Abstract
Background and Purpose:
MRI studies have demonstrated magnetic susceptibility in multiple sclerosis (MS) lesions is dependent on lesion age. The objective of this study was to utilize quantitative susceptibility mapping (QSM) to determine whether lesions with hyperintense rim, indicative of iron-laden inflammatory cells (rim+), follow a unique time-dependent trajectory of susceptibility change compared to those without (rim−).
Materials and Methods:
MS patients with at least one new gadolinium (Gd)-enhancing lesion and at least three longitudinal QSM scans ranging between 1.1 to 6.1 years. Lesions were classified as rim+ if a hyperintense rim appeared on QSM at any time. A multilevel growth curve model compared longitudinal susceptibility among rim+ and rim− lesions.
Results:
Thirty-two new Gd-enhancing lesions from 19 MS patients were included and 16 lesions (50%) were identified as rim+. QSM rim+ lesions were larger than rim− lesions at Gd-enhancement (p<0.0001). Among all lesions, susceptibility increased sharply after enhancement to a peak between 1 to 2 years followed by a decrease. The overall susceptibility curve height for rim− lesions was 4.27 ppb lower than that for rim+ lesions (p=0.0097). Rim− lesions demonstrated higher linear slope relative to rim+ lesions (p=0.0232) but faster cubic decay relative to rim+ lesions (p=0.0053). Rim− lesions started decaying approximately 2 years earlier as compared to rim+ lesions.
Conclusion:
There was a marked difference in susceptibility temporal trajectory between rim+ and rim− lesions over the first six years of lesion formation. The majority of rim+ lesions retain iron for years after initial lesion appearance.
INTRODUCTION
Quantitative susceptibility mapping (QSM)1 provides efficient in vivo quantification of susceptibility changes related to iron deposition and helps identify lesions harboring iron-laden inflammatory cells.2 It has been widely used in studying multiple sclerosis (MS), and can demonstrate the retention of iron among a subset of chronic lesions.3–5 Enhancing MS lesions identified on post-Gadolinium (Gd) T1w images in the routine MRI surveillance are representative of breakdown of the blood brain barrier (BBB) and acute disease activity.6 As the BBB closes, lesions transition to the chronic stage. However, a subset of lesions may retain a rim of iron-enriched inflammatory cells with on-going damage. Chronic active MS lesions, characterized by a hyperintense rim on QSM, have been shown to contain iron-enriched, activated microglia and macrophages on histopathology5 and have been linked to greater tissue damage on in vivo MRI.7, 8 Identifying lesions likely to retain chronic inflammation would be useful for potential therapeutic targeting. Accordingly, it would be valuable to study MS lesion evolution trajectories from the time of enhancement through to the chronic lesion stage.
QSM is a phase-based magnetic field deconvolution technique that overcomes blooming artifacts and provides accurate quantification and localization of the magnetic sources.1, 9, 10 Previous QSM studies demonstrated that MS lesion susceptibility increases significantly as the acute enhancing MS lesion transitions to the non-enhancing stage, reaches a peak in the chronic active stage, and eventually decays away in the final stage of a glia scar.11–14 Although these studies identified a unique time-dependent trajectory in susceptibility on QSM, these studies are limited given the cross-sectional design or short longitudinal follow-up. In addition, a number of recent gradient echo (GRE) imaging studies have identified a unique sub-population of chronic MS lesions with a hypointense rim on phase image or a hyperintense rim on QSM. These studies have utilized histopathologic validation7, 15–17 and more recently, PET imaging,18 to validate that these lesions have persistent inflammation as represented by iron-laden microglia and macrophages. Initial detection and expected life-span of lesions with hyperintense rim appearance on QSM (rim+) as well as the time-dependent susceptibility changes among these lesions as compared to those without rim (rim−) lesions have yet to be explored.
The aim of this study was to assess longitudinal tissue susceptibility changes in new Gd-enhancing lesions for up to six years after first identification and to determine if lesion trajectories depend on the development of a hyperintense rim on QSM.
MATERIALS AND METHODS
Patient selection
This was a retrospective study of a cohort of 19 relapsing-remitting (RR) MS patients selected from an ongoing, prospective MS MRI and clinical database for which annual MRI scans (including QSM) were collected over the course of six years. Patients were selected for this study if they met the following inclusion criteria: 1) had at least one new Gd-enhancing (Gd+) MS lesion on routine annual MRI scan, 2) had at least three longitudinal QSM scans (including at the time of Gd+ lesion detection), 3) had at least one MRI performed more than one year after Gd+ lesion detection and 4) had a prior MRI to ensure that Gd+ lesions were newly formed lesions and not re-enhancement of older lesions. MRI scans were acquired on two different imaging platforms over the course of the six years (GE and Siemens, details below). Clinical data collected for patients included: age, gender, expanded disability status scale (EDSS), disease duration, and treatment duration. This study was approved by our local institutional review board and written informed consent was obtained from each subject.
MRI protocol and image processing
Brain MRIs (from year 2011 to year 2018) were performed on 3T MRI scanners (Signa HDxt; GE Healthcare, Waukesha, WI, USA, a product eight-channel head coil; Magnetom Skyra, Siemens Medical Solutions USA, Malvern, PA, a product twenty-channel head/neck coil). The scanning protocol consisted of standard 3D T1-weighted (T1w), 2D T2-weighted (T2w), and 3D T2w FLAIR sequences for anatomical structure, multi-echo 3D GRE imaging for QSM, as well as gadolinium-enhanced 3D T1w imaging (T1w+Gd) to detect blood-brain barrier disruption. The acquisition parameters for multi-echo GRE were: field of view = 24 cm, TR = 49–58 ms, TE1/ΔTE = 4.5–6.7/4.1–4.8 ms, last TE = 47.7 ms, acquisition matrix= 320–416×205–320, readout bandwidth = 244–260 Hz/pixel, axial slice thickness = 3 mm, flip angle = 15–20°, acceleration factor = 2, and number of averages = 1. The scan time was around 4.5 min (48 slices), varying slightly with brain superior-inferior dimension. This QSM imaging protocol was harmonized for both scanner manufacturers and was demonstrated to be a reproducible across manufacturers.19, 20 QSM was reconstructed from complex GRE images using a fully automated Morphology Enabled Dipole Inversion (MEDI+0) method zero-referenced to the ventricular cerebrospinal fluid (CSF)21. All the conventional images (T1w, T1w+Gd, T2w, T2w FLAIR) and the follow-up QSM images were co-registered to the baseline GRE magnitude images using the FMRIB’s Linear Image Registration Tool algorithm.22
Lesion susceptibility and volume measurements
New Gd+ MS lesions were identified on T1w+Gd images and visually classified on QSM as rim+ or rim−8 by two independent reviewers (SZ, neuroradiologist, 7 years of experience; SG, MS neurologist, 16 years of experience).8 A lesion was designated as rim+ if QSM was hyperintense at the edge of the lesion at any of the longitudinal time points. In addition, at each time point, newly identified Gd+ lesions were dated as 0 years. Lesions were also classified as either “nodular” or “shell” enhancing to estimate stage of lesion enhancement, i.e. early or late stage, respectively.12, 23 In the case of a rare (4 lesions) disagreement, a third neuroradiologist (I.K., 22 years of experience) was called on to determine the lesion type. Region-of-interest (ROI) analysis was performed using ITK-SNAP software (version 3.6.0; available at: http://www.itksnap.org/) to obtain regional volume and QSM measurements within the identified lesions. To assess change in lesion volume, lesion ROIs were drawn on raw T2w FLAIR images, which had isotropic 1 mm high-resolution images, at all time points. To assess longitudinal susceptibility change, ROIs were first created on co-registered T2w FLAIR images, and then overlaid on the QSM images at the time of initial lesion detection. When necessary, these ROIs were manually edited to better match lesion geometry on QSM and removal of the central veins (vessel-like structures with hyperintense QSM appearance). The edited ROI were overlaid onto QSM images from all other subsequent time points. The susceptibility value of the adjacent normal appearing white matter (NAWM) was subtracted from the lesion susceptibility to offset the influence of local fiber orientation.
Statistical analysis
A regression model with orthogonal time polynomials was used to analyze the longitudinal evolution of lesion volumes for rim+ and rim− lesions at the lesion level while adjusting for multiple lesions per patient. The final model included a third order orthogonal polynomial, the fixed conditional effect was lesion group, and patient was the random effect.
A multilevel growth curve model with orthogonal time polynomials was used to analyze the longitudinal evolution of QSM values for rim+ and rim− lesions while adjusting for patient level covariates (fixed effects: individual lesion volume, patient age, EDSS, disease duration and treatment duration) and multiple lesions per patient (random effects). An eighth-order orthogonal polynomial model was necessary to capture the upward and downward evolution of QSM values within lesion groups. Orthogonal polynomials are transformations that make the original time terms independent. They allow for a precise and robust evaluation of QSM longitudinal differences. Our orthogonal polynomial was defined based on the lesion age octiles (8-quantiles). This approach accounts for the sample lesion age distribution. The statistical analysis was performed using R (R Core Team, 2017).
RESULTS
Patient and lesion characteristics
Nineteen RRMS patients (15 women and 4 men, aged 36.3 ± 6.4 years) met the inclusion criteria with a total of 32 new T1w+Gd lesions: 9 nodular enhancing (28%, susceptibility 6.87 ± 5.80 ppb) and 23 shell enhancing (72%, 12.10 ± 8.95 ppb). Central veins were appreciated in 10 (31%) of the lesions. Patients had a mean disease duration of 4.8 ± 3.2 years and EDSS of 1.4 ± 1.7. The average time from initial MRI to last MRI was 3.6 ± 1.4 years (range 1.1–6.1 years). Patients were treated with various disease modifying therapies and at the time of lesion identification, the cohort was on therapy for a mean duration of 3.5 ± 3.0 years.
Lesions with hyperintense rim on QSM
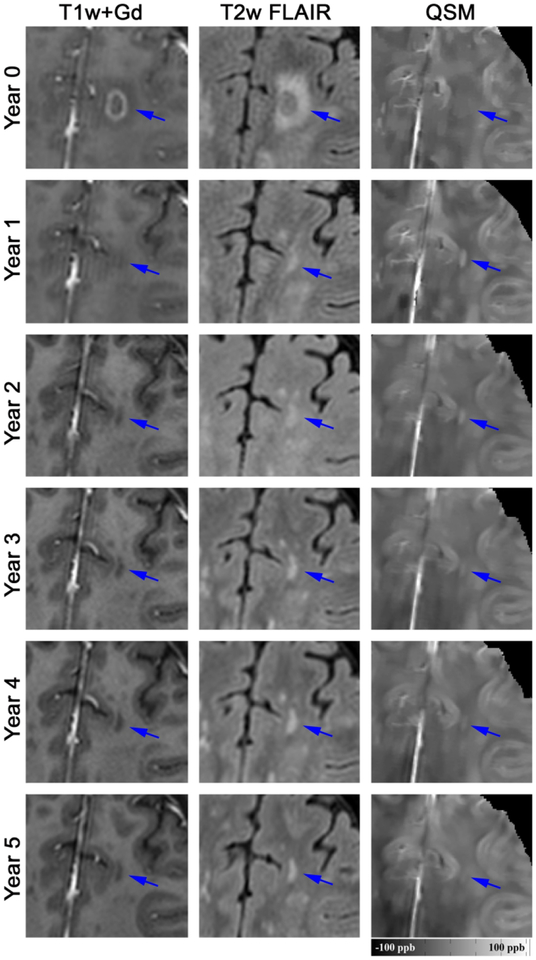
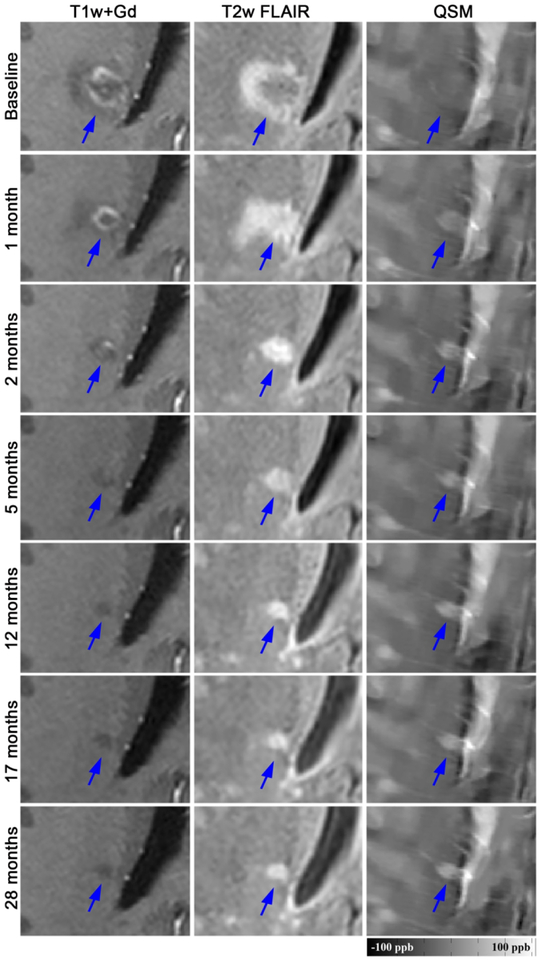
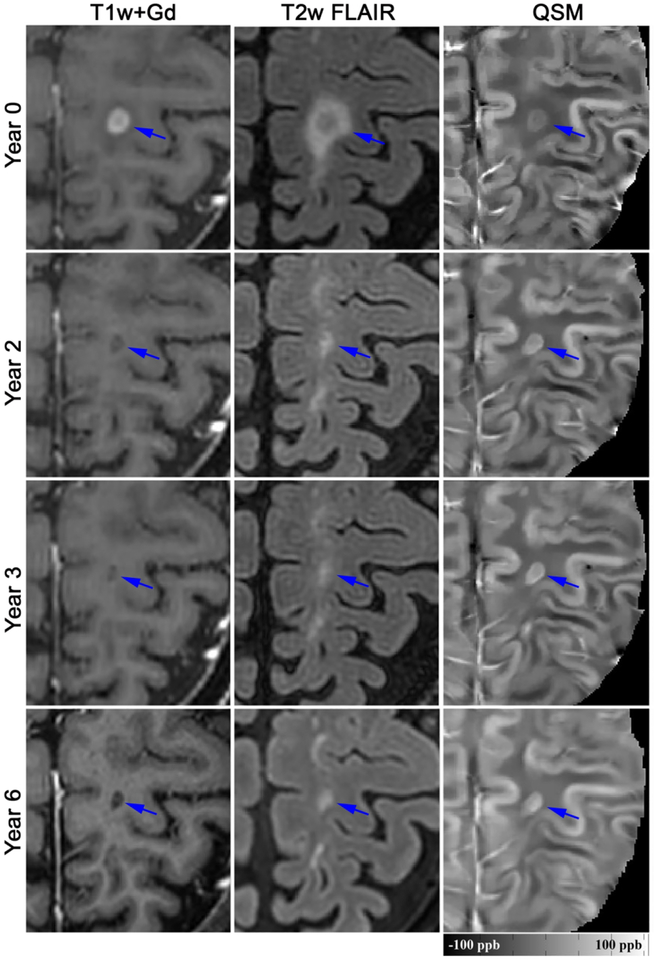
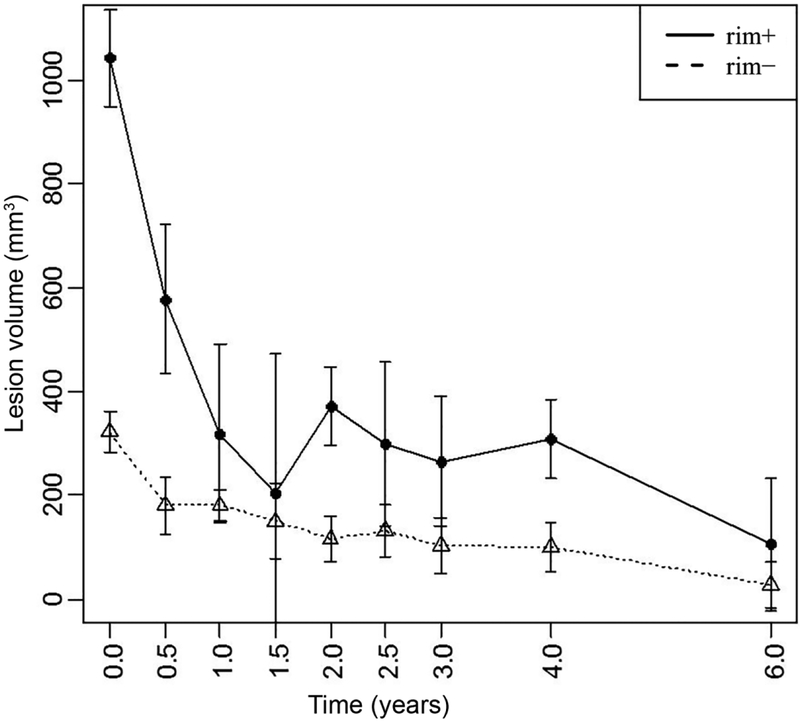
Among the 32 new Gd+ lesions, 16 lesions (50%) were identified as QSM rim+ and evidence of the rim was appreciated at time of enhancement for the majority (81%) of these lesions. Qualitatively, rim+ lesions were visualized on QSM scans longer than rim− lesions (Fig.1–3) and once identified, the hyperintense rim was consistently appreciated on all subsequent scans in 14 (88%) of the rim+ lesion (Fig.2 and 3). Although susceptibility values were lowest at the time of Gd-enhancement, subtle evidence of a rim could be appreciated in 13 (81%) of lesions at that time. The longitudinal lesion evolution of lesion volume adjusting for multiple lesions per patient and conditioning on lesion group is presented in Figure 4. Fitted volumes derived from the regression model demonstrated that rim+ lesions (1042.45mm3) were significantly larger than rim− lesions (322.34 mm3) at the time of Gd-enhancement, p<0.0001. Following the start of a decline in volume after Gd-enhancement, rim+ lesions remained larger than rim− lesions, and maintained a significant difference at lesion ages 0.5, 2 and 4 years (all p<0.0001), Figure 4.
Figure 1.
Longitudinal QSM and T2w FLAIR images of a new Gd-enhancing MS lesion without QSM rim appearance (rim-). At baseline, the enhancing lesion was isointense on QSM (mean lesion susceptibility = −3.31 ppb), became most hyperintense at year 1 (22.50 ppb), and gradually disappeared in subsequent years (−1.18 ppb at year 5).
Figure 3.
Longitudinal QSM and T2w FLAIR images of a new Gd-enhancing MS lesion with QSM rim appearance (rim+). This lesion was isointense on QSM at baseline and demonstrated an increase in susceptibility and rim appearance on subsequent QSM scans. The lesion susceptibility increased continually from −0.34 ppb at baseline to 26.35 ppb at 28 months. Gd-enhancement remained in this lesion until month 2.
Figure 2.
Longitudinal QSM and T2w FLAIR images of a new Gd-enhancing MS lesion with QSM rim appearance (rim+). This lesion was slightly hyperintense on QSM at time of Gd-enhancement (mean lesion susceptibility = 12.74 ppb), became most hyperintense at year 3 (34.28 ppb), and remained hyperintense at year 6 (25.15 ppb).
Figure 4.
Longitudinal lesion volume evolution changes among QSM rim+ and rim− lesions. Rim+ lesions were statistically larger at Gd-enhancement (Time=0), 0.5, 2 and 4 years of age (all p<0.0001).
Time-dependent susceptibility change in QSM rim+ and rim− lesions
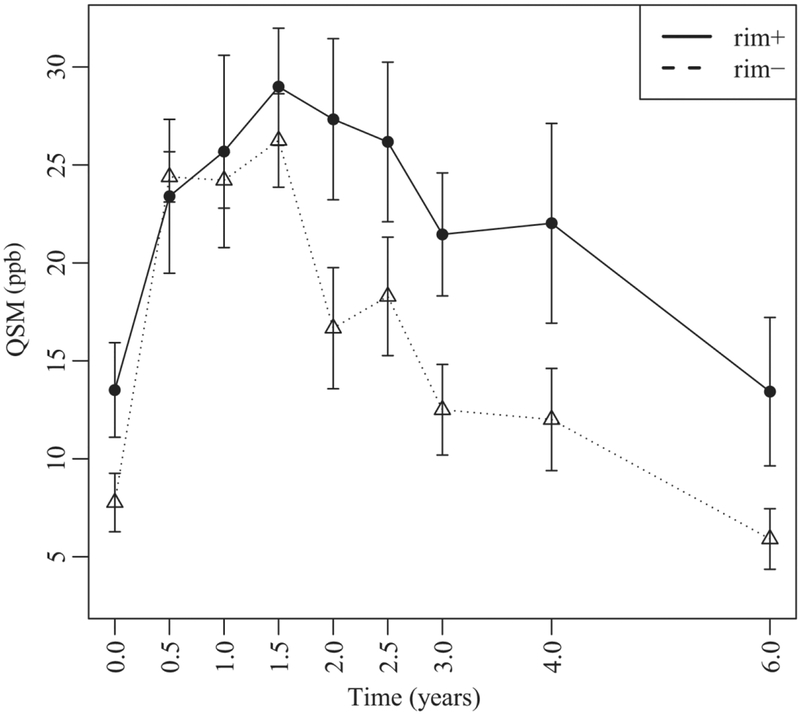
The final model for the longitudinal lesion evolution of QSM values included T2w FLAIR lesion volume and EDSS at baseline as patient-level fixed effects (all p-values<0.05). All lesions demonstrated a continued increase in susceptibility until a peak between years 1 and 2 which was followed by a reduction over the subsequent years. There was a significant effect of lesion group (rim+ versus rim−) on the intercept term, indicating lower overall QSM values for the rim− lesions relative to the rim+ (Estimate = −4.27, SE=1.62, p= 0.0097), Fig.5. There was also a significant effect of lesion group on the slope and cubic terms, indicating faster linear growth rate for rim− lesions relative to rim+ lesions (Estimate = 8.42, SE = 3.66, p = 0.0232) as well as significantly faster cubic decay for rim− lesions relative to that of rim+ lesions (Estimate = −10.64, SE= 3.75, p = 0.0053). All other effects of lesion group conditioning on time decay were not significantly different between the two groups. When considering lesion volume change, as opposed to baseline lesion volume, as a covariate in the model, a significant association was found between QSM change and volume change, p = 0.0022 (for every 1 mm3 decrease in volume, there was 0.014 ppb reduction in QSM). The relationship between susceptibility and volume change was similar among rim+ and rim−lesions (p>0.2726).
Figure 5.
Longitudinal lesion age-dependent susceptibility time course of QSM rim+ and rim− MS lesions. Rim+ lesions demonstrate a higher peak QSM value and significantly slower decay rate compared to rim−(see text).
Table 1 summarizes the model fitted mean susceptibility along with their 95% confidence intervals. The estimated susceptibility means for rim− lesions demonstrated a decay after 1.5 years with 95% confidence, while the estimated means for rim+ lesions remained persistently elevated from lesion age 0.5 to 4 years. A decline in susceptibility is appreciated in rim+ lesions only after the fourth year.
Table 1.
The mean susceptibility of rim+ and rim− lesions derived from the regression model
| Time (year) | Rim+ | Rim− | ||||
|---|---|---|---|---|---|---|
| No of lesions | Mean (ppb) | 95% CI | No of lesions | Mean (ppb) | 95% CI | |
| 0 | 16 | 13.51 | 11.09, 15.93 | 16 | 7.77 | 6.27, 9.26 |
| 0.5 | 10 | 23.40 | 19.47, 27.33 | 9 | 24.39 | 23.11, 25.67 |
| 1 | 7 | 25.69 | 20.78, 30.60 | 11 | 24.22 | 22.79, 25.64 |
| 1.5 | 5 | 28.99 | 26.00, 31.98 | 5 | 26.25 | 23.86, 28.63 |
| 2 | 6 | 27.33 | 23.22, 31.44 | 7 | 16.66 | 13.57, 19.76 |
| 2.5 | 8 | 26.17 | 22.10, 30.25 | 10 | 18.29 | 15.27, 21.31 |
| 3 | 7 | 21.45 | 18.31, 24.59 | 9 | 12.50 | 10.19, 14.81 |
| 4 | 8 | 22.02 | 16.92, 27.12 | 10 | 12.00 | 9.40, 14.61 |
| 6 | 5 | 13.42 | 9.64, 17.21 | 6 | 5.90 | 4.36, 7.45 |
DISCUSSION
The current study is the longest longitudinal lesion-based susceptibility study examining the time-dependent susceptibility changes quantified on QSM. Our study indicates that QSM rim+ lesions have a unique time-dependent trajectory. As compared to QSM rim− lesions, rim+ lesions start with a higher susceptibility and larger volume and importantly, retain a high susceptibility value for a number of years after initial detection. This study provides further insight into a distinct subgroup of MS lesions, those that retain a rim of iron-laden inflammatory cells and have the potential for continued tissues damage.7, 8, 17
QSM provides a noninvasive way to quantify the susceptibility change in MS lesions. The susceptibility increase observed in our study is consistent with previous studies, in which a jump in lesion susceptibility occurs as an enhancing lesion evolves to the chronic, non-enhancing state.11–14 The initial rise in susceptibility, occurring within weeks, in active lesions may be related to myelin digestion24 and the subsequent increase, which occurs over the course of months, is more likely related to removal of the myelin debris within macrophages25 and the release of iron.12 A subset of chronic MS lesions, identified as chronic active or slowly expanding lesions have been described as having a hypocellular lesion center and a rim of activated pro-inflammatory microglia and macrophages (m/M).17, 26, 27 These lesions demonstrate evidence of active demyelination and axonal destruction at their rim and are felt to contribute to long-term, ongoing tissue damage in MS.8, 17, 26–28 A number of studies have demonstrated that the majority of the m/M found at the rim of chronic active MS lesions contain iron.7, 15, 17, 29–32 The source of the released iron is presumed to be derived from damaged myelin and dying oligodendrocytes in acute lesions31 and functions to promote polarization of m/M cells to a pro-inflammatory state.30 Our data suggests that the development of rim+ lesions may be related to a higher level of iron release, given the higher susceptibility peak among these lesions, and that the extent of iron release potentially contributes to development of chronic inflammation. In addition, we found that rim+ lesions were much larger at the time of Gd-enhancement, which supports the concept of a larger inflammatory event leading to more demyelination and iron release. Studying the physiological mechanisms driving iron release within the acute lesion could identify therapeutic targets aimed at decreasing the occurrence of chronic active MS lesions.
Reduction of susceptibility in both rim+ and rim− lesions was related to volume loss, however the relationship was similar and suggests pathological differences may explain our observed differences in decay rate. The relatively abrupt reduction in susceptibility found among the QSM rim− lesions, would be consistent with either loss of iron from the lesion or more likely, a higher potential for remyelination among these lesions.5 Remyelination occurring in rim− lesions would be consistent with histological observations indicating that iron-enriched m/M are not found at the rim of remyelinated or shadow plaques17 and in vivo MRI studies demonstrating less tissue damage in lesions without QSM rim.7, 8 The slow decay of susceptibility in rim+ lesions and retention of the hyperintense rim suggest that these lesions retain iron for a number of years, thus have the potential for ongoing damage over more extended period.17 Although iron is retained, there is an eventual reduction in susceptibility, which suggests that the majority of these lesions have a life-span of only a few years before iron loss and probable transition to a chronic inactive state or glia scar.25 Histologically, chronic active lesions have been found to be associated with longer disease duration and to predominantly occur in progressive disease, where new Gd+ lesions are infrequently found.27 However, given aforementioned MRI GRE studies, these lesions can occur frequently within the relapsing phase of the disease and consistent with others,7 we found that lesions that become chronic active lesions can show subtle evidence of a rim at the Gd+ stage. These combined observations suggest QSM rim+ lesions could serve as an early-stage imaging biomarker for disease prognosis and warrants further exploration.
QSM has been shown to provide a high level of diagnostic accuracy to predicting Gd+ lesions.13 In this study, the majority of the lesions were shell-enhancing lesions, which are felt to be slightly older enhancing lesions and tend to demonstrate a slightly higher susceptibility.12 Importantly, the average susceptibility at the time of enhancement of all lesions (nodular and shell enhancing) was comparable to previous work12, and below the cutoff value of 13.5 ppb for predicting Gd+ MS lesions.13 Furthermore, MRI GRE imaging is being explored to improve the diagnostic accuracy of MS based upon the identification of a central vein or central vein sign (CVS). Interestingly, we found that only one-third of the lesions had a central vein on QSM, which is lower than previous suggested utilizing a combined T2w FLAIR and T2* sequence,33 and suggests more research is required to assess the frequency of CVS in MS lesions.
There are limitations in this study. Our lesion sample size is relatively small and, more importantly, not all lesions were measured at each time point or followed through to all six years. We will continue to identify Gd+ lesions from our ongoing database to expand upon our observations and provide more data to each lesion-age year. This expansion will allow further exploration into the effect of individual lesion size as well as patient specific covariates such as disease duration, disability status and treatment effect. As mentioned above, very early changes in susceptibility are likely due to a number of pathophysiological mechanisms at play, thus imaging the early staged lesions with frequent and short-interval QSM, with the addition of myelin imaging,34 would allow a more detailed analysis of the early rise in susceptibility. Similarly, a serial MRI study with both QSM and myelin imaging during the decay stage can evaluate the loss of iron versus remyelination in rim− lesions. Lastly, our study focused on white matter MS lesions and excluded cortical gray matter lesions, which are known to occur quite frequently in MS and require ultra-high field imaging to appreciate.35 Interestingly, a high-field 7T study utilizing QSM identified cortical lesions as having a much lower susceptibility as compared to white matter lesions, suggesting less iron in cortical lesions as compared to white matter lesions.36 Thus, as we move forward with 7T QSM MRI, we intend to explore and compare the time-dependent susceptibility changes among smaller regions of within the lesion (i.e. regions of tissue enhancement) as well as lesions located within the cortex.
In conclusion, we identified unique trajectories of lesion time-dependent change in susceptibility among different sub-types of MS lesions. These observations are consistent with the presence of iron-laden inflammatory cells present within the rim of a select subset of chronic lesions retain iron for a number of years and slowly transition to an inactive state. This study supports the use of serial QSM to provide information regarding the current state of inflammation within chronic MS lesions.
Acknowledgments
This work was supported in part by grants from the National Institutes of Health (R01NS090464, R01NS104283, R01NS105144, S10 OD021782), the National Multiple Sclerosis Society (RG-1602-07671) and grant (UL1 TR000456-06) from the Weill Cornell Clinical and Translational Science Center (CTSC).
Abbreviations:
- MS
multiple sclerosis
- QSM
quantitative susceptibility mapping
- Gd+
Gd-enhancing
- NAMW
normal-appearing white matter
- rim+
rim positive
- rim−
rim negative
- BBB
blood brain barrier
- GRE
gradient echo
- m/M
microglia and macrophages
- CVS
central vein sign
REFERENCES
- 1.de Rochefort L, Liu T, Kressler B, et al. Quantitative Susceptibility Map Reconstruction from MR Phase Data Using Bayesian Regularization: Validation and Application to Brain Imaging. Magnetic Resonance in Medicine 2010; 63:194–206 [DOI] [PubMed] [Google Scholar]
- 2.Wang Y, Spincemaille P, Liu Z, et al. Clinical quantitative susceptibility mapping (QSM): Biometal imaging and its emerging roles in patient care. J Magn Reson Imaging 2017; 46:951–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Langkammer C, Liu T, Khalil M, et al. Quantitative susceptibility mapping in multiple sclerosis. Radiology 2013; 267:551–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stuber C, Pitt D, Wang Y. Iron in Multiple Sclerosis and Its Noninvasive Imaging with Quantitative Susceptibility Mapping. Int J Mol Sci 2016; 17:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wisnieff C, Ramanan S, Olesik J, et al. Quantitative susceptibility mapping (QSM) of white matter multiple sclerosis lesions: Interpreting positive susceptibility and the presence of iron. Magn Reson Med 2015; 74:564–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Filippi M, Rocca MA, Ciccarelli O, et al. MRI criteria for the diagnosis of multiple sclerosis: MAGNIMS consensus guidelines. Lancet Neurol 2016; 15:292–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Absinta M, Sati P, Schindler M, et al. Persistent 7-tesla phase rim predicts poor outcome in new multiple sclerosis patient lesions. J Clin Invest 2016; 126:2597–2609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yao Y, Nguyen TD, Pandya S, et al. Combining Quantitative Susceptibility Mapping with Automatic Zero Reference (QSM0) and Myelin Water Fraction Imaging to Quantify Iron-Related Myelin Damage in Chronic Active MS Lesions. AJNR Am J Neuroradiol 2018; 39:303–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li JQ, Chang SX, Liu T, et al. Reducing the object orientation dependence of susceptibility effects in gradient echo MRI through quantitative susceptibility mapping. Magnetic Resonance in Medicine 2012; 68:1563–1569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou D, Liu T, Spincemaille P, et al. Background field removal by solving the Laplacian boundary value problem. Nmr in Biomedicine 2014; 27:312–319 [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y, Gauthier SA, Gupta A, et al. Longitudinal change in magnetic susceptibility of new enhanced multiple sclerosis (MS) lesions measured on serial quantitative susceptibility mapping (QSM). Journal of Magnetic Resonance Imaging 2016; 44:426–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y, Gauthier SA, Gupta A, et al. Quantitative Susceptibility Mapping and R2* Measured Changes during White Matter Lesion Development in Multiple Sclerosis: Myelin Breakdown, Myelin Debris Degradation and Removal, and Iron Accumulation. AJNR Am J Neuroradiol 2016; 37:1629–1635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang S, Nguyen TD, Zhao Y, et al. Diagnostic accuracy of semiautomatic lesion detection plus quantitative susceptibility mapping in the identification of new and enhancing multiple sclerosis lesions. Neuroimage Clin 2018; 18:143–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen W, Gauthier SA, Gupta A, et al. Quantitative susceptibility mapping of multiple sclerosis lesions at various ages. Radiology 2014; 271:183–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bagnato F, Hametner S, Yao B, et al. Tracking iron in multiple sclerosis: a combined imaging and histopathological study at 7 Tesla. Brain 2011; 134:3599–3612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yao B, Ikonomidou VN, Cantor FK, et al. Heterogeneity of Multiple Sclerosis White Matter Lesions Detected With T2*-Weighted Imaging at 7.0 Tesla. Journal of neuroimaging : official journal of the American Society of Neuroimaging 2015;25:799–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dal-Bianco A, Grabner G, Kronnerwetter C, et al. Slow expansion of multiple sclerosis iron rim lesions: pathology and 7 T magnetic resonance imaging. Acta Neuropathol 2017; 133:25–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaunzner UW, Kang Y, Zhang S, et al. Quantitative susceptibility mapping identifies inflammation in a subset of chronic multiple sclerosis lesions. Brain 2019; 142:133–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deh K, Kawaji K, Bulk M, et al. Multicenter reproducibility of quantitative susceptibility mapping in a gadolinium phantom using MEDI+0 automatic zero referencing. Magn Reson Med 2019; 81:1229–1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deh K, Nguyen TD, Eskreis-Winkler S, et al. Reproducibility of quantitative susceptibility mapping in the brain at two field strengths from two vendors. J Magn Reson Imaging 2015; 42:1592–1600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu Z, Spincemaille P, Yao Y, et al. MEDI+0: Morphology enabled dipole inversion with automatic uniform cerebrospinal fluid zero reference for quantitative susceptibility mapping. Magn Reson Med 2018; 79:2795–2803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jenkinson M, Bannister P, Brady M, et al. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 2002; 17:825–841 [DOI] [PubMed] [Google Scholar]
- 23.Gaitan MI, Shea CD, Evangelou IE, et al. Evolution of the blood-brain barrier in newly forming multiple sclerosis lesions. Ann Neurol 2011; 70:22–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deh K, Ponath GD, Molvi Z, et al. Magnetic susceptibility increases as diamagnetic molecules breakdown: Myelin digestion during multiple sclerosis lesion formation contributes to increase on QSM. J Magn Reson Imaging 2018; 48:1281–1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuhlmann T, Ludwin S, Prat A, et al. An updated histological classification system for multiple sclerosis lesions. Acta Neuropathologica 2017; 133:13–24 [DOI] [PubMed] [Google Scholar]
- 26.Frischer JM, Bramow S, Dal-Bianco A, et al. The relation between inflammation and neurodegeneration in multiple sclerosis brains. Brain 2009; 132:1175–1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prineas JW, Kwon EE, Cho ES, et al. Immunopathology of secondary-progressive multiple sclerosis. Ann Neurol 2001; 50:646–657 [DOI] [PubMed] [Google Scholar]
- 28.Singh S, Dallenga T, Winkler A, et al. Relationship of acute axonal damage, Wallerian degeneration, and clinical disability in multiple sclerosis. J Neuroinflammation 2017; 14:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stephenson E, Nathoo N, Mahjoub Y, et al. Iron in multiple sclerosis: roles in neurodegeneration and repair. Nat Rev Neurol 2014; 10: 459–468 [DOI] [PubMed] [Google Scholar]
- 30.Mehta V, Pei W, Yang G, et al. Iron Is a Sensitive Biomarker for Inflammation in Multiple Sclerosis Lesions. Plos One 2013; 8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hametner S, Wimmer I, Haider L, et al. Iron and neurodegeneration in the multiple sclerosis brain. Ann Neurol 2013; 74:848–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lassmann H The Pathologic Substrate of Magnetic Resonance Alterations in Multiple Sclerosis. Neuroimag Clin N Am 2008; 18:563–576 [DOI] [PubMed] [Google Scholar]
- 33.Maggi P, Absinta M, Grammatico M, et al. Central vein sign differentiates Multiple Sclerosis from central nervous system inflammatory vasculopathies. Ann Neurol 2018; 83:283–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nguyen TD, Deh K, Monohan E, et al. Feasibility and reproducibility of whole brain myelin water mapping in 4 minutes using Fast Acquisition with Spiral Trajectory and adiabatic T2prep (FAST-T2) at 3 Tesla. Magn Reson Med 2016; 76:456–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Magliozzi R, Reynolds R, Calabrese M. MRI of cortical lesions and its use in studying their role in MS pathogenesis and disease course. Brain Pathol 2018; 28:735–742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bian W, Tranvinh E, Tourdias T, et al. In Vivo 7T MR Quantitative Susceptibility Mapping Reveals Opposite Susceptibility Contrast between Cortical and White Matter Lesions in Multiple Sclerosis. AJNR Am J Neuroradiol 2016; 37:1808–1815 [DOI] [PMC free article] [PubMed] [Google Scholar]