Abstract
Expression of inhibitors of apoptosis protein (IAP) family members is associated with poor prognosis in cancer patients. Immunity to ML-IAP (livin) and survivin have been well studied in patients with a variety of tumors. XIAP, the most potent inhibitor of apoptosis, is widely expressed in melanoma. To better define its potential role as an immunogenic target, cellular and humoral responses to XIAP were investigated in patients with advanced melanoma. An overlapping peptide library covering the full length of the XIAP protein was used to screen T cell responses of peripheral blood mononuclear cells (PBMC) from stage-IV melanoma patients treated with or without anti-CTLA4 (ipilimumab). The screen identified an array of peptides that predominantly induced CD4+ T cell responses. XIAP epitope-specific CD4+ T cells revealed proliferative responses to melanoma cells that express XIAP. Humoral responses to XIAP were also explored. Cellular and humoral responses to XIAP were associated with beneficial clinical outcomes after ipilimumab-based treatment, supporting XIAP as a potential therapeutic target.
Keywords: Melanoma, XIAP, CTLA4 blockade, PD-1 blockade
Introduction
Inhibitors of apoptosis protein family members are characterized with evolutionarily conserved baculoviral inhibitor of apoptosis repeat (BIR) domains [1]. Because they prevent caspase-dependent apoptosis, these proteins have become attractive therapeutic targets of chemotherapy and immunotherapy of cancer [2–5]. Immunity to IAP family members, ML-IAP and survivin, have been studied previously, and both cellular and humoral responses to ML-IAP and survivin have been detected in cancer patients [6–10]. Furthermore, GM-CSF-based vaccination has been reported to induce cellular and humoral responses to ML-IAP [11]. Increases in sera titers to ML-IAP and beneficial clinical responses were observed in a subset of melanoma patients receiving ipilimumab [9].
XIAP is the most potent inhibitor of apoptosis and is highly expressed in melanoma cell lines and tumors [12, 13]. Elevation in XIAP expression correlates with tumor progression and chemotherapy resistance [14]. In contrast, loss of XIAP in XIAP knock-out mice results in reduced stability of SOC1 and Foxp3, and decreased suppression of T cells by Treg [15]. As a result, there remains interest in evaluating XIAP as an immunogenic target.
Materials and methods
PBMC and Sera of melanoma patients
PBMC and sera samples were obtained from melanoma patients participating in IRB-approved protocols at the Dana-Farber/Harvard Cancer Center. PBMC and plasma were obtained from heparinized peripheral blood by gradient centrifugation using Ficoll-Paque Plus (GE Healthcare Bio-Sciences, Piscataway, NJ). PBMC from 35 stage-IV melanoma patients without any treatment were used for screening T cell responses to XIAP peptides by ELISPOT. Posttreatment PBMC from seven, stage-IV melanoma patients receiving ipilimumab were used to screen T cell responses to XIAP peptides. Studies of cellular and humoral responses to XIAP were primarily based on four cohorts of advanced melanoma patients who were treated with ipilimumab and/or anti-PD1 (i.e., ipilimumab alone; ipilimumab plus bevacizumab; anti-PD-1 alone; or ipilimumab plus anti-PD1) as standard of care or through participation in clinical trials. Among these cohorts, most patients had cutaneous melanoma. In 36 ipilimumab-treated patients, one had ocular disease; in 63 patients treated with P-1, three had ocular and ten had mucosal disease; in 58 patients treated with ipilimumab plus anti-P-1, two had ocular and eleven had mucosal disease.
Cell lines
A375, K008, K028, K029, K033, M13, and M23 melanoma cell lines were grown in DMEM medium containing 10% fetal bovine serum.
SDS-PAGE and immunoblotting
For XIAP expression in cell line studies, melanoma cell lines were cultured in complete DMEM medium. After washing with PBS three times, the cells were harvested and further lysed in RIPA lysis buffer. 100 μg of purified proteins were subjected to 10% SDS-polyacrylamide gel electrophoresis (PAGE) and transferred onto PVDF membranes. The membranes were immunoblotted overnight at 4°C with a mouse anti-human XIAP antibody at 0.25 μg/ml (BD Transduction Lab), and further incubated with an HRP-conjugated, anti-mouse secondary antibody for two hours at room temperature. The protein bands were detected by chemiluminescence.
ELISPOT Assay
T cell responses of melanoma patients to XIAP were examined using overlapping peptides (Fig. 1) that were screened as described previously [16]. Briefly, 1 × 106 PBMC were sensitized with either overlapping peptide pools or individual peptides in the presence of 10 U/ml IL-2 for one week, and the cells were re-stimulated with the same peptides and autologous PBMC overnight during the assay. L11(LLFGYPVYV), an HIV peptide, was used as a negative control.
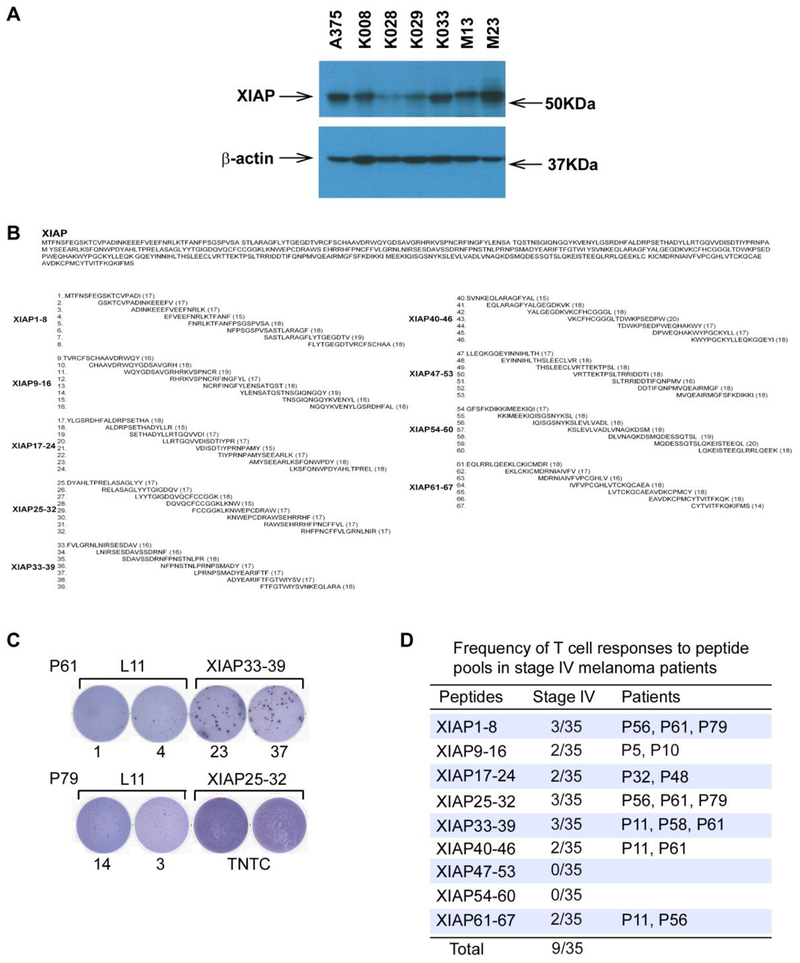
Figure 1.
Spontaneous cellular immunity to XIAP in patients with metastatic melanoma. (A) XIAP expression in melanoma cells. XIAP expression in melanoma cells was detected by SDS-PAGE and immunoblotting assays, β-actin was used as an internal loading control. (B) Peptide sequence of XIAP protein with corresponding overlapping peptides generated. (C) Examples of T cell responses to overlapping peptide pools by ELISPOT analyses. Patients P61 and P79 showed responses to peptide pools XIAP33–39 and XIAP25–32, respectively. TNTC: too numerous to count. (D) Frequency of T cell responses to overlapping peptide pools in stage-IV melanoma patients. PBMC were cultured with overlapping peptide pools of XIAP for one week in the presence of 10 U/ml IL-2, and further re-stimulated with either L11 peptide (negative control) or the same peptide pools in ELISPOT assays. Reported is the number of patients with positive responses to the depicted peptide pool. The total refers to the number of patients that underwent peptide pool screening.
Generation of Antigen Specific T cells
Antigen-specific CD4+ T cells were generated as previously described [9]. Briefly, naïve CD4+ T cells were stimulated with irradiated autologous PBMC in the presence of 10 μg/ml peptide, and the antigen-specific T cells were enriched with IFN-γ selection and further expanded with PHA, irradiated PBMC, and IL-2. To study haplotype specificity, 10 μg/ml of anti-HLA-DR (clone L243, BD Biosciences), anti-HLA-DP (clone B7/21, Abeam, Cambridge, MA), and anti-HLA-DQ (clone SPVL3, Beckman Coulter, Miami, FL) antibodies were utilized to block the interactions between CD4+ T cells and autologous APC/melanoma cell lines.
HLA Typing
HLA-DRB1 and HLA-DPB1 alleles of patients and melanoma cell lines were typed by a combination of sequence-specific oligonucleotide (SSO) probes and sequence-specific primer-based methods using commercially available kits (One Lambda, Canoga Park, CA) at the Tissue Typing Laboratory, Brigham and Women’s Hospital, Boston, MA.
Proliferation assay
As previously described [17], T cells were co-cultured with irradiated melanoma cells for two days. Cell proliferation was measured using [3H] thymidine.
ELISA
Sera titers to XIAP were examined by ELISAs as previously described [16]. Approximately 1.7 pmol/well of His-tagged recombinant XIAP (R & D systems, Minneapolis, MN) and HIS peptide (ATVIDHHHHHHSSNG) as control were coated and detected with patient sera and an HRP-conjugated, goat anti-human IgG antibody. Plates were further treated with H2O2 and biotinyl-tyramide and developed with TMB substrate. All samples were examined in duplicate. Measurements were reported as the mean of the sample minus the mean of His peptides.
Statistical Analyses
All ELISPOT and ELISA samples were run in duplicate. Three-fold increases between pre- and post-treatment were considered positive responses in ELISPOT, and at least 1.26-fold increases were considered meaningful responses in ELISA. The dichotomous cut point relating sera titer fold-changes between pre-treatment and 4-months post-treatment with overall survival was estimated using leave-one-out jack-knife resampling of the algorithm of Contal-O’Quigley. To address the potential guarantee-time bias introduced by the 4-month time point, a conditional landmark approach was used. The distributions of overall survival were summarized using the method of Kaplan-Meier with 95% confidence intervals estimated using log(−log) methodology. Survival of high versus low fold-change groups were compared using stratified log-rank tests. The hazard ratios of high versus low fold-change groups were estimated using Cox proportional hazards regression models of OS and are presented with 95% Wald confidence intervals. The prognostic model for ipilimumab-containing therapies was stratified by treatment to allow for differences in the underlying baseline hazard. Comparisons of clinical response were performed using Fisher’s exact tests. The associations between sex and melanoma subtype and response to XIAP or overall survival were investigated using multivariable logistic regression models and multivariable Cox proportional hazards regression models. Each model contained XIAP fold-change, sex, and melanoma type (cutaneous vs. other) as independent predictors. All testing was two-sided, and P < 0.05 was considered statistically significant.
Results
XIAP expression in melanoma cells
XIAP expression is known to be frequently up-regulated in melanoma [13]. The expression level of XIAP in melanoma cell lines was examined by SDS-PAGE and immunoblotting. Consistent with previous findings [13], XIAP was found to be widely expressed in melanoma cell lines at variable expression levels (Fig. 1A).
T cell responses to XIAP peptides
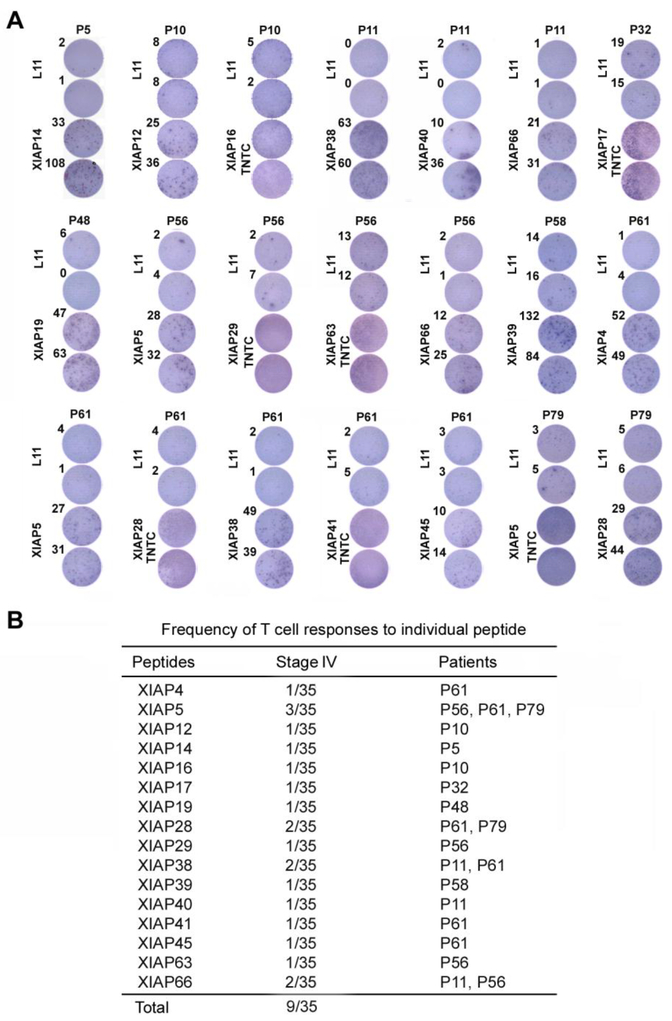
A total of 67 peptides were divided into nine peptide pools (Fig. 1B). PBMC from 35 stage-IV melanoma patients were examined with the peptide pools. As shown in Figs. 1C and 1D, and Supplementary Fig. 1, XIAP peptide pools induced T cell responses. Further evaluation of individual peptides showed that 18 induced T cell responses (Fig. 2A), and 9 of 35 melanoma patients exhibited T cell responses to one or more of the individual peptides. Three patients (P56, P61, and P79) showed responses to multiple peptides (Fig. 2B); 86% of them are located in the functional BIR or RING finger domains (Supplementary Fig. 2). ELISPOT assays were conducted to determine the relevant contributions of CD4+ and CD8+ T cells to response and CD4+/CD8+ T cell depletion. T cell responses to the peptides were mainly due to CD4+ T cell activation (Fig. 3A).
Figure 2.
T cell responses of melanoma patient PBMC to XIAP individual peptides. (A) T cell responses to individual XIAP peptides by ELISPOT analyses. PBMC were cultured with individual peptides of XIAP for one week in the presence of 10 U/ml IL-2, and further re-stimulated with either L11 peptide (negative control) or the same peptides in ELISPOT assays. TNTC: too numerous to count. (B) Frequency of T cell responses to individual peptides in stage-IV melanoma patients.
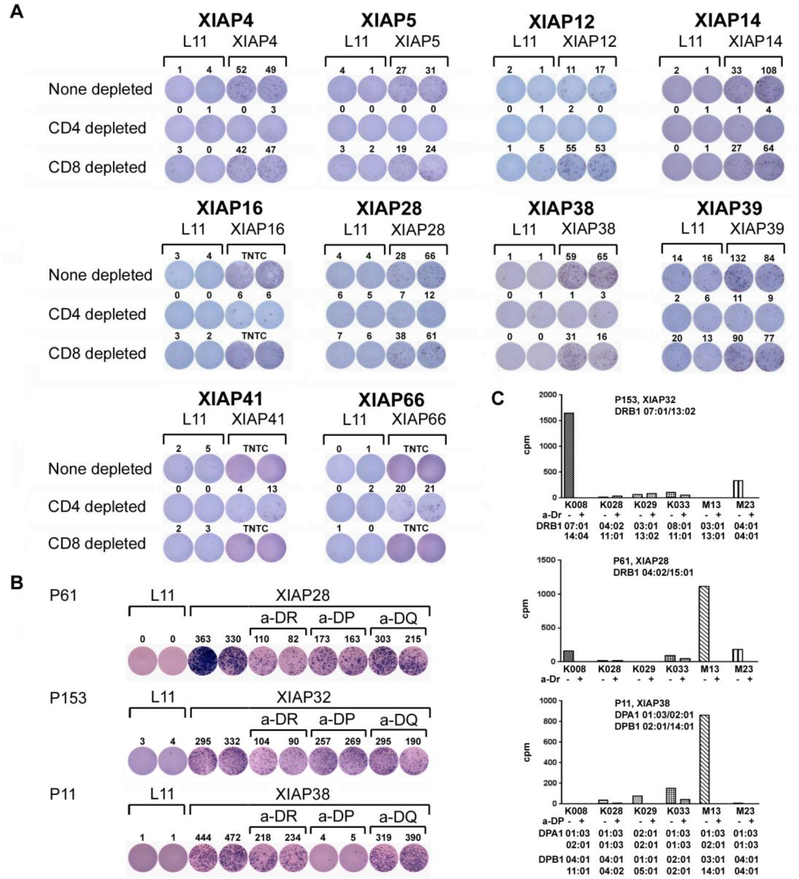
Figure 3.
Responses to XIAP peptides primarily involve CD4+ T cells. (A) Responses to XIAP peptides are CD4+ T cell dominant. T cell responses induced by individual XIAP peptides were further investigated for CD4+ and CD8+ T cell responses. PBMC from patients were cultured with 10 μg/ml individual peptides for one week. CD4+ or CD8+ T cells were isolated from the peptide-treated patient PBMC by relevant depletion, and these cells were re-stimulated with 10 μg/ml of either L11 or the individual peptides for ELISPOT analyses. Responses for none depleted, CD4+ depleted, and CD8+ depleted are presented. TNTC: too numerous to count. (B) Generation of XIAP28, XIAP32, and XIAP38 specific CD4+ T cells from melanoma patients. CD4+ T cells from the patients underwent multiple rounds of stimulation with autologous PBMC in the presence of 10 μg/ml relevant peptide. ELISPOT assay for IFN-γ production proved the specificities of the epitope specific CD4+ T cells with the addition of 10 μg/ml of anti-HLA DR, HLA DP, or HLA DQ antibody. (C) Proliferative responses of epitope specific CD4+ T cells to melanoma cells. Proliferative responses of the CD4+ T cells to melanoma cells were assessed by [3H] thymidine incorporation assay in the absence or presence of 10 μg/ml of anti-HLA antibody as indicated.
Generation and functional characterization of antigen specific CD4+ T cells
CD4+ T cells responsive to peptides XIAP28, XIAP32, and XIAP38 were further isolated and expanded from PBMC of melanoma patients. Specificity of the CD4+ T cells to the peptides was examined by anti-HLA antibody blocking and ELISPOT assays. As shown in Fig. 3B, responses to XIAP28 and XIAP32 peptides were HLA-DR dependent, whereas responses to XIAP38 peptide were HLA-DP dependent.
Reactivities of the three XIAP-specific CD4+ T cells to melanoma cells were studied further. XIAP32-specific CD4+ T cells of patient P153 proliferated in response to the K008 melanoma cells (Fig. 3C). The addition of an anti-HLA-DR antibody abrogated the proliferative response, suggesting the response to be HLA-DR dependent. Genotyping of the patient and K008 cells revealed that both were HLA-DRB1*07:01 positive. XIAP28-specific CD4+ T cells of patient P61 proliferated in response to M13 melanoma cells (Fig. 3C). The addition of anti-HLA-DR antibody blocked the response, suggesting that the response was HLA-DR dependent. However, genotyping of patients P61 and M13 indicated different haplotypes of HLA-DRB1. It is possible that other DR alleles were involved in the response or that the response represents promiscuity of the peptides to binding to HLA class II. XIAP38 CD4+ T cells of patient P11 proliferated in response to M13 (Fig. 3C). The addition of the anti-HLA-DP antibody inhibited the response, suggesting an HLA-DP dependence. Genotyping indicated that patients P11 and M13 were HLA-DPA1 *01:03/02:01 and DPB1* 14:01 positive. These suggest that endogenous XIAP epitopes are naturally presented by HLA on melanoma cells.
Cellular responses to XIAP in patients receiving ipilimumab
To better understand the clinical significance of immunity to XIAP, T cell responses of seven, stage IV patients receiving ipilimumab were examined. Six of the seven patients showed T cell responses to XIAP peptides in peptide pools (Table 1A and Supplementary Fig. 3A). Seven individual peptides to which the patient T cells responded were identified from these pools and patient PI30 showed response to multiple peptides (Table 1B and Supplementary Fig. 3B). The frequency of T cell responses in the ipilimumab-treated patients was significantly higher compared to 35 stage-IV untreated patients (Fisher’s exact p = 0.005, Supplementary Fig. 3C). Furthermore, five of six XIAP responders showed favorable clinical responses (i.e., complete response, partial response, or stable disease lasting at least six months), and four XIAP responders showed continued responses after four months of treatment and long-term survival (Table 1B).
Table 1.
T cell responses of melanoma patients to XIAP following ipilimumab treatment. (A) Frequency of T cell responses to XIAP peptide pools in patients receiving ipilimumab. (B) Frequency of T cell responses to individual XIAP peptides in patients receiving ipilimumab. Periods of treatment represent months of treatment. Partial responses (PR), complete responses (CR), and stable disease for 6 months (SD) are considered favorable clinical responses.
| A Frequency of T cell responses to peptide pools in melanoma patients treated with ipilimumab | ||
|---|---|---|
| Peptides | Stage IV | Patients |
| XIAP1–8 | 1/7 | P130 |
| XIAP9–16 | 0/7 | |
| XIAP17–24 | 2/7 | P27, P102 |
| XIAP25–32 | 2/7 | P110, P130 |
| XIAP33–39 | 1/7 | P124 |
| XIAP40–46 | 0/7 | |
| XIAP47–53 | 1/7 | P140 |
| XIAP54–60 | 0/7 | |
| XIAP61–67 | 0/7 | |
| Total | 6/7 | |
| B Frequency of T cell responses to individual peptides in melanoma patients treated with ipilimumab | ||||
|---|---|---|---|---|
| Peptides | Stage IV | Patients | Clinical responses | Period of treatment (m) |
| XIAP5 | 1/7 | P130 | PD | 1.4 |
| XIAP19 | 1/7 | P27 | CR | 13.9 |
| XIAP20 | 1/7 | P102 | PR | 5.7 |
| XIAP28 | 1/7 | P130 | ||
| XIAP32 | 1/7 | P110 | PR | 9.7 |
| XIAP39 | 1/7 | P124 | SD | 7.1 |
| XIAP48 | 1/7 | P140 | CR | 2.2 |
| Total | 6/7 | |||
Humoral responses to XIAP in patients treated with check-point blockade
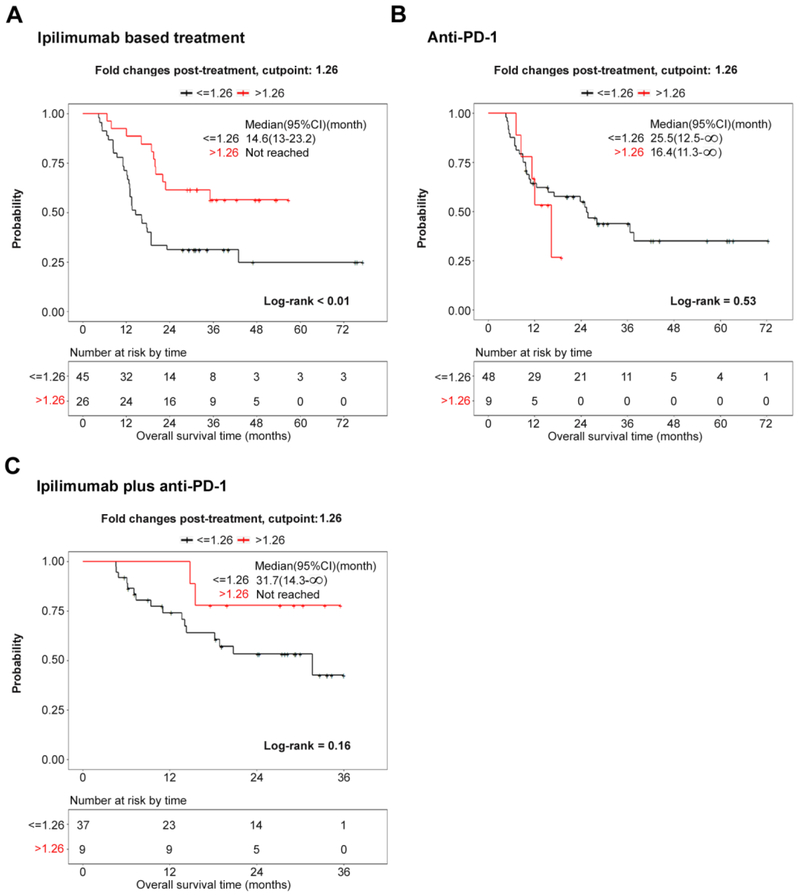
Serologic responses to XIAP in 71 stage-IV melanoma patients receiving either ipilimumab or ipilimumab plus bevacizumab were assessed by ELISA. The samples were divided into two groups based on a fold-change of 1.26, which was identified as the optimum cut point for OS. Changes in sera titers to XIAP within four months of starting treatment were found to be significantly associated with overall survival and favorable clinical responses (Hazard ratio >1.26 vs. <1.26: 0.32 (95% CI: 0.15 − 0.66, p = 0.002; Fig. 4A, Supplementary Table 1A). There were significant associations between XIAP change and survival in patients receiving ipilimumab plus bevacizumab, but not ipilimumab alone (Supplementary Fig. 4A and 4B).
Figure 4.
Associations between serologic responses of melanoma patients to recombinant XIAP and survival after check-point blockade. Associations between sera titer to recombinant XIAP and survival in melanoma patients receiving either (A) ipilimumab-based treatment including ipilimumab and ipilimumab plus bevacizumab or (B) anti-PD-1, or (C) ipilimumab plus anti-PD-1. Pre-treatment and post-treatment sera samples were examined by ELISA. Survival of high versus low fold-change (FC) groups were compared using stratified log-rank tests. P < 0.05 was considered statistically significant.
To investigate whether the increases in antibody titers against XIAP were not restricted to ipilimumab-based treatment, samples from 57 patients treated with anti-PD-1 (pembrolizumab or nivolumab) and 46 patients treated with ipilimumab (anti-CTLA-4) plus nivolumab (anti-PD-1) were examined. The samples were divided into two groups based on a fold-change of 1.26 that was previously identified as the “optimum” division point for OS. Based on this division, changes in sera titer to XIAP within four months of starting treatment were found not to be significantly associated with OS or clinical response (Fig. 4B and 4C, Supplementary Table 1B and 1C).
We observed that among 23 patients in all four trials with either long-term or delayed increases (> 4 months) in sera titers, 20 (87%) had favorable clinical responses or survival of greater than 12 months. In addition, there were delayed sera increases among six clinical responders to anti-PD-1 (CR/PR/SD) (Supplementary Fig. 5, 6, 7 and 8).
T cell responses to 19 identified individual XIAP peptides were further studied with pre-and post-treatment samples from 18 patients receiving ipilimumab. Two patients, P177 and P164, showed increases in T cell response to XIAP48 and XIAP38, respectively, after ipilimumab treatment (Supplementary Fig. 9A). P177, with 12-month survival, failed to show sera response to these XIAP peptides, and patient PI64, with 43-month survival, showed increases in sera response to XIAP14 and XIAP48 (Supplementary Fig. 9B and 9C). Due to different sets of patients and diversity of HLA, different rates of T cell responses to identified individual peptides were observed. Sera response to XIAP peptides were investigated in 9 patients who showed increases in sera titer to XIAP protein by ELISA after treatment with either ipilimumab or ipilimumab plus bevacizumab or anti-PDl. Six peptides that induced a high frequency of CD4+ T cell responses or T cell responses from patients with favorable clinical responses after check-point blockade were selected for further analyses. Five of 9 patients showed titer increases to 3 peptides (XIAP 12, XIAP 14, and XIAP28) but not to the remaining 3 peptides (XIAP5, XIAP38, and XIAP41) (Supplementary Fig. 10). These suggest that there are simultaneous cellular and humoral responses to XIAP after ipilimumab treatment.
Finally, the associations between sex and melanoma subtype and either response to XIAP or overall survival were investigated. The results showed no significant associations between sex/subtype and response to XIAP or overall survival (Supplementary Table 2 and 3).
Discussion
Among IAP proteins, XIAP is important because of its association with tumor progression and patient survival [18–21]. In the present study, CD4+ T cell responses to XIAP peptides were analyzed in melanoma patients. HLA class II restricted epitopes were identified, which are naturally presented by melanoma cells. Cellular and humoral responses to XIAP were observed in patients receiving ipilimumab or ipilimumab plus bevacizumab or ipilimumab plus anti-PD-1. Responses to XIAP were found to be associated with longer survival and favorable clinical outcomes in patients receiving ipilimumab-based treatment.
Antigenic epitopes are naturally presented on the cells by MHC class I and II. This process involves protein expression, proteasome digestion, transport by TAP, and peptide affinity to MHC molecules [22]. XIAP expression in melanoma cells and spontaneous responses of T cells to XIAP in melanoma patients were detected. In addition, XIAP-specific CD4+ T cells were found to be responsive to HLA-matched melanoma cells, and the responses could be abrogated by HLA blocking antibodies. Taken together, the processing of XIAP epitopes in cancer cells may provide an opportunity to target XIAP.
Autoreactive T cells and antibodies against a variety of antigens have been found in both healthy donors and cancer patients [7, 9, 17, 23, 24], Our data showed that T cell responses to XIAP peptides exist in untreated patients, and pre-existing antibody titers against XIAP were also observed. This observation is consistent with our previous studies which showed that the immune response for autoantigen ML-IAP occurred in healthy donors and was elevated in melanoma patients. Antibodies against autoantigens are frequent and associated with anti-tumor response in cancer patients receiving vaccination [9, 23]. The current study further demonstrates that simultaneous responses of cellular and humoral immunity against XIAP can occur after treatment with check-point blockade. Furthermore, increases in sera titers against XIAP are associated with improved overall survival and favorable clinical responses to ipilimumab-based treatment. Taken together, these suggest that the autoantigen XIAP may be involved in antitumor immunity. CTLA-4 and PD-1 signaling can impact B cell response and antibody production via Tfh and Tfr cell populations [25, 26]. It is possible that CD4+ T cell response to XIAP by check-point blockade further helps improve humoral immunity.
The presence of CD4+ T cells facilitates functional development of memory CD8+ T cells [27]. Furthermore, CD4+ T cells play crucial roles in the induction of protective immunity against tumor and tumor eradication [28–30]. Adoptive transfer of tumor-reactive CD4+ T cells exerts cytotoxic activity and eradicates tumor in a mouse melanoma model and in a patient with melanoma [31, 32], Previous studies also showed that T cell and antibody responses can be induced by check-point blockade [9, 11, 17, 23, 33, 34], Furthermore, tumor antigens that trigger T cell and antibody responses in concert improve efficacy of vaccination [35]. The current study revealed CD4+ T cell responses to XIAP peptides following treatment with ipilimumab. Notably, these epitopes are mainly located in the functional domains of BIR and RING finger, suggesting the immunogenicity of the epitopes and ability of the immune system to recognize and mount an immune response against functional protein motifs. These may provide therapeutic strategies targeting functional domains of XIAP alone, or in combination with immune check-point blockade. Further investigation is warranted to better define epitopes and coordination of immunity to XIAP as an autoantigen.
Given its role in inhibition of apoptosis and its immunogenic characteristics, XIAP is a target worthy of further therapeutic investigation in treatment of cancer.
Supplementary Material
Funding
This study was supported by the Sharon Crowley Martin Memorial Fund for Melanoma Research and the Malcolm and Emily Mac Naught Fund for Melanoma Research at Dana-Farber Cancer Institute.
Abbreviation
- ATCC
American Type Culture Collection
- BIR
Baculoviral inhibitor of apoptosis repeat
- Foxp3
Forkhead box P3 protein
- GM-CSF
Granulocyte-macrophage colony stimulating factor
- HLA
Human leukocyte antigen
- ML-IAP
Melanoma inhibitor of apoptosis protein
- SOC1
Suppressor of over-expression of contans 1
- Treg
T regulatory cells
- XIAP
X-Linked inhibitor of apoptosis protein
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of interest: Dr. Hodi reports grant funding from Bristol-Myers Squibb to institution; personal fees from Bristol-Myers Squibb, Merck, EMD Serono, Novartis, Celldex, Amgen, Genentech/Roche, Incyte, Apricity, Bayer, Aduro, Partners Therapeutics, Sanofi, Pfizer, Pionyr, 7 Hills Pharma, Verastem, Torque, Compass Therapeutics, Takeda; In addition, Dr. Hodi has a patent Methods for Treating MICA-Related Disorders (#20100111973) with royalties paid, a patent Tumor antigens and uses thereof (#7250291) issued, a patent Angiopoiten-2 Biomarkers Predictive of Anti-immune check-point response (#20170248603) pending, a patent Compositions and Methods for Identification, Assessment, Prevention, and Treatment of Melanoma using PD-L1 Isoforms (#20160340407) pending, a patent Therapeutic peptides (#20160046716) pending, a patent Therapeutic Peptides (#20140004112) pending, a patent Therapeutic Peptides (#20170022275) pending, a patent Therapeutic Peptides (#20170008962) pending, a patent THERAPEUTIC PEPTIDES Therapeutic Peptides patent number: 9402905 issued, and a patent METHODS OF USING PEMBROLIZUMAB AND TREBANANIB pending. The authors declare that there are no other conflicts of interest.
Ethical approval and ethical standards: All tumor and blood samples were collected in accordance with the Dana-Farber Harvard Cancer Center Institutional Review Board (Disapproved protocol (IRB # 05–042). Informed consent was obtained from melanoma patients to collect and analyze blood and tumor samples for the study of immune function. The informed consent outlines specific uses for blood and tumor, which include but are not limited to PBMC processing and analysis, cell line generation. Informed consent was waived for healthy donors since they are anonymous.
Cell line authentication: K008, K028, K029, K033, M13, and M23 melanoma cell lines were generated from tumor biopsies of melanoma patients in accordance with Dana-Farber/Harvard Cancer Center Institutional Review Board approved protocols. A375 cells were obtained from the ATCC. Expression of MITF in all melanoma cell lines were confirmed. Comparisons of short tandem repeat profiling with cell line DNA profiles were conducted to authenticate the cell line after the project was completed.
References
- 1.Schimmer AD, Inhibitor of apoptosis proteins: translating basic knowledge into clinical practice. Cancer Res, 2004. 64(20): p. 7183–90. [DOI] [PubMed] [Google Scholar]
- 2.Schimmer AD, et al. , Small-molecule antagonists of apoptosis suppressor XIAP exhibit broad antitumor activity. Cancer Cell, 2004. 5(1): p. 25–35. [DOI] [PubMed] [Google Scholar]
- 3.Idenoue S, et al. , A potent immunogenic general cancer vaccine that targets survivin, an inhibitor of apoptosis proteins. Clin Cancer Res, 200511(4): p. 1474–82. [DOI] [PubMed] [Google Scholar]
- 4.Vucic D and Fairbrother WJ, The inhibitor of apoptosis proteins as therapeutic targets in cancer. Clin Cancer Res, 2007. 13(20): p. 5995–6000. [DOI] [PubMed] [Google Scholar]
- 5.Vanneman M and Dranoff G, Combining immunotherapy and targeted therapies in cancer treatment. Nat Rev Cancer, 201212(4): p. 237–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Piesche M, et al. , Identification of a promiscuous HLA DR-restricted T-cell epitope derived from the inhibitor of apoptosis protein survivin. Hum Immunol, 2007. 68(7): p. 572–6. [DOI] [PubMed] [Google Scholar]
- 7.Grube M, et al. , CD8+ T cells reactive to survivin antigen in patients with multiple myeloma. Clin Cancer Res, 200713(3): p. 1053–60. [DOI] [PubMed] [Google Scholar]
- 8.Casati C, et al. , The apoptosis inhibitor protein survivin induces tumor-specific CD8+ and CD4+ T cells in colorectal cancer patients. Cancer Res, 2003. 63(15): p. 4507–15. [PubMed] [Google Scholar]
- 9.Zhou J, et al. , Immunity to the melanoma inhibitor of apoptosis protein (ML-IAP; livin) in patients with malignant melanoma. Cancer Immunol Immunother, 2012. 61(5): p. 655–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andersen MH, et al. , The melanoma inhibitor of apoptosis protein: a target for spontaneous cytotoxic Tcell responses. J Invest Dermatol, 2004122(2): p. 392–9. [DOI] [PubMed] [Google Scholar]
- 11.Schmollinger JC, et al. , Melanoma inhibitor of apoptosis protein (ML-IAP) is a target for immune-mediated tumor destruction. Proc Natl Acad Sei USA, 2003100(6): p. 3398–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Emanuel PO, et al. , Immunohistochemical detection of XIAP in melanoma. J Cutan Pathol, 2008. 35(3): p. 292–7. [DOI] [PubMed] [Google Scholar]
- 13.Kluger HM, et al. , The X-linked inhibitor of apoptosis protein (XIAP) is up-regulated in metastatic melanoma, and XIAP cleavage by Phenoxodiol is associated with Carboplatin sensitization. J Transi Med, 2007. 5: p. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burstein DE, et al. , Immunohistochemical detection of the X-linked inhibitor of apoptosis protein (XIAP) in cervical squamous intraepithelial neoplasia and squamous carcinoma. Ann Diagn Pathol, 2008. 12(2): p. 85–9. [DOI] [PubMed] [Google Scholar]
- 15.Hsieh WC, et al. , IL-6 receptor blockade corrects defects ofXIAP-deficient regulatory Tcells. Nat Commun, 2018. 9(1): p. 463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou J, et al. , Immunity to the melanoma inhibitor of apoptosis protein (ML-IAP; livin) in patients with malignant melanoma. Cancer Immunol Immunother, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou J, et al. , Immunity to the vacuolar ATPase complex accessory unitATP6Sl in patients with malignant melanoma. Cancer Immunol Res, 2015. 3(1): p. 59–67. [DOI] [PubMed] [Google Scholar]
- 18.Ramp U, et al. , XIAP expression is an independent prognostic marker in clear-cell renal carcinomas. Hum Pathol, 2004. 35(8): p. 1022–8. [DOI] [PubMed] [Google Scholar]
- 19.Flanagan L, et al. , High levels of X-linked Inhibitor-of-Apoptosis Protein (XIAP) are indicative of radio chemotherapy resistance in rectal cancer. Radiat Oncol, 2015. 10: p. 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paschall AV, et al. , Ceramide targets xlAP and cl API to sensitize metastatic colon and breast cancer cells to apoptosis induction to suppress tumor progression. BMC Cancer, 201414: p. 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferreira CG, et al. , Expression of X-linked inhibitor of apoptosis as a novel prognostic marker in radically resected non-small cell lung cancer patients. Clin Cancer Res, 2001. 7(8): p. 2468–74. [PubMed] [Google Scholar]
- 22.Yewdell JW and Bennink JR, Immunodominance in major histocompatibility complex class I-restricted Tlymphocyte responses. Annu Rev Immunol, 1999. 17: p. 51–88. [DOI] [PubMed] [Google Scholar]
- 23.Sittler T, et al. , Concerted potent humoral immune responses to autoantigens are associated with tumor destruction and favorable clinical outcomes without autoimmunity. Clin Cancer Res, 2008. 14(12): p. 3896–905. [DOI] [PubMed] [Google Scholar]
- 24.Valmori D, et al. , Naturally occurring human lymphocyte antigen-A2 restricted CD8+ T-cell response to the cancer testis antigen NY-ESO-1 in melanoma patients. Cancer Res, 2000. 60(16): p. 4499–506. [PubMed] [Google Scholar]
- 25.Sage PT, et al. , The coinhibitory receptor CTLA-4 controls B cell responses by modulating T follicular helper, Tfollicular regulatory, and T regulatory cells. Immunity, 2014. 41(6): p. 1026–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sage PT, et al. , The receptor PD-1 controls follicular regulatory T cells in the lymph nodes and blood. Nat Immunol, 2013. 14(2): p. 152–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shedlock DJ and Shen H, Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science, 2003. 300(5617): p. 337–9. [DOI] [PubMed] [Google Scholar]
- 28.Toes RE, et al. , CD4 T cells and their role in antitumor immune responses. J Exp Med, 1999. 189(5): p. 753–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hung K, et al. , The central role ofCD4(+) T cells in the antitumor immune response. J Exp Med, 1998. 188(12): p. 2357–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ossendorp F, et al. , Specific T helper cell requirement for optimal induction of cytotoxic T lymphocytes against major histocompatibility complex class II negative tumors. J Exp Med, 1998. 187(5): p. 693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quezada SA, et al. , Tumor-reactive CD4(+) Tcells develop cytotoxic activity and eradicate large established melanoma after transfer into lymphopénie hosts. J Exp Med, 2010. 207(3): p. 637–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hunder NN, et al. , Treatment of metastatic melanoma with autologous CD4+ T cells against NY-ESO-1. N Engl J Med, 2008. 358(25): p. 2698–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hodi FS, et al. , ATP6S1 elicits potent humoral responses associated with immune-mediated tumor destruction. Proc Natl Acad Sei USA, 2002. 99(10): p. 6919–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kitano S, et al. , Enhancement of tumor-reactive cytotoxic CD4+ T cell responses after ipilimumab treatment in four advanced melanoma patients. Cancer Immunol Res, 2013. 1(4): p. 235–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Srinivasan R and Wolchok JD, Tumor antigens for cancer immunotherapy: therapeutic potential of xenogeneic DNA vaccines. J Transi Med, 2004. 2(1): p. 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.