Abstract
BACKGROUND:
Advanced imaging techniques have allowed for earlier and more accurate detection of cerebral deep medullary vein thrombosis and infarction. Our objective was to develop an MR imaging scoring system to evaluate the severity of white matter injury in neonates with deep medullary vein thrombosis and infarction.
METHODS:
Retrospective study of infants born ≥32 weeks gestation (2000–2016), diagnosed with deep medullary vein thrombosis and infarction on neuroimaging in the first 30 days of life. A 102-point deep medullary vein white matter injury global severity score was developed. MR scans were scored by two pediatric radiologists. Subject clinical data, regional and global severity scores were recorded.
RESULTS:
51 patients (mean gestational age 37.3±2.2 weeks; mean birth weight 3182±720g) were included with mean age at diagnosis via MR imaging postnatal day 10.1±6.1. Global severity scores ranged from 1 to 53, with median score 11 [IQR 5-25]. Lesions were more common in frontal and parietal regions and less common in occipital and temporal regions. Fifty-five percent of the group had neonatal seizures. No difference in perinatal risk factors (gestational age, birthweight, 5-minute APGAR, chorioamnionitis, delivery room resuscitation, ventilator or inotrope requirement) was observed between severity score quartiles.
CONCLUSION:
A MR imaging scoring system provides a comprehensive and objective classification of WM injury after deep medullary vein thrombosis and infarction in late preterm and term neonates. The global severity score is independent of gestational age and other antenatal risk factors, consistent with presentation in previously healthy appearing newborns.
INTRODUCTION
Cerebral sinovenous thrombosis is an important cause of morbidity and mortality in children, with an incidence of 0.67 cases per 100,000 children with nearly half of cases occurring in neonates.1,2 Neonates are at increased risk of developing thrombosis secondary to immature hemostatic systems.3,4 Other recognized predisposing risks include maternal and perinatal factors unique to the neonatal time period, such as maternal preeclampsia, diabetes and chorioamnionitis, fetal distress, birth asphyxia, sepsis, cardiac defects and inherited prothrombotic abnormalities.1–3
The incidence of cerebral sinovenous thrombosis may be underestimated in neonates: cranial ultrasound is still the most routinely used neuroimaging modality in this age group, yet it is insufficient to precisely identify deep or diffuse thromboses. However, central nervous system imaging using MRI, and in particular SWI, allows accurate localization of deep vein thrombosis or infarction in children and adults.5–7 In particular, linear, radially oriented, fan-shaped lesions in the periventricular WM are indicative of deep medullary vein (DMV) involvement. This particular imaging pattern is explained by the anatomic distribution of the WM venous drainage.8 The characteristic pattern of DMV thrombosis and ensuing infarction of the surrounding tissue has been recognized on neuroimaging with increasing frequency in the past decade.5,6,9
Despite increasing recognition of DMV thrombosis and infarction on neuroimaging, there is no published standardized approach to evaluate or grade these lesions in neonates. Clinical outcome data related to DMV pathology in neonates or children exist only in isolated case reports of motor impairment.9 Fetal diagnosis of DMV thrombosis and infarction has been described; however, all resulted in in-utero fetal demise or neonatal death shortly after delivery10,11. Similar lesions are described more frequently in preterm neonates with germinal matrix hemorrhage and congestion and obstruction of the terminal or medullary veins with subsequent parenchymal hemorrhage. However, in term neonates, germinal matrix hemorrhage is much less frequent but DMV congestion, thrombosis, infarction and hemorrhage are also seen. It has been hypothesized that DMV lesions in term infants may be related to transient hypoperfusion or impairment in cerebral blood flow.5 No current system classifies this problem in infants during the perinatal period.
Therefore, the aim of our study was to develop a scoring system for conventional MR imaging that comprehensively and objectively defines brain injury after DMV thrombosis and infarction, in order to facilitate future prospective studies of developmental outcomes. We hypothesized that we could develop a semi-quantitative system to characterize both extent and the topography of insults to the neonatal brain after DMV thrombosis and infarction. To test this hypothesis, we leveraged a large retrospective neuroimaging database at our institution and graded DMV lesions while characterizing perinatal risk factors consistent with cerebral sinovenous thrombosis.
METHODS
Study Design and Participants
We retrospectively reviewed patients with a diagnosis of DMV thrombosis and infarction in a pediatric neuroradiology database. All subjects were admitted to the neonatal intensive care unit or other hospital unit at our institution between 2000 and 2016. Included in the review were infants born at ≥32 weeks of gestation, diagnosed with DMV thrombosis and infarction on neuroimaging during the first 30 days after birth. We excluded infants with congenital CNS anomalies or major congenital syndromes. The electronic medical record was reviewed and all pertinent clinical, laboratory, imaging and follow-up data was collected. This study was approved by the hospital’s institutional review board for human research. Study data were managed using REDCap electronic data capture tools hosted at our institution.12
MR Imaging Acquisition
All brain MRI scans were performed on either 1.5T or 3T MRI scanners (EXCITE HDXT 1.5T and DISCOVERY 3T General Electric, Milwaukee, WI, USA, and ESPREE 1.5T and SKYRA 3T Siemens AG, Munich, Germany). Due to the retrospective study design, there was variation in imaging protocols, sequences, and parameters obtained. Scans included a combination of T1, T2, T1 FLAIR, DWI, and SWI sequences. Twenty patients also had a 2D time-of-flight MR venogram available for review.
MR Imaging Assessment
While all scans for all subjects were reviewed, the MRI scan obtained at the time of initial radiologic diagnosis of DMV thrombosis and infarction was used for scoring purposes. A standardized scoring system was developed a priori based on topography of venous supply to the WM8, in order to describe the severity of the cerebral WM injury after DMV thrombosis and infarction.
The DMV WM injury severity scoring system identified and assigned points for degree of injury for focal lesions within the right and left frontal, parietal, occipital and temporal lobes, for focal infarction within the corpus callosum, and for diffuse WM cerebral edema (Table 1). Focal infarction and diffuse cerebral swelling were identified as focal or diffuse WM increased T2 and/or restricted diffusion signal abnormality respectively and scored as present in cerebral WM either unilateral, bilateral symmetric or bilateral asymmetric. Regions were then scored separately for any punctate, linear and/or cavitary lesions. Punctate and linear lesions, including those in a radial pattern, were identified as T1 bright and T2 dark with hemorrhage confirmed on SWI and scored as mild, moderate or severe based on number of lesions. Cavitary lesions were more heavily weighted to account for their larger and more severe tissue injury. All regional scores were added to the corpus callosum and cerebral edema scores to yield a global severity score (0-102 points). A diagrammatic representation of the scoring system is shown (Figure 1-4). Two pediatric radiologists independently scored the scans, and then agreed on final consensus scoring after review of any discrepancies.
Table 1.
DMV White Matter Injury Severity Scoring System
| Points | Scoring | |
|---|---|---|
| Cerebral edema | 0-3 | None = 0, right or left = 1, bilateral asymmetric = 2, bilateral symmetric = 3 |
| Corpus callosum | 0-3 | 1 point each for genu, body, and/or splenium lesions |
| RIGHT cerebral WM | Score each cerebral hemisphere region for punctate, linear and/or cavitary lesions | |
| Frontal | 0-12 | Punctate: mild (1-3 lesions)=1, moderate (4-6 lesions)=2, severe (>6 lesions)=3 |
| Parietal | 0-12 | Linear: mild (1-3 lesions)=1, moderate (4-6 lesions)=2, severe (>6 lesions)=3 |
| Occipital | 0-12 | Cavitary: mild (1-3 lesions, <15mm)=2, moderate (4-6 lesions, <15mm)=4 |
| Temporal | 0-12 | severe (>6 lesions, <15mm OR 1+ lesion >15mm)=6 |
| LEFT cerebral WM | ||
| Frontal | 0-12 | |
| Parietal | 0-12 | |
| Occipital | 0-12 | |
| Temporal | 0-12 | |
| 0-102 | GLOBAL SEVERITY SCORE |
Figure 1 A-E.
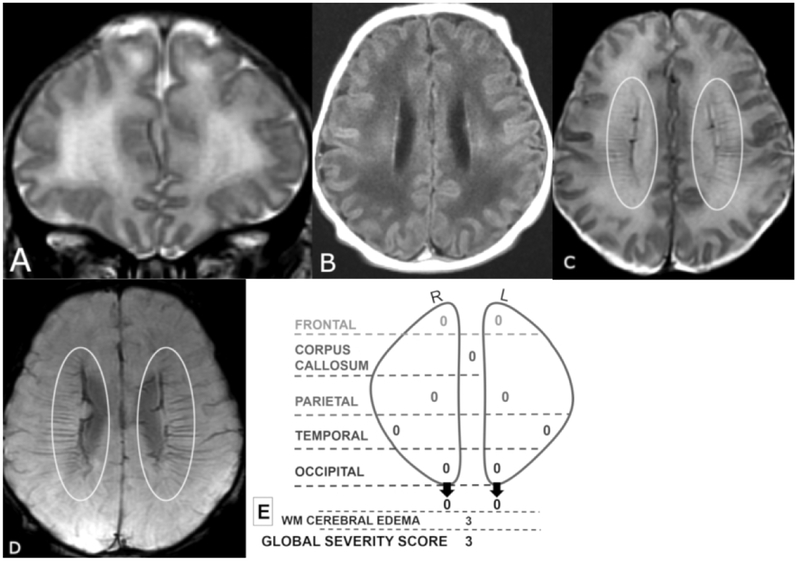
Coronal T2 (A) and axial T1 FLAIR (B), T2 (C), and SWI (D) MR images of a 6-day old male. Global Severity Score = 3 (first quartile) (E). There is diffuse bilateral WM signal abnormality of cerebral edema without infarction (3 pts.) (A,B). The DMV are prominent bilaterally, seen on T2 (C) and SWI (D) (ovals). No WM lesions are visible.
Figure 4 A-F.
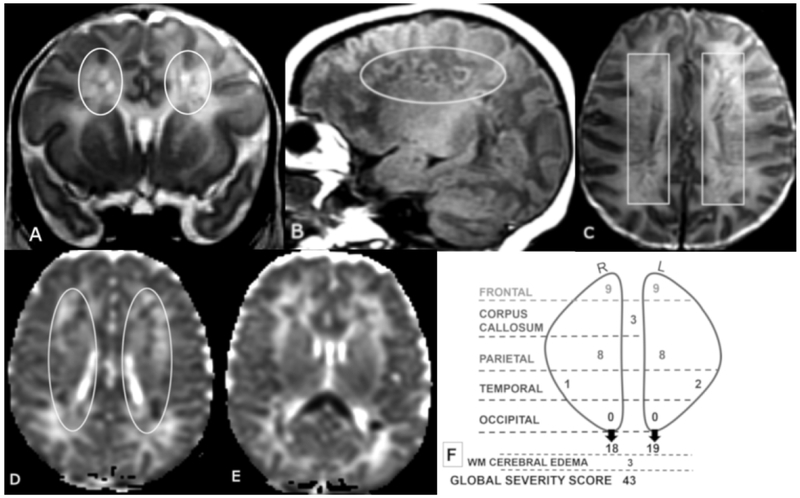
Coronal T2 (A) and sagittal T1 (B), axial T2 (C) and axial ADC (D, E) MR images of a 12-day old male. Global Severity Score = 43 (fourth quartile) (F). There is diffuse bilateral WM cerebral edema (3 pts) and multiple bilateral hemorrhagic WM lesions with restricted diffusion (A-F). There are symmetric bilateral frontal and parietal lesions: severe linear lesions (3 pts, C, boxes) and severe cavitary lesions withrestricted diffusion (6 pts, A, B, D, ovals), There are bilateral temporal lesions: right (mild punctate, 1 pt.) and left (moderate punctate, 2 pts, not shown). There is restricted diffusion throughout the corpus callosum (3 pts, E). No occipital WM lesions are visible.
Statistical Analysis
Continuous variables were summarized using the mean ± standard deviation or median (interquartile range). The DMV WM injury severity scoring system is a sum of scaled scores with a continuous composite. Categorical variables were summarized using percentiles. ANOVA was used to compare variance in means between the four quartiles in continuous variables. Adjustment for multiple comparisons was through Bonferroni correction. The Kruskal Wallis test for non-parametric variables, was used to assess frequency distribution of categorical variables. Significance was assigned at P < 0.05. All analyses were performed using SPSS (IBM Corp. Released 2016. IBM SPSS Statistics for MacIntosh, Version 24.0. Armonk, NY: IBM Corp.).
RESULTS
Our cohort consisted of 51 patients. Infants were born at a mean gestational age of 37 weeks (range 32 to 41 weeks) and mean birth weight of 3182±720 grams. Sixty-three percent of the group was male. On average, infants were admitted to our hospital on day of life 4.7±4.9 with only 37% admitted within the first day of life. The most commonly identified maternal risk factors were pre-eclampsia or hypertension (33%), diabetes (12%) and a maternal pro-thrombotic disorder (6%). Fifty-five percent of the infants had no identified maternal risk factors. One-third of infants demonstrated signs of fetal distress during the labor and delivery process (decelerations or a non-reassuring biophysical profile). Twenty percent were intubated and 4% required chest compressions or epinephrine during the post-delivery resuscitation. The average 5-minute APGAR score was 7.8±1.7. Neonatal comorbidities included encephalopathy (20%), neonatal infection (18%), hypoglycemia (35%), complex congenital heart disease (8%) and prothrombotic disorders (8%). Forty-three percent of infants required mechanical ventilation and 18% required inotropes in the first week of life. Infants presented on day of life 4.5±5.3 with seizures (55%), apnea (53%), lethargy (49%) and poor feeding (35%). The average age at referral of these infants to our institution was 4.7±4.8 days and the average age at diagnosis via MR imaging was postnatal day 10.1±6.1.
DMV WM injury global severity scores ranged from 1 to 53, and were divided into quartiles. The median score for the cohort was 11 [interquartile range (25th-75th percentile) 5-25]. Seventy-six percent and 73% of patients had lesions in the frontal and parietal WM respectively. Twelve percent and 41% of patients had lesions in the occipital and temporal WM respectively. Acute parenchymal and corpus callosum lesions showed restricted diffusion. Representative MR images from a patient in each quartile with corresponding diagrammatic scoring are shown in Figures 1-4, illustrating the range of severity and morphology of the various lesions. Table 2 demonstrates the distribution of injury severity scores by region. Only occipital regional severity scores and WM cerebral edema scores were not significantly different between quartiles. Perinatal clinical variables were compared with global severity score quartile (Table 3). Perinatal variables included factors associated with increased risk of perinatal morbidity and mortality such as 5-minute APGAR, chorioamnionitis, small for gestational age, neonatal infection, ventilator requirement, inotrope use and seizures. We observed no significant difference in perinatal risk factors between the severity score quartiles.
Table 2.
Injury Severity Scores by Region
| Score 0 | Score 1 | Score 2 | Score 3 | ||
|---|---|---|---|---|---|
| Regions | Number of patients (%) | P value | |||
| WM cerebral edema | 4 (8) | 2 (4) | 9 (18) | 36 (71) | .52 |
| Corpus callosum | 26 (51) | 10 (20) | 8 (16) | 7 (14) | <.001 |
| Score 0-2 | Score 3-5 | Score 6-8 | Score >9 | ||
|---|---|---|---|---|---|
| Regions | Number of patients (%) | P value | |||
| Right frontal | 24 (47) | 14 (28) | 8 (16) | 5 (10) | <.001 |
| Right parietal | 33 (65) | 8 (16) | 9 (18) | 1 (2) | <.001 |
| Right occipital | 50 (98) | 1 (2) | 0 (0) | 0 (0) | .68 |
| Right temporal | 43 (84) | 6 (12) | 2 (4) | 0 (0) | .006 |
| Left frontal | 31 (61) | 10 (20) | 7 (14) | 3 (6) | <.001 |
| Left parietal | 37 (73) | 5 (10) | 7 (14) | 2 (4) | <.001 |
| Left occipital | 49 (96) | 0 (0) | 2 (4) | 0 (0) | .67 |
| Left temporal | 43 (84) | 7 (14) | 1 (2) | 0 (0) | <.001 |
Table 3.
Global Severity Score and Perinatal Variables
| 1st quartile (score 1-5) n = 14 |
2nd quartile (score 6-11) n = 13 |
3rd quartile (score 12-25) n = 12 |
4th quartile (score >26) n = 12 |
P
value |
|
|---|---|---|---|---|---|
| Gestation at birth (weeks) | 38.2 ± 1.3 | 36.3 ± 2.4 | 36.7 ± 2.5 | 38 ± 1.9 | 0.051 |
| Birth weight (g) | 3445.8 ± 618 | 3066 ± 989 | 2947 ± 619 | 3247 ± 570 | 0.341 |
| Male sex | 8 (57) | 8 (62) | 9 (75) | 7 (58) | 0.872 |
| 5 minute APGAR | 7.8 ± 1.6 | 7.9 ± 1.4 | 8 ± 1.5 | 7.4 ± 2.4 | 0.921 |
| Compressions Epinephrinea | 1 (7) | 0 (0) | 0 (0) | 1 (8) | 0.972 |
| Chorioamnionitis | 0 (0) | 0 (0) | 0 (0) | 1 (8) | 0.982 |
| Small for gestational age | 0 (0) | 2 (15) | 3 (25) | 2 (17) | 0.752 |
| Neonatal infectionb | 1 (7) | 2 (15) | 3 (25) | 3 (25) | 0.842 |
| Ventilator requirement | 2 (14) | 7 (54) | 6 (50) | 7 (58) | 0.182 |
| Inotrope use | 0 (0) | 3 (23) | 2 (17) | 4 (33) | 0.522 |
| Seizures | 7 (50) | 5 (38) | 6 (50) | 10 (83) | 0.252 |
Note: Data are mean ± SD or number (%)
Compressions and/or epinephrine given in the delivery room during resuscitation
Neonatal infection defined as systemic/serious infection in the first week of life
ANOVA used to measure variance in means between 4 quartiles in continuous variables
Kruskal-Wallis test used for frequency distribution of categorical variables
DISCUSSION
By leveraging the largest cohort to date with imaging for DMV thrombosis and infarction, we developed a neuroimaging scoring system, allowing a comprehensive and objective classification of WM injury in term and late preterm neonates. In our cohort of infants with DMV thrombosis and infarction, WM injury global severity scores were independent of gestational age at birth and other perinatal risk factors for morbidity and mortality. While the average age at referral of these infants to our institution was 4.7 days, the average age at MRI diagnosis was 10.1 days, suggesting this entity is either under recognized or that patients are not referred for immediate imaging. This may be due lack of MR imaging availability in most regional nurseries, necessitating transport to a higher level of care NICU.
We compared regional WM injury scores to the global severity score and found that the occipital WM scores and WM cerebral edema scores were not significantly different between quartiles and therefore do not seem to impact the global severity score. Scores for all other regions were significantly different between quartiles. Given this, a modified, simpler score that excludes occipital and WM cerebral edema scores might be considered in future studies. This lesional distribution concentrated in the frontoparietal area has been described previously.5,10 It has been hypothesized that the higher frequency of transcerebral veins in temporal and occipital regions and the thinner WM and shorter medullary veins in the temporal region are protective factors, and thus contribute to the predominantly frontoparietal pattern seen in our patients and others studied.5
We noted a difference in gestational age at birth between global severity score quartiles with infants of a slightly younger gestational age (36-37 weeks) clustered around the median in the second and third quartiles and infants born at an older gestational age (38 weeks) in the first and fourth quartiles. This U-shaped distribution of gestational age and global severity score quartile is likely not clinically significant but may suggest that other unidentified factors affect severity.
We observed no difference between global severity score quartile and selected perinatal variables (Table 3). We considered using a neonatal disease severity scoring system (such as Scores for Neonatal Acute Physiology-SNAP) to stratify our patients by clinical disease severity at the time of presentation. However, most scoring systems are based on physiologic variables or treatments in the first 24 hours of life.13 Most of our patients were well-appearing at birth and did not come to medical attention until postnatal day 4-5, making use of these scoring systems inappropriate.
Limitations of this study included the retrospective, non-harmonized imaging data. However, the scoring system is relatively simple and can be applied to routine MR neuroimaging sequences. We also acknowledge the sample size, which may have contributed to the lack of significance of some perinatal risk factors. However, this remains the largest cohort examined to date.
There is no method to predict prognosis for term and late preterm infants with perinatal DMV thrombosis and infarction. This standardized scoring system allows us to stratify patients by severity of objective radiological findings at time of diagnosis. Further investigation is needed to compare severity scores at diagnosis to outcome data. Correlation of long-term effects of lesions on motor, cognitive and behavioral performance may lead to a predictive model to help guide parental counseling, recommend therapies and early intervention.
No clinical outcome data related to DMV pathology in neonates or children exists beyond few isolated case reports. In another cohort, follow-up MR imaging studies demonstrated evolution of the lesions over time with WM necrosis, development of cystic spaces, volume reduction and hyperintense signal intensity of the periventricular WM on T2-weighted and FLAIR images giving a pattern with similar appearance to periventricular leukomalacia.5 It is likely that for DMV pathology, like for periventricular leukomalacia14,15, a scoring system could be used to evaluate the long-term associations between perinatal lesions on motor, cognitive and behavioral performance in prospectively acquired datasets. However, unlike the published periventricular leukomalacia scoring system, the current DMV thrombosis and infarction classification can only be used in late preterm or term infants, as this insult is not related in pathophysiology to encephalopathy of prematurity.
CONCLUSIONS
A 102-point MR imaging scoring system provides a comprehensive and objective classification of WM injury after DMV thrombosis and infarction in late preterm and term neonates. The DMV WM injury global severity score is independent of gestational age and other antenatal risk factors. This standardized scoring system can be used to stratify patients into severity groups for future work on the neurodevelopmental outcomes of patients after DMV thrombosis and infarction.
Figure 2 A-G.
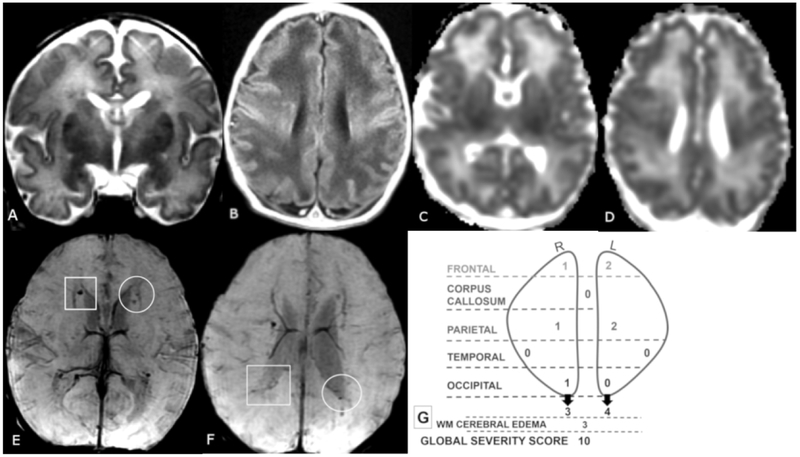
Coronal T2 (A), axial T1 (B), ADC (C,D) and SWI (E, F) MR images of a 7-day old female. Global Severity Score = 10 (second quartile) (G). There is diffuse bilateral WM cerebral edema (3 pts.) (A, B) and multiple acute bilateral, asymmetric (left > right) hemorrhagic WM lesions (T1 bright and T2 dark) without restricted diffusion (C-F). On the right, there are mild punctate lesions in the frontal (1 pt.), parietal (1 pt.) and occipital WM (1 pt., not shown) (E, F, boxes), and on the left there are moderate punctate lesions in the frontal (2 pts.) and parietal WM (2 pts.) (E, F, circles) No corpus callosum or temporal region WM lesions are visible.
Figure 3 A-E.
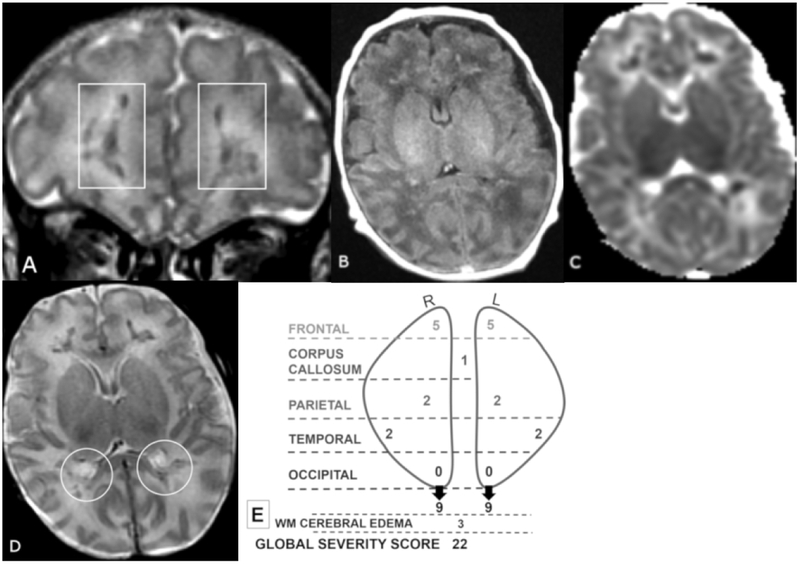
Coronal T2 (A) and axial TI FLAIR (B), ADC (C), and T2 (D), MR images of a 21-day old male. Global Severity Score = 22 (third quartile) (E). There is diffuse bilateral WM cerebral edema (3 pts.) (A-D) and multiple acute bilateral symmetric hemorrhagic WM lesions (T1 bright and T2 dark): frontal (moderate punctate, 2 pts and severe linear, 3 pts.) (A, boxes), parietal (moderate punctate, 2 pts.) (D, circles) and temporal region (moderate punctate, 2 pts, not shown). There are no visible occipital WM lesions.
Acknowledgments
Grant support: This work was supported by 1R01HD081120-01A1 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development to N.L.M. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding organizations.
Abbreviations:
- DMV
deep medullary veins
Footnotes
Accepted for poster presentation at 2018 Midwest Society for Pediatric Research Annual Meeting (Oct 2018) and 2018 Children’s Hospital Neonatal Consortium Annual Symposium (Oct 2018)
REFERENCES
- 1.deVeber G, Andrew M, Adams C, et al. Cerebral Sinovenous Thrombosis in Children. N Engl J Med. 2001;345(6):417–423. doi: 10.1056/NEJM200108093450604 [DOI] [PubMed] [Google Scholar]
- 2.Moharir MD, Shroff M, Pontigon A-MM, et al. A Prospective Outcome Study of Neonatal Cerebral Sinovenous Thrombosis. J Child Neurol. 2011;26(9):1137–1144. doi: 10.1177/0883073811408094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang JYK, Chan AKC, Callen DJA, Paes BA. Neonatal Cerebral Sinovenous Thrombosis: Sifting the Evidence for a Diagnostic Plan and Treatment Strategy. Rev Artic Pediatr. 2010;126(3):693. doi: 10.1542/peds.2010-1035 [DOI] [PubMed] [Google Scholar]
- 4.Saxonhouse MA. Thrombosis in the Neonatal Intensive Care Unit. Clin Perinatol. 2015;42(3):651–673. doi: 10.1016/j.clp.2015.04.010 [DOI] [PubMed] [Google Scholar]
- 5.Arrigoni F, Parazzini C, Righini A, et al. Deep medullary vein involvement in neonates with brain damage: An MR imaging study. Am J Neuroradiol. 2011. ;32(11):2030–2036. doi: 10.3174/ajnr.A2687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taoka T, Fukusumi A, Miyasaka T, et al. Structure of the Medullary Veins of the Cerebral Hemisphere and Related Disorders. RadioGraphics. 2017;37(1):281–297. doi: 10.1148/rg.2017160061 [DOI] [PubMed] [Google Scholar]
- 7.Friedman DP. Abnormalities of the deep medullary white matter veins: MR imaging findings. Am J Roentgenol. 1997;168(4):1103–1108. doi: 10.2214/ajr.168.4.9124123 [DOI] [PubMed] [Google Scholar]
- 8.Okudera T, Huang YP, Fukusumi A, Nakamura Y, Hatazawa J, Uemura K. Micro-angiographical studies of the medullary venous system of the cerebral hemisphere. Neuropathology. 1999; 19(1):93–111. doi: 10.1046/j.1440-1789.1999.00215.x [DOI] [PubMed] [Google Scholar]
- 9.Vilan A, Ribeiro JM, Reis C, Sampaio L. Deep Medullary Veins and Brain Injury. J Pediatr. 2018;200:290–290.e1. doi: 10.1016/j.jpeds.2018.03.051 [DOI] [PubMed] [Google Scholar]
- 10.Doneda C, Righini A, Parazzini C, Arrigoni F, Rustico M, Triulzi F. Prenatal MR imaging detection of deep medullary vein involvement in fetal brain damage. Am J Neuroradiol. 2011;32(8):E146–9. doi: 10.3174/ajnr.A2249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Konanki R, Varma DR, Ratha C, Lingappa L, Shah N. Teaching NeuroImages: Fetal deep medullary vein thrombosis presenting as progressive intracerebral hemorrhage. Neurology. 2015;85(1):e5–6. doi: 10.1212/WNL.0000000000001719 [DOI] [PubMed] [Google Scholar]
- 12.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)-A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dorling JS. Neonatal disease severity scoring systems. Arch Dis Child - Fetal Neonatal Ed. 2005;90(1):F11–F16. doi: 10.1136/adc.2003.048488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martinez-Biarge M, Groenendaal F, Kersbergen KJ, et al. MRI based preterm white matter injury classification: The importance of sequential imaging in determining severity of injury. PLoS One. 2016;11(6). doi: 10.1371/journal.pone.0156245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Vries LS, Van Haastert ILC, Rademaker KJ, Koopman C, Groenendaal F. Ultrasound abnormalities preceding cerebral palsy in high-risk preterm infants. J Pediatr. 2004;144(6):815–820. doi: 10.1016/j.jpeds.2004.03.034 [DOI] [PubMed] [Google Scholar]


