Abstract
Body fatness and its distribution are strongly and independently associated with peripheral insulin action. However, these associations are limited in their ability to predict the independent nature of hepatic and peripheral insulin resistance, especially in obese women. To define the relationships more precisely between regional fat distribution and adiponectin, and hepatic and peripheral insulin resistance, we studied 22 obese (43 ± 0.1%) women who underwent a dual-energy X-ray absorptiometry scan and a computed tomography scan at the L4–L5 level. An octreotide (60 ng · kg−1 · min−1), glucagon (0.65 ng · kg−1 · min−1), and two-step insulin (0.25 mU · kg−1 · min−1 and 1.0 mU · kg−1 · min−1) infusion was performed to quantify insulin-mediated suppression of hepatic glucose production (SGP) and insulin-stimulated glucose disposal (ISGD) in a simultaneous fashion. Hepatic glucose production (HGP) was measured using a primed, constant infusion of [6,62H2] glucose. Mean plasma insulin increased from 5.6 ± 0.1 μU/mL at baseline to 15.1 ± 1.5 μU/mL in the first stage, and to 80.7 ± 0.5 μU/mL in the second stage. Although there was no significant relationship between visceral adipose tissue (VAT) and basal HGP (r = 0.34, p = 0.117), there was a significant inverse correlation (r = −0.67, p = 0.003) between VAT and SGP. There was a significant correlation (r = 0.55, p = 0.008) between adiponectin and ISGD. In conclusion, these data support: (1) the inability of basal glucose metabolism to accurately reflect hepatic insulin resistance, (2) the deleterious role of VAT in the development of insulin resistance in the liver, and (3) provide additional support for the positive influence of adiponectin against peripheral insulin resistance in obese, postmenopausal women.
Introduction
Obesity and the increased risk of type 2 diabetes are characterized by the impaired suppression of hepatic glucose production (HGP), reduced peripheral glucose transport, and defects in insulin secretion.1 In addition, the menopause-induced reduction in estrogen promotes abdominal obesity in women and may disproportionately increase their risk of metabolic disease.2
Approximately two thirds of women between the ages of 50 and 69 are either overweight (body mass index [BMI] = 25–29.9 kg/m2) or obese (BMI > 30 kg/m2).3 Although obesity confers an increased risk for the development of type 2 diabetes, this provides little information on the potential effects of changes in body composition on the etiology of hepatic and peripheral insulin resistance. It is also known that whereas premenopausal women have significantly less abdominal fat than men,4 obese, postmenopausal women are more likely to accumulate fat into the abdominal region.3 This is important because the accumulation of visceral adipose tissue (VAT) of more than 110 cm2 as derived from computed tomography (CT) scans represents a critical point in the onset of metabolic abnormalities.5 As such, the contribution of VAT to excess free fatty acid (FFA) delivery into the portal and systemic circulation may be elevated in women,6 and would likely influence the development of hepatic insulin resistance. Despite compelling evidence outlining the existence of hepatic insulin resistance under prandial insulin concentrations7 in obese males and females, the link between hepatic insulin resistance and visceral fat has not been established under mild hyperinsulinemic conditions (i.e., two-fold above baseline). Given the range of basal insulin concentrations, and the shift of adipose tissue from gluteal/femoral regions to abdominal depots associated with menopause,8 the contribution of VAT toward hepatic insulin resistance in this population requires investigation.
In addition to potential relevance of regional adipose tissue deposition on systemic insulin resistance, decreased adiponectin may potentiate metabolic dysregulation and exacerbate lipid-induced insulin resistance in the liver,9 and skeletal muscle.10–12 Taken together, menopause accentuates the storage of excess abdominal fat and may augment the development of hepatic insulin resistance,5 whereas diminished adiponectin levels linked to increased adipose tissue13 may be associated with the onset of both hepatic and peripheral insulin resistance.14
In obese, postmenopausal women, we hypothesized that: (1) an inverse correlation would exist between visceral fat and insulin-mediated suppression of HGP (SGP, suppression of hepatic glucose production) and insulin-stimulated glucose disposal (ISGD), and that (2) adiponectin would be positively correlated with SGP and ISGD. To test these two hypotheses, SGP and ISGD were evaluated using an octreotide, multistage insulin infusion with basal glucagon replacement following a controlled eucaloric diet and an overnight fast. In addition, we used CT-derived scans of visceral, abdominal subcutaneous, and thigh fat, as well as enzyme-linked immunosorbent assay (ELISA)-derived measurements of plasma adiponectin.
Methods
Subjects
Twenty-two female obese subjects with a mean body mass index (BMI) of 30 ± 1 kg/m2 were studied. Subjects were recruited according to age (50–60 years) and BMI (26–40 kg/m2). No subpopulation was targeted for recruitment, and subjects were recruited without regard to sex, race, or ethnic status. Patients with a history of cardiovascular disease, diabetes, or cancer were excluded. Nonetheless, it is important to realize that obese volunteers within our stated BMI range often have other medical conditions. Although it was impractical to exclude all patients taking any type of medication, subjects taking fibrates, niacin, statins, or other pharmaceuticals that might have potential effects on carbohydrate or lipid metabolism were excluded.
All research volunteers were recruited from the greater Little Rock, Arkansas, area using local newspaper advertisements and flyers posted around the community and within The University of Arkansas for Medical Sciences (UAMS). Potential volunteers were screened over the telephone according to the above-mentioned exclusion criteria. Upon arrival for testing according to eligibility criteria, all volunteers signed a screening consent and completed a medical exam. Those deemed eligible for the study were fully informed of the experimental procedures and possible risks associated with our research project, and were required to sign an informed consent form prior to participation. All study procedures and materials were approved by the Human Research Advisory Committee at UAMS and the Central Arkansas Veterans Healthcare System.
Oral glucose tolerance test
A standard 2-hour oral glucose tolerance test (OGTT) using 75 grams of glucose was completed during the medical screening to determine subject eligibility. On the basis of the results of the OGTT, subjects with diabetes (i.e., fasting plasma glucose ≥ 126 mg/dL and/or 2-hour plasma glucose of ≥ 200 mg/dL) were excluded.15 Among the 22 subjects in the study, 7 had normal glucose tolerance (i.e., plasma glucose of 100–139 mg/dL at 120 min of the OGTT) and 15 had impaired glucose tolerance (plasma glucose of 140–199 mg/dL at 120 min of the OGTT).
Body weight and composition
Total body mass was measured to the nearest 0.1 kg using an electronic scale (Ohaus Corp, USA). ISGD data was calculated relative to lean tissue mass using dual-energy X-ray absorptiometry (DEXA).
Computed tomography
VAT and subcutaneous abdominal adipose tissue (SAT) were determined from CT scans. The images from these scans allowed fat, lean tissue, and bone to be clearly identified and quantified. The scans were completed using a GE High-Speed Advantage scanner (General Electric Medical Systems, Milwaukee, WI). A lateral scout was used to identify the L4–L5 vertebral disc space, and a cross-sectional 10-mm scan was obtained using 280 mA. Total VAT was determined using public domain imaging software (National Institutes of Health [NIH] Image was available by FTP at http://zippy.nimh.nih.com). VAT was highlighted and computed using an attenuation range of −190 to −30 Hounsfield units (HU). Total SAT and thigh fat (TF) cross-sectional area was determined by identifying the area between the skin and the external-most aspect of the abdominal or thigh muscle wall. The CT images obtained were digitized by HU density to separate fat, muscle, and bone compartments using the NIH image program on a Macintosh Centris 660av computer. In our laboratory, the coefficient of variation for the measurement of fat area is 1.0–1.5%.
Blood samples for determination of plasma adiponectin were drawn after an overnight fast and immediately prior to the MSI procedure. Blood plasma was immediately frozen and stored at −80°C, then later analyzed for plasma adiponectin by ELISA (Linco Research, Inc., St. Charles, MO). This assay has a sensitivity of 0.01 mg/dL and intra- and interassay coefficients of variation of <8%.
SGP and ISGD
To measure SGP and ISGD in a sequential fashion within a single experiment, we utilized an octreotide, multistage insulin infusion with basal glucagon replacement. To measure HGP, a priming dose (3.27mg/kg of [6,6 2H2]glucose) was administered followed by an infusion of 0.22 μmol · kg−1 · min−1 from time = −120–120 minutes, and then increased to 0.44 μmol · kg−1 · min−1 from time = 120–240 minutes to minimize changes in enrichment. To ensure precise control of pancreatic hormone levels, infusions of octreotide (60 ng · kg−1 · min−1), and glucagon (0.65 ng · kg−1 · min−1) were started at t = 0 min and continued throughout the clamp. Insulin was infused at a rate of 0.25 mU · kg−1 · min−1 from t = 4– 120 minutes (first stage) to evaluate SGP, and and 1.0 mU · kg−1 · min−1 from t = 120–240 minutes in the second stage to evaluate ISGD [Basu, 2001 #1454]. We used a 4-minute delay in the insulin infusion to minimize changes in peripheral insulin concentration that might induce subtle changes in glycemia. Euglycemia was maintained by a variable 20% dextrose infusion spiked with 8 mg of [6,6 2H2]glucose/gram of dextrose to prevent an underestimation of HGP.16 The initial insulin infusion rate represented insulin levels that were approximately two-fold greater than fasting levels, and provided an ideal scenario to measure SGP by mild hyperinsulinemia. Following the initial insulin infusion rate, the rate was increased fourfold,17 allowing us the opportunity to examine ISGD. All glucose enrichments were determined using gas chromatography/mass spectrometry.
HGP was estimated for the basal period, and modified for non-steady-state estimations18 using the original equations of Steele.19 SGP was calculated as 1 − (mild hyperinsulinemia glucose Ra/[insulin])/basal glucose Ra/[insulin]) × 100, and indicates the degree of HGP suppressed under basal glucagon/mild hyperinsulinemic conditions. The M value (adjusted for lean tissue mass), or ISGD, was determined during the last 30 minutes of the 120-minute clamp by subtracting HGP from the exogenous glucose infusion rate. Indirect calorimetry was used to calculate oxidative and nonoxidative disposal.20
Statistical analysis
Reported values are mean ± standard error of the mean (SEM). We used Pearson correlation coefficients to assess the associations between fat distribution and plasma adiponectin, and the indices of glucose metabolism (HGP, SGP, and ISGD).
Results
Subject characteristics
A total of 22 older, obese, postmenopausal females (56 ± 1 years old; BMI = 30 ± 1 kg/m2) were recruited for participation in the study (Table 1). On the basis of the results of an OGTT performed during the screening process, volunteers fell within the range of normal to impaired glucose tolerance (7 had a plasma glucose of 100–139 mg/dL at 120 min of the OGTT and 15 had a plasma glucose of 140–199 mg/dL at 120 min of the OGTT).
Table 1.
Clinical Characteristics, Indices of Glucose Metabolism, and Regional Fat Distribution of Obese, Postmenopausal Women
| Characteristic | Women (n = 22) |
|---|---|
| Age (years) | 56 ± 1 |
| Weight | 84 ± 2 |
| BMI (kg/m2) | 31 ± 1 |
| Percentage body fat (%) | 43 ± 1 |
| Adiponectin (μg/mL) | 16.9 ± 2.5 |
| Triglycerides (mg/dL) | 119 ± 12 |
| Total cholesterol (mg/dL) | 203 ± 5 |
| HDL (mg/dL) | 55 ± 2 |
| LDL (mg/dL) | 117 ± 6 |
| HbA1c (%) | 5.5 ± 0.1 |
| Fasting plasma glucose | 102 ± 4 |
| OGTT (120-minute plasma glucose) | 130 ± 5 |
| Suppression (%) of HGP during 1st stage of MSI | 68 ± 3 |
| Basal glucose Ra (mg · kg−1 · min−1) | 3.1 ± 0.2 |
| ISGD (mg · kgFEM−1 · min−1) | 7.4 ± 0.5 |
| Visceral fat (cm2) | 203 ± 17 |
| Subcutaneous abdominal adipose tissue (cm2) | 390 ± 25 |
| Thigh fat (cm2) | 159 ± 13 |
Note: HDL, high-density lipoprotein; LDL, low-density lipoprotein; HbA1c, glycosylated hemoglobin; OGTT, oral glucose tolerance test; ISDG, insulin-stimulated glucose disposal.
Pancreatic hormone plasma concentrations and glucose kinetics
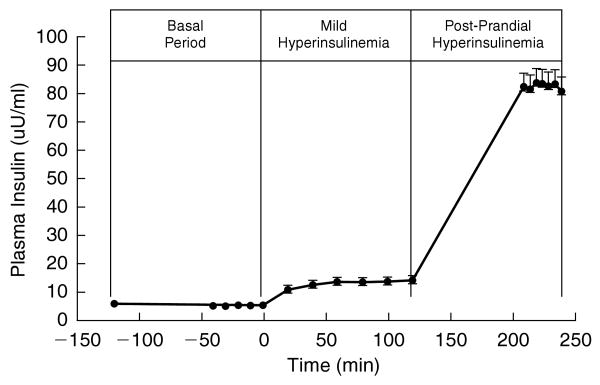
Mean plasma insulin concentrations rose from 5.6 ± 0.7 μU/mL in the basal period to 14.5 ± 1.1 μU/mL in the first stage of the MSI (Fig. 1). As a result, mean HGP fell from 3.1 ± 0.2 mg · kg−1 · min−1 in the basal period to 2.5 ± 0.2 mg · kg−1 · min−1 under mild hyperinsulinemic conditions, indicating the variability of SGP (Table 1). In the second stage of the MSI, mean plasma insulin concentrations rose to 81.2 ± 4.8 μU/mL (Fig. 1), and ISGD increased to 7.4 ± 0.5 mg · kg FFM−1 · min−1 (Table 1). In the presence of the octreotide infusion and basal glucagon replacement, plasma glucagon concentrations remained similar during the basal state (43.2 ± pg/mL), and the first stage of the MSI (44.6 ± 2.8 pg/mL). Although basal glucagon replacement continued during the second stage of the MSI, glucagon was not measured during the second stage of the MSI.
FIG. 1.
Plasma insulin levels during the basal, mild hyperinsulinemia (first stage), and postprandial hyperinsulinemia (second stage) periods of the MSI.
Adipose tissue and insulin resistance in the liver
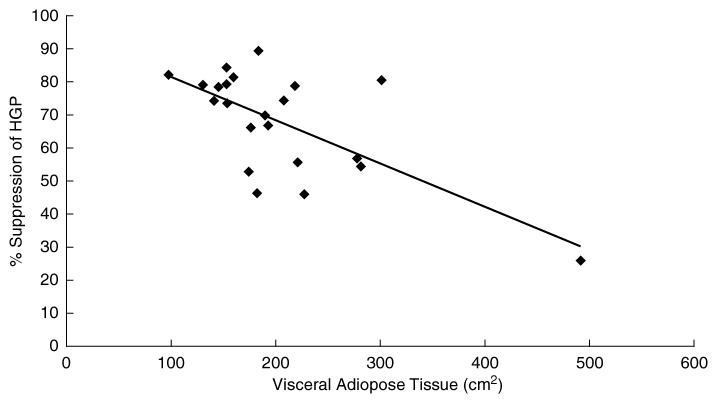
There were no significant relationships between BMI, VAT, SAT, TF, plasma adiponectin, and basal HGP (Table 2). There was an inverse trend between BMI and SGP (r = −0.305, p = 0.08), but it was not significant within the range of BMI of this study. However, there was an inverse relationship between VAT and SGP (r = −0.673, p = 0.003), and this indicates a direct correlation between VAT and hepatic insulin resistance (Fig. 2). On the contrary, there was no relationship between SAT and SGP or TF and SGP (Table 2).
Table 2.
Relationships between Fat Deposition and Plasma Adiponectin and Glucose Metabolism: Correlation Coefficients and Probability Values
| HGP | SGP | ISGD | |
|---|---|---|---|
| Body mass index (BMI), kg/m2 | −0.280 ± 0.21 | −0.305 ± 0.080 | 0.130 ± 0.282 |
| Visceral adipose tissue (VAT), cm2 | 0.344 ± 0.117 | −0.673 ± 0.003* | −0.101 ± 0.328 |
| Subcutaneous abdominal adipose tissue (SAT), cm2 | −0.378 ± 0.083 | −0.098 ± 0.332 | −0.127 ± 0.573 |
| Thigh fat (TF), cm2 | −0.185 ± 0.411 | −0.171 ± 0.224 | 0.168 ± 0.455 |
| Adiponectin, μg/mL | 0.397 ± 0.070 | 0.191 ± 0.396 | 0.547 ± 0.008* |
Statistical comparisons were made between BMI, VF, AF, TF, and adiponectin, and indices of glucose metabolism.
p < 0.05.
FIG. 2.
Relationship between visceral fat and the percent suppression of hepatic glucose production. The trendline indicates significant inverse correlation coefficient between VF and SGP in obese, postmenopausal women (r = −0.673, p = 0.003). The inverse correlation coefficient remains significant (r = −0.4124, p = 0.03) without the inclusion of visceral fat data point of 492 cm2.
Adipose tissue and insulin resistance in the muscle
Among this relatively homogeneous group of obese volunteers, there were no significant relationships between BMI, VAT, SAT, TF, and ISGD (Table 2).
Plasma adiponectin and insulin resistance in liver and muscle
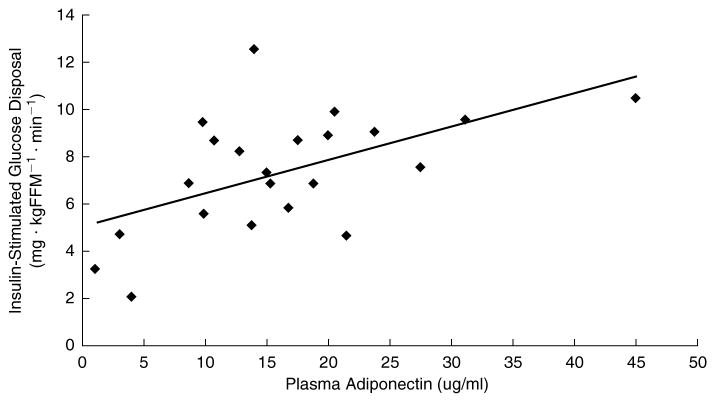
Among this group of obese women there was no significant correlation between plasma adiponectin and basal HGP or SGP (Table 2). On the contrary, there was a very strong positive correlation between plasma adiponectin and ISGD (Fig. 3).
FIG. 3.
Relationships between plasma adiponectin and insulin-stimulated glucose disposal in obese, postmenopausal woman. The trendline indicates significant correlation coefficient between plasma adiponectin and insulin-stimulated glucose disposal in females (r = 0.547, p = 0.008).
Discussion
The data presented in this study demonstrate a strong link between the amount of VAT and the SGP, a direct measurement of hepatic insulin resistance. These data further demonstrate that BMI and/or total body fat content do not have a strong relationship with hepatic insulin resistance. In these obese women, body fat distribution is a better predictor of insulin-mediated SGP. Although it is well accepted that elevations in BMI across a wide range of individuals are associated with an increased risk of type 2 diabetes, this provides little direct information about the severity of insulin resistance in liver and skeletal muscle. Moreover, the majority of studies in the scientific literature that describe the importance of adipose tissue or adipokines have focused on peripheral insulin resistance. Our study examined insulin resistance that develops in both hepatic and peripheral tissues, and it described the relationships to regional adipose tissue as well as plasma adiponectin in obese, postmenopausal women. The lack of a significant relationship between VAT and HGP strongly demonstrates that basal glucose metabolism alone is not sufficient to examine hepatic insulin resistance. In addition, the strong relationship between adiponectin and ISGD underscore the importance of this adipokine in skeletal muscle fuel metabolism.
Combined with the significant link between VAT and SGP, our data coincide with the increased risk of adiposity and metabolic diseases in older women.21 Unlike VAT, there was no relationship between SAT or TF, and hepatic insulin resistance. The reason for this discrepancy may be largely due to well-established link between VAT and the site-specific delivery of FFA into the portal circulation.22 This is supported by evidence describing greater degrees of FFA turnover and lipolysis rates in VAT compared to SAT.23 In addition, VAT may be less sensitive to the antilipolytic influence of insulin.23
Congruent with this scenario, elevated levels of VAT could lead to chronic elevations in FFA delivery into the portal circulation promoting enhanced gluconeogenic activity,24 hepatic lipogenesis,25 and increased liver triglyceride content.26 In turn, this would contribute to decreased hepatic insulin extraction.27 It is also quite possible that elevated VAT directly promotes inflammation through increases in tumor necrosis factor-α28 and resistin29 while diminishing the protective role of adiponectin.9 Moreover, reductions in resistin have been shown to result in improvements in SGP (0.24–0.06 mg · kg−1 · min−1) under moderate hyperinsulinemic conditions in patients with type 2 diabetes.30 Taken together, VAT-derived elevations in FFA released into the portal circulation as well as the dysregulation of inflammatory cytokines may be jointly responsible for the development of hepatic insulin resistance in obese women.
Unlike previous studies linking regional fat distribution to peripheral insulin resistance,31–33 our data did not identify any significant relationships between VAT, SAT, or TF, and peripheral insulin resistance. Previous studies from our group in a somewhat older female population reached the same conclusion.34 The reason for this discrepancy may be due to relatively homogeneous range of regional fat distribution in the present study compared to other studies utilizing a wider range of volunteers.31 In addition, it should be noted that while significant correlations have been identified between VAT and metabolic variables in pre- and post-menopausal women,33,35 these conclusions were reached using OGTT-derived data to assess insulin sensitivity. Unfortunately, hepatic and peripheral insulin sensitivity cannot be differentiated using data from the OGTT.36 Therefore, the use of this particular method for measuring insulin sensitivity limits the interpretation of these studies. In fact, the significant relationship between VAT and SGP in the present study may outline the importance of visceral fat in modulating rates of HGP during the OGTT. This could potentially contribute to hyperglycemia that might not even be associated with large impairments in ISGD.
Although conclusions from studies linking regional fat distribution to peripheral insulin sensitivity using OGTT-derived data are limited, investigators have also linked reductions in peripheral insulin sensitivity as measured by the hyperinsulinemic, euglycemic clamp to regional fat deposition.31,37 Although these links may be strong in studies comparing individuals within a wide BMI range (i.e., ∼20–41 kg/m) and ISGD range (i.e., ∼3–17 mg · kgFFM−1 · min−1), the associations between VAT, SAT, and TF and ISGD become weaker among a relatively narrow homogeneous range of women.34 As such, wider BMI and ISGD ranges, as well as gender differences may be responsible for the discrepancies between our findings and other studies that did not differentiate for gender and utilized a more heterogeneous population of individuals.
No significant relationships between VAT, SAT, or TF and ISGD were noted in our obese women. However, we did find a significant, seemingly protective relationship between plasma adiponectin and ISGD. This corroborates our previous findings, and it is interesting to note that the positive relationship was quite similar in the present study (i.e., r = 0.55; p = 0.008) to our previously reported data in older women (i.e., r = 0.48; p = 0.01).34 Similar findings have also been reported in a wide range of individuals,38–40 and our findings in women as well as others,41 and confirm that the relationship between plasma adiponectin and ISGD is well established in obese women.
No significant correlation was noted between adiponectin and SGP in the present study. In clinical studies where individuals with type 2 diabetes were given pioglitazone for 16 weeks, plasma adiponectin increased three-fold and correlated negatively with residual HGP during the hyperinsulinemic, euglycemic clamp. In so far as the results of the studies from Bajaj et al.,9 hepatic insulin resistance was characterized by the degree of residual HGP under almost postprandial insulin concentrations in individuals with type 2 diabetes. This experimental scenario differs considerably from that of the present study in that our insulin concentrations were only about two-fold greater than basal levels (roughly one third of the previously mentioned study) and glucagon concentrations were maintained at baseline. Furthermore, our population was restricted to only obese women who fell within the range of normal to impaired glucose tolerance. Although adiponectin may play a more decisive role as metabolic dysregulation persists in the presence of type 2 diabetes, it is difficult to make comparisons between our results and the studies of Bajaj et al. due to differences in experimental design and the health status of the individuals.
Even though elevations in BMI are useful toward evaluating the overall risk of type 2 diabetes in the general population, our data demonstrate the specific relevance of VAT and plasma adiponectin in the modulation of insulin resistance in the liver and muscle obese, postmenopausal women, respectively. Using a well-controlled MSI procedure to manipulate insulin and glucagon concentrations precisely, we were able to demonstrate a powerful association between VAT and hepatic insulin resistance in obese, postmenopausal women. Although this technique has obvious advantages when evaluating insulin resistance in liver and muscle, it should be mentioned that portal insulin concentrations would be significantly higher and may undergo more rapid fluctuations under conditions of daily living. In fact, the dynamic nature of portal insulin concentrations highlights the potential importance of SGP toward the regulation of glycemia, especially in obese, postmenopausal women. This is supported by large- scale, multisite investigations that revealed a significant relationship between the accumulation of VAT and elevations in fasting insulin.42 Although this association may be associated with peripheral insulin resistance, our data support a more site-specific role for VAT in the development of hepatic insulin resistance. Also, decrements in plasma adiponectin are consistently associated with peripheral insulin resistance. In summary, the evaluation of basal HGP does not provide sufficient data to evaluate insulin resistance in the liver, VAT is closely linked to hepatic insulin resistance, and the strong inverse relationship between adiponectin and ISGD demonstrates its primary role against the development of peripheral insulin resistance.
Acknowledgments
This research was supported by National Institutes of Health grants KO1 DK 64716-01 (R.H.C.) and AHA grant SDA 0335172N (R.H.C.). We also acknowledge the support of the University of Arkansas for Medical Sciences General Clinical Research Center funded through grant M01 RR14288.
References
- 1.Ivy JL, Zderic TW, Fogt DL. Prevention and treatment of non-insulin-dependent diabetes mellitus. Exerc Sport Sci Rev. 1999;27:1–35. [PubMed] [Google Scholar]
- 2.Tchernof A, Desmeules A, Richard C, Laberge P, Daris M, Mailloux J, Rheaume C, Dupont C. Ovarian hormone status and abdominal visceral adipose tissue metabolism. J Clin Endocrinol Metab. 2004;89:3425–3430. doi: 10.1210/jc.2003-031561. [DOI] [PubMed] [Google Scholar]
- 3.Carey VJ, Walters EE, Colditz GA, Solomon CG, Willett WC, Rosner BA, Speizer FE, Manson JE. Body fat distribution and risk of non-insulin-dependent diabetes mellitus in women. The Nurses' Health Study. Am J Epidemiol. 1997;145:614–619. doi: 10.1093/oxfordjournals.aje.a009158. [DOI] [PubMed] [Google Scholar]
- 4.Lemieux S, Prud'homme D, Bouchard C, Tremblay A, Despres JP. Sex differences in the relation of visceral adipose tissue accumulation to total body fatness. Am J Clin Nutr. 1993;58:463–467. doi: 10.1093/ajcn/58.4.463. [DOI] [PubMed] [Google Scholar]
- 5.Williams MJ, Hunter GR, Kekes-Szabo T, Trueth MS, Snyder S, Berland L, Blandeau T. Intra-abdominal adipose tissue cut-points related to elevated cardiovascular disease risk in women. Int J Obes Relat Metab Disord. 1996;20:613–617. [PubMed] [Google Scholar]
- 6.Nielsen S, Guo Z, Johnson CM, Hensrud DD, Jensen MD. Splanchnic lipolysis in human obesity. J Clin Invest. 2004;113:1582–1588. doi: 10.1172/JCI21047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bock G, Chittilapilly, Basu R, Toffolo G, Cobelli C, Chandramouli, Landau B, Rizza RA. Contribution of hepatic and extrahepatic insulin resistance to the pathogenesis of impaired fasting glucose: role of increased rates of gluconeogenesis. Diabetes. 2007;56:1703–1709. doi: 10.2337/db06-1776. [DOI] [PubMed] [Google Scholar]
- 8.Folsom AR, Kushi LH, Anderson KE, Mink PJ, Olson JE, Hong CP, Sellers TA, Lazovich D, Prineass RJ. Associations of general and abdominal obesity with multiple health outcomes in older women. Arch Intern Med. 2000;160:2117–2128. doi: 10.1001/archinte.160.14.2117. [DOI] [PubMed] [Google Scholar]
- 9.Bajaj M, Suraamornkul S, Piper P, Hardies LJ, Glass L, Cersosimo E, Pratipanawatr T, Miyazaki Y, Defronzo RA. Decreased plasma adiponectin concentrations are closely related to hepatic fat content and hepatic insulin resistance in pioglitazone-treated type 2 diabetic patients. J Clin Endocrinol Metab. 2004;89:200–206. doi: 10.1210/jc.2003-031315. [DOI] [PubMed] [Google Scholar]
- 10.Fruebis J, Tsao TS, Javorschi S, Ebbets-Reed D, Erickson MR, Yen FT, Bihain BE, Lodish HF. Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proc Natl Acad Sci USA. 2001;98:2005–2010. doi: 10.1073/pnas.041591798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berg AH, Combs TP, Du X, Brownlee M, Scherer PE. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med. 2001;7:947–953. doi: 10.1038/90992. [DOI] [PubMed] [Google Scholar]
- 12.Tomas E, Tsao TS, Saha AK, Murrey HE, Zhang CC, Itani SI, Lodish HF, Rudernman NB. Enhanced muscle fat oxidation and glucose transport by ACRP30 globular domain: acetyl-CoA carboxylase inhibition and AMP-activated protein kinase activation. Proc Natl Acad Sci USA. 2002;10:16309–16313. doi: 10.1073/pnas.222657499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rolland YM, Perry HMr, Patrick P, Banks WA, Morley JE. Leptin and adiponectin levels in middle-aged postmenopausal women: associations with lifestyle habits, hormones, and inflammatory markers—a cross-sectional study. Metabolism. 2006;55:1630–1636. doi: 10.1016/j.metabol.2006.07.026. [DOI] [PubMed] [Google Scholar]
- 14.Goodarzi MT, Babaahmadi-Rezaei H, Kadkhodaei-Eliaderani M, Haddainezhad S. Relationship of serum adiponectin with blood lipids, HbA(1)c, and hs-CRP in type II diabetic postmenopausal women. J Clin Lab Anal. 2007;21:197–200. doi: 10.1002/jcla.20175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Association AD. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2006;29:S43–48. [PubMed] [Google Scholar]
- 16.Finegood DT, Bergman RN, Vranic M. Estimation of endogenous glucose production dring hyperinsulinemic-euglycemic glucose clamps. Diabetes. 1987;36:914–924. doi: 10.2337/diab.36.8.914. [DOI] [PubMed] [Google Scholar]
- 17.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237:E214–E223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- 18.Toth MJ, Sites CK, Cefalu WT, Matthews DE, Poehlman ET. Determinants of insulin-stimulated glucose disposal in middle aged, premenopausal women. Am J Physiol Endocrinol Metab. 2001;281:E113–E121. doi: 10.1152/ajpendo.2001.281.1.E113. [DOI] [PubMed] [Google Scholar]
- 19.Steele R. Influences of glucose loading and injected insulin on hepatic glucose output. Ann NY Acad Sci. 1959;82:420–430. doi: 10.1111/j.1749-6632.1959.tb44923.x. [DOI] [PubMed] [Google Scholar]
- 20.Starling RD, Toth MJ, Matthews DE, Poehlman ET. Energy requirements and physical activity of older free-living African-Americans: a doubly labeled water study. J Clin Endocrinol Metab. 1998;83:1529–1534. doi: 10.1210/jcem.83.5.4811. [DOI] [PubMed] [Google Scholar]
- 21.Bouchard C, Despres JP, Mauriege P. Genetic and non-genetic determinants of regional fat distribution. Endocr Rev. 1993;14:72–93. doi: 10.1210/edrv-14-1-72. [DOI] [PubMed] [Google Scholar]
- 22.Bjorntorp P. Metabolic abnormalities in visceral obesity. Ann Med. 1992;24:3–5. doi: 10.3109/07853899209164137. [DOI] [PubMed] [Google Scholar]
- 23.Ostman J, Arner P, Engfeldt P, Kager L. Regional differences in the control of lipolysis in human adipose tissue. Metabolism. 1979;28:1198–1205. doi: 10.1016/0026-0495(79)90131-8. [DOI] [PubMed] [Google Scholar]
- 24.Williamson JR. Mechanism for the stimulation in vivo of hepatic gluconeogenesis by glucacgon. Biochem J. 1966;101:11C–14C. doi: 10.1042/bj1010011c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clarke SD. Polyunsaturated fatty acid regulation of gene transcription: a mechanism to improve energy balance and insulin resistance. Br J Nutr. 2000;83:S59–S66. doi: 10.1017/s0007114500000969. [DOI] [PubMed] [Google Scholar]
- 26.Oakes ND, Cooney GJ, Camilleri M, Chisholm DJ, Kraegen EW. Mechanisms of liver and muscle insulin resistance induced by chronic high-fat feeding. Diabetes. 1997;46:1768–1774. doi: 10.2337/diab.46.11.1768. [DOI] [PubMed] [Google Scholar]
- 27.Mittelman SD, Van Citters GW, Kim P, Davis DA, Dea MK, Hamilton-Wessler M, Bergman RN. Longitudinal compensation for fat-induced insulin resistance includes reduced insulin clearance and enchanced beta-cell response. Diabetes. 2000;49:2116–2125. doi: 10.2337/diabetes.49.12.2116. [DOI] [PubMed] [Google Scholar]
- 28.Hotamisligil GS. Mechanisms of TNF-alpha-induced insulin resistance. Exp Clin Endocrinol Diabetes. 1999;107:119–125. doi: 10.1055/s-0029-1212086. [DOI] [PubMed] [Google Scholar]
- 29.Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, Patel HR, Ahima RS, Lazar MA. The hormone resistin links obesity to diabetes. Nature. 2001;409:307–312. doi: 10.1038/35053000. [DOI] [PubMed] [Google Scholar]
- 30.Bajaj M, Suraamornkul S, Piper P, Hardies LJ, Glass L, Cersosimo E, Pratipanawatr T, Miyachi H, Defronzo RA. Decreased plasma adiponectin concentrations are closely related to hepatic fat content and hepatic insulin resistance in pioglitazone-treated type 2 diabetic patients. J Clin Endocrinol Metab. 2004;89:200–206. doi: 10.1210/jc.2003-031315. [DOI] [PubMed] [Google Scholar]
- 31.Goodpaster BH, Thaete FL, Simoneau JA, Kelley DE. Subcutaneous abdominal fat and thigh muscle composition predict insulin sensitivity independent of visceral fat. Diabetes. 1997;46:1579–1585. doi: 10.2337/diacare.46.10.1579. [DOI] [PubMed] [Google Scholar]
- 32.Ross R, Fortier L, Hudson R. Separate associations between visceral and subcutaneous adipose tissue distribution, insulin and glucose levels in obese women. Diabetes Care. 1996;19:1404–1411. doi: 10.2337/diacare.19.12.1404. [DOI] [PubMed] [Google Scholar]
- 33.Despres JP, Nadeau A, Tremblay A, Ferland M, Mooriani S, Lupien PJ, Theriault G, Pinault S, Bouchard C. Role of deep abdominal fat in the association between regional adipose tissue distribution and glucose tolerance in obese women. Diabetes. 1989;38:304–309. doi: 10.2337/diab.38.3.304. [DOI] [PubMed] [Google Scholar]
- 34.Yeo SE, Hays NP, Dennis RA, Kortebein PM, Sullivan DH, Evans WJ, Coker RH. Fat distribution and glucose metabolism in older, obese men and women. J Gerontol A Biol Sci Med Sci. 2007;62:1393–1401. doi: 10.1093/gerona/62.12.1393. [DOI] [PubMed] [Google Scholar]
- 35.Zamboni M, Armellini F, Milani MP, DeMarchi M, Todesco T, Robbi R, Bergamo-Andreis IA, Bosello O. Body fat distribution in pre- and post-menopausal women: metabolic and anthropometric variables and their inter-relationships. Int J Obes Relat Metab Disord. 1992;16:495–504. [PubMed] [Google Scholar]
- 36.Monzillo LU, Hamby O. Evaluation of insulin sensitivity in clinical practice and in research settings. Nutrit Rev. 2003;61:397–412. doi: 10.1301/nr.2003.dec.397-412. [DOI] [PubMed] [Google Scholar]
- 37.Kelley DE, Thaete FL, Troost F, Huwe T, Goodpaster BH. Subdivisions of subcutaneous abdominal adipose tissue and insulin resistance. Am J Physiol Endocrinol Metab. 2000;278:E941–E948. doi: 10.1152/ajpendo.2000.278.5.E941. [DOI] [PubMed] [Google Scholar]
- 38.Yokoyama H, Emoto M, Mori K, Araki T, Teramura M, Koyama H, Shoji T, Inaba M, Nishizawa Y. Plasma adiponectin level is associated with insulin-stimulated nonoxidative glucose disposal. J Clin Endocrinol Metab. 2006;91:290–294. doi: 10.1210/jc.2004-2549. [DOI] [PubMed] [Google Scholar]
- 39.Abbasi F, Chu JW, Lamendola C, McLaughlin T, Hayden J, Reaven GM, Reaven PD. Discrimination between obesity and insulin resistance in the relationship with adiponectin. Diabetes. 2004;53:585–590. doi: 10.2337/diabetes.53.3.585. [DOI] [PubMed] [Google Scholar]
- 40.Kern PA, DiGregorio GB, Lu T, Rasouli N, Ranganathan G. Adiponectin expression from human adipose tissue: relation to obesity, insulin resistance, and tumor necrosis factor-alpha expression. Diabetes. 2003;52:1779–1785. doi: 10.2337/diabetes.52.7.1779. [DOI] [PubMed] [Google Scholar]
- 41.Ryan AS, Berman DM, Nicklas BJ, Sinha M, Gingerich RL, Meneilly GS, Egan JM, Elahi D. Plasma adiponectin and leptin levels, body composition, and glucose utilization in adult women with wide rangs of age and obesity. Diabetes Care. 2003;26:2383–2388. doi: 10.2337/diacare.26.8.2383. [DOI] [PubMed] [Google Scholar]
- 42.Goodpaster BH, Krishnaswami S, Resnick H, Kelley DE, Haggerty C, Harris TB, Schwartz AV, Kritchevsky S, Newman AB. Association between regional adipose tissue distribution and both type 2 diabetes and impaired glucose tolerance in elderly men and women. Diabetes Care. 2003;26:372–379. doi: 10.2337/diacare.26.2.372. [DOI] [PubMed] [Google Scholar]