Summary
Convection-enhanced delivery (CED) is a novel drug-delivery technique that uses positive hydrostatic pressure to deliver a fluid containing a therapeutic substance by bulk flow directly into the interstitial space within a localized region of the brain parenchyma. CED circumvents the blood-brain barrier and provides a wider, more homogenous distribution than bolus deposition (focal injection) or other diffusion-based delivery approaches. A potential use of CED is for the local delivery of antiseizure agents, which would provide an epilepsy treatment approach that avoids the systemic toxicities of orally administered antiepileptic drugs and bystander effects on non-epileptic brain regions. Recent studies have demonstrated that brief CED infusions of nondiffusible peptides that inhibit the release of excitatory neurotransmitters, including ω-conotoxins and botulinum neurotoxins, can produce long-lasting (weeks to months) seizure protection in the rat amygdala-kindling model. Seizure protection is obtainable without detectable neurological or behavioral side effects. Although conventional diffusible antiepileptic drugs do confer seizure protection when administered locally by CED, the effect is transitory. CED is a potential approach for seizure protection that could represent an alternative to resective surgery in the treatment of focal epilepsies that are resistant to orally-administered antiepileptic drugs. The prolonged duration of action of nondiffusible toxins would allow seizure protection to be maintained chronically with infrequent reinfusions.
Keywords: Convection-enhanced delivery, drug delivery, ω-conotoxin, botulinum neurotoxin, kindling, antiepileptic drug, epilepsy, seizure
INTRODUCTION
A variety of approaches have been investigated for the delivery of anticonvulsant substances directly into the CNS to treat epilepsy.1,2 These approaches provide the potential to use substances for epilepsy therapy, such as peptides, that are not orally active or blood-brain barrier permeable. Some direct delivery approaches involve targeted application into brain regions responsible for the initiation or spread of seizures. A major factor limiting the utility of systemically administered antiepileptic drugs (e.g., by the oral or intravenous routes) is that nonepileptic brain regions are exposed to the therapeutic agent, with the possibility of side effects due to actions on these bystander brain regions. Targeted delivery overcomes this limitation. Traditional CNS delivery methods, such as intrathecal or intraventricular administration, do not provide targeted delivery.3 Conventionally, focal intraparenchymal injection (bolus deposition) is used for targeted delivery in the brain.4 However, this delivery approach provides only limited control over the volume of brain that is exposed to the therapeutic agent. Moreover, within the volume of exposure, the concentration distribution is highly nonuniform (FIG. 1). With intraparenchymal bolus deposition, the active agent is distributed by diffusion so that this method is mainly useful for diffusible substances, which include traditional small molecule antiepileptic drugs (AEDs). When transport is dominated by diffusion, the concentration decays exponentially so that there is a large concentration gradient from the site of deposition to the margins of the distribution volume. Even for diffusible substances, tissue exposure is limited to the brain volume within several millimeters of the site of deposition because of capillary clearance.5 Since diffusible agents rapidly dissipate from the injection site, they must be infused continuously to obtain the sustained tissue concentrations necessary for chronic seizure protection. Antiseizure agents can also be applied to the brain surface via the epidural or subdural spaces, so that delivery occurs by the transmeningeal route.6-8 This mode of delivery, which is particularly applicable for neocortical epilepsies, requires therapeutic molecules that can penetrate the meninges and diffuse into the underlying neocortex to at least a limited extent. To date, this approach has been studied with agents that would require continuous delivery to maintain therapeutic concentrations.
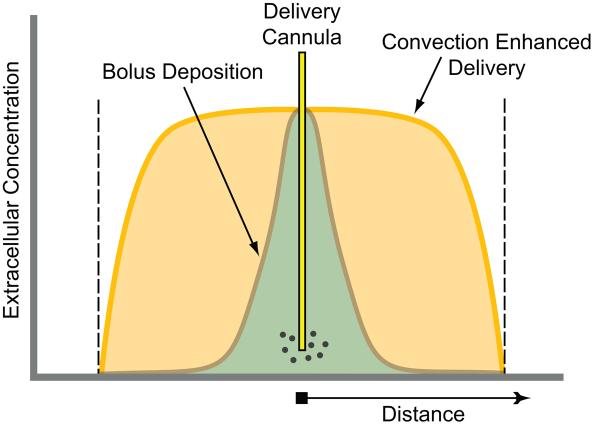
FIG. 1.
Comparison of the extracellular fluid concentration for intraparenchymal bolus deposition (focal injection) and intraparenchymal convection-enhanced delivery. The region perfused is delimited by the dashed lines. With bolus deposition, solute is distributed by diffusion resulting in a rapidly declining concentration gradient throughout the region perfused. In contrast, convection-enhanced delivery provides a uniform concentration throughout the region perfused with a sharp drop-off in concentration at the borders.
As an alternative to continuous delivery, an antiseizure agent could be injected or delivered to the brain surface when needed by a self-contained, implanted device.8 Delivery would be triggered by a seizure prediction or detection system. This approach, which would require a reliable prediction method coupled to a fluid delivery device and a sufficiently large drug reservoir (or a method to allow convenient refilling of the implanted reservoir), presents daunting technical challenges. Drug-eluting polymer wafers or bioceramic implants are delivery methods of potential utility for diffusible compounds.9-12 However, such implants are bulky and cause greater local tissue deformation and damage than fine fluid delivery catheters. Moreover, as with focal injection, the drug concentration profile is highly nonuniform, and the extent of delivery is limited as with all diffusion-based approaches, so that multiple implants are likely to be necessary. Because the capacity of such carriers is limited, they are preferred for delivering exceedingly potent therapeutic agents, such as some growth factors or hormones. However, prolonged activity of polymers loaded with conventional AEDs, such as phenytoin and valproic acid, has been obtained.11,12 Finally, there has been significant interest in gene therapy approaches with vectors expressing, for example galanin or neuropeptide Y, or in the use of transplanted engineered cells or even stem cells.13-17 All of these approaches have promise but there are substantial scientific and technical hurdles. An alternative approach to achieve targeted CNS exposure is convection-enhanced delivery (CED).
CED
The term convection is most commonly used in reference to the bulk movement of thermal energy (heat) in fluids, but more generally refers to the mass transfer of molecules within flowing fluids. Formally, convection is the superposition of transport by the random motion of the molecules (diffusion) and the bulk motion of the fluid (advection). CED uses advection to distribute a therapeutic agent homogeneously throughout clinically significant volumes of brain parenchyma.18-21 This approach offers a greater volume of distribution than simple diffusion and is designed to distribute a drug uniformly throughout a specific target region, with a steep concentration drop-off at the advancing margin of bulk flow (FIG. 1). It has been empirically observed that agents deposited in the brain in a small volume do not readily disperse from the infusion site.22 Therefore, the sharp border occurs because diffusion is limited in the brain parenchyma. In other words, the dissolved molecules are carried along with the flow of fluid to the fluid front, but they do not travel substantially beyond. CED can provide distribution volumes in brain that are several thousand-fold greater than the predicted distribution for diffusion from a point source.23 It has been shown experimentally that the extent of delivery is proportional to the infusion volume regardless of the molecular weight of the solute. Because of this relationship, the solute distribution can be controlled by adjusting the infusion volume. CED thus provides greater control over the distribution of a therapeutic agent than other local delivery techniques. In general, CED causes minimal structural damage to the infused tissue, except at the site of the catheter track.
In a typical CED application, the therapeutic agent is dissolved in saline solution and delivered by slow infusion under positive pressure through a fine cannula whose tip is positioned stereotaxically in the target zone (FIG. 2). It is believed that such positive-pressure infusions cause the fluid carrying the therapeutic agent to be forced through the interstitial space. Because flow in brain parenchyma is highly tortuous, drug molecules, in theory, are spread throughout the infusion volume. However, inhomogeneities in the tissue may cause non-ideal behavior. For example, flow is limited by pial surfaces.
FIG. 2.
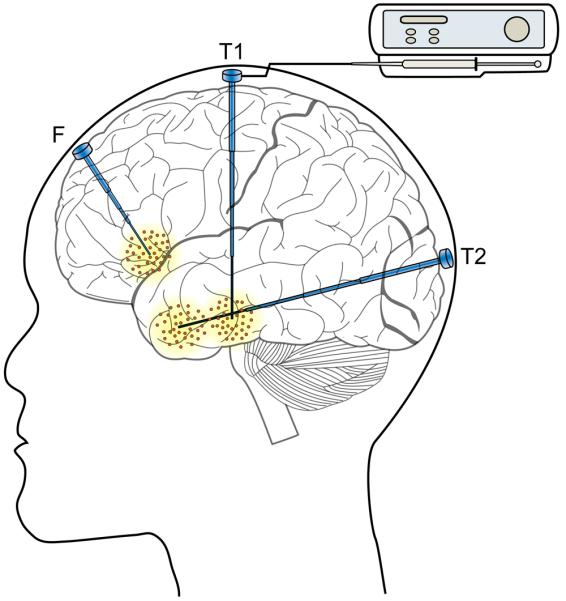
Schematic illustration of proposed convection-enhanced delivery infusion system for the treatment of focal epilepsy with possible catheter trajectories to access frontal lobe (F) and temporal lobe sites (T1 and T2). The step catheter design prevents reflux along the catheter track. In a possible application, the infusion catheter is fully implanted with a transdermal port that is internally sealed and filtered to prevent bacterial ingress. At the time of treatment, an infusion pump would be attached to the port (shown for the T1 catheter only). Multiple catheters are shown to illustrate possible trajectories. An individual patient would ordinarily have a single catheter, but more than one catheter might be necessary in some situations to achieve a sufficient distribution volume or for anatomically distinct foci.
There are a variety of ways in which CED could be applied in epilepsy therapy. For example, one or more permanently implanted cannulas could be placed stereotactically through cranial burr holes and the therapeutic agent delivered via a microinfusion pump, which would be external to the body and connected to the cannula through a transdermal port (FIG. 2). Alternatively, a pump-cannula system could be implanted entirely within the body. In this case, a solution of the therapeutic agent would be loaded into a reservoir within the pump assembly. Stability of the therapeutic agent for prolonged periods at body temperature would need to be verified. Delivery could be actuated by an external signal.
Clinical Experience with CED
It is estimated that more that 1000 patients have received CED infusions in clinical trials. The majority of these have been in studies of malignant glioma.24-27 Although the trials have shown some promise, unequivocal evidence of efficacy is lacking. Gliomas are inherently difficult to treat by CED because their cellular heterogeneity confers resistance to any specific therapeutic agent and because neoplastic cells are often widely dispersed from the tumor as visualized on neuroimaging. In addition, mechanical properties of tumor tissue and surgical cavities serve as impediments to convective flow. In most of the glioma treatment studies, the distribution of the therapeutic agent was not monitored and it is not known whether there was adequate distribution throughout the tissue. In recent studies that have begun to follow the distribution of the therapeutic agent in real time by co-infusion of a tracer (such as 123I-albumin, which is visualized by single-photon emission computed tomography), it has been found that there is often leakage into CSF subarachnoid spaces, which leads to delivery failure.28 This often occurs when the delivery catheter crosses a deep sulcus. Overall, optimal drug delivery may be achieved in as few as 20% of patients. Given these disappointing results, there has been an interest in the development of computational methods to aid in planning CED treatments. One experimental approach uses a computational algorithm in conjunction with presurgical diffusion tensor magnetic resonance imaging (MRI) to predict the volume of distribution and to exclude catheter trajectories that are unlikely to be effective in delivering the therapeutic agent to the desired anatomical region.29 A software platform (iPlan Flow from BrainLAB AG, Feldkirchen, Germany) to carry out these computations is commercially available.
CED has also been used to deliver growth factors, such as glial cell line-derived neurotrophic factor, for the treatment of Parkinson's disease with mixed results.30-32 In addition, direct brain infusion has been used to deliver gene therapy vectors in early clinical trials.33,34
Catheters
Although CED is a minimally traumatic approach, catheters do cause local tissue damage along the insertion track. Ultrafine catheters (0.2 mm, outside diameter at tip) are in development that minimize such tissue injury.35-37 The catheters can be fabricated of materials such as plastic, Teflon, or fused silica that provide bio-compatibility to permit long implantable lifetimes (so as to allow repeated infusions). Such catheters can also be fabricated to provide MRI compatibility. The utility of CED to treat focal epilepsies will depend on the ability to restrict delivery of the therapeutic agent to the intended brain region. Experience with direct brain infusion has demonstrated that prolonged infusions using conventional delivery catheters is associated with leakage and widespread solute distribution beyond the infusion zone.37-39 Leakage is a concern because uncontrolled release of the therapeutic agent (e.g., into the CSF) can cause toxicity. In addition, leakage prevents the maintenance of the pressure head necessary to drive bulk fluid flow into the expected distribution volume.5 Reflux along the catheter track is a common route of leakage. New catheter designs have been developed that prevent reflux over a broad range of flow rates. These include a step-design cannula36 and a microfabricated probe with a side-positioned exit port.37 CED typically uses flow rates of 0.1 to 10 μL/min. Backflow can occur with flow rates >1 μL/min (using a typical 32–20-gauge [0.2 to 0.9 mm O.D.] stainless steel cannula). The newer catheters permit flow rates of up to 5 μL/min, which make coverage of large volumes in the human brain feasible. In addition to minimizing trauma to the brain parenchyma, the new catheter designs are associated with a low incidence of hemorrhage.40
Agents for CED Administration in Epilepsy Therapy
Because CED relies on bulk flow of the therapeutic material through the extracellular space,18 it can be used to deliver agents of much larger molecular size than conventional small molecule AEDs. Indeed, CED is particularly applicable to slowly diffusing substances of high molecular weight that have limited capillary permeability. Theoretically, “bleeding” of such substances beyond the fluid front would be restricted because of their low diffusivity, allowing the spatial extent of delivery to be better controlled than with diffusible agents. In addition, since capillary efflux is limited, such substances would not be washed from the site of infusion, so that prolonged durations of action are possible. Among the large molecular size substances that have been convected into brain in experimental studies are peptides and protein complexes, nucleic acids (such as antisense and siRNA), viral particles, and liposomes containing various pay-loads.22 All of these classes of substances could be of potential utility in epilepsy, either for the objective of directly reducing excitability (as occurs with conventional AEDs) or causing disease-modifying effects, such as anti-epileptogenesis or neuroprotection.
In our proof-of-concept studies examining CED as an epilepsy treatment approach, we have focused on agents that inhibit fast neurotransmission and would therefore be expected to suppress seizure generation and spread. The agents we have chosen are more suitable for CED delivery than conventional AEDs because they are nondiffusible and hydrophilic, so that capillary efflux is limited. Nevertheless, local infusion or surface application of conventional AEDs has been investigated in animal models of focal epilepsy and found to rapidly reduce electrographic and behavioral seizures.6,41-45 Most conventional AEDs are moderately lipophilic (estimated octanol:water partition coefficient values of older AEDs by XLogP is in the range of 1.4–2.9), so that they easily cross cell membranes and are able to leave the site of deposition by diffusion into capillaries. Therefore, their action dissipates rapidly. For local delivery of such agents to be practical in epilepsy therapy, administration on a “when needed” basis (i.e., coupled to a seizure prediction or detection system) would be necessary. In addition to AEDs, small molecule inhibitory substances, such as GABA, muscimol, or adenosine, have been investigated,4,7,8,46 but they generally have the same limitations as small molecule AEDs and may be more prone to inactivation by uptake transporters or local metabolism. As an alternative, we have investigated hydrophilic peptides that ordinarily would not be useful for epilepsy therapy because they are not orally active and do not cross the blood brain barrier.45 These peptides are too toxic to be delivered throughout the brain. However, they have physiochemical properties that are favorable for CED. We have found that local administration by CED confers long-lasting seizure protection without CNS side effects. To date, we have studied the N-type calcium channel antagonists ω-conotoxin GVIA and ω-conotoxin MVIIA and botulinum neurotoxins A and B.
Conotoxin N-type Calcium Channel Antagonists
ω-Conotoxin GVIA and ω-conotoxin MVIIA are 2.6 and 3.0 kDa disulphide-rich peptides that are highly hydrophilic (XLogP, −16.5 and −10.3, respectively) and act as selective N-type calcium channel antagonists.47 N-type calcium channels are present on dendrites and some synaptic terminals throughout the brain, including regions relevant to epilepsy, such as the cerebral cortex, hippocampus, and amygdala.48 Inhibition of N-type calcium channels by ω-conotoxin GVIA and ω-conotoxin MVIIA depresses calcium influx into presynaptic nerve terminals that express the channels and thereby potently suppresses synaptic transmission. In rat neocortical slices in vitro, blockade of N-type calcium channels with ω-conotoxin potently inhibits spontaneous and evoked epileptiform discharges.49 Blockade of other types of calcium channels, including L-type and probably also T-type channels, is largely inactive in this model, which has a pharmacological profile similar to human partial epilepsies (epiletiform discharges are inhibited by phenytoin). Because of their physiochemical properties, the ω-conotoxins are not orally active and they distribute poorly in the brain by diffusion. However, they do have anticonvulsant activity when administered at high doses intraventricularly, but with installation into the CSF, seizure protection is accompanied by generalized tremors.50-52 In the rat amygdala-kindling model, we recently demonstrated that localized CED infusion of ω-conotoxin GVIA and ω-conotoxin MVIIA into the amygdala (20 min at 0.25 μL/min via a 33-gauge stainless steel cannula) produced a long-lasting elevation in the afterdischarge threshold of fully kindled rats.45 The toxins also caused a dose-dependent reduction in seizure parameters, including afterdischarge duration and duration of behavioral limbic seizure activity. The protective effects of the toxins reached a maximum at 48 h postinfusion, and then gradually resolved over the next 5 days. In contrast, the AED carbamazepine was active at 20 min but not at 24 h after the infusion. Except for transient tremor in some rats receiving supratherapeutic toxin doses, no adverse effects were observed. In control experiments, inactivated (proteolyzed) ω-conotoxin MVIIA failed to affect the seizure parameters. It is noteworthy that while the infusion duration was only 20 minutes, seizure protection was obtained for nearly 1 week.
ω-Conotoxin MVIIA (ziconotide) was approved by the United States Food and Drug Administration in 2004 for the management of severe chronic pain. It is marketed as a 0.9% saline solution for intrathecal administration. Systemic (intravenous) administration is associated with hypotension but this side effect is avoided by the intrathecal delivery route (as would presumably also be the case for CED delivery).53 A high incidence of CNS adverse events, including dizziness, confusion, abnormal gait and ataxia, and memory impairment have been observed in clinical trials with intrathecal ziconotide. Although ziconotide is cleared from the CSF within about 24 hours, CNS side effects can persist for up to 2 weeks after discontinuation of the infusion. This long-lasting action is reminiscent of the prolonged action observed with CED and may reflect uptake and sequestration in brain. Ziconotide is not metabolized by the CSF, but it is slowly absorbed into the systemic circulation where it is bound to plasma proteins and degraded by proteolytic enzymes in peripheral organs.53 In the treatment of chronic pain, many patients have received ziconotide by continuous infusion for periods of more than one year, demonstrating that it can be safely administered over long periods of time. If the animal studies are predictive, administration of ziconotide by localized CED infusion should allow CNS side effects to be avoided.
Botulinum Neurotoxins
Botulinum neurotoxins are 150 kDa bacterial metalloprotease enzymes that act on nerve terminals to cause a long-lasting inhibition of neurotransmitter release. The toxins cleave SNARE proteins that form a complex that mediates synaptic vesicle fusion with the plasma membrane; botulinum neurotoxin A and E cleaves SNAP-25, whereas botulinum neurotoxin B cleaves synaptobrevin 2 (VAMP2). Botulinum neurotoxin A was first isolated and crystallized in 1946, and it was discovered to block neuromuscular transmission in 1949. Botulinum neurotoxins A and B were approved by the Food and Drug Administration for clinical applications in 1989 and 2000. The approved clinical uses involve intradermal and intramuscular injection. Despite the long history, it has only recently become recognized that botulinum neurotoxins also act on neurotransmitter release in the CNS. Indeed, for botulinum neurotoxins A and E that act on SNAP-25, there is evidence that the toxins are more effective in blocking the release of the excitatory neuro-transmitter glutamate than the inhibitory neurotransmitter GABA.54-56
The ability of botulinum toxins to selectively inhibit glutamate release raises the possibility that they could be useful to treat conditions of neuronal hyperexcitability, including epilepsy. Unilateral intrahippocampal bolus injections of botulinum neurotoxin E have been shown to attenuate electrographic seizure activity in rats produced by intrahippocampal kainic acid.57,58 The toxin also reduced behavioral limbic seizures resulting from systemic kainic acid injection. In these experiments, kainic acid was administered 2 days after the toxin injections. Uni-lateral intrahippocampal botulinum injection reduced activation of the ipsilateral hippocampus as assessed by c-fos induction, but induction in the contralateral hippocampus was not significantly reduced. Remarkably, however, the unilateral botulinum neurotoxin treatment prevented the hippocampal seizure activity induced by systemic kainate acid (as assessed by c-fos induction) from spreading throughout the entire brain, indicating that the toxin inhibits focal seizures locally as well as their generalization. (Recruitment of both hippocampi seems to be required for seizure generalization.) Toxin treatment was also associated with a preservation of hippocampal neurons and a reduction in the memory deficit that results from systemic kainic acid-induced seizures, indicating a neuroprotective action. In other studies, the toxin inhibited the afterdischarge during hippocampal kindling.
For CED to be useful clinically in the treatment of focal epilepsy, it would be desirable for the treatment agent to have a sufficiently prolonged duration of action so that patients would not be inconvenienced by the need to return for reinfusion at short intervals. Therefore, we investigated the time course of action of botulinum neurotoxins with the aim of determining the duration of action when administered by CED as in the experiments with conotoxins (M. Gasior, R. Tang, N. White and M.A.R., unpublished). Fully amygdala-kindled rats received 20-min CED infusions of botulinum neurotoxins A and B (1-10 ng) into the amygdala, which resulted in significant, dose-dependent elevations in afterdischarge threshold and decreases in afterdischarge duration from 3 to 35 days post infusion. With the highest doses, the effect appeared to persist for more than 64 days. Despite significant changes in kindling parameters, animals receiving CED infusions of the neurotoxins gained weight normally and appeared healthy. The magnitude of the effect of botulinum neurotoxin B on the electrophysiological values may have been somewhat greater than that of botulinum neurotoxin A, and it also appeared to cause a greater suppression of behavioral seizure activity.
Clinical Application
Anticonvulsant toxins delivered locally using the CED technique can produce long-lasting elevations in seizure threshold in an experimental epilepsy model. This indicates that CED may be useful therapeutically to treat partial epilepsies in patients with defined seizure foci. Given the high success rate achieved with resective surgery in appropriately selected patients with intractable epilepsy,59 it is reasonable to hypothesize that silencing the brain region to be resected could be similarly efficacious while allowing patients the opportunity to avoid permanent loss of brain tissue. In addition to its use in patients who are candidates for resective surgery, the approach may also be of value in patients who are not surgical candidates because their focus is near an eloquent brain region; the patient is not sufficiently medically stable to undergo major brain surgery, or the patient wishes to avoid the risks of craniotomy and brain resection. The specific ways in which CED would be used in practice remain to be defined. Advanced CED technologies, as discussed in this review, are especially applicable to epilepsy treatment. For example, ultrafine, tissue compatible, antireflux catheters that produce minimal tissue damage would be useful in epilepsy therapy in which the catheters would remain implanted for long periods. In some epilepsy treatment situations, it may be necessary to use multiple infusion catheters to obtain adequate coverage of the epileptic zone. Multi-lumen connectors and manifolds that permit fluid distribution to multiple catheters are under development (NeuroRoot, Renishaw, UK). The initial infusion could be performed while the patient is being monitored with scalp or depth EEG, with suppression of interictal activity as a marker of biological effect. Incorporation of imaging tracers (such as gadolinium for MRI) would allow the distribution of the therapeutic agent to be monitored in real time during the infusion providing an opportunity to adjust the delivery volume and catheter placement to achieve targeting of the desired brain volume without leakage.5 Patients would be reinfused through the implanted catheters at intervals, either through a percutaneous port or via an implanted pump. Reinfusion could be accelerated if breakthrough behavioral seizures occurred or by EEG criteria. A major advantage of the CED approach over resective surgery is that it is reversible and could be discontinued if ineffective or if unacceptable side effects occurred.
In conclusion, CED provides an opportunity to treat epilepsy with a broad diversity of therapeutic substances that are inactive when administered systemically or unsuitable for systemic administration because of their toxicity. In the near term, agents that suppress seizures are likely to receive the most attention. However, advancements in the understanding of mechanisms of epileptogenesis will undoubtedly suggest molecules that could permanently interrupt or reverse the epileptic process. Studies of such agents will be of considerable interest. Another potential use of CED is to deliver neural toxins (such as the excitotoxin ibotenate) to chemically ablate an epileptic focus.60 Because of the risks of damage to brain regions outside the epileptic focus and (in the case of excitotoxins) for proepileptogenic effects, this strategy must be approached with caution. Targeted, cell-specific neural toxins may eventually provide an improved approach to “chemical neurosurgery” for epilepsy.61 In addition to its therapeutic applications, CED could also be used diagnostically as part of a presurgical evaluation along with depth electrode recording to localize the seizure focus and to define the critical extent of tissue to be removed in a definitive surgical procedure. The wide range of potential applications for CED in epilepsy has only begun to be appreciated.
Acknowledgments
The author's research was supported by the National Institute of Neurological Disorders and Stroke and the Epilepsy Research Foundation. The contribution of Dr. Maciej Gasior to the studies described herein is gratefully acknowledged.
Footnotes
Disclosure
The author is a co-inventor of a patent application claiming the use of CED to treat focal epilepsies, and is a consultant to the licensee, MedGenesis Therapeutix Inc.
REFERENCES
- 1.Nilsen KE, Cock HR. Focal treatment for refractory epilepsy: hope for the future? Brain Res Brain Res Rev. 2004;44:141–153. doi: 10.1016/j.brainresrev.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 2.Fisher RS, Chen DK. New routes for delivery of anti-epileptic medications. Acta Neurol Taiwan. 2006;15:225–231. [PubMed] [Google Scholar]
- 3.Barcia JA, Gallego JM. Intraventricular and intracerebral delivery of anti-epileptic drugs in the kindling model. Neurotherapeutics. 2009;6:337–343. doi: 10.1016/j.nurt.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anschel DJ, Ortega EL, Kraus AC, Fisher RS. Focally injected adenosine prevents seizures in the rat. Exp Neurol. 2004;190:544–547. doi: 10.1016/j.expneurol.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 5.Fiandaca MS, Forsayeth JR, Dickinson PJ, Bankiewicz KS. Image-guided convection-enhanced delivery platform in the treatment of neurological diseases. Neurotherapeutics. 2008;5:123–127. doi: 10.1016/j.nurt.2007.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ludvig N, Kuzniecky RI, Baptiste SL, et al. Epidural pentobarbital delivery can prevent locally induced neocortical seizures in rats: the prospect of transmeningeal pharmacotherapy for intractable focal epilepsy. Epilepsia. 2006;47:1792–1802. doi: 10.1111/j.1528-1167.2006.00642.x. [DOI] [PubMed] [Google Scholar]
- 7.John JE, Baptiste SL, Sheffield LG, et al. Transmeningeal delivery of GABA to control neocortical seizures in rats. Epilepsy Res. 2007;75:10–17. doi: 10.1016/j.eplepsyres.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 8.Ludvig N, Baptiste SL, Tang HM, et al. Localized transmeningeal muscimol prevents neocortical seizures in rats and nonhuman primates: therapeutic implications. Epilepsia. 2008 Dec 2; doi: 10.1111/j.1528-1167.2008.01914.x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 9.Kokaia M, Aebischer P, Elmér E, et al. Seizure suppression in kindling epilepsy by intracerebral implants of GABA- but not by noradrenaline-releasing polymer matrices. Exp Brain Res. 1994;100:385–394. doi: 10.1007/BF02738399. [DOI] [PubMed] [Google Scholar]
- 10.Kubek M, Liang D, Byrd K, Domb A. Prolonged seizure suppression by a single implantable polymeric-TRH microdisk preparation. Brain Res. 1998;809:189–197. doi: 10.1016/s0006-8993(98)00860-9. [DOI] [PubMed] [Google Scholar]
- 11.Tamargo RJ, Rossell LA, Kossoff EH, Tyler BM, Ewend MG, Aryanpur JJ. The intracerebral administration of phenytoin using controlled-release polymers reduces experimental seizures in rats. Epilepsy Res. 2002;48:145–155. doi: 10.1016/s0920-1211(01)00330-8. [DOI] [PubMed] [Google Scholar]
- 12.López T, Ortiz E, Quintana P, González RD. A nanostructured titania bioceramic implantable device capable of drug delivery to the temporal lobe of the brain. Colloids Surf A: Physicochem Eng Asp. 2007;300:3–10. [Google Scholar]
- 13.McCown TJ. Adeno-associated virus-mediated expression and constitutive secretion of galanin suppresses limbic seizure activity in vivo. Mol Ther. 2006;14:63–68. doi: 10.1016/j.ymthe.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 14.Noè F, Pool AH, Nissinen J, et al. Neuropeptide Y gene therapy decreases chronic spontaneous seizures in a rat model of temporal lobe epilepsy. Brain. 2008;131(pt 6):1506–1515. doi: 10.1093/brain/awn079. [DOI] [PubMed] [Google Scholar]
- 15.Carpentino JE, Hartman NW, Grabel LB, Naegele JR. Region-specific differentiation of embryonic stem cell-derived neural progenitor transplants into the adult mouse hippocampus following seizures. J Neurosci Res. 2008;86:512–524. doi: 10.1002/jnr.21514. [DOI] [PubMed] [Google Scholar]
- 16.Noé F, Frasca1 A, Balducci C, et al. Neuropeptide Y overexpression using recombinant adenoassociated viral vectors. Neurotherapeutics. 2009;6:300–306. doi: 10.1016/j.nurt.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCown TJ. Adeno-associated virus vector-mediated expression and constitutive secretion of galanin suppresses limbic seizure activity. Neurotherapeutics. 2009;6:307–311. doi: 10.1016/j.nurt.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bobo RH, Laske DW, Akbasak A, Morrison PF, Dedrick RL, Oldfield EH. Convection-enhanced delivery of macromolecules in the brain. Proc Natl Acad Sci USA. 1994;91:2076–2080. doi: 10.1073/pnas.91.6.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lieberman DM, Laske DW, Morrison PF, Bankiewicz KS, Oldfield EH. Convection-enhanced distribution of large molecules in gray matter during interstitial drug infusion. J Neurosurg. 1995;82:1021–1029. doi: 10.3171/jns.1995.82.6.1021. [DOI] [PubMed] [Google Scholar]
- 20.Haroun RI, Brem H. Local drug delivery. Curr Opin Oncol. 2000;12:187–193. doi: 10.1097/00001622-200005000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Lonser RR, Walbridge S, Garmestani K, et al. Successful and safe perfusion of the primate brainstem: in vivo magnetic resonance imaging of macromolecular distribution during infusion. J Neurosurg. 2002;97:905–913. doi: 10.3171/jns.2002.97.4.0905. [DOI] [PubMed] [Google Scholar]
- 22.MacKay JA, Deen DF, Szoka FC., Jr Distribution in brain of liposomes after convection enhanced delivery; modulation by particle charge, particle diameter, and presence of steric coating. Brain Res. 2005;1035:139–153. doi: 10.1016/j.brainres.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 23.Morrison PF, Laske DW, Bobo H, Oldfield EH, Dedrick RL. High-flow microinfusion: tissue penetration and pharmacodynamics. Am J Physiol. 1994;266(1 pt 2):R292–R305. doi: 10.1152/ajpregu.1994.266.1.R292. [DOI] [PubMed] [Google Scholar]
- 24.Sampson JH, Raghavan R, Brady ML, et al. Clinical utility of a patient-specific algorithm for simulating intracerebral drug infusions. Neuro Oncol. 2007;9:343–353. doi: 10.1215/15228517-2007-007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laske DW, Youle RJ, Oldfield EH. Tumor regression with regional distribution of the targeted toxin TF-CRM107 in patients with malignant brain tumors. Nat Med. 1997;3:1362–1368. doi: 10.1038/nm1297-1362. [DOI] [PubMed] [Google Scholar]
- 26.Rand RW, Kreitman RJ, Patronas N, Varricchio F, Pastan I, Puri RK. Intratumoral administration of recombinant circularly permuted interleukin-4-Pseudomonas exotoxin in patients with high-grade glioma. Clin Cancer Res. 2000;6:2157–2165. [PubMed] [Google Scholar]
- 27.Voges J, Reszka R, Gossmann A, et al. Imaging-guided convection-enhanced delivery and gene therapy of glioblastoma. Ann Neurol. 2003;54:479–487. doi: 10.1002/ana.10688. [DOI] [PubMed] [Google Scholar]
- 28.Lidar Z, Mardor Y, Jonas T, et al. Convection-enhanced delivery of paclitaxel for the treatment of recurrent malignant glioma: a phase I/II clinical study. J Neurosurg. 2004;100:472–479. doi: 10.3171/jns.2004.100.3.0472. [DOI] [PubMed] [Google Scholar]
- 29.Sampson JH, Akabani G, Archer GE, et al. Intracerebral infusion of an EGFR-targeted toxin in recurrent malignant brain tumors. Neuro Oncol. 2008;10:320–329. doi: 10.1215/15228517-2008-012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gill SS, Patel NK, Hotton GR, et al. Direct brain infusion of glial cell line-derived neurotrophic factor in Parkinson disease. Nat Med. 2003;9:589–595. doi: 10.1038/nm850. [DOI] [PubMed] [Google Scholar]
- 31.Lang AE, Gill S, Patel NK, et al. Randomized controlled trial of intraputamenal glial cell line-derived neurotrophic factor infusion in Parkinson disease. Ann Neurol. 2006;59:459–466. doi: 10.1002/ana.20737. [DOI] [PubMed] [Google Scholar]
- 32.Patel NK, Gill SS. GDNF delivery for Parkinson's disease. Acta Neurochir Suppl. 2007;97(pt 2):135–154. doi: 10.1007/978-3-211-33081-4_16. [DOI] [PubMed] [Google Scholar]
- 33.Kaplitt MG, Feigin A, Tang C, et al. Safety and tolerability of gene therapy with an adeno-associated virus (AAV) borne GAD gene for Parkinson's disease: an open label, phase I trial. Lancet. 2007;369:2097–2105. doi: 10.1016/S0140-6736(07)60982-9. [DOI] [PubMed] [Google Scholar]
- 34.Marks WJ, Jr, Ostrem JL, Verhagen L, et al. Safety and tolerability of intraputaminal delivery of CERE-120 (adeno-associated virus sero-type 2-neurturin) to patients with idiopathic Parkinson's disease: an open-label, phase I trial. Lancet Neurol. 2008;7:400–408. doi: 10.1016/S1474-4422(08)70065-6. [DOI] [PubMed] [Google Scholar]
- 35.Kaplitt MG, During MJ. Infusion device and method for infusing material into the brain of a patient. US patent application. 2006:0129126. A1 (application 11/280,965) [Google Scholar]
- 36.Krauze MT, Saito R, Noble C, et al. Reflux-free cannula for convection-enhanced high-speed delivery of therapeutic agents. J Neurosurg. 2005;103:923–939. doi: 10.3171/jns.2005.103.5.0923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Neeves KB, Lo CT, Foley CP, Saltzman WM, Olbricht WL. Fabrication and characterization of microfluidic probes for convection enhanced drug delivery. J Control Release. 2006;111:252–362. doi: 10.1016/j.jconrel.2005.11.018. [DOI] [PubMed] [Google Scholar]
- 38.Ai Y, Markesbery W, Zhang Z, Grondin R, Elseberry D, Gerhardt GA, Gash DM. Intraputamenal infusion of GDNF in aged rhesus monkeys: distribution and dopaminergic effects. J Comp Neurol. 2003;461:250–261. doi: 10.1002/cne.10689. [DOI] [PubMed] [Google Scholar]
- 39.Bennewitz MF, Saltzman WM. Nanotechnology for the delivery of drugs to the brain for epilepsy. Neurotherapeutics. 2009;6:323–336. doi: 10.1016/j.nurt.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Richardson RM, Larson PS, Bankiewicz KS. Gene and cell delivery to the degenerated striatum: status of preclinical efforts in primate models. Neurosurgery. 2008;63:629–644. doi: 10.1227/01.NEU.0000325491.89984.CE. [DOI] [PubMed] [Google Scholar]
- 41.Smith DC, Krahl SE, Browning RA, Barea EJ. Rapid cessation of focally induced generalized seizures in rats through micro-infusion of lidocaine hydrochloride into the focus. Epilepsia. 1993;34:43–53. doi: 10.1111/j.1528-1157.1993.tb02374.x. [DOI] [PubMed] [Google Scholar]
- 42.Eder HG, Stein A, Fisher RS. Interictal and ictal activity in the rat cobalt/pilocarpine model of epilepsy decreased by local perfusion of diazepam. Epilepsy Res. 1997;29:17–24. doi: 10.1016/s0920-1211(97)00061-2. [DOI] [PubMed] [Google Scholar]
- 43.Eder HG, Jones DB, Fisher RS. Local perfusion of diazepam attenuates interictal and ictal events in the bicuculline model of epilepsy in rats. Epilepsia. 1997;38:516–521. doi: 10.1111/j.1528-1157.1997.tb01134.x. [DOI] [PubMed] [Google Scholar]
- 44.Stein AG, Eder HG, Blum DE, Drachev A, Fisher RS. An automated drug delivery system for focal epilepsy. Epilepsy Res. 2000;39:103–114. doi: 10.1016/s0920-1211(99)00107-2. [DOI] [PubMed] [Google Scholar]
- 45.Gasior M, White NA, Rogawski MA. Prolonged attenuation of amygdala-kindled seizure measures in rats by convection-enhanced delivery of the N-type calcium channel antagonists ω-conotoxin GVIA and ω-conotoxin MVIIA. J Pharmacol Exp Ther. 2007;323:458–468. doi: 10.1124/jpet.107.125047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Heiss JD, Walbridge S, Morrison P, et al. Local distribution and toxicity of prolonged hippocampal infusion of muscimol. J Neurosurg. 2005;103:1035–1045. doi: 10.3171/jns.2005.103.6.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bowersox SS, Luther R. Pharmacotherapeutic potential of ω-conotoxin MVIIA (SNX-111), an N-type neuronal calcium channel blocker found in the venom of Conus magus. Toxicon. 1998;36:1651–1658. doi: 10.1016/s0041-0101(98)00158-5. [DOI] [PubMed] [Google Scholar]
- 48.Westenbroek RE, Hell JW, Warner C, Dubel SJ, Snutch TP, Catterall WA. Biochemical properties and subcellular distribution of an N-type calcium channel α1 subunit. Neuron. 1992;9:1099–1115. doi: 10.1016/0896-6273(92)90069-p. [DOI] [PubMed] [Google Scholar]
- 49.Boulton CL, O'Shaughnessy CT. The effect of calcium channel antagonists on spontaneous and evoked epileptiform activity in the rat neocortex in vitro. Eur J Neurosci. 1991;3:992–1000. doi: 10.1111/j.1460-9568.1991.tb00035.x. [DOI] [PubMed] [Google Scholar]
- 50.Jackson HC, Scheideler MA. Behavioural and anticonvulsant effects of Ca2+ channel toxins in DBA/2 mice. Psychopharmacology (Berl) 1996;126:85–90. doi: 10.1007/BF02246415. [DOI] [PubMed] [Google Scholar]
- 51.van Luijtelaar G, Wiaderna D, Elants C, Scheenen W. Opposite effects of T- and L-type Ca2+ channels blockers in generalized absence epilepsy. Eur J Pharmacol. 2000;406:381–389. doi: 10.1016/s0014-2999(00)00714-7. [DOI] [PubMed] [Google Scholar]
- 52.Wang S, Ding M, Wu D, Zhan J, Chen Z. ω-Conotoxin MVIIA inhibits amygdaloid kindled seizures in Sprague-Dawley rats. Neurosci Lett. 2007;413:163–167. doi: 10.1016/j.neulet.2006.11.049. [DOI] [PubMed] [Google Scholar]
- 53.Williams JA, Day M, Heavner JE. Ziconotide: an update and review. Expert Opin Pharmacother. 2008;9:1575–1583. doi: 10.1517/14656566.9.9.1575. [DOI] [PubMed] [Google Scholar]
- 54.Verderio C, Pozzi D, Pravettoni E, et al. SNAP-25 modulation of calcium dynamics underlies differences in GABAergic and glutamatergic responsiveness to depolarization. Neuron. 2004;41:599–610. doi: 10.1016/s0896-6273(04)00077-7. [DOI] [PubMed] [Google Scholar]
- 55.Matteoli M, Pozzi D, Grumelli C, et al. The synaptic split of SNAP-25: Different roles in glutamatergic and GABAergic neurons? Neuroscience. 2009;158:223–230. doi: 10.1016/j.neuroscience.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 56.Verderio C, Grumelli C, Raiteri L, et al. Traffic of botulinum toxins A and E in excitatory and inhibitory neurons. Traffic. 2007;8:142–153. doi: 10.1111/j.1600-0854.2006.00520.x. [DOI] [PubMed] [Google Scholar]
- 57.Costantin L, Bozzi Y, Richichi C, et al. Antiepileptic effects of botulinum neurotoxin E. J Neurosci. 2005;25:1943–1951. doi: 10.1523/JNEUROSCI.4402-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bozzi Y, Costantin L, Antonucci F, Caleo M. Action of botulinum neurotoxins in the central nervous system: antiepileptic effects. Neurotox Res. 2006;9:197–203. doi: 10.1007/BF03033939. [DOI] [PubMed] [Google Scholar]
- 59.Cohen-Gadol AA, Wilhelmi BG, Collignon F, et al. Long-term outcome of epilepsy surgery among 399 patients with nonlesional seizure foci including mesial temporal lobe sclerosis. J Neurosurg. 2006;104:513–524. doi: 10.3171/jns.2006.104.4.513. [DOI] [PubMed] [Google Scholar]
- 60.Pace JR, Lonser RR, Kirkby RD, Jeffries N, Rogawski MA, Oldfield EH. Epileptiform activity extinguished by amygdala infusion of the neurotoxin ibotenate in a rat model of temporal lobe epilepsy. J Neurosurg. 2002;97:450–454. doi: 10.3171/jns.2002.97.2.0450. [DOI] [PubMed] [Google Scholar]
- 61.Wiley RG, Kline IV RH. Neuronal lesioning with axonally transported toxins. J Neurosci Methods. 2000;103:73–82. doi: 10.1016/s0165-0270(00)00297-1. [DOI] [PubMed] [Google Scholar]