Abstract
Forward genetic screens in genetically accessible invertebrate organisms such as Drosophila melanogaster have shed light on transcription factors that specify formation of neurons in the vertebrate central nervous system. However, invertebrate models have, to date, been uninformative with respect to genes that specify formation of the vertebrate glial lineages. All recent insights into specification of vertebrate glia have come via monitoring the spatial and temporal expression patterns of individual transcription factors during development. In studies described here, we have taken this approach to the genome scale with an in silico screen of the Mahoney pictorial atlas of transcription factor expression in the developing CNS. From the population of 1445 known or probable transcription factors encoded in the mouse genome we identify 12 novel transcription factors that are expressed in glial lineage progenitor cells. Entry-level screens for biological function establish one of these transcription factors, Klf15, as sufficient for genesis of precocious GFAP positive astrocytes in spinal cord explants. Another transcription factor, Tcf4, plays an important role in maturation of oligodendrocyte progenitors.
Keywords: Transcription factor, Glia, Development, Oligodendrocyte, Astrocyte, Spinal Cord
Introduction
The spatial and temporal expression patterns of transcription factors during development shed light on the specification of vertebrate glia. For example, the bHLH transcription factors Olig1 and Olig2, which promote formation of oligodendrocytes, were identified via their initial expression in the pMN domain of the embryonic spinal cord (Lu et al., 2000; Zhou et al., 2000), which gives rise to spinal cord oligodendrocytes (Lu et al., 2002; Takebayashi et al., 2002; Zhou and Anderson, 2002). Olig2 knockout mice fail to develop oligodendrocytes and motor neurons in the spinal cord whereas Olig1 knockout mice show slight delays in oligodendrocyte maturation as well as impaired remyelination (Arnett et al., 2004; Xin et al., 2005). In a similar vein, gliogenic functions of the Scl and Nfia transcription factors were initially suggested by their developmentally regulated expression in neural progenitors that give rise to astrocytes (Muroyama et al., 2005; Deneen et al., 2006). Scl specifies formation of V2b interneurons and astrocytes in a regionally restricted area (p2) of the ventral spinal cord. Nfia is expressed in Olig2+ cells at the onset of gliogenesis in the embryonic spinal cord. Both Scl and Nfia exhibit antagonistic interactions with Olig2.
In studies described here, we have taken the spatial and temporal expression pattern approach to the genome scale with an in silico screen of the Mahoney pictorial atlas of transcription factor expression in the developing CNS (Gray et al., 2004). From an entry-level list of more than 1,400 candidates, we have identified 87 transcription factors that are expressed at early times in the germinal zone of developing spinal cord, 12 of which are novel transcription factors that i) show sustained expression in spinal cord white matter and ii) have not hitherto been linked to glial development. We identify 3 novel transcription factors related to the astrocyte lineage and 9 transcription factors related to the oligodendrocyte lineage. Entry-level screens for biological function establish one of the astrocyte-related transcription factors, Klf15, as sufficient for genesis of precocious GFAP positive astrocytes in spinal cord explants, whereas the oligodendrocyte-related transcription factor, Tcf4, plays an important role in maturation of oligodendrocyte progenitors.
Materials and Methods
Animals
Timed-pregnancy CD-1 mice were ordered from Charles River. The genotypes of Tcf4−/−, Nkx6.1−/−, Nkx2.2−/−, Sox10−/−, Klf15−/−, Klf15+/− LacZ, Olig1−/− and Olig2−/− mice were determined by PCR of the genomic DNA, which has been reported previously (Korinek et al., 1998; Qi et al., 2001; Lu et al., 2002; Stolt et al., 2002; Liu et al., 2003; Fisch et al., 2007; Gray et al., 2007).
In situ Hybridization and Immunostaining
In situ hybridization (ISH) and immunofluorescent (IF) procedures were performed as described previously (Fu et al., 2002; Gray et al., 2004). Double staining was performed by first conducting ISH followed by IF. ISH images were pseudocolored using Adobe Photoshop, then combined with IF images to obtain double staining images. Antibodies used in this study include: anti-Tcf4 (Upstate, 1:200), NG2 (1:200, Dr. William Stallcup), Sox10 (1:10, Dr. David Anderson), Olig1 [1:500 (Arnett et al., 2004)], Olig2 [1:10000 (Arnett et al., 2004)], MBP (1:200, Chemicon), CNPase (1:100, Sigma), GFAP (1:200, Sigma), β-Catenin (1:200, BD Biosciences), Ki67 (1:200, Novocastra), Hmgb1 (1:100, Abcam), Hmgb2 (1:500, Abcam), Erf (1:200, Abcam), Rxra (1:100, R&D Systems), and Tcf12 (1:50, Novus).
Mixed glia culture
The cortices of neonatal (P1–P2) rat pups were used for mixed glia culture (McCarthy and de Vellis, 1980). Cortices were physically dissociated and plated on poly-L-ornithine coated coverslips, grown in vitro for 13 days (DMEM/F12, 10%FBS, Pen/Strep). Coverslips were fixed in 4% paraformaldehyde for immunostaining.
Spinal Cord Explant Culture and Electroporation
The spinal cords of E11.5 mouse embryos were dissected out and only the cervical region was used for tissue culture. The cervical spinal cord was transferred onto 0.8µM nitrocellulose membrane (Millipore) with the ventricular side facing up. After 2 hours in culture media (DMEM, 5% FBS, Pen/Strep) in 37°C incubator, plasmids were electroporated onto the ventricular side of the spinal cord explant culture. After 6 days in culture, the explants were fixed and sectioned for GFAP immunostaining. Full-length mouse Olig2, Klf15 (Open Biosystems), and Scl (from Dr. David Rowitch) cDNA were subcloned into the pIRES2-acGFP1 (BD Biosciences) expression vector for electroporation.
Results
An in silico Screen for Transcription Factors that Direct Formation of Glia
The Mahoney atlas was generated by using computer algorithms to search for protein sequences encoded in the existing public and private databases (Sonnhammer et al., 1998; Matys et al., 2003; Thomas et al., 2003; Wheeler et al., 2004). Open reading frames were classified as transcription factors only if their predicted protein sequence included a Protein Families Database (Pfam)-defined DNA binding domain. By this definition, a total of 1445 known or probable transcription factors were shown to be encoded in the mouse genome. Colorimetric in situ hybridization was used to map the expression of over 1000 of these transcription factors and transcription factor-coregulator genes in the brains of developing mice (Gray et al., 2004).
We screened this atlas for transcription factors that may play a role in the regulation of glial development. We chose to focus on expression in the spinal cord for our in silico screen on the basis of its relatively simple anatomical structure. Our entry-level screen was for transcription factors that are expressed within the germinal zone of the spinal cord at a time (E13.5) that coincides roughly with the neural/glial switch (Fig. 1). A comprehensive list of 87 transcription factors that showed germinal zone-restricted expression at E13.5 is provided in Table 1.
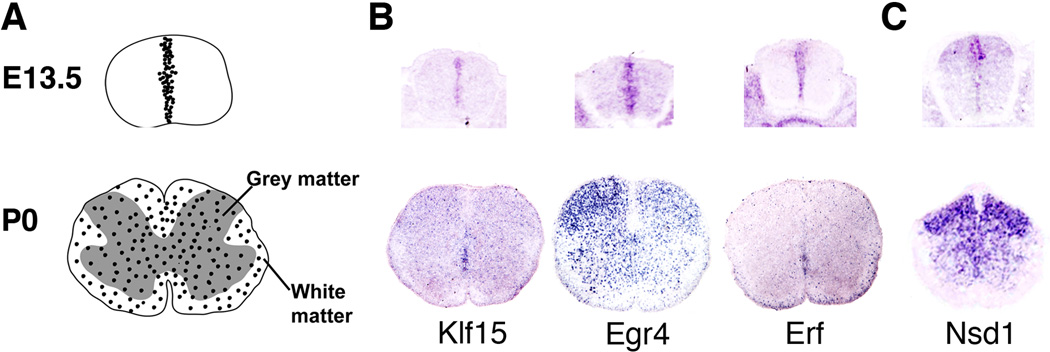
Figure 1. An in silico Screen for Transcription Factors that Direct Formation of Glia.
(A) Gray scale drawings are schematic views of glial progenitors in the developing spinal cord. At E13.5, glial progenitors are sequestered within the ventricular zone. At P0, these progenitors have migrated throughout the gray and white matter of the spinal cord. (B) Color micrographs: Three representative examples of transcription factors from the Mahoney database that show spatial/temporal expression patterns consistent with a role in specification/maturation of glia. (C) Example of a transcription factor with a predominantly grey matter expression pattern at P0. See Results for details.
Table 1.
Transcription factors with germinal zone-restricted expression at E13.5. Genes with "*" are previously known to be regulators for development of glia.
| Gene name | Family | LocusID | Gene name | Family | LocusID |
|---|---|---|---|---|---|
| Arntl | bhlh | 11865 | Hmgb1 | HMG | 15289 |
| Neurod4 | bhlh | 11923 | Sox14 | HMG | 20669 |
| Hes5* | bhlh | 15208 | Bbx | hmg | 70508 |
| Id2* | bhlh | 15902 | Irx1 | homeobox | 16371 |
| Id1* | bhlh | 15901 | Irx3 | homeobox | 16373 |
| Id3* | bhlh | 15903 | Lhx2 | homeobox | 16870 |
| Srebf1 | bhlh | 20787 | lhx4 | homeobox | 16872 |
| Tcf12 | bhlh | 21406 | LHP/Lhx8 | homeobox | 16875 |
| Tcf4 | bHLH | 21413 | Nkx 2.2* | homeobox | 18088 |
| E47 | bhlh | 21423 | Nkx6.1* | homeobox | 18096 |
| Tfeb | bHLH | 21425 | Otx2 | homeobox | 18424 |
| Olig2* | bHLH | 50913 | Pax3 | homeobox | 18505 |
| Olig1* | bHLH | 50914 | Pax7 | homeobox | 18509 |
| Mxd3 | bHLH | 17121 | Six5 | homeobox | 20475 |
| Smarcc1 | bromo | 20588 | Tgif2 | homeobox | 228839 |
| Brd7 | bromo | 26992 | Hoxd3 | homeobox | 15434 |
| Crebl1 | bZIP | 12915 | Pknox1 | homeobox | 18771 |
| Atf4 | bzip | 11911 | Pdlim4 | lim | 30794 |
| Tsc22d4 | bZIP | 78829 | Csrp2 | lim | 13008 |
| Cited4 | cited | 56222 | Prickle3 | Lim | 54630 |
| Nfia* | CTF/NF-I | 18027 | Rest | Myb | 104383 |
| Nfib* | CTF/NF-I | 18028 | Btf3 | Nac | 218490 |
| Nfix | CTF/NF-I | 18032 | Rxra | nucrec | 20181 |
| E2f1 | e2f | 13555 | Rxrb | nucrec | 20182 |
| Tfdp1 | e2f | 21781 | Nr1h2 | nucrec | 22260 |
| E2f6 | e2f | 50496 | Nr2e1 | nucrec | 21907 |
| E2f2 | e2f | 242705 | Trp53 | p53 | 22059 |
| E2f3 | e2f | 13557 | Per2 | PAS | 18627 |
| E2f5 | e2f | 13559 | Rfx3 | RFX | 19726 |
| Erf | ETS | 13875 | Rfx4 | RFX | 71137 |
| Foxo4 | Forkhead | 54601 | Nfatc3 | rhd | 18021 |
| FoxM1 | Forkhead | 14235 | Tbpl1 | TBP | 237336 |
| FoxJ1 | Forkhead | 15223 | Tead2/Klf3 | TEA | 21677 |
| FoxK1 | Forkhead | 17425 | Ciao1 | WD40 | 26371 |
| Gtf2i | GTF2I | 14886 | Sall2 | zinc | 50524 |
| Hmg20b | HMG | 15353 | Egr4 | ZN C2H2 | 13656 |
| Sox10* | HMG | 20665 | Sp1 | ZN C2H2 | 20683 |
| Sox2 | hmg | 20674 | Zfp26 | ZN C2H2 | 22688 |
| Sox9* | HMG | 20682 | Klf15 | ZN C2H2 | 66277 |
| Tcf3 | HMG | 21415 | Zkscan3 | ZN C2H2 | 72739 |
| Tcf7l2 | HMG | 21416 | Nsd1 | Zn PhD | 18193 |
| Smarce1 | HMG | 57376 | Phf21a | ZN PHD | 192285 |
| Hmgb2 | HMG | 97165 | Mid1 | ZN RING B BOX | 17318 |
| Sox21 | HMG | 223227 |
As a secondary screen, we monitored the 87 transcription factors listed in Table 1 for sustained expression at later times in the white matter of the spinal cord. By P0, the majority of the 87 transcription factors detected at E13.5 were no longer expressed or were expressed exclusively in gray matter (Fig.1C). Transcription factors expressed exclusively in gray matter were discounted. However, 20 members of the initial set of 87 were still expressed at P0 in white matter exclusively or in white matter and gray matter together. Some representative images from the P0 screen are shown in Fig. 1 and a comprehensive list of the P0 transcription factors is included in Table 2. It should be noted that all of the transcription factors in this subset are also expressed in neurons. Furthermore, the design of this screen was geared towards identification of transcription factors expressed in glial progenitors. As such, it is possible that some of these transcription factors are involved in maintaining an immature phenotype rather than promoting terminal differentiation. Similarly, the experimental design would naturally exclude transcription factors that are expressed exclusively in gray matter astrocytes.
Table 2.
Transcription factors with glial expression pattern at P0. Genes with "*" are previously known to be regulators for development of glia.
| Gene name | Family | LocusID |
|---|---|---|
| Olig2• | bHLH | 50913 |
| Olig1• | bHLH | 50914 |
| Klf15 | ZN C2H2 | 66277 |
| Rxra | nucrec | 20181 |
| Rxrb | nucrec | 20182 |
| Nkx 2.2• | homeobox | 18088 |
| Tcf7l2 | HMG | 21416 |
| Hmgb2 | HMG | 97165 |
| Hes5• | bhlh | 15208 |
| Id2• | bhlh | 15902 |
| Id3• | bhlh | 15903 |
| Sox10• | HMG | 20665 |
| Sox9• | HMG | 20682 |
| E2f2 | e2f | 242705 |
| Erf | ETS | 13875 |
| E2f1 | e2f | 13555 |
| Six5 | homeobox | 20475 |
| Hmgb1 | HMG | 15289 |
| Egr4 | ZN C2H2 | 13656 |
| Tcf12 | bhlh | 21406 |
Note that the E13.5 and P0 inventory of spatially restricted transcription factors in Table 1 and Table 2 includes 12 transcription factors that have been previously linked to the formation of glia including Olig1, Olig2, Scl, Nfia, Sox9. The presence of these factors serves in part to validate our screening strategy. In addition, Table 2 lists 12 novel transcription factors that show sustained expression in white matter at P0 and have not previously been associated with glial development. These 12 transcription factors were singled out for further study.
Segregation into oligodendrocyte and astrocyte-related subgroups
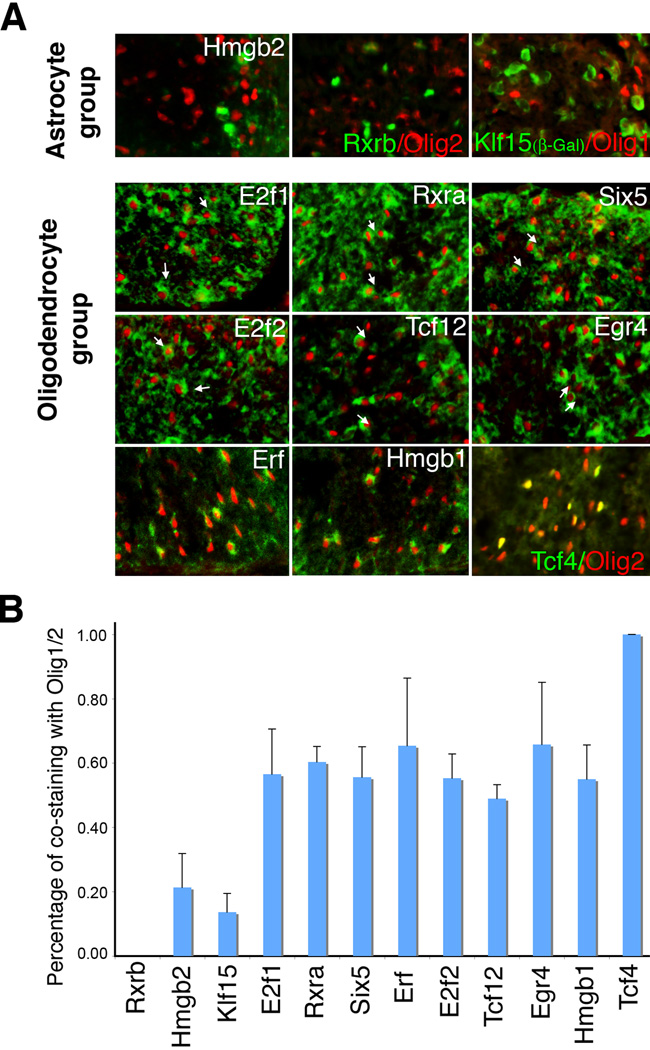
The CNS white matter contains both oligodendrocytes and GFAP-positive fibrous astrocytes (Privat and Rataboul, 1986). To resolve the cell types marked by novel transcription factors in our P0 “short list”, we performed in situ hybridization for the various transcription factors together with immunofluorescence with antibodies to Olig1 or Olig2, a pair of bHLH transcription factors that show >95% overlap in expression (and thus were treated as interchangeable for our purposes here) and are restricted to the nuclei of oligodendrocytes and oligodendrocyte progenitors in the P0 spinal cord (Lu et al., 2000; Takebayashi et al., 2000; Zhou et al., 2000). Transcription factors that were mainly precluded from Olig2-positive cells were considered to be astrocyte-related (Fig. 2A and 2B). Transcription factors that largely colocalized with Olig2 were considered to be in an oligodendrocyte-related group (Fig. 2A and 2B). To see if P0 expression patterns reflect adult patterning, we performed expression analysis on Tcf4 and Klf15 (Supplemental Fig. 1). In the postnatal spinal cord, Tcf4 expression is exclusive to oligodendrocyte lineage cells and decreases as myelination proceeds, whereas Klf15 is mainly expressed in the astrocyte lineage. In the brain, Tcf4 is also expressed in neurons, especially in the thalamus. Overall, a group of 9 transcription factors are oligodendrocyte-related by this criterion and 3 are astrocyte-related.
Figure 2. Segregation of glia-related transcription factors into an astrocyte group and oligodendrocyte group.
A) Expression assays in P0 spinal cords. Oligodendrocytes are imaged by immunofluorescence with antibodies to Olig2 or (in the case of Klf15) Olig1. Transcription factors from the Mahoney database are imaged by in situ hybridization (green pseudocolor) except for Klf15 (imaged by β-galactosidase) and Rxrb (imaged by immunofluorescence). In the astrocyte group of transcription factors, there is minimal overlap with Olig2 or Olig1. In the oligodendrocyte group, cells double labeled with Olig2 antibodies (arrows) are far more common. B) Quantification of double-stained cells, presented as percent overlap with Olig1/2. Klf15 was double-stained with Olig1. All other transcription factors were double-stained with Olig2. Error bars indicate standard deviation (n: Rxrb=3, Hmgb2=14, Klf15=7, E2f1=7, Rxra=5, Six5=6, Erf=9, E2f2=6, Tcf12=3, Egr4=5, Hmgb1=12, Tcf4=9).
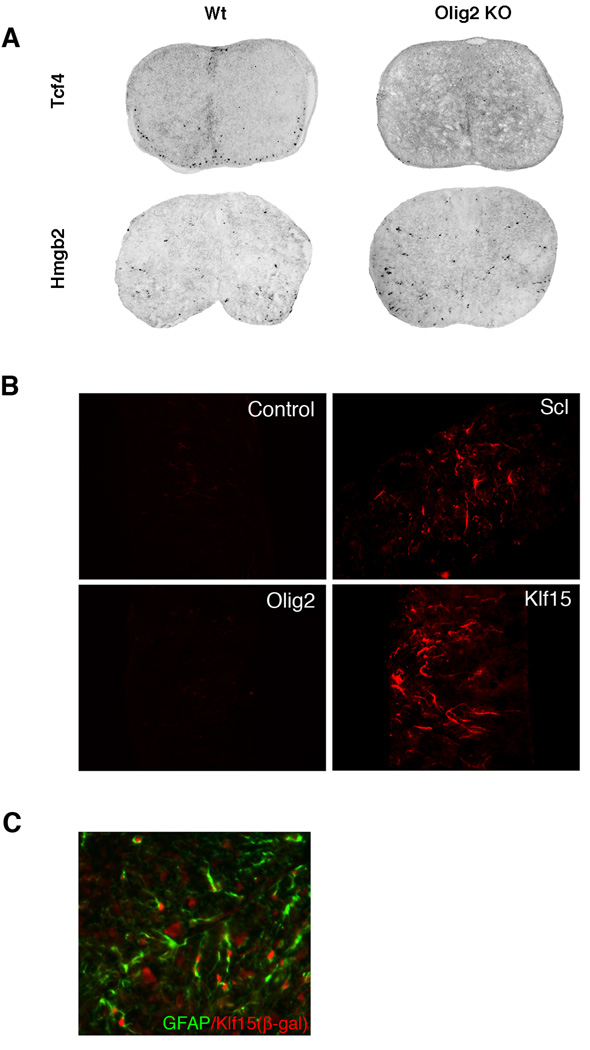
Our classification of the astrocyte-related transcription factors was further validated by examining the spinal cords of Olig2 knockout mice and by mis-expression studies. Targeted disruption of Olig2 leads to complete ablation of the oligodendrocyte lineage throughout the spinal cord while formation of astrocytes is unperturbed (Lu et al., 2002). All three of the astrocyte-related transcription factors are expressed equally well in wild type and Olig2 knockout spinal cords. Representative in situ images of Hmgb2 (astrocyte group) and Tcf4 (oligodendrocyte group) in the spinal cords of wild type and Olig2 knockout mice are shown in Fig. 3A (see also Supplemental Fig. 2). With the exception of Tcf4, no striking differences were seen in the oligodendrocyte-related group of transcription factors within the white matter (Supplemental Fig. 3). Since Tcf4 is the only transcription factor with 100% overlap with Olig2, and the other oligodendrocyte-related transcription factors are also expressed in neurons, this result is not surprising. Tcf12 and Rxra expression is lost in the motor neuron region, consistent with previously reports of motor neuron loss in the Olig2 knockout mice.
Figure 3. Analysis of astrocyte-related transcription factors.
A) At E18.5, Hmgb2 has very similar expression in Olig2 KO spinal cords and in wild type littermates, while Tcf4 expression is ablated in Olig2 KO spinal cords. Tcf4 is used as a negative control. B) Klf15, an astrocyte group gene, induces ectopic GFAP expression in mouse spinal cord explant cultures. No GFAP expression is found in Olig2 or empty vector control transfected spinal cord explants. GFAP expression is also observed when the astrocyte-promoting transcription factor Scl is electroporated into the spinal cord. C) Double immunostaining showed colocalization of GFAP and Klf15 (β-gal) in P14 spinal cord of Klf15 heterozygotes (with β-gal knockin at the Klf15 locus).
Functional analysis of an astrocyte-related transcription factor
From commercial sources, we were able to obtain full-length cDNA for two of the transcription factors in the astrocyte-related group. For comparative purposes, we also obtained full-length cDNA clones corresponding to two of the nine novel transcription factors in the oligodendrocyte-related group. After validating the nucleic acid sequences, these full-length cDNAs were placed into expression vectors and electroporated into E11.5 “open book” mouse spinal cord explant cultures as described in Materials and Methods. At day six after electroporation, the explant cultures were fixed, sectioned and immunostained for GFAP. As a positive control for the open book cultures, we used Scl, a bHLH transcription factor that has been previously shown to be both necessary and sufficient to promote formation of astrocytes in a regionally restricted domain of the ventral spinal cord (Muroyama et al., 2005). As a negative control we used the oligodendrocyte-specific bHLH transcription factor Olig2, which specifies formation of oligodendrocytes.
As shown in Fig. 3B, Scl and Klf15 promoted formation of precocious GFAP positive cells, whereas Hmgb2 did not (data not shown). In contrast, neither Olig2 nor the two novel transcription factors from the oligodendrocyte-related group (Tcf4, Tcf12) displayed this activity (Fig. 3B and data not shown). Physiologic relevance of Klf15 function in these spinal explant cultures was validated using a Klf15 β-galactosidase knockin mouse (Fisch et al., 2007) for fate mapping. As indicated in Fig. 3C, we observed excellent overlap between β-gal expression and GFAP staining (> 60%) and poor overlap with β-gal expression and Olig1 staining (< 15%) in the postnatal spinal cord (quantification not shown). As a technical aside, our spinal cord explants routinely express GFAP positive astrocytes at day 7 in vitro. Since explant cultures are harvested one day earlier (day 6 in vitro), we were unable to determine if overexpression of oligodendrocyte-related transcription factors results in repression of GFAP expression. Overexpression of the oligodendrocyte transcription factors (even control Olig2) did not stimulate expression of late stage oligodendrocyte markers in spinal cord explants, suggesting that additional transcription factors [e.g., Nkx2.2 (Zhou et al., 2001)] are necessary for oligodendrocyte progenitors to progress to the MBP-expressing developmental stage.
Tcf4 is an oligodendrocyte-related transcription factor
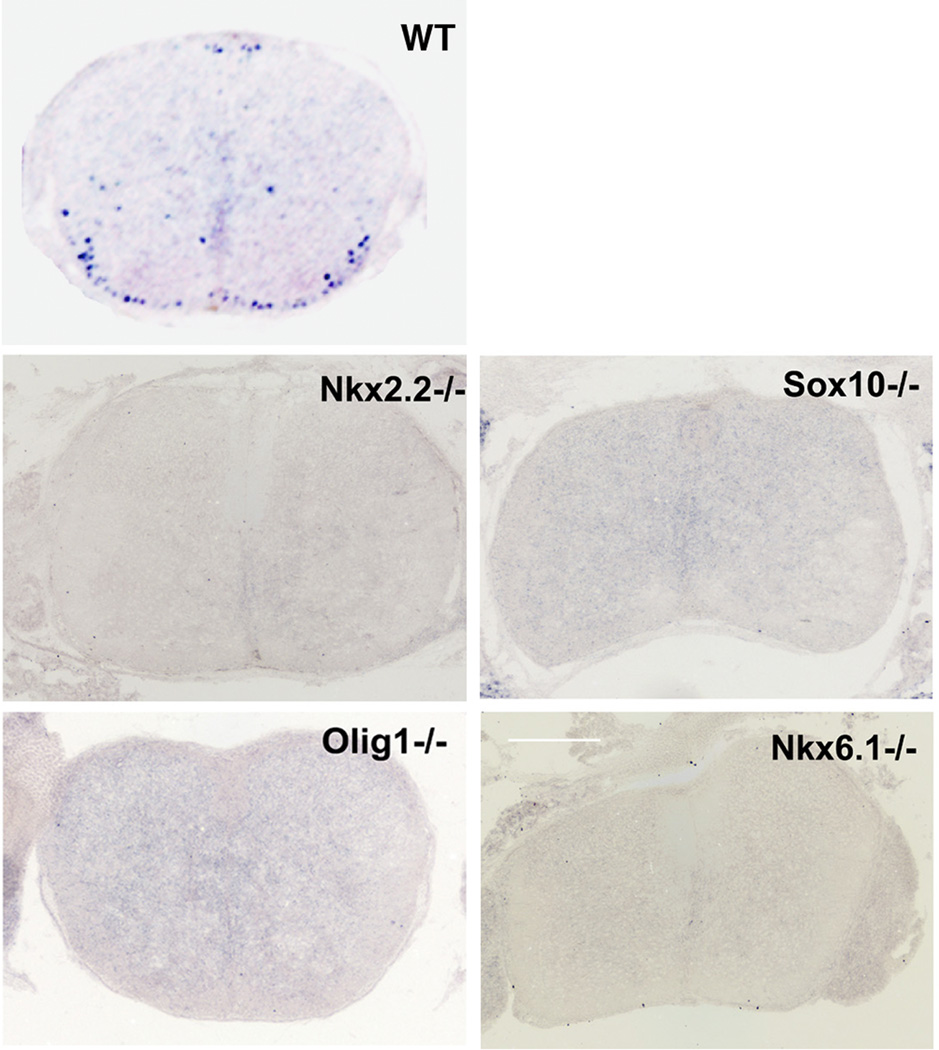
We chose to pursue Tcf4 for detailed study from our collection of potential pro-oligodendrocyte lineage genes because i) it was the only transcription factor that showed 100% overlap with Olig2, ii) it was the only oligodendrocyte transcription factor whose expression was disrupted in the Olig2 knockout spinal cord, and iii) because of its provocative relationship to the Wnt/β-catenin signaling pathway. A functional overlap between Tcf4 and Olig2 suggested by the phenotype of Olig2 knockout spinal cords (Fig. 3A) was further resolved with a series of knockout mouse models that feature a moderate-to-severe impact on myelin development. The list includes Nkx6.1 and Sox10 (severe phenotype) and Olig1 and Nkx2.2 (moderate phenotype) (Qi et al., 2001; Lu et al., 2002; Stolt et al., 2002; Takebayashi et al., 2002; Zhou and Anderson, 2002; Liu et al., 2003). As shown in Fig. 4, Tcf4 expression is ablated or severely delayed in all four of these hypomyelination mouse models. Olig1 knockout mice are viable and some Nkx2.2 animals survive to P7. Low levels of Tcf4 expression were seen at P5 in Olig1 and Nkx2.2 knockout animals (data not shown).
Figure 4. Tcf4 expression is ablated or a ttenuated in multiple hypomyelination mouse models.
All of the in situ images shown are from E18.5 spinal cords.
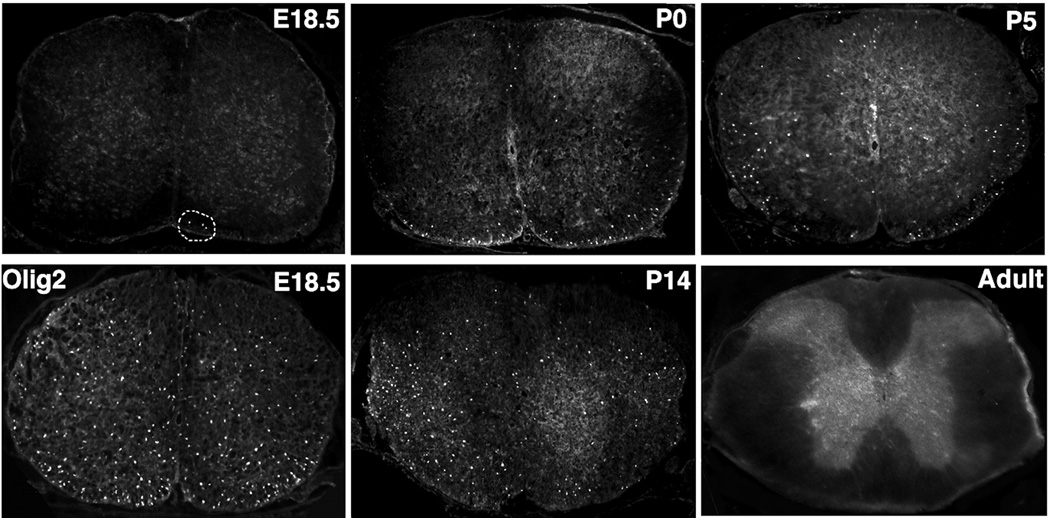
Physiologic relevance of Tcf4 ablation in the various hypomyelination models is suggested by Tcf4 immunostaining experiments at intervals ranging from E18.5 to adult life (Fig. 5). As indicated in Fig. 2, all Tcf4-positive cells express the oligodendrocyte lineage marker Olig2. At E18.5, Tcf4 protein is detectable but only within a minor subset of cells in the ventral spinal cord (compare E18.5 Tcf4 image to E18.5 Olig2 in Fig. 5). Between P0 and P14, Tcf4 positive cells become more abundant and they are dispersed throughout the gray and white matter of the spinal cord. However, in adult mice, Tcf4 positive cells can no longer be detected in white matter of the spinal cord. Likewise, Tcf4 protein is not expressed in adult corpus callosum (data not shown). Note that Tcf4 protein expression does not strictly overlap the expression of Tcf4 mRNA (compare Fig. 5 weak Tcf4 protein expression at E18.5 to robust mRNA expression in Mahoney atlas). The disconnect between mRNA and protein expression may reflect some level of translational or post-translational regulation of Tcf4.
Figure 5. Temporal expression of Tcf4 in developing spinal cord.
In timed mating experiments, Tcf4 protein is barely detectable prior to E18.5 in the spinal cord (three positive cells are circled). For comparative purpose the expression pattern of Olig2 in E18.5 mouse spinal cord is shown below the Tcf4 image. Between P0 and P14, Tcf4 protein is readily detectable. In mature white matter of the adult spinal cord, however, Tcf4 is no longer expressed.
Tcf4 Marks Post-Mitotic/Pre-Myelinating Oligodendrocytes
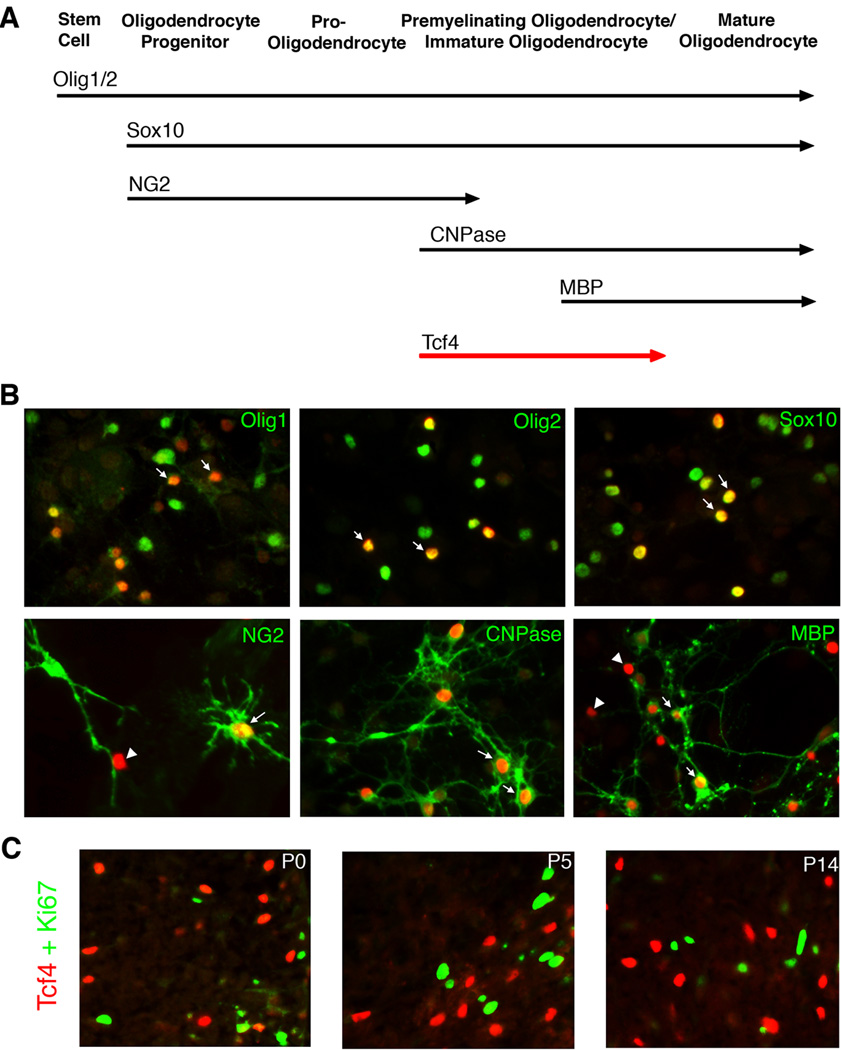
The relationship between expression of the Tcf4 and Olig2 proteins is not symmetric. Although all Tcf4 positive cells express Olig2, many Olig2 positive cells do not express Tcf4. To identify the subset of oligodendrocyte progenitors marked by Olig2 and Tcf4, we turned to mixed glial cultures from neonatal rat cortex. Oligodendrocyte progenitors in mixed glial cultures differentiate into myelin positive oligodendrocytes over a two-week period of time. Differentiation is asynchronous so that at any given time, a mixed population of immature, partially mature and mature oligodendrocytes can be observed (McCarthy and de Vellis, 1980). A cellular “deletion map” of maturational stages in these cultures can be obtained with a set of marker proteins that are expressed at timed intervals as shown (Fig. 6A).
Figure 6. Tcf4 Marks Post-Mitotic/Pre-Myelinating Oligodendrocytes.
A) Schematic drawing of stage-specific markers for oligodendrocyte development. B) Double immunofluorescence of Tcf4 (red) with stage-specific markers (green) in mixed glia cultures. Arrows point to the double-labeled cells, while arrowheads point to cells that are only Tcf4+. As expected, Tcf4 is expressed in a subpopulation of Olig1+, Olig2+, and Sox10+ cells because these markers are detectable at all stages of oligodendrocyte formation [36% ± 11% (n=9), 40% ± 12% (n=3) and 35% ± 8% (n=7), respectively]. However Tcf4 shows a near 90% overlap with CNPase [88% ± 2% (n=9)] and only partially overlap with NG2 [47% ± 20% (n=6)] or MBP [36% ± 12% (n=5)]. C) Double immunofluorescence with antibodies to Tcf4 (red) and Ki67 (green), a marker for dividing cells. As indicated, there is no overlap between Tcf4 and Ki67.
As shown in Fig. 6B, Tcf4 protein is expressed in a subset of the cells with the sustained early markers (Olig1, Olig2 or Sox10). Tcf4 is also expressed in a subset of cells marked by proteins that are expressed at early (NG2) or late (MBP) stages of the maturational process. However, the marker most closely linked with Tcf4 (roughly 90% co-expression) is CNPase - a protein expressed as oligodendrocytes exit the cell cycle for terminal differentiation (Barradas et al., 1995). The linkage between Tcf4 expression and cell cycle exit is further confirmed by double immunostaining with antibodies to Ki67, a marker for dividing cells. As indicated (Fig. 6C), there is no overlap between Tcf4 and Ki67 at any stage of development, showing that Tcf4 is expressed only in postmitotic cells.
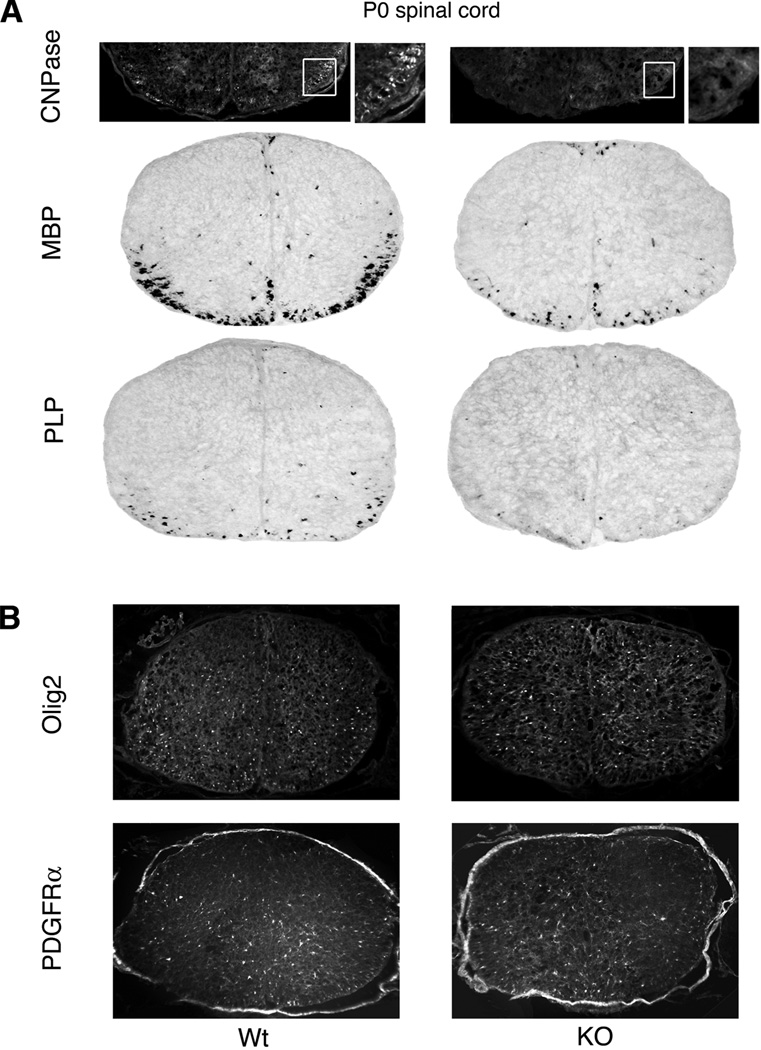
Tcf4 expression is required for maturation of oligodendrocyte progenitors but is dispensable for their initial specification
Ablation of white matter by targeted disruption of pro-myelin transcription factors leads to loss of Tcf4 positive cells (Fig. 3A and Fig. 4). However, these data do not address the role of Tcf4 itself in formation of myelin. As shown in Fig. 7B, the spinal cords of Tcf4 knockout mice show little difference in the number of cells that express early markers of the oligodendrocyte lineage such as Olig2 or PDGFRα when examined at P0. Thus Tcf4 is dispensable for the initial specification of oligodendrocyte progenitors (see schematic in Fig. 6A). However, as shown in Fig. 7A, there are significant differences in expression of later markers for oligodendrocytes. For example, CNPase is almost nonexistent in Tcf4 knockout mice at P0. Moreover, the mRNA levels of the late marker proteins MBP and PLP are decreased to approximately one-third the levels in Tcf4 knockout mice relative to their wild type littermates at P0. Thus Tcf4 promotes the maturation of oligodendrocyte progenitors – a function also ascribed to the bHLH transcription factor Olig1 (Arnett et al., 2004; Xin et al., 2005).
Figure 7. Tcf4 expression is required for maturation of oligodendrocyte progenitors but is dispensable for their initial specification.
A) At P0, expression of late markers of the oligodendrocyte lineage (CNPase, MBP and PLP) is reduced or delayed in the Tcf4 knockout mice relative to wild type littermates [MBP: KO=29% ± 3% (n=3)]. CNPase is detected by immunofluorescence with insets (white boxes) shown at higher magnification. MBP and PLP are detected by in situ hybridization. B) In contrast to late markers, early markers of the oligodendrocyte lineage (Olig2 and PDGFRα) are unaffected by Tcf4 knockout at P0.
One important remaining question is whether Tcf4 expression must be extinguished prior to full maturation of oligodendrocytes. Unfortunately Tcf4 knockout mice die immediately after birth. Since spinal cord explant cultures are not technically feasible for postnatal stages and oligodendrocyte cultures are difficult to transfect and mature in vitro, further biological tools, including the development of inducible Tcf4 overexpression mouse models, will be required to fully define the role of Tcf4 in development and repair of CNS white matter.
Tcf4 probably functions as a transcription activator in oligodendrocyte progenitors
As a downstream effector of the Wnt signaling axis, Tcf4 can function either as a repressor or an activator of gene expression depending upon the activation state of β-catenin (Clevers, 2006). In the absence of Wnt signaling, β-catenin is phosphorylated by the serine/threonine kinase GSK3 and rapidly proteolysed in the cell cytosol. Under these conditions, Tcf4 is a transcription repressor. In the presence of Wnt signaling, GSK3 function is silenced and the stabilized β-catenin moves into the cell nucleus. Under these conditions, Tcf4 is a transcription activator.
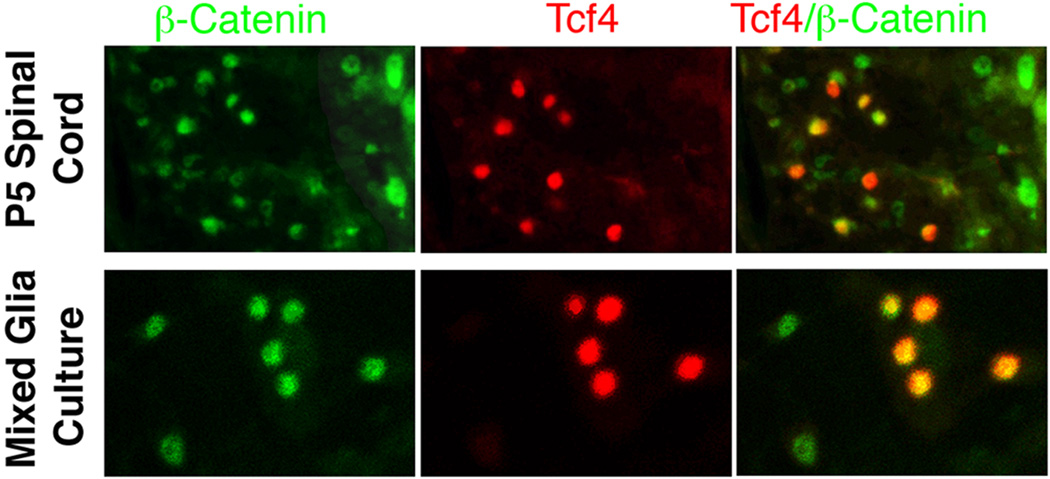
To obtain insight into the molecular mechanism whereby Tcf4 promotes the timely maturation of oligodendrocyte progenitors (Fig. 7A), we conducted double immunostaining with antibodies to Tcf4 and β-catenin. As shown in Fig. 8, all of the Tcf4-positive cells in the P5 spinal cord and also in our mixed glial cultures show β-catenin within their nuclei. Accordingly, Tcf4 probably functions as a transcription activator during the process of oligodendrocyte maturation.
Figure 8. Tcf4 probably functions as a transcription activator in oligodendrocyte progenitors.
Double immunofluorescence with antibodies to Tcf4 (red) and β-catenin (green) shows that Tcf4 is co-expressed with nuclear β-catenin both in P5 spinal cord white matter (upper row) and mixed glia cell cultures (lower row). Thus Tcf4 is most likely functioning as a transcription activator in these cells. See Results for details.
Discussion
Transcription factors that specify formation of glia in genetically accessible organisms such as Drosophila and nematode worms have been largely displaced and reassigned to different functions in vertebrate animals (Wegner and Riethmacher, 2001). For vertebrate CNS development, a short list of gliogenic transcription factors have been identified via spatially restricted expression patterns in developing spinal cord including Olig1, Olig2, Scl, Sox 9, Sox10, Nfia and Nfib (Lu et al., 2000; Zhou et al., 2000; Stolt et al., 2002; Stolt et al., 2003; Muroyama et al., 2005; Deneen et al., 2006). We show here how a visual database can be used to expand upon this gene discovery strategy.
From an entry-level list of more than 1,400 candidates, we identified 87 transcription factors that are expressed at early times in the germinal zone of the developing spinal cord. From this spatially restricted cohort of 87 candidates, we identified a subset of 12 novel transcription factors that i) show sustained expression in spinal cord white matter and ii) have not hitherto been linked to glial development. Both of the gene sets contain chromatin remodeling proteins and regulators of cell division. More detailed studies of those genes could lead to a greater understanding of chromatin modification during glial development and possibly the molecular mechanisms coupling cell division with cell differentiation. Knockout mice for many of the 12 novel genes exist. Most of them can be categorized into two groups, embryonic lethal and those with little or no phenotype in the CNS. The former genes will require conditional knockout strategies, while the latter may benefit from combinatorial knockouts.
One caveat to an entry-level screen based upon in situ expression data is that the presence of mRNA does not always equate to the expression of protein. Tcf4 mRNA, for example, is seen in oligodendrocyte progenitors as early as E13.5 by in situ hybridization. However, Tcf4 protein, as detected by immunostaining, is not seen until E18.5. In contradistinction, Tcf4 mRNA expression in neurons correlates well with expression of the protein (data not shown). We and others have noted a similar disconnect in Olig1, which has been linked to maturation of oligodendrocyte progenitors. Expression of Olig1 mRNA can be seen at times as early as E9 in developing spinal cord (Lu et al., 2000). However, multiple different antibody preparations fail to detect Olig1 protein until E18.5 when oligodendrocyte progenitors have begun to mature (J.A. Alberta and C.D. Stiles, unpublished observations). It seems likely that the function of these transcription factors is regulated in part by post-transcriptional mechanisms. Sequence analysis of Olig1 and Tcf4 mRNA transcripts reveals multiple potential binding sites for microRNAs (MiRBase, http://microrna.sanger.ac.uk).
From a cluster of novel transcription factors with an oligodendrocyte-like expression pattern (Fig. 2), Tcf4 was chosen for functional analysis in part because of its known involvement in the Wnt signaling pathway (Kuhl et al., 2000). A broad body of literature documents Tcf4 functions in the development of intestinal stem cells as well as in colorectal tumor formation (Korinek et al., 1997; Morin et al., 1997; Korinek et al., 1998). More recently a strong link between Tcf4 and type 2 diabetes has been described (Grant et al., 2006). Tcf4 expression in the developing CNS, however, correlates strongly with oligodendrocyte maturation. Relative to their wild type littermates, Tcf4 knockout mice express much less CNPase protein and MBP and PLP mRNA at P0 (Fig. 7). Moreover, Tcf4 expression is attenuated in several different hypomyelination models (Fig. 3 and Fig. 4). Preliminary results culturing Tcf4 knockout cells suggest that oligodendrocyte progenitors are present in similar numbers but show a developmental delay in maturation that mimics the in vivo situation. It is unclear whether these cells would eventually catch up to their wild type counterparts. In other studies, we have seen that Tcf4 is upregulated during the remyelination stage in the cuprizone model of relapsing/remitting multiple sclerosis (data not shown), further suggesting Tcf4 plays a role in oligodendrocyte maturation. However, in silico analysis of the 10kb upstream promoter regions of CNPase, MBP and PLP genes failed to discover any Tcf4 binding sites.
He et al. have shown that targeted disruption of the Ying Yang 1 (YY1) transcription factor leads to overexpression of Tcf4 and an accumulation of immature oligodendrocytes (He et al., 2007). This YY1 knockout phenotype would be predicted from our own observations that Tcf4 is expressed in pre-myelinating oligodendrocyte progenitors but not in mature oligodendrocytes. He et al. also showed that ectopic Tcf4 suppresses the expression of myelin reporter constructs. However it is well known that Tcf4 by itself is a transcription inhibitor. Co-expression with activated β-catenin is required to convert Tcf4 into a transcription activator (Clevers and van de Wetering, 1997). We find that all Tcf4 positive spinal cord cells express β-catenin in their nuclei, suggesting that Wnt signaling is important for this stage of oligodendrocyte differentiation (Fig. 8). Our results appear to contradict previous reports suggesting Wnt signaling prevents oligodendrocyte differentiation (Shimizu et al., 2005; Kim et al., 2008). However, both studies address the role of Wnt signaling during the window of oligodendrocyte specification and/or proliferation, a time prior to Tcf4 protein expression. Furthermore, the pharmacological activation of Wnt signaling resulted in increased apoptosis and few surviving oligodendrocytes, making it difficult to interpret the role of Wnt signaling on differentiation. While this manuscript was under review Fancy et al. published a survey of transcription factors involved in the repair of demyelinated lesions in the postnatal CNS and Ye et al. characterized the role of Wnt pathway signaling in oligodendrocyte development (Fancy et al., 2009; Ye et al., 2009). Both of these studies converged on Tcf4 as a central component of oligodendrocyte progenitor maturation thus providing independent support of the conclusions drawn here.
To this point, there are only a few examples of transcription factors that have been shown to be both necessary and sufficient for the genesis of vertebrate astrocytes. The Sox9 and Nfia/b gene products appear to play broad roles in astrocyte development. However, Sox9 and Nfia/b are both expressed in oligodendrocytes as well as astrocytes and the formation of these two cell types is mutually exclusive. For example bone morphogenic proteins promote astrocytes and inhibit oligodendrocytes (Gross et al., 1996; Mekki-Dauriac et al., 2002; Gomes et al., 2003), while Olig2 promotes formation of oligodendrocytes and inhibits formation of astrocytes (Zhou and Anderson, 2002; Fukuda et al., 2004; Setoguchi and Kondo, 2004). Accordingly, the pivotal functions of Sox9 and Nfia/b in formation of astrocytes may be to inhibit neuronal fate choice making the resulting cells permissive for glial development.
We identified three novel transcription factors that meet our criteria for being astrocyte-specific. Klf15 is biologically active in a spinal cord explant assay for the formation of ectopic astrocytes and is expressed in astrocytes of the postnatal spinal cord (Fig. 3). We note, in addition, that recent work in the Barres laboratory has identified Klf15 as one of the best candidates for an astrocyte-specific transcription factor using expression array data obtained from highly purified astrocytes (Cahoy et al., 2008).
Overexpression of Klf15 in spinal cord explants leads to overexpression of GFAP. Loss-of-function studies with Klf15 (data not shown) were uninformative. The Klf15 knockout mice are viable and fertile with no outward signs of a CNS defect such as tremor, seizure or ataxia (Fisch et al., 2007; Gray et al., 2007). Klf15 knockout mice show no differences in glial marker expression (GFAP, Glast, Glutamate synthase, and S-100β) from wild type littermates between E13.5 and P14, the critical stages in the development of astrocytes (data not shown). Klf15 is a member of the Kruppel-like family (Klf) of transcription factors, which play functional roles in cell growth and differentiation in a wide range of mammalian cell types (Black et al., 2001; Pearson et al., 2008). It is thus possible that other members of the Klf family are functionally redundant with Klf15 in the specification, maturation or survival of astrocytes in the vertebrate CNS.
Several pictorial atlases of gene expression in the vertebrate CNS have appeared in recent years (Gong et al., 2003; Gray et al., 2004; Visel et al., 2004; Christiansen et al., 2006; Magdaleno et al., 2006; Lein et al., 2007). The content of these newer databases transcends transcription factors to include a wide range regulatory gene classes. Expression screens of these data sets along lines described here might identify novel growth factors, morphogenic proteins, cell surface receptors and cytoplasmic signal generating proteins involved in the genesis of glial cells. In the fullness of time, identification of the agents that regulate glial development could have practical overtones for the therapy of a wide range of neurological disease states including multiple sclerosis, hypoxic injury and seizure disorders.
Supplementary Material
Acknowledgements
We would like to thank Dr. William Stallcup for NG2 antibody and Dr. David Anderson for Sox10 antibody. We also thank Xuemei Hu for technical support. This research was supported by grant NS059893 from the National Institutes of Health and by NO30860131 from the National Natural Science Foundation of China.
References
- Arnett HA, Fancy SP, Alberta JA, Zhao C, Plant SR, Kaing S, Raine CS, Rowitch DH, Franklin RJ, Stiles CD. bHLH transcription factor Olig1 is required to repair demyelinated lesions in the CNS. Science. 2004;306:2111–2115. doi: 10.1126/science.1103709. [DOI] [PubMed] [Google Scholar]
- Barradas PC, Gomes SS, Cavalcante LA. CNPase expression in the developing opossum brain stem and cerebellum. Neuroreport. 1995;6:289–292. doi: 10.1097/00001756-199501000-00016. [DOI] [PubMed] [Google Scholar]
- Black AR, Black JD, Azizkhan-Clifford J. Sp1 and kruppel-like factor family of transcription factors in cell growth regulation and cancer. J Cell Physiol. 2001;188:143–160. doi: 10.1002/jcp.1111. [DOI] [PubMed] [Google Scholar]
- Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, Xing Y, Lubischer JL, Krieg PA, Krupenko SA, Thompson WJ, Barres BA. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci. 2008;28:264–278. doi: 10.1523/JNEUROSCI.4178-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen JH, Yang Y, Venkataraman S, Richardson L, Stevenson P, Burton N, Baldock RA, Davidson DR. EMAGE: a spatial database of gene expression patterns during mouse embryo development. Nucleic Acids Res. 2006;34:D637–D641. doi: 10.1093/nar/gkj006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Clevers H, van de Wetering M. TCF/LEF factor earn their wings. Trends Genet. 1997;13:485–489. doi: 10.1016/s0168-9525(97)01305-x. [DOI] [PubMed] [Google Scholar]
- Deneen B, Ho R, Lukaszewicz A, Hochstim CJ, Gronostajski RM, Anderson DJ. The transcription factor NFIA controls the onset of gliogenesis in the developing spinal cord. Neuron. 2006;52:953–968. doi: 10.1016/j.neuron.2006.11.019. [DOI] [PubMed] [Google Scholar]
- Fancy SP, Baranzini SE, Zhao C, Yuk DI, Irvine KA, Kaing S, Sanai N, Franklin RJ, Rowitch DH. Dysregulation of the Wnt pathway inhibits timely myelination and remyelination in the mammalian CNS. Genes Dev. 2009;23:1571–1585. doi: 10.1101/gad.1806309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisch S, Gray S, Heymans S, Haldar SM, Wang B, Pfister O, Cui L, Kumar A, Lin Z, Sen-Banerjee S, Das H, Petersen CA, Mende U, Burleigh BA, Zhu Y, Pinto YM, Liao R, Jain MK. Kruppel-like factor 15 is a regulator of cardiomyocyte hypertrophy. Proc Natl Acad Sci U S A. 2007;104:7074–7079. doi: 10.1073/pnas.0701981104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu H, Qi Y, Tan M, Cai J, Takebayashi H, Nakafuku M, Richardson W, Qiu M. Dual origin of spinal oligodendrocyte progenitors and evidence for the cooperative role of Olig2 and Nkx2.2 in the control of oligodendrocyte differentiation. Development. 2002;129:681–693. doi: 10.1242/dev.129.3.681. [DOI] [PubMed] [Google Scholar]
- Fukuda S, Kondo T, Takebayashi H, Taga T. Negative regulatory effect of an oligodendrocytic bHLH factor OLIG2 on the astrocytic differentiation pathway. Cell Death Differ. 2004;11:196–202. doi: 10.1038/sj.cdd.4401332. [DOI] [PubMed] [Google Scholar]
- Gomes WA, Mehler MF, Kessler JA. Transgenic overexpression of BMP4 increases astroglial and decreases oligodendroglial lineage commitment. Dev Biol. 2003;255:164–177. doi: 10.1016/s0012-1606(02)00037-4. [DOI] [PubMed] [Google Scholar]
- Gong S, Zheng C, Doughty ML, Losos K, Didkovsky N, Schambra UB, Nowak NJ, Joyner A, Leblanc G, Hatten ME, Heintz N. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature. 2003;425:917–925. doi: 10.1038/nature02033. [DOI] [PubMed] [Google Scholar]
- Grant SF, et al. Variant of transcription factor 7-like 2 (TCF7L2) gene confers risk of type 2 diabetes. Nat Genet. 2006;38:320–323. doi: 10.1038/ng1732. [DOI] [PubMed] [Google Scholar]
- Gray PA, et al. Mouse brain organization revealed through direct genome-scale TF expression analysis. Science. 2004;306:2255–2257. doi: 10.1126/science.1104935. [DOI] [PubMed] [Google Scholar]
- Gray S, Wang B, Orihuela Y, Hong EG, Fisch S, Haldar S, Cline GW, Kim JK, Peroni OD, Kahn BB, Jain MK. Regulation of gluconeogenesis by Kruppel-like factor 15. Cell Metab. 2007;5:305–312. doi: 10.1016/j.cmet.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross RE, Mehler MF, Mabie PC, Zang Z, Santschi L, Kessler JA. Bone morphogenetic proteins promote astroglial lineage commitment by mammalian subventricular zone progenitor cells. Neuron. 1996;17:595–606. doi: 10.1016/s0896-6273(00)80193-2. [DOI] [PubMed] [Google Scholar]
- He Y, Dupree J, Wang J, Sandoval J, Li J, Liu H, Shi Y, Nave KA, Casaccia-Bonnefil P. The transcription factor Yin Yang 1 is essential for oligodendrocyte progenitor differentiation. Neuron. 2007;55:217–230. doi: 10.1016/j.neuron.2007.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Kim SH, Kim H, Chung AY, Cha YI, Kim CH, Huh TL, Park HC. Frizzled 8a function is required for oligodendrocyte development in the zebrafish spinal cord. Dev Dyn. 2008;237:3324–3331. doi: 10.1002/dvdy.21739. [DOI] [PubMed] [Google Scholar]
- Korinek V, Barker N, Moerer P, van Donselaar E, Huls G, Peters PJ, Clevers H. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat Genet. 1998;19:379–383. doi: 10.1038/1270. [DOI] [PubMed] [Google Scholar]
- Korinek V, Barker N, Morin PJ, van Wichen D, de Weger R, Kinzler KW, Vogelstein B, Clevers H. Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC−/− colon carcinoma. Science. 1997;275:1784–1787. doi: 10.1126/science.275.5307.1784. [DOI] [PubMed] [Google Scholar]
- Kuhl M, Sheldahl LC, Park M, Miller JR, Moon RT. The Wnt/Ca2+ pathway: a new vertebrate Wnt signaling pathway takes shape. Trends Genet. 2000;16:279–283. doi: 10.1016/s0168-9525(00)02028-x. [DOI] [PubMed] [Google Scholar]
- Lein ES, et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445:168–176. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- Liu R, Cai J, Hu X, Tan M, Qi Y, German M, Rubenstein J, Sander M, Qiu M. Region-specific and stage-dependent regulation of Olig gene expression and oligodendrogenesis by Nkx6.1 homeodomain transcription factor. Development. 2003;130:6221–6231. doi: 10.1242/dev.00868. [DOI] [PubMed] [Google Scholar]
- Lu QR, Sun T, Zhu Z, Ma N, Garcia M, Stiles CD, Rowitch DH. Common developmental requirement for Olig function indicates a motor neuron/oligodendrocyte connection. Cell. 2002;109:75–86. doi: 10.1016/s0092-8674(02)00678-5. [DOI] [PubMed] [Google Scholar]
- Lu QR, Yuk D, Alberta JA, Zhu Z, Pawlitzky I, Chan J, McMahon AP, Stiles CD, Rowitch DH. Sonic hedgehog--regulated oligodendrocyte lineage genes encoding bHLH proteins in the mammalian central nervous system. Neuron. 2000;25:317–329. doi: 10.1016/s0896-6273(00)80897-1. [DOI] [PubMed] [Google Scholar]
- Magdaleno S, Jensen P, Brumwell CL, Seal A, Lehman K, Asbury A, Cheung T, Cornelius T, Batten DM, Eden C, Norland SM, Rice DS, Dosooye N, Shakya S, Mehta P, Curran T. BGEM: an in situ hybridization database of gene expression in the embryonic and adult mouse nervous system. PLoS Biol. 2006;4:e86. doi: 10.1371/journal.pbio.0040086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matys V, et al. TRANSFAC: transcriptional regulation, from patterns to profiles. Nucleic Acids Res. 2003;31:374–378. doi: 10.1093/nar/gkg108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy KD, de Vellis J. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J Cell Biol. 1980;85:890–902. doi: 10.1083/jcb.85.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekki-Dauriac S, Agius E, Kan P, Cochard P. Bone morphogenetic proteins negatively control oligodendrocyte precursor specification in the chick spinal cord. Development. 2002;129:5117–5130. doi: 10.1242/dev.129.22.5117. [DOI] [PubMed] [Google Scholar]
- Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B, Kinzler KW. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science. 1997;275:1787–1790. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- Muroyama Y, Fujiwara Y, Orkin SH, Rowitch DH. Specification of astrocytes by bHLH protein SCL in a restricted region of the neural tube. Nature. 2005;438:360–363. doi: 10.1038/nature04139. [DOI] [PubMed] [Google Scholar]
- Pearson R, Fleetwood J, Eaton S, Crossley M, Bao S. Kruppel-like transcription factors: a functional family. Int J Biochem Cell Biol. 2008;40:1996–2001. doi: 10.1016/j.biocel.2007.07.018. [DOI] [PubMed] [Google Scholar]
- Privat A, Rataboul P. Fibrous and Protoplasmic astrocytes. In: Fedoroff S, Vernadakis A, editors. Astrocytes: Development, Morphology and Regional Specialization of Astrocytes. Orlando: Academic Press; 1986. pp. 105–130. [Google Scholar]
- Qi Y, Cai J, Wu Y, Wu R, Lee J, Fu H, Rao M, Sussel L, Rubenstein J, Qiu M. Control of oligodendrocyte differentiation by the Nkx2.2 homeodomain transcription factor. Development. 2001;128:2723–2733. doi: 10.1242/dev.128.14.2723. [DOI] [PubMed] [Google Scholar]
- Setoguchi T, Kondo T. Nuclear export of OLIG2 in neural stem cells is essential for ciliary neurotrophic factor-induced astrocyte differentiation. J Cell Biol. 2004;166:963–968. doi: 10.1083/jcb.200404104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu T, Kagawa T, Wada T, Muroyama Y, Takada S, Ikenaka K. Wnt signaling controls the timing of oligodendrocyte development in the spinal cord. Dev Biol. 2005;282:397–410. doi: 10.1016/j.ydbio.2005.03.020. [DOI] [PubMed] [Google Scholar]
- Sonnhammer EL, Eddy SR, Birney E, Bateman A, Durbin R. Pfam: multiple sequence alignments and HMM-profiles of protein domains. Nucleic Acids Res. 1998;26:320–322. doi: 10.1093/nar/26.1.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolt CC, Lommes P, Sock E, Chaboissier MC, Schedl A, Wegner M. The Sox9 transcription factor determines glial fate choice in the developing spinal cord. Genes Dev. 2003;17:1677–1689. doi: 10.1101/gad.259003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolt CC, Rehberg S, Ader M, Lommes P, Riethmacher D, Schachner M, Bartsch U, Wegner M. Terminal differentiation of myelin-forming oligodendrocytes depends on the transcription factor Sox10. Genes Dev. 2002;16:165–170. doi: 10.1101/gad.215802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takebayashi H, Nabeshima Y, Yoshida S, Chisaka O, Ikenaka K, Nabeshima Y. The basic helix-loop-helix factor olig2 is essential for the development of motoneuron and oligodendrocyte lineages. Curr Biol. 2002;12:1157–1163. doi: 10.1016/s0960-9822(02)00926-0. [DOI] [PubMed] [Google Scholar]
- Takebayashi H, Yoshida S, Sugimori M, Kosako H, Kominami R, Nakafuku M, Nabeshima Y. Dynamic expression of basic helix-loop-helix Olig family members: implication of Olig2 in neuron and oligodendrocyte differentiation and identification of a new member, Olig3. Mech Dev. 2000;99:143–148. doi: 10.1016/s0925-4773(00)00466-4. [DOI] [PubMed] [Google Scholar]
- Thomas PD, Campbell MJ, Kejariwal A, Mi H, Karlak B, Daverman R, Diemer K, Muruganujan A, Narechania A. PANTHER: a library of protein families and subfamilies indexed by function. Genome Res. 2003;13:2129–2141. doi: 10.1101/gr.772403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visel A, Thaller C, Eichele G. GenePaint.org: an atlas of gene expression patterns in the mouse embryo. Nucleic Acids Res. 2004;32:D552–D556. doi: 10.1093/nar/gkh029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegner M, Riethmacher D. Chronicles of a switch hunt: gcm genes in development. Trends Genet. 2001;17:286–290. doi: 10.1016/s0168-9525(01)02275-2. [DOI] [PubMed] [Google Scholar]
- Wheeler DL, Church DM, Edgar R, Federhen S, Helmberg W, Madden TL, Pontius JU, Schuler GD, Schriml LM, Sequeira E, Suzek TO, Tatusova TA, Wagner L. Database resources of the National Center for Biotechnology Information: update. Nucleic Acids Res. 2004;32:D35–D40. doi: 10.1093/nar/gkh073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin M, Yue T, Ma Z, Wu FF, Gow A, Lu QR. Myelinogenesis and axonal recognition by oligodendrocytes in brain are uncoupled in Olig1-null mice. J Neurosci. 2005;25:1354–1365. doi: 10.1523/JNEUROSCI.3034-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye F, Chen Y, Hoang T, Montgomery RL, Zhao XH, Bu H, Hu T, Taketo MM, van Es JH, Clevers H, Hsieh J, Bassel-Duby R, Olson EN, Lu QR. HDAC1 and HDAC2 regulate oligodendrocyte differentiation by disrupting the beta-catenin-TCF interaction. Nat Neurosci. 2009;12:829–838. doi: 10.1038/nn.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Anderson DJ. The bHLH transcription factors OLIG2 and OLIG1 couple neuronal and glial subtype specification. Cell. 2002;109:61–73. doi: 10.1016/s0092-8674(02)00677-3. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Wang S, Anderson DJ. Identification of a novel family of oligodendrocyte lineage-specific basic helix-loop-helix transcription factors. Neuron. 2000;25:331–343. doi: 10.1016/s0896-6273(00)80898-3. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Choi G, Anderson DJ. The bHLH transcription factor Olig2 promotes oligodendrocyte differentiation in collaboration with Nkx2.2. Neuron. 2001;31:791–807. doi: 10.1016/s0896-6273(01)00414-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.