Abstract
Chloroplast genetic engineering offers several advantages, including high levels of transgene expression, transgene containment via maternal inheritance, and multi-gene expression in a single transformation event. Oral delivery is facilitated by hyperexpression of vaccine antigens against cholera, tetanus, anthrax, plague, or canine parvovirus (4%–31% of total soluble protein, TSP) in transgenic chloroplasts (leaves) or non-green plastids (carrots, tomato) as well as the availability of antibiotic free selectable markers or the ability to excise selectable marker genes. Hyperexpression of several therapeutic proteins, including human serum albumin (11.1% TSP), somatotropin (7% TSP), interferon-alpha (19% TSP), interferon-gamma (6% TSP), and antimicrobial peptide (21.5% TSP), facilitates efficient and economic purification. Also, the presence of chaperones and enzymes in chloroplasts facilitates assembly of complex multisubunit proteins and correct folding of human blood proteins with proper disulfide bonds. Functionality of chloroplast-derived vaccine antigens and therapeutic proteins has been demonstrated by several assays, including the macrophage lysis assay, GM1-ganglioside binding assay, protection of HeLA cells or human lung carcinoma cells against encephalomyocarditis virus, systemic immune response, protection against pathogen challenge, and growth or inhibition of cell cultures. Purification of human proinsulin has been achieved using novel purification strategies (inverse temperature transition property) that do not require expensive column chromatography techniques. Thus, transgenic chloroplasts are ideal bioreactors for production of functional human and animal therapeutic proteins in an environmentally friendly manner.
Introduction
Vaccines and therapeutic proteins are the great successes of modern medicine, which have been used for several decades to prevent diseases and eradicate them. The uses of vaccines and therapeutic proteins have great potential but are limited by their cost of production, distribution, and delivery. Modified mammalian cells are used for producing therapeutic proteins, which have the advantage of resulting in products that are similar to their natural counterparts. These cells can be cultured on a limited scale but production is quite expensive. Bacteria can be used for large-scale production of proteins, but the products differ from the natural products considerably. For example, the proteins that are usually glycosylated in humans are not glycosylated by bacteria. Moreover, many proteins are expressed in Escherichia coli on a large scale, but sometimes they may differ in conformation with eventual precipitation due to a lack of proper folding and disulfide bridges (Daniell et al. 2001d).
For ages, humans have been using plants as a source of food, clothing, medicine, and building materials. Plants have been of immense help in the past and continue to be so. Plants are now one of the new hosts for the production of biopharmaceuticals, polymers, vaccines, enzymes, plasma proteins, and antibodies. There are many advantages in production of recombinant proteins in plants. Primarily, plant systems are more economical in that they can be produced on a large scale rather than using industrial methods (fermentation of bacteria, yeast or cultured animal or human cell lines) that are very expensive. Also, there is no need to maintain the cold chain as the plant parts expressing the vaccine or plant extracts can be stored and transported at room temperature. Plants have the ability to carry out posttranslational modifications similar to naturally occurring systems. There is also minimized risk of contamination from potential human pathogens, as plants are not hosts for human infectious agents (Giddings et al. 2000). If therapeutic proteins are delivered orally, then the purification step from plants can be eliminated. Other advantages of foodbased vaccines include the low cost of raw material and convenient storage; the cost of syringes and needles in delivery of vaccines is eliminated, which thereby eliminates blood-borne pathogens.
Plant Expression System
Many different proteins with applications for human or animal vaccines, biopharmaceuticals, and biopolymers have been expressed in transgenic plants. With a few exceptions, most often very low expression levels of foreign proteins (less than 1% of the total soluble protein) were observed in nuclear transgenic plants (Daniell et al. 2001d), including the B subunit of enterotoxigenic E. coli (0.01% TSP; Haq et al. 1995), hepatitis B virus envelope surface protein (0.01% TSP; Mason et al. 1992; Thanavala et al. 1995), human cytomegalovirus glycoprotein B (0.02% TSP; Tackaberry et al. 1999), and transmissible gastroenteritis coronavirus glycoprotein S (0.06% TSP; Gomez et al. 1998). Also, gene silencing can occur in nuclear transformation, which results in lower expression of recombinant proteins. For commercial exploitation of the therapeutic proteins and vaccine antigens, high and reliable levels of expression are required, which could be achieved by alternative approaches.
Chloroplast Expression System
The highest level of protein expression of Bacillus thuringiensis (Bt) cry2Aa2 in plants was achieved at 46.1% TSP in transgenic tobacco chloroplasts (De Cosa et al. 2001). Besides such high expression levels, there are several other advantages of chloroplast genetic engineering over nuclear transformation. Chloroplast genomes defy the laws of Mendelian inheritance in that they are maternally inherited in most crops (Zhang et al. 2003) and thus minimize out-crossing of transgenic pollen with related weeds or crops. Even if the pollen from plants that exhibit maternal inheritance contains metabolically active plastids, the plastid DNA is lost during pollen maturation and is not transmitted to the next generation (Daniell 2002). Therefore, the chloroplast expression system is an environmentally friendly approach. Also, chloroplasts have the ability to express multiple genes in a single transformation event. Expression of polycistrons in transgenic chloroplasts is a unique feature, which facilitates the expression of entire pathways in a single transformation event (De Cosa et al. 2001; Daniell and Dhingra 2002). For the first time, a complete bacterial operon was successfully expressed in transgenic chloroplasts, resulting in the formation of stable cry2Aa2 crystals (De Cosa et al. 2001). This should facilitate expression of polyvalent vaccines or multisubunit proteins in transgenic chloroplasts.
In addition, the position effect seen in nuclear transgenics can be eliminated as the transgenes are targeted into spacer regions at precise locations in the chloroplast genome by homologous recombination of chloroplast DNA flanking sequences. The problem of gene silencing has not been observed in transgenic chloroplasts in spite of higher expression levels than in nuclear transgenic plants. It has been observed that there is no gene silencing in spite of high translation levels, up to 46.1% TSP (De Cosa et al. 2001) or transcription in transgenic chloroplasts, despite accumulation of transcripts 169-fold and 150-fold higher than nuclear transgenics (Lee et al.2003; Dhingra et al. 2004).
Chloroplasts also have the ability to process eukaryotic proteins, including correct folding of subunits and formation of disulfide bridges (Daniell et al. 2001d). Functional assays showed that chloroplast-synthesized cholera toxin-B subunit binds to the intestinal membrane GM1-ganglioside receptor, thereby confirming the correct folding and disulfide bond formation (Staub et al. 2000; Daniell et al. 2001b; Molina et al. 2004). Chaperones present in chloroplasts facilitate correct folding and assembly of monoclonal antibody in transgenic chloroplasts (Daniell et al. 2004a) and also result in fully functional human therapeutic proteins, as seen in interferon alpha and gamma (Falconer 2002; Leelavathi and Reddy 2003). Chloroplasts can be a good place to store the biosynthetic products that could otherwise be harmful when accumulated in cytosol (Bogorad 2000). This was demonstrated when cholera toxin B subunit was accumulated in large quantities in transgenic chloroplasts and it had no toxic effect (Daniell et al. 2001b), whereas when accumulated in the cytosol in very small quantities, CTB was toxic (Mason et al. 1998). Similarly, trehalose, which is used as a preservative in the pharmaceutical industry, was toxic when accumulated in cytosol but was nontoxic when compartmentalized within chloroplasts (Lee et al. 2003). Several therapeutic proteins and vaccine antigens expressed via the chloroplast genome are listed in Tables 1 and 2.
Table 1.
List of vaccine antigen proteins expressed via the chloroplast genome
| Vaccine antigens | Gene | Site of integration |
Promoter | 5′/3′ Regulatory elements |
% TSP expression |
Functionality assay | Lab/year |
|---|---|---|---|---|---|---|---|
| Cholera toxin | CtxB | TrnI/trnA | Prrn | ggagg/ TpsbA | 4% | GM—1 ganglioside binding assay |
Daniell 2001 |
| Tetanus toxin |
TetC (bacterial and synthetic) |
TrnV/rps12/7 | Prrn | T7 gene 10a, atpBb / TrbcL |
25% a, 10% b | Positive systemic immune response |
Maliga 2003 |
| Canine parvovirus (CPV) | CTB—2L21/GFP—2L21 | TrnI/trnA | Prrn | PpsbA/TpsbA | 31.1%, 22.6% | Immune response | Daniell, Veramandi 2004 |
| Anthrax protective antigen | Pag | TrnI/trnA | Prrn | PpsbA/TpsbA | 4%–5% | Macrophage lysis assay | Daniell 2004 |
| Plague vaccine | CaF1~LcrV | TrnI/trnA | Prrn | PpsbA/TpsbA | 14.8% | ND | Daniell 2003 |
Table 2.
List of biopharmaceutical proteins expressed via the chloroplast genome
| Biopharmaceutical proteins |
Gene | Site of Integration |
Promoter | 5′/3′ Regulatory elements |
% TSP expression | Functionality assay | Lab, year |
|---|---|---|---|---|---|---|---|
| Elastin-derived polymer | EG121 | trnI/trnA | Prrn | T7gene10/TpsbA | ND | Inverse temp. transition assay | Daniell 2000 |
| Human somatotropin | hST | trnV/rps12/7 | Prrna, P psbAb | T7gene10a or psbAb/ Trps16 |
7.0% a and 1.0% b | Positive growth response of Nb2 cell line |
Monsanto 2000 |
| Antimicrobial peptide | MSI—99v | trnI/trnA | Prrn | ggagg/TpsbA | 21.5% – 43% | Minimum inhibitory conc. (MIC) of P. aeruginosa |
Daniell 2001 |
| Insulin-like growth factor | IGF—1 | trnI/trnA | Prrn | PpsbA/ TpsbA | 33% | ND | Daniell 2002 |
| Interferon-α 5 | INFα 5 | trnI/trnA | Prrn | PpsbA/TpsbA | ND | ND | Daniell 2002 |
| Interferon-α 2b | INFα 2B | trnI/trnA | Prrn | PpsbA/TpsbA | 18.8% | Protection of HeLA cells against cytopathic effects of EMC virus |
Daniell 2002 |
| Human serum albumin | hsa | trnI/trnA | Prrna, PpsbAb | ggagga, psbAb/TpsbA | 0.02%a, 11.1% b | ND | Daniell 2003 |
| Interferon-γ | IFN-g | rbcL/accD | PpsbA | PpsbA/TpsbA | 6% | Protection of human lung carci- noma cells against infection by EMC virus |
Reddy 2003 |
| Monoclonal antibodies | Guy’s 13 | trnI/trnA | Prrn | ggagg/ TpsbA | ND | ND | Daniell 2001 |
Novel Purification Strategies
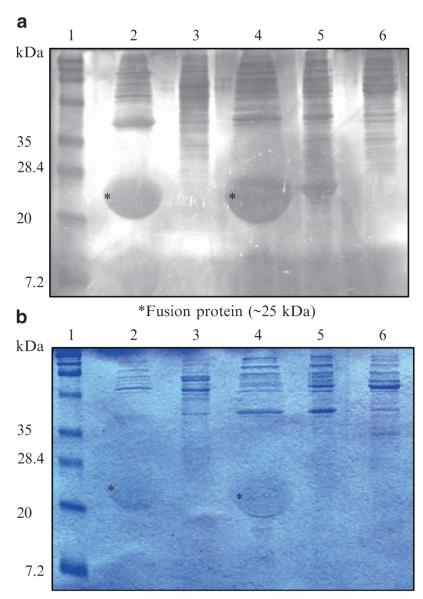
The main reason for the high cost of pharmaceutical protein production is purification of recombinant proteins. Therefore, novel protein purification strategies can be used that do not require the use of expensive column chromatography. For instance, a synthetic protein-based polymer gene (GVGVP) 121 has been expressed in E. coli and a very high expression level was achieved such that polymer inclusion bodies were formed that occupied nearly 90% of the cell volume (Daniell et al. 1997; Guda et al. 2000). (GVGVP) 121 exhibits inverse temperature transition properties, making it soluble in water below room temperature, but aggregates into a more ordered, viscoelastic state, called a coacervate, at 37°C (Daniell et al. 1997; Guda et al. 2000). The inverse temperature transition property makes purification easier and less expensive in aqueous solutions simply by raising the temperature. In addition, this property makes (GVGVP) 121 an ideal fusion protein for purification. To demonstrate this, the proinsulin gene was fused to a smaller version of this biopolymer (GVGVP) 40 with inverse temperature transition properties and was expressed in E. coli (Daniell et al. 2004a). At 4°C, the biopolymer exists as an extended molecule, but when incubated at 42°C, it folds into dynamic structures called β-spirals that further aggregate by hydrophobic association to form twisted filaments (Urry et al. 1996). Therefore, this principle was successfully used to purify the fusion protein simply by raising the temperature to 42°C and was analyzed on SDS-polyacrylamide gel. The presence of polymer in the proinsulin-polymer fusion protein was confirmed by negatively staining the SDS-PAGE gels with 0.3 M Cucl2 (Fig. 1a). Copper stained gels, when illuminated obliquely, show dark bands against a light, semiopaque background. The same gel was stained with Coomassie R-250. The sulphonic acid groups in the dye (Coomassie R-250) form ion pairs with lysine and arginine in the protein, which are not present in the polymer but are present in proinsulin. Since the Coomassie R-250 stained the polypeptide, it confirms the presence of fusion protein (Fig. 1b). After purification, the fusion protein can be cleaved with suitable enzymes and the polymer can be discarded in the pellet fraction by another round of polymerization at 42°C followed by centrifugation. This novel purification strategy should significantly decrease the purification cost because of the elimination of column chromatography.
Fig. 1.
a, b Expression and purification of insulin-polymer fusion protein detected in copper- (a) and Coomassie- (b) stained gels. Two rounds of purification through thermally reversible phase transition were performed. The same gel was first stained with copper, destained, and restained with Coomassie R-250. Lane 1: Prestained marker; lane 2: purified extract of polymer-insulin fusion protein from the chloroplast vector pSBL-OC-40Pris; lane 3: reverse orientation of fusion protein from pSBL-OC-40Pris; lane 4: purified extract of pLD-OC-40Pris; lane 5: reverse orientation of pLD-OC-40Pris; lane 6: purified extract of E. coli strain XL-1 blue containing no plasmid
Oral Delivery of Vaccine Antigens
Oral delivery of vaccines is an attractive alternative because of the ease of administration and low costs. Also, oral delivery of the recombinant protein could reduce the production costs by almost 90%. As pointed out above, chloroplast genetic engineering is most suitable for hyperexpression of vaccine antigens and therapeutic proteins. However, oral delivery of vaccine antigens could significantly reduce the cost of their production, purification, storage, and transportation, thereby eliminating the need to maintain the cold chain. To achieve oral delivery of vaccines, one of the important requirements is the production of therapeutic proteins in antibiotic free selection.
Most transformation techniques use an antibiotic resistance gene along with the gene of interest such that only the transgenic lines containing the desired trait are selected. But once the trait has been established, the antibiotic resistance gene has only one purpose: producing its products. One major concern is horizontal transfer of the antibiotic resistance genes to other related or unrelated organisms. However, now chloroplasts can be genetically modified without the use of antibiotic resistance genes. There are several ways of doing this. For example, the spinach betaine aldehyde dehydrogenase (BADH) gene has been developed as a selectable marker to transform the chloroplast genome (Daniell et al. 2001c, e). The toxic betaine aldehyde (BA) is converted to nontoxic glycine betaine by the chloroplast BADH enzyme. This glycine betaine also serves as an osmoprotectant and confers salt tolerance (Kumar et al. 2004). The BADH enzyme is present in only a few plant species that are adapted to salty and dry environments.
In addition, the selectable marker genes can be removed using short, 174-bp DNA repeats, but the gene of interest could be retained outside the direct repeats, thereby producing marker-free plants (Iatham and Day 2000). Another method of eliminating the selectable marker is by employing a transiently co-integrated vector with a single homologous flank (Klaus et al. 2004). The co-integrates are lost rapidly by recombination when the selection pressure is removed.
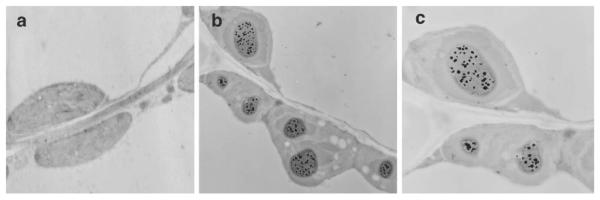
For oral delivery of vaccines, another important requirement is the expression of the vaccine antigens in plastids of non-green tissues. For the first time, stable and highly efficient plastid transformation of carrot using non-green tissues as explants, regenerated via somatic embryogenesis, has been reported (Kumar et al. 2004). A useful plant trait (salt tolerance) has been expressed for the first time in a non-solanaceous crop via the chloroplast genome. The carrotspecific plastid transformation vector pDD-Dc-aadA/BADH was constructed and carrot callus was bombarded. The bombarded callus was plated on selection medium containing 150 mg/l spectinomycin to obtain spectinomycin-resistant transgenic lines. The BADH enzyme activity was assayed in crude extracts of protein from both transformed and untransformed carrot cell cultures. BADH enzyme in the presence of betaine aldehyde converts NAD+ to NADH and the reaction rate was measured by an increase in absorbance at 340 nm due to the reduction of NAD+ (Kumar et al. 2004). Crude extracts from transgenic plastids showed higher levels of BADH activity when compared to untransformed tissue (Fig. 2a). High BADH activity was observed in leaves, tap roots, and cells in suspension culture but the difference in activity may be due to variation in plastid genome copy numbers. The high BADH activity in carrot taproot may be due to a large number of chromoplasts, which is evident by the orange color (Fig. 2b). Also, the protein expression in carrot cells, root, and shoot was tested using Western blot analysis (Fig. 2c). These results were consistent with the BADH enzyme activity. Also, glycine betaine is found in many higher plants, bacteria, and mammalian animals, which they accumulate under conditions of water or salt stress and acts as an osmoprotectant (Figueroa-Soto et al. 1999). In plants and bacteria, glycine betaine can build up the cytoplasmic osmotic strength without preventing any cellular functions. BADH was reported to be present in porcine kidneys localized in both cortex and medulla (Figueroa-Soto et al. 1999).
Fig. 2.
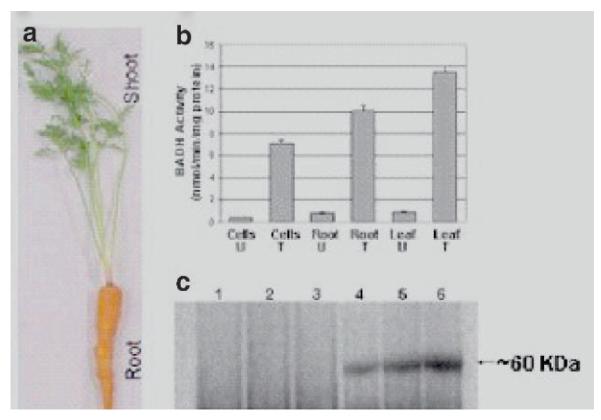
a—c BADH enzyme activity and BADH expression in control and pDD-Dc-aadA/BADH lines. a BADH activity in untransformed (U) and transformed (T) cell suspension, root and leaf. b A pDD-Dc-aadA/BADH transgenic line shown with taproot and shoot. c Western blot using polyclonal anti-BADH serum. Antigenic peptides were detected using horseradish peroxidase-linked secondary antibody. Lanes 1, 2, 3, untransformed cell culture, root and leaf and lanes 4, 5, 6, transformed cell culture, root and leaf
Carrot is a biennial plant with a vegetative phase in the 1st year and the reproductive phase in the 2nd year. Harvesting the crop containing the transgene at the end of 1st year prevents gene flow through seeds or pollen because there is no reproductive system. Also, maternal inheritance of carrot chloroplast genomes adds to the environmentally friendly approach (Vivek et al. 1999). Somatic embryos of carrot are single-cell-derived and multiply through recurrent embryogenesis, which provides a uniform source of cell culture and a homogeneous single source, which is a requirement for the production of human therapeutic proteins. Oral delivery of therapeutic proteins via edible carrots preserves the structural integrity of the proteins, as no cooking is needed. Encapsulated embryos, which are viable for many years in culture, can be used for cryopreservation. Because of all these advantages, carrot can be used as an ideal system for oral delivery.
Chloroplast Derived Vaccine Antigens
Cholera Toxin B Antigen
Cholera toxin B subunit (CTB) of Vibrio cholerae, a candidate vaccine antigen, has been expressed in transgenic chloroplasts, and this resulted in accumulation of up to 4.1% total soluble protein as functional oligomers (Daniell et al. 2001b). Higher expression levels (up to 31.1% TSP) were obtained when CTB-2L21 fusion protein was expressed in transgenic chloroplasts (Molina et al. 2004). The difference in the expression levels of the CTB gene was due to the presence of a ribosome-binding site (GGAGG, 4.1% TSP) or the 5′ UTR of the psbA gene (31.1% TSP). CTB synthesized from transgenic chloroplasts in both investigations assembled into functional oligomers and were antigenically identical to purified native CTB. CTB has the highest affinity to bind with gangliosides (GM1), which are the natural toxin receptors in the intestinal epithelial cells. The functionality of CTB was determined by the GM1-ganglioside ELISA binding assay (Fig. 3), where the plates were coated first with GM1-gangliosides and BSA, which was then plated with the total soluble protein from the transformed and the untransformed plants and bacterial CTB. Then the absorbance of the GM1—ganglioside—CTB-antibody complex was measured. The bacterial CTB and chloroplast-synthesized CTB showed a strong affinity for the GM1—ganglioside complex, confirming that the antigenic sites necessary for the binding of CTB to GM1 were conserved. With such high levels of expression of an efficient transmucosal carrier molecule such as CTB in chloroplasts, fusion proteins can be synthesized and also plant-derived vaccines can be commercialized.
Fig. 3.
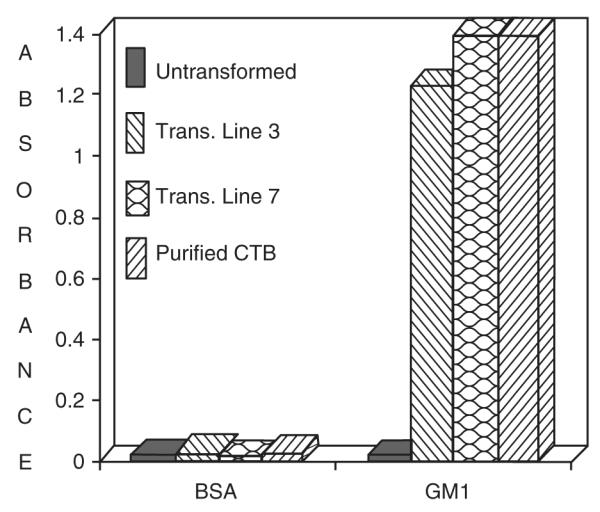
CTB-GM1-ganglioside binding ELISA assay. Plates, coated first with GM1-ganglioside and bovine serum albumin (BSA), respectively, were irrigated with total soluble plant protein from chloroplast transgenic lines (3, 7) and 300 ng of purified bacterial CTB. The absorbance of the GM1-ganglioside-CTB-antibody complex in each case was measured at 405 nm. Total soluble protein from untransformed plants was used as the negative control
Anthrax Protective Vaccine Antigen
The Center for Disease Control (CDC) lists Bacillus anthracis as a category A agent and estimates the cost of an anthrax attack to exceed $26 billion per 100,000 exposed individuals (Kaufmann et al. 1997). The present vaccine for anthrax is produced by cell-free filtrate of toxigenic, nonencapsulated strain of B. anthracis (Baillie 2001). In addition to the protective antigen (PA), the immunogenic portion, trace amounts of lethal factor and edema factor are present in the filtrate, which are considered to be toxic and cause side effects (Ivins et al. 1995; Baillie 2001; Joellenback et al. 2002). The PA heptamerizes and binds itself to the host cell receptor. This can now bind to lethal factor or edema factor. When bound to lethal factor, the toxin stimulates the release of interleukins-1β by macrophages and other cytokines that lead to sudden death. For the above reasons and also because of the limited supply of the current vaccine, biothrax (formerly anthrax vaccine adsorbed, or AVA), there is an urgent need for an improved and pure vaccine. Therefore, the B. anthracis 83-kDa PA was expressed in transgenic tobacco chloroplasts. The PA gene was cloned into the chloroplast transformation vector (pLD-JW1) along with psbA regulatory signals to enhance translation. The crude plant extracts contained up to 2.5 mg PA/g fresh weight (Daniell et al. 2004b). The PA protein as a percentage of total soluble protein expressed in pLD-JW1 plants was determined for 3 and 5 days of continuous illumination. A maximum 18.1% and 13.4% PA in the total soluble protein was achieved (Fig. 4). The maximum levels of expression were observed in mature leaves under continuous illumination. Also, Western blots were performed using two different detergents in the extraction buffer, CHAPS and SDS, and both extracted PA equally well. After storage for 2 days at 4°C and −20°C, the PA in the crude extracts was quite stable. Powdered leaf was stored at −80°C for several months and then Western blots were performed, showing no noticeable decrease in PA quantity or functionality. This should facilitate the long-term storage of harvested leaves before PA is extracted for vaccine production. Trypsin, chymotrypsin, and furin proteolytic sites present in PA expressed in transgenic chloroplasts were protected in that only PA 83 was observed. The functionality of the antigen was determined by its ability to internalize the lethal factor and cause the lysis of cultured macrophage cells, which is termed as the macrophage lysis assay (Fig. 5). The percentage macrophage viability was determined using the ability of live but not dead cells to reduce a water-soluble yellow dye, MTT (3-[4,5-dimethylthiazol-2-yl [-2,5-diphenyltetrazolium bromide) to an insoluble purple formazan product (Daniell et al. 2004b). The PA also showed heptamerization and cleaved properly. With an average yield of 172 mg of PA per plant using the experimental transgenic cultivar grown in greenhouse, 400 million doses of vaccine (free of contaminants) could be produced per acre. The yield could increase further by 18-fold when a commercial cultivar is used rather than the experimental cultivar.
Fig. 4.
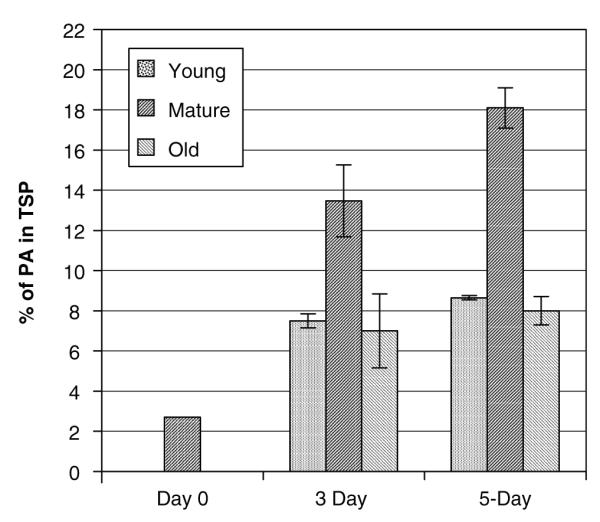
Quantification of anthrax protective antigen in JW1 plants of T1 generation using ELISA. Histogram of percentage of PA in the total soluble protein in fresh tissue of young, mature, and old leaves of plant in 16 h light and 8 h dark (day 0), 3-day continuous illumination and 5-day continuous illumination
Fig. 5.
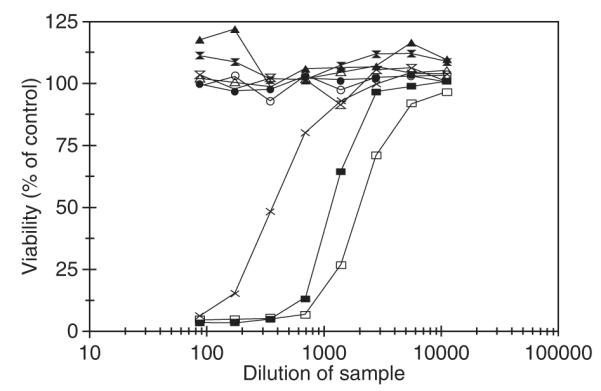
Macrophage cytotoxic assays for extracts from transgenic plants. Supernatant samples from T1 pLD-JW1 tested (proteins extracted in buffer containing no detergent and MTT added after 5 h). pLD-JW1 (extract stored 2 days); pLD-JW1 (extract stored 7 days); PA 5 μg/ml; control wild type (extract stored 2 days); control wild type (extract stored 7 days); control wild type no LF (extract stored 2 days); control wild type no LF (extract stored 7 days); control pLD-JW1 no LF (extract stored 2 days); control pLD-JW1 no LF (extract stored 7 days)
Plague Vaccine Antigen
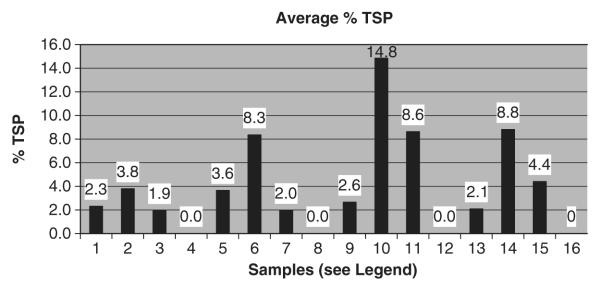
Yersinia pestis, a Gram-negative bacterium, is the causative agent of plague and has been listed by Center for Disease Control (CDC 2003) as one of the six category A biological agents. There are three different forms of plague. Bubonic plague, the most common form of plague, is caused by infected fleas. When it enters the lymph nodes, the bacterium causes swelling of the nodes and forms buboes; the other forms are septicemic plague and pneumatic plague. The current vaccine available is a killed whole subunit vaccine, which is moderately effective against bubonic plague and ineffective against the other two forms of the plague (Titball and Williamson 2001). Several subunit vaccines have been evaluated for immunogenicity against Y. pestis. CaF1 and LcrV are the most effective subunit vaccines so far against Y. pestis. F1 is a capsular protein located on the surface of the bacterium with antiphagocytic properties. The V antigen is a component of the Y. pestis type III secretion system and it may form part of an injectosome. The fusion protein of F1-V expressed in E. coli has been shown to be safe and immunogenic when mice were challenged with Y. pestis (Williamson et al., 1997). The fusion protein F1-V was expressed in transgenic chloroplasts consisting of the F1 antigen fused at its carboxy terminus to the amino terminus of the V antigen. Western blot and ELISA were performed and samples were collected from plants under continuous illumination from days 0–5 from young, mature, and old leaves (Fig. 6). The maximum expression levels were observed in mature leaves, which is as high as 14.8% of the total soluble protein (Arlen et al. 2008). Further studies need to be done to confirm immunogenicity. Since Y. pestis is one of the category A biological warfare agents and there is no proper vaccine, improved vaccine is a high priority.
Fig. 6.
Enzyme linked immunoassays expressed, with protein yields expressed as % TSP and micrograms of F1 V per gram of fresh leaf material. For the continuous illumination experiment, leaf material was sampled on days 0, 1, 3, and 5. Young, mature, and old samples were taken for each experiment. The figure shows average levels of F1 V antigen for samples 1–4 [day 0: (1) young, (2) mature, (3) old, and (4) wild-type leaf samples]; samples 5–8 [day 1: (5) young, (6) mature, (7) old and (8) wild-type leaf samples]; samples 9–12 [day 3: (9) young, (10) mature, (11) old and (12) wild-type leaf samples]; samples 13–16 [day 5: (13) young, (14) mature, (15) old, (16) wild-type leaf samples]
Canine Parvovirus VP2 Antigen
Canine parvovirus (CPV) infects dogs and other Canidae such as wolves, South American dogs, and Asiatic raccoon dogs, producing hemorrhagic gastroenteritis and myocarditis (CPV vaccination, Cornell University) .The 2L21 synthetic peptide, coupled to KLH carrier protein, was studied extensively and has been shown to be effective in protecting dogs and minks against parvovirus (Langeveld et al. 1994, 1995). The 2L21 peptide, which confers protection to dogs against virulent canine parvovirus (CPV), was expressed in tobacco chloroplasts as a fusion protein with cholera toxin B (CTB) and with the green fluorescent protein (GFP). The maximum levels of protein expression were achieved with CTB-2L21, 7.49 mg/g fresh weight (equivalent to 31.1% total soluble protein) and with GFP-2L21, 5.96 mg/g fresh weight (equivalent to 22.6% of total soluble protein, Molina et al. 2004; Fig. 7). The expression levels were also dependent on the age of the plant. Mature plants accumulated the highest amounts of protein when compared to the young and senescent plants. This shows the importance of the plant’s harvesting time. Also, the chimeric protein retained the pentamerization and G M1-ganglioside-binding properties of the native CTB. When mice were immunized intraperitoneally with the leaf extracts from CTB-2L21, mice immunized with CTB-2L21 elicited anti-2L21 antibodies able to recognize VP2 protein from CPV (Fig. 8). This is the first report of an animal vaccine and viral antigen expressed in transgenic chloroplasts.
Fig. 7.
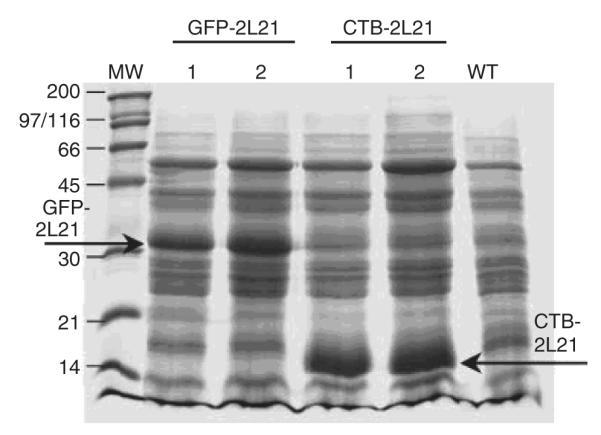
Coomassie blue-stained sodium dodecylsulphate-polyacrylamide gel electrophoresis (SDS-PAGE) gel of plant samples, 60 days after transplanting. Two independent lines (1, 2) for each construction were analyzed. Recombinant proteins GFP-2L21 and CTB-2L21 are marked. Fifty micrograms of plant total protein was loaded per well. 2L21 epitope from the VP2 protein of the canine parvovirus, CTB cholera toxin B, GFP green fluorescent protein, MW molecular weight marker, WT wild-type Petit Havana plant
Fig. 8.
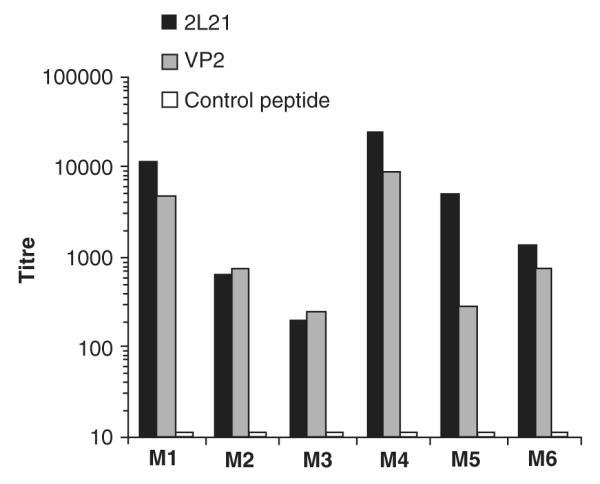
Titers of antibodies at day 50 induced by plant-derived CTB-2L21 recombinant protein. Balb/c mice were intraperitoneally immunized with leaf extract from CTB-2L21 transgenic plants. Animals were boosted at days 21 and 35. Each mouse received 20 μg of CTB-2L21 recombinant protein. Individual mice sera were titrated against 2L21 synthetic peptide, VP2 protein and control peptide (amino acids 122–135 of hepatitis B virus surface antigen). Titers were expressed as the highest serum dilution to yield twice the absorbance mean of preimmune sera. M1—M6 mouse 1–6, 2L21 epitope from the VP2 protein of the canine parvovirus, CTB cholera toxin B, VP2 protein of the canine parvovirus that includes the 2L21 epitope
Chloroplast-Derived Therapeutic Proteins
Several human therapeutic proteins that have been expressed in transgenic chloroplasts are listed in Table 2. The expression levels depend on the site of integration, regulatory elements used to enhance transcription/translation efficiency, and the stability of the foreign protein. Genes encoding for proteins as small as 20 amino acids (magainin; DeGray et al. 2001) or as large as 83 kDa (PA; Daniell et al. 2004b) have been expressed in transgenic chloroplasts. A few illustrations from the Daniell laboratory are described in the following sections.
Human Serum Albumin
Human serum albumin (HSA), the most widely used intravenous protein, is obtained by fractionation of blood serum and accounts for approximately 60% of the total protein in the blood (Fernández-San Millán et al. 2003). The world needs more than 500 tons annually, representing a market value of more than $1.5 billion. HSA was produced in a wide variety of microbial systems but no system is commercially feasible yet. Expression of functional HSA in a transgenic chloroplast expression system is advantageous because of the inexpensive production costs and absence of human pathogens. This protein was expressed in transgenic chloroplasts under the translational control of a Shine Dalgarno sequence, 5′psbA region or the cry2Aa2 UTR. Different expression levels were obtained in transgenic plants with different regulatory sequences; for example, potted transgenic plants regulated by Shine-Dalgarno sequence showed 0.02% TSP, while 7.2% and 0.08% TSP HSA was observed in plants regulated by chloroplast 5′ psbA or heterologous cry2Aa2 UTR, respectively. Expression in seedlings with Shine-Dalgarno control was 0.8% total protein of HSA, while 1.6% TP was observed with 5′ psbA control and 5.9% TP under cry2Aa2 UTR control. The low levels of HSA accumulation in mature plants under the control of SD may be due to excessive proteolytic degradation and poor rates of translation. As 5′ psbA region is light-regulated, under continuous light illumination for 50 h, the HSA quantity in mature leaves was maximum: 11.1% of the total soluble protein (Fernández-San Millán et al. 2003). This is 500-fold greater expression than the previous reports of nuclear expression.
HSA was observed to form inclusion bodies in transgenic chloroplasts, thus offering protection from proteolytic degradation (Fig. 9a—c). Inclusion bodies can easily be separated from the majority of the cellular proteins by centrifugation, thereby eliminating the need for expensive affinity columns and chromatographic techniques. Properly folded HSA can be recovered from the inclusion bodies after denaturation for complete solubilization and in vitro refolding (Fernández-San Millán et al. 2003).
Fig. 9.
a—c Study of HSA accumulation in transgenic chloroplasts (a—c) Electron micrographs of immunogold labelled tissues from untransformed (a) and transformed mature leaves with the chloroplast vector pLDApsbAHSA (b, c). Magnifications: A×10,000; B×5,000; C×6,300
Human Interferon Alpha
Human interferon (IFNα 2b) is used in the treatment of malignant carcinoid tumors and was shown to be very effective in the reduction of tumor size. It has other therapeutic values such as inhibition of viral replication, cell proliferation, and enhancement of immune response, and recently, in treatment of patients suffering from West Nile virus infection. At present, IFNα 2b protein is produced in E. coli for commercial use and also requires in vitro processing and purification. The nuclear expression of this protein also resulted in very low expression (0.000017% TSP) in tobacco (Elderbaum et al. 1992). This has altogether made the treatment with interferon very expensive, which on average costs $26,000–$40,000 per year.
Recombinant IFNα 2b was expressed in transgenic chloroplasts using the chloroplast transformation vector integrated with the gene cassette that included IFNα2b gene along with polyhistidine purification tag and a thrombin cleavage site (Fig. 10). Western blots were performed to detect the multimers and monomer of IFNα 2b using interferon alpha monoclonal antibodies and confirm formation of disulfide bonds. Integration of the gene cassette into chloroplast genome was confirmed by Southern blots. ELISA was performed to quantify the IFNα 2b protein and expression up to 18.8% of total soluble protein was obtained (Arlen et al., 2007).
Fig. 10.
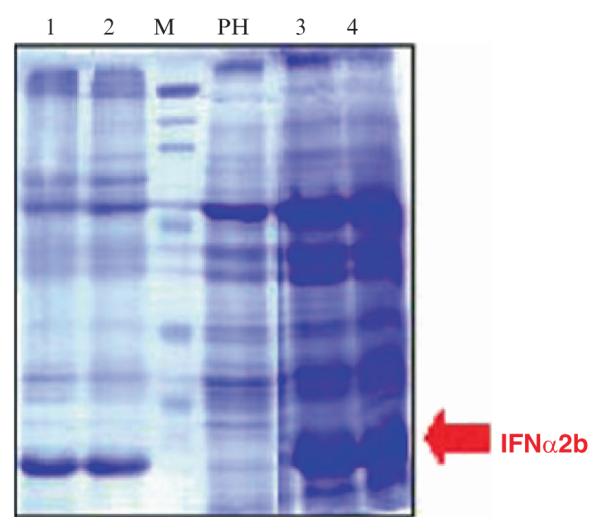
Coomassie-stained SDS-polyacrylamide gel showing chloroplast transgenic lines expressing IFN α 2b. Lanes 1 and 2: total soluble protein; lanes PH, 3 and 4: total protein
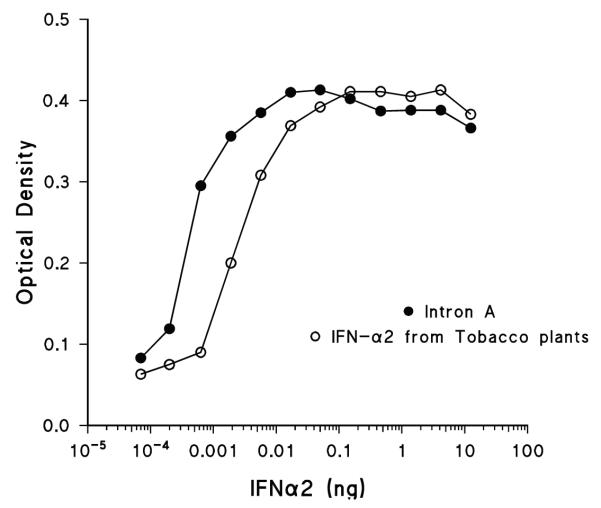
The functionality of IFNα 2b was investigated by its ability to protect HeLa cells against the cytopathic effect of encephalomyocarditis virus (EMC) and through the identification of interferon-induced transcripts (Fig. 11). The chloroplast derived IFNα 2b is found to have the same activity as commercially produced Intron A. The mRNA levels of two genes induced by IFNα 2b (2′-5′ oligoadenylate synthase and STAT-2) were tested by RT-PCR using primers specific for each gene. Chloroplast-derived IFNα 2b induced the expression of both genes in a manner similar to commercial IFNα 2b. This confirms that chloroplast derived IFNα 2b is as active as commercially produced Intron A.
Fig. 11.
Demonstration of IFNα 2b functionality by the ability of IFNα 2b to protect HeLa cells against the cytopathic effect of encephalomyocarditis virus. Note that chloroplast derived IFNα 2b is as active as commercially produced Intron A
Human Insulin-Like Growth Factor-1
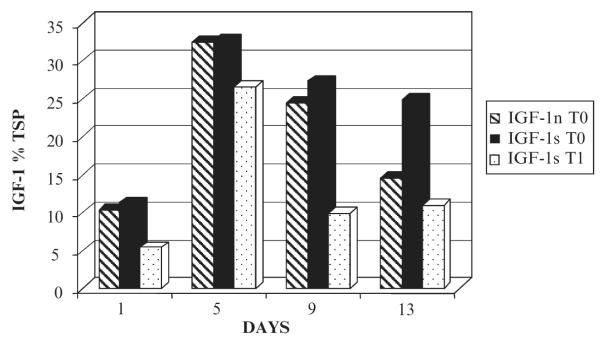
Human insulin-like growth factor (IGF-1) has therapeutic value not only in mediating the growth of muscle and other tissues, but its therapeutic value is being currently evaluated in diabetes, IGF-I induced neuroprotection, and in promoting bone healing. The IGF-1 is a naturally occurring single-chain polypeptide with three disulfide bonds, produced in the liver (Torrado and Carrascosa 2003). When produced in E. coli, IGF-1 cannot produce the mature form, as disulfide bonds cannot be formed in E. coli cytoplasm. Since the IGF-1 has codons suitable for the eukaryotic environment, codon was optimized for chloroplast to increase the levels of expression in transgenic chloroplasts. PCR and Southern blot analysis confirmed the chloroplast integration of IGF-1 gene. ELISA was performed to quantify the expression levels of IGF-1 from both native and synthetic genes in transgenic chloroplasts and the expression levels were as high as 32% of the total soluble protein (Ruiz 2002; Fig. 12). However, quantification of expression of IGF-1 was complicated by the zz-tag used for purification and levels of expression should be verified using antibodies that do not cross-react with the zz-tag. These observations suggest that unlike bacterial translational machinery, chloroplast translation machinery may be quite flexible.
Fig. 12.
Expression of IGF-1 in transgenic chloroplasts after continuous illumination for 13 days. IGF-1 expression is shown as a percentage of total soluble protein. IGF-1n is the native gene and IGF-1 s is the chloroplast codon-optimized gene
Anti-Microbial Peptide
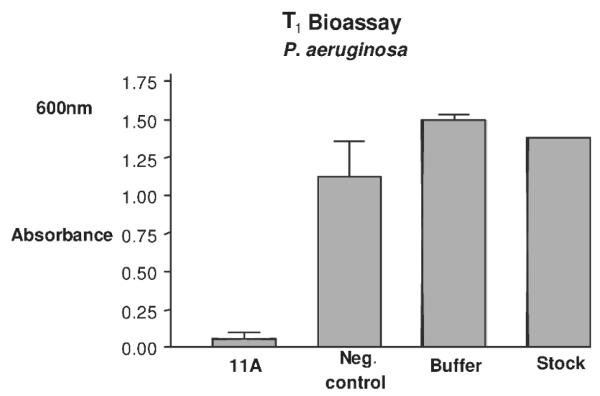
Anti-microbial peptides (AMPs) are a common component of the innate defense mechanisms in the animal kingdom, which help to combat pathogens and to control normal microbial flora. Magainin, secreted from the skin of the African clawed frog (Xenopus laevis), is a broad-spectrum topical agent, a systemic antibiotic and a wound-healing and anticancer agent (Zasloff 1987; Jacob and Zasloff 1994; DeGray et al. 2001). Magainin has affinity for the negatively charged phospholipids in the outer leaflet of the prokaryotic membrane. A magainin analog, MSI-99, was expressed in transgenic chloroplasts and expression levels up to 21.5% of total soluble protein were obtained (DeGray et al. 2001; Daniell et al. 2001a). The effectiveness of the lytic peptide expressed in transgenic chloroplasts was tested with a multidrug resistant Gram-negative bacterium, Pseudomonas aeruginosa, and this resulted in 96% growth inhibition of the pathogen (Fig. 13). MSI-99 was most effective against Pseudomonas syringae requiring only 1 μg/1,000 bacteria based on the study of minimum inhibitory concentration of MSI-99.
Fig. 13.
In vitro bioassay for T1 generation chloroplast transgenic line against P. aeruginosa. Bacterial cells from an overnight culture were diluted to A600 0.1–0.3 and incubated for 2 h at 25°C with 100 μ g of total protein extract. Of LB, 1 ml was added to each sample and incubated overnight at 26°C. Absorbance was recorded at 600 nm. Data were analyzed using Graph Pad Prism
Chloroplast-Derived Human Antibody
Monoclonal antibodies for passive immunotherapy have been the most widely studied therapeutic proteins produced in transgenic plants. Though large numbers of therapeutic proteins have been produced in plants, only a few have entered clinical trials. The anti-Streptococcus mutans secretory antibody for the prevention of dental caries is the only plant-derived antibody currently in phase II clinical trials (Larrick and Thomas 2001). Based on chloroplast’s ability to form fully active and assembled proteins, a codon-optimized and humanized gene encoding a chimeric monoclonal antibody (IgA/G, Guy’s 13) under the control of a specific 5′-untranslated region, was used to synthesize monoclonal antibodies in transgenic chloroplasts. Guy’s 13 was developed to prevent dental caries, which is caused by Streptococcus mutans (Daniell and Wycoff 2001; Daniell et al. 2001a; Daniell 2004). Integration of the chimeric antibody gene into chloroplast genome was confirmed by PCR and Southern blot analysis. Western blot analysis showed the expression of heavy and light chains as well as fully assembled antibody (Fig. 14), suggesting the presence of chaperones for proper protein folding and enzymes for formation of disulfide bond in transgenic chloroplasts. However, expression levels should be enhanced further to facilitate commercialization.
Fig. 14.
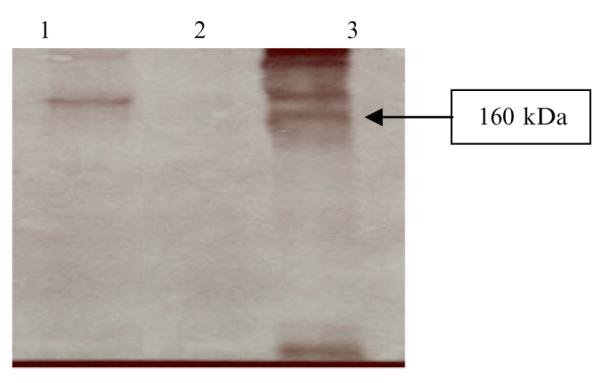
Western blot analysis of transgenic lines showing the expression of an assembled Guy’s 13 monoclonal antibody in transgenic chloroplasts. Lane 1: extract from a chloroplast transgenic line; lane 2: extract from an untransformed plant; lane 3: positive control (human IgA). The gel was run under nonreducing conditions. The antibody was detected with AP-conjugated goat anti-human kappa antibody
Conclusion
The chloroplast genetic engineering approach is ideal for economical production of vaccine antigens and biopharmaceuticals in an environmentally friendly manner. The proven functionality and the high expression levels of vaccine antigens and therapeutic proteins in transgenic chloroplasts hold the promise for unlimited quantities of production, but the cost of purification is still prohibitive. This can be overcome by the oral delivery of vaccine antigens and therapeutic proteins or by using the novel purification strategies described in this review.
Acknowledgements
Results of investigations from the Daniell laboratory are supported in part by the United States Department of Agriculture 3611–21000–017–00D and the National Institutes of Health R01 GM 63879 grants.
References
- Arlen PA, Singleton M, Adamovicz JJ, Ding Y, Davoodi-Semiromi A, Daniell H. Effective plague vaccination via oral delivery of plant cells expressing F1-V antigens in chloroplasts. Infect. Immun. 2008;76:3640–3650. doi: 10.1128/IAI.00050-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arlen PA, Falconer R, Cherukumilli S, Cole A, Cole AM, Oishi KK, Daniell H. Field production and functional evaluation of chloroplast-derived interferon-alpha2b. Plant Biotechnol. J. 2007;5:511–525. doi: 10.1111/j.1467-7652.2007.00258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baillie L. The development of new vaccines against Bacillus anthracis. J Appl Microbiol. 2001;91:609–613. doi: 10.1046/j.1365-2672.2001.01498.x. [DOI] [PubMed] [Google Scholar]
- Bogorad L. Engineering chloroplasts: an alternative site for foreign genes, proteins, reactions and products. Trends Biotechnol. 2000;18:257–263. doi: 10.1016/s0167-7799(00)01444-x. [DOI] [PubMed] [Google Scholar]
- Daniell H. Molecular strategies for gene containment in transgenic crops. Nat Biotechnol. 2002;20:581–586. doi: 10.1038/nbt0602-581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniell H. Medical Molecular Pharming. In: Goodman RM, editor. The encyclopedia of plant and crop sciences. Marcel Dekker; New York: 2004. pp. 705–710. [Google Scholar]
- Daniell H, Dhingra A. Multigene engineering: dawn of an exciting new era in biotechnology. Curr Opin Biotechnol. 2002;13:136–141. doi: 10.1016/s0958-1669(02)00297-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniell H, Wycoff K. 01–64929. WO Patent. 2001 2001.
- Daniell H, Guda C, McPherson DT, Xu J, Zhang X, Urry DW. Hyperexpression of an environmentally friendly synthetic polymer gene. Methods Mol Biol. 1997;63:359–371. doi: 10.1385/0-89603-481-X:359. [DOI] [PubMed] [Google Scholar]
- Daniell H, Dhingra A, Fernández-San Millán A. Chloroplast transgenic approach for production of antibodies, biopharmaceuticals and edible vaccines. 12th International Congress on Photosynthesis Vol. S40–04, CSIRO Publishing; Brisbane, Australia. 2001a. pp. 1–6. [Google Scholar]
- Daniell H, Lee SB, Panchal T, Wiebe P. Expression of native cholera toxin B sub-unit gene and assembly as functional oligomers in transgenic tobacco chloroplasts. J Mol Biol. 2001b;311:1001–1009. doi: 10.1006/jmbi.2001.4921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniell H, Muthukumar B, Lee SB. Marker free transgenic plants: engineering the chloroplast genome without the use of antibiotic selection. Curr Genet. 2001c;39:109–116. doi: 10.1007/s002940100185. [DOI] [PubMed] [Google Scholar]
- Daniell H, Streatfield SJ, Wycoff K. Medical molecular farming: production of antibodies, biopharmaceuticals and edible vaccines in plants. Trends Plant Sci. 2001d;6:219–226. doi: 10.1016/S1360-1385(01)01922-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniell H, Wiebe PO, Millan AF. Antibiotic-free chloroplast genetic engineering: an environmentally friendly approach. Trends Plant Sci. 2001e;6:237–239. doi: 10.1016/s1360-1385(01)01949-5. [DOI] [PubMed] [Google Scholar]
- Daniell H. Medical Molecular Pharming. In: Goodman RM, editor. The encyclopedia of plant and crop sciences. Marcel Dekker; New York: 2004. pp. 705–710. [Google Scholar]
- Daniell H, Carmona-Sanchez O, Burns B. Chloroplast derived antibodies, biopharmaceuticals and edible vaccines. In: Fischer R, Schillberg S, editors. Molecular farming. Wiley-VerlagVCH; Weinheim, Germany: 2004a. pp. 113–133. [Google Scholar]
- Daniell H, Watson J, Koya V, Leppla SH. Expression of Bacillus anthracis protective antigen in transgenic chloroplasts of tobacco, a non-food/feed crop. Vaccine. 2004b;22:4374–4384. doi: 10.1016/j.vaccine.2004.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Cosa B, Moar W, Lee SB, Miller M, Daniell H. Over expression of the cry2Aa2 operon in chloroplasts leads to formation of insecticidal crystals. Nat Biotechnol. 2001;19:71–74. doi: 10.1038/83559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGray G, Rajasekaran K, Smith F, Sanford J, Daniell H. Expression of an antimicrobial peptide via the chloroplast genome to control phytopathogenic bacteria and fungi. Plant Physiol. 2001;127:852–862. [PMC free article] [PubMed] [Google Scholar]
- Dhingra A, Portis AR, Daniell H. Enhanced translation of a chloroplast-expressed RbcS gene restores small subunit levels and photosynthesis in nuclear RbcS antisense plants. Proc Natl Acad Sci U S A. 2004;101:6315–6320. doi: 10.1073/pnas.0400981101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elderbaum O, Stein D, Holland N, Gafni Y, Livneh O, Novick D, Rubinstein M, Sele I. Expression of active human interferon beta in transgenic plants. J Interferon Res. 1992;12:449–453. doi: 10.1089/jir.1992.12.449. [DOI] [PubMed] [Google Scholar]
- Falconer R. M.S. thesis. University of Central Florida; 2002. Expression of Interferon alpha 2b in transgenic chloroplasts of a low-nicotine tobacco. [Google Scholar]
- Fernández-San Millán A, Mingo-Castel A, Miller M, Daniell H. A chloroplast transgenic approach to hyper-express and purify Human Serum Albumin, a protein highly susceptible to proteolytic degradation. Plant Biotech J. 2003;1:71–79. doi: 10.1046/j.1467-7652.2003.00008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa-Soto CG, Guillermo LC, Valenzuela-Soto EM. Immunolocalization of betaine aldehyde dehydrogenase in porcine kidneys. Biochem Biophys Res Commun. 1999;258:732–736. doi: 10.1006/bbrc.1999.0584. [DOI] [PubMed] [Google Scholar]
- Giddings G, Allison G, Brooks D, Carter A. Transgenic plants as factories for biopharmaceuticals. Nat Biotechnol. 2000;18:1151–1155. doi: 10.1038/81132. [DOI] [PubMed] [Google Scholar]
- Gomez N, Carrillo C, Salinas J, Parra F, Borca MV, Escribano JM. Expression of immunogenic glycoprotein S polypeptides from transmissible gastroenteritis corona virus in transgenic plants. Virology. 1998;249:352–358. doi: 10.1006/viro.1998.9315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guda C, Lee SB, Daniell H. Stable expression of a protein based polymer in tobacco chloroplasts. Plant Cell Rep. 2000;19:257–262. doi: 10.1007/s002990050008. [DOI] [PubMed] [Google Scholar]
- Haq TA, Mason HS, Clements JD, Arntzen CJ. Oral immunization with a recombinant bacterial antigen produced in transgenic plants. Science. 1995;268:714–716. doi: 10.1126/science.7732379. [DOI] [PubMed] [Google Scholar]
- Iatham S, Day A. Removal of antibiotic resistance genes from transgenic tobacco plastids. Nat Biotechnol. 2000;18:1172–1176. doi: 10.1038/81161. [DOI] [PubMed] [Google Scholar]
- Ivins B, Fellows P, Pitt L, Estep J, Farchaus J, Friedlander A, et al. Experimental anthrax vaccines: efficacy of adjuvants combined with protective antigen against an aerosol Bacillus anthracis spore challenge in guinea pigs. Vaccine. 1995;13:1779–1783. doi: 10.1016/0264-410x(95)00139-r. [DOI] [PubMed] [Google Scholar]
- Jacob L, Zasloff M. Potential therapeutic applications of magainins and other microbial; agents animal origin: antimicrobial peptides. Ciba Found Symp. 1994;186:197–223. doi: 10.1002/9780470514658.ch12. [DOI] [PubMed] [Google Scholar]
- Joellenback LM, Zwanziger LL, Durch JS, Strom BL, editors. Is it safe? Does it work? National Academy; Washington, DC: 2002. “Anthrax vaccine manufacture” in the anthrax vaccine; pp. 180–197. [PubMed] [Google Scholar]
- Kaufmann AF, Meltzer MI, Schmid GP. The economic impact of a bioterrorist attack: are prevention and post attack intervention programs justifiable? Emerg Infect Dis. 1997;3:83–94. doi: 10.3201/eid0302.970201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaus SM, Huang FC, Golds TJ, Koop HU. Generation of marker-free plastid transformants using a transiently cointegrated selection gene. Nat Biotechnol. 2004;22:225. doi: 10.1038/nbt933. [DOI] [PubMed] [Google Scholar]
- Kumar S, Dhingra A, Daniell H. Plastid-expressed betaine aldehyde dehydrogenase gene in carrot cultured cells, roots, and leaves confers enhanced salt tolerance. Plant Physiol. 2004;136:2843–2854. doi: 10.1104/pp.104.045187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langeveld JP, Casal JI, Osterhaus AD, Cortes E, de Swart R, Vela C, Dalsgaard K, Puijk WC, Schaaper WM, Meloen RH. First peptide vaccine providing protection against viral infection in the target animal: studies of canine parvovirus in dogs. J Virol. 1994;68:4506–4513. doi: 10.1128/jvi.68.7.4506-4513.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langeveld JP, Kamstrup S, Uttenthal A, Strandbygaard B, Vela C, Dalsgaard K, Beekman NJ, Meloen RH, Casal JI. Full protection in mink against enteritis virus with new generation canine parvovirus vaccines based on synthetic peptide or recombinant protein. Vaccine. 1995;13:1033–1037. doi: 10.1016/0264-410x(95)00021-r. [DOI] [PubMed] [Google Scholar]
- Larrick JW, Thomas DW. Producing protein in transgenic plants and animals. Curr Opin Biotechnol. 2001;12:411–418. doi: 10.1016/s0958-1669(00)00236-6. [DOI] [PubMed] [Google Scholar]
- Lee SB, Kwon HB, Kwon SJ, Park SC, Jeong MJ, Han SE, Byun MO, Daniell H. Accumulation of trehalose within transgenic chloroplasts confers drought tolerance. Mol Breed. 2003;11:1–13. [Google Scholar]
- Leelavathi S, Reddy VS. Chloroplast expression of His-tagged GUS-fusions: a general strategy to overproduce and purify foreign proteins using transplastomic plants as bioreactors. Mol Breed. 2003;11:49–58. [Google Scholar]
- Mason HS, Lam D, Arntzen CJ. Expression of hepatitis B surface antigen in transgenic plants. Proc Natl Acad Sci U S A. 1992;89:11745–11749. doi: 10.1073/pnas.89.24.11745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason HS, Haq TA, Clements JD, Arntzen CJ. Edible vaccine protects mice against Escherichia coli heat-labile enterotoxin (LT): potatoes expressing a synthetic LT-B gene. Vaccine. 1998;16:1336–1343. doi: 10.1016/s0264-410x(98)80020-0. [DOI] [PubMed] [Google Scholar]
- Molina A, Hervas-Stubbs S, Daniell H, Mingo-Castel AM, Veramendi J. High yield expression of a viral peptide animal vaccine in transgenic tobacco chloroplasts. Plant Biotechnol. 2004;2:141–153. doi: 10.1046/j.1467-7652.2004.00057.x. [DOI] [PubMed] [Google Scholar]
- Ruiz G. MS thesis. University of Central Florida; 2002. Optimization of codon composition and regulatory elements for expression of the human IGF-1 in transgenic chloroplasts. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton ML. MS thesis. University of Central Florida; 2003. Expression of CaF1 and LcrV as a fusion protein for a vaccine against Yersinia pestis via chloroplast genetic engineering. [Google Scholar]
- Tackaberry E, Dudani A, Prior F, Tocchi M, Sardana R, Altosaar I, Ganz PR. Development of biopharmaceuticals in plant expression systems: cloning, expression and immunological reactivity of human cytomegalovirus glycoprotein B (UL55) in seeds of transgenic tobacco. Vaccine. 1999;17:3020–3029. doi: 10.1016/s0264-410x(99)00150-4. [DOI] [PubMed] [Google Scholar]
- Thanavala Y, Yang Y, Lyon P, Mason HS, Arntzen C. Immunogenicity of transgenic plant-derived hepatitis B surface antigen. Proc Natl Acad Sci U S A. 1995;92:3358–3361. doi: 10.1073/pnas.92.8.3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titball RW, Williamson ED. Vaccination against bubonic and pneumonic plague. Vaccine. 2001;19:4175–4184. doi: 10.1016/s0264-410x(01)00163-3. [DOI] [PubMed] [Google Scholar]
- Torrado J, Carrascosa C. Pharmacological characteristics of parenteral IGF-I administration. Curr Pharm Biotechnol. 2003;4:123–140. doi: 10.2174/1389201033489865. [DOI] [PubMed] [Google Scholar]
- Urry DW, McPherson DT, Xu J, Daniell H, Guda C, Gowda DC, Jing N, Parker TM. Protein based polymeric materials: synthesis and properties. In: Salamone JC, editor. The polymeric materials encyclopedia: synthesis, properties and applications. CRC Press; Boca Raton, FL: 1996. pp. 2645–2699. [Google Scholar]
- Vivek BS, Ngo QA, Simon PW. Evidence for maternal inheritance of the chloroplast genome in cultivated carrot (Daucus carota L. ssp. sativus) Theor Appl Genet. 1999;98:669–672. [Google Scholar]
- Williamson ED, Eley SM, Stagg AJ, Green M, Russell P, Titball RW. A sub-unit vaccine elicits IgG in serum, spleen cell cultures and bronchial washings and protects immunized animals against plague. Vaccine. 1997;15:1079–1084. doi: 10.1016/s0264-410x(96)00303-9. [DOI] [PubMed] [Google Scholar]
- Zasloff M. Magainins, a class of antimicrobial peptides from Xenopus skin: isolation, characterization of two active forms, and partial cDNA sequence of a precursor. Proc Natl Acad Sci U S A. 1987;84:5449–5953. doi: 10.1073/pnas.84.15.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Liu Y, Sodmergen Examination of the cytoplasmic DNA in male reproductive cells to determine the potential for cytoplasmic inheritance in 295 angiosperm species. Plant Cell Physiol. 2003;44:941–951. doi: 10.1093/pcp/pcg121. [DOI] [PubMed] [Google Scholar]