Abstract
PD-1 targeted therapy has dramatically changed advanced cancer treatment. However many questions remain, including specificity of T cells activated by PD-1 therapy and how peripheral blood analysis correlates to effects at tumor sites. In this study, we utilized TCR sequencing to dissect the composition of peripheral blood CD8 T cells activated upon therapy, comparing it with tumor-infiltrating lymphocytes. We report on a nonagenarian melanoma patient who showed a prominent increase in peripheral blood Ki-67+ CD8 T cells following brain stereotactic radiation and anti-PD-1 immunotherapy. Proliferating CD8 T cells exhibited an effector-like phenotype with expression of CD38, HLA-DR and Granzyme B, as well as expression of the positive costimulatory molecules CD28 and CD27. TCR sequencing of peripheral blood CD8 T cells revealed a highly oligoclonal repertoire at baseline with one clonotype accounting for 30%. However, the majority of dominant clones - including a previously identified cytomegalovirus-reactive clone - did not expand following treatment. In contrast, expanding clones were present at low frequencies in the peripheral blood but were enriched in a previously resected liver metastasis. The patient has so far remained recurrence-free for 36 months, and several CD8 T cell clones that expanded after treatment were maintained at elevated levels for at least 8 months. Our data show that even in a nonagenarian individual with oligoclonal expansion of CD8 T cells, we can identify activation of tumor-infiltrating CD8 T cell clones in peripheral blood following anti-PD-1-based immunotherapies.
Keywords: PD-1, immunotherapy, CD8 T cells, T cell repertoire, melanoma
Précis:
T cell receptor sequencing in an elderly melanoma patient reveals that most CD8 T cells expanding in peripheral blood following anti-PD-1 and brain stereotactic radiation are not high frequency, but rather clones enriched in tumor compared to blood.
Introduction
The management of patients with advanced melanoma has improved considerably in the last few years. Agents that block the inhibitory receptor PD-1 (programmed cell death-1) offer higher response rates and improved overall survival outcome for advanced melanoma patients compared to conventional cytotoxic chemotherapy or anti-CTLA-4 (cytotoxic T lymphocyte associated antigen 4) therapy [1]. Recent data show that PD-1 targeted therapy can also benefit patients with resected advanced melanoma in an adjuvant setting [2]. In addition, there is increasing evidence that PD-1 therapy can result in objective responses in patients with brain metastases [3–5]. Stereotactic radiosurgery (SRS) to brain metastases has potentially immunostimulatory effects likely through antigen release from radiated lesions, and how PD-1 targeted therapy can be combined effectively and safely with brain SRS is currently being evaluated in prospective studies [6,7].
Despite the success of PD-1 targeted therapies, response rates are seldom above 50% and durable complete responses are rare. Although elderly patients represent most of the cancer diagnoses by age group, the number of elderly patients included in most clinical trials of immune checkpoint inhibitors is rather small and thus the impact of age on the efficacy of PD-1 targeted therapies is controversial [8–10]. The composition of the CD8 T cell repertoire is dramatically altered with age and infection history and elderly individuals accumulate expanded CD8 T cell clones, many of those specific for cytomegalovirus (CMV)[11,12]. Furthermore, the functionality of the adaptive immune system including the ability to efficiently respond to vaccination declines with age [13]. However, how age-related changes in the CD8 T cell compartment affect PD-1 directed immunotherapy and biomarkers of response remains to be addressed.
In this study, we report the successful treatment of a nonagenarian melanoma patient who received anti-PD-1 and brain stereotactic radiation (SRS) therapy and provide a detailed analysis of the elicited CD8 T cell response. Using T cell receptor (TCR)β sequence information derived from formalin-fixed paraffin-embedded (FFPE) samples of a previously resected liver metastasis and sorted peripheral blood CD8 T cells, we identified tumor-infiltrating CD8 T cell clones expanding in the blood in response to therapy. We show that this patient had an oligoclonal repertoire of CD8 T cells in peripheral blood, which is frequently observed in the elderly. The majority of CD8 T cell clones that were found at high frequencies in peripheral blood were also present in the tumor, but at much lower frequencies. Furthermore, high frequency CD8 T cells clones did not respond to therapy, since there were only minimal changes in the prevalence of the 10 most abundant clones in peripheral blood before and after therapy. We show that CD8 T cell clones which expanded in peripheral blood following PD-1 and SRS brain therapy acquired activation markers consistent with effector cell differentiation such as HLA-DR, CD38 and granzyme B, and expressed the positive costimulatory molecules CD28 and CD27, and were more prevalent in tumor than blood. Our findings provide further guidance on strategies to monitor immune responses in patients receiving PD-1 combination therapies, with particular importance for elderly cancer patients.
Materials and Methods
Study design
The patient was treated according to a standard of care at Winship Cancer Institute at Emory University Hospital. Sample collection was conducted under a protocol approved by the Institutional Review Board of Emory University. Written informed consent was obtained from the donor for the collection and storage of archived tissue and blood, and permission to review medical records and laboratory data.
Flow cytometry and cell sorting
Peripheral blood samples were collected, processed and stained as previously described [14]. Samples were acquired with LSR II flow cytometer (BD) and analyzed using Flow Jo software (Tree Star). CD8 T cells were sorted on BD Aria II cell sorter.
TCR repertoire analysis
DNA was extracted from sorted T cells using the Qiagen AllPrep DNA/RNA Micro Kit. DNA extraction from FFPE slides as well as amplification, library preparation, sequencing and preliminary data processing of all samples was performed at Adaptive Biotechnologies using the immunoSEQ platform (TCRβ survey level). The germline-like score of the TCRβ CDR3 sequences was calculated as described previously [15].
Immunohistochemistry
Immunohistochemistry (IHC) for T cell markers CD4 and CD8 was performed using a polymer-based detection system with mouse monoclonal antibodies according to the manufacturer’s instructions. Positive controls and negative controls with primary antibody replaced by Tris-buffered saline were run with the patient’s slides. The percentage of cells showing cytoplasmic CD4 or CD8 labeling was evaluated.
Results
Clinical Evaluation
A nonagenarian patient with no history of melanoma was found to have a 1.3 × 1.1 cm liver mass during cardiac MRI in 2015 that was not present on previous abdominal MRI imaging in 2009. This was confirmed by MRI of the abdomen and PET/CT scan, which showed no other disease. About 3 months later, the patient underwent resection of this mass yielding a 1.7 cm epithelioid non-pigmented melanoma with moderate heterogeneous lymphocytic response and evident mitotic activity. No evidence of primary tumor was found upon examination of the skin and eyes. The patient’s lactate dehydrogenase was consistently normal. Per 7th edition of the AJCC staging system the patient was staged as having TxN0M1c (or T0N0M1c) Stage IV malignant melanoma. 8 days post surgery, a brain MRI revealed 4 subcentimeter lesions compatible with brain metastases. 16 days post surgery, the patient received the first dose of pembrolizumab (200 mg IV flat dose) followed the next day by single fraction SRS to all brain lesions. The SRS was well tolerated, and the patient subsequently received 8 additional doses of pembrolizumab 3 weeks apart. Pembrolizumab was discontinued because the patient was without evidence of disease and noted to have increasing myalgia, arthralgia, and fatigue. The patient remains alive and with no melanoma recurrence now 36 months since diagnosis.
CD8 T cell activation in peripheral blood after treatment initiation
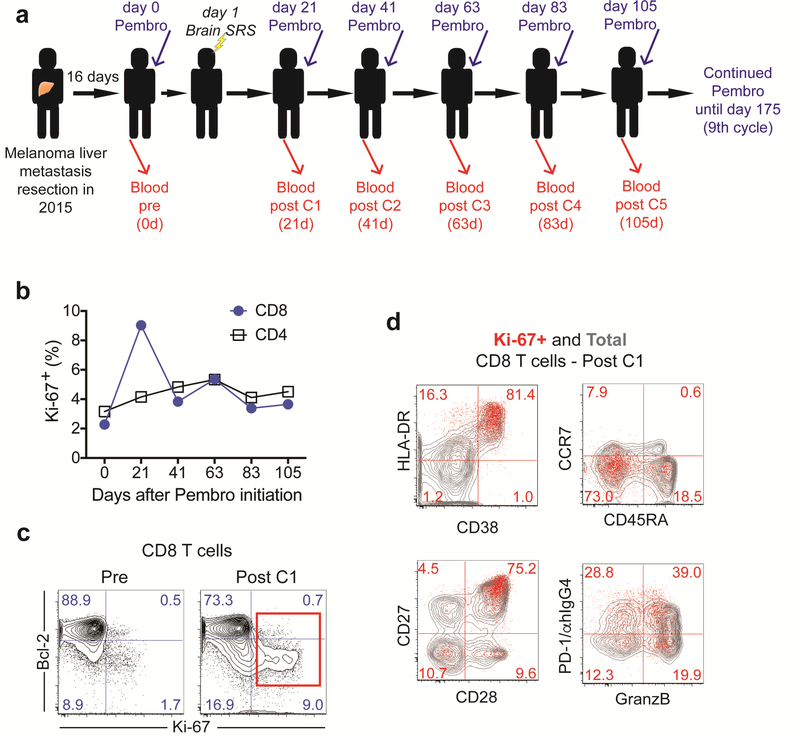
To study the immunological effects from pembrolizumab and brain SRS treatment, we collected peripheral blood prior to treatment initiation and at each pembrolizumab treatment cycle (Fig. 1a). We isolated PBMC from peripheral blood and examined T cell proliferation by analyzing Ki-67 expression. We observed a 4-fold increase in the frequency of proliferating CD8 T cells following treatment initiation (Fig. 1b, c). The frequency of CD8 T cells in cell cycle peaked at the first blood draw 21 days after infusion with close to 10% of total CD8 T cells expressing Ki-67. In contrast, the frequency of Ki-67+ CD4 T cells increased only slightly after treatment (Fig.1b). Hence, consistent with previous observations in advanced cancer patients in which CD8 T cell responses were best detected in peripheral blood 2–3 weeks after PD-1 targeted therapy initiation [16,14,17], in this melanoma patient with undetectable extra-cranial metastasis, pembrolizumab and brain SRS treatment resulted in CD8 T cell proliferation in peripheral blood that peaked at about 3 weeks after treatment initiation. Of note, until about 80 days post-treatment initiation, the frequency of Ki-67+ CD8 T cells remained elevated compared to baseline levels.
Fig. 1:
CD8 T cell activation in peripheral blood. (a) Scheme shows treatment and blood sampling schedule. (b) Proliferation of CD4 and CD8 T cells (c) Ki-67 and Bcl-2 expression on CD8 T cells prior to treatment and post cycle 1. Numbers represent frequency among total CD8 T cells. (d) Phenotypic analysis of proliferating CD8 T cells on day 21 post treatment initiation. Numbers represent frequency among Ki-67+ CD8 T cells. Contour plots show total CD8 T cells (grey contour plot) and Ki-67+ CD8 T cells (red dots). C: cycle, Pembro: Pembrolizumab, d: days
To characterize the CD8 T cells responding to treatment in the peripheral blood, we analyzed several markers associated with effector cell differentiation that we previously detected on responding CD8 T cells induced by blockade of the PD-1 pathway in patients with advanced non-small cell lung cancer (NSCLC) [14]. Consistent with our previous findings in NSCLC, dividing CD8 T cells showed reduced Bcl-2 expression (Fig. 1c). In addition, the majority of proliferating CD8 T cells upregulated the activation markers HLA-DR and CD38, were negative for CD45RA and CCR7, and co-expressed the positive costimulatory molecules CD28 and CD27 (Fig. 1d). About 60% of Ki-67+ CD8 T cells also expressed granzyme B, indicative of cytotoxic ability (Fig. 1d). Finally, about 70% of proliferating CD8 T cells expressed PD-1 [14]. Our data show that in this patient with metastases only detectable in the brain, treatment with pembrolizumab and brain SRS induces activation of CD8 T cells with similar characteristics as CD8 T cells reinvigorated by blockade of the PD-1 pathway in advanced stage melanoma and NSCLC [14,17].
Analysis of the TCR repertoire in the liver metastasis
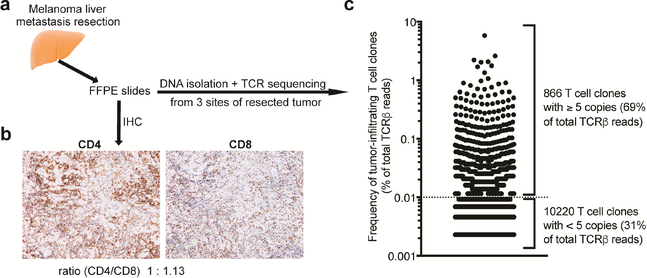
Since the liver metastasis resected before treatment initiation had been preserved, we had the opportunity to characterize tumor-infiltrating T cells. Immunohistochemical analysis showed a heterogeneous pattern of T cell infiltration with highly as well as mildly infiltrated tumor sites (Fig. 2a, b and Supplementary figure 1). Both CD4 and CD8 T cells were detectable within and around the tumor, with a 1.13:1 ratio of CD8 to CD4 T cells. We next analyzed the TCR repertoire and clonal diversity of tumor-infiltrating T cells. To assess and account for intra-tumoral heterogeneity we analyzed the TCR repertoire using FFPE slides obtained from three spatially distinct tumor sites. We detected a high degree of similarity between the TCR repertoires sampled from the three tumor sites (Morisita’s overlap indices of 0.888 – 0.953, where 1 indicates complete overlap). Hence, for analysis purposes we combined the TCR repertoires of the different tumor sites resulting in the identification of 11,086 unique T cell clones (Fig. 2c). 866 clones were present with at least 5 copies and accounted for 69% of the productive reads.
Fig. 2:
Analysis of tumor-infiltrating T cells in liver metastasis. (a) Experimental scheme. (b) Immunohistochemical (IHC) analysis of tumor infiltrating CD4 and CD8 T cells in a highly-infiltrated tumor site (hotspot). (c) Frequency of unique T cell clones. Dotted line indicates cut-off of 5 TCRβ copies.
The 10 most prevalent clones comprised 21.6% of T cells present in the tumor with the most prevalent clone accounting for 5.8%. The clonality score, a widely-used measure of repertoire diversity which can range from 0 (completely polyclonal) to 1 (monoclonal), was 0.2324. Overall, the liver metastasis presented significant infiltration of CD4 and CD8 T cells and the TCR repertoire analysis indicated expansion of T cell clones, both consistent with a pre-existing anti-tumor immune response
TCR repertoire analysis of CD8 T cells in the peripheral blood
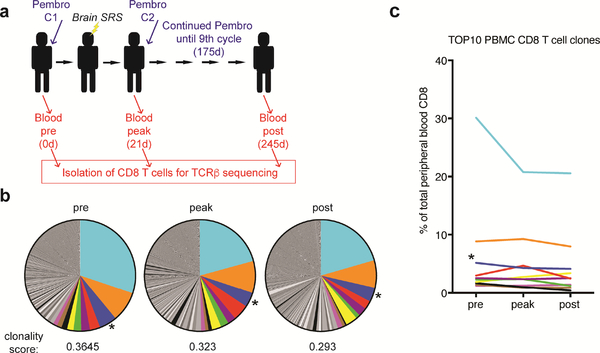
Our data show an increase in proliferating CD8 T cells in the peripheral blood upon treatment. To analyze changes in the CD8 T cell repertoire we performed TCR sequencing of sorted CD8 T cells from the peripheral blood prior to treatment initiation, at the peak of CD8 T cell proliferation, as well as post treatment cessation (Fig. 3a). TCR analysis revealed oligoclonal expansions: at baseline the most prevalent CD8 T cell clone represented 30% of all CD8 T cells and the 10 most prevalent clones accounted for about 58% of CD8 T cells (Fig. 3b). The clonality of CD8 T cells is known to increase with age and oligoclonal expansions of CMV-specific CD8 T cells have been previously reported [12]. Of note, the third most prevalent clone with a frequency of 5.1% prior to treatment initiation had been previously identified to recognize the CMV-derived epitope pp65265–275 [18]. Interestingly, only one of the 10 most prevalent clones increased at least 1.5-fold in frequency at the peak of the CD8 T cell response whereas the remaining clones - including the CMV-reactive clone - remained stable or decreased in frequency (Fig. 3c). The sole expanding clone among the 10 most prevalent clones, represented 3% of CD8 T cells prior to treatment initiation and increased to about 4.7% at the peak of CD8 T cell proliferation and returned to about 2.5% post treatment (Fig. 3c, in red ). In summary, our data show that this melanoma patient presented a striking oligoclonal peripheral blood CD8 T cell repertoire. However, the majority of dominant clones did not respond to treatment with pembrolizumab and brain SRS.
Fig. 3:
TCR repertoire analysis in peripheral blood CD8 T cells. (a) Scheme shows treatment and sampling schedule. (b) Clonal composition and clonality score of peripheral blood CD8 T cells at the indicated time points. (c) Frequency of the 10 most prevalent CD8 T cell clones at baseline. Asterisk indicates TCRβ previously shown to recognize the CMV-derived epitope pp65265–275. C: cycle, Pembro: Pembrolizumab, d: days
Identification and tracking of tumor-infiltrating CD8 T cell clones in peripheral blood
To address whether tumor-infiltrating T cell clones identified in the liver metastasis could be detected in the peripheral blood and whether these clones were expanding upon treatment, we combined the TCR sequence information obtained from FFPE tumor tissue and from peripheral blood CD8 T cells. Since expanded T cell clones infiltrating the tumor are more likely to be tumor-specific [19], we focused our analysis on the 866 most abundant tumor-derived T cell clones accounting for 69% of productive reads (Fig. 2c).
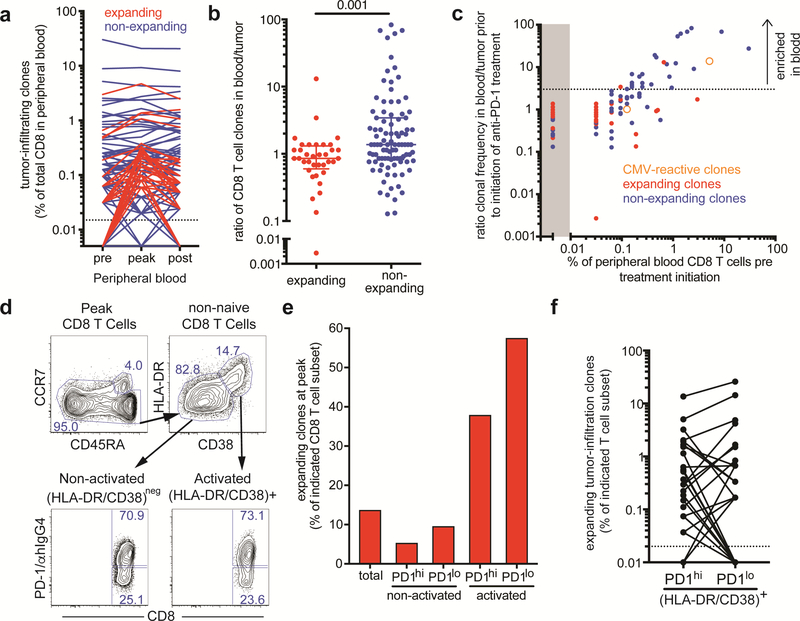
131 of the 866 most abundant tumor-infiltrating T cell clones could be identified among peripheral blood CD8 T cells. The remaining tumor-infiltrating T cell clones are likely to be predominantly CD4 T cells, but also CD8 T cell clones either not present in the blood or present at low frequencies below our limit of detection. We next tracked tumorinfiltrating CD8 T cell clones in the peripheral blood and analyzed changes in frequency in response to treatment. Whereas the majority of tumor-infiltrating CD8 T clones either remained stable or decreased in frequency, 36 tumor-infiltrating CD8 T cell clones increased in frequency at least 1.5-fold when we compared baseline to day 21 post treatment initiation (Fig. 4a). Even 8 months after treatment initiation, the frequencies of many expanding tumor-infiltrating CD8 T cell clones remained above baseline levels, indicative of durable treatment effects.
Fig. 4:
Expanding tumor-infiltrating clones are enriched in tumor and among activated peripheral blood CD8 T cells. (a) Frequency of tumor-infiltrating CD8 T cell clones in peripheral blood. Expanding clones (red) increased in frequency at least 1.5-fold upon treatment initiation, whereas non-expanding clones (blue) showed less than 1.5-fold increase in frequency. Dotted line indicates limit of detection. (b) Ratio of expanding and non-expanding CD8 T cell clones in blood to tumor. p value was calculated using Mann-Whitney test. (c) Relationship of the ratio of clonal frequency in blood to tumor prior to treatment initiation and peripheral blood frequency of 131 tumor-infiltrating CD8 T cell clones. Expanding T cell clones are shown as red dots, non-expanding clones as blue dots, and previously identified CMV-reactive clones are depicted as orange open circles. Dotted line indicates a suggested blood/tumor ratio cut-off of 3 that would separate mainly non-expanding clones enriched in the peripheral blood. (d) Gates used for sorting of activated (HLA-DR/CD38)+ and non-activated (HLA-DR/CD38)- CD8 T cells and subsequent separation based on PD-1 expression on day 21 post treatment initiation (post cycle 1). (e) Cumulative frequency of expanding tumor-infiltrating clones among the indicated CD8 T cell populations in the peripheral blood on day 21 post treatment initiation. (f) Frequency of expanding tumor-infiltrating clones in PD-1hi and PD-1lo activated CD8 T cell subsets.
We next compared the frequency of the expanding and the non-expanding tumorinfiltrating CD8 T cell clones in the resected tumor and in peripheral blood prior to treatment initiation. In order to calculate the blood/tumor ratio of individual CD8 T cell clones, the frequencies of FFPE-derived sequences were multiplied by a factor of 2 to account for the equivalent presence of CD4 and CD8 T cells in the resected liver metastasis (Fig. 2a) and the fact that TCR sequencing cannot distinguish between CD4 and CD8 subsets. Overall, expanding tumor-infiltrating clones were present at comparable or higher frequencies in the tumor compared to the peripheral blood (ratio of blood/tumor ≤1), whereas non-expanding clones tended to be overrepresented in the peripheral blood (ratio of blood/tumor >1) (Fig. 4b). In this patient with an oligoclonal CD8 T cell repertoire this analysis was particularly revealing: The 10 most prevalent peripheral blood CD8 T cell clones could also be found in the tumor but were 10–100-fold more prominent in the blood compared to the tumor suggesting that these blood-enriched clones might not be tumor-specific (Fig. 4C). For example, the third most prevalent clone, previously identified to recognize the CMV-derived pp65265–275 epitope, was present in the tumor but at about 14-fold lower frequency compared to the peripheral blood. These data support the notion that T cell clones irrespective of their specificity can be found in the tumor [20,21], but also suggest that clones more prevalent in the blood than tumor are less likely to be tumor-specific.
Of note, we did not detect any significant differences in CDR3 length or germlinelikeness between expanding and non-expanding tumor-infiltrating CD8 T cell clones even when blood-enriched clones were filtered out (Supplementary figure 2). Our data suggest that applying a blood/tumor ratio cut-off may help to reduce the number of non-tumor-specific CD8 T cell clones, especially in situations of oligoclonal expansions as frequently observed in the elderly.
Tumor-infiltrating expanding CD8 T clones in peripheral blood are more likely to have an activated phenotype after pembrolizumab
Our phenotypic analysis showed the highest proliferation of peripheral blood CD8 T cells at the first blood draw post-treatment (three weeks after treatment initiation). The majority of proliferating CD8 T cells expressed high levels of the activation markers HLA-DR and CD38 (Fig. 1d and Supplementary figure 3a). CD8 T cells responding to the therapy defined either by Ki-67 or HLA-DR/CD38 expression appeared similar with regards to the expression of CD45RA and PD-1 (Supplementary figure 3b). To address how phenotypic changes observed following treatment initiation in peripheral blood CD8 T cells are related to immune responses against the tumor, we examined the TCR repertoire of CD8 T cells expressing the activation markers HLA-DR and CD38. High PD-1 expression has been previously shown to enrich for tumor-specific CD8 T cells in the peripheral blood of melanoma patients [19]. Therefore, we purified activated HLADR/CD38+ and non-activated HLA-DR/CD38neg CD8 T cells at the peak of CD8 T cell proliferation, and further separated those populations according to PD-1 expression level for TCR repertoire analysis (Fig. 4d). About 70% of CD8 T cells expressed high levels of PD-1 and about 25% expressed low to undetectable levels of PD-1 in both activated as well as non-activated CD8 T cell subsets. Tumor-infiltrating expanding CD8 T cell clones were highly enriched among activated CD8 T cells and accounted for about 38% and 57% of the PD-1hi and PD-1lo subsets, respectively (Fig. 4e). The frequency of tumor-infiltrating expanding CD8 T cell clones was about 3–4-fold higher in activated CD8 T cells compared to total CD8 T cells. In contrast, tumor-infiltrating expanding CD8 T cell clones comprised a lower proportion of non-activated CD8 T cells compared to total CD8 T cells. Thus, tumor-infiltrating expanding CD8 T cell clones are highly enriched among activated CD8 T cells found in the peripheral blood following treatment initiation. Interestingly, PD-1 expression did not further increase the enrichment for tumor-infiltrating expanding CD8 T cell clones among HLA-DR/CD38+ CD8 T cells. However, we observed significant clonal overlap between the TCR repertoires of the analyzed CD8 T cell subsets. For example, most tumor-infiltrating expanding CD8 T cell clones present among activated CD8 T cells were found in both PD-1hi and PD-1lo subsets, and could even be detected among non-activated CD8 T cells, although at much lower frequencies (Fig. 4f and data not shown).
Discussion
For this nonagenarian patient, we have shown that brain SRS and anti-PD-1 therapy resulted in activation of CD8 T cells in peripheral blood. The kinetics of the CD8 T cell response and the phenotype of the activated T cells were consistent with other studies of PD-1 targeted therapy in advanced melanoma and NSCLC [14,17]. These data show that similar peripheral blood analyses can be used to monitor T cell responses in a broad range of cancer types, stages and treatments.
TCR repertoire analysis of CD8 T cells in the peripheral blood showed that before treatment initiation this melanoma patient had an oligoclonal CD8 T cell repertoire. Oligoclonal CD8 T cell expansions have been reported to occur in the elderly and CMV has been implicated as a major contributing factor [12]. Interestingly, 9 out of the 10 most prevalent clones including a CMV-reactive clone did not expand upon treatment initiation. We reported previously that EBV-specific CD8 T cells were not activated by PD-1 targeted therapy in advanced NSCLC patients [14]. These data suggest that PD-1 targeted therapies do not indiscriminately expand CD8 T cells and that CD8 T cells specific to latent viruses may not be affected by blockade of the PD-1 pathway. [22]
We show that tumor-infiltrating CD8 T cell clones that expanded in peripheral blood following treatment initiation were enriched among CD8 T cells with an activated phenotype at the peak of the response. Overall, responding CD8 T cell clones were present at very low frequencies in the blood prior to treatment initiation and at least as prevalent in the tumor as in the blood, whereas non-responding clones were more prevalent in blood than tumor. We thus propose that comparing the frequency in tumor and blood will be helpful for future studies using TCRβ sequencing to study T cell responses in cancer patients. This analysis will be important to avoid blood contaminants or T cells recruited by bystander activation, and may be especially relevant when patients have CD8 oligoclonal expansion, as it is commonly observed in the elderly population.
Synchronous melanoma metastases may have unique neoantigens and TCR clones [23]. It is thus likely that T cells infiltrating the melanoma brain lesions of this patient possessed a slightly different TCR repertoire compared to T cells present in the resected liver metastasis. Hence, our findings that potential tumor-specific CD8 T cells enriched in the resected liver metastasis accounted for about 34% of the PD-1hi and 57% of the PD-1lo activated CD8 T cells in peripheral blood may actually be an underestimation of tumor-specific clones responding to PD-1 therapy.
It is important to note that we observed a high overlap between the repertoire of PD-1hi and PD-1lo CD8 T cells, and that clones were even shared between activated and nonactivated CD8 T cells at the same time point. These data show that CD8 T cells with a particular specificity can display distinct differentiation and activation states within the same individual. CD8 T cells undergo dynamic changes, especially during T cell activation. PD-1 expression is controlled by antigen levels [24,25], and some CD8 T cells with an activated phenotype may assume a PD-1neg/lo phenotype when T cells cease to receive cognate antigen signals – which may be particularly likely when cognate antigen levels are not high. Therefore, even though PD-1 expression can help identify tumor-specific CD8 T cells in the tumor as well as in the peripheral blood of patients with advanced disease, PD-1 expression is expected to be more variable in patients with low tumor burden, such as the melanoma patient described here. This is an important principle that would need to be taken into account, for example in studies of anti-PD-1 as adjuvant therapy.
Following SRS and anti-PD-1 therapy, this melanoma patient has remained disease free for 36 months. The immunological response observed in the peripheral blood of this patient was comparable to advanced melanoma and NSCLC patients undergoing single agent PD-1 targeted therapies [14,17] suggesting that anti-PD-1 therapy most likely was the main driver of the observed immunological response. However, the SRS itself was most likely curative for the treated brain lesions. The combination of SRS and anti-PD-1 therapy has shown clinical benefit in retrospective studies [26], however, we can only speculate about potential synergistic effects of SRS and anti-PD-1 therapy preventing recurrence in this patient. Our data warrant larger prospective studies on SRS and anti-PD-1 therapy with immunological monitoring in peripheral blood as well as further evaluation of anti-PD-1 therapy as adjuvant therapy, including patients undergoing brain SRS.
Supplementary Material
Acknowledgments:
We thank Winship Cancer Institute personnel involved in this patient’s care and sample collection, with special thanks to Cabell Pietras. We thank Patrick Raber (Adaptive Biotechnologies) for his assistance during TCR analysis.
Funding:
This research project was supported in part by a Winship Cancer Institute Melanoma Research Pilot Grant and the Emory University School of Medicine Flow Cytometry Core.
Abbreviations:
- AJCC
American Joint Committee on Cancer
- CDR3
Complementarity-determining region 3
- EBV
Epstein-Barr virus
- FFPE
Formalin-fixed paraffin-embedded
- PET/CT
Positron Emission Tomography/Computed Tomography
- SRS
Stereotactic Radiosurgery
Footnotes
Compliance with ethical standards
Conflict of interest:
Rafi Ahmed is an inventor on patents held by Emory University that cover the topic of PD-1–directed immunotherapy. All other authors declare no potential conflicts of interest.
Ethical approval and ethical standards:
The sample collection and research aspects of this trial were conducted under a study approved by the Institutional Review Board of Emory University, Atlanta, Georgia, USA.
Informed consent:
Informed consent for collection of samples was signed by the patient prior to any study related procedures.
Note on previous publication:
This work was presented as a poster at the Keystone Symposium “Immunological Memory: Innate, Adaptive, and Beyond (X1)” on Feb. 28th, 2018 in Austin, TX, USA.
References
- 1.Luke JJ, Flaherty KT, Ribas A, Long GV (2017) Targeted agents and immunotherapies: optimizing outcomes in melanoma. Nat Rev Clin Oncol 14 (8):463–482. doi: 10.1038/nrclinonc.2017.43 [DOI] [PubMed] [Google Scholar]
- 2.Weber J, Mandala M, Del Vecchio M, Gogas HJ, Arance AM, Cowey CL, Dalle S, Schenker M, Chiarion-Sileni V, Marquez-Rodas I, Grob JJ, Butler MO, Middleton MR Maio M, Atkinson V, Queirolo P, Gonzalez R, Kudchadkar RR, Smylie M, Meyer N, Mortier L, Atkins MB, Long GV, Bhatia S, Lebbe C, Rutkowski P, Yokota K, Yamazaki N, Kim TM, de Pril V, Sabater J, Qureshi A, Larkin J, Ascierto PA, CheckMate C (2017) Adjuvant Nivolumab versus Ipilimumab in Resected Stage III or IV Melanoma. N Engl J Med 377 (19):1824–1835. doi: 10.1056/NEJMoa1709030 [DOI] [PubMed] [Google Scholar]
- 3.Goldberg SB, Gettinger SN, Mahajan A, Chiang AC, Herbst RS, Sznol M, Tsiouris AJ, Cohen J, Vortmeyer A, Jilaveanu L, Yu J, Hegde U, Speaker S, Madura M, Ralabate A, Rivera A, Rowen E, Gerrish H, Yao X, Chiang V, Kluger HM (2016) Pembrolizumab for patients with melanoma or non-small-cell lung cancer and untreated brain metastases: early analysis of a non-randomised, open-label, phase 2 trial. Lancet Oncol 17 (7):976–983. doi: 10.1016/S1470-2045(16)30053-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parakh S, Park JJ, Mendis S, Rai R, Xu W, Lo S, Drummond M, Rowe C, Wong A, McArthur G, Haydon A, Andrews MC, Cebon J, Guminski A, Kefford RF, Long GV, Menzies AM, Klein O, Carlino MS (2017) Efficacy of anti-PD-1 therapy in patients with melanoma brain metastases. Br J Cancer 116 (12):1558–1563. doi: 10.1038/bjc.2017.142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schadendorf D, Ascierto PA, Haanen JBAG, Espinosa E, Demidov LV, Garbe C, Lorigan P, Gogas H, Hoeller C, Guren TK, Rorive A, Rutkowski P, Munoz-Couselo E, Dummer R, Carneiro A, Hospers G, Grigoryeva EB, Bhore R, Nathan P (2017) Efficacy and safety of nivolumab (NIVO) in patients with advanced melanoma (MEL) and poor prognostic factors who progressed on or after ipilimumab (IPI): Results from a phase II study (CheckMate 172). ASCO Annual Meeting 2017. J Clin Oncol 35 (suppl; Abstract 9524) [Google Scholar]
- 6.Ahmed KA, Abuodeh YA, Echevarria MI, Arrington JA, Stallworth DG, Hogue C, Naghavi AO, Kim S, Kim Y, Patel BG, Sarangkasiri S, Johnstone PA, Sahebjam S, Khushalani NI, Forsyth PA, Harrison LB, Yu M, Etame AB, Caudell JJ (2016) Clinical outcomes of melanoma brain metastases treated with stereotactic radiosurgery and anti-PD-1 therapy, anti-CTLA-4 therapy, BRAF/MEK inhibitors, BRAF inhibitor, or conventional chemotherapy. Ann Oncol 27 (12):2288–2294. doi: 10.1093/annonc/mdw417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Formenti SC, Demaria S (2009) Systemic effects of local radiotherapy. Lancet Oncol 10 (7):718–726. doi: 10.1016/S1470-2045(09)70082-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daste A, Domblides C, Gross-Goupil M, Chakiba C, Quivy A, Cochin V, de Mones E, Larmonier N, Soubeyran P, Ravaud A (2017) Immune checkpoint inhibitors and elderly people: A review. Eur J Cancer 82:155–166. doi: 10.1016/j.ejca.2017.05.044 [DOI] [PubMed] [Google Scholar]
- 9.Marrone KA, Forde PM (2017) Cancer Immunotherapy in Older Patients. Cancer J 23 (4):219–222. doi: 10.1097/PPO.0000000000000268 [DOI] [PubMed] [Google Scholar]
- 10.Nishijima TF, Muss HB, Shachar SS, Moschos SJ (2016) Comparison of efficacy of immune checkpoint inhibitors (ICIs) between younger and older patients: A systematic review and meta-analysis. Cancer Treat Rev 45:30–37. doi: 10.1016/j.ctrv.2016.02.006 [DOI] [PubMed] [Google Scholar]
- 11.Qi Q, Liu Y, Cheng Y, Glanville J, Zhang D, Lee JY, Olshen RA, Weyand CM, Boyd SD, Goronzy JJ (2014) Diversity and clonal selection in the human T-cell repertoire. Proc Natl Acad Sci U S A 111 (36):13139–13144. doi: 10.1073/pnas.1409155111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khan N, Shariff N, Cobbold M, Bruton R, Ainsworth JA, Sinclair AJ, Nayak L, Moss PA (2002) Cytomegalovirus seropositivity drives the CD8 T cell repertoire toward greater clonality in healthy elderly individuals. J Immunol 169 (4):1984–1992 [DOI] [PubMed] [Google Scholar]
- 13.Goronzy JJ, Weyand CM (2017) Successful and Maladaptive T Cell Aging. Immunity 46 (3):364–378. doi: 10.1016/j.immuni.2017.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kamphorst AO, Pillai RN, Yang S, Nasti TH, Akondy RS, Wieland A, Sica GL, Yu K, Koenig L, Patel NT, Behera M, Wu H, McCausland M, Chen Z, Zhang C, Khuri FR, Owonikoko TK, Ahmed R, Ramalingam SS (2017) Proliferation of PD-1+ CD8 T cells in peripheral blood after PD-1-targeted therapy in lung cancer patients. Proc Natl Acad Sci U S A 114 (19):4993–4998. doi: 10.1073/pnas.1705327114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu X, Almeida JR, Darko S, van der Burg M, DeRavin SS, Malech H, Gennery A, Chinn I, Markert ML, Douek DC, Milner JD (2014) Human syndromes of immunodeficiency and dysregulation are characterized by distinct defects in T-cell receptor repertoire development. J Allergy Clin Immunol 133 (4):1109–1115. doi: 10.1016/j.jaci.2013.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger SN, Kohrt HE, Horn L, Lawrence DP, Rost S, Leabman M, Xiao Y, Mokatrin A, Koeppen H, Hegde PS, Mellman I, Chen DS, Hodi FS (2014) Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 515 (7528):563–567. doi: 10.1038/nature14011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang AC, Postow MA, Orlowski RJ, Mick R, Bengsch B, Manne S, Xu W, Harmon S, Giles JR, Wenz B, Adamow M, Kuk D, Panageas KS, Carrera C, Wong P, Quagliarello F, Wubbenhorst B, D’Andrea K, Pauken KE, Herati RS, Staupe RP, Schenkel JM, McGettigan S, Kothari S, George SM, Vonderheide RH, Amaravadi RK, Karakousis GC, Schuchter LM, Xu X, Nathanson KL, Wolchok JD, Gangadhar TC, Wherry EJ (2017) Tcell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature 545 (7652):60–65. doi: 10.1038/nature22079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brennan RM, Petersen J, Neller MA, Miles JJ, Burrows JM, Smith C, McCluskey J, Khanna R, Rossjohn J, Burrows SR (2012) The impact of a large and frequent deletion in the human TCR beta locus on antiviral immunity. J Immunol 188 (6):2742–2748. doi: 10.4049/jimmunol.1102675 [DOI] [PubMed] [Google Scholar]
- 19.Gros A, Parkhurst MR, Tran E, Pasetto A, Robbins PF, Ilyas S, Prickett TD, Gartner JJ, Crystal JS, Roberts IM, Trebska-McGowan K, Wunderlich JR, Yang JC, Rosenberg SA (2016) Prospective identification of neoantigen-specific lymphocytes in the peripheral blood of melanoma patients. Nat Med 22 (4):433–438. doi: 10.1038/nm.4051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Erkes DA, Smith CJ, Wilski NA, Caldeira-Dantas S, Mohgbeli T, Snyder CM (2017) Virus-Specific CD8(+) T Cells Infiltrate Melanoma Lesions and Retain Function Independently of PD-1 Expression. J Immunol 198 (7):2979–2988. doi: 10.4049/jimmunol.1601064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simoni Y, Becht E, Fehlings M, Loh CY, Koo SL, Teng KWW, Yeong JPS, Nahar R, Zhang T, Kared H, Duan K, Ang N, Poidinger M, Lee YY, Larbi A, Khng AJ, Tan E, Fu C, Mathew R, Teo M, Lim WT, Toh CK, Ong BH, Koh T, Hillmer AM, Takano A, Lim TKH, Tan EH, Zhai W, Tan DSW, Tan IB, Newell EW (2018) Bystander CD8(+) T cells are abundant and phenotypically distinct in human tumour infiltrates. Nature 557 (7706):575–579. doi: 10.1038/s41586-018-0130-2 [DOI] [PubMed] [Google Scholar]
- 22.Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V, West AN, Carmona M, Kivork C, Seja E, Cherry G, Gutierrez AJ, Grogan TR, Mateus C, Tomasic G, Glaspy JA, Emerson RO, Robins H, Pierce RH, Elashoff DA, Robert C, Ribas A (2014) PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 515 (7528):568–571. doi: 10.1038/nature13954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reuben A, Spencer CN, Prieto PA, Gopalakrishnan V, Reddy SM, Miller JP, Mao X, De Macedo MP, Chen J, Song X, Jiang H, Chen PL, Beird HC, Garber HR, Roh W, Wani K, Chen E, Haymaker C, Forget MA, Little LD, Gumbs C, Thornton RL, Hudgens CW, Chen WS, Austin-Breneman J, Sloane RS, Nezi L, Cogdill AP, Bernatchez C, Roszik J, Hwu P, Woodman SE, Chin L, Tawbi H, Davies MA, Gershenwald JE, Amaria RN, Glitza IC, Diab A, Patel SP, Hu J, Lee JE, Grimm EA, Tetzlaff MT, Lazar AJ, Wistuba II, Clise-Dwyer K, Carter BW, Zhang J, Futreal PA, Sharma P, Allison JP, Cooper ZA, Wargo JA (2017) Genomic and immune heterogeneity are associated with differential responses to therapy in melanoma. NPJ Genom Med 2. doi: 10.1038/s41525-017-0013-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blattman JN, Wherry EJ, Ha SJ, van der Most RG, Ahmed R (2009) Impact of epitope escape on PD-1 expression and CD8 T-cell exhaustion during chronic infection. J Virol 83 (9):4386–4394. doi: 10.1128/JVI.02524-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schildberg FA, Klein SR, Freeman GJ, Sharpe AH (2016) Coinhibitory Pathways in the B7-CD28 Ligand-Receptor Family. Immunity 44 (5):955–972. doi: 10.1016/j.immuni.2016.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahmed KA, Stallworth DG, Kim Y, Johnstone PA, Harrison LB, Caudell JJ, Yu HH, Etame AB, Weber JS, Gibney GT (2016) Clinical outcomes of melanoma brain metastases treated with stereotactic radiation and anti-PD-1 therapy. Ann Oncol 27 (3):434–441. doi: 10.1093/annonc/mdv622 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.