Abstract
Purpose of review:
In humans, only one independent immunologic correlate of reduced risk of HIV infection has been identified: a robust antibody (Ab) response to the V1V2 domain of the gp120 Envelope (Env) protein. In recent years, the presence and level of V1V2-specific Abs has also been correlated with protection from SIV and SHIV infections. Here we review the multitude of studies showing the in vivo protective effects of V1V2 Abs and review their immunologic characteristics and anti-viral functions.
Recent findings:
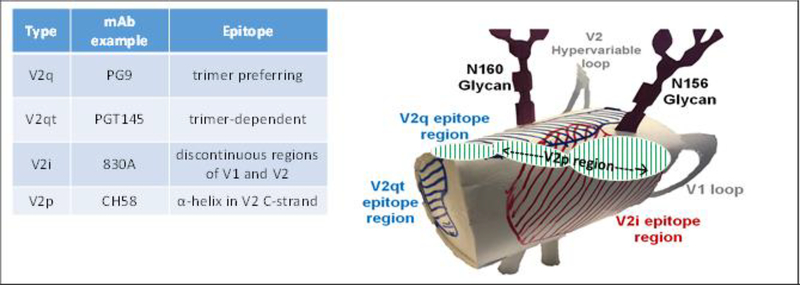
Structural and immunologic studies have defined four epitope families in the V1V2 domain: one epitope family, V2q, which preferentially present as a quaternary structure of the Env trimer, and another epitope family (V2qt) which requires the quaternary trimeric Env structure; these two epitope types are recognized by two families of monoclonal Abs (mAbs)―V2q- and V2qt-specific mAbs―which display broad and potent neutralizing activity. A third epitope family, V2i, is present as a discontinuous conformational structure that overlays the α4β7 integrin binding motif, and a fourth epitope family (V2p) exists on V2 peptides. Antibodies specific for V2i and V2p epitopes display only poor neutralizing activity but effectively mediate other anti-viral activities and have been correlated with control of and/or protection from HIV, SIV and SHIV. Notably, V2q and V2qt Abs have not been induced by any vaccines, but V2p and V2i Abs have been readily induced with various vaccines in non-human primates and humans.
Summary:
The correlation of vaccine-induced V2p and V2i Abs with protection from HIV, SIV and SHIV suggests that these Ab types are extremely important to induce with prophylactic vaccines.
Keywords: vaccines, V2, antibodies, non-human primates, epitope, anti-viral functions
INTRODUCTION
The key to the rational design of an effective HIV vaccine depends on the identification of immune correlates of protection and immunologic mechanisms that prevent HIV acquisition. The first independent correlate of reduced risk of HIV infection in humans was identified by studies of participants in the RV144 clinical vaccine trial: a robust antibody (Ab) response to the V1V2 region of the virus gp120 envelope (Env) glycoprotein. Similar correlations were subsequently identified in studies of non-human primates (NHPs) between protection from SIV and SHIV and V2 Ab levels. Here, we summarize these human and NHP findings and the V2 Abs that are involved in the control of and/or protection from HIV, SIV and SHIV.
Structural and immunologic characterization of the V1V2 domain.
Studies of polyclonal sera from HIV-infected individuals have established that, over time, infection generates different “humoral fingerprints” (1). This is true for patterns of Ab specificities, subclasses, and anti-viral activities (2–6). Similar findings pertain to Abs induced by vaccines targeting SIV (7, 8) and SHIV (9–12). Given the association between V2 Abs and protection in human and animal models, it is critical to understand the complexity of the V1V2 domain of the virus Env and the Ab response to it.
Conformational complexity of V1V2.
In HIV, the V1V2 domain, like the rest of gp120, exhibits marked conformational flexibility. The V1V2 domain serves as the “trimer association domain” at the apex of the closed trimeric Env, but the V1V2 domain of each of the three gp120 protomers opens out when gp120 interacts with CD4 (13–16). The C-strand of V2, composed of amino acids (AAs) 170–176, one of the five strands composing the V1V2 beta-barrel, exists in different conformations, varying between a beta-strand and an alpha-helix (17–19), where the beta-strand configuration is preferentially present in the closed, structurally constrained trimeric Env while the alpha-helical conformation is preferred where there is less structural constraint when the Env is fully open. The preferred configuration is undoubtedly affected by (a) the sequence of V1 and V2, (b) substitutions at key residues, (c) the molecular context in which the V1V2 domain is placed, and (d) the intra- and inter-protomer interactions of V1V2 within the Env trimer (20).
Alternative V2 conformational epitopes.
As a result of this configurational complexity, there are at least four types of epitopes in the V1V2 region as shown in Figure 1: (a) V2q epitopes which preferentially recognize structures formed by the quaternary interaction of the three gp120 protomers and are glycan-dependent; V2q is recognized by V2q mAbs such as PG9 and PG16 (17, 21–24), (b) V2qt epitopes which recognize quaternary, trimer-dependent V2 epitopes at the apical center of Env are recognized by several V2qt mAbs exemplified by PGT145 (25), (c) V2i epitopes which overlay the α4β7 integrin binding site in V2 and are recognized by V2i mAbs such as 830A and 2158 (26–28), and (d) V2p epitopes which are presented by V2 linear and cyclic peptides, and recognized by V2p mAbs such as CH58 and CAP228–16H (19, 29, 30). The V2q, V2qt and V2i mAbs preferentially recognize their various epitopes when the C-strand of V2 is in the beta-strand conformation. In contrast, V2p mAbs recognize the C-strand in its alpha-helical configuration (19). These four families of mAbs that recognize the various V1V2 epitopes display distinct patterns of reactivity; an example is shown in Figure 2 in which the patterns of reactivity of V2p and V2i mAbs are shown vs. a panel of eight V2 bearing antigens.
Figure 1.
Diagram of the position of the four epitope classes in the V1V2 region (V2p, V2i, V2q and V2qt), and examples of mAbs that target each of these epitopes.
Figure. 2.
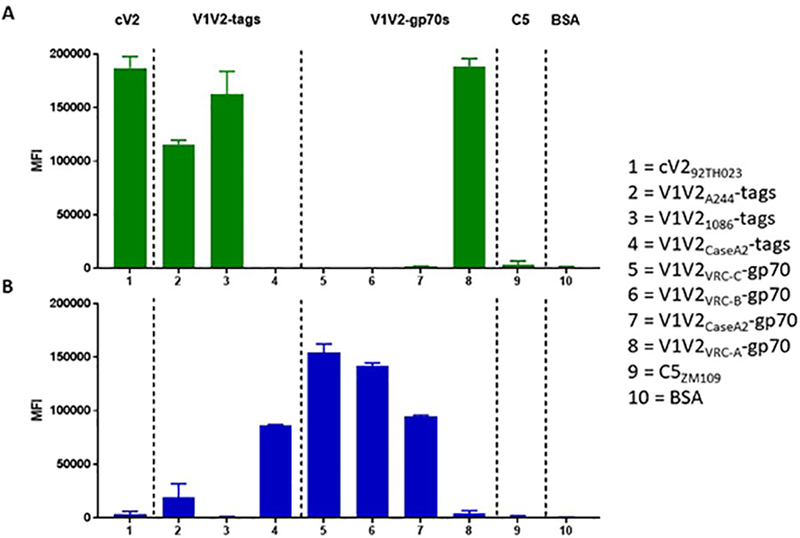
Luminex reactivity of V2p mAb 8490 (A) and V2i mAb 697–30D (B) vs. cyclic V2 peptide (column 1), V1V2-tags (columns 2–4), V1V2-gp70 fusion proteins (columns 5–8), C5 peptide (column 9) and BSA (column 10). The negative control is shown at right.
The correlates of reduced risk in RV144.
RV144 is the only clinical vaccine trial to date which provided a marginal but statistically significant reduced rate of infection (31) as well as an independent correlate of risk (COR) of HIV infection: an inverse relationship between the incidence of infection and the level of Abs binding to a V1V2CaseA2-gp70 fusion protein (32–34). The V1V2CaseA2-gp70 fusion protein preferentially reacts with V2i mAbs, and indeed, several V2i mAbs have been selected from the cells of HIV-infected individuals using this reagent, and these V2i mAbs are highly cross-clade reactive (35, 36). In this context, it is noteworthy that while V1V2CaseA2-gp70 carries the V1V2 sequence from a clade B strain (44), infections occurring in the RV144 participants were primarily due to clade AE (CRF01_AE), the predominant circulating strain in Thailand, and this supports the hypothesis that the V2 Abs implicated in the inverse COR were cross-clade reactive.
Notably, in human vaccine studies other than RV144, the induction of highly reactive and functional V2-specific Abs has not been strong, e.g., in studies such as VAX003, VAX004 and HVTN100 (5, 37, 38). It is hypothesized that the gp120 of the clade AE A244 strain used in RV144 is unusual in its ability to efficiently induce V2 Abs. Thus, for example, the immunogens used in HVTN100 (ALVAC-HIV [vCP2438] and bivalent Subtype C gp120s [1086 and TV1]), which was the precursor of the on-going Phase III HVTN702 study in South Africa, induced a markedly poorer V2 response than that attained in RV144 (39). These findings are of particular interest in the context of recent studies in which robust V2i and V2p Ab responses have been elicited using V2-targeting vaccine constructs in rabbits (40, 41) and NHPs (42). Notably, in the latter study, the use of a trimeric V1V2A244-scaffold fusion protein as part of an immunogen cocktail appeared to be particularly effective in inducing broadly reactive and functional V2 Abs.
In addition to the inverse COR with V2i Abs, a similar role for V2p Abs has been documented. Thus, studies with plasma from RV144 vaccinees demonstrated an inverse COR in terms of the Ab response to linear V2 peptides tested by microarray (43), and the correlations of Abs cross-reactive with V2 peptides representing different HIV clades were at least as significant as the correlation seen with the primary variable generated using the V1V2CaseA2-gp70 fusion protein. As noted, linear and cyclic V2 (cV2) peptides preferentially assume a structure when complexed with specific V2p mAbs in which the C-strand is in an alpha-helical configuration (19, 29, 30), and two such V2p mAbs were isolated from circulating cells of an individual receiving the RV144 vaccine regimen (19). Thus, we know from polyclonal and mAb studies emanating from RV144 that V2p Abs recognizing the alpha-helix in the C-strand of V2 were induced by the RV144 vaccine and that they constitute an inverse COR ((43) and Table 1). Additional V2p mAbs have recently been isolated from individuals infected with clade C (29, 30). All of these V2p mAbs have been crystallized and reveal the targeted epitope in the V2 C-strand as an alpha-helix or helix-loop, and, like the plasma V2p polyclonal Abs in RV144 vaccinees (33), these mAbs are cross-clade reactive (Figure 2A). (44)
Table 1.
Studies showing statistically significant correlation of V2-specific antibodies that confer protection from and/or control of HIV, SIV and SHIV
| Year | Protection and/or control of | Correlation with Abs specific for | Immunization | Reference |
|---|---|---|---|---|
| 2012 | HIV | V2i | Active | Haynes et al (32) |
| SIVmac251 | V2p | Active | Barouch et al (48) | |
| 2013 | HIV | V2p | Active | Gottardo et al (43) |
| SIVmac251 | V2p | Active | Pegu et al (51) | |
| 2014 | SIVmac251 | V2p | Active | Gordon et al (52) |
| 2015 | SIVE660 | V2p | Active | Roederer et al (49) |
| 2016 | SIVmac251 | V2p | Active | Vaccari et al (50) |
| 2018 | SHIVBaL | V2i | Passive | Hessell et al (56) |
| SIVsmE66 | V2p | Active | Singh et al (53) | |
| SHIVBaL | V2 | Active | Hessell et al (54) | |
| SHIVBaL | V2p | Active | Weiss et al (55) |
The role of V2 Abs in the control of and protection from SIV and SHIV infections in non-human primates.
The original observation of an inverse COR in RV144 has been supported by many subsidiary studies of the RV144 data (3, 4, 43, 45, 46). Nonetheless, there are critics who remain skeptical of the RV144 correlates analyses (47). This skepticism is now tempered by both active and passive immunization studies from many labs showing correlates of protection from SIV and SHIV infection with the presence of V2 Abs (Table 1).
V2p-specific Abs protect against SIV infection.
In an NHP vaccine study using vaccine regimens consisting of Ad26 and/or modified vaccinia Ankara (MVA) vector-based vaccines expressing SIVSME543 gag, pol and Env antigens with subsequent intra-rectal (i.r.) challenges with SIVmac251, there was a ≥80% reduction in per-exposure probability of infection (48). The strength of Ab binding to a biotinylated cyclic V2 peptide from SIVSME543 correlated positively with the number of challenges required to establish infection (p< 0.0001).
Another NHP study utilized priming with gp160 DNA from SIVmac239 and boosting with recombinant Ad5SIVmac239 with subsequent multiple mucosal challenges with SIVE660 (49). Among the significant humoral response correlates identified was the strength of binding of plasma IgG Abs to a V1V2SIVmac239 linear peptide with time to infection (p=0.009). In this study, a sieving effect was also noted at a glycosylation site in V1V2 that conferred neutralization resistance. This is similar to the sieving signature identified in the RV144 human trial (45).
Additional studies in NHPs also support the role of V2-specific Abs in protection from or control of SIV. With sera from animals immunized with a regimen similar to that used in RV144, Ab assays revealed that the reduced risk of SIVmac251 acquisition correlated with the presence of mucosal IgG to cyclic V2 (p=0.0018) (50, 51). In yet another study, where animals were immunized with gp120 protein and human papilloma pseudoviruses expressing SIVmac251 genes +/− ALVAC-SIVmac251, a significant correlation was found between the number of challenges to achieve persistent infection with SIVmac251 and the avidity index for V1V2 Abs in blood (p=0.014) (52). And most recently, in NHPs immunized with SIVmac251-derived env plasmids and monomeric M766 gp120 protein followed by challenge with SIVE660, inverse correlations were identified for (a) plasma and mucosal V1V2 responses with peak viral load (p=0.05 and p=0.01, respectively), (b) responses to cyclic V2 peptides with post-peak and chronic viremia (p=0.01 for each), and (c) V2-specific responses with delayed virus acquisition and post-infection control (53). Each of these experiments suggests that Abs that hampered SIV infection were of the V2p type which recognize the alpha-helical V2 C-strand configuration
V2p Abs protect against SHIV infection.
Most recently, an RV144-like vaccine regimen was tested in NHPs that were challenged with SHIVBaL (54). All three unimmunized animals were infected after two i.r. challenges, but in five of the nine immunized macaques, tight control of viremia was noted as reflected by only transient and low plasma viral load measurements, with no measurable virus in tissues at necropsy 13 weeks after challenge. Luminex studies of the plasma from these animals showed a correlate of protection from SHIVBaL with Abs of the V2p type that were reactive with V1V21086-tags (19), a reagent in which the V2 C-strand preferentially adopts an alpha-helical conformation as shown by circular dichroism (55).
The role of V2i Abs in SHIV viral control.
To date, only indirect, correlative data have linked V2 Abs with protection in human and NHP experiments. The first direct in vivo test of the hypothesis emanating from RV144—that V2 Abs could reduce the risk of infection—was reported recently in experiments investigating the role of a passively administered V2i mAb in protection from, and/or control of infection in NHPs challenged i.r. with SHIVBaL (56). NHPs received three weekly doses at 10 mg/kg of the IgG1) isoform of the V2i mAb 830A (n= 12, while control animals (n=12) received a dengue-specific mAb (DEN3) at the same times and doses. Animals were challenged with SHIVBaL twice during each week that they received the passively transferred mAb. Blood specimens were drawn at regular intervals at the time of and after challenge, and animals were sacrificed and necropsied six weeks after infection. On the basis of SIVgag RNA copies/ml in plasma, 11/12 control animals were infected by the sixth and final SHIVBaL challenge dose, and while the twelfth animal had no detectable plasma viral load (PVL), SHIV was detected in its liver at necropsy. Of the 12 animals receiving V2i mAb 830A, three had no detectable PVL at necropsy and no SIVgag DNA was detected in their tissues. Of these latter three macaques, one was plasma aviremic after all challenge doses and two had only low and transient positive PVLs at a single time point. Compared in a grouped analysis, the PVL in the 830A recipients was significantly lower than that in the DEN3 controls (p = 0.031). The cell-associated viral load (CAVL) DNA in peripheral blood mononuclear cells was assessed as virus copies collected during the course of the experiment, and a statistical analysis revealed a significant difference in CAVLs between controls and the 830A group (p = 0.038). In addition, copy numbers of viral DNA in 13 different tissues were measured at the time of necropsy and revealed significant differences in the viral DNA loads between viremic 830A-treated and control macaques (p = 0.017). Copy numbers of SIVgag DNA associated with each tissue sampled from the 830A-treated macaques were compared individually to the corresponding tissue from animals in the control group and again significant differences in copy numbers were found in iliosacral, axillary and inguinal lymph nodes and from mixed tissues from the reproductive tract. Thus, while too few animals remained uninfected in the treated group to achieve statistical significance in terms of the risk of infection, the data demonstrate that the presence of the passively administered V2i 830A mAb had significant effects against SHIV challenge in macaques by reducing the viral infectious titer so that animals were either not infected or experienced lower level virus production in blood and tissues resulting in significantly delayed infection, reduced plasma virus load, and decreased viral DNA in lymphoid tissues. This is the first direct demonstration showing the ability of V2i Abs to impede SHIV infection (56).
The biologic functions of V2 antibodies.
Neutralizing Abs were not an inverse COR in the RV144 vaccine trial, suggesting that non-neutralizing Ab effects were critical. These effects could be mediated by either the Fab portion of the Ab which binds to antigens on the surface of the virus or virus-infected cell and/or by the Fc portion of the Ab which binds to Fc receptors (FcRs) after the Fab fragment binds to its antigen.
Anti-viral activities mediated by the Fab fragment of antibodies.
Neutralization of virus infectivity is the most frequently measured anti-viral activity, resulting as a function of the attachment of Abs to virions. However, several other phenomena belong to this category of anti-viral functions and appear to play a critical role in vivo (57). These include:
Virus Aggregation. Abs can bind to virions; when they do, they can aggregate viruses, enhancing their destruction via phagocytosis. It has been shown that there is a clear association between virus aggregation and virus phagocytosis (58), and this may play a role in reducing the virus inoculum upon exposure to the virus, resulting in a reduced risk of infection. Similarly, this mechanism may assist in the clearance of circulating virus, which, perhaps as a result, has a half-life in circulation of only approximately 0.3 days (59). Monoclonal IgG and dimeric IgA Abs, as well as polyclonal purified serum IgA from RV144 participants, have been shown to aggregate HIV virions, and the specificities of the Abs used to do so include mAbs to various epitopes of both gp120 and gp41 (60).
Virus Capture. This in vitro assay has been used to demonstrate that the RV144 vaccine-elicited gp120-specific Abs of multiple specificities (V3, V2, and C1) can bind to virions (61). These studies were extended to show that C1- and V2-specific mAbs derived from vaccinees acted in synergy to capture virions (46).
Inhibition of virus binding to cell-surface α4β7: It was shown in 2008 that a tripeptide in the V2 loop of gp120 (at AA 179–181) constitutes a motif that mimics structures presented by the natural ligands of the α4β7 integrin, a gut-homing receptor, and that the HIV-1 envelope protein gp120 bound to an activated form of α4β7 (62). In a recent follow-up to the initial findings, the V2 domain of gp120 was shown to preferentially engage extended forms of α4β7 in a cation-sensitive manner. A 15-mer V2-derived peptide (AA 168–181) can bind to α4β7, and V2p-specific mAbs derived from vaccinated and infected subjects recognize this peptide and block the V2/α4β7 interaction (63). Other labs have reproduced and extended these findings (64) and have shown that purified IgG from some uninfected RV144 vaccinees can also inhibit the V2/α4β7 interaction. These data suggest that anti-V2 Abs may play a role in vivo, blocking the gp120/α4β7 interaction and thus preventing HIV acquisition and/or controlling early targeting of the gut lymphoid tissues by the virus.
Fc-dependent effector functions.
Many Abs result in anti-viral effects due to their ability to bind to infected cells and/or virions, leading to conformational changes in the Fc fragment which allow it to bind to FcRs on the surface of various cell types such as T cells, monocyte/macrophages, polymorphonuclear granulocytes, dendritic cells, etc. These Abs include both bnAbs and Abs that are poor or non-neutralizers, can be specific for various regions of the gp120 and gp41 Env proteins (Figure 3 and (19, 30, 40, 46, 65–71)), and can bind to the Env trimer in its different states (closed, partially open, or fully open (16). The Fc/FcR interaction initiates anti-viral activities that include Ab-dependent cellular phagocytosis (ADCP) and Ab-dependent cellular cytotoxicity (2, 4, 46, 72–74) as well as complement virolysis (38, 75–77). Many of these mechanisms have been associated with reduced risk of infection. For example, ADCP has been associated with reduced HIV infection risk in humans (38, 78) and with SIV infection in NHPs (12), and Ab-dependent complement activation and deposition (79, 80) as well as Ab-dependent cell-mediated viral inhibition have been shown to contribute to control of SIV and SHIV (81–84).
Figure 3.
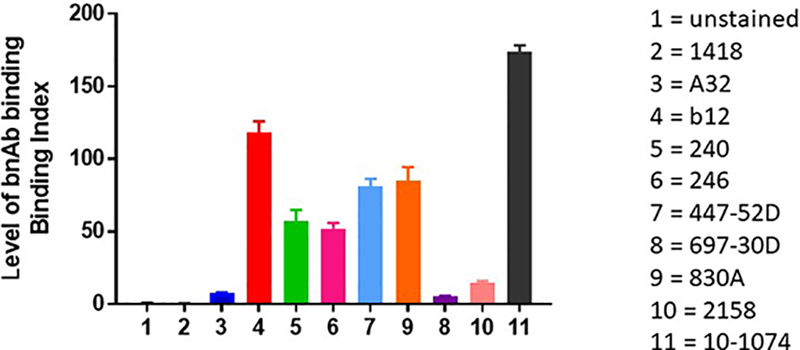
Level of binding of mAbs to native-like Env. Results shown are for binding of mAbs to tetherinhi Jurkat cells nucleofected with an mCherry+ NL4–3 reporter construct as described by Alvarez et al (71). Monoclonal Abs used (and the epitope for which they are specific) include: human anti-parvovirus mAb (1418), A32 (anti-C1), b12 (CD4bs), 240 & 246 (anti-gp41, cluster I), 447–52D (V3 crown), 697–30D (V2i), 830A (V2i), 2158 (V2i), and 10–1074 (V3-glycan).
CONCLUSIONS
The first and only independent correlate of reduced risk of HIV infection in humans was identified by studies of participants in the RV144 clinical vaccine trial: a robust Ab response to the V1V2 region of the virus gp120 Env glycoprotein. Subsequent to this observation, several active and passive immunization studies in NHPs identified the presence and level of V2 Abs as correlates of protection from SIV and SHIV infections. Currently, 11 vaccine studies in humans and NHPs (summarized in Table 1) support the role of V1V2-specific Abs in protection. In each case, the Abs involved displayed little or no neutralizing activity but mediated other anti-viral activities. Protection was documented against viruses heterologous to the strains used in the vaccines. These studies suggest a new paradigm for vaccine development: protection from and/or control of infection can be achieved with Abs that: (i) are induced by existing vaccine constructs, (ii) are effective against heterologous viruses, (iii) do not display broad and potent neutralizing activity, and (iv) mediate a variety of non-neutralizing Fab- and Fc-mediated anti-viral activities.
KEY POINTS.
V2-specific non-neutralizing Abs are involved in the control of and/or protection from HIV, SIV and SHIV.
The V2 Abs that correlate with protection after active or passive immunization are specific for the continuous epitope in the C-strand of V2 and/or for a discontinuous epitope that includes residues in V1 and V2, including the tripeptide α4β7 integrin binding motif in V2 (V2p and V2i Abs, respectively).
Fc-mediated anti-viral Ab activities play an important role in control of and/or protection from HIV, SIV, and SHIV infection.
ACKNOWLEDGEMENTS
Acknowledgements. The authors thank Drs. David Easterhof and Barton Haynes for the generation use of V2p mAb 8490.
Financial support. Partial support provided by a grant from the U. S. National Institute of Allergy and Infectious Diseases (NIH grant P01 AI100151) and by the Department of Medicine, Icahn School of Medicine at Mount Sinai, New York, NY.
Funding received for this work came from the U. S. National Institutes of Allergy and Infectious Diseases (NIH grant P01 AI100151) and funds from the Division of Infectious Diseases, Department of Medicine, Icahn School of Medicine at Mount Sinai, New York, NY 10029, USA.
Conflicts of interest. None
REFERENCES
(*denotes recent references discussed in detail herein)
- 1.Liu P, Overman RG, Yates NL, Alam SM, Vandergrift N, Chen Y, et al. Dynamic antibody specificities and virion concentrations in circulating immune complexes in acute to chronic HIV-1 infection. J Virol. 2011;85(21):11196–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chung AW, Kumar MP, Arnold KB, Yu WH, Schoen MK, Dunphy LJ, et al. Dissecting Polyclonal Vaccine-Induced Humoral Immunity against HIV Using Systems Serology. Cell. 2015;163(4):988–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yates NL, Liao HX, Fong Y, deCamp A, Vandergrift NA, Williams WT, et al. Vaccine-induced Env V1-V2 IgG3 correlates with lower HIV-1 infection risk and declines soon after vaccination. Sci Transl Med. 2014;6(228):228ra39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chung AW, Ghebremichael M, Robinson H, Brown E, Choi I, Lane S, et al. Polyfunctional Fc-effector profiles mediated by IgG subclass selection distinguish RV144 and VAX003 vaccines. Sci Transl Med. 2014;6(228):228ra38. [DOI] [PubMed] [Google Scholar]
- 5.Balasubramanian P, Williams C, Shapiro MB, Sinangil F, Higgins K, Nadas A, et al. Functional Antibody Response Against V1V2 and V3 of HIV gp120 in the VAX003 and VAX004 Vaccine Trials. Sci Rep. 2018;8(1):542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *6.Yates NL, deCamp AC, Korber BT, Liao HX, Irene C, Pinter A, et al. HIV-1 Envelope Glycoproteins from Diverse Clades Differentiate Antibody Responses and Durability among Vaccinees. J Virol. 2018;92(8). [DOI] [PMC free article] [PubMed] [Google Scholar]; *Analysis of vaccine-elicited V1V2 binding antibody in longitudinal samples from the RV144 clinical trial revealed a striking heterogeneity among individual vaccinees in maintaining durable responses. These data support the idea that a major goal for vaccine development is to improve antibody levels, breadth, and durability at the population level.
- *7.Ackerman ME, Das J, Pittala S, Broge T, Linde C, Suscovich TJ, et al. Route of immunization defines multiple mechanisms of vaccine-mediated protection against SIV. Nat Med. 2018;24(10):1590–8. [DOI] [PMC free article] [PubMed] [Google Scholar]; *The route of immunization plays a critical role for phagocytic Fc-effector Ab activity in protection from SIV, driving Fc activity via distinct innate effector cells and antibody isotypes. The same correlates predict protection from SHIV infection. Thus functional humoral mechanisms initiated by distinct vaccination routes and immunization strategies are pivotal for inducing multiple, potentially complementary correlates of immunity.
- 8.Miller-Novak LK, Das J, Musich TA, Demberg T, Weiner JA, Venzon DJ, et al. Analysis of Complement-Mediated Lysis of Simian Immunodeficiency Virus (SIV) and SIV-Infected Cells Reveals Sex Differences in Vaccine-Induced Immune Responses in Rhesus Macaques. J Virol. 2018;92(19). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bradley T, Pollara J, Santra S, Vandergrift N, Pittala S, Bailey-Kellogg C, et al. Pentavalent HIV-1 vaccine protects against simian-human immunodeficiency virus challenge. Nat Commun. 2017;8:15711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Srivastava IK, Stamatatos L, Kan E, Vajdy M, Lian Y, Hilt S, et al. Purification, characterization, and immunogenicity of a soluble trimeric envelope protein containing a partial deletion of the V2 loop derived from SF162, an R5-tropic human immunodeficiency virus type 1 isolate. J Virol. 2003;77(20):11244–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barnett SW, Srivastava IK, Kan E, Zhou F, Goodsell A, Cristillo AD, et al. Protection of macaques against vaginal SHIV challenge by systemic or mucosal and systemic vaccinations with HIV-envelope. AIDS. 2008;22(3):339–48. [DOI] [PubMed] [Google Scholar]
- 12.Barouch DH, Stephenson KE, Borducchi EN, Smith K, Stanley K, McNally AG, et al. Protective efficacy of a global HIV-1 mosaic vaccine against heterologous SHIV challenges in rhesus monkeys. Cell. 2013;155(3):531–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kwon YD, Finzi A, Wu X, Dogo-Isonagie C, Lee LK, Moore LR, et al. Unliganded HIV-1 gp120 core structures assume the CD4-bound conformation with regulation by quaternary interactions and variable loops. Proc Natl Acad Sci U S A. 2012;109(15):5663–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mao Y, Wang L, Gu C, Herschhorn A, Xiang S-H, Haim H, et al. Subunit organization of the membrane-bound HIV-1 envelope glycoprotein trimer. Nat Struct Mol Biol. 2012;19(9):893–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Munro JB, Gorman J, Ma X, Zhou Z, Arthos J, Burton DR, et al. Conformational dynamics of single HIV-1 envelope trimers on the surface of native virions. Science. 2014;346(6210):759–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *16.Wang H, Barnes CO, Yang Z, Nussenzweig MC, Bjorkman PJ. Partially Open HIV-1 Envelope Structures Exhibit Conformational Changes Relevant for Coreceptor Binding and Fusion. Cell Host Microbe. 2018;24(4):579–92 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]; *Cryo-EM structures of SOSIP with various Abs and/or CD4 reveals the structure of the displaced V1V2 and reveals its structures.
- 17.McLellan JS, Pancera M, Carrico C, Gorman J, Julien JP, Khayat R, et al. Structure of HIV-1 gp120 V1/V2 domain with broadly neutralizing antibody PG9. Nature. 2011;480(7377):336–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pan R, Gorny MK, Zolla-Pazner S, Kong XP. The V1V2 Region of HIV-1 gp120 Forms a Five-Stranded Beta Barrel. J Virol. 2015;89(15):8003–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liao H-X, Bonsignori M, Alam SM, McLellan Jason S, Tomaras Georgia D, Moody MA, et al. Vaccine Induction of Antibodies against a Structurally Heterogeneous Site of Immune Pressure within HIV-1 Envelope Protein Variable Regions 1 and 2. Immunity. 2013;38:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Powell RLR, Totrov M, Itri V, Liu X, Fox A, Zolla-Pazner S. Plasticity and Epitope Exposure of the HIV-1 Envelope Trimer. J Virol. 2017;91(17). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gorny MK, Stamatatos L, Volsky B, Revesz K, Williams C, Wang XH, et al. Identification of a new quaternary neutralizing epitope on human immunodeficiency virus type 1 virus particles. J Virol. 2005;79(8):5232–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kimura T, Wang XH, Williams C, Zolla-Pazner S, Gorny MK. Human monoclonal antibody 2909 binds to pseudovirions expressing trimers but not monomeric HIV-1 envelope proteins. Hum Antibodies. 2009;18(1–2):35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pancera M, Zhou T, Druz A, Georgiev IS, Soto C, Gorman J, et al. Structure and immune recognition of trimeric pre-fusion HIV-1 Env. Nature. 2014;514(7523):455–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walker LM, Phogat SK, Chan-Hui PY, Wagner D, Phung P, Goss JL, et al. Broad and Potent Neutralizing Antibodies from an African Donor Reveal a New HIV-1 Vaccine Target. Science. 2009;326(5950):285–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee JH, Andrabi R, Su CY, Yasmeen A, Julien JP, Kong L, et al. A Broadly Neutralizing Antibody Targets the Dynamic HIV Envelope Trimer Apex via a Long, Rigidified, and Anionic beta-Hairpin Structure. Immunity. 2017;46(4):690–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mayr LM, Cohen S, Spurrier B, Kong X-P, Zolla-Pazner S. Epitope mapping of conformational V2-specific Anti-HIV Human Monoclonal Antibodies Reveals an Immunodominant Site in V2. PLoS One. 2013;8(7):e70859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gorny MK, Moore JP, Conley AJ, Karwowska S, Sodroski J, Williams C, et al. Human anti-V2 monoclonal antibody that neutralizes primary but not laboratory isolates of HIV-1. J Virol. 1994;68:8312–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nyambi PN, Mbah HA, Burda S, Williams C, Gorny MK, Nadas A, et al. Conserved and exposed epitopes on intact, native, primary human immunodeficiency virus type 1 virions of group M. J Virol. 2000;74(15):7096–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *29.Wibmer CK, Richardson SI, Yolitz J, Cicala C, Arthos J, Moore PL, et al. Common helical V1V2 conformations of HIV-1 Envelope expose the alpha4beta7 binding site on intact virions. Nat Commun. 2018;9(1):4489. [DOI] [PMC free article] [PubMed] [Google Scholar]; *Structural studies of the epitope recognized by new V2p mAbs derived from
- *30.van Eeden C, Wibmer CK, Scheepers C, Richardson SI, Nonyane M, Lambson B, et al. V2-Directed Vaccine-like Antibodies from HIV-1 Infection Identify an Additional K169-Binding Light Chain Motif with Broad ADCC Activity. Cell Rep. 2018;25(11):3123–35 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]; *First description of V2p mAbs from HIV-infected individuals
- 31.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, et al. Vaccination with ALVAC and AIDSVAX to Prevent HIV-1 Infection in Thailand. N Engl J Med. 2009;361(23):2209–20. [DOI] [PubMed] [Google Scholar]
- 32.Haynes BF, Gilbert PB, McElrath MJ, Zolla-Pazner S, Tomaras GD, Alam SM, et al. Immune Correlates Analysis of the ALVAC-AIDSVAX HIV-1 Vaccine Efficacy Trial. N Engl J Med. 2012;366(14):1275–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zolla-Pazner S, deCamp AC, Cardozo T, Karasavvas N, Gottardo R, Williams C, et al. Analysis of V2 Antibody Responses Induced in Vaccinees in the ALVAC/AIDSVAX HIV-1 Vaccine Efficacy Trial. PLos One. 2013;8(1):e53629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zolla-Pazner S, DeCamp AC, Gilbert PB, Williams C, Yates NL, Williams WT, et al. Vaccine-induced IgG Antibodies to V1V2 Regions of Multiple HIV-1 Subtypes Correlate with Decreased Risk of HIV-1 Infection. Plos One. 2014;9(2):e87572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gorny MK, Pan R, Williams C, Wang XH, Volsky B, O’Neal T, et al. Functional and immunochemical cross-reactivity of V2-specific monoclonal antibodies from human immunodeficiency virus type 1-infected individuals. Virology. 2012;427:198–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pinter A, Honnen WJ, He Y, Gorny MK, Zolla-Pazner S, Kayman SC. The V1/V2 domain of gp120 is a global regulator of sensitivity of primary human immunodeficiency virus type 1 isolates to neutralization by antibodies commonly induced upon infection. J Virol. 2004;78(10):5205–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xiaoying Shen ZM, Arthur McMillan, Derrick Goodman,, Nicole Yates RS, Nicole Grunenberg, Peter Gilbert,, Sanjay Phogat CD, Fatima Laher, Linda-Gail Bekker GG, Larry Corey, Julie McElrath, Guido Ferrari,, Georgia Tomaras. V1V2 IgG and Antibody Fc Effector Functions in a Subtype C ALVAC-HIV and Bivalent Subtype C gp120/MF59 HIV-1 Vaccine Trial in South Africa. HIV Research for Prevention 2018; Abstract OA02.03. [Google Scholar]
- 38.Perez LG, Martinez DR, deCamp AC, Pinter A, Berman PW, Francis D, et al. V1V2-specific complement activating serum IgG as a correlate of reduced HIV-1 infection risk in RV144. PLoS One. 2017;12(7):e0180720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xiaoying Shen ZM, Arthur McMillan, Derrick Goodman,, Nicole Yates RS, Nicole Grunenberg, Peter Gilbert,, Sanjay Phogat CD, Fatima Laher, Linda-Gail Bekker GG, Larry Corey, Julie McElrath, Guido Ferrari,, Georgia Tomaras HaHST. V1V2 IgG and Antibody Fc Effector Functions in a Subtype C ALVAC-HIV and Bivalent Subtype C gp120/MF59 HIV-1 Vaccine Trial in South Africa. HIV Research for Prevention 2018; Madrid. [Google Scholar]
- 40.Zolla-Pazner S, Powell R, Yahyaei S, Williams C, Jiang X, Li W, et al. Rationally-designed Vaccines Targeting the V2 Region of HIV-1 gp120 Induce a Focused, Cross Clade-reactive Biologically Functional Antibody Response. J Virol. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jiang X, Totrov M, Li W, Sampson JM, Williams C, Lu H, et al. Rationally Designed Immunogens Targeting HIV-1 gp120 V1V2 Induce Distinct Conformation-Specific Antibody Responses in Rabbits. J Virol. 2016;90(24):11007–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *42.Hessell AP R; Jiang X; Luo C Weiss S, Dussupt V; Itri V; Fox A; Shapiro M; Pandey S; Cheever T; Henderson H; Fuller DH; Park B; Krebs SJ; Totrov M; Haigwood NL; Kong X-P; Zolla-Pazner S Multimeric Epitope-scafold HIV Vaccines Target V1V2 and Differentially Tune Polyfunctional Antibody Responses. Cell Report. 2019;in press. [DOI] [PMC free article] [PubMed] [Google Scholar]; *Description of the immunogenicity of V1V2-scaffold protein immunogens in non-human primates and of the patterns of the V1V2-focused Ab responses induced.
- 43.Gottardo R, Bailer RT, Korber BT, Gnanakaran S, Phillips J, Shen X, et al. Plasma IgG to Linear Epitopes isn the V2 and V3 Regions of HIV-1 gp120 as Correlates of Infection Risk in the RV144 Vaccine Efficacy Trial. PLoS One. 2013;8(9):e75665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pinter A, Honnen WJ, Kayman SC, Trochev O, Wu Z. Potent neutralization of primary HIV-1 isolates by antibodies directed against epitopes present in the V1/V2 domain of HIV-1 gp120. Vaccine. 1998;16(19):1803–11. [DOI] [PubMed] [Google Scholar]
- 45.Rolland M, Edlefsen PT, Larsen BB, Tovanabutra S, Sanders-Buell E, Hertz T, et al. Increased HIV-1 vaccine efficacy against viruses with genetic signatures in Env V2. Nature. 2012;490(7420):417–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pollara J, Bonsignori M, Moody MA, Liu P, Alam SM, Hwang K-K, et al. HIV-1 vaccine-induced C1 and V2 Env-specific antibodies synergize for increased antiviral activities. J Virol. 2014;88(14):7715–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Desrosiers RC. Protection against HIV Acquisition in the RV144 Trial. J Virol. 2017;91(18). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barouch DH, Liu J, Li H, Maxfield LF, Abbink P, Lynch DM, et al. Vaccine protection against acquisition of neutralization-resistant SIV challenges in rhesus monkeys. Nature. 2012;482(7383):89–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roederer M, Keele BF, Schmidt SD, Mason RD, Welles HC, Fischer W, et al. Immunological and virological mechanisms of vaccine-mediated protection against SIV and HIV. Nature. 2014;505(7484):502–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vaccari M, Gordon SN, Fourati S, Schifanella L, Liyanage NP, Cameron M, et al. Adjuvant-dependent innate and adaptive immune signatures of risk of SIV acquisition. Nat Med. 2016;22(7):762–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pegu P, Vaccari M, Gordon S, Keele BF, Doster M, Guan Y, et al. Antibodies with high avidity to the gp120 envelope protein in protection from simian immunodeficiency virus SIV(mac251) acquisition in an immunization regimen that mimics the RV-144 Thai trial. J Virol. 2013;87(3):1708–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gordon SN, Doster MN, Kines RC, Keele BF, Brocca-Cofano E, Guan Y, et al. Antibody to the gp120 V1/V2 loops and CD4+ and CD8+ T cell responses in protection from SIVmac251 vaginal acquisition and persistent viremia. J Immunol. 2014;193(12):6172–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Singh S, Ramirez-Salazar EG, Doueiri R, Valentin A, Rosati M, Hu X, et al. Control of Heterologous Simian Immunodeficiency Virus SIVsmE660 Infection by DNA and Protein Coimmunization Regimens Combined with Different Toll-Like-Receptor-4-Based Adjuvants in Macaques. J Virol. 2018;92(15). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hessell AJ DM, Philip Barnette, Shilpi Pandey,William Sutton, Lily Liu, Liuzhe Li, Xiang-Peng Kong, Haigwood Nancy L., Gorny Miroslaw K.. Tight Control of SHIV BaL.P4 Challenge in Rhesus Macaques Co-immunized With DNA and Protein HIV Vaccine Regimen. HIV Research for Prevention; Madrid 2018. p. OA09.4. [Google Scholar]
- *55.Weiss S, Vincenza Itri, Ruimin Pan, Xungqing Jiang, Xiang-Peng Kong, Nancy Haigwood, Ann Hessell, Miroslaw K. Gorny, Susan Zolla-Pazner. Tight Control of SHIVBaL in Rhesus Macaques Immunized With gp160 DNA + gp120 Proteins (Clades E and B) Correlates With V2p Antibodies. HIV Research for Prevention; Madrid 2018. p. OA14.06LB. [Google Scholar]; *First description of a correlate of protection from SHIV infection with the presence of V2p Abs
- *56.Hessell AJ, Shapiro MB, Powell R, Malherbe DC, McBurney SP, Pandey S, et al. Reduced cell-associated DNA and improved viral control in macaques following passive transfer of a single anti-V2 monoclonal antibody and repeated SHIV challenges. J Virol. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]; *Efficacy of V2i mAbs passively administered to non-human primates and challenged with SHIVBaL.
- 57.Mayr LM, Su B, Moog C. Non-Neutralizing Antibodies Directed against HIV and Their Functions. Front Immunol. 2017;8:1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gach JS, Bouzin M, Wong MP, Chromikova V, Gorlani A, Yu KT, et al. Human immunodeficiency virus type-1 (HIV-1) evades antibody-dependent phagocytosis. PLoS Pathog. 2017;13(12):e1006793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Perelson AS, Neumann AU, Markowitz M, Leonard JM, Ho DD. HIV-1 dynamics in vivo: virion clearance rate, infected cell life-span, and viral generation time. Science. 1996;271(5255):1582–6. [DOI] [PubMed] [Google Scholar]
- 60.Alexander MR, Sanders RW, Moore JP, Klasse PJ. Short Communication: Virion Aggregation by Neutralizing and Nonneutralizing Antibodies to the HIV-1 Envelope Glycoprotein. AIDS Res Hum Retroviruses. 2015;31(11):1160–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu P, Yates NL, Shen X, Bonsignori M, Moody MA, Liao HX, et al. Infectious virion capture by HIV-1 gp120-specific IgG from RV144 vaccinees. J Virol. 2013;87(14):7828–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Arthos J, Cicala C, Martinelli E, Macleod K, Van Ryk D, Wei D, et al. HIV-1 envelope protein binds to and signals through integrin alpha4beta7, the gut mucosal homing receptor for peripheral T cells. Nat Immunol. 2008;9(3):301–9. [DOI] [PubMed] [Google Scholar]
- *63.Lertjuthaporn S, Cicala C, Van Ryk D, Liu M, Yolitz J, Wei D, et al. Select gp120 V2 domain specific antibodies derived from HIV and SIV infection and vaccination inhibit gp120 binding to alpha4beta7. PLoS Pathog. 2018;14(8):e1007278. [DOI] [PMC free article] [PubMed] [Google Scholar]; *HIV and SIV V2 mAbs elicited by both vaccination and infection block the interaction of V2 and α4β7.
- 64.Peachman Kristina K, Karasavvas N, Chenine A-L, McLinden R, Rerks-Ngarm S, Jaranit K, et al. Identification of new regions in HIV-1 gp120 variable 2 and 3 loops that bind to α4β7 integrin receptor. Retrovirology. 2015;Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Alvarez RA, Hamlin RE, Monroe A, Moldt B, Hotta MT, Rodriguez Caprio G, et al. HIV-1 Vpu antagonism of tetherin inhibits antibody-dependent cellular cytotoxic responses by natural killer cells. J Virol. 2014;88(11):6031–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Holl V, Peressin M, Decoville T, Schmidt S, Zolla-Pazner S, Aubertin AM, et al. Nonneutralizing antibodies are able to inhibit human immunodeficiency virus type 1 replication in macrophages and immature dendritic cells. J Virol. 2006;80(12):6177–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Musich T, Li L, Liu L, Zolla-Pazner S, Robert-Guroff M, Gorny MK. Monoclonal Antibodies Specific for the V2, V3, CD4-Binding Site, and gp41 of HIV-1 Mediate Phagocytosis in a Dose-Dependent Manner. J Virol. 2017;91(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mayr LM, Decoville T, Schmidt S, Laumond G, Klingler J, Ducloy C, et al. Non-neutralizing Antibodies Targeting the V1V2 Domain of HIV Exhibit Strong Antibody-Dependent Cell-mediated Cytotoxic Activity. Sci Rep. 2017;7(1):12655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chung AW, Crispin M, Pritchard L, Robinson H, Gorny MK, Yu X, et al. Identification of antibody glycosylation structures that predict monoclonal antibody Fc-effector function. AIDS. 2014;28:2523–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Burton DR, Hessell AJ, Keele BF, Klasse PJ, Ketas TA, Moldt B, et al. Limited or no protection by weakly or nonneutralizing antibodies against vaginal SHIV challenge of macaques compared with a strongly neutralizing antibody. Proc Natl Acad Sci U S A. 2011;108(27):11181–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Alvarez RA, Maestre AM, Law K, Durham ND, Barria MI, Ishii-Watabe A, et al. Enhanced FCGR2A and FCGR3A signaling by HIV viremic controller IgG. JCI Insight. 2017;2(4):e88226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bonsignori M, Pollara J, Moody MA, Alpert MD, Chen X, Hwang KK, et al. Antibody-dependent cellular cytotoxicity-mediating antibodies from an HIV-1 vaccine efficacy trial target multiple epitopes and preferentially use the VH1 gene family. J Virol. 2012;86(21):11521–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gomez-Roman VR, Patterson LJ, Venzon D, Liewehr D, Aldrich K, Florese R, et al. Vaccine-elicited antibodies mediate antibody-dependent cellular cytotoxicity correlated with significantly reduced acute viremia in rhesus macaques challenged with SIVmac251. J Immunol. 2005;174(4):2185–9. [DOI] [PubMed] [Google Scholar]
- 74.Excler JL, Ake J, Robb ML, Kim JH, Plotkin SA. Nonneutralizing Functional Antibodies: a New “Old” Paradigm for HIV Vaccines. Clin Vaccine Immunol. 2014;21(8):1023–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Aasa-Chapman MM, Holuigue S, Aubin K, Wong M, Jones NA, Cornforth D, et al. Detection of antibody-dependent complement-mediated inactivation of both autologous and heterologous virus in primary human immunodeficiency virus type 1 infection. J Virol. 2005;79(5):2823–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Spear GT, Landay AL, Sullivan BL, Dittel B, Lint TF. Activation of complement on the surface of cells infected by human immunodeficiency virus. J Immunol. 1990;144(4):1490–6. [PubMed] [Google Scholar]
- 77.Spear GT, Sullivan BL, Landay AL, Lint TF. Neutralization of HIV-1 by complement occurs by viral lysis. J Virol. 1990;64(12):5869–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sips M, Krykbaeva M, Diefenbach TJ, Ghebremichael M, Bowman BA, Dugast AS, et al. Fc receptor-mediated phagocytosis in tissues as a potent mechanism for preventive and therapeutic HIV vaccine strategies. Mucosal Immunol. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ackerman ME, Mikhailova A, Brown EP, Dowell KG, Walker BD, Bailey-Kellogg C, et al. Polyfunctional HIV-Specific Antibody Responses Are Associated with Spontaneous HIV Control. PLoS Pathog. 2016;12(1):e1005315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *80.Alter G, Dowell KG, Brown EP, Suscovich TJ, Mikhailova A, Mahan AE, et al. High-resolution definition of humoral immune response correlates of effective immunity against HIV. Mol Syst Biol. 2018;14(3):e7881. [DOI] [PMC free article] [PubMed] [Google Scholar]; *Systemic serological analysis of a cohort of HIV-infected subjects with varying degrees of viral control revealed multifaceted and coordinated contributions of polyclonal antibodies to diverse antiviral responses and suggests key biophysical features predictive of viral control.
- 81.Forthal DN, Gilbert PB, Landucci G, Phan T. Recombinant gp120 vaccine-induced antibodies inhibit clinical strains of HIV-1 in the presence of fc receptor-bearing effector cells and correlate inversely with HIV infection rate. J Immunol. 2007;178(10):6596–603. [DOI] [PubMed] [Google Scholar]
- 82.Hidajat R, Xiao P, Zhou Q, Venzon D, Summers LE, Kalyanaraman VS, et al. Correlation of vaccine-elicited systemic and mucosal nonneutralizing antibody activities with reduced acute viremia following intrarectal simian immunodeficiency virus SIVmac251 challenge of rhesus macaques. J Virol. 2009;83(2):791–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Florese RH, Demberg T, Xiao P, Kuller L, Larsen K, Summers LE, et al. Contribution of nonneutralizing vaccine-elicited antibody activities to improved protective efficacy in rhesus macaques immunized with Tat/Env compared with multigenic vaccines. J Immunol. 2009;182(6):3718–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xiao P, Zhao J, Patterson LJ, Brocca-Cofano E, Venzon D, Kozlowski PA, et al. Multiple vaccine-elicited nonneutralizing antienvelope antibody activities contribute to protective efficacy by reducing both acute and chronic viremia following simian/human immunodeficiency virus SHIV89.6P challenge in rhesus macaques. J Virol. 2010;84(14):7161–73. [DOI] [PMC free article] [PubMed] [Google Scholar]