Abstract
The roof plate is an organizing center in the dorsal central nervous system that controls specification and differentiation of adjacent neurons through secretion of the BMP and WNT signaling molecules. Lmx1a is expressed in the roof plate and its progenitors at all axial levels of the CNS and is necessary and sufficient for roof plate formation in the spinal cord. In the anterior CNS, however, a residual roof plate develops in the absence of Lmx1a. Lmx1b, a highly related gene required for the development of the isthmic organizer, is co-expressed with Lmx1a in dorsal rhombomere 1 roof plate. Although Lmx1b null mice do not show a substantial deficiency in hindbrain roof plate formation, Lmx1a/Lmx1b compound null mutants fail to generate hindbrain roof plate. This observation indicates that both genes act in concert to direct normal hindbrain roof plate formation. Since the requirement of Lmx1b function for normal isthmic organizer at the mid-hindbrain boundary complicates analysis of a distinct dorsal patterning role of this gene, we also used a conditional knockout strategy to specifically delete dorsal midline Lmx1b expression. Phenotypic analysis of single and compound conditional mutants confirmed overlapping roles for Lmx1 genes in regulating hindbrain roof plate formation and growth and also revealed roles in regulating adjacent cerebellar morphogenesis. Our data provides the first evidence of overlapping function of the Lmx1 genes during embryonic CNS development.
Keywords: dorsal, patterning, cerebellum, hindbrain, genetics, development
INTRODUCTION
During hindbrain development, the isthmic organizer (IsO) at the mid/hindbrain junction determines anterior/posterior patterning signals (Sillitoe and Joyner, 2007) while the 4th ventricle roof plate provides dorsal positional information (Chizhikov et al., 2006). The hindbrain roof plate differentiates into the epithelium of the choroid plexus, a structure with multiple physiological functions including the secretion of cerebrospinal fluid (Currle et al., 2005). The genetic cascades driving IsO formation and function have been extensively studied (Liu and Joyner, 2001; Wang and Zoghbi, 2001; Wurst and Bally-Cuif, 2001; Nakamura et al., 2005). Far less is understood regarding hindbrain roof plate and choroid plexus formation.
Previously, roof plate studies have primarily concentrated on the spinal cord, where roof plate progenitors are induced at the lateral edges of the neural plate through a Bmp-Lmx1a signaling pathway (Liem et al., 1997; Lee et al., 1998; Lee and Jessell, 1999; Lee et al., 2000; Chizhikov and Millen, 2004a, 2005). Although loss of Lmx1a completely abolishes roof plate induction in the spinal cord, a residual roof plate still forms in rhombomere 1 (rh1), the most anterior segment of the hindbrain in Lmx1a−/− (dreher) mice. We hypothesized that Lmx1b, a protein with 64% amino acid identity to mouse Lmx1a (100% identity in the homeodomain and 67% and 83% in each LIM domain), functions redundantly with Lmx1a. Although Lmx1b is expressed in the IsO, it is also expressed in the anterior hindbrain roof plate, in a domain which closely correlates with the residual roof plate in Lmx1a−/− embryos suggesting redundant function to Lmx1a. Further, Lmx1b can induce roof plate when overexpressed in chick spinal cord (Chizhikov and Millen, 2004b). The function of Lmx1b in the developing IsO is well characterized (Adams et al., 2000; Matsunaga et al., 2002; Guo et al., 2007). The specific role of Lmx1b in hindbrain roof plate development, however, has never been assessed.
Here we show that Lmx1a and Lmx1b are individually dispensable for hindbrain roof plate induction. Complete loss of both genes, however, abolishes hindbrain roof plate demonstrating that both genes have overlapping roles in inducing this critical embryonic signaling center. Since Lmx1b is required for IsO maintenance (Adams et al., 2000; Matsunaga et al., 2002; Guo et al., 2007), the loss of IsO in the Lmx1b null background complicated our analysis. To isolate the anterior/posterior patterning functions from the dorsal/ventral patterning role of Lmx1b, we used a conditional knockout strategy to specifically delete Lmx1b in the dorsal midline without disturbing its isthmic expression. These Lmx1b conditional mutant mice were also bred with Lmx1a mutant mice to further reduce Lmx1 gene dosage. Lmx1a; Lmx1b conditional compound mutant mice showed severe 4th ventricle roof plate size reduction, confirming overlapping roles for the dorsally expressed Lmx1 genes in roof plate formation. The viability of these compound conditional mutant mice with small roof plate further allowed us to demonstrate that rh1 roof plate directs multiple aspects of cerebellar morphogenesis, including cerebellar anlage proliferation. Together, these experiments provide the first evidence for overlapping function of Lmx1a and Lmx1b in the central nervous system.
MATERIALS AND METHODS
Mice
Lmx1a null (Lmx1a−/−) (Lmx1adr-J Jackson Laboratories strain #000636), Lmx1b null (Lmx1b−/−)(Chen et al., 1998), Lmx1b floxed (Lmx1bf/f) (Zhao et al., 2006), and Lmx1a-cre transgenic mice (Chizhikov et al., 2006) were genotyped as previously described. Cre activity was discerned by crossing Lmx1a-cre transgenic mice to ROSA26 floxed LacZ reporter mice (129S-Gt(ROSA)26Sortm1Sor/J; Jackson Laboratories; strain #003310) (Soriano, 1999). Compound conditional mutant mice (Lmx1a−/−;Lmx1bcko/−mice) were generated by intercrossing Lmx1bf/f; Lmx1a+/− mice and Lmx1a-cre; Lmx1a+/−; Lmx1b+/− mice. Noon of the observation of the vaginal plug was considered as embryonic day (E) 0.5.
The Lmx1a-cre BAC transgene includes wild-type exon 2, necessitating adjustment of the standard exon 2 Lmx1a genotyping protocol (Chizhikov et al. 2006) in mice carrying the transgene. Restriction analysis of the wild-type exon 2 amplified allele using HpyCh4V yielded 127bp, 14bp, 51bp, 33bp in 5′ to 3′ order. The dr-J point mutation abolishes the HpyCh4V site between the 51bp and 33bp fragments resulting in a 84 bp fragment. Restriction digests of the exon 2 amplicon from Lmx1a−/− mice carrying the Lmx1a-cre transgene produced the 84bp dr-J band and the wild-type 51bp band. To distinguish these mice from heterozygous Lmx1a+/− mice carrying the Lmx1a-cre transgene, we compared the band intensities of the 51bp wild-type vs. 84bp mutant band. Genotyping conditions and primers are available upon request. All mice were maintained on mixed genetic backgrounds. All mouse procedures followed the policies of the University of Chicago and NIH Guidelines on Care and Use of Laboratory Animals.
Tissue analysis
In situ hybridization was performed as previously described (Chizhikov et al., 2006). Probes used were mouse Lmx1a, Lmx1b, Gdf7 (T. Jessell), Wnt1 (A. McMahon), Math1 (J. Johnson), Ptf1a (C. Wright), and Otp (A. Simeone). For the Lmx1b homeodomain-specific probe, exons 4, 5, and 6 were PCR amplified and cloned into PCRII-TOPO (Invitrogen), linearized with EcoRV, and antisense transcribed with SP6. Immunohistochemistry and BrdU analysis was performed as previously described (Chizhikov et al., 2006). Primary antibodies used were: Lmx1a (M. German), Lmx1b (T. Perlmann), BrdU (DSHB and Abcam), and Calbindin (Swant). Appropriate species-specific secondary antibodies were used (Jackson Immunological). X-gal staining and histology were performed as previously described (Chizhikov et al., 2006).
Measurements and statistical analysis
All embryos were photographed on a LEICA MZFLIII microscope using a Spot II camera. The areas of 4th ventricle roof plate were measured using the Spot version 4.6 software in whole mount embryos. Three individual embryos of each genotype were measured three times (unit μm2) to obtain an average. Areas of sagittal sections of the cerebellar vermis were measured using four individual mice of each genotype. For quantitative analysis of the BrdU positive cells, three mice of each genotype were examined. The mitotic index of the cerebellar ventricular zone was calculated as the number of BrdU positive cells divided by the total number of cells (DAPI) in a set area (a total of 500 DAPI cells along the ventricular zone,100μm × 10μm in area, were counted). Two tailed t-tests were used to determine statistical significance. * indicates p<0.01, ** indicates p<0.001.
RESULTS
Lmx1a and Lmx1b are expressed in the rh1 roof plate and its derivative, the choroid plexus
Extending previous studies from chick (Adams et al., 2000) and zebrafish (O'Hara et al., 2005; Elsen et al., 2008), we carefully examined the developmental expression profile of Lmx1b in dorsal rh1 of the developing mouse (Fig. 1). We first observed Lmx1b expression at E8.0, along the lateral edges of the closing neural plate (Fig. 1B), the region that gives rise to rh1 roof plate (Awatramani et al., 2003; Chizhikov et al., 2006; Hunter and Dymecki, 2007). After neural tube closure, Lmx1b was highly expressed in rh1 roof plate and its later derivative, the choroid plexus epithelium, at all stages examined (Fig. 1D,G,J and data not shown). In the mid/hindbrain region, we also observed Lmx1b expression in the IsO (Fig. 1D arrow), where Lmx1b has a critical function in IsO maintenance (Adams et al., 2000; Matsunaga et al., 2002; Guo et al., 2007). Similar to Lmx1b, Lmx1a was expressed at the lateral edges of the mid/hindbrain neural plate but its expression was initiated slightly later than Lmx1b, at E 8.5 (Failli et al., 2002; Millen et al., 2004) (Fig. 1A). Lmx1a was also expressed in rh1 roof plate and choroid plexus epithelium during embryonic and postnatal development (Fig. 1C,F and (Chizhikov et al., 2006)). However, beginning from E12.5, Lmx1a expression was further initiated in the adjacent rhombic lip (Fig. 1F arrow). In contrast, Lmx1b expression remained restricted to the choroid plexus at both embryonic and postnatal stages (Fig. 1G,H,J). Given that both genes have overlapping expression domains, we hypothesized that both are involved in rh1 roof plate development and may function together.
Figure 1. Expression of Lmx1a and Lmx1b in rh1 roof plate and its derivative, the choroid plexus epithelium.
(A-D) Whole mount in situ hybridization using probes for Lmx1a and Lmx1b. (A and B) Dorsal views of E8.5 embryos. Lmx1a and Lmx1b were expressed in roof plate progenitor cells at the lateral edges of the neural tube (arrowheads). Lmx1b was also expressed across the isthmic organizer (IsO) at the mid/hindbrain junction (arrow in (B)). (C and D) Lateral view of E10.5 embryos. Brackets indicate rhombomere 1 (rh1). (C) Lmx1a was expressed in the roof plate along the anterior posterior axis of the embryo including rh1 (arrowhead). (D) Lmx1b was expressed in the anterior roof plate (arrowhead) and in the IsO (arrow). (E) Schematic of dorsal view of rh1 and paramedial sagittal section through rh1 at E10.5. Boxed region is equivalent to sagittal sections in (F-H). (F) Immunohistochemistry at E12.5 showed expression of Lmx1a in choroid plexus (CP) and in the rhombic lip (RL) (arrow). (G and H) Arrowhead shows the CP/RL boundary. (G) Lmx1b expression was restricted to the CP. (H) Merged image of (F) and (G). (I) Schematic of mid-sagittal section of P6 cerebellum. Boxed region identifies the posterior lobe and the CP shown in the next panel. (J) Lmx1b was expressed in the CP but not in the cerebellum. EGL, external granule layer.
Lmx1a and Lmx1b have overlapping roles in hindbrain roof plate development
Although the role of Lmx1b in IsO is well documented (Adams et al., 2000; Matsunaga et al., 2002; O'Hara et al., 2005; Guo et al., 2007), its specific role in rh1 roof plate development has never been assessed. We therefore examined rh1 roof plate in Lmx1b null (Lmx1b−/−) mice. Despite considerable disruption of the mid/hindbrain junction caused by loss of Lmx1b function in the IsO (Adams et al., 2000; Matsunaga et al., 2002; O'Hara et al., 2005; Guo et al., 2007), Lmx1b−/− embryos had a relatively normal rh1 roof plate based on its size, shape and normal expression of multiple roof plate markers, including Gdf7, Wnt1, and Msx1 at E10.5 (n=3 for each marker) (Fig. 2C,D and data not shown). These data indicate that despite its significant role in IsO maintenance, Lmx1b is largely dispensable for rh1 roof plate development. This is in contrast to Lmx1a since its loss results in severe reduction of rh1 roof plate (Fig. 2E,F, (Millonig et al., 2000; Millen et al., 2004; Chizhikov et al., 2006)).
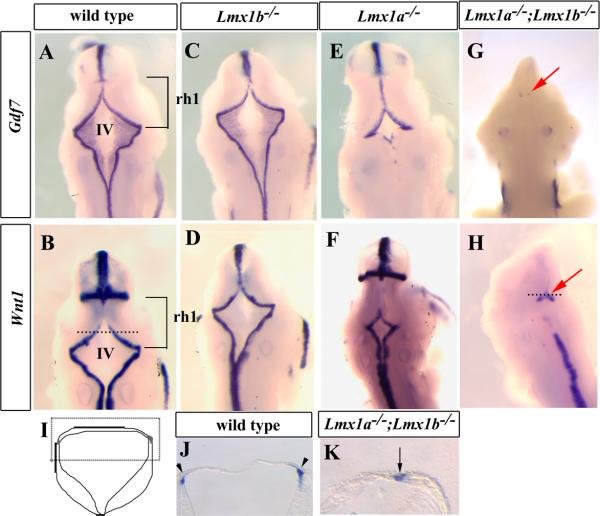
Figure 2. Development of the hindbrain roof plate is dependent on overlapping functions of Lmx1a and Lmx1b.
(A-H) Dorsal view of whole mount in situ hybridization of E10.5 embryos using probes for Gdf7 and Wnt1. The brackets in (A) and (B) indicate the position of rh1 and the future cerebellum. Line in (B) specifies level of transverse section in (I). (C and D) In Lmx1b−/− embryos, the 4th ventricle roof plate was nearly normal despite the loss of the IsO. (E and F) The 4th ventricle roof plate was reduced in Lmx1a−/− embryos. (G and H) Lmx1a−/−;Lmx1b−/− embryos had little or no residual 4th ventricle roof plate. Line in (H) indicates the transverse section in (K). (I) Schematic of a transverse section of the 4th ventricle. Boxed region indicates equivalent region in (J) and (K). (J) In wild-type embryos, the roof plate covered the 4th ventricle. (K) This region was lost in Lmx1a−/−;Lmx1b−/− embryos, although residual, lateral non-roof plate Wnt1 expression remained. IV; 4th ventricle.
To test if Lmx1b cooperates with Lmx1a during roof plate development, we next examined the rh1 roof plate phenotype of double null (Lmx1a−/−;Lmx1b−/−) mutant embryos. Strikingly, at E10.5, expression of Gdf7 was completely missing or extremely reduced in double null mutant embryos (Fig. 2G) (n=3). In addition, we detected abnormal expression of Wnt1 in dorsal rh1 of E10.5 Lmx1a−/−;Lmx1b−/− embryos (n=3). In wild-type embryos, Wnt1 is expressed in the rhombic lip adjacent to hindbrain roof plate (Awatramani et al., 2003; Hunter and Dymecki, 2007). We confirmed this expression in rh1 of wild-type embryos (Fig. 2B,J arrowheads), but observed fused medial expression in Lmx1a−/−;Lmx1b−/− embryos at E10.5 (Fig. 2H,K arrow), further demonstrating the lack of rh1 roof plate.
We next tested whether dorsal midline cells in Lmx1a−/−;Lmx1b−/− embryos retained any properties of roof plate. We and others have shown that rh1 roof plate signaling is required to induce Math1 in the adjacent cerebellar rhombic lip (Alder et al., 1999; Chizhikov et al., 2006). In Lmx1b−/− mice, despite IsO disruption, rh1 Math1 expression was similar to wild-type embryos (Fig. 3B). In Lmx1a−/− mice, although the roof plate was small, Math1 was still induced (Fig. 3C). However, no Math1 expression was detected in Lmx1a−/−;Lmx1b−/− mice (n=4) (Fig. 3D) indicating complete loss of roof plate signaling. Importantly, Ptf1a, a marker of cerebellar ventricular zone whose induction is not dependent on roof plate signaling (Chizhikov et al., 2006), was still present in Lmx1a−/−;Lmx1b−/− embryos (Fig. 3H). This indicates that the cerebellar ventricular zone which forms just ventral to the rhombic lip within dorsal rh1 was still present. Ptf1a expression was reduced however, since the roof plate is required to expand the ventricular zone progenitor pool that expresses this marker (Chizhikov et al., 2006). At an earlier stage (E10.5), Otp, a ventral marker, was expressed normally in all mutant embryos (Fig. 3I-L), arguing that there was no perturbation of the alar/basal plate boundary in Lmx1a−/−;Lmx1b−/− embryos and that dorsal/ventral patterning phenotype in rh1 phenotype restricted to very dorsal cell types. This confirms our previous finding that roof plate-dependent cell fate specification is limited to just the adjacent rhombic lip in rh1 (Chizhikov et al., 2006). Together, both our morphological and functional data indicate that Lmx1b and Lmx1a have overlapping roles in the development of rh1 roof plate.
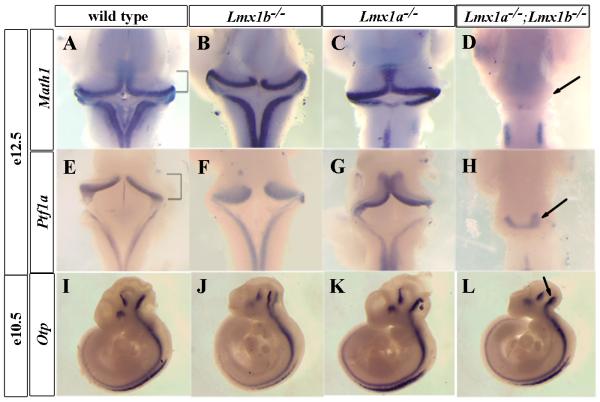
Figure 3. Math1+ rhombic lip cells are lost in Lmx1a−/−;Lmx1b−/− embryos.
(A-H) Dorsal view of whole mount in situ hybridization of sub-dissected E12.5 brains using probes for Math1 (A-D) and Ptf1a (E-H). Brackets in (A) and (E) indicate the developing cerebellum. (A-C) Math1 expression, induced by roof plate signaling, was retained in Lmx1b−/− (B), and Lmx1a−/− (C) embryos. However, Math1 expression was completely lost in Lmx1a−/−;Lmx1b−/− embryos (arrow in (D)) confirming complete loss of roof plate function in rh1. (E-H) Ptf1a expression is independent of roof plate signaling, and was present in all genotypes, although the domain of expression was reduced in Lmx1a−/−;Lmx1b−/− mutants (arrow in (H)). (I-L) Otp expression was not disturbed in Lmx1a−/−;Lmx1b−/− mutants (arrow in (L)) compared to the wild-type (I) or single mutant embryos (J and K), indicating that alar/basal boundary is not perturbed.
Generation of mice with conditional loss of Lmx1b only in the roof plate where it is co-expressed with Lmx1a
Our analysis of Lmx1a−/−;Lmx1b−/− embryos suggested involvement of Lmx1b in rh1 roof plate development. The mid/hindbrain region of Lmx1a−/−;Lmx1b−/− mice however, was severely mispatterned since Lmx1b expression at the mid/hindbrain is required to maintain the IsO (Adams et al., 2000; Matsunaga et al., 2002; Guo et al., 2007). This anterior/posterior mispatterning significantly complicated our assessment of the role of Lmx1b in rh1 roof plate formation. To more specifically examine the role of Lmx1b just in roof plate development, we designed a conditional knockout strategy to delete Lmx1b only in the dorsal midline, without disturbing its expression in the IsO.
The mice used to create the desired mutant embryos carried a floxed allele of Lmx1b (Lmx1bf) (Zhao et al., 2006) and a null allele of Lmx1b (Lmx1b−) (Chen et al., 1998). In the deleter mouse strain used, cre recombinase expression was controlled by Lmx1a regulatory elements (Lmx1a-cre) (Fig. 4A) (Chizhikov et al., 2006). A series of control experiments were initially conducted to determine the efficacy of the strategy. First, we verified that the Lmx1bf allele was not hypomorphic by assessing postnatal (P) 20 cerebellar morphology in Lmx1bf/− mice, since adult cerebellar morphology is sensitive to perturbations of IsO (Sato et al., 2004; Sillitoe and Joyner, 2007). As expected, we observed normal cerebellar morphology in Lmx1bf/− mutants, demonstrating that embryonic IsO was undisturbed (data not shown). We next verified cre activity in rh1 roof plate by assessing LacZ activity in embryos from transgenic Lmx1a-cre mice mated with ROSA26 floxed LacZ reporter mice (Soriano, 1999). Weak LacZ activity was initially detected at E9.0, 0.5 days later than endogenous Lmx1a expression and approximately 1 day later than endogenous Lmx1b expression (Fig. 4B). By E9.5, however, robust LacZ activity was readily detected in the majority of rh1 roof plate cells (Fig. 4C).
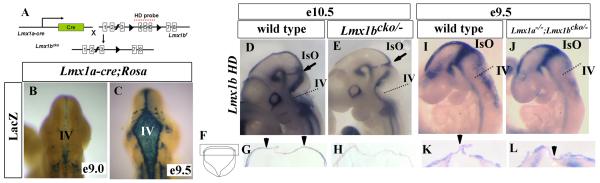
Figure 4. A conditional knockout strategy to delete Lmx1b only in the roof plate where it is co-expressed with Lmx1a.
(A) Schematic diagram demonstrating the Lmx1b conditional knockout strategy. Lmx1a-cre transgenic mice were bred to mice carrying a floxed allele of Lmx1b (Lmx1bf), leading to excision of Lmx1bf only within the Lmx1a expression domain in the roof plate. (B and C) β-gal activity in the progeny of Lmx1a-cre mice mated to ROSA26 floxed LacZ reporter mice. (B) Only a few LacZ positive cells are visible in the 4th ventricle at E9.0. (C) LacZ expression is clearly visible at the 4th ventricle at E9.5.
(D-L) In situ hybridization using the Lmx1b homeodomain (HD) probe indicated in (A). (D and E) Whole mount at E10.5 of wild-type (D) and Lmx1bcko/− embryos (E). Lmx1b HD expression was reduced in the 4th ventricle roof plate but remained intact in the IsO in Lmx1bcko/− embryos. (F) Schematic of a transverse section of the 4th ventricle at E10.5. Boxed region indicates equivalent region in (G-H and K-L). (G and H) Transverse sections at E10.5 showed that Lmx1b HD expression is present in the wild-type (G) but lost in Lmx1bcko/− embryos (H). Arrowheads indicate Lmx1b HD expression in the wild-type. (I and J) Whole mount at E9.5 showed that Lmx1b HD is expressed in both the wild-type (I) and Lmx1a−/−; Lmx1bcko/− embryos (J). (K and L) Transverse sections through the 4th ventricle at E9.5 showed that Lmx1b HD expression is present in both the wild-type (K) and Lmx1a−/−; Lmx1bcko/− embryos (L). Arrowheads indicate Lmx1b HD expression.
To specifically delete the dorsal expression domain of Lmx1b, we crossed Lmx1bf/− mice with Lmx1b+/− mice carrying the Lmx1a-cre transgene (the Lmx1bf/−;Lmx1a-cre genotype referred to as Lmx1bcko/−). To verify that Lmx1b expression was eliminated only in the roof plate in Lmx1bcko/− double mutant mice, we conducted in situ hybridization using a probe specific for the exons flanked by loxP sites. As expected, Lmx1b expression was detected in the IsO and rh1 roof plate of E10.5 wild-type embryos (Fig. 4D). In Lmx1bcko/− embryos, we detected normal expression of Lmx1b in the IsO but none in the rh1 roof plate (Fig. 4E). Transverse sections through the rh1 further verified that expression in 4th ventricle roof plate was specifically deleted in Lmx1bcko/− embryos (Fig. 4 F-H). Finally, we assessed the roof plate phenotype of Lmx1bcko/− mice. The normal rh1 roof plate in these embryos (data not shown) confirmed our earlier observation in Lmx1b−/− embryos that partial or complete loss of Lmx1b alone has minimal effect on rh1 roof plate development.
Both Lmx1 genes are required for normal rh1 roof plate growth
To further reduce hindbrain roof plate Lmx1 gene dosage we crossed Lmx1bcko/− mice with Lmx1a−/− mice, to generate Lmx1a−/−;Lmx1bcko/− double mutants. At E10.5, we observed dramatic abnormalities in 4th ventricle roof plate in Lmx1a−/−;Lmx1bcko/− embryos (n=4). Patches of Gdf7-negative medial cells, not found in Lmx1a−/− or wild-type embryos were detected (Fig. 5C, arrowheads). Additionally, 4th ventricle roof plate size was significantly smaller in Lmx1a−/−;Lmx1bcko/− mice than Lmx1a−/− and wild-type mice at E10.5 (Fig. 5D), although the size was equivalent at E9.5 in Lmx1a−/− and Lmx1a−/−;Lmx1bcko/− mice (Fig. 5 B′,C′). Notably, the E10.5 conditional double mutant phenotype was not as severe as that observed in Lmx1a−/−;Lmx1b−/− double null mutants (compare Fig. 2 G,H with 5C). We predicted that this was due to transient, residual expression of Lmx1b in these conditional mutants since LacZ expression in Lmx1a-cre;ROSA embryos, demonstrated that cre-activity in the roof plate was only robust from E9.5. Indeed, at E9.5, we observed residual Lmx1b expression in Lmx1a−/−;Lmx1bcko/− double mutant mice (Fig. 4I-L). Thus, our analysis of Lmx1a−/−;Lmx1bcko/− embryos show that Lmx1b expression from E8.0 until at least E9.5, even in the absence of Lmx1a, is sufficient to normally induce rh1 roof plate. Our analysis of these double conditional mutants however also revealed partially overlapping roles for Lmx1 genes in later regulation of hindbrain roof plate growth.
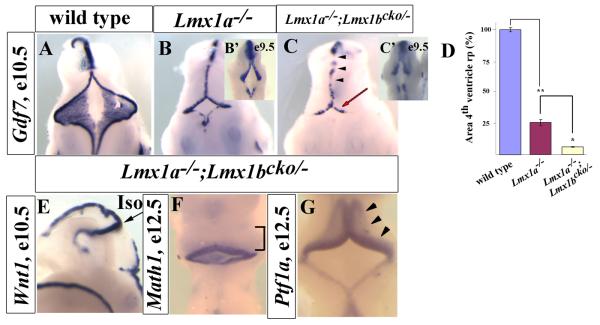
Figure 5. An intermediate roof plate phenotype is observed in conditional double knockout embryos.
(A-C) Dorsal view of E10.5 embryos hybridized with a Gdf7 in situ probe. (B) Lmx1a−/− embryos had a small 4th ventricle roof plate compared to the wild-type. (C) Lmx1a−/−; Lmx1bcko/− retained the roof plate (unlike the double null embryos) but developed an even smaller 4th ventricle roof plate compared to Lmx1a−/− embryos (red arrow). In addition, Gdf7 expression was patchy (arrowheads). Insets in panels B and C show roof plate at E9.5 in Lmx1a−/− embryos (B′) and Lmx1a−/−; Lmx1bcko/− embryos (C′). (D) Quantification of 4th ventricle roof plate area in wt, Lmx1a−/−, and Lmx1a−/−; Lmx1bcko/− embryos at E10.5 (n=3). Y-axis is normalized to wt. * indicates p<0.01; ** indicates p<0.001. Bars indicate standard error (se). (E) Lateral view of E10.5 embryo stained with Wnt1 in situ probe. IsO is normally present in Lmx1a−/−; Lmx1bcko/− embryos. (F and G) Dorsal view of E12.5 Lmx1a−/−; Lmx1bcko/− embryos stained with Math1 (F) and Ptf1a (G) in situ probes. Variability of these markers was not detected, despite reduction in size of the 4th ventricle roof plate.
Lmx1 dependent roof plate signaling is required for normal cerebellar growth and patterning
We next examined cerebellar anlage patterning in Lmx1a−/−;Lmx1bcko/− embryos. Hybridization with a Wnt1 in situ probe determined that the IsO was still intact (Fig. 5E), Additionally, Math1 was still expressed in the developing cerebellar anlage of the Lmx1a−/−;Lmx1bcko/− embryos (Fig. 5F), indicating that the severely reduced 4th ventricle roof plate was still able to initiate rhombic lip development. Ptf1a, expressed in the ventricular zone of the developing cerebellar anlage, was also still expressed relatively normally in the Lmx1a−/−;Lmx1bcko/− embryos (Fig. 5G, arrowheads). Thus we conclude that despite dramatic roof plate defects, early embryonic cerebellar anlage patterning is not markedly affected in Lmx1a−/−;Lmx1bcko/− embryos.
To date, it has been difficult to comprehensively define the role of rh1 roof plate signaling on adjacent cerebellar morphogenesis. Previously we used a diphtheria toxin ablation strategy to demonstrate that rh1 roof plate signaling regulates adjacent cerebellar anlage proliferation, cell fate specification and patterning (Chizhikov et al., 2006). However Gdf7-DTA roof plate ablated mice do not survive past E12.5, long before the completion of the cerebellar morphogenesis. In addition, cerebellar anlage abnormalities in Gdf7-DTA mice were confounded by an open neural tube defect (Monuki et al., 2001; Chizhikov et al., 2006). Importantly, Lmx1a−/− and Lmx1a−/−; Lmx1bcko/− mice survive past weaning making it possible to analyze the consequences of graded roof plate reduction on later stages of cerebellar morphogenesis.
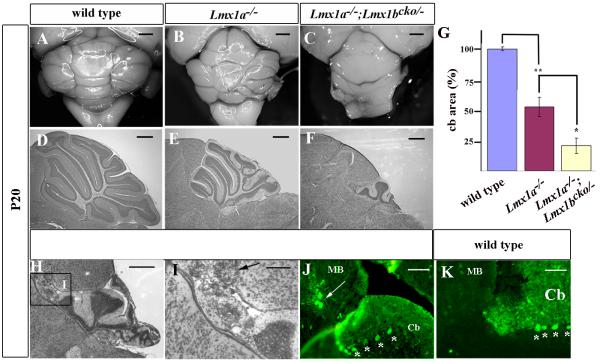
Since our mutations exist on a mixed genetic background, we first developed a grading system to assess the variable adult Lmx1a−/− cerebellar phenotypes generated in this study (Suppl. Fig. 1). We assigned each individual Lmx1a−/− cerebellum to one of five phenotypic groups. Group 1 cerebella were nearly normal, while those in group 5 were poorly developed. This system allowed us to establish a quantitative means of assessing the phenotypic effects of loss of dorsal Lmx1b expression on the Lmx1a mutant background. While the cerebellar phenotypes of Lmx1a−/− mice were almost equally distributed between our 5 phenotypic groups, all Lmx1a−/−; Lmx1bcko/− mice analyzed in this study (n=8) had very severe cerebellar defects and were assigned to groups 4 and 5, a significant skewing of severity (P<0.017) (Fig. 6C,F). The phenotypic severity of Lmx1a−/−; Lmx1bcko/− mice was confirmed by reduced cerebellar vermis area based on mid-sagittal sections (p<0.001) (Fig. 6G). Despite the severe foliation patterning disruptions, Purkinje and granule cells were still present and a relatively normal cerebellar lamination pattern was evident in Lmx1a−/−; Lmx1bcko/− mice (Fig. 6F). However, in two of these mice, we detected ectopic Purkinje and granule cells anterior to the cerebellum within the inferior colliculus of the midbrain (Fig. 6H-K). Together, these data indicate that Lmx1-dependent rh1 roof plate signaling is critical for establishing the proper size and appropriate three-dimensional structure of the adult cerebellum.
Figure 6. Cerebellar morphogenesis is dependent on Lmx1 dependent roof plate signaling.
(A-C) Whole mount and (D-F) nissl stained mid-sagittal sections of P20 cerebella demonstrating that loss of both Lmx1b and Lmx1a had significant effects on cerebellar development. The cerebella of Lmx1a−/− mice were small, but those of Lmx1a−/−; Lmx1bcko/− mice were even smaller. (G) Quantification of mid-sagittal sections of P20 cerebella (n=4). Area of wild-type is indicated as 100%. * indicates p<0.01; ** indicates p<0.001. Bars indicate standard error. (H-J, L) Ectopic cerebellar cells were observed in n=2/8 Lmx1a−/−; Lmx1bcko/− cerebella in the midbrain are evident in nissl stained sections. (I) Higher magnification of the boxed region in (H) showing ectopic cerebellar cells in the midbrain. (J) White arrows indicate calbindin-positive Purkinje cells present in the midbrain (MB) with cerebellar (Cb) Purkinje cells are indicated by *, in similar sections. (K) An equivalent wild-type calbindin stained section. Scale bars are (A-C) 1 mm, (D-F) 500 μm, (H) 500 μm, (I-K) 250 μm.
Graded loss of roof plate signaling results in reduced embryonic cerebellar anlage proliferation
Since we have previously demonstrated that roof plate signaling drives adjacent cerebellar anlage proliferation (Chizhikov et al., 2006), we predicted that the size reduction of adult cerebella in Lmx1a−/− and Lmx1a−/−; Lmx1bcko/− mice would correlate with reduced cell division in the cerebellar anlage due to reduced roof plate size. BrdU labeling at E12.5 demonstrated graded reduction in proliferation in the ventricular zone of the mutants compared to wild-type (Fig. 7). Therefore, our data suggests that reduced proliferation in early cerebellar anlage mediated by reduced roof plate size significantly contributes to the reduced cerebellar size in Lmx1a−/−; Lmx1bcko/− mice.
Figure 7. Reduced proliferation in the cerebellar anlage correlates with reduced roof plate size.
(A-C) Midsagittal sections of BrdU (red) and DAPI (blue) stained wild-type (A), Lmx1a−/− (B), and Lmx1a−/−; Lmx1bcko/− (C) cerebella anlage at E12.5. Arrowheads show BrdU staining in the ventricular zone. Proliferation was reduced in Lmx1a−/− and Lmx1a−/−; Lmx1bcko/− embryos.
(D) Mitotic index of E12.5 ventricular zone (BrdU/DAPI). Lmx1a−/−; Lmx1bcko/− embryos showed significant reduction compared to Lmx1a−/− embryos, which was reduced compared to wild-type embryos. * indicates p<0.01; ** indicates p<0.001. Bars indicate standard error.
DISCUSSION
Lmx1b have overlapping function to Lmx1a in rh1 roof plate formation
To test the hypothesis that Lmx1 genes have overlapping functions in the development of the hindbrain roof plate, we compared the embryonic roof plate phenotypes of Lmx1a−/−, Lmx1b−/−, and Lmx1a−/−; Lmx1b−/− mice. We showed that roof plate development is largely normal in Lmx1b−/− mutants, indicating no unique role for Lmx1b in hindbrain roof plate development. However, we observed complete deletion of the hindbrain roof plate in Lmx1a−/−; Lmx1b−/− double null mutants. This conclusion is based not only on loss of all roof plate markers in double null embryos, but also our demonstration that there is complete loss of roof plate function in these embryos. No induction of Math1, a marker of rhombic lip, occurs. Our data therefore provides genetic proof that Lmx1b function is overlapping to Lmx1a in roof plate development.
Lmx1b is expressed in the IsO where it is required to maintain the IsO, an important anterior-posterior signaling center at the mid/hindbrain junction (Adams et al., 2000; Matsunaga et al., 2002; Guo et al., 2007). Thus, Lmx1b−/− and Lmx1a−/−; Lmx1b−/− double null mutants had disrupted mid/hindbrain patterning along the anterior/posterior axis. Others have shown that levels of Fgf signaling from the IsO influence anterior rh1 roof plate (Alexandre et al., 2006; Basson et al., 2008). Anterior rh1 roof plate lies adjacent to the IsO and occupies a narrow medial domain while the posterior rh1 roof plate widens over the expanse of the fourth ventricle (Chizhikov et al., 2006). The only roof plate phenotype that we observed in Lmx1b−/− mice was loss of the anterior rh1 roof plate. Posterior rh1 roof plate was unaffected in these mice. We conclude that the anterior roof plate defect is a direct result of loss of Lmx1b-dependent Fgf 8 IsO expression and not Lmx1b dorsal roof plate expression, since Lmx1b is required to maintain Wnt1 IsO expression, which in turn is required for IsO Fgf8 maintenance (Adams et al., 2000; Matsunaga et al., 2002).
To dissect dorsal roof plate function of Lmx1b from IsO function of this gene, we used Lmx1a-cre mice (Chizhikov et al., 2006) to delete a floxed Lmx1b allele just in the roof plate. Notably, the roof plate phenotype of Lmx1a−/−; Lmx1bcko/− mice was less severe than the Lmx1a−/−; Lmx1b−/− mice. Two possibilities can explain this observation. Either Lmx1b IsO has a role in roof plate formation or there is a delay in excision of Lmx1b in the roof plate in Lmx1a−/−;Lmx1bcko/− mice. Since the roof plate is near normal in Lmx1b null mice, our data strongly argue that the IsO plays no role in development of most of the 4th ventricle roof plate. Rather our data suggest that a delay in excision of dorsal midline Lmx1b is a more probable explanation of the intermediate roof plate phenotype and that transient expression of Lmx1b in the double conditional mutant embryos is sufficient to induce roof plate even in the absence of Lmx1a. Notably, although the roof plate was present, Lmx1a−/−; Lmx1bcko/− mice had a smaller 4th ventricle roof plate compared to Lmx1a−/− mice at E10.5, revealing overlapping roles for these genes in roof plate growth and confirming that dorsal Lmx1b on the dorsal midline acts together with Lmx1a to direct normal roof plate formation.
Our mutant analyses demonstrate that Lmx1b function is completely redundant to Lmx1a in roof plate development. However, the reverse is not true. Lmx1a has both unique and overlapping function with Lmx1b. We speculate that structural differences in these two highly related proteins may lead to differences in DNA-binding or co-factor interactions which may drive these functional differences.
Cerebellar morphogenesis is dependent on redundant, Lmx1-dependent roof plate signaling
Based on our analysis of Lmx1a−/− mice, we demonstrated that reduced signaling from the small roof plate contributes to the small and mispatterned cerebellar phenotype in adult mice (Millonig et al., 2000). Here we have shown that the adult Lmx1a−/−; Lmx1bcko/− cerebellum was even smaller than that of Lmx1a−/− mutants. We did not observe gross abnormalities of the E12.5 cerebellar anlage patterning in Lmx1a−/−; Lmx1bcko/− embryos. However, we observed smaller 4th ventricle roof plate in Lmx1a−/−; Lmx1bcko/− embryos, We have previously demonstrated that signaling from rh1 roof plate controls cerebellar anlage proliferation (Chizhikov et al., 2006). Indeed, BrdU analysis confirmed that the small rh1 roof plate in Lmx1a−/−; Lmx1bcko/− embryos resulted in dramatically reduced proliferation within the cerebellar anlage at E12.5, where neither Lmx1 gene is expressed. Thus, although the roof plate and choroid plexus may have additional signaling roles later during cerebellar development, we hypothesize that the small adult cerebellar size in the Lmx1a−/−; Lmx1bcko/− mutants is partially attributable to an early growth failure of the mutant cerebellar anlage.
Although Lmx1a−/−; Lmx1bcko/− mice had a very small cerebellum, cerebellar lamination was essentially normal in these mutants, indicating that although reduced in number, most cerebellar cell types were still generated and migrated to appropriate final positions. We did, however, detect ectopic midbrain Purkinje cells in these mice which were never observed in Lmx1a−/− animals. Since Lmx1b expression was restricted to the rh1 roof plate and its derivative, the choroid plexus epithelium during embryogenesis and early post-natal development, our data indicate that roof plate signaling regulates positioning of the cerebellar cells.
Lmx1 gene redundancy – more than just the roof plate?
Our study provides the first detailed description of the unique and overlapping functions of Lmx1a and Lmx1b in the developing dorsal CNS where these genes are co-expressed. Notably, there are other regions of the CNS where both Lmx1 genes likely interact. Lmx1a and Lmx1b are co-expressed in ventral midbrain dopaminergic (DA) neuronal progenitors. Lmx1a−/− mice have reduced numbers of midbrain DA neurons (Ono et al., 2007), and RNAi experiments in chick have demonstrated that Lmx1a is required by DA neuron progenitors (Andersson et al., 2006). Lmx1b−/− mice have DA neurons with different molecular expression, indicating that these cells are not properly differentiated (Smidt et al., 2000). Our demonstration of genetic interactions between Lmx1a and Lmx1b in the dorsal hindbrain suggests that detailed analysis of ventral midbrain development in Lmx1a−/−; Lmx1b−/− mice will reveal novel and severe DA abnormalities. There may also be Lmx1 genetic redundancy in the developing inner ear where both genes are expressed and Lmx1a has been shown to be expressed and Lmx1a has a critical developmental role (Huang et al., 2008; Nichols et al., 2008).
Supplementary Material
The grading system used to classify the phenotypic variation of Lmx1a−/− cerebella phenotypes on the mixed genetic background used in this study.
(A) Representative cerebella showing the five grades of phenotypic severity.
(B) Table describing the defining characteristics of each grade.
(C) Distribution of phenotypes in Lmx1a−/− vs. Lmx1a−/−; Lmx1bcko/− mice, showing significantly more severe phenotype in Lmx1a−/−; Lmx1bcko/− mice. Chi-square test show significant difference. p<0.017 with df=4. n=20 for Lmx1a−/− mice and n=8 for Lmx1a−/−; Lmx1bcko/− mice.
ACKNOWLEDGEMENTS
We would like to thank Michael German, Tom Jessell, Jane Johnson, Andrew McMahon Thomas Perlmann, Antonio Simeone, and Chris Wright for providing reagents. This work was supported by NIH grant RO1 NS044262 to KJM.
REFERENCES
- Adams KA, Maida JM, Golden JA, Riddle RD. The transcription factor Lmx1b maintains Wnt1 expression within the isthmic organizer. Development. 2000;127:1857–1867. doi: 10.1242/dev.127.9.1857. [DOI] [PubMed] [Google Scholar]
- Alder J, Lee KJ, Jessell TM, Hatten ME. Generation of cerebellar granule neurons in vivo by transplantation of BMP-treated neural progenitor cells. Nat Neurosci. 1999;2:535–540. doi: 10.1038/9189. [DOI] [PubMed] [Google Scholar]
- Alexandre P, Bachy I, Marcou M, Wassef M. Positive and negative regulations by FGF8 contribute to midbrain roof plate developmental plasticity. Development. 2006;133:2905–2913. doi: 10.1242/dev.02460. [DOI] [PubMed] [Google Scholar]
- Andersson E, Tryggvason U, Deng Q, Friling S, Alekseenko Z, Robert B, Perlmann T, Ericson J. Identification of intrinsic determinants of midbrain dopamine neurons. Cell. 2006;124:393–405. doi: 10.1016/j.cell.2005.10.037. [DOI] [PubMed] [Google Scholar]
- Awatramani R, Soriano P, Rodriguez C, Mai JJ, Dymecki SM. Cryptic boundaries in roof plate and choroid plexus identified by intersectional gene activation. Nat Genet. 2003;35:70–75. doi: 10.1038/ng1228. [DOI] [PubMed] [Google Scholar]
- Basson MA, Echevarria D, Petersen Ahn C, Sudarov A, Joyner AL, Mason IJ, Martinez S, Martin GR. Specific regions within the embryonic midbrain and cerebellum require different levels of FGF signaling during development. Development. 2008;135:889–898. doi: 10.1242/dev.011569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Lun Y, Ovchinnikov D, Kokubo H, Oberg KC, Pepicelli CV, Gan L, Lee B, Johnson RL. Limb and kidney defects in Lmx1b mutant mice suggest an involvement of LMX1B in human nail patella syndrome. Nat Genet. 1998;19:51–55. doi: 10.1038/ng0598-51. [DOI] [PubMed] [Google Scholar]
- Chizhikov VV, Millen KJ. Mechanisms of roof plate formation in the vertebrate CNS. Nat Rev Neurosci. 2004a;5:808–812. doi: 10.1038/nrn1520. [DOI] [PubMed] [Google Scholar]
- Chizhikov VV, Millen KJ. Control of roof plate development and signaling by Lmx1b in the caudal vertebrate CNS. J Neurosci. 2004b;24:5694–5703. doi: 10.1523/JNEUROSCI.0758-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chizhikov VV, Millen KJ. Roof plate-dependent patterning of the vertebrate dorsal central nervous system. Dev Biol. 2005;277:287–295. doi: 10.1016/j.ydbio.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Chizhikov VV, Lindgren AG, Currle DS, Rose MF, Monuki ES, Millen KJ. The roof plate regulates cerebellar cell-type specification and proliferation. Development. 2006;133:2793–2804. doi: 10.1242/dev.02441. [DOI] [PubMed] [Google Scholar]
- Currle DS, Cheng X, Hsu CM, Monuki ES. Direct and indirect roles of CNS dorsal midline cells in choroid plexus epithelia formation. Development. 2005;132:3549–3559. doi: 10.1242/dev.01915. [DOI] [PubMed] [Google Scholar]
- Elsen GE, Choi LY, Millen KJ, Grinblat Y, Prince VE. Zic1 and Zic4 regulate zebrafish roof plate specification and hindbrain ventricle morphogenesis. Dev Biol. 2008;314:376–392. doi: 10.1016/j.ydbio.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Failli V, Bachy I, Retaux S. Expression of the LIM-homeodomain gene Lmx1a (dreher) during development of the mouse nervous system. Mech Dev. 2002;118:225–228. doi: 10.1016/s0925-4773(02)00254-x. [DOI] [PubMed] [Google Scholar]
- Guo C, Qiu HY, Huang Y, Chen H, Yang RQ, Chen SD, Johnson RL, Chen ZF, Ding YQ. Lmx1b is essential for Fgf8 and Wnt1 expression in the isthmic organizer during tectum and cerebellum development in mice. Development. 2007;134:317–325. doi: 10.1242/dev.02745. [DOI] [PubMed] [Google Scholar]
- Huang M, Sage C, Li H, Xiang M, Heller S, Chen ZY. Diverse expression patterns of LIM-homeodomain transcription factors (LIM-HDs) in mammalian inner ear development. Dev Dyn. 2008;237:3305–3312. doi: 10.1002/dvdy.21735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter NL, Dymecki SM. Molecularly and temporally separable lineages form the hindbrain roof plate and contribute differentially to the choroid plexus. Development. 2007;134:3449–3460. doi: 10.1242/dev.003095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KJ, Jessell TM. The specification of dorsal cell fates in the vertebrate central nervous system. Annu Rev Neurosci. 1999;22:261–294. doi: 10.1146/annurev.neuro.22.1.261. [DOI] [PubMed] [Google Scholar]
- Lee KJ, Mendelsohn M, Jessell TM. Neuronal patterning by BMPs: a requirement for GDF7 in the generation of a discrete class of commissural interneurons in the mouse spinal cord. Genes Dev. 1998;12:3394–3407. doi: 10.1101/gad.12.21.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KJ, Dietrich P, Jessell TM. Genetic ablation reveals that the roof plate is essential for dorsal interneuron specification. Nature. 2000;403:734–740. doi: 10.1038/35001507. [DOI] [PubMed] [Google Scholar]
- Liem KF, Jr., Tremml G, Jessell TM. A role for the roof plate and its resident TGFbeta-related proteins in neuronal patterning in the dorsal spinal cord. Cell. 1997;91:127–138. doi: 10.1016/s0092-8674(01)80015-5. [DOI] [PubMed] [Google Scholar]
- Liu A, Joyner AL. Early anterior/posterior patterning of the midbrain and cerebellum. Annu Rev Neurosci. 2001;24:869–896. doi: 10.1146/annurev.neuro.24.1.869. [DOI] [PubMed] [Google Scholar]
- Matsunaga E, Katahira T, Nakamura H. Role of Lmx1b and Wnt1 in mesencephalon and metencephalon development. Development. 2002;129:5269–5277. doi: 10.1242/dev.129.22.5269. [DOI] [PubMed] [Google Scholar]
- Millen KJ, Millonig JH, Hatten ME. Roof plate and dorsal spinal cord dl1 interneuron development in the dreher mutant mouse. Dev Biol. 2004;270:382–392. doi: 10.1016/j.ydbio.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Millonig JH, Millen KJ, Hatten ME. The mouse Dreher gene Lmx1a controls formation of the roof plate in the vertebrate CNS. Nature. 2000;403:764–769. doi: 10.1038/35001573. [DOI] [PubMed] [Google Scholar]
- Monuki ES, Porter FD, Walsh CA. Patterning of the dorsal telencephalon and cerebral cortex by a roof plate-Lhx2 pathway. Neuron. 2001;32:591–604. doi: 10.1016/s0896-6273(01)00504-9. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Katahira T, Matsunaga E, Sato T. Isthmus organizer for midbrain and hindbrain development. Brain Res Brain Res Rev. 2005;49:120–126. doi: 10.1016/j.brainresrev.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Nichols DH, Pauley S, Jahan I, Beisel KW, Millen KJ, Fritzsch B. Lmx1a is required for segregation of sensory epithelia and normal ear histogenesis and morphogenesis. Cell Tissue Res. 2008;334:339–358. doi: 10.1007/s00441-008-0709-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hara FP, Beck E, Barr LK, Wong LL, Kessler DS, Riddle RD. Zebrafish Lmx1b.1 and Lmx1b.2 are required for maintenance of the isthmic organizer. Development. 2005;132:3163–3173. doi: 10.1242/dev.01898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono Y, Nakatani T, Sakamoto Y, Mizuhara E, Minaki Y, Kumai M, Hamaguchi A, Nishimura M, Inoue Y, Hayashi H, Takahashi J, Imai T. Differences in neurogenic potential in floor plate cells along an anteroposterior location: midbrain dopaminergic neurons originate from mesencephalic floor plate cells. Development. 2007;134:3213–3225. doi: 10.1242/dev.02879. [DOI] [PubMed] [Google Scholar]
- Sato T, Joyner AL, Nakamura H. How does Fgf signaling from the isthmic organizer induce midbrain and cerebellum development? Dev Growth Differ. 2004;46:487–494. doi: 10.1111/j.1440-169x.2004.00769.x. [DOI] [PubMed] [Google Scholar]
- Sillitoe RV, Joyner AL. Morphology, molecular codes, and circuitry produce the three-dimensional complexity of the cerebellum. Annu Rev Cell Dev Biol. 2007;23:549–577. doi: 10.1146/annurev.cellbio.23.090506.123237. [DOI] [PubMed] [Google Scholar]
- Smidt MP, Asbreuk CH, Cox JJ, Chen H, Johnson RL, Burbach JP. A second independent pathway for development of mesencephalic dopaminergic neurons requires Lmx1b. Nat Neurosci. 2000;3:337–341. doi: 10.1038/73902. [DOI] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Wang VY, Zoghbi HY. Genetic regulation of cerebellar development. Nat Rev Neurosci. 2001;2:484–491. doi: 10.1038/35081558. [DOI] [PubMed] [Google Scholar]
- Wurst W, Bally-Cuif L. Neural plate patterning: upstream and downstream of the isthmic organizer. Nat Rev Neurosci. 2001;2:99–108. doi: 10.1038/35053516. [DOI] [PubMed] [Google Scholar]
- Zhao ZQ, Scott M, Chiechio S, Wang JS, Renner KJ, Gereau RWt, Johnson RL, Deneris ES, Chen ZF. Lmx1b is required for maintenance of central serotonergic neurons and mice lacking central serotonergic system exhibit normal locomotor activity. J Neurosci. 2006;26:12781–12788. doi: 10.1523/JNEUROSCI.4143-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The grading system used to classify the phenotypic variation of Lmx1a−/− cerebella phenotypes on the mixed genetic background used in this study.
(A) Representative cerebella showing the five grades of phenotypic severity.
(B) Table describing the defining characteristics of each grade.
(C) Distribution of phenotypes in Lmx1a−/− vs. Lmx1a−/−; Lmx1bcko/− mice, showing significantly more severe phenotype in Lmx1a−/−; Lmx1bcko/− mice. Chi-square test show significant difference. p<0.017 with df=4. n=20 for Lmx1a−/− mice and n=8 for Lmx1a−/−; Lmx1bcko/− mice.