Abstract
Introduction:
This study investigates whether the middle frontal gyrus (MFG) can be used as an indicator for language hemispheric dominance in brain tumor patients using task-free resting state fMRI (rsfMRI). We hypothesize no significant difference in language lateralization between the MFG and Broca’s Area (BA) and that the MFG can serve as a simple and reliable means of measuring language laterality.
Methods:
51 patients with glial neoplasms. Using rsfMRI, the MFG was compared to BA for voxel activation, language laterality index (LI) and the effect of tumor grade on LI. LI derived by rsfMRI and task-based fMRI was compared in a subset of 40 patients.
Results:
Voxel activations in the left MFG and left BA were positively correlated (r = 0.47, p<0.001). Positive correlations were seen between the LI of BA and LI of MFG regions (r = 0.56, p < 0.0005). 27/40 patients (67.5%) showed concordance of LI based on BA using rsfMRI with LI based on a language task. 30/40 patients (75%) showed concordance of LI based on the MFG using rsfMRI with LI based on a language task.
Conclusion:
MFG is comparable to BA in its ability to determine hemispheric dominance for language using rsfMRI. Our results suggest the addition of rsfMRI of MFG to the list of noninvasive modalities that could be used in glioma patients to evaluate hemispheric dominance of language prior to tumor resection. In patients who cannot participate in traditional task-based fMRI, rsfMRI offers a task-free alternate to presurgically map eloquent cortex.
Introduction
The determination of hemispheric language dominance is a critical part of presurgical evaluation of patients with brain tumors. Studies have shown blood-oxygen-level-dependent task-based functional MRI (fMRI) to be an excellent noninvasive alternative to the intracarotid amobarbitol procedure or WADA test1–4. However, the usefulness and reliability of task-based fMRI is limited in cognitively impaired patients, in pediatric patients and in patients with language barriers in whom task completion poses a major challenge. In order to overcome this limitation, resting state fMRI (rsfMRI) is emerging as an alternative paradigm-free extraction of brain networks, including language networks, using low-frequency fluctuations in BOLD signal5.
Wernicke’s area and Broca’s are (BA) are considered primary language centers, and activations in these regions are commonly evaluated for language laterality1,6,7. However, the presence of abnormal tumor neovasculature and resultant neurovasculature uncoupling, can contribute to false negative signals from the disruption and subsequent diversion of BOLD signals ipsilateral to the tumor8,9. To overcome this problem, studies are investigating the utilization of secondary language areas supplementary to, or as substitutes of, primary language areas to declare hemispheric dominance10. The middle frontal gyrus (MFG) is one of the secondary language areas implicated in nuances of language expression such as semantics, grammar and syntax, verbal fluency and verbal working memory among other cognitive functions, including attention orientation11–19. Prior research has noted that the MFG consistently activates during fMRI language tasks4,20,21 and a recent study using task-based fMRI demonstrated MFG is comparable to BA in its ability to indicate hemispheric dominance for language using a measure of verbal fluency18.
To the best of our knowledge, no previous study has utilized rsfMRI to estimate language laterality using the MFG. The purpose of this study, therefore, is to investigate whether the MFG can be used as an indicator for language hemispheric dominance in brain tumor patients using paradigm free resting state fMRI. We hypothesize no significant difference in language lateralization between the MFG and BA and that the MFG can serve as a simple and reliable means of measuring language laterality.
Methods:
Subjects
The study was approved by the Institutional Review Board. Fifty-one patients with brain tumors (age range: 22 to 78 years, mean ± SD = 51 ± 14.2 years; 31 males) referred for presurgical functional mapping by functional Magnetic Resonance Imaging (fMRI) were included in this retrospective study. All patients were native English speakers and had no preexisting language impairment per chart review. Handedness was determined by the Edinburgh Handedness Inventory (Oldfield, 1971) with 47 patients determined to be right-handed and 4 determined to be left handed. All patients had subsequently pathologically-confirmed intra-axial primary glial tumors. Pathology revealed 20 low-grade (WHO I and II) and 31 high-grade (WHO III and IV) tumors. In 28 patients, the lesion was located in the left hemisphere (7 in the temporal lobe, 15 in the frontal and 6 in the parietal lobe). In 23 patients, the lesion was located in the right hemisphere (5 in the temporal lobe, 17 in the frontal and 1 in the parietal lobe).
Data Acquisition
Each patient received resting state fMRI scan as part of the routine presurgical workup. Scanning was performed on 3-T scanners (GE Healthcase, Milwaukee, Wisconsin) using an eight-channel head coil. For task based and rsfMRI, T2*-weighted images were acquired with a single-shot gradient echo echo-planar imaging (EPI) sequence in the axial orientation (repetition time/echo time (TR/TE) = 2500/30 ms, flip angle = 80°, slice thickness = 4 mm, field-of-view (FOV) = 240 mm2, matrix = 64×64) covering the whole brain. Three-dimensional T1-weighted images were acquired with a spoiled gradient recalled sequence (TR/TE = 22/4 ms, matrix = 256 × 256 matrix, flip angle = 30°, slice thickness = 1.5 mm). For the resting state fMRI scan, patients were instructed to leave their eyes open, focus on looking at a crosshair, and not think about anything during the scan. A total of 160 volumes were acquired. Of the 51 patients included in the study, 40 also performed a silent word generation task in the same session as part of presurgical language mapping. The language tasks were used to determine language laterality (right dominant, left dominant or bihemispheric dominance) using methodology previously used in Dong et al. and was documented in the patients final presurgical mapping report18.
Resting state fMRI data analysis
In the current study, we implemented a data processing scheme as outlined in Gohel et. al.22. Data processing was based on SPM12 (http://www.fil.ion.ucl.ac.uk/spm/software/spm12/). In the first step, we discarded the first five time-points from the functional MRI data to allow for T1relaxation. Next head motion correction was performed to reduce the effect of within scan head motion. During head motion correction, we extracted the motion parameters, which describe the subject motion in six different directions. Following motion correction, each of the subject’s fMRI data was coregistered with the subject specific anatomical images to improve normalization into MNI space. Following coregistration, we segmented each subject’s anatomical images into grey matter, white matter and cerebrospinal fluid images. During the segmentation procedure, deformation fields were derived to transfer functional images into MNI standard space. We performed segmentation using the new segment procedures in SPM12. Each subject’s segmentation maps were manually inspected to ensure successful segmentation.
Finally, we used this subject specific deformation field to transform the functional images into standard space images. In the next step, we implemented a linear regression to remove the effects of motion related noises from the BOLD fMRI data. A general linear model based regression approach was implemented using a total of 24 motion regressors. This consisted of 6 motion parameters derived in the motion correction step, 6 squared of the original motion parameters, 6 one-time-points delayed version of the motion parameters and finally 6 squared of the delayed motion parameters. No regressors from cerebrospinal fluid or white matter region were included in the regression model. Following regression, residual data was derived and a temporal filtering between the frequency bands of 0.01–0.1 Hz was applied. Finally, 6 mm spatial smoothing was applied to the filtered fMRI data.
Spatial extent of MFG and BA
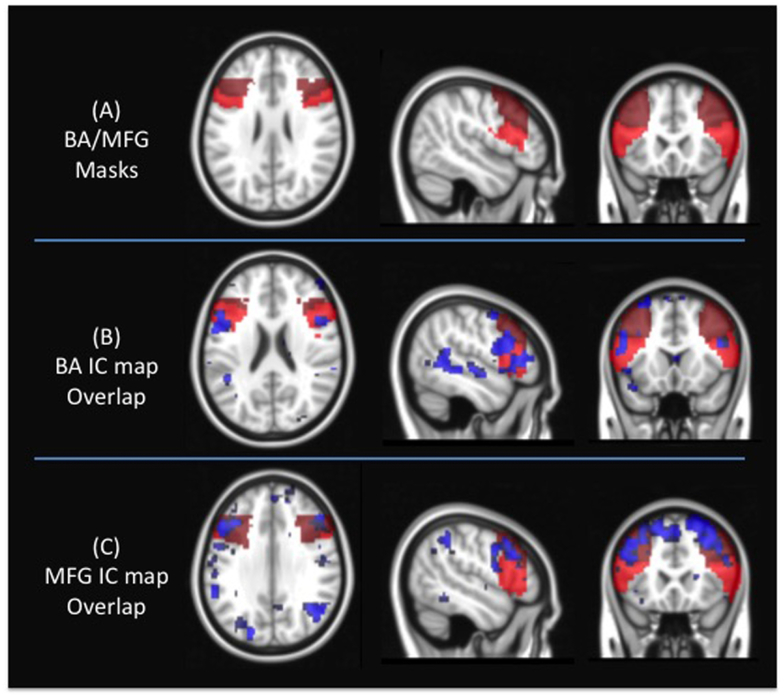
In order to identify active voxels in the BA and MFG regions, we implemented an independent component analysis (ICA) based approach. In the first step, we performed a single subject ICA on the filtered fMRI data. For each of the subjects, we performed a separate ICA and extracted 40 independent components. In order to identify ICs representing MFG and BA, we implemented a two-step process. First, we calculated DICE coefficients between each of the independent components and the BA and MFG mask derived from Harvard Oxford Atlas23,24. Next, we identified the top 5 independent components with the highest DICE coefficient. Each of these five independent components was then manually inspected using consensus viewing by two neuroradiologists with 4 to 15 years of fMRI experience to identify independent components representing the functional connectivity of MFG and BA. After identifying these components, active voxels within the left-MFG was calculated as the overlap between the independent component representing MFG and the MFG region derived through Harvard-Oxford atlas. The same process was repeated for the right MFG, as well as the left and right BA to calculate the active voxels for each of the ROIs. Figure 1 illustrates the masks for BA and MFF regions, as well as independent components from a representative patient. The mean activated voxels in the MFG and BA were calculated for each patient. In order to account for differences in the size of MFG and BA masks, we divided active voxels by the number of voxels in the Harvard-Oxford atlas. We compared the ratio of active voxels using a paired t-test. The possible relationship of voxel count between the left MFG and the left BA was displayed using a scatterplot, and correlation was tested using the Pearson correlation analysis. For all statistical analyses, a significance level of 0.001 was used.
Figure 1.
(A) Broca’s Area (BA) and middle frontal gyrus (MFG) masks overlaid on the MNI standard brain (B) IC maps representing BA network overlaid on the BA/MFG masks for a representative subject 42, (C) IC maps representing MFG network overlaid on the BA/MFG masks for a representative subject.
Laterality index in MFG and BA
The laterality index (LI) for MFG and BA was calculated using the standard LI formula2,7,25: LI = L-R/L+R, where L and R are the number of active voxels in given ROIs (MFG and BA) in the left and right hemispheres respectively. The LI ranged from −1 (complete right dominance) to +1 (complete left dominance). Consistent with prior studies26–28, we defined right hemisphere laterality as −1 ≤ LI < −0.2, bilaterality as −0.2 ≤ LI ≤ 0.2, and left hemispheric language laterality as 0.2 < LI ≤1.
In addition, we also calculated functional connectivity between the MFG and BA ROIs. For each of the subjects, filtered fMRI signal was extracted from the active voxels. Pearson’s correlation coefficient was calculated between these time-series to derive functional connectivity between MFG and BA ROIs.
Effect of tumor grade and location on LI
Tumors in each of the left and right hemispheres were categorized into those with high-grade (WHO grades III and IV) or low-grade (WHO grades I and II) tumors. For each hemisphere, LIs of BA and MFG were compared between the high-grade versus low-grade tumor groups. Differences in LI of BA and MFG between tumors in the left versus right hemisphere were also compared. LI differences were assessed using two-sample t-test with significance level set at < 0.05.
Comparison of LI between rsfMRI and task-based fMRI
Forty of the 51 patients included in this study had both rsfMRI and language task-based data obtained in the same session as part of presurgical language mapping. For each of these 40 patients LI of MFG and BA based on rsfMRI was compared with LI based on task based fMRI, such that a match (e.g. left versus left) was scored as 100% accurate, while a mismatch (e.g. left versus right) was scored as 0% accurate.
Results:
Spatial extent of MFG and BA
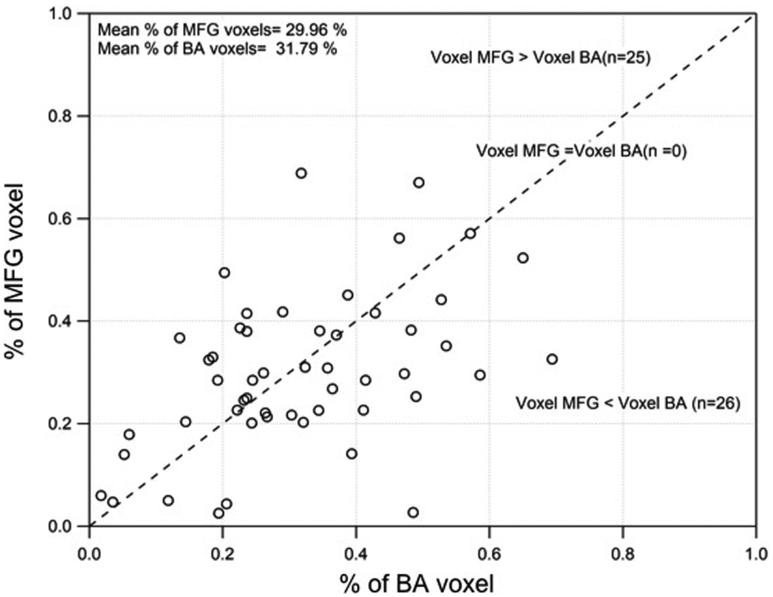
In the first step, we compared the number of active voxels between the left BA and left MFG regions. The scatterplot between the number of active-voxels for left BA and the left middle frontal gyrus is displayed in Figure 2. We observed significant correlation for the number of active voxels between the left MFG and left BA (r = 0.47, p<0.001). The number of active voxels was found to be significantly higher in MFG regions (376.92) compared to the BA region (218.09); however, when correcting for the size of the MFG and BA masks, these differences were not statistically significant. Similarly, significantly higher number of voxels was found in the right MFG region (218.09) compared to the right BA (99.35); however these differences were also not significant when correcting for the size of the MFG and BA masks. (Figure 2).
Figure 2.
Scatter plot between percentage of Broca’s Area (BA) voxels and percentage of middle frontal gyrus (MFG) voxels across participants. Significant correlation was observed between percentage of MFG and percentage of BA voxels across patients
Laterality index in MFG and BA
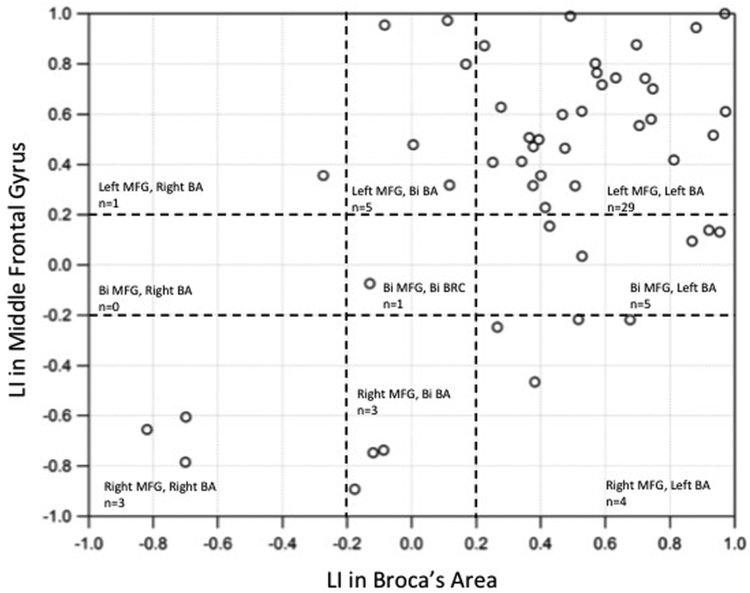
Based on the values for number of active voxels, we calculated laterality indices for both MFG and BA regions. Figure 3 displays the LI for each of the subjects calculated using these active voxels. We observed significant correlation (r = 0.56, p < 0.0005) between the LI of BA and LI of MFG regions. As is evident from Figure 3, a large number of patients were identified as left lateralized for both MFG and BA while only 3 patients were found to be right lateralized in both the groups.
Figure 3.
Scatterplot of LIs in middle frontal gyrus (MFG) versus Broca’s Area (BA). Significant positive correlation was observed between LI of MFG and BA regions.
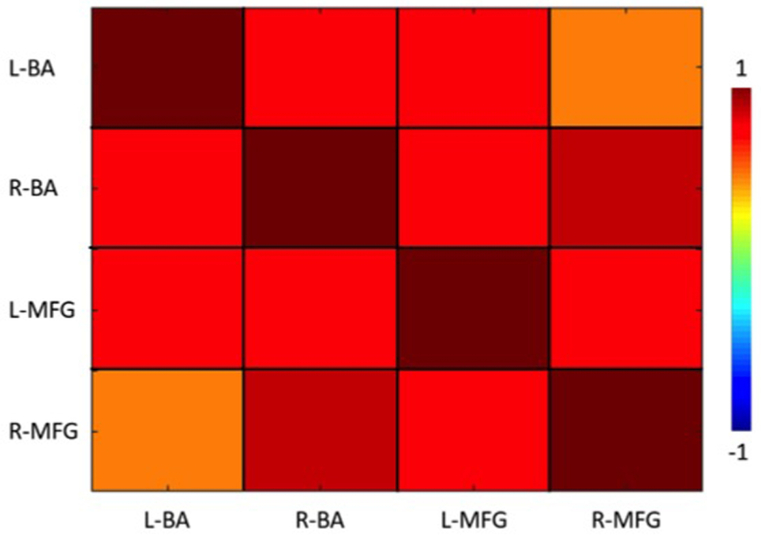
We observed significantly higher functional connectivity between left and right BAs with the left MFG, while the functional connectivity between the left BA regions and the right MFG was the lowest. Significantly high functional connectivity between right BA regions and right MFG was noted. Figure 4 depicts group level functional connectivity between the BA and the MFG regions.
Figure 4.
Group level functional connectivity between bilateral Broca’s Area (BA) and middle frontal gyrus (MFG) regions across patients. Positive functional connectivity was observed between bilateral BA and bilateral MFG regions.
Effect of tumor grade and location on LI
In patients with tumors in the left hemisphere, the mean LI in the MFG was 0.06 ± 0.50 for lowgrade tumors and 0.26 ± 0.52 for high-grade tumors (p = 0.3577, two sample t-test) while the mean LI in the BA was 0.26 ± 0.45 for low-grade tumors and 0.30 ± 0.47 for high-grade tumors (p = 0.8383, two sample t-test). For right hemisphere tumors, the mean LI in MFG was 0.38 ± 0.60 for low grade tumors and 0.58 ± 0.33 for high grade tumors (p = 0.3353, two sample t-test), while the mean LI in BA was 0.56 ± 0.37 for low grade tumors and 0.43 ± 0.35 for high grade tumors (p = 0.3917, two sample t-test).
Tumor location in the left versus right hemisphere regardless of grade did not significantly contribute to LI in both BA and MFG. The mean LI in the BA for left hemisphere tumors was 0.29 ± 0.46 and 0.49 ± 0.36 for right hemisphere tumors (p = 0.09, two sample t-test). The mean LI in the MFG for left hemisphere tumors was 0.19 ± 0.52 and 0.48 ± 0.49 for right hemisphere tumors (p = 0.04, two sample t-test). However, tumor location in the left hemisphere (frontal versus non-frontal) contributed to differences in the LI in BA and MFG. The mean LI in BA for left frontal tumors was 0.12 ± 0.53 and 0.47 ± 0.28 for left non-frontal tumors (p = 0.0472, two sample t-test). The mean LI of MFG for left frontal tumors was −0.02 ± 0.49 and 0.44 ± 0.42 for left non-frontal tumors (p = 0.0113, two sample t-test). Tumor location in the right hemisphere (frontal versus non-frontal) also contributed to differences in the LI in BA but not in MFG. The mean LI in BA for right frontal tumors was 0.58 ± 0.26 and 0.14 ± 0.47 for right non-frontal tumors (p = 0.0119, two sample t-test). The mean LI in MFG for right frontal tumors was 0.56 ± 0.37 and 0.18 ± 0.74 for right non-frontal tumors (p = 0.12, two sample t-test).
Comparison of LI between rsfMRI and task-based fMRI
In order to derive reliability of the laterality index derived through resting state fMRI, we directly compared it with the task-based laterality index. We observed overlap between the laterality index derived by rsfMRI and task-based fMRI. Specifically when we derived the LI based on BA using rsfMRI, 27/40 patients (67.5%) showed concordance with laterality index based on a silent word generation task. Similarly when we derived the LI using the MFG using rsfMRI, 30/40 patients (75%) showed concordance with laterality index based on a silent word generation task.
Discussion
This study is the first to demonstrate that the MFG is a reliable means of lateralizing language networks using rsfMRI in patients with brain tumors. Our study demonstrated that voxel activation in the MFG correlated to that in BA. Similarly, the LIs in MFG correlated to LIs in BA such that the greater the LI was in BA, the greater it was in the MFG. While the MFG had slightly higher average LI than BA, we found no statistically significant differences in language lateralization between MFG and BA. Importantly, LIs calculated from rsfMRI showed significant overlap with LI determined from task-based fMRI.
Although the MFG is a well-known secondary language area29–31research characterizing its exact role in presurgical mapping is limited11,12,14,32,33. In a study of patients with temporal lobe epilepsy, Lehericy et. Al. demonstrated that asymmetric activation in the MFG correlated with hemispheric dominance determination based on WADA testing4. Task-based fMRI has shown similar activation patterns of MFG in brain tumor patients who were provided language tasks20,21. Results of our study support these prior findings and suggest that the MFG can be used as an additional indicator in determining language hemispheric dominance in clinically difficult cases where a brain tumor could result in false negative activation in the BA.
In contrast to prior studies that used task-based paradigms to study the MFG, this study provided a comparative measure of MFG’s utility relative to BA in determining language lateralization using task-free resting state fMRI. In those patients with significant physical or cognitive deficits, performing a task can be challenging; rsfMRI has risen as a promising alternate. This study adds to the growing body of evidence that suggest that rsfMRI can be potentially useful for presurgical mapping of eloquent cortices5,34–42. Further, in contrast to prior studies that have used a priori seed-based methods for pre-surgical mapping of language functions with rsfMRI, this study uses a model-free ICA approach that avoided some of the pitfalls of seed-based analysis including subjective expertise in seed placement.
Our study found no significant effects of tumor grade on language lateralization. However, not unexpectedly tumor location contributed to language lateralization such that patients with left hemisphere tumors that were located in the frontal region had lower lateralization compared to non-frontal left hemisphere tumors. Previous studies have suggested that tumor neovasculature diminished fMRI activation in the tumor hemisphere8,43,44 and consequently affected the fMRI determination of true lateralization for language in brain tumor patients. Our findings are consistent with previous literature that demonstrated that right handed patients with neoplasms affecting language areas in the left hemisphere had lower LIs compared to normal controls45.
There are limitations to this study that merit further research. First, the sample size was small especially in terms of patients with atypical or right-sided language laterality. Second, although we excluded patients with prior surgery from this study, we acknowledge that this method may not be generalizable to post-surgical patients or patients with major structural lesions that may affect consistency of standardized anatomic templates. Third we used a modelfree ICA approach; specific parameters such as number of ICA components could have influenced the results of ICA-based functional MR imaging analyses.
Prior to surgery, it is important to map the eloquent areas close to a tumor to avoid damaging those areas. Generally, DCS has been the method of choice for making this assessment, but it is limited to detecting mainly the cortical surface areas. Moreover, DCS determination of language hemispheric dominance is not perfect. There is no one method that can provide a completely accurate lateralization of language. Therefore, multiple modalities must be utilized in order to determine lateralization of language with much accuracy and certainty as possible. This is especially important in cases where lateralization based on BA can be misleading and therefore additional markers are needed. Our results suggest the addition of rsfMRI of MFG to the list of non-invasive modalities that could be used in glioma patients to evaluate hemispheric dominance of language prior to tumor resection.
Conclusion
Activation in MFG parallels that in BA in non-task based rsfMRI assessing hemispheric dominance of language. Task-based and rsfMRI comparisons of Broca’s and MFG are similar. Therefore, clinical utilization of rsfMRI for language lateralization, specifically by assessing MFG activity, should be considered in brain tumor patients.
Acknowledgments
This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748. Research reported in this study was also supported by the National Cancer Institute of the National Institutes of Health under Award Number R25CA020449 and in part by a grant to (PI: MEL) from the Department of Radiology at Memorial Sloan Kettering Cancer Center for an ESOR Visiting Scholarship in Oncologic Imaging. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Health.
References:
- 1.Janecek JK, Swanson SJ, Sabsevitz DS, et al. Language lateralization by fMRI and Wada testing in 229 patients with epilepsy: rates and predictors of discordance. Epilepsia. 2013;54(2):314–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Desmond JE, Sum JM, Wagner AD, et al. Functional MRI measurement of language lateralization in Wada-tested patients. Brain. 1995;118 (Pt 6):1411–1419. [DOI] [PubMed] [Google Scholar]
- 3.Dym RJ, Burns J, Freeman K, Lipton ML. Is functional MR imaging assessment of hemispheric language dominance as good as the Wada test?: a meta-analysis. Radiology. 2011;261:446–455. [DOI] [PubMed] [Google Scholar]
- 4.Lehericy S, Cohen L, Bazin B, et al. Functional MR evaluation of temporal and frontal language dominance compared with the Wada test. Neurology. 2000;54:1625–1633. [DOI] [PubMed] [Google Scholar]
- 5.Tie Y, Rigolo L, Norton IH, et al. Defining language networks from resting-state fMRI for surgical planning--a feasibility study. Hum Brain Mapp. 2014;35(3):1018–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frost JA, Binder JR, Springer JA, et al. Language processing is strongly left lateralized in both sexes. Evidence from functional MRI. Brain. 1999;122 (Pt 2):199–208. [DOI] [PubMed] [Google Scholar]
- 7.Szaflarski JP, Binder JR, Possing ET, McKiernan KA, Ward BD, Hammeke TA. Language lateralization in left-handed and ambidextrous people fMRI data. Neurology. 2002;59:238–244. [DOI] [PubMed] [Google Scholar]
- 8.Ulmer JL, Hacein-Bey L, Mathews VP, et al. Lesion-induced pseudo-dominance at functional magnetic resonance imaging: implications for preoperative assessments. Neurosurgery. 2004;55(3):569–579; discussion 580–561. [DOI] [PubMed] [Google Scholar]
- 9.Hou BL, Bradbury M, Peck KK, Petrovich NM, Gutin PH, Holodny AI. Effect of brain tumor neovasculature defined by rCBV on BOLD fMRI activation volume in the primary motor cortex. Neuroimage. 2006;32(2):489–497. [DOI] [PubMed] [Google Scholar]
- 10.Sanai N, Mirzadeh Z, Berger MS. Functional outcome after language mapping for glioma resection. N Engl J Med. 2008;358(1):18–27. [DOI] [PubMed] [Google Scholar]
- 11.Brown S, Martinez MJ, Parsons LM. Music and language side by side in the brain: a PET study of the generation of melodies and sentences. Eur J Neurosci. 2006;23(10):27912803. [DOI] [PubMed] [Google Scholar]
- 12.Wang S, Zhu Z, Zhang JX, et al. BA plays a role in syntactic processing during Chinese reading comprehension. Neuropsychologia. 2008;46(5):1371–1378. [DOI] [PubMed] [Google Scholar]
- 13.Abrahams S, Goldstein LH, Simmons A, et al. Functional magnetic resonance imaging of verbal fluency and confrontation naming using compressed image acquisition to permit overt responses. Hum Brain Mapp. 2003;20(1):29–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leung HC, Gore JC, Goldman-Rakic PS. Sustained mnemonic response in the human middle frontal gyrus during on-line storage of spatial memoranda. J Cogn Neurosci. 2002;14(4):659–671. [DOI] [PubMed] [Google Scholar]
- 15.Smolker HR, Depue BE, Reineberg AE, Orr JM, Banich MT. Individual differences in regional prefrontal gray matter morphometry and fractional anisotropy are associated with different constructs of executive function. Brain Struct Funct. 2015;220(3):12911306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steinmann E, Schmalor A, Prehn-Kristensen A, et al. Developmental changes of neuronal networks associated with strategic social decision-making. Neuropsychologia. 2014;56:37–46. [DOI] [PubMed] [Google Scholar]
- 17.Brennan NP, Peck KK, Holodny A. Language Mapping Using fMRI and Direct Cortical Stimulation for Brain Tumor Surgery: The Good, the Bad, and the Questionable. Top Magn Reson Imaging. 2016;25(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dong JW, Brennan NM, Izzo G, Peck KK, Holodny AI. fMRI activation in the middle frontal gyrus as an indicator of hemispheric dominance for language in brain tumor patients: a comparison with BA. Neuroradiology. 2016;58(5):513–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Japee S, Holiday K, Satyshur MD, Mukai I, Ungerleider LG. A role of right middle frontal gyrus in reorienting of attention: a case study. Front Syst Neurosci. 2015;9:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roux FE, Boulanouar K, Lotterie JA, Mejdoubi M, LeSage JP, Berry I. Language functional magnetic resonance imaging in preoperative assessment of language areas: correlation with direct cortical stimulation. Neurosurgery. 2003;52(6):1335–1345; discussion 1345–1337. [DOI] [PubMed] [Google Scholar]
- 21.Kamada K, Sawamura Y, Takeuchi F, et al. Expressive and receptive language areas determined by a non-invasive reliable method using functional magnetic resonance imaging and magnetoencephalography. Neurosurgery. 2007;60(2):296–305; discussion 305–296. [DOI] [PubMed] [Google Scholar]
- 22.Bharath RD, Munivenkatappa A, Gohel S, et al. Recovery of resting brain connectivity ensuing mild traumatic brain injury. Front Hum Neurosci. 2015;9:513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taylor PA, Gohel S, Di X, Walter M, Biswal BB. Functional covariance networks: obtaining resting-state networks from intersubject variability. Brain Connect. 2012;2(4):203–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taylor PA, Saad ZS. FATCAT: (an efficient) Functional and Tractographic Connectivity Analysis Toolbox. Brain Connect. 2013;3(5):523–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Binder JR, Sabsevitz DS, Swanson SJ, Hammeke TA, Raghavan M, Mueller WM. Use of preoperative functional MRI to predict verbal memory decline after temporal lobe epilepsy surgery. Epilepsia. 2008;49(8):1377–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seghier ML. Laterality index in functional MRI: methodological issues. Magn Reson Imaging. 2008;26(5):594–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deblaere K, Boon PA, Vandemaele P, et al. MRI language dominance assessment in epilepsy patients at 1.0 T: region of interest analysis and comparison with intracarotid amytal testing. Neuroradiology. 2004;46(6):413–420. [DOI] [PubMed] [Google Scholar]
- 28.Springer JA, Binder JR, Hammeke TA, et al. Language dominance in neurologically normal and epilepsy subjects: a functional MRI study. Brain. 1999;122(Pt 11):20332046. [DOI] [PubMed] [Google Scholar]
- 29.Rogalski E, Cobia D, Harrison TM, et al. Anatomy of language impairments in primary progressive aphasia. J Neurosci. 2011;31(9):3344–3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fertonani A, Rosini S, Cotelli M, Rossini PM, Miniussi C. Naming facilitation induced by transcranial direct current stimulation. Behav Brain Res. 2010;208(2):311–318. [DOI] [PubMed] [Google Scholar]
- 31.Grossman M, Cooke A, DeVita C, et al. Age-related changes in working memory during sentence comprehension: an fMRI study. Neuroimage. 2002;15(2):302–317. [DOI] [PubMed] [Google Scholar]
- 32.Acheson DJ, MacDonald MC. Verbal working memory and language production: Common approaches to the serial ordering of verbal information. Psychol Bull. 2009;135(1):50–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baddeley A Working memory and language: an overview. Journal of Communication Disorders. 2003;36(3):189–208. [DOI] [PubMed] [Google Scholar]
- 34.DeSalvo MN, Douw L, Takaya S, Liu H, Stufflebeam SM. Task-dependent reorganization of functional connectivity networks during visual semantic decision making. Brain Behav. 2014;4(6):877–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mitchell TJ, Hacker CD, Breshears JD, et al. A novel data-driven approach to preoperative mapping of functional cortex using resting-state functional magnetic resonance imaging. Neurosurgery. 2013;73(6):969–982; discussion 982–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosazza C, Aquino D, D’Incerti L, et al. Preoperative mapping of the sensorimotor cortex: comparative assessment of task-based and resting-state FMRI. PLoS One. 2014;9(6):e98860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Doucet GE, Pustina D, Skidmore C, Sharan A, Sperling MR, Tracy JI. Resting-state functional connectivity predicts the strength of hemispheric lateralization for language processing in temporal lobe epilepsy and normals. Hum Brain Mapp. 2015;36(1):288–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang D, Johnston JM, Fox MD, et al. Preoperative sensorimotor mapping in brain tumor patients using spontaneous fluctuations in neuronal activity imaged with functional magnetic resonance imaging: initial experience. Neurosurgery. 2009;65(6 Suppl):226–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu H, Buckner RL, Talukdar T, Tanaka N, Madsen JR, Stufflebeam SM. Task-free presurgical mapping using functional magnetic resonance imaging intrinsic activity. J Neurosurg. 2009;111(4):746–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kokkonen SM, Nikkinen J, Remes J, et al. Preoperative localization of the sensorimotor area using independent component analysis of resting-state fMRI. Magn Reson Imaging. 2009;27(6):733–740. [DOI] [PubMed] [Google Scholar]
- 41.Shimony JS, Zhang D, Johnston JM, Fox MD, Roy A, Leuthardt EC. Resting-state spontaneous fluctuations in brain activity: a new paradigm for presurgical planning using fMRI. Acad Radiol. 2009;16(5):578–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leuthardt EC, Roland J, Breshears JD, Bandt SK, Shimony JS. Listening to the brain: new techniques in intraoperative brain mapping. Clinical Neurosurgery. 2013;60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Holodny AI, Schulder M, Liu W-C, Wolko J, Maldjian JA, Kalnin AJ. The effect of brain tumors on BOLD functional MR imaging activation in the adjacent motor cortex: implications for image-guided neurosurgery. AJNR Am J Neuroradiol. 2000;21:14151422. [PMC free article] [PubMed] [Google Scholar]
- 44.Schreiber A, Hubbe U, Ziyeh S, Hennig J. The influence of gliomas and nonglial spaceoccupying lesions on blood-oxygen-dependent contrast enhancement. AJNR Am J Neuroradiol. 2000;21:1055–1063. [PMC free article] [PubMed] [Google Scholar]
- 45.Partovi S, Jacobi B, Rapps N, et al. Clinical standardized fMRI reveals altered language lateralization in patients with brain tumor. AJNR Am J Neuroradiol. 2012;33(11):2151–2157. [DOI] [PMC free article] [PubMed] [Google Scholar]