, especially at concentrations below 20 µM (Fig. 5A). We then treated the paired HCT116 cell lines with As(III) and/or paclitaxel and examined their gross morphologies. As expected, treatment with paclitaxel caused significant rounding-up in both cell lines (Fig. 5B). Co-treatment with As(III) had a little effect on modulating the detached (rounded-up) phenotype of HCT116 (p53+/+) cells caused by paclitaxel; however, As(III) greatly attenuated paclitaxel-induced cell rounding/detachment in HCT116 (p53−/−) cells (Fig. 5B). Our subsequent flow cytometric analyses revealed that As(III) significantly suppressed paclitaxel-induced mitotic arrest in HCT116 (p53−/−) cells but not in HCT116 (p53+/+) cells (Fig. 5C). Combined, our studies strongly suggest that p53-deficiency cooperates with As(III) in compromising the spindle checkpoint function and suppressing mitotic arrest induced by paclitaxel.
Figure 5. Suppression of paclitaxel-induced mitotic arrest by As2O3 is p53-dependent.
(A) Isogenic HCT116 (p53+/+) and HCT116 (p53−/−) cells were treated with various concentrations of As(III) for 24 h. Cell survival was measured using the MTT assay. Data were summarized from three independent experiments. (B) Paired HCT116 cells were cultured in the presence or absence of As(III) (5 µM) and/or paclitaxel (10 nM) for 24 h. Representative cell images were shown. (C) Paired HCT116 cells were cultured in the presence or absence of As(III) (5 µM) and/or paclitaxel (10 nM) for 24 h. Cells of various treatments were then processed for flow cytometry analysis. Representative data were shown. The percent of G2/M cell population in each treatment was indicated.
DISCUSSION
Our current study shows that As(III) interferes with paclitaxel-induced activation of the spindle checkpoint as well as mitotic arrest in vitro. We also demonstrate that attenuation of paclitaxel-induced mitotic arrest by As(III) is primarily due to slowed cell cycle progression during S phase, but not enhanced mitotic exit. It is rather intriguing to observe that As(III) attenuates paclitaxel-induced mitotic arrest. The clinical efficacy of paclitaxel is associated with its ability to induce mitotic arrest and subsequent mitotic catastrophe. Given the wide clinical applications of these two compounds in cancer treatment, it is essential for us to understand the mechanism by which As(III) suppresses the spindle checkpoint activation and attenuates mitotic arrest induced by paclitaxel.
As(III) directly interferes with the function of tubulins (Ling et al. 2002a;Carre et al. 2002b;Ochi, Nakajima, and Nasui 1999;Li and Broome 1999b), thus potentially affecting the integrity of microtubules and mitotic spindles. We and others have documented that As(III) treatment alone induces features often found in cells treated with microtubule-stabilizing or destabilizing agents such as taxol and nocodazole (Cai et al. 2003;Halicka et al. 2002;Ling et al. 2002b). Indeed, As(III) inhibits GTP-induced formation of microtubules in vitro as a non-competitive inhibitor (Li and Broome 1999a). It has been hypothesized that As(III) is capable of cross-linking two vicinal cysteine residues (Cys-12 and Cys-213), which inactivates the GTP binding site (Li and Broome 1999a). It has also been shown that As(III) antagonizes the effect of taxol on tubulin and microtubules and that it binds to SH group and blocks stoichiometric interaction of paclitaxel with tubulin (Carre et al. 2002a). Despite differences in its exact mode of action, As(III) significantly affects the dynamics of microtubule in vivo, thus sharing similar properties with many well-known microtubule disrupting agents. This may account for the fact that As(III) blocks cell cycle progression and induces mitotic arrest, frequently followed by mitotic catastrophe (Halicka et al. 2002;Kligerman and Tennant 2007).
It remains unclear how As(III) interferes with the action of paclitaxel. Despite As(III) and taxol affect microtubule dynamics they exhibit no synergistic effect on blocking cell proliferation and inducing apoptosis. An early study shows that when they are used in combination, As(III) and paclitaxel behave antagonistically (Carre et al. 2002a). As(III) suppresses paclitaxel-induced perturbation of microtubule structures and mitotic arrest; likewise, paclitaxel reduces the inhibitory effect of As(III) on tubulin polymerization (Carre et al. 2002a). Interestingly, the interaction between As(III) with SH groups of tubulin is not affected by the binding of paclitaxel to tubulin (Carre et al. 2002a), suggesting the antagonism is not simply due to stereo-hindrance between these compounds blocking access of each of them to tubulin.
The p53 status is known to affect arsenite-induced genomic instability and apoptosis. p53 is induced or activated upon treatment with As(III) (Yih and Lee 2000;Salazar et al. 1997). A series of in vitro studies reveal that cell lines with p53 mutations or p53 deficiency are more sensitive to arsenite-induced apoptosis than those cell lines with wild-type p53 (Taylor et al. 2006;Salazar et al. 1997). A recent study demonstrates that the activation of cell cycle checkpoints including G1 and G2 by arsenite does not require p53; however, arsenite-induced mitotic catastrophe occurs preferentially in cells without functional p53 (Taylor et al. 2006), suggesting that p53 is involved in guarding certain aspects of normal mitotic progression and initiating mitotic catastrophe when they are deregulated. Supporting this, p53 appears to influence mitotic exit induced by arsenite; different from p53 positive cells, p53 negative cells exit from mitosis more slowly when they exposed to arsenite (McNeely et al. 2006), consistent with the notion that persistent mitotic arrest can result in mitotic catastrophe. In the current study, we have shown that p53 deficiency somewhat compromises mitotic arrest induced by paclitaxel (Fig. 5C). This may partly explain why As(III) has a preferential effect on attenuating paclitaxel-induced mitotic arrest.
The observation that p53 deficiency greatly facilitate the suppression of paclitaxel-induced spindle checkpoint activation and mitotic arrest by As(III) should have a profound clinical implication. For example, mutations in or inactivation of TP53, the gene encoding p53, are found in at least 50% of all human cancers. Moreover, deficiency in, or haploinsufficiency of, spindle check point genes often results in enhanced tumorigenesis (Wassmann and Benezra 2001). In fact, many tumor cells harbor deficiencies in spindle checkpoint control (Cahill et al. 1998). If the observed antagonism between As(III) and paclitaxel also occurs in vivo, it is imperative for us to take into consideration of the spindle checkpoint status/integrity and the p53 status of tumor cells when the patients undergo chemotherapy with arsenic compounds.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Katherine Xie for her assistance in the current study and co-workers in the laboratory for discussions. This work was supported in part by grants from the National Institutes of Health to WD (CA074229, CA090658).
REFERENCES
- 1.Cahill DP, Lengauer C, Yu J, Riggins GJ, Willson JK, Markowitz SD, Kinzler KW, Vogelstein B. Mutations of mitotic checkpoint genes in human cancers. Nature. 1998;392:300–303. doi: 10.1038/32688. [DOI] [PubMed] [Google Scholar]
- 2.Cai X, Shen YL, Zhu Q, Jia PM, Yu Y, Zhou L, Huang Y, Zhang JW, Xiong SM, Chen SJ, Wang ZY, Chen Z, Chen GQ. Arsenic trioxide-induced apoptosis and differentiation are associated respectively with mitochondrial transmembrane potential collapse and retinoic acid signaling pathways in acute promyelocytic leukemia. Leukemia. 2000;14:262–270. doi: 10.1038/sj.leu.2401650. [DOI] [PubMed] [Google Scholar]
- 3.Cai X, Yu Y, Huang Y, Zhang L, Jia PM, Zhao Q, Chen Z, Tong JH, Dai W, Chen GQ. Arsenic trioxide-induced mitotic arrest and apoptosis in acute promyelocytic leukemia cells. Leukemia. 2003;17:1333–1337. doi: 10.1038/sj.leu.2402983. [DOI] [PubMed] [Google Scholar]
- 4.Carre M, Carles G, Andre N, Douillard S, Ciccolini J, Briand C, Braguer D. Involvement of microtubules and mitochondria in the antagonism of arsenic trioxide on paclitaxel-induced apoptosis. Biochem. Pharmacol. 2002b;63:1831–1842. doi: 10.1016/s0006-2952(02)00922-x. [DOI] [PubMed] [Google Scholar]
- 5.Carre M, Carles G, Andre N, Douillard S, Ciccolini J, Briand C, Braguer D. Involvement of microtubules and mitochondria in the antagonism of arsenic trioxide on paclitaxel-induced apoptosis. Biochem. Pharmacol. 2002a;63:1831–1842. doi: 10.1016/s0006-2952(02)00922-x. [DOI] [PubMed] [Google Scholar]
- 6.Castedo M, Perfettini JL, Roumier T, Andreau K, Medema R, Kroemer G. Cell death by mitotic catastrophe: a molecular definition. Oncogene. 2004;23:2825–2837. doi: 10.1038/sj.onc.1207528. [DOI] [PubMed] [Google Scholar]
- 7.Chen GQ, Zhu J, Shi XG, Ni JH, Zhong HJ, Si GY, Jin XL, Tang W, Li XS, Xong SM, Shen ZX, Sun GL, Ma J, Zhang P, Zhang TD, Gazin C, Naoe T, Chen SJ, Wang ZY, Chen Z. In vitro studies on cellular and molecular mechanisms of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia: As2O3 induces NB4 cell apoptosis with downregulation of Bcl-2 expression and modulation of PML-RAR alpha/PML proteins. Blood. 1996;88:1052–1061. [PubMed] [Google Scholar]
- 8.Guo W, Tang XD, Tang S, Yang Y. Preliminary report of combination chemotherapy including Arsenic trioxide for stage III osteosarcoma and Ewing sarcoma. Zhonghua Wai Ke. Za Zhi. 2006;44:805–808. [PubMed] [Google Scholar]
- 9.Halicka HD, Smolewski P, Darzynkiewicz Z, Dai W, Traganos F. Arsenic trioxide arrests cells early in mitosis leading to apoptosis. Cell Cycle. 2002;1:201–209. [PubMed] [Google Scholar]
- 10.Huang C, Li J, Ding M, Wang L, Shi X, Castranova V, Vallyathan V, Ju G, Costa M. Arsenic-induced NFkappaB transactivation through Erks- and JNKs-dependent pathways in mouse epidermal JB6 cells. Mol Cell Biochem. 2001;222:29–34. [PubMed] [Google Scholar]
- 11.Huang X. The revival of the ancient drug--arsenic. Chin Med. J. (Engl.) 2003;116:1637–1638. [PubMed] [Google Scholar]
- 12.Jordan MA, Wilson L. Microtubules as a target for anticancer drugs. Nat. Rev. Cancer. 2004;4:253–265. doi: 10.1038/nrc1317. [DOI] [PubMed] [Google Scholar]
- 13.Kitamura K, Yoshida H, Ohno R, Naoe T. Toxic effects of arsenic (As3+) and other metal ions on acute promyelocytic leukemia cells. Int. J Hematol. 1997;65:179–185. doi: 10.1016/s0925-5710(96)00547-6. [DOI] [PubMed] [Google Scholar]
- 14.Kligerman AD, Tennant AH. Insights into the carcinogenic mode of action of arsenic. Toxicol. Appl. Pharmacol. 2007;222:281–288. doi: 10.1016/j.taap.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 15.Larochette N, Decaudin D, Jacotot E, Brenner C, Marzo I, Susin SA, Zamzami N, Xie Z, Reed J, Kroemer G. Arsenite induces apoptosis via a direct effect on the mitochondrial permeability transition pore. Exp. Cell Res. 1999;249:413–421. doi: 10.1006/excr.1999.4519. [DOI] [PubMed] [Google Scholar]
- 16.Li YM, Broome JD. Arsenic targets tubulins to induce apoptosis in myeloid leukemia cells. Cancer Res. 1999b;59:776–780. [PubMed] [Google Scholar]
- 17.Li YM, Broome JD. Arsenic targets tubulins to induce apoptosis in myeloid leukemia cells. Cancer Res. 1999a;59:776–780. [PubMed] [Google Scholar]
- 18.Ling YH, Jiang JD, Holland JF, Perez-Soler R. Arsenic trioxide produces polymerization of microtubules and mitotic arrest before apoptosis in human tumor cell lines. Mol. Pharmacol. 2002a;62:529–538. doi: 10.1124/mol.62.3.529. [DOI] [PubMed] [Google Scholar]
- 19.Ling YH, Jiang JD, Holland JF, Perez-Soler R. Arsenic trioxide produces polymerization of microtubules and mitotic arrest before apoptosis in human tumor cell lines. Mol. Pharmacol. 2002b;62:529–538. doi: 10.1124/mol.62.3.529. [DOI] [PubMed] [Google Scholar]
- 20.Maeda H, Hori S, Nishitoh H, Ichijo H, Ogawa O, Kakehi Y, Kakizuka A. Tumor growth inhibition by arsenic trioxide (As2O3) in the orthotopic metastasis model of androgen-independent prostate cancer. Cancer Res. 2001;61:5432–5440. [PubMed] [Google Scholar]
- 21.McNeely SC, Xu X, Taylor BF, Zacharias W, McCabe MJ, Jr, States JC. Exit from arsenite-induced mitotic arrest is p53 dependent. Environ. Health Perspect. 2006;114:1401–1406. doi: 10.1289/ehp.8969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murgo AJ. Clinical trials of arsenic trioxide in hematologic and solid tumors: overview of the National Cancer Institute Cooperative Research and Development Studies. Oncologist. 2001;6(Suppl 2):22–28. doi: 10.1634/theoncologist.6-suppl_2-22. [DOI] [PubMed] [Google Scholar]
- 23.Ochi T, Nakajima F, Nasui M. Distribution of gamma-tubulin in multipolar spindles and multinucleated cells induced by dimethylarsinic acid, a methylated derivative of inorganic arsenics, in Chinese hamster V79 cells. Toxicology. 1999;136:79–88. doi: 10.1016/s0300-483x(99)00061-x. [DOI] [PubMed] [Google Scholar]
- 24.Ouyang B, Pan H, Lu L, Li J, Stambrook P, Li B, Dai W. Human Prk is a conserved protein serine/threonine kinase involved in regulating M phase functions. J Biol. Chem. 1997;272:28646–28651. doi: 10.1074/jbc.272.45.28646. [DOI] [PubMed] [Google Scholar]
- 25.Park JW, Choi YJ, Jang MA, Baek SH, Lim JH, Passaniti T, Kwon TK. Arsenic Trioxide Induces G2/M Growth Arrest and Apoptosis after Caspase-3 Activation and Bcl-2 Phosphorylation in Promonocytic U937 Cells. Biochemical and Biophysical Research Communications. 2001;286:726–734. doi: 10.1006/bbrc.2001.5416. [DOI] [PubMed] [Google Scholar]
- 26.Ramirez P, Eastmond DA, Laclette JP, Ostrosky-Wegman P. Disruption of microtubule assembly and spindle formation as a mechanism for the induction of aneuploid cells by sodium arsenite and vanadium pentoxide. Mutat. Res. 1997;386:291–298. doi: 10.1016/s1383-5742(97)00018-5. [DOI] [PubMed] [Google Scholar]
- 27.Rousselot P, Labaume S, Marolleau JP, Larghero J, Noguera MH, Brouet JC, Fermand JP. Arsenic trioxide and melarsoprol induce apoptosis in plasma cell lines and in plasma cells from myeloma patients. Cancer Res. 1999;59:1041–1048. [PubMed] [Google Scholar]
- 28.Salazar AM, Ostrosky-Wegman P, Menendez D, Miranda E, Garcia-Carranca A, Rojas E. Induction of p53 protein expression by sodium arsenite. Mutat. Res. 1997;381:259–265. doi: 10.1016/s0027-5107(97)00207-8. [DOI] [PubMed] [Google Scholar]
- 29.Shen ZX, Chen GQ, Ni JH, Li XS, Xiong SM, Qiu QY, Zhu J, Tang W, Sun GL, Yang KQ, Chen Y, Zhou L, Fang ZW, Wang YT, Ma J, Zhang P, Zhang TD, Chen SJ, Chen Z, Wang ZY. Use of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia (APL): II. Clinical efficacy and pharmacokinetics in relapsed patients. Blood. 1997a;89:3354–3360. [PubMed] [Google Scholar]
- 30.Shen ZY, Shen J, Cai WJ, Hong C, Zheng MH. The alteration of mitochondria is an early event of arsenic trioxide induced apoptosis in esophageal carcinoma cells. Int. J Mol Med. 2000;5:155–158. doi: 10.3892/ijmm.5.2.155. [DOI] [PubMed] [Google Scholar]
- 31.Shen ZX, Chen GQ, Ni JH, Li XS, Xiong SM, Qiu QY, Zhu J, Tang W, Sun GL, Yang KQ, Chen Y, Zhou L, Fang ZW, Wang YT, Ma J, Zhang P, Zhang TD, Chen SJ, Chen Z, Wang ZY. Use of Arsenic Trioxide (As2O3) in the Treatment of Acute Promyelocytic Leukemia (APL): II. Clinical Efficacy and Pharmacokinetics in Relapsed Patients. Blood. 1997b;89:3354–3360. [PubMed] [Google Scholar]
- 32.Siu KP, Chan JY, Fung KP. Effect of arsenic trioxide on human hepatocellular carcinoma HepG2 cells: inhibition of proliferation and induction of apoptosis. Life Sci. 2002;71:275–285. doi: 10.1016/s0024-3205(02)01622-3. [DOI] [PubMed] [Google Scholar]
- 33.Soignet SL, Maslak P, Wang ZG, Jhanwar S, Calleja E, Dardashti LJ, Corso D, DeBlasio A, Gabrilove J, Scheinberg DA, Pandolfi PP, Warrell RP. Complete Remission after Treatment of Acute Promyelocytic Leukemia with Arsenic Trioxide. N Engl J Med. 1998;339:1341–1348. doi: 10.1056/NEJM199811053391901. [DOI] [PubMed] [Google Scholar]
- 34.Taylor BF, McNeely SC, Miller HL, Lehmann GM, McCabe MJ, Jr, States JC. p53 Suppression of Arsenite-Induced Mitotic Catastrophe Is Mediated by p21CIP1/WAF1. J Pharmacol Exp Ther. 2006;318:142–151. doi: 10.1124/jpet.106.103077. [DOI] [PubMed] [Google Scholar]
- 35.Uslu R, Sanli UA, Sezgin C, Karabulut B, Terzioglu E, Omay SB, Goker E. Arsenic trioxide-mediated cytotoxicity and apoptosis in prostate and ovarian carcinoma cell lines. Clin. Cancer Res. 2000;6:4957–4964. [PubMed] [Google Scholar]
- 36.Wassmann K, Benezra R. Mitotic checkpoints: from yeast to cancer. Curr. Opin. Genet. Dev. 2001;11:83–90. doi: 10.1016/s0959-437x(00)00161-1. [DOI] [PubMed] [Google Scholar]
- 37.Yih LH, Lee TC. Arsenite Induces p53 Accumulation through an ATM-dependent Pathway in Human Fibroblasts. Cancer Res. 2000;60:6346–6352. [PubMed] [Google Scholar]
- 38.Zhang TD, Chen GQ, Wang ZG, Wang ZY, Chen SJ, Chen Z. Arsenic trioxide, a therapeutic agent for APL. Oncogene. 2001;20:7146–7153. doi: 10.1038/sj.onc.1204762. [DOI] [PubMed] [Google Scholar]
- 39.Zhu XH, Shen YL, Jing YK, Cai X, Jia PM, Huang Y, Tang W, Shi GY, Sun YP, Dai J, Wang ZY, Chen SJ, Zhang TD, Waxman S, Chen Z, Chen GQ. Apoptosis and growth inhibition in malignant lymphocytes after treatment with arsenic trioxide at clinically achievable concentrations. J Natl. Cancer Inst. 1999;91:772–778. doi: 10.1093/jnci/91.9.772. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.