Abstract
Objective
We investigated whether levels of signaling pathways and inflammatory adipokines in epicardial fat regulate cardiovascular risks in humans and mice.
Methods
Epicardial fat was obtained from the hearts of patients with heart failure requiring coronary artery bypass surgery and signaling pathways were compared with visceral fat. The genetic profile of epicardial and visceral fat from humans were also compared to genetic profiles of epicardial and visceral fat in obese mice. Left ventricular fractional shortening (LVF) in obese mice after treatment with inducers of mitochondrial signaling; HO-1-PGC1α. An RNA array/heatmap on 88 genes that regulate adipose tissue function was used to identify a target gene network.
Results
Human epicardial fat gene profiling expressed decreased levels of mitochondrial signaling HO-1-PGC1α and an increase of the inflammatory adipokine NOV. Similar observations were seen in epicardial and visceral fat of obese mice. Improvement in LV function was linked to the increase in mitochondrial signaling in epicardial fat of obese mice.
Conclusions
There is a link between cardiac ectopic fat deposition and cardiac function in humans that is similar to that which is described in obese mice. An increase of mitochondrial signaling pathway genes in epicardial fat attenuates cardiometabolic dysfunction and LV fractional shortening in obese mice.
Keywords: inflammation, oxidative stress, cardiomyopathy, metabolic syndrome, hypertension, myocardial biology
Introduction
Obesity is a significant risk factor for heart failure with a prevalence of 70% in individuals who were morbidly obese for 20 years and 90% in those were morbidly obese for 30 years (1). Adipose tissue is an active endocrine organ which secretes multiple signaling molecules, among them leptin, adiponectin, and resistin (2). Pericardial thickness had a direct association with inappropriately high Left ventricular (LV) mass and inversely with fraction shortening (FS) (3) and decreases LV function (2, 4). In contrast, echordiographic assessment of epicardial fat may be a reliable markers of cardiovascular risk, at least in female (2).
Enlarged white adipose cells secrete a number of inflammatory cytokines (5, 6). Elevated adipokine nephroblastoma over expressed CCN3 (NOV) which is a multifamily protein of the CCN family, and is elevated in obesity. Elevated NOV levels are associated with inflammation and tissue damage (7). NOV is a matricellular protein that regulates multiple cellular activities including cell adhesion, migration, proliferation, differentiation, and survival. Induction of NOV increased adipose tissue deposition and enhanced cholesterol and increased insulin resistance and sleep apnea (8). In particular, epicardial fat is a distinct source of adipokines, reactive oxygen species (ROS), and inflammatory cytokines and has been tied to significant cardiac remodeling due to a decrease in the levels of heme oxygenase (HO-1) (9, 10) and peroxisome proliferator-activated receptor gamma coactivator (PGC-1α) (11, 12). HO-1 is regarded as the first line of defense against oxidative stress, given that oxidative stress is a strong inducer of HO, which comprises both an inducible and a constitutive form (HO-1 and HO-2, respectively) and catalyzes the oxidative degradation of heme into iron, biliverdin and carbon monoxide (CO). An increase in HO-1 expression triggers an increase in heme degradation and in the production of CO and bilirubin, an antiapoptotic and an antioxidant respectively, and both of which decrease cardiac remodeling (10, 13, 14). An increase in mouse epicardial fat deposition is associated with a decrease of fat in HO-1 levels and a resultant increase in ROS (9, 11). Importantly, perturbations in the levels of HO-1 regulate mitochondrial biogenesis, (15) levels of mitochondria carriers and their function (16).
PGC-1α, a regulator of PPARα in brown adipose tissue rich with mitochondria, has a critical role in thermogenesis (17) in maintaining mitochondrial biogenesis and function as well as cellular energy metabolism (18). Transgenic mice with mildly elevated levels of muscle PGC-1α are resistant to age-related obesity and diabetes suggesting that PGC-1α stimulates the secretion of factors that affect the function of other tissues (19). Adipose-specific PGC-1α deficiency decreased mitochondrial biogenesis, glucose uptake and the development of insulin resistance (20). Moreover, HO-1 expression is mediated via PGC-1α activation, and, indirectly, can further increase PGC-1α (21). Overexpression of PGC-1α in cardiac tissue of mice activated mitochondrial biogenesis and proliferation (22, 23). Conversely, mice lacking PGC-1α in adipose tissue and fed a high fat diet (HFD) develop insulin resistance and increased levels of circulating lipids (20). PGC-1α along with the transcriptional regulator PR domain containing 16 (PRDM16), are the major facilitators of adipocyte browning and are responsible for thermogenic activation of brown fat (24, 25). In humans, adipose tissue is located beneath the skin (subcutaneous fat), around internal organs (visceral fat), in bone marrow (yellow bone marrow), intramuscularly (muscular system) and in breast tissue. Adipose tissue is found in specific locations, one such area of visceral adiposity is epicardial fat, located around the heart, which is a major determinant of cardiovascular risk.
We examined signaling pathways in adipocyte markers of epicardial fat from diseased hearts afflicted with advanced heart failure and requiring coronary artery bypass surgery, and compared them with visceral fat obtained from age and sex matched individuals undergoing abdominal surgery. Alterations in levels of inflammatory mediators, and cytoprotective/antioxidant autacoids are related to, and appear to be responsible for, the development of heart failure in the obese mouse. Specifically, local adipose tissue appears to have a more pronounced effect on the myocardium than other visceral adipose tissue.
To identify novel relevant epicardial fat adipokines, we combined a genome-wide and heatmap analysis of gene expression in adipocytes that allowed us to identify levels of novel regulatory genes in epicardial and visceral fat. The results indicate that in both humans and mice HO-1, PGC-1α and adiponectin genes expression follow the same pattern. HO-1-PGC1α-UCP1 correlate positively with adiponectin and each other suggesting networks for these message expressions. Further, an increase of HO-1-PGC-1α is associated with a decrease in inflammatory adipokines including NOV and improvement in LV function.
Materials and Methods
Animal Protocols
All mouse experimental protocols were performed following an IACUC of New York Medical College approved protocol in accordance with the NIH Guide for the Care and Use of Laboratory Animals. We used db/db mice as a model of obesity induced diabetic cardiomyopathy as these mice develop insulin resistance, hyperglycemia and fail to control gluconeogenesis, leading to peripheral neuropathy and myocardial disease. Sixteen week old male db/db mice, on C57BL/6J background (Jackson Labs, Bar Harbor, ME), were divided into two groups, 6 mice per group, following a 16-week acclimatization period weighing approximately 54 g at the start of the experiment 1) Control and 2) group treated with EET-agonist; an inducer of HO-1, injected intraperitoneal twice/week for eight weeks at a dose of 1.5 mg/kg body weight and ECHO determined as described (26, 27).
Patient Population
Following informed consent, epicardial fat specimens were obtained from 16 sequential overweight patients undergoing coronary artery bypass surgery. Visceral fat specimens were obtained from age and sex matched controls of 6 donors, 3 females and 3 males, undergoing abdominal surgery. Written informed consent was received from participants prior to inclusion in the study. The human specimens were obtained at St. Mary’s Medical Center, Huntington, WV. The protocol and informed consent were approved by the local IRB.
Human Echocardiograms
Echocardiograms were performed on a Philips IE 33 machine. All echocardiograms were performed by a standard protocol in a laboratory accredited by the Intersocietal Accreditation Commission on Echocardiography Laboratories. The measurements were performed according to the recommendations set forth by the American Society of Echocardiography (28).
Sample Collection
Informed consent was obtained prior to planned surgery. During surgery, samples of epicardial fat and 2 tubes of blood were obtained, placed on ice, and stored at −80 °C. Samples were shipped on ice to New York Medical College for further analysis.
Echocardiogram Measurement of Fractional Shortening (FS)
Echocardiography was performed using a 12-MHz probe on isoflurane anesthetized mice. Images of the left ventricular (LV) diameter was obtained in M-mode and used to measure the end-diastolic and end-systolic diameters from which the LV fractional shortening was calculated (29).
Real-time quantitative PCR
Pooled the pericardial and visceral fat data from both the human (Figure 3) and mouse (Figure 6). The correlations are between the different gene expressions within the specific subjects are presented. PCR array analysis of mRNAs was carried out in randomly selected subjects as well as in mice. Total RNA was extracted from epicardial and visceral adipose tissue with TRIzol (Ambion, Austin, TX). A Biotek plate reader and the Take3 plate (Biotek Winooski, VT) were used to determine RNA at an absorbance of 260 nm (A260). RNA measurements were subsequently evaluated by the A260-to-A280 ratio. A High-Capacity cDNA Reverse Transcription Kit (Applied BioSystems) was used to synthesize cDNA from total RNA (Applied Bio Systems). TaqMan Fast Universal Master Mix (2×) on a 7500 HT Fast Real-Time PCR System (Applied BioSystems) was used to perform real-time PCR. Specific TaqMan Gene Expression Assay probes for mouse HO-1, PGC-1α, NOV, Mfn2, TNF-α, IL-4, PRDM16, adiponectin, and GAPDH were used as previously described (9, 11).
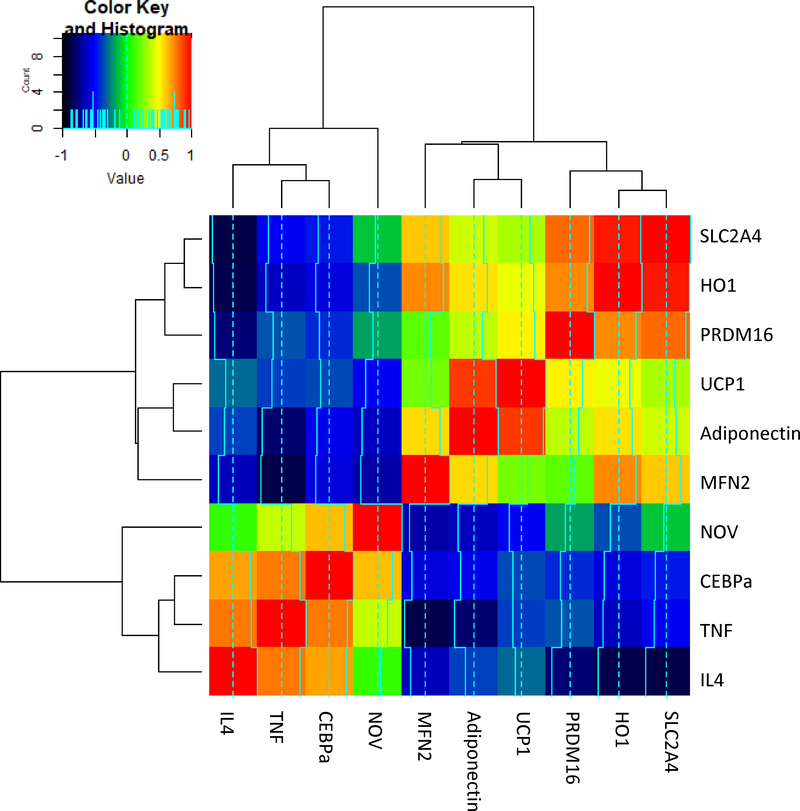
Figure 3.
RNA array analyses-changes in correlation coefficients gene expression of SLC2C4, HO1, PRDM16, UCP1, Adiponectin, MFN2, NOV, CEBPα, TNFα and IL4 both in Human epicardial (P) and visceral fat (V). Data from pericardial and visceral fat were compared using heatmap. Results are mean ± SE, n=4, *p<0.05 vs epicardial fat
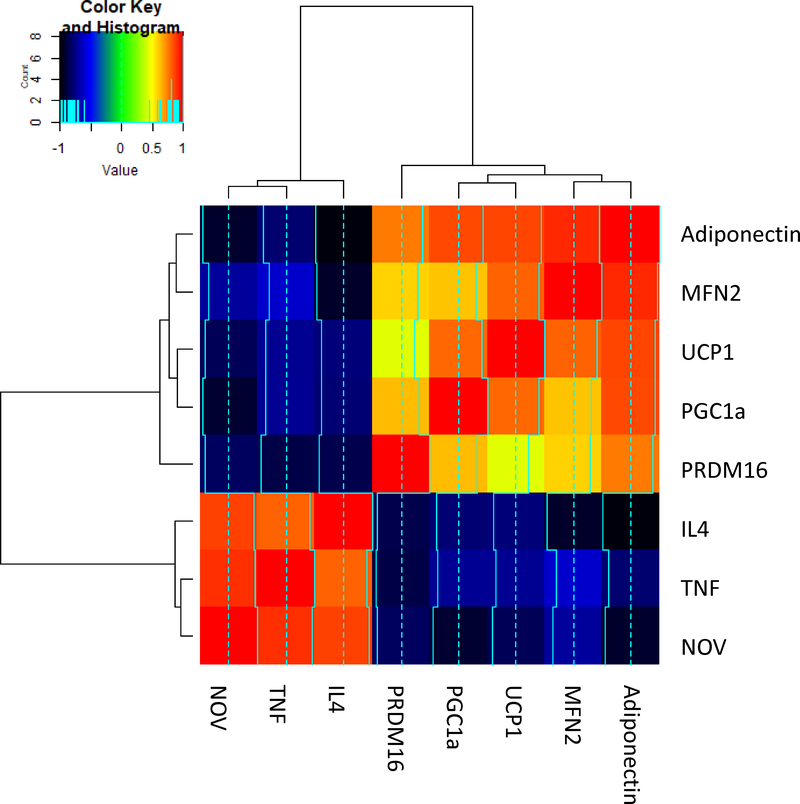
Figure 6.
FS in lean and obese mice and RNA array analyses-changes in correlation coefficients gene expression of adiponectin, MFN2, UCP1, PGC1α, PRMD16, IL4, TNF and NOV in mice epicardial (P) and visceral (V) fat. Data from pericardial and visceral fat were compared using heatmap. Results are mean ± SE, n=4, *p<0.05 vs epicardial fat..
ScienCell’s GeneQuery™ Human Preadipocyte Cell Biology qPCR Array Kit (GQHPAD) Catalog #GK103 was used as an array to find expression of target genes. GeneQuery™ qPCR array kits are qPCR ready in a 96-well plate format, with each well containing one primer set that can specifically recognize and efficiently amplify a target gene’s cDNA. Each GeneQuery™ plate contains eight controls. Five target housekeeping genes (β-actin, GAPDH, LDHA, NONO, and PPIH) enable normalization of data. Pre-adipocyte cell markers: ADIPOQ, LEP, HOXC8/9, TBX1, TMEM26, UCP1, CIDEA, PRDM16, SLC7A10, P2RX5 • Proliferation: CCND1, PROKR1, LYRM1, FTO, SLC2A4, PDGFA, IL4, IGF1, FGF5, MAPK1/3, TNFSF10 • Differentiation and adipogenesis: PPARG, CEBPA/B, KLF2/5/15, SOCS1/3, INSR, DLK1, ADD1, SREBF1 • ECM and cytoskeleton: MMP1/2/3, FAP, DPP4, TGFB1, COL1A1/2, SMAD2/4 • Lipid metabolism: FABP5/7, LBP, LPL, PLIN2, ACSL1, LIPC • Obesity: MC4R, UCP3, ADRB2/3, POMC, FFAR4, CARTPT • Liposarcoma: FUS, MDM2, HMGA2, DDIT3, CDK4, EWSR1, DES, VIM. Aforesaid genes were analyzed in both epicardial and visceral adipose tissue (9, 21).
Gene expression is presented as a heatmap of log10 transformed mean values (n=3) relative to the mean values (n=16) expressed in the human and mice pericardial vs visceral subgroup. The color code appears as a legend in the upper left hand corner of the graph. Values of relative expression ranged from 0.15 to 16.5 or log10 transformed values of −0.83 to 1.22. Genes are ordered from highest increase in expression to greatest decrease in expression in high fat subgroup. Note that the subgroup of enhanced visceral fat demonstrates marked attenuation of the changes compared to pericardial fat. In the short list, we selected those 10 genes that had the highest increase in expression and those 10 genes which had the greatest decrease in expression in visceral vs pericardial fat. Again, the color code for the log10 transformed data is shown in the upper left hand corner of the graph, and again, pericardial fat vs visceral fat changes. The correlation plot shows the Pearson correlation between different gene expressions in the entire set of data (e.g., visceral fat vs pericardial fat). We note that substantial positive and negative correlations of gene expressions exist. As we perform network analysis, we see that to a large degree, there is a group of genes (about 1/2) that correlated positively with each other and negatively with the remaining genes (again, about 1/2). This is illustrated by choosing the genes focused on in the “short” list of top 10 upregulated and top 10 downregulated genes. Very clearly, the top 10 upregulated genes correlated strongly positively with each other (pericardial fat) and the top 10. Details of this method is well described (30, 31)
Statistical analysis
Data analysis was performed using the R programming language with network analysis performed on correlation plots using the gplots package (30, 31). Data values are expressed as means ± SE. Individual group means compared using unpaired Student’s t-test. The null hypothesis was rejected at P < 0.05.
Results
Baseline characteristics are shown in Table 1. The mean body mass index (BMI) of the participants was 28.3 ± 6.8. The mean age was 65.8 years ± 9.9 years. We enrolled 16 sequential patients undergoing a planned cardiac surgery, 15 coronary bypass surgeries alone and one coronary bypass plus valve replacement. The mean age was 65.8 years, 50% were female. The mean BMI was 28. Data regarding cardiac risk factors was collected. All participants were hypertensive, 50% had diabetes. Chronic kidney disease was present in 25%, and 37.5% were smokers.
TABLE 1.
Visceral fat specimens were obtained from age and sex matched controls undergoing abdominal surgery.
| Age years | 65.8 ± 9.9 |
| Female | 8/16 (50%) |
| BMI kg/m2 | 28 ± 6.8 |
| Hypertension | 16/16 (100%) |
| Diabetes | 8/16 (50%) |
| Smoking | 6/16 (37.5%) |
| Chronic Kidney Disease | 4/16 (25%) |
| Total Cholesterol mg/dl | 135.8 ± 36 |
| HDL mg/dl | 43.1 ± 21.4 |
| LDL mg/dl | 78.9 ± 30.4 |
| Triglycerides mg/dl | 122.8 ± 24.5 |
| Fasting glucose mg/dl | 126.3 ± 35.5 |
| Epicardial fat thickness by echo mm | 6.7 ± 1.8 |
| Ejection fraction % | 49.7 ± 8.8 |
| Fractional Shortening % | 23.6 ± 8 |
Listed as mean ± standard deviation. BMI = Body mass index.
Lab data was available for lipid studies in 14 of 16 participants. The average total cholesterol was 135.8 mg/dl. The average LDL cholesterol was 78.9 mg/dl and the mean HDL was 43 mg/dl. Average triglyceride level among participants was 122.8 mg/dl. The lipid data may be affected by fluctuations in cholesterol levels during acute events and by treatment with statins. The mean fasting glucose level was 126.3 mg/dl.
Echocardiographic data was obtained prior to surgery in all participants. The average thickness of epicardial fat by echocardiography was 6.7 mm. The mean ejection fraction was 49.7%, with a mean fractional shortening of 23.6%.
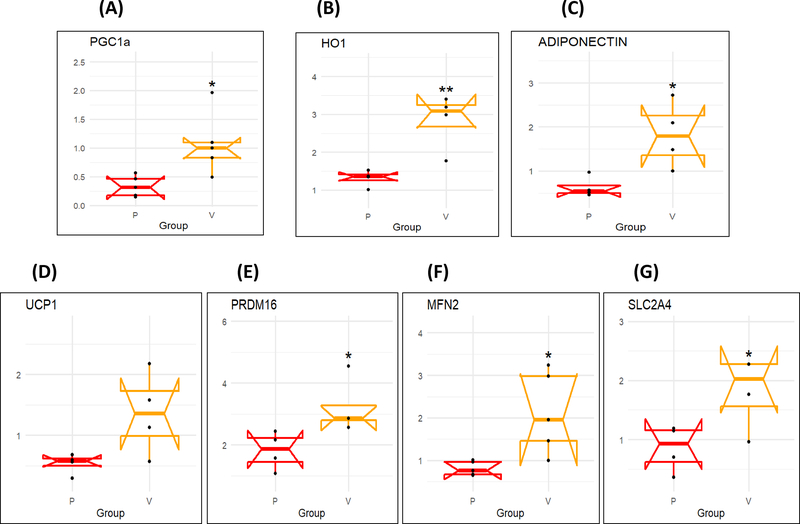
Human epicardial fat and visceral fat exhibit similar differences in the expression levels of PGC1α, HO-1, and adiponectin, UCP1, PRDM16 and MFN2
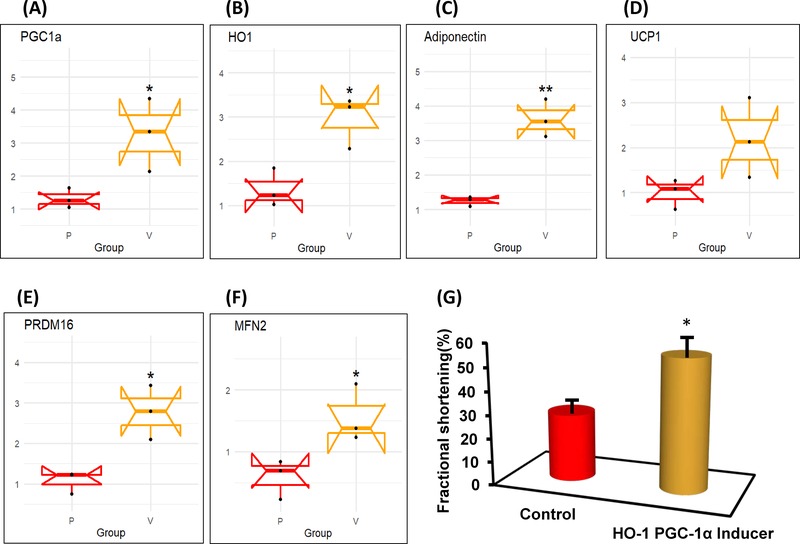
The mRNA expression levels of PGC1α, HO-1 and adiponectin in human epicardial fat were significantly (p < 0.05) reduced as compared to visceral fat during RT-PCR analysis (Figure 1A, B and C). Similarly, uncoupling gene UCP1, thermogenesis gene PRDM16 and mitochondrial dynamics related mitofusion gene Mfn2 were also significantly (p < 0.05) decreased in epicardial compared to visceral fat (Figure 1D, E and F). Furthermore, Glut4 expression was decreased in epicardial fat (Figure 1G).
Figure 1.
mRNA expression of (A) PGC-1α, (B) HO-1, (C) Adiponectin, (D) UCP1, (E) PRDM16, (F) MFN2 and (G) SLC2A4 in Human epicardial (P) vs Visceral adipose (V) tissues. Results are mean ± SE, n=4, *p<0.05 vs epicardial fat, **p<0.02 vs epicardial fat.
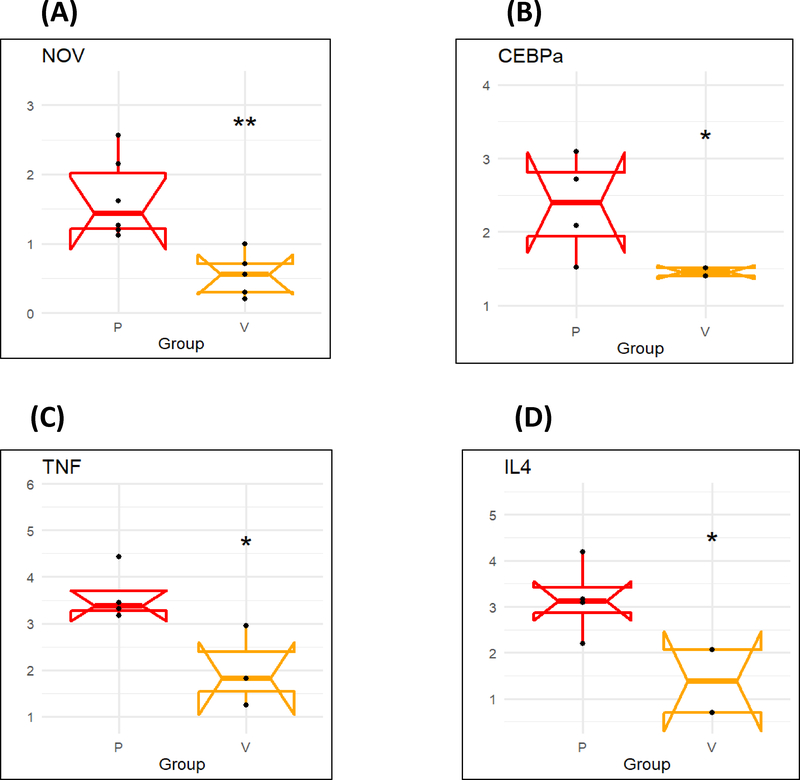
Human epicardial fat displays increased expression of adipogenic and inflammatory markers
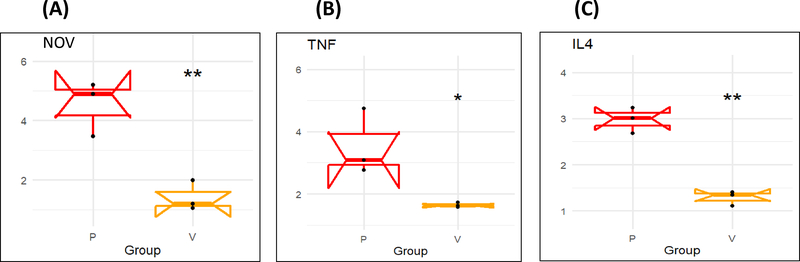
There was higher mRNA expression of inflammatory adipokine CCN3/NOV in epicardial fat (Figure 2A) compared to visceral fat. Moreover, the mRNA expression of adipogenic CEBPα (p< 0.05) was significantly upregulated in human epicardial fat compared to visceral fat (Figure 2B), and expression of inflammatory TNF-α and IL-4 mRNA expression was significantly higher (p<0.05) in epicardial fat compared to visceral fat (P < 0.05) (Figure 2C and D).
Figure 2.
mRNA expression of inflammatory markers (A) NOV, (B) CEBPα, (C) TNFα, and (D) IL4 in Human epicardial (P) vs visceral adipose (V) tissues. Results are mean ± SE, n=4, *p<0.05 vs epicardial fat, **p<0.02 vs epicardial fat.
RNA array analyses identify changes in correlation coefficients of gene expression in human epicardial and visceral fat
Examination of the upper part of the graph indicates a color code for the correlation coefficients positive, as yellow to red and negative is blue to black. In human genes (Figure 3), it is clear that IL4 correlates with TNFα and CEBPα, those latter two, correlate very tightly with each other and also positively with NOV. This group of four correlates negatively with MFN2, adiponectin, UCP1, PRDM16, HO-1 and SLC2A4 genes of which HO-1 and SLC2A4 correlate closely with each other. UCP1 and adiponectin correlate positively with each other suggesting networks for these message expressions. MFN2 correlates with HO-1 and SLC2A4 but not as tightly as these two correlate with each other. UCP1 is red but almost as red with adiponectin and is less strong with MFN2 almost in the no correlation range. PRDM16 is very tightly connected to HO-1 and SLC2A4.
HO-1, PGC-1α in mouse epicardial fat vs visceral fat and left ventricular fractional shortening
Decreased levels of PGC-1α and HO-1 were found in epicardial fat compared to visceral fat p<0.05), Figure 4 A and B. The expression of adiponectin mRNA was decreased in epicardial vs visceral fat (p<0.05) (Figure 4 C). Similarly, levels of the mitochondrial uncoupling gene UCP1 and PRDM16, a thermogenic gene, are decreased in epicardial fat compared to the levels found in visceral fat, p<0.05 (Figure 4 D and E). The decrease in HO-1 and PGC-1α was associated with a decrease in the mitochondrial biogenesis gene Mfn2. The levels of Mfn2 in epicardial adipose tissue are less than 50% than in visceral fat p<0.05) (Figure 4F). Further, we examined the effect of the increase of HO-1 PGC1-α levels on cardiac function in mice. Cardiac function, measured by echocardiography and calculation of left ventricular fractional shortening (FS) was impaired (p<0.05 in db/db mice when compared to lean mice). Mice (db/db) were administered with an inducer of HO-1,-PGC1-α using EET agonist, for 8 weeks; followed by measuring FS (as described (10, 11)). As depicted on Figure 4G, FS was reduced (p<0.05) in untreated obese mice while the treated mice with HO-1 PGC1-α inducer (EET-agonist ) exhibited improved heart function as measured by increased FS. FS in wild type obese mice treated with EET-agonist mice as measured by M-mode echocardography was 55% in lean mice, while the FS in obese mice was reduced to 29% (Figure 4 G).
Figure 4.
mRNA expression of (A) PGC-1α, (B) HO-1, (C) Adiponectin, (D) UCP1, (E) PRDM16, (F) MFN2 and in mice epicardial (P) vs Visceral adipose (V) tissues. Results are mean ± SE, n=4, *p<0.05 vs epicardial fat, **p<0.02 vs epicardial fat. (G) Increase of HO-1-PGC-1α using EET-A as an inducer, improved FS. Results mean ± SE, n=5, *p<0.05 vs db/db/ (saline) mice. Echocardiogram measured LV and FS as previously described by Singh et al, (11).
Differential levels of adiponectin and inflammatory adipokines in mouse visceral fat and epicardial fat
Inflammatory adipokine NOV and IL4 and TNF-α in epicardial fat of obese mice are markedly elevated (p<0.05) compared to levels found in visceral fat. (Figure 5A, B and C).
Figure 5.
mRNA expression of inflammatory markers (A) NOV, (B) TNFα, (C) IL4, in mice epicardial (P) vs Visceral adipose (V) tissues. Results are mean ± SE, n=4, *p<0.05 vs epicardial fat, **p<0.02 vs epicardial fat.
RNA array analyses identify similar changes in correlation coefficients of gene expression in mouse epicardial and visceral fat
Although identifying fewer genes, the mouse heatmap demonstrates similarities to the human in that HO-1, PGC1α and adiponectin correlate closely with high levels of TNF, NOV and IL4 (Figure 6). Red represents a complete correlation, while the color orange in this figure represents an R value of 0.65 – 0.7. Conversely, UCP1 correlates negatively with IL 4 and TNFα represented by blue and black. IL4 and MFN2 are negatively correlated (Figure 6).
Discussion
In the present report, we describe signaling pathways in the epicardial fat of overweight and obese patients with coronary disease that correspond to pathways that we have previously described in epicardial fat of an obese mouse model. In addition, we describe adverse signaling expression pathways in the epicardial fat of overweight humans with vascular disease that are significantly more prominent than those found in visceral fat, another observation in concert with that seen in our mouse model (9, 11, 12). This suggests that the accumulation of inflammatory cytokines and chemokines in epicardial fat has a significantly greater toxic effect on the heart than those emanating from visceral fat, both in human and mice. Additionally, epicardial fat NOV is highly expressed compared to visceral fat. More importantly, we identify PGC-1α and HO-1 as candidate markers for attenuation of LV dysfunction and, as such, may be the basis for the development of personalized therapeutic approaches to this problem due to obesity. The correlations are between different gene expressions within the specific subjects are presented. For example, that SLC2A4 gene expression correlates extremely well with HO1 gene expression between visceral and pericardial fat. HO-1-PGC1α-UCP1 correlate positively with adiponectin and each other suggesting networks for these message expressions. Further, an increase of HO-1-PGC-1α is associated with a decrease in inflammatory adipokines including NOV and improvement in LV function.
Adipose tissue exerts powerful effects on both vascular and cardiac function (32, 33). In particular, excess epicardial fat is associated with cardiac remodeling and cardiomyopathy (29, 34). Epicardial fat is anatomically adjacent to the epicardium and is not separated by any fascial layer (35). The lack of a significant barrier between epicardial fat and the myocardium allows for the transport of adipokines into the heart with relative ease by a number of mechanisms, including a concentration gradient. This is suggested by our prior observation of a greater expression of NOV in the epicardial fat than in the myocardium of obese mice (9).
HO-1 reduces adiposity and vascular dysfunction in obese mice by a number of mechanisms, among which is the increased expression of adiponectin through an increase in bilirubin and carbon monoxide, products of HO-1 mediated heme degradation, known to attenuate vascular dysfunction in hypertension (36, 37, 38, 39, 40). In the heart, the anti-inflammatory effects of bilirubin include increasing nitric oxide bioavailability (41). Similarly, bilirubin decreased levels of inflammatory cytokines as well as markers of endoplasmic reticulum stress (42). Reduction in HO-1 expression is associated with an increase in ROS resulting in organ damage in both humans and mice (43, 44).
We have previously shown that ablation of HO-1 is associated with a reduction in PGC-1 α, which, itself, is responsible for a decrease in Mfn2 and a resulting increase in white fat over brown-like fat (6, 45). As noted above, HO-1 expression is also mediated via PGC-1α activation, and, indirectly, can further increase PGC-1α by enhancing EET activity. As PGC-1α is downstream of EET, it becomes clear that HO-1, EET, and PGC-1α together constitute a feedback loop that has critical ramifications for cardiac function (Figure 7).
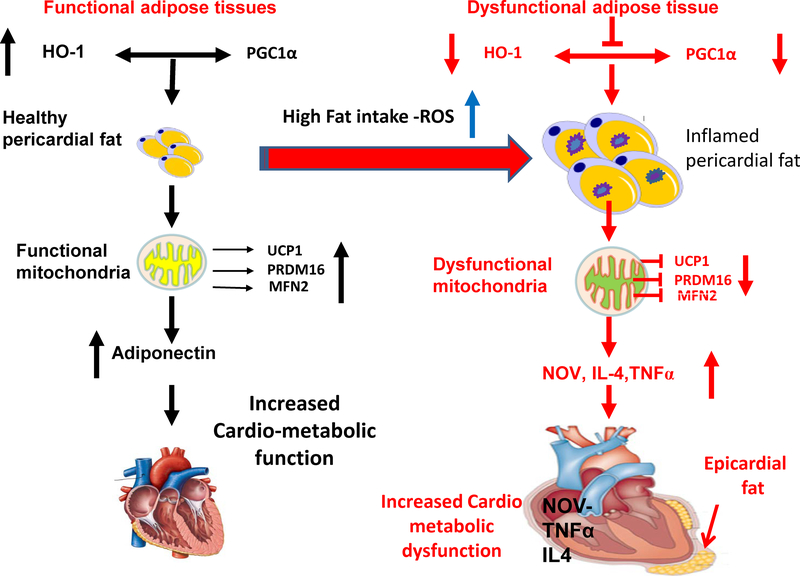
Figure 7.
Schematic depiction showed in the epicardial fat of humans and mice with coronary disease, there is a significant reduction in HO-1, PGC-1α, adiponectin, Mfn2, and PRDM16 when compared to the visceral fat of age and sex matched controls as well in lean obese mice. This is accompanied by a reciprocal increase in NOV and the inflammatory marker, TNF-α in the epicardial fat.
Inhibition of PGC-1α is accompanied by a reduction in mitochondrial biogenesis as manifested by a concurrent reduction in Mfn2 and thermogenic genes such as PRDM16. This, in turn, results in cardiac remodeling and cardiomyopathy as well as reduced energy expenditure and conversion of brown to white adipose tissue. This process can be reversed in the mouse by induction of the increase in HO-1-PGC-1α axis. Increased levels of HO-1, and PGC1α are essential in restoring LV function (9, 10, 11).
The increase of FS in obese mice treated with EET agonist significantly decreases NOV protein expression in epicardial fat, which concurrently upregulated HO-1-PGC1α downstream signaling and restores ejection fraction and heart function. The observed reduction of HO-1 and PGC-1α in untreated obese mice was associated with a marked increase in NOV indicating that inflammatory markers NOV is located downstream of either HO-1 or PGC-1α. Moreover, downregulation of NOV is associated with a reduction of other inflammatory adipokines (9, 11, 12). We have previously demonstrated that NOV is interrelated with HO-1 and PGC-1α in the obese db/db mouse and that it is located downstream of PGC-1α in the signaling pathway (9).
In conclusion, we demonstrate in the epicardial fat of humans with coronary disease, there is a significant reduction in HO-1, PGC-1α, adiponectin, Mfn2, and PRDM16 when compared to the visceral fat of age and sex matched controls. This is accompanied by a reciprocal increase in NOV, TNF-α in the epicardial fat. This latter observation is identical to that reported in the mouse, (11) as is the reduction in the activity of the HO-1-PGC-1α levels. Moreover, reduction in the activity of the HO-1-PGC-1α levels in the epicardial fat appears to promote myocardial dysfunction and cardiomyopathy (Figure 4). As seen on Figure 3 and 6 pooled the pericardial and visceral fat data from both the human and mouse. The correlations are between the different gene expressions within the specific subjects as in obese mice vs lean are presented. For example, that SLC2A4 gene expression correlates extremely well with HO-1 gene expression between visceral and pericardial fat. This study provides the first evidence for a difference in activity of the HO-1-PGC-1α levels between epicardial fat and visceral fat, and demonstrates for the first time that these signaling pathways previously described in the mouse appear to be active in human epicardial fat as well. Stimulation of HO-1-PGC-1α in the mouse is associated with significant improvement in the ejection fraction of the hearts of mice with obesity and diabetic cardiomyopathy (9, 11). Hence, the current observations open the way toward additional human studies that may further elucidate these pathways and consequently lead to novel targets for the treatment both of obesity itself and its related cardiomyopathy syndromes.
What is already known about this subject?
Site specific pericardial fat affects ventricular function
Pericardial and epicardial fat metabolic profile affects cardiac structure
Adipose tissue surrounding the heart can have local toxic effects
What does this study add?
The signaling profile of epicardial fat reveals an increase in inflammatory adipokines and a reduction in inducers of mitochondrial biogenesis compared to visceral fat in both overweight humans and mice with heart failure.
RNA arrays and heatmap identifies target genes that may be essential to LV function in humans and mice.
An increase of mitochondrial signaling genes correlates closely with a decrease in inflammatory adipokines in epicardial fat and restoration of LV fractional shortening in obese mice.
Acknowledgments
All authors have read the manuscript and agree entirely with its contents. We thank Mrs. Jennifer Brown for her editorial assistance in preparing the manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
FUNDING
This work was supported by the National Institutes of Health (Grant 1R56HL139561; NGA).
Footnotes
CONFLICT OF INTEREST
The authors declared there are no conflicts of interests regarding the submitted work which was not carried out in the presence of any personal, professional or financial relationships that could potentially be construed as such.
References
- 1.Alpert MA, Terry BE, Mulekar M, Cohen MV, Massey CV, Fan TM, et al. Cardiac morphology and left ventricular function in normotensive morbidly obese patients with and without congestive heart failure, and effect of weight loss. Am J Cardiol 1997;80: 736–740. [DOI] [PubMed] [Google Scholar]
- 2.Fernandez Munoz MJ, Basurto Acevedo L, Cordova Perez N, Vazquez Martinez AL, Tepach Gutierrez N, Vega Garcia S, et al. Epicardial adipose tissue is associated with visceral fat, metabolic syndrome, and insulin resistance in menopausal women. Revista espanola de cardiologia (English ed) 2014;67: 436–441. [DOI] [PubMed] [Google Scholar]
- 3.Pucci G, Battista F, de VS, Boni M, Scavizzi M, Ricci MA, et al. Pericardial fat, insulin resistance, and left ventricular structure and function in morbid obesity. Nutr Metab Cardiovasc Dis 2014;24: 440–446. [DOI] [PubMed] [Google Scholar]
- 4.Rosito GA, Massaro JM, Hoffmann U, Ruberg FL, Mahabadi AA, Vasan RS, et al. Pericardial fat, visceral abdominal fat, cardiovascular disease risk factors, and vascular calcification in a community-based sample: the Framingham Heart Study. Circulation 2008;117: 605–613. [DOI] [PubMed] [Google Scholar]
- 5.Peterson SJ, Shapiro JI, Thompson E, Singh S, Liu L, Weingarten JA, et al. Oxidized HDL, Adipokines, and Endothelial Dysfunction: A Potential Biomarker Profile for Cardiovascular Risk in Women with Obesity. Obesity (Silver Spring) 2019;27: 87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh S, Grant I, Meissner A, Kappas A, Abraham N. Ablation of adipose-HO-1 expression increases white fat over beige fat through inhibition of mitochondrial fusion and of PGC1alpha in female mice. Horm Mol Biol Clin Investig 2017;31. [DOI] [PubMed] [Google Scholar]
- 7.Pakradouni J, Le GW, Calmel C, Antoine B, Villard E, Frisdal E, et al. Plasma NOV/CCN3 levels are closely associated with obesity in patients with metabolic disorders. PLoS One 2013;8: e66788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weingarten J, Bellner L, Peterson S, Zaw M, Chadha P, Singh S, et al. The association of NOV/CCN3 with obstructive sleep apnea (OSA): preliminary evidence of a novel biomarker in OSA. Horm Mol Biol Clin Investig 2017;31. [DOI] [PubMed] [Google Scholar]
- 9.Cao J, Singh SP, McClung J, Joseph G, Vanella L, Barbagallo I, et al. EET Intervention on Wnt1, NOV and HO-1 Signaling Prevents Obesity-Induced Cardiomyopathy in Obese Mice. Am J Physiol Heart Circ Physiol 2017;313: H368–H380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Monu SR, Pesce P, Sodhi K, Boldrin M, Puri N, Fedorova L, et al. HO-1 induction improves the type-1 cardiorenal syndrome in mice with impaired angiotensin II-induced lymphocyte activation. Hypertension 2013;62: 310–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh SP, McClung JA, Bellner L, Cao J, Waldman M, Schragenheim J, et al. CYP-450 Epoxygenase Derived Epoxyeicosatrienoic Acid Contribute To Reversal of Heart Failure in Obesity-Induced Diabetic Cardiomyopathy via PGC-1 alpha Activation. Cardiovascular Pharmacology (Open Access) 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schragenheim J, Bellner L, Cao J, Singh SP, Bamshad D, McClung JA, et al. EET enhances renal function in obese mice resulting in restoration of HO-1-Mfn1/2 signaling, and decrease in hypertension through inhibition of sodium chloride co-transporter. Prostaglandins Other Lipid Mediat 2018;137: 30–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abraham NG, Rezzani R, Rodella L, Kruger A, Taller D, Li VG, et al. Overexpression of human heme oxygenase-1 attenuates endothelial cell sloughing in experimental diabetes. Am J Physiol Heart Circ Physiol 2004;287: H2468–H2477. [DOI] [PubMed] [Google Scholar]
- 14.Abraham NG, Junge JM, Drummond GS. Translational Significance of Heme Oxygenase in Obesity and Metabolic Syndrome. Trends Pharmacol Sci 2016;37: 17–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Piantadosi CA, Carraway MS, Babiker A, Suliman HB. Heme oxygenase-1 regulates cardiac mitochondrial biogenesis via Nrf2-mediated transcriptional control of nuclear respiratory factor-1. Circ Res 2008;103: 1232–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Di Noia MA, Van DS, Palmieri F, Yang LM, Quan S, Goodman AI, et al. Heme oxygenase-1 enhances renal mitochondrial transport carriers and cytochrome C oxidase activity in experimental diabetes. J Biol Chem 2006;281: 15687–15693. [DOI] [PubMed] [Google Scholar]
- 17.Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 1998;92: 829–839. [DOI] [PubMed] [Google Scholar]
- 18.Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 1999;98: 115–124. [DOI] [PubMed] [Google Scholar]
- 19.Wenz T, Rossi SG, Rotundo RL, Spiegelman BM, Moraes CT. Increased muscle PGC-1alpha expression protects from sarcopenia and metabolic disease during aging. Proc Natl Acad Sci U S A 2009;106: 20405–20410. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.Kleiner S, Mepani RJ, Laznik D, Ye L, Jurczak MJ, Jornayvaz FR, et al. Development of insulin resistance in mice lacking PGC-1alpha in adipose tissues. Proc Natl Acad Sci U S A 2012;109: 9635–9640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singh SP, Schragenheim J, Cao J, Falck JR, Abraham NG, Bellner L. PGC-1 alpha regulates HO-1 expression, mitochondrial dynamics and biogenesis: Role of epoxyeicosatrienoic acid. Prostaglandins Other Lipid Mediat 2016;125: 8–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arany Z, He H, Lin J, Hoyer K, Handschin C, Toka O, et al. Transcriptional coactivator PGC-1 alpha controls the energy state and contractile function of cardiac muscle. Cell Metab 2005;1: 259–271. [DOI] [PubMed] [Google Scholar]
- 23.Rowe GC, Jiang A, Arany Z. PGC-1 coactivators in cardiac development and disease. Circ Res 2010;107: 825–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seale P, Bjork B, Yang W, Kajimura S, Chin S, Kuang S, et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature 2008;454: 961–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hondares E, Rosell M, Diaz-Delfin J, Olmos Y, Monsalve M, Iglesias R, et al. Peroxisome proliferator-activated receptor alpha (PPARalpha) induces PPARgamma coactivator 1alpha (PGC-1alpha) gene expression and contributes to thermogenic activation of brown fat: involvement of PRDM16. J Biol Chem 2011;286: 43112–43122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schreyer SA, Wilson DL, LeBoeuf RC. C57BL/6 mice fed high fat diets as models for diabetes-accelerated atherosclerosis. Atherosclerosis 1998;136: 17–24. [DOI] [PubMed] [Google Scholar]
- 27.Surwit RS, Kuhn CM, Cochrane C, McCubbin JA, Feinglos MN. Diet-induced type II diabetes in C57BL/6J mice. Diabetes 1988;37: 1163–1167. [DOI] [PubMed] [Google Scholar]
- 28.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography 2005;18: 1440–1463. [DOI] [PubMed] [Google Scholar]
- 29.Fuster JJ, Ouchi N, Gokce N, Walsh K. Obesity-Induced Changes in Adipose Tissue Microenvironment and Their Impact on Cardiovascular Disease. Circ Res 2016;118: 1786–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Team RC. (2018) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: URL https://wwwR-projectorg/. [Google Scholar]
- 31.Gregory R Warnes BB, Bonebakker Lodewijk, Gentleman Robert, Huber Wolfgang Liaw Andy, Lumley Thomas, Maechler Martin, Magnusson Arni, Moeller Steffen, Schwartz Marc and Venables Bill. (2019) gplots: Various R Programming Tools for Plotting Data R package version 3011 https://CRANR-projectorg/package=gplots.
- 32.Akoumianakis I, Tarun A, Antoniades C. Perivascular adipose tissue as a regulator of vascular disease pathogenesis: identifying novel therapeutic targets. Br J Pharmacol 2017;174: 3411–3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Antonopoulos AS, Antoniades C. The role of epicardial adipose tissue in cardiac biology: classic concepts and emerging roles. J Physiol 2017;595: 3907–3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Graner M, Pentikainen MO, Nyman K, Siren R, Lundbom J, Hakkarainen A, et al. Cardiac steatosis in patients with dilated cardiomyopathy. Heart 2014;100: 1107–1112. [DOI] [PubMed] [Google Scholar]
- 35.Iacobellis G, Corradi D, Sharma AM. Epicardial adipose tissue: anatomic, biomolecular and clinical relationships with the heart. Nature clinical practice Cardiovascular medicine 2005;2: 536–543. [DOI] [PubMed] [Google Scholar]
- 36.Hosick PA, Stec DE. Heme oxygenase, a novel target for the treatment of hypertension and obesity? Am J Physiol Regul Integr Comp Physiol 2012;302: R207–R214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kawamura K, Ishikawa K, Wada Y, Kimura S, Matsumoto H, Kohro T, et al. Bilirubin from heme oxygenase-1 attenuates vascular endothelial activation and dysfunction. Arterioscler Thromb Vasc Biol 2005;25: 155–160. [DOI] [PubMed] [Google Scholar]
- 38.Li M, Kim DH, Tsenovoy PL, Peterson SJ, Rezzani R, Rodella LF, et al. Treatment of obese diabetic mice with a heme oxygenase inducer reduces visceral and subcutaneous adiposity, increases adiponectin levels, and improves insulin sensitivity and glucose tolerance. Diabetes 2008;57: 1526–1535. [DOI] [PubMed] [Google Scholar]
- 39.Nicolai A, Li M, Kim DH, Peterson SJ, Vanella L, Positano V, et al. Heme oxygenase-1 induction remodels adipose tissue and improves insulin sensitivity in obesity-induced diabetic rats. Hypertension 2009;53: 508–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Braud L, Pini M, Muchova L, Manin S, Kitagishi H, Sawaki D, et al. Carbon monoxide-induced metabolic switch in adipocytes improves insulin resistance in obese mice. JCI Insight 2018;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bakrania B, Du Toit EF, Ashton KJ, Kiessling CJ, Wagner KH, Headrick JP, et al. Hyperbilirubinemia modulates myocardial function, aortic ejection, and ischemic stress resistance in the Gunn rat. Am J Physiol Heart Circ Physiol 2014;307: H1142–1149. [DOI] [PubMed] [Google Scholar]
- 42.Dong H, Huang H, Yun X, Kim DS, Yue Y, Wu H, et al. Bilirubin increases insulin sensitivity in leptin-receptor deficient and diet-induced obese mice through suppression of ER stress and chronic inflammation. Endocrinology 2014;155: 818–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ohta K, Yachie A, Fujimoto K, Kaneda H, Wada T, Toma T, et al. Tubular injury as a cardinal pathologic feature in human heme oxygenase-1 deficiency. Am J Kidney Dis 2000;35: 863–870. [DOI] [PubMed] [Google Scholar]
- 44.Poss KD, Tonegawa S. Reduced stress defense in heme oxygenase 1-deficient cells. Proc Natl Acad Sci U S A 1997;94: 10925–10930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vanella L, Sodhi K, Kim DH, Puri N, Maheshwari M, Hinds TD Jr., et al. Increased heme-oxygenase 1 expression decreases adipocyte differentiation and lipid accumulation in mesenchymal stem cells via upregulation of the canonical Wnt signaling cascade. Stem Cell Res Ther 2013;4: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]