Abstract
Objective:
Klotho and Fibroblast Growth Factor (FGF)-23 were recently postulated as candidate biomarkers and/or therapeutic targets in acute kidney injury (AKI). We examined whether urine Klotho and serum intact FGF23 levels were differentially and independently associated with major adverse kidney events (MAKE) in critically ill patients with and without AKI.
Design:
Single-center, prospective, case-control study.
Setting:
ICU in a tertiary medical center.
Patients:
54 AKI patients and 52 controls without AKI admitted to the ICU.
Interventions:
None.
Measurements and Main Results:
AKI was defined by KDIGO criteria and included only AKI stage ≥2. Controls were matched by age, gender, and baseline eGFR. Paired serum and urine samples were obtained 24–48h after AKI diagnosis (cases) or ICU admission (controls). The primary outcome was 90-day MAKE, which was the composite of all-cause death, dependence on renal replacement therapy or a 50% or higher decrease in eGFR from baseline. Forty-four (41.5%) patients developed MAKE-90. Patients who developed MAKE-90 had more comorbidity, higher acuity of illness scores and more prevalent AKI. Levels of urine Klotho adjusted by creatinine (Cr) were lower and serum intact FGF23 levels were higher in AKI patients vs. ICU controls. In adjusted models, the highest vs. lowest tertile of urine Klotho/Cr was independently associated with an overall 95% lower risk of MAKE-90 (81% lower risk in patients with AKI). The highest vs. lowest tertile of serum intact FGF23 was associated with >300% higher risk of MAKE-90.
Conclusions:
Urine Klotho/Cr levels were significantly lower and serum intact FGF23 levels significantly higher in critically ill patients with AKI vs. matched-controls without AKI. When measured in the first 48h of ICU admission or AKI diagnosis, urine Klotho/Cr independently associated with major adverse kidney events, particularly in patients with AKI. These results show promise for testing these biomarkers –individually or in combination– as part of novel risk-prediction models of renal outcomes in the ICU.
Keywords: Klotho, FGF23, acute kidney injury, critical illness, ICU, major adverse kidney events
Introduction
Acute kidney injury (AKI) is a detrimental syndrome that occurs in approximately 50% of critically ill patients admitted to intensive care units (ICU).(1, 2) AKI is associated with high morbidity, mortality(3, 4) and risk of chronic kidney disease (CKD)(5, 6) and end-stage renal disease (ESRD).(7) Discovery and validation of AKI biomarkers have mostly focused on early AKI recognition(8, 9) and little is known about their utility to predict AKI recovery or subsequent CKD/ESRD risk post-AKI. Kidney function examination within 90 days post-discharge in survivors of AKI associates with subsequent risk of ESRD and has been proposed as a surrogate end point for ESRD after AKI.(10)
αKlotho (referred to as Klotho) is primarily expressed in the kidney(11) and has emerged as a potential therapeutic agent for AKI in preclinical studies.(12) Transmembrane Klotho is a co-receptor for fibroblast growth factor (FGF)-23. The extracellular domain of transmembrane Klotho is cleaved by proteases(13–15) and released into the circulation (soluble Klotho) and can be measured in blood and urine.(16, 17) Soluble Klotho is not filtered by the glomerulus but traffics via transcytosis across the renal tubules, from the basolateral to the luminal side, exerts actions in the urine, and is then excreted in the urine.(17) Preclinical data have shown that Klotho protein replacement or transgenic overexpression(18–20) can attenuate AKI, promote recovery, and prevent CKD post-AKI.(18, 21)
Elevation of plasma FGF23 levels has been described in AKI(22, 23) and associates with adverse outcomes post-AKI in critically ill and cardiac surgery patients.(24–26) Impaired renal clearance or increased production from bone or other organs are potential origins of this elevation.(27)
The purpose of the present study was to examine whether urine Klotho and serum intact FGF23 levels are different in critically ill patients with vs. without AKI and if these biomarker levels associate with major adverse kidney events. To our knowledge, this is the first study in critically ill patients in which concomitant measurements of urine Klotho and serum intact FGF23 levels were done.
Methods
Study Design and Participants
Single-center, prospective study of 54 AKI patients and 52 matched-controls without AKI admitted to the ICU at the UT Southwestern Hospital from January 2015 through January 2016. AKI was defined by KDIGO criteria.(28) Only patients with AKI stage ≥2 were included in the study as cases. Controls were frequency-matched by age (10-year intervals), gender and 2-category baseline estimated glomerular filtration rate (eGFR, calculated using CKD EPI equation, ≥90 and 60–89 ml/min/1.73 m2).(29) Baseline serum creatinine (SCr) was defined as the most recent SCr within the 6-month period before ICU admission.
Inclusion criteria included: adults ≥18 years of age, admission to the ICU, and baseline eGFR ≥60 ml/min/1.73 m2. Exclusion criteria consisted of prior kidney or any other solid organ transplant, ESRD, evidence of AKI before ICU admission, or the presence of uroepithelial tumors. The protocol was approved by the Institutional Review Board. Informed consent was obtained for all study participants.
Sample Collection
A single-timepoint paired serum and urine samples were obtained 24–48 hours after AKI diagnosis (cases) or ICU admission (controls). Standardized techniques for serum and urine collection, transport, processing, and storage were employed. Biospecimens were centrifuged at 1000 g, 4°C for 10 minutes. Serum and urine supernatant were aliquoted in codified non-siliconized cryovials and stored at −80°C until biomarker measurements were done by laboratory personnel blinded to the study design and data. Urine samples were not obtained in 7 patients due to anuria.
Laboratory Analyses
Urine Klotho was measured by immunoblotting urine with monoclonal anti-Kl1 antibody KM2076 (TransGenic Inc., Kobe, Japan) with calibration against Klotho standards.(30) Urine Klotho was normalized to creatinine concentration in the same spot urine sample. The intra-assay coefficient of variation is 8%. Serum intact FGF23 was measured using the human Intact FGF23 ELISA kit (Immutopics-Quidel, San Diego, CA). Urine creatinine was measured by capillary electrophoresis.(31) Other reported analytes were measured as part of standard of care.
Clinical Data
Data pertaining to demographics, kidney function, comorbidity, Charlson index, and critical illness parameters were obtained. Acute Physiology and Chronic Health Evaluation II (APACHE II) and Sequential Organ Failure Assessment (SOFA) scores were calculated integrating data from the first day of ICU admission. For both APACHE II and SOFA, the points related to SCr were subtracted from the total score. Anemia was defined as admission hematocrit <39% for men and <36% for women.
Study Outcome
The observation period was from study enrollment until death or 90 days for survivors. The primary outcome was 90-day major adverse kidney events (MAKE-90), which consisted of the composite of all-cause death, renal replacement therapy (RRT)-dependence or the decrease in eGFR of ≥50% from baseline.(32) Secondary outcomes consisted of mechanical ventilation days and hospital days and were examined only in hospital survivors to offset the competing risk of death.
Statistical Analysis
Categorical data were reported as percentages and continuous data as means ± SD or median [25th-75th percentile]. Comparisons across biomarker tertiles for categorical variables were made using Fisher’s exact test. For continuous variables, ANOVA was used for Gaussian and Kruskal-Wallis test for non-Gaussian distributed data. Comparisons between AKI vs. no-AKI and MAKE-90 vs. no MAKE-90 for categorical variables were made using Fisher’s exact test. For continuous variables, t-test was used for Gaussian and Wilcoxon rank-sum test for non-Gaussian distributed data. Biomarker data were non-Gaussian distributed and were therefore natural log-transformed.
Multivariable logistic regression models were constructed for MAKE-90 as the dependent variable and to evaluate the following biomarkers as independent variables: urine Klotho, urine Klotho adjusted by urine creatinine (urine Klotho/Cr), serum intact FGF23, and serum intact FGF23 divided by urine Klotho adjusted by urine creatinine (FGF23-to-Klotho/Cr ratio). Model 1 included age, sex and Charlson index. Model 2 included variables in Model 1 plus non-renal APACHE II score. Only 1 of 2 acuity of illness scores (APACHE II or SOFA) was included due to collinearity between variables. Secondary outcomes (hospital days and mechanical ventilation days) were examined only in those that survived the hospitalization utilizing negative binomial regression adjusted for Model 2. The two-way interaction between AKI status and each study biomarker for its association with MAKE-90 was evaluated and found to be non-significant (p >0.1 for all biomarkers).
Spearman correlation analysis was performed for each study biomarker and pre-selected biochemical parameters measured less than 48 hours apart from the study biomarkers. Two-sided p values of less than 0.05 indicated statistical significance. SAS 9.4 (SAS Institute, Cary, NC) was used for statistical analyses.
Sensitivity Analyses
We examined an alternative and more sensitive definition for the primary outcome of MAKE-90, which consisted of the composite of all-cause death, RRT-dependence or the decrease in eGFR of ≥25% from baseline (rather than 50%).(32)
Results
Clinical Characteristics
A total of 106 patients were included in the study, 54 with AKI stage ≥2 and 52 without AKI (controls). When comparing characteristics according to tertiles of urine Klotho/Cr, we observed that patients in the lowest tertile of urine Klotho/Cr had more frequently diabetes, anemia, and dipstick proteinuria ≥30 mg/dl. Concordantly, patients in the lowest tertile of urine Klotho/Cr had higher Charlson index scores and higher SOFA and APACHE II scores, along with worse critical illness parameters when compared with their counterparts (Table 1). As depicted in Supplementary Table 1, patients in the highest tertile of FGF23 had more frequently anemia and dipstick proteinuria ≥30 mg/dl, higher Charlson scores, and higher APACHE II scores.
Table 1.
Characteristics of patients according to tertiles of Urine Klotho/Cr
| Tertile 1 | Tertile 2 | Tertile 3 | ||
|---|---|---|---|---|
| Range, fmol/mg | ≤11.4 | 11.7 to 21.2 | 22.3 to 92.8 | P-value |
| Number of patients | 33 | 33 | 33 | |
| Demographics | ||||
| Age, years ± SD | 56.9 ± 17.3 | 61.0 ± 14.6 | 53.5 ± 15.3 | 0.16 |
| Women, n (%) | 16 (48.5) | 11 (33.3) | 15 (45.5) | 0.44 |
| White race, n (%) | 23 (69.7) | 22 (66.7) | 24 (72.7) | 0.77 |
| BMI, kg/m2, years ± SD | 30.4 ± 9.8 | 27.5 ± 6.8 | 27.3 ± 7.5 | 0.23 |
| Baseline kidney function | ||||
| eGFR, ml/min/1.73m2, (median IQR) | 90.2 [70.1, 100.2] | 91.9 [78.9, 100.2] | 96.6 [83.7, 103.0] | 0.49 |
| SCr, mg/dl, (median IQR) | 0.9 [0.7, 1.0] | 0.9 [0.6, 1.0] | 0.9 [0.7, 1.0] | 0.82 |
| Dipstick proteinuria >30 mg/dl, n (%) | 24 (72.7) | 11 (33.3) | 7 (21.2) | <0.0001 |
| Comorbidity | ||||
| Diabetes, n (%) | 13 (39.4) | 6 (18.2) | 6 (18.2) | 0.09 |
| Hypertension, n (%) | 20 (60.6) | 16 (48.5) | 19 (57.6) | 0.67 |
| Congestive heart failure, n (%) | 11 (33.3) | 10 (30.3) | 9 (27.3) | 0.96 |
| COPD, n (%) | 6 (18.2) | 3 (9.1) | 6 (18.2) | 0.54 |
| Liver disease, n (%) | 11 (33.3) | 6 (18.2) | 4 (12.1) | 0.14 |
| Anemia, n (%) | 29 (87.9) | 18 (54.6) | 23 (69.7) | 0.01 |
| Cancer, n (%) | 15 (45.5) | 16 (50.0) | 14 (42.4) | 0.85 |
| Charlson Index, (median IQR) | 5 [2, 8] | 3 [2, 5] | 2 [2, 4] | 0.008 |
| Critical illness parameters | ||||
| CFB 72 hours, liters, (median IQR) | 5.0 [2.1, 8.0] | 1.7 [−0.1, 4.3] | 1.0 [−0.3, 2.9] | 0.004 |
| CFB ICU stay, liters, (median IQR) | 8.5 [0.9, 14] | 1.7 [−0.1, 4.6] | 1.0 [−2, 3.5] | 0.003 |
| Pressor or inotrope, n (%) | 21 (63.6) | 14 (42.4) | 11 (33.3) | 0.04 |
| Mechanical ventilation, n (%) | 22 (66.7) | 15 (45.5) | 11 (33.3) | 0.03 |
| PRBC transfusion, n (%) | 21 (63.6) | 13 (39.4) | 9 (27.3) | 0.01 |
| Non-renal APACHE II score, (median IQR) | 18 [15, 22] | 18 [12, 20] | 11 [7, 17] | 0.004 |
| Non-renal SOFA score, (median IQR) | 8 [4, 11] | 6 [1, 8] | 2 [1, 6] | 0.0008 |
APACHE II = Acute Physiology and Chronic Health Evaluation II (the points related to SCr were subtracted from the total score); BMI = body mass index; CFB = cumulative fluid balance; COPD = chronic obstructive pulmonary disease; eGFR = estimated glomerular filtration rate (CKD-EPI equation); PRBC = packed red blood cell; SCr = serum creatinine; SOFA = Sequential Organ Failure Assessment (the points related to SCr were subtracted from the total score)
Characteristics according to AKI status are reported in Supplementary Table 2. There were no differences in age, gender or baseline eGFR as per study design. However, patients with AKI had more frequently anemia, liver disease and higher Charlson scores. Similarly, patients with AKI had higher SOFA and APACHE II scores along with worse critical illness parameters when compared with those without AKI.
Study Outcomes
A total of 44 (41.5%) patients developed MAKE-90: 33 (75%) died, 3 (6.8%) became RRT-dependent, and 8 (18.2%) survived, were RRT-independent but had a decrease in eGFR of ≥50% from baseline by 90 days post-enrollment. Patients that developed MAKE-90 had higher Charlson scores and higher critical illness scores (Supplementary Table 3). MAKE-90 occurred more frequently in patients that suffered from AKI (70.4% vs 29.6%, p<0.0001).
Study Biomarkers according to AKI status
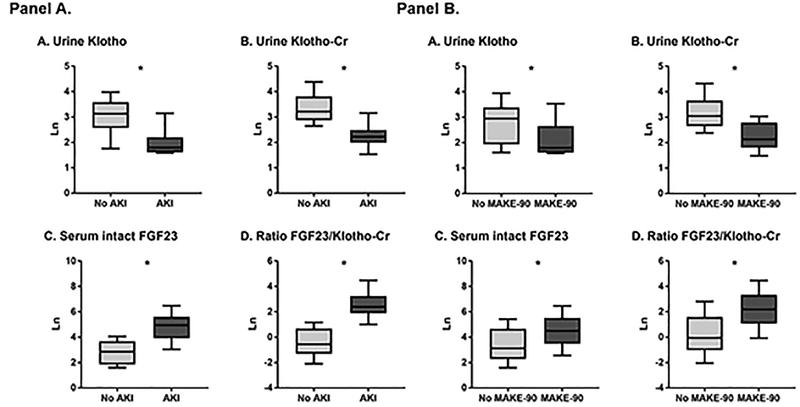
Urine Klotho (pM) and urine Klotho/Cr (fmol/mg of Cr) levels were significantly lower in patients with AKI vs. without AKI: median (25th-75th percentile), 6.0 (4.9–45.0) vs. 23.0 (5.0–67.4), p<0.0001 and 9.2 (3.0–33.6) vs. 25.0 (6.0–92.8), p<0.0001, respectively). Serum intact FGF23 (pg/ml) was significantly higher in patients with AKI vs. without AKI: 139.4 (10.5–4844.4) vs. 17.4 (4.9–196.0), p<0.0001. Similarly, FGF23-to-Klotho/Cr ratios were significantly higher in patients with AKI vs. without AKI (Figure 1).
Figure 1.
Study biomarkers according to Panel A: AKI status, and Panel B: MAKE-90 outcome. All biomarker measurements are natural log-transformed.
Association of Study Biomarkers with MAKE-90
Urine Klotho (pM) and urine Klotho/Cr (fmol/mg of Cr) levels were significantly lower in patients who had MAKE-90 vs. those who did not: median (25th-75th percentile), 6.0 (4.9–54.6) vs. 19.0 (4.9–67.4), p=0.0008 and 8.4 (3.0–28.7) vs. 21.2 (4.7–92.8), p<0.0001, respectively). Serum intact FGF23 (pg/ml) was significantly higher in patients who had vs. did not have MAKE-90: 89.2 (4.9–3151.0) vs. 22.9 (4.9–4844.4), p=0.0004. Similarly, FGF23-to-Klotho/Cr ratios were significantly higher in patients who had vs. did not have MAKE-90 (Figure 1).
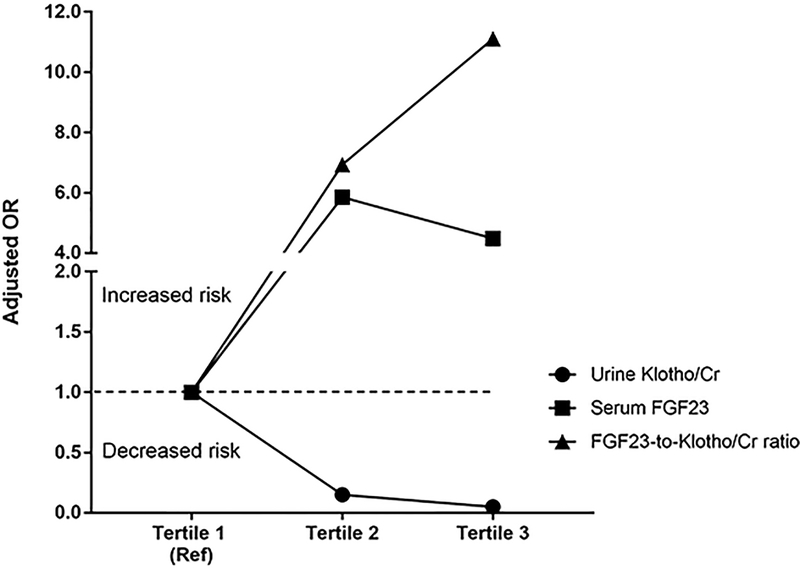
In adjusted models, each 1-fold higher urine Klotho/Cr levels was associated with an 83% (95% CI: 60–93%) lower risk of developing MAKE-90. In contrast, each 1-fold higher serum intact FGF23 levels was associated with a 90% (95% CI: 17–210%) higher risk of developing MAKE-90. Similarly, each 1-fold increase in the FGF23-to-Klotho/Cr ratio was associated with a 172% (95% CI: 49–396%) higher risk of developing MAKE-90 (Table 2). When biomarker levels were stratified in tertiles, the highest tertile of urine Klotho/Cr (vs. the lowest tertile) was independently associated with a 95% (95% CI: 75–99%) lower risk of developing MAKE-90. In contrary, the highest tertile of both serum intact FGF23 and the FGF23-to-Klotho/Cr ratio (vs. the lowest tertile) were associated with higher risk of MAKE-90 (Table 2 and Figure 2).
Table 2.
Association of biomarkers with MAKE-90 outcome (death, RRT-dependency, 50% decrease in eGFR)
| Tertile 1 | Tertile 2 | Tertile 3 | Per 1-fold higher | |||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |||||
| Urine Klotho | Range, pM | ≤6.0 | 6.8 to 21.1 | 22.0 to 67.4 | ||||
| No. of patients | 36 | 30 | 33 | |||||
| No. of MAKE-90 | 22 | 9 | 7 | |||||
| Model 1 | 1.00 (Ref) | 0.20 (0.06, 0.66) | 0.21 (0.07, 0.67) | 0.52 (0.32, 0.87) | ||||
| Model 2 | 1.00 (Ref) | 0.23 (0.07, 0.82) | 0.21 (0.06, 0.71) | 0.50 (0.30, 0.86) | ||||
| Urine Klotho/Cr | Range, fmol/mg | ≤11.4 | 11.7 to 21.2 | 22.3 to 92.8 | ||||
| No. of patients | 33 | 33 | 33 | |||||
| No. of MAKE-90 | 25 | 10 | 3 | |||||
| Model 1 | 1.00 (Ref) | 0.18 (0.05, 0.56) | 0.04 (0.01, 0.19) | 0.17 (0.07, 0.39) | ||||
| Model 2 | 1.00 (Ref) | 0.15 (0.04, 0.55) | 0.05 (0.01, 0.25) | 0.17 (0.07, 0.40) | ||||
| Serum FGF23 | Range, pg/ml | ≤21.5 | 22.5 to 100.9 | 104.3 to 4844.4 | ||||
| No. of patients | 35 | 36 | 35 | |||||
| No. of MAKE-90 | 6 | 18 | 20 | |||||
| Model 1 | 1.00 (Ref) | 3.74 (1.18, 11.79) | 4.30 (1.34, 13.84) | 1.86 (1.17, 2.96) | ||||
| Model 2 | 1.00 (Ref) | 5.85 (1.62, 21.18) | 4.48 (1.29, 15.53) | 1.90 (1.17, 3.10) | ||||
| FGF23-to-Klotho/Cr ratio | Range, loge x | −2.93 to −0.14 | −0.07 to 2.00 | 2.01 to 6.22 | ||||
| No. of patients | 33 | 33 | 33 | |||||
| No. of MAKE-90 | 3 | 13 | 22 | |||||
| Model 1 | 1.00 (Ref) | 5.29 (1.29, 21.65) | 12.81 (3.03, 54.11) | 2.81 (1.58, 5.02) | ||||
| Model 2 | 1.00 (Ref) | 6.93 (1.54, 31.10) | 11.11 (2.41, 51.29) | 2.72 (1.49, 4.96) | ||||
Model 1: Adjust for age, sex, Charlson Comorbidity Index
Model 2: Adjusted for age, sex, Charlson Comorbidity Index and non-renal APACHE II score
Figure 2.
Association of biomarkers with MAKE-90 outcome (death, RRT-dependency, 50% decrease in eGFR). The adjusted odds ratios for the higher tertiles (Tertiles 2 and 3) of each biomarker are represented in reference to the lowest tertile (Tertile 1).
There was no statistical interaction between the AKI status and the association between the study biomarkers and MAKE-90 (p >0.1 for each biomarker). Notably, urine Klotho/Cr was the only biomarker significantly associated with MAKE-90 in patients with AKI (Supplementary Table 4). There were 16 (25.8%) patients who had AKI but did not develop MAKE-90. In these patients, urine Klotho/Cr was significantly higher and serum intact FGF23 lower, when compared to those who developed MAKE-90: median (25th-75th percentile), 26.3 (12.4–92.8) vs. 8.4 (3.0–28.7), p <0.0001 and 17.4 (4.9–196.0) vs. 89.2 (4.9–3151.0), p <0.0001, respectively.
Association of Study Biomarkers with Secondary Outcomes
In adjusted models, urine Klotho and urine Klotho/Cr levels were inversely correlated with total hospital days and mechanical ventilation days in hospital survivors. In contrast, serum intact FGF23 and the FGF23-to-Klotho/Cr ratio were positively correlated with total hospital days and mechanical ventilation days in hospital survivors (Table 3). The median length of hospital stay was 10.5 (1–63) days for the 82 patients that survived the hospitalization.
Table 3.
Associations of biomarkers with secondary outcomes
| Biomarker | Outcome | β (95% CI) | P-value |
|---|---|---|---|
| Urine Klotho | |||
| Hospital days | −0.18 (−0.36 to −0.0004) | 0.05 | |
| MV days | −0.62 (−1.05 to −0.19) | 0.005 | |
| Urine Klotho/Cr | |||
| Hospital days | −0.27 (−0.47 to −0.08) | 0.006 | |
| MV days | −0.98 (−1.57 to −0.38) | 0.002 | |
| Serum FGF23 | |||
| Hospital days | 0.23 (0.13 to 0.32) | <0.0001 | |
| MV days | 0.45 (0.17 to 0.73) | 0.0001 | |
| FGF23-to-Klotho/Cr ratio | |||
| Hospital days | 0.17 (0.10 to 0.25) | <0.0001 | |
| MV days | 0.33 (0.13 to 0.54) | 0.002 |
Hospital survivors only (n=82)
Model 2: Adjusted for age, sex, Charlson Comorbidity Index and non-renal APACHE II score
Correlation between Study Biomarkers and Biochemical Parameters
Urine Klotho/Cr levels were positively correlated with serum calcium, serum albumin, and serum bicarbonate levels (r 0.047 to 0.184, p <0.05) and negatively correlated with serum phosphate and serum magnesium. In contrast, serum intact FGF23 levels were positively correlated with serum phosphate levels (r=0.379, p <0.001) and negatively correlated with serum albumin, serum bicarbonate and arterial pH (Supplementary Figure 1).
Sensitivity Analyses
Similar results were obtained when the MAKE-90 definition was modified to the composite of all-cause death, RRT-dependence or the decrease in eGFR of ≥25% (rather than 50%) from baseline. In adjusted models, each 1-fold higher urine Klotho/Cr levels was associated with an 83% (95% CI: 60–93%) lower risk of MAKE-90. Each 1-fold higher serum intact FGF23 levels was associated with a 110% (95% CI: 26–251%) higher risk of MAKE-90. Similar associations to the primary analysis were observed when biomarker levels were stratified in tertiles (Supplementary Table 5).
Discussion
The new finding of our study is that both urine Klotho/Cr and serum intact FGF23 levels associate with MAKE-90 in critically ill patients admitted to the ICU. Specifically, we found that higher urine Klotho/Cr levels were associated with a decreased risk of MAKE-90 while higher serum intact FGF23 levels were associated with an increased risk of MAKE-90 in this subset of critically ill patients. These findings are timely and relevant because increased circulating FGF23 levels(33–35) and reduced Klotho expression(18, 36–38) have been consistently described in experimental models of AKI. While higher circulating FGF23 levels have been previously associated with human AKI(22, 23) and increased morbidity and mortality in critically ill patients,(25, 26, 39) data related to soluble Klotho levels are scarce.(40) Our study builds upon evidence that serum intact FGF23 and urine Klotho/Cr levels may inform novel risk-prediction models focusing on renal outcomes in critically ill patients with and without AKI. In addition, our study underpins the relevance of ongoing experimental work targeting therapies to modify circulating levels or downstream effects of FGF23 and Klotho pathways in AKI and critical illness.
Low renal Klotho mRNA and protein levels have been described in experimental AKI.(18, 36–38) Interestingly, in murine models of post-ischemia reperfusion injury (IRI) compounded by high-phosphate diet starting 2 weeks after IRI (CKD was detected functionally and histologically ~22 weeks post-IRI), renal Klotho mRNA and protein expression showed a subacute and progressive decline starting at day 14 post-IRI, particularly in mice exposed to prolonged ischemia.(41) In the latter model, administration of recombinant Klotho protein given for 4 consecutive days beginning 24 hours after IRI accelerated kidney recovery, reduced kidney fibrosis, enhanced endogenous renal Klotho mRNA and protein expression, and attenuated the occurrence of CKD.(41) Multiple mechanisms of Klotho-related renoprotection have been postulated such as: suppression of apoptosis(38, 42) and cell senescence,(43, 44) anti-fibrosis,(37, 45–48) and upregulation of autophagy(41, 49) in renal tubular cells.
Elevated plasma FGF23 levels have been reported in murine models of AKI.(33–35, 50) Nonetheless, the mechanisms of FGF23 elevation in AKI are not known. Impaired excretion or catabolism of FGF23 by the kidneys or increased production from bone or other organs could be reasonable explanations for this elevation.(33–35, 50–52) Measurement of intact FGF23 –the biologically active form– is less represented than FGF23 assays with C-terminal reagents (non-discriminatory between full length and C-terminal fragments) in human AKI literature. Elevation of FGF23 in AKI can augment myofibroblast activation and promote fibrosis via activation of TGF-β pathways.(53)
Data related to urine Klotho measurements in human AKI are scarce. Hu et al. demonstrated lower urine Klotho/Cr levels in hospitalized AKI patients when compared to healthy volunteers (4.85 ± 1.69 vs. 25.38 ± 4.08 fmol per mg of Cr, p <0.01).(18) In contrast, Torregosa et al. reported that urine Klotho/Cr levels were not different in patients with AKI (cardiac surgery or coronary angiography-related) vs. no AKI.(54) It is important to note that the Klotho assays utilized were different. Nonetheless, concerns regarding a reliable soluble Klotho assay have precluded large scale studies.(30)
Elevation of plasma FGF-23 levels has been described in human AKI.(22, 23) In a cohort of 250 adult patients undergoing cardiac surgery, Leaf et al. reported that plasma C-terminal FGF23 levels were differentially elevated starting at the end of cardiopulmonary bypass in patients who did vs. did not develop postoperative AKI.(23) In critically ill patients, elevated levels of C-terminal FGF23 have been also reported to be independently associated with incident AKI.(26, 39) In a recent post-hoc analysis, critically ill patients with the highest vs. lowest quartiles of plasma C-terminal and intact FGF23 were found to have higher 60-day mortality.(25)
Our study has important strengths that need to be delineated. First, we included patients with well characterized AKI (KDIGO stage ≥2) and controls in the ICU that were matched on clinical parameters that can potentially affect both the independent and dependent variables in the analysis. Second, we utilized SCr and urine output criteria to define AKI, which is particularly appropriate in the ICU setting. Third, we adjusted our multivariable analyses for appropriate confounders, including objective and comprehensive comorbidity and critical illness scores. Fourth, we measured –for the first time– serum intact FGF23 and urine Klotho/Cr using paired biospecimens of critically ill patients, which highlights their bidirectional relationship in human AKI and their association with adverse outcomes. Fifth, we measured urine Klotho using a previously reported and validated method that is available and can be replicated.(30) Finally, our study tested a relevant 90-day renal outcome which is a recommended endpoint because it relates to the risk of CKD/ESRD post-AKI.(32)
Our study also has limitations. First, although our study sample is representative of a heterogeneous ICU population, our study design is also susceptible to selection bias as we included patients with AKI stage ≥2 and corresponding matched-controls for pre-specified clinical characteristics. Further, our sample size is relatively small for the examination of associations of study biomarkers with MAKE-90 according to AKI status. Nonetheless, we did not find a significant interaction between study biomarkers and AKI status for MAKE-90. Second, we did not clinically adjudicate etiology of AKI which can also provide important information for risk-stratification of adverse outcome. Third, although we adjusted for confounders by comprehensive comorbidity and critical illness scores, residual confounding by unmeasured covariates may not have been completely eliminated. Finally, our study did not specifically test the utility (performance) of urine Klotho/Cr or serum intact FGF23 for the prediction of MAKE-90 but identified an important bidirectional relationship between the levels of these biomarkers measured early during the course of ICU admission or AKI diagnosis and subsequent adverse renal outcomes.
Conclusions
Urine Klotho/Cr levels were significantly lower and serum intact FGF23 levels significantly higher in critically ill patients with AKI vs. matched-controls without AKI. When measured in the first 48 hours of ICU admission or AKI diagnosis, urine Klotho/Cr independently associated with major adverse kidney events, particularly in patients with AKI. These results show promise for testing these biomarkers –individually or in combination– as part of novel risk-prediction models of renal outcomes in the ICU. Nonetheless, our results need to be validated in a larger sample of critically ill patients with and without AKI to further underpin the impact of Klotho for diagnostics and therapeutics in AKI and critical illness.
Supplementary Material
Supplementary Table 1.Characteristics of patients according to tertiles of serum intact FGF23
Supplementary Table 2.Characteristics of patients according to AKI status
Supplementary Table 3.Characteristics of patients according to MAKE-90 outcome (death, RRT-dependency, 50% decrease in eGFR)
Supplementary Table 4.Associations of biomarkers with MAKE-90 (death, RRT-dependency, 50% decrease in eGFR) by AKI status
Supplementary Table 5.Association of biomarkers with MAKE-90 outcome (death, RRT-dependency, 25% decrease in eGFR)
Supplementary Figure 1.Correlations between i) urine Klotho/Cr and ii) serum FGF23 and standard-of-care biochemical parameters. All measurements are natural log-transformed.
Financial Support
Research reported in this publication was supported by the University of Texas (UT) Southwestern Medical Center O’Brien Kidney Research Core Center (P30 DK079328–06), the National Center for Advancing Translational Sciences (UL1TR001105), and the National Institutes of Health (R01 DK092461–04S1). The content is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health or the UT Southwestern. JAN was supported by the Ben J. Lipps Research Fellowship Program of American Society of Nephrology Foundation for Kidney Research, the Truelson Fellowship Fund and the Seldin-Pak Center of Metabolic Research at UT Southwestern Charles and Jane Pak Center of Mineral Metabolism and Clinical Research. JAN is currently supported by an Early Career Pilot Grant from the National Center for Advancing Translational Sciences, National Institutes of Health, through grant UL1TR001998.
Footnotes
Disclosures
The authors declare that they have no relevant financial interests.
References
- 1.Mandelbaum T, Scott DJ, Lee J, et al. Outcome of critically ill patients with acute kidney injury using the Acute Kidney Injury Network criteria. Crit Care Med 2011;39(12):2659–2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neyra JA, Manllo J, Li X, et al. Association of de novo dipstick albuminuria with severe acute kidney injury in critically ill septic patients. Nephron Clinical practice 2014;128(3–4):373–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Waikar SS, Liu KD, Chertow GM. Diagnosis, epidemiology and outcomes of acute kidney injury. Clinical journal of the American Society of Nephrology: CJASN 2008;3(3):844–861. [DOI] [PubMed] [Google Scholar]
- 4.Lafrance JP, Miller DR. Acute kidney injury associates with increased long-term mortality. Journal of the American Society of Nephrology: JASN 2010;21(2):345–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chawla LS, Amdur RL, Amodeo S, et al. The severity of acute kidney injury predicts progression to chronic kidney disease. Kidney international 2011;79(12):1361–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney international 2012;81(5):442–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wald R, Quinn RR, Adhikari NK, et al. Risk of chronic dialysis and death following acute kidney injury. The American journal of medicine 2012;125(6):585–593. [DOI] [PubMed] [Google Scholar]
- 8.Kashani K, Al-Khafaji A, Ardiles T, et al. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit Care 2013;17(1):R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bihorac A, Chawla LS, Shaw AD, et al. Validation of cell-cycle arrest biomarkers for acute kidney injury using clinical adjudication. Am J Respir Crit Care Med 2014;189(8):932–939. [DOI] [PubMed] [Google Scholar]
- 10.Grams ME, Sang Y, Coresh J, et al. Candidate Surrogate End Points for ESRD after AKI. Journal of the American Society of Nephrology: JASN 2016;27(9):2851–2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kato Y, Arakawa E, Kinoshita S, et al. Establishment of the anti-Klotho monoclonal antibodies and detection of Klotho protein in kidneys. Biochem Biophys Res Commun 2000;267(2):597–602. [DOI] [PubMed] [Google Scholar]
- 12.Hu MC, Moe OW. Klotho as a potential biomarker and therapy for acute kidney injury. Nature reviews Nephrology 2012;8(7):423–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen CD, Tung TY, Liang J, et al. Identification of cleavage sites leading to the shed form of the anti-aging protein klotho. Biochemistry 2014;53(34):5579–5587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bloch L, Sineshchekova O, Reichenbach D, et al. Klotho is a substrate for alpha-, beta- and gamma-secretase. FEBS Lett 2009;583(19):3221–3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen CD, Podvin S, Gillespie E, et al. Insulin stimulates the cleavage and release of the extracellular domain of Klotho by ADAM10 and ADAM17. Proc Natl Acad Sci U S A 2007;104(50):19796–19801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mencke R, Harms G, Moser J, et al. Human alternative Klotho mRNA is a nonsense-mediated mRNA decay target inefficiently spliced in renal disease. JCI Insight 2017;2(20). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu MC, Shi M, Zhang J, et al. Renal Production, Uptake, and Handling of Circulating alphaKlotho. Journal of the American Society of Nephrology: JASN 2016;27(1):79–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu MC, Shi M, Zhang J, et al. Klotho deficiency is an early biomarker of renal ischemia-reperfusion injury and its replacement is protective. Kidney international 2010;78(12):1240–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sugiura H, Yoshida T, Mitobe M, et al. Klotho reduces apoptosis in experimental ischaemic acute kidney injury via HSP-70. Nephrol Dial Transplant 2010;25(1):60–68. [DOI] [PubMed] [Google Scholar]
- 20.King GD, Chen C, Huang MM, et al. Identification of novel small molecules that elevate Klotho expression. The Biochemical journal 2012;441(1):453–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shi M, Flores B, Gillings N, et al. alphaKlotho Mitigates Progression of AKI to CKD through Activation of Autophagy. Journal of the American Society of Nephrology : JASN 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brown JR, Katz R, Ix JH, et al. Fibroblast growth factor-23 and the long-term risk of hospital-associated AKI among community-dwelling older individuals. Clinical journal of the American Society of Nephrology: CJASN 2014;9(2):239–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leaf DE, Christov M, Juppner H, et al. Fibroblast growth factor 23 levels are elevated and associated with severe acute kidney injury and death following cardiac surgery. Kidney international 2016;89(4):939–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leaf DE, Wolf M, Waikar SS, et al. FGF-23 levels in patients with AKI and risk of adverse outcomes. Clin J Am Soc Nephrol 2012;7(8):1217–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leaf DE, Siew ED, Eisenga MF, et al. Fibroblast Growth Factor 23 Associates with Death in Critically Ill Patients. Clinical journal of the American Society of Nephrology: CJASN 2018;13(4):531–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leaf DE, Jacob KA, Srivastava A, et al. Fibroblast Growth Factor 23 Levels Associate with AKI and Death in Critical Illness. Journal of the American Society of Nephrology: JASN 2017;28(6):1877–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neyra JA, Moe OW, Hu MC. Fibroblast growth factor 23 and acute kidney injury. Pediatr Nephrol 2015;30(11):1909–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Inter Suppl 2012;2:1–138. [Google Scholar]
- 29.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150(9):604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barker SL, Pastor J, Carranza D, et al. The demonstration of alphaKlotho deficiency in human chronic kidney disease with a novel synthetic antibody. Nephrol Dial Transplant 2015;30(2):223–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zinellu A, Caria MA, Tavera C, et al. Plasma creatinine and creatine quantification by capillary electrophoresis diode array detector. Anal Biochem 2005;342(2):186–193. [DOI] [PubMed] [Google Scholar]
- 32.Kellum JA, Zarbock A, Nadim MK. What endpoints should be used for clinical studies in acute kidney injury? Intensive Care Med 2017;43(6):901–903. [DOI] [PubMed] [Google Scholar]
- 33.Christov M, Waikar SS, Pereira RC, et al. Plasma FGF23 levels increase rapidly after acute kidney injury. Kidney international 2013;84(4):776–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Toro L, Barrientos V, Leon P, et al. Erythropoietin induces bone marrow and plasma fibroblast growth factor 23 during acute kidney injury. Kidney international 2018. [DOI] [PubMed] [Google Scholar]
- 35.Mace ML, Gravesen E, Nordholm A, et al. Kidney fibroblast growth factor 23 does not contribute to elevation of its circulating levels in uremia. Kidney international 2017;92(1):165–178. [DOI] [PubMed] [Google Scholar]
- 36.Sugiura H, Yoshida T, Tsuchiya K, et al. Klotho reduces apoptosis in experimental ischaemic acute renal failure. Nephrol Dial Transplant 2005;20(12):2636–2645. [DOI] [PubMed] [Google Scholar]
- 37.Sugiura H, Yoshida T, Shiohira S, et al. Reduced Klotho expression level in kidney aggravates renal interstitial fibrosis. American journal of physiology Renal physiology 2012;302(10):F1252–1264. [DOI] [PubMed] [Google Scholar]
- 38.Panesso MC, Shi M, Cho HJ, et al. Klotho has dual protective effects on cisplatin-induced acute kidney injury. Kidney international 2014;85(4):855–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rygasiewicz K, Hryszko T, Siemiatkowski A, et al. C-terminal and intact FGF23 in critical illness and their associations with acute kidney injury and in-hospital mortality. Cytokine 2018;103:15–19. [DOI] [PubMed] [Google Scholar]
- 40.Neyra JA, Hu MC. Potential application of klotho in human chronic kidney disease. Bone 2017;100:41–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shi M, Flores B, Gillings N, et al. alphaKlotho Mitigates Progression of AKI to CKD through Activation of Autophagy. Journal of the American Society of Nephrology: JASN 2016;27(8):2331–2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hu MC, Shi M, Zhang J, et al. Klotho deficiency causes vascular calcification in chronic kidney disease. Journal of the American Society of Nephrology: JASN 2011;22(1):124–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kuro-o M Klotho as a regulator of oxidative stress and senescence. Biol Chem 2008;389(3):233–241. [DOI] [PubMed] [Google Scholar]
- 44.Liu F, Wu S, Ren H, et al. Klotho suppresses RIG-I-mediated senescence-associated inflammation. Nat Cell Biol 2011;13(3):254–262. [DOI] [PubMed] [Google Scholar]
- 45.Doi S, Zou Y, Togao O, et al. Klotho inhibits transforming growth factor-beta1 (TGF-beta1) signaling and suppresses renal fibrosis and cancer metastasis in mice. The Journal of biological chemistry 2011;286(10):8655–8665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou L, Li Y, Zhou D, et al. Loss of Klotho contributes to kidney injury by derepression of Wnt/beta-catenin signaling. Journal of the American Society of Nephrology: JASN 2013;24(5):771–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guan X, Nie L, He T, et al. Klotho suppresses renal tubulo-interstitial fibrosis by controlling basic fibroblast growth factor-2 signalling. The Journal of pathology 2014;234(4):560–572. [DOI] [PubMed] [Google Scholar]
- 48.Huang JS, Chuang CT, Liu MH, et al. Klotho attenuates high glucose-induced fibronectin and cell hypertrophy via the ERK1/2-p38 kinase signaling pathway in renal interstitial fibroblasts. Mol Cell Endocrinol 2014;390(1–2):45–53. [DOI] [PubMed] [Google Scholar]
- 49.Bian A, Neyra JA, Zhan M, et al. Klotho, stem cells, and aging. Clin Interv Aging 2015;10:1233–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mace ML, Gravesen E, Hofman-Bang J, et al. Key role of the kidney in the regulation of fibroblast growth factor 23. Kidney international 2015;88(6):1304–1313. [DOI] [PubMed] [Google Scholar]
- 51.Hassan A, Durlacher K, Silver J, et al. The fibroblast growth factor receptor mediates the increased FGF23 expression in acute and chronic uremia. American journal of physiology Renal physiology 2016;310(3):F217–221. [DOI] [PubMed] [Google Scholar]
- 52.Smith ER, Tan SJ, Holt SG, et al. FGF23 is synthesised locally by renal tubules and activates injury-primed fibroblasts. Sci Rep 2017;7(1):3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smith ER, Holt SG, Hewitson TD. FGF23 activates injury-primed renal fibroblasts via FGFR4-dependent signalling and enhancement of TGF-beta autoinduction. Int J Biochem Cell Biol 2017;92:63–78. [DOI] [PubMed] [Google Scholar]
- 54.Torregrosa I, Montoliu C, Urios A, et al. Urinary Klotho measured by ELISA as an early biomarker of acute kidney injury in patients after cardiac surgery or coronary angiography. Nefrologia 2015;35(2):172–178. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1.Characteristics of patients according to tertiles of serum intact FGF23
Supplementary Table 2.Characteristics of patients according to AKI status
Supplementary Table 3.Characteristics of patients according to MAKE-90 outcome (death, RRT-dependency, 50% decrease in eGFR)
Supplementary Table 4.Associations of biomarkers with MAKE-90 (death, RRT-dependency, 50% decrease in eGFR) by AKI status
Supplementary Table 5.Association of biomarkers with MAKE-90 outcome (death, RRT-dependency, 25% decrease in eGFR)
Supplementary Figure 1.Correlations between i) urine Klotho/Cr and ii) serum FGF23 and standard-of-care biochemical parameters. All measurements are natural log-transformed.