Abstract
In the neonatal intensive care unit (NICU), heart rate, respiratory rate, and oxygen saturation are vital signs (VS) that are continuously monitored in infants, while blood pressure is often monitored continuously immediately after birth, or during critical illness. Although changes in VS can reflect infant physiology or circadian rhythms, persistent deviations in absolute values or complex changes in variability, can indicate acute or chronic pathology. Recent studies demonstrate that analysis of continuous VS trends can predict sepsis, necrotizing enterocolitis, brain injury, bronchopulmonary dysplasia, cardiorespiratory decompensation, and mortality. Subtle changes in continuous VS patterns may not be discerned even by experienced clinicians reviewing spot VS data or VS trends captured in the monitor. In contrast, objective analysis of continuous VS data can improve neonatal outcomes by allowing heightened vigilance or preemptive interventions. In this review, we provide an overview of the studies that have used continuous analysis of single or multiple VS, their interactions, and combined VS and clinical analytic tools, to predict or detect neonatal pathophysiology. We make the case that big-data analytics are promising, and with continued improvements, can become a powerful tool to mitigate neonatal diseases in the 21st century.
Introduction
Continuous assessment of cardiorespiratory stability is a critical component of neonatal intensive care in preterm infants. Vital signs including heart rate (HR), respiratory rate (RR), and pulse oximetry (SpO2) are continuously monitored throughout hospital stay, while blood pressure (BP) may be continuously monitored in the first days after birth and during acute critical illness. Temperature is another important VS for assessing illness (1), but is not considered here, as its measurement is confounded by use of external heat sources. VS are under complex physiological control, and while patterns of variation often reflect normal physiology such as circadian rhythms, they may also represent the earliest signs of decompensation triggered by pathological states (2–6). While clinical care is strongly influenced by interval assessments of VS displayed continuously on bedside monitors and periodic nursing assessments, the majority of continuous VS data is not utilized. Furthermore, periodic reporting of VS lends itself to bias as well as under-or over reporting of abnormal values, which might adversely impact clinical decisions (7). Recent studies in neonates suggest that cumulative analysis of monitor-recorded continuous vital signs trends can predict impending clinical deterioration or disease such as sepsis, and may also predict long-term neurological or respiratory outcomes (2–4, 8–12). In this review, we highlight studies that have evaluated the utility of continuous heart rate (HR), respiratory rate (RR), pulse oximetry (SpO2), and blood pressure (BP) data analytics to predict disease, and thereby potentially prevent adverse outcomes. Incorporating continuous VS analytics into the clinical record may improve outcomes for preterm infants in the 21st century NICU.
Methods
We searched MEDLINE, CENTRAL, and CINAHL from 1990 to March 2019. Select studies were included in which continuous VS data on heart rate, respiratory rate, pulse oximetry, and/or blood pressure were captured and analyzed in neonates during their stay in the neonatal intensive care unit, for any period of time. Outcomes of interest were analytics of VS physiologic variability, comparisons between continuous and interval VS measurements, and associations with short-term or long-term clinical outcomes.
Continuous heart rate monitoring and analytics
Physiologic determinants of heart rate monitoring
HR is continuously monitored from birth until discharge for all NICU infants. The electrocardiogram (ECG) signal is obtained from three electrodes, two on the chest and one on the abdomen, and the bedside monitor displays one lead of ECG. HR can be calculated from the inter-beat interval (R to R wave of the QRS complexes), and in most NICU monitors the numerical HR displayed represents a moving average of several QRS complexes. Pulse rate (PR) calculated from the pulse oximetry signal is also continuously displayed. A significant discrepancy between the pulse oximetry-derived PR and the ECG-derived HR often indicates poor pulse oximetry signal quality, and therefore, the HR is generally more reliable than the PR (13). Normative data on HR in preterm NICU patients have recently been published (14).
Using HRV patterns to detect disease
HR variability (HRV), or normal beat to beat fluctuations in HR, is regulated by the sympathetic and parasympathetic nervous systems (15). In healthy preterm infants, HR accelerations and decelerations reflect high variability and are indicative of a functioning, adaptable autonomic nervous system, while decreased HRV suggests a stressed or pathological state. More than a century ago, obstetricians recognized that low HR variability punctuated by HR decelerations was a sign of fetal compromise (16), often associated with acidemia, and sometimes with chorioamnionitis and a fetal inflammatory response (17, 18). A similar pattern of low variability with superimposed decelerations was observed in preterm neonates with sepsis and replicated in a preclinical model (19–21). Data from mice suggest that low HRV upon exposure to pathogens is mediated in part by cytokines released during sepsis. HR decelerations, in the same model, were mediated both by direct and indirect pathogen effects on the vagus nerve, as part of the cholinergic anti-inflammatory response (22).
In preterm infants, the most significant cause of abnormal heart rate characteristics (HRC) is sepsis. Late-onset sepsis (LOS, >3 days of age) and necrotizing enterocolitis (NEC) can be life-threatening, especially if not diagnosed until the infant is displaying VS and clinical instability. The use of continuous HR analytics for earlier detection and treatment could prevent mortality and long-term morbidity (23–25) by alerting clinicians, and prompting a more careful clinical examination to support decisions about testing and treatment. The first commercially available device that serves as an early warning system for sepsis in the NICU is the HRC index monitor (HeRO monitor). A mathematical algorithm was developed by Moorman et al. to measure the observed phenomenon of decreased HRV punctuated by HR decelerations during systemic inflammation, and thereby detect an early signature of sepsis in premature infants (26). The algorithm incorporates measures of HRV, and measures of sample asymmetry and entropy (lack of predictability) to detect pathologic decelerations (27, 28). The HRC index, which is the fold-increased risk of sepsis diagnosis within 24 hours, is continuously calculated from the previous 12 hours of monitored ECG signal and an hourly updated value between zero and 7 is displayed, together with the HR values and the five-day HRC index trend.
HRC in sepsis and infection-related outcomes
The impact of the HRC index monitor was tested in a multicenter, randomized controlled trial of 3,003 VLBW infants. The overall incidence of LOS was not significantly different among infants with or without HRC display. However, infants whose HRC index was displayed to clinicians had a 22% relative reduction in all-cause mortality in the 120 days after randomization, and a 40% reduction in death within 30 days of septicemia compared to infants whose HRC index was not made available to clinicians (29, 30). Although the HRC index was designed as a risk score for sepsis, subsequent analyses have demonstrated its utility in predicting other outcomes, including necrotizing enterocolitis (31). In another small study, decreased high-frequency HRV in the first week after birth, suggesting reduced parasympathetic nervous system activity, was associated with later NEC diagnosis (32). Such early risk stratification could be useful for heightened vigilance or preventative bundles aimed at reducing incidence or severity of common preterm morbidities.
HRC in respiratory and neurologic morbidities
A limitation of HRC monitoring for LOS and NEC is that infants with acute or chronic lung or brain injury often have abnormal HRC (33, 34). An analysis of large increases, or “spikes” in the HRC index for infants in the control arm of the RCT in one NICU showed that about half of the spikes were associated with suspected or proven infection and about a third were associated with a significant acute respiratory deterioration in absence of infection (35). In the setting of acute (or acute on chronic) respiratory distress, a high or rising HRC index may reflect repetitive apnea (with associated HR deceleration), lung inflammation, or effects of hypoxemia or acidosis on HRV. Another study found that, of multiple cardiorespiratory signs tested, the most useful analytic predicting respiratory deterioration requiring urgent intubation was the HRC index (36). A subsequent study found that the HRC index assisted in determining extubation readiness in preterm infants (37). This suggests that continuous display of a HR or cardiorespiratory index might alert clinicians to preterm infants’ need for either more or less respiratory support. Abnormal heart rate patterns are associated with acute brain injury presumably due to autonomic dysfunction and perhaps reflecting a systemic inflammatory response associated with neuroinflammation (34–39). Severe IVH has been shown to lead to a chronically high HRC index for the first month after birth, followed by normalization (38). Another study found that among neonates undergoing therapeutic hypothermia for hypoxic-ischemic encephalopathy, low HRV was associated with worse brain MRI changes and neurological outcomes (39). Use of HR analytics for IVH prediction has also been explored using such methods as detrended fluctuation analysis (40) and sample entropy (41).
HRC analytics combined with clinical assessment tools and other VS trends
Even with advanced analytics, abnormal HR patterns must be interpreted in the context of clinical variables and other VS trends. Griffin et al. developed a clinical risk score to use in conjunction with HRC monitoring for early detection of LOS (42). Adding respiratory analysis to HRC analysis may improve diagnostic accuracy of HRC analysis. This is especially true for sepsis-related complications as increased apneas and periodic breathing frequently accompany abnormal HR patterns, in part related to endogenous prostaglandins release (43–45). Apnea and periodic breathing analysis for sepsis detection is discussed in the respiratory section (46, 47). The “PhysiScore” incorporating HRV and other continuously monitored VS in the first hours after birth, was shown in preterm infants to predict later sepsis and other adverse outcomes (48). The NeoNEEDS score that incorporates clinical evaluation of behavior and abdominal examination along with HR, blood pressure, FiO2 changes, and respiratory rate, was shown to decrease the rate of NEC in a small single-center study (46). The RALIS (Integralis-medical.com) system allows clinicians to input 6 monitor VS every 2 hours to generate an alarm for possible onset of sepsis in subsequent days (49).
In summary, although display of the HRC index or other HR-based early warning systems for sepsis has the potential to improve outcomes, clinicians in most NICUs continue to rely solely on observation of ECG HR trends on bedside monitors to make determinations about clinical status, and the need for escalation of care. Ongoing research aims to further improve sepsis early warning systems by incorporating other VS, clinical signs, and biomarkers to develop decision support tools to reduce sepsis and NEC-associated outcomes.
Continuous pulse oximetry monitoring and analytics
Physiological determinants and importance of oxygen saturation monitoring
Pulse oximetry allows for transcutaneous estimation of oxygen saturation in the blood using principles of spectrophotometry (50). The pulse oximetry probe contains two light emitting diodes positioned on one side of the tissue (generally the hand or foot), and a photodetector on the opposing side. The probe measures the differential light absorption of deoxyhemoglobin and oxyhemoglobin at 600 nm (red) and 940 nm (infrared) wavelengths, to determine their respective concentrations in the blood passing through tissue, and calculate the percentage of oxyhemoglobin (SpO2). The final SpO2 determination is made by comparing the absorbance during pulsatile and non-pulsatile flow to isolate the light absorption of blood (51, 52).
While fetal growth occurs in a relatively hypoxic intrauterine environment (PaO2 20–28 torr), premature infants are exposed to a hyperoxic environment ex-utero. This poses the distinct challenge of maintaining optimal oxygenation to ensure adequate growth and development, while limiting free-radical damage in an oxygen-rich milieu (53, 54). SpO2 monitoring allows continuous assessment of both hypoxemia and hyperoxemia (53, 54). The importance of SpO2 targeting is established by the meta-analysis of large clinical trials (SUPPORT, BOOST, and COT) that enrolled over 5000 infants <1250 grams birthweight (55–58). Pooled data from these trials demonstrated that targeting SpO2 between 85–89% was associated with a higher risk of mortality and necrotizing enterocolitis, whereas targeting higher SpO2 between 91–95% was associated with higher risk of retinopathy of prematurity.
Maintenance of SpO2 in the intended target range
Most NICUs have guidelines to keep preterm infants’ SpO2 within certain target limits based on gestational and chronologic age, and disease state. SpO2 analytics have been used to evaluate the precision of oxygen targeting. In a study involving premature infants receiving supplemental oxygen at fourteen centers, SpO2 was maintained in the intended range only 48% of the time, being above and below the range 36% and 16% of the time, respectively (59). Similar findings were observed in another study for infants on continuous positive airway pressure, where SpO2 was maintained in the targeted range only 31% of the time (60). Recent studies also suggest that bedside recognition of both hypoxemia and hyperoxemia plays a role in achieving targeted SpO2 range (3, 4, 12).
Pulse oximetry analytics in prediction of clinical outcomes
Analysis of continuous SpO2 trends, either alone or in combination with other VS parameters has been shown to predict short-term clinical deterioration as well as long-term outcomes such as BPD. In one study involving 150 babies born with a gestational age (GA) <34 weeks, the mean, baseline and residual SpO2 variability was combined with HR and RR patterns in the first 3 hours of life, to develop a probability score (PhysiScore) for predicting adverse outcomes (48). PhysiScore was more accurate in predicting overall morbidity with 86% sensitivity and 96% specificity compared to other scoring systems such as APGAR (61), SNAP-II (62), SNAPPE-II (62), and CRIB (63). Using Bayesian modeling, the investigators demonstrated that overall morbidity was significantly associated with mean SpO2 < 92% and prolonged time spent (>5% of time) at SpO2 less than 85%. PhysiScore also achieved almost 100% specificity for 2 major combined outcome categories; infection (NEC, sepsis, UTI and pneumonia; with 90% sensitivity) and cardiopulmonary complication (including BPD, hemodynamic instability, pulmonary hypertension and pulmonary hemorrhage with 96% sensitivity). In another study, the mean, standard deviation, kurtosis and skewness of SpO2 data was combined with continuous HR trends in the first 12 hours, and first 7 days of life to develop a pulse oximetry predictive score (POPS) in 800 VLBW infants (64). POPS is a combination of pulse oximetry data score and a clinical predictive score. The predictive performance of POPS was most significant for BPD (AUC: 0.935) and mortality (AUC: 0.864), but was also significant for severe IVH, NEC, treated ROP, and length of stay. Associations between a lower mean SpO2 in the first 12 hours and mortality, and lower SpO2 in the first 7 days and BPD were also found. In another study of 137 extremely preterm infants, BPD was associated with increasing intermittent hypoxemias in the first 4 weeks of life (65). A recent study of 645 VLBW infants at 3 NICUs found that early severe hypoxemia burden (time spent with SpO2 <70%) was significantly higher in infants with severe grade 3–4 IVH (66).
Retinopathy of prematurity (ROP) is known to be associated with both hypoxemia and hyperoxemia, and a study of 56 ELBW infants found that an increase in weekly mean SpO2 fluctuations over the first 28 days of life was associated with moderate to severe ROP (8). In another study of 63 infants, severe ROP was associated with early intermittent hypoxemia events (67). Overall, data from these studies reveal that analyzing trends in continuous SpO2 is a useful tool to predict several preterm diseases.
Prediction of imminent clinical deterioration using continuous SpO2 data
In a two-center study of 1065 VLBW infants, mean, SD, and cross-correlation of SpO2, HR and RR, were analyzed for 1.15 million hours to detect correlations with imminent diagnosis LOS and NEC (68). In one center, a small but statistically significant decrease in mean SpO2 preceded LOS or NEC, whereas a decrease in SD of SpO2 preceded LOS or NEC in another center. Importantly, the cross-correlation of HR and SpO2, measuring co-trending of the two VS, was the single best predictor of illness at both centers. The best combined model (mean SpO2, SD HR, and cross correlation of HR-SpO2) provided additive value to the HRC index for illness prediction (net improvement 0.25, 95% CI 0.113, 0.328). SpO2 data has also been used in predicting short term respiratory outcomes. In a study of 31 premature infants, failure to wean from nasal positive pressure support to nasal cannula was correlated with the proportion of time spent with saturations< 86% pre-wean (69). In another study of 100 premature infants, daily mean, and SD of SpO2 data, along with cumulative time of hypoxemia was analyzed to detect impending respiratory deterioration (4). Analysis of > 3 million data points from relatively stable infants on noninvasive respiratory support or room air showed a 3.7-fold increase in odds of respiratory support escalation within the next 3 days if hypoxemia was present for 5–10% of total time. This study also showed a significant correlation between the hypoxemia time and decreased Fenton weight z-scores (4). Additionally, nursing documentation of hypoxemia did not correlate with short-term respiratory deterioration. Interestingly, similar associations between hypoxemia and impaired growth outcomes has also been shown in animal models (70). These studies highlight the potential for utilizing the continuous pulse oximetry data in prediction of adverse outcomes such as LOS, respiratory deterioration and growth failure.
In summary, analysis of continuous SpO2 data can improve clinicians’ ability to maintain preterm neonates in a target range, and provide high resolution data to supplement bedside assessments in predicting clinical deterioration. Many newer pulse oximeters and bedside monitors have the option to display cumulative SpO2 histograms or other data displays to inform caregivers about percentage of time spent in lower and higher SpO2 ranges. In one quality improvement study, educating caregivers about the risks of hyperoxemia and the importance of reviewing monitor data and improving oximetry targeting, resulted in significant decrease in severe ROP (71).
In light of the inherent difficulty for ICU nurses and respiratory therapists to titrate supplemental oxygen to keep infants in safe target ranges, efforts have been directed over the past several decades toward automated closed-loop FiO2 titration systems. Several studies have shown these sophisticated systems to be more effective than manual control of oxygen delivery to achieve optimal oxygen targeting in preterm infants (72–78). Since NICU nurses have many responsibilities in addition to oxygen titration, the real-world NICU is likely to use automated FiO2 titration in the 21st century. However, well designed trials are needed to establish the efficacy of these approaches to improve clinical outcomes.
Continuous respiratory rate monitoring and analytics
Physiological determinants and methods of respiratory rate (RR) monitoring
In most NICU monitors, RR rate is measured by detection of alterations in thoracic impedance between two electrodes placed on the chest and abdomen. Due to the differential impedance of air and tissue, the electrodes detect a change in impedance as the air-to-tissue ratio increases during inspiration, thereby allowing display of a RR or absence of respiratory effort during central apnea (79, 80). Thoracic impedance measurement of respiratory rate, despite being universally used in NICUs, has a number of drawbacks. Obstructive apnea is not detected since chest wall impedance changes during airway obstruction. Obstructive apnea can be monitored using a nasal cannula thermistor detecting warm exhaled breath or capnography detecting exhaled carbon dioxide, but these are not performed in routine clinical care. Another drawback of chest impedance monitoring is that it can provide an inaccurate RR due to the tendency of infants to have irregular and sometimes shallow respirations and due to motion and cardiac artifact (81). Respiratory inductance plethysmography measures RR more reliably than impedance monitoring but requires placement of multiple electrodes circling the chest, and as such is not practicable for monitoring infants in the NICU (82). Other promising methodologies, including devices based on monitoring breath sounds in the neck (83), and using electrocardiogram or photoplethysmography waveform data to determine RR (84) have not be evaluated in neonates.
Importance of apnea and periodic breathing (PB) and improved methods for their quantitation
Respiratory physiology in premature infants has unique characteristics when compared to older children, and assessment of respiratory function in the NICU has been largely limited to nursing documentation of oxygen saturation, respiratory rate, and apnea events. Prolonged apneic pauses may be associated with decreases in heart rate and/or SpO2 (77, 78) (85, 86). Although apnea and periodic breathing predominantly reflect normal physiologic immaturity of respiratory control (87–90), pathological states such as LOS and NEC can also predispose preterm infants to increased number or severity of apnea spells (91, 92). It is well known that interval monitoring and documentation of apnea/periodic breathing in the medical record do not accurately capture trends in breathing patterns or apnea (4, 9, 10, 93, 94). To address this challenge, an automated apnea detection system for preterm NICU patients was developed. This algorithm analyzes the waveform chest impedance signal, after removing motion and cardiac artifact, for episodes of very low variance indicative of central apneic pauses (81). Multiple studies have been published using this algorithm, quantitating and describing “ABD” events, central apnea with associated decline in HR and SpO2 (93, 95, 96). An algorithm to quantitate periodic breathing (PB), which if prolonged, can be pathological has also been developed. This algorithm which analyzes chest impedance apneic pauses was useful for defining normal PB, and identifying NICU patients with severely exaggerated PB who later died of SIDS or sepsis-like illness (97). Algorithms for continuous evaluation of central ABD and PB are not yet available for clinical use.
Continuous RR monitoring and clinical outcomes
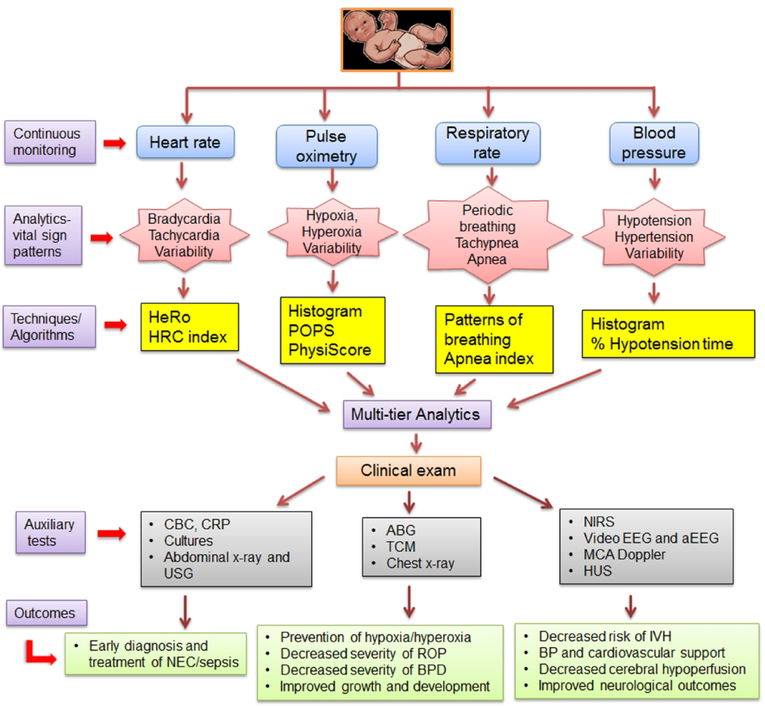
Limited studies suggest that continuously monitored RR data can be used for prediction or prognostication in preterm infants. The PhysiScore study previously cited in the HRV section also analyzed RR mean and variability in a small cohort of preterm infants in the first 3 hours of life, and showed that an average RR of less than 35 or greater than 75 breaths per minute was associated with higher mortality. In contrast to low HRV being associated with adverse outcomes, for RR the effect was bimodal, with both high and low variability associated with higher risk of morbidity (48). In another study of 100 preterm infants, analysis of continuous RR data was able to predict impending worsening of respiratory status. Among infants on non-invasive respiratory support or room air, infants who were tachypneic (RR>70) for >30% of time had 2.8-fold increased odds of requiring escalation of respiratory support in the subsequent 24–72 h window (4). In the same study, a significant correlation between time spent per day with RR>70 and decreased weight gain was reported in infants on RA or non-invasive respiratory support. In a prospective study of 33 ELBW infants, continuous chest impedance, electrocardiographic waveforms and SpO2 data were collected for apnea, bradycardia and desaturations (98). Blood stream infection was noted in 42% of the study participants, and a significant increase in apnea events was noted prior to the onset of infection. In a large retrospective study of 1211 premature infants (GA<35 weeks), a 2-fold increase in apnea, bradycardia and desaturations (ABD) events was noted in about one-third cases of NEC and almost half of the cases with LOS 24 h prior to diagnosis (43). In addition to apneas, periodic breathing (PB), commonly defined as short repetitive cycles of respiratory pauses may also be a harbinger of disease (99, 100). The association between extreme PB, defined, in this study, as PB for >10% of time and higher than 6 standard deviations above the mean for GA, and clinical outcomes were evaluated retrospectively in the above cohort (44). PB was found in 76 of 1211 infants, and was associated 45% of the time with infection or NEC, immunizations, or caffeine discontinuation. Analysis of continuous cardiorespiratory data has also been able to shed some light on the impact of caffeine for treatment of apnea of prematurity. In another retrospective study of 300 preterm infants (GA< 32 weeks), caffeine loading was associated with a significant decrease in ABDs events (101). While these early studies suggest that variation in RR or apnea/PB events can portend adverse events, the sensitivity and specificity for predicting outcomes appear modest. Moreover, unlike HRC-based algorithms, standardized criteria to delineate normal from abnormal ranges have not been tested in randomized clinical trials. Whether addition of continuous RR and apnea monitoring into complex algorithms including other VS would increase sensitivity or specificity for predicting clinical outcomes remains to be answered (Figure 2).
Figure 2:
21st century analytics of continuous vital sign data to prevent diseases in neonates. This illustration depicts a three step-approach combining: a) single or multi-tier analytics of vital signs, b) careful clinical assessment, and c) auxiliary blood and other imaging studies to prevent and decrease morbidity from life-threatening illnesses. HeRo, heart rate characteristics; HRC index, heart rate characteristics index; POPS, pulse oximetry predictive score; BP, blood pressure; CBC, complete blood count; CRP, C-reactive protein; USG, ultrasonography; ABG, arterial blood gas; TCM, transcutaneous carbon dioxide monitoring; NIRS, near infrared spectroscopy; EEG, electroencephalogram; HUS, head ultrasound; NEC, necrotizing enterocolitis; BPD, bronchopulmonary dysplasia; ROP, retinopathy of prematurity; IVH, intraventricular hemorrhage; MCA Doppler, middle cerebral artery Doppler
Continuous Blood pressure (BP) monitoring
Physiological determinants and monitoring of blood pressure
BP is determined by the product of cardiac output and systemic vascular resistance and increases with birth weight, higher GA, and with postnatal adaptation to extra-uterine life (102, 103). BP in preterm infants is influenced by factors such as restricted myocardial compliance and contractility, poor tolerance to high SVR, antenatal steroids, chorioamnionitis, relative adrenal insufficiency, PDA, and mechanical ventilation (104–111). While BP is used as a surrogate to monitor systemic blood flow and tissue perfusion, it is impacted by the presence of ductal and atrial shunts, and thus may not accurately reflect cardiac output or tissue perfusion (112, 113). BP is measured non-invasively by cuffs that estimate blood pressure by quantifying oscillometric changes in the arterial wall generated by pulsatile blood flow (114). Although easy to use, this method can overestimate pressures, especially in sick, hypotensive preterm infants (115–118). Invasive arterial BP measurement, allows continuous direct monitoring of the BP generated by every heartbeat. In the immediate postnatal period, an umbilical artery catheter may be inserted into the abdominal or thoracic aorta and connected to a transducer to allow continuous measurement of BP (114). Later in the NICU stay, arterial BP may be continuously monitored through a peripheral arterial catheter during times of critical illness.
In preterm neonates, low mean arterial blood pressure (MBP) in the first week of life has been associated with higher risk of mortality, and worse neurodevelopmental outcomes (119, 120). Continuous BP studies have primarily focused on the first week of life because of a putative “window” of the enhanced vulnerability of preterm brain to perfusion-related brain injury and ready availability of umbilical arterial catheter data. Studies utilizing continuous BP trends have been used to glean normative blood pressure data in preterm infants, assess cerebral perfusion, and study patterns of brain injury and neurological outcomes (121–124).
Defining normative BP
A study of 35 infants with mean GA of 25 weeks, analyzed >11 million BP values generated from continuous sampling every 2 seconds in the first 72 hours of life (111). Optimum MBP estimated from this data was higher with a narrow range than previously published, at around 33 ± 3 mmHg. In a retrospective study, where mean BP was averaged over 1 min in 232 VLBW over the first 7 days of life, similar values were generated (102). The discrepancies between normative data generated by continuous BP sampling from published studies may be due to the use of interval data collection with different sampling periods, and inconsistent methodology such as utilizing both non-invasive oscillometer and intra-arterial manometric measurements (125–127).
Association of early post-natal hypotension with neurological outcomes
Computerized analysis of continuous BP data suggests that early low MBP can predict adverse events in preterm infants. In a study of infants with GA<31 weeks, MBP <30 mmHg for over an hour was associated with severe IVH, ischemic cerebral lesions, and mortality within 48 hours (121). A similar relationship between low BP and grade II+ IVH was observed 100 VLBW infants (122). In this study IVH was also associated with wider swings in MBP. In a study of 98 infants with GA<34 weeks, continuous HR, BP, and transcutaneous oxygen tension (PaO2) data from the first 4 days of life were analyzed (124). Among infants with hypotension and hypoxemia, 53% had either postmortem evidence of brain injury or abnormal neurological outcome at one year of age, compared to an 8% incidence without hypotension or hypoxemia. In contrast to the findings in these studies, several studies (128–135) including the large ELGAN study (136, 137) did not find an association between early hypotension and adverse neurological outcomes. These different findings may be attributable to analysis of intermittent data either from monitors or medical records, and mixed use of cuff-based and invasive BP measurements.
Immature autoregulation of cerebral blood flow in preterm infants leads to a pressure passive state, a known risk factor for cerebrovascular injury (138–140). In past studies, BP variability alone has produced conflicting results related to IVH prediction (102, 141), but showed a strong association when combined with changes in cerebral blood-flow velocity in the anterior cerebral artery (142). The unreliability of isolated MBP monitoring is exemplified by a study in 90 VLBW infants where both continuous MBP and cerebral perfusion using near-infrared spectroscopy (NIRS) in the first 5 days was measured (143). Although this study did find a strong correlation between pressure passivity and hypotension, MBP and NIRS were not invariably associated with each other. Another study of 44 infants with median GA of 25 weeks suggested that identifying individualized optimum MBP based on the correlation of cerebrovascular reactivity (estimated from HR and NIRS tissue oxygenation index) can better predict IVH (144). In a study of 25 preterm infants continuous MBP and electrical cortical activity by spectral analysis using continuous multichannel EEG recordings was measured (145). This study found increased mortality if there was a stronger directionality of interaction between MBP and aEEG leading to decreasing spectral power of EEG with decreasing MBP. Although mean BP alone was predominantly used in these prior studies, recently BP parameters such as systolic (SBP), diastolic (DBP), and pulse pressure (PP) are also being explored. In a study of 485 neonates with mean GA of 28 weeks, left ventricular output was positively associated with SBP and PP, and not MBP and DBP (146).
In summary, continuous BP data, rather than intermittent values, have the potential to predict short and long-term adverse neurological outcomes. However, rapid hemodynamic changes occurring in the first week after birth decrease sensitivity of BP as a tool to monitor cardiac output and tissue perfusion. Ideally, BP analysis should be combined with other measurements such as functional echocardiography, NIRS, aEEG, and Doppler studies of regional blood flow to predict and potentially prevent adverse outcomes (112, 113, 147–150).
Conclusion
It is increasingly evident that intermittent assessment of VS from review of standard bedside monitor data or medical record documentation fails to capture trends that portend impending pathophysiology, and that analysis of continuous VS data can improve outcomes of infants in the NICU. Display to clinicians of a score based on continuous analysis of HR characteristics has been shown in a large randomized clinical trial to decrease sepsis-associated mortality in preterm VLBW infants. While various analytics of continuous SpO2, RR, apnea, and BP data either alone or in combination show promise for disease prevention or mitigation, they require more validation in randomized clinical trials. The prospect of computerized, artificial intelligence-based multi-tier analysis (Fig. 2) incorporating several vital signs will likely offer a plethora of actionable data to guide bedside management. A three-tiered approach incorporating VS analytics, careful consideration of clinical variables, complimented by laboratory testing and imaging represents a “trifecta” for using readily accessible continuous VS data to prevent neonatal diseases in the 21st century.
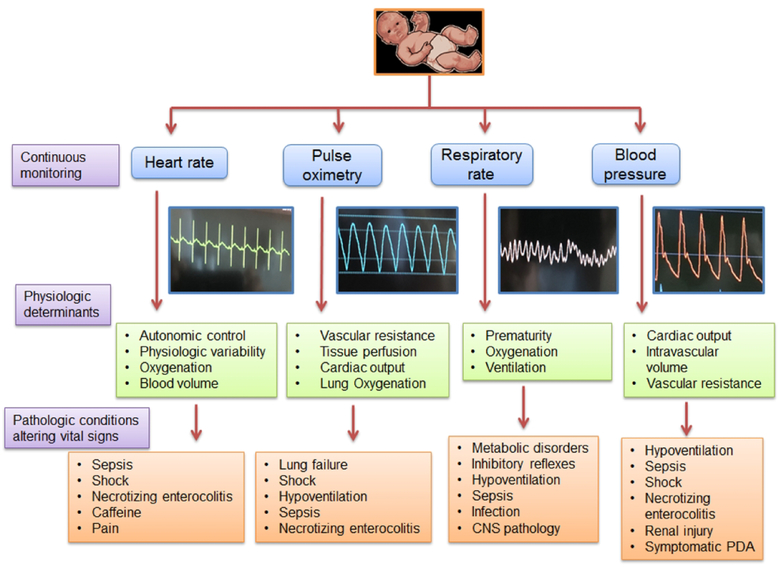
Figure 1 -.
Physiologic and pathologic factors regulating vital signs. Diagram depicting how HR, BP, RR, and SpO2 are physiologically regulated in the preterm neonate, and patterns of vital signs changes that can be associated with pathophysiological states.
Table 1:
Continuous heart rate monitoring analytics and outcome studies.
| Investigators | Study N Site(s) |
Inclusion criteria | Results/Conclusions |
|---|---|---|---|
| Griffin et al (19) | 633 Two centers |
27 0/7 – 37 weeks | Abnormal HRC (reduced variability, decelerations) is a valid tool for early diagnosis of impending LOS or sepsis like illness. |
| Griffin et al (42) | 337 Single center |
27 0/7 – 35 weeks | HRC along with clinical signs of illness can add independent information in the diagnosis of LOS. |
| Moorman et al (29) | 3003 Multicenter |
<1500 grams | Display of HRC index is associated with 22% decreased mortality and trend towards increased ventilator free days in comparison to control subjects with recording of HRC index without display. |
| Fairchild et al (30) | 2989 Multicenter |
<1500 grams | 40% decreased mortality within 30 days of septicemia with HRC index display due to earlier diagnosis. |
| Stone et al (31) | 97 Multicenter |
<1500 grams | Abnormal HRC occurs prior to diagnosis of medical and surgical NEC and can help with early diagnosis and treatment. |
| Addison et al (33) | 65 Single center |
<1500 grams | Cumulative frequency of abnormal HRC score is associated with increased risk of cerebral palsy and delayed early cognitive development. |
| Fairchild et al (68) | 1065 Two Center |
<1500 grams | Best metric for predicting LOS/NEC was cross correlation(X-Corr)-HR-SpO2 and a 3-variable vital sign model. |
| Fairchild et al (46) | 629 Single center |
<1500 grams | High cross correlation (X-Corr)-HR-SpO2 is associated with apnea and adverse events including NEC and LOS. |
| Sullivan et al (34) | 566 Single center |
<1500 grams | HRC index on day of life 1 and 7 compares favorably to established risk scores to predict death and morbidities (LOS, BPD, severe IVH, NEC and severe ROP). |
| Doheny et al (32) | 70 Single center |
28 0/7 –34 6/7 weeks | HF-HRV can be a potential non-invasive biomarker for NEC diagnosis. |
| Fairchild et al (38) | 384 Single center |
<1000 grams | Increased HRC index is associated with abnormal brain imaging and can predict adverse neurologic outcomes. |
| Vergales et al (39) | 37–64 Single center |
Term gestation | Low HRV (and corresponding high HRC index) is associated with worse EEG, MRI and 2 year Bayley neurodevelopmental outcomes. |
| Goel et al (37) | 102 Single center |
All Ventilated infants | HRC index is higher in infants with extubation failures compared to infants with successful extubation. Infants with failed extubation had lower gestational age, extubated at lower corrected gestational age, longer duration of ventilation prior to extubation and culture positive sepsis. |
| Sullivan et al (35) | 274 Single center |
<1500 grams | Sepsis, respiratory deterioration, surgical procedures and other infectious or inflammatory conditions are associated with large increase in HRC index. |
HRC, heart rate characteristics; LOS, late onset sepsis; NEC, necrotizing enterocolitis; X-Corr-HR-SpO2, cross correlation of heart rate and oxygen saturation; BPD, bronchopulmonary dysplasia; ROP, retinopathy of prematurity; IVH, intraventricular hemorrhage; HUS, head ultrasound; MRI, magnetic resonance imaging
Table 2:
Continuous pulse oximetry monitoring analytics and outcome studies.
| Investigators | Study N Site(s) |
Inclusion criteria | Results/Conclusions |
|---|---|---|---|
| Di Fiore et al (58) | 1316 Multicenter |
24 0/7 – 27 6/7 weeks | SGA infants in lower target SpO2 (85–89%) range achieved lowest oxygen saturation and higher incidence of intermittent hypoxemia in comparison with higher target SpO2 (91–95%) during first 3 days of life. Lowest quartile for saturation (≤ 92%) in the first 3 days of life is associated with increased 90-day mortality in AGA and SGA infants. |
| Poet et al (57) | 1019 Multicenter |
23 0/7 – 27 6/7 weeks | Prolonged hypoxemic episodes in first 2–3 months of life increased risk of death or cognitive/motor impairment at 18 months of age. Bradycardia did not alter the prognostic value of hypoxemia. |
| Sullivan et al (64) | 778 Two center |
<1500 gram | Pulse oximetry predictive score (POPS) performed better for prediction of death, IVH and BPD compared to HRC alone. |
| Raffay et al (65) | 137 Single center |
<28 weeks | More frequent, longer and elevated intermittent Hypoxemia (IH) nadirs with increased oxygen and pressure exposure in first 28 days of life increases the risk of BPD. Early IH patterns may contribute to the development of BPD and may help identify infants at risk. |
| Di Fiore et al (67) | 63 Single center |
24 0/7 – 27 6/7 weeks | Increased variability, longer duration, and lower nadir of hypoxemia is associated with increased risk of severe ROP. Identification of spectral component of SpO2 waveform may aid in identification of infants at risk for severe ROP. |
| Fairchild et al (68) | 1065 Two center |
<1500 gram | HR-SpO2 cross correlation model performed better than HR or SpO2 for preclinical detection of NEC and sepsis. |
| Warburton et al (4) | 94 Single center |
<36 weeks gestation | % of time with SpO2 <90% is associated with risk of respiratory support escalation and decreased weight gain. |
| Das et al (8) | 56 Single center |
<1000 gram | Increased SpO2 fluctuation during first 4 weeks of life was higher in infants with severe ROP. No association was found if the time spent was in the target range of SpO2. |
| Mascoll- Robertson et al (69) | 31 Single center |
24 0/7 – 32 6/7 weeks | Pulse oximetry histogram may be helpful in determining readiness of weaning from CPAP/HFNC to LFNC/oxyhood/RA transition. |
| Vesoulis et al (66) | 645 Multicenter |
<32 weeks or <1500 gram | Greater hypoxemia burden in first week of life was significantly associated with grade III/IV IVH. |
| Bizzarro et al (71) | 700 Single center |
<1500 gram | Significant reduction in severe ROP and ROP requiring surgery after staff education and implementation of signal extraction technology to quantify success with achieved target oxygen saturations. |
SGA, small for gestational age; AGA, appropriate for gestational age; SpO2, oxygen saturation; POPS, pulse oximetry predictive scores; IVH, intraventricular hemorrhage; BPD, bronchopulmonary dysplasia; HRC, heart rate characteristics; ROP, retinopathy of prematurity; NEC, necrotizing enterocolitis; CPAP, continuous positive airway pressure; HFNC, high flow nasal cannula; LFNC, low flow nasal cannula; RA, room air; HUS, head ultrasound
Table 3:
Continuous respiratory rate, effort and blood pressure data analysis and outcome studies.
| Investigators (Respiratory Rate) |
Study N Site(s) |
Inclusion criteria | Results/Conclusions |
| Hofstetter et al (98) | 33 Single center |
23 0/7 –27 6/7 weeks | Apnea/hypopnea, bradycardia and hypoxemia episodes decreased with age, but continued at term equivalent, and even after hospital discharge. Infection increased apnea/hypopnea and hypoxemia events. |
| Fairchild et al (43) | 1211 Single center |
<35 weeks | Number and duration of apnea events decreased with increasing gestational age. ABD events has higher frequency in <31 weeks infants but not increased in infants with severe ROP, BPD and severe IVH after adjusting for GA. ABD events increased before the diagnosis of LOS and NEC. |
| Tabacaru et al (101) | 302 Single center |
<32 weeks | Intermittent caffeine boluses and discontinuation at 33 weeks PMA were associated with small changes in ABD events. |
| Patel et al (44) | 1211 Single center |
<35 weeks | Periodic breathing (PB) increases with gestational age and the highest amount was between 30–33 weeks and 2 weeks chronological age. Extreme PB is associated with infection, NEC, caffeine discontinuation and immunizations. |
| Warburton et al (4) | 94 Single center |
<36 weeks | Tachypnea (RR>70) is associated with poor growth and respiratory support escalation. >30% tachypnea/day is associated with increased respiratory support in subsequent 3 days. |
| Mohr et al (97) | 70 Single center |
All infants in the NICU | For 32 weeks gestation infants, PB peaked 7–14 days after birth (6.5%). Infant with death (SIDS) had 40% PB each day and her twin had 15% PB each day. |
| Investigators (Blood Pressure) |
Study N Site(s) |
Inclusion criteria | Results/Conclusions |
| Goldstein et al (119) | 191 Single center |
<1500 grams | Metabolic acidosis and respiratory acidosis are related to adverse cognitive, motor and neurologic outcome at 6 months of age, while only metabolic component is related to adverse outcomes at 24 months age. |
| Miall- Allen et al (121) | 33 Single center |
<31 weeks | Hypotension (<30mm Hg) for over an hour was associated with IVH, ischemic cerebral lesions and death (within 48 hours). |
| Miall- Allen et al (141) | 22 Single center |
<31 weeks | No association was found between blood pressure fluctuation and IVH in first 36 hours of life. |
| Low et al (124) | 98 Single center |
<34 weeks | Combination of hypotension and hypoxemia in first 96 hours of life significantly increased the risk of brain damage and poor outcomes. |
| Cunningham et al (102) | 232 Single center |
<1500 grams | IVH was associated with low or variable BP. PVL and ROP were not associated with BP. BP variability was associated with death. |
| Bada et al (122) | 100 Single center |
<1500 grams | Infants with periventricular IVH had a greater minute to minute BP variability compared to infants with no periventricular IVH. |
| Perlman et al (142) | 50 Single center |
<1500 grams | Fluctuating cerebral blood flow velocity in infants with RDS increases the risk of IVH. |
| Soul et al (143) | 90 Two centers |
<1500 grams | Cerebral pressure passivity is associated with gestational age and low birth weight, systemic hypotension and maternal hemodynamic factors. |
| Dacosta et al (144) | 44 Single center |
23 0/7–26 6/7 weeks | Defining the MAP with strongest cerebrovascular activity is feasible and deviations in that increased the risk of IVH and death. |
| Semenova et al (145) | 25 Single center |
<32 weeks | Normal well-being is associated with non-linear association between EEG and BP. Presence of weak association with distinctive directionality of information flow is associated with increased mortality. |
| Hoffman et al (138) | 61 Single center |
23 0/7–28 6/7 weeks | More time with impaired cerebral auto regulation and less time with cerebral reactivity was associated with grade 3–4 IVH. |
ABD, apnea bradycardia desaturation; BPD, bronchopulmonary dysplasia; ROP, retinopathy of prematurity; IVH, intraventricular hemorrhage; LOS, late onset sepsis; NEC, necrotizing enterocolitis; GA, gestational age; PMA, post menstrual age; MAP, mean arterial pressure; EEG, electroencephalogram; RDS, respiratory distress syndrome; PVL, periventricular leukomalacia; SIDS, sudden infant death syndrome
Acknowledgments
Statement of financial support: Children’s Mercy Research Institute (VS); NIH HD072071 (KF), Translational Health Research Institute of Virginia Scholars Award (BS)
Footnotes
Conflict of interest. The authors declare no conflict of interest.
References
- 1.Leante-Castellanos JL, Martínez-Gimeno A, Cidrás-Pidré M, Martínez-Munar G, García-González A, Fuentes-Gutiérrez C. Central-peripheral Temperature Monitoring as a Marker for Diagnosing Late-onset Neonatal Sepsis. The Pediatric infectious disease journal 2017;36(12):e297. [DOI] [PubMed] [Google Scholar]
- 2.Fairchild KD, Schelonka RL, Kaufman DA, et al. Septicemia mortality reduction in neonates in a heart rate characteristics monitoring trial. Pediatric research 2013;74(5):570–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ruiz TL, Trzaski JM, Sink DW, Hagadorn JI. Transcribed oxygen saturation vs oximeter recordings in very low birth weight infants. Journal of Perinatology 2014;34(2):130–135 [DOI] [PubMed] [Google Scholar]
- 4.Warburton A, Monga R, Sampath V, Kumar N. Continuous pulse oximetry and respiratory rate trends predict short-term respiratory and growth outcomes in premature infants. Pediatric research 2019 [DOI] [PubMed] [Google Scholar]
- 5.Cardona‐Morrell M, Prgomet M, Turner RM, Nicholson M, Hillman K. Effectiveness of continuous or intermittent vital signs monitoring in preventing adverse events on general wards: a systematic review and meta‐analysis. International Journal of Clinical Practice 2016;70(10):806–824 [DOI] [PubMed] [Google Scholar]
- 6.Chapman SM, Maconochie IK. Early warning scores in paediatrics: an overview. Archives of Disease in Childhood 2018:314807 [DOI] [PubMed] [Google Scholar]
- 7.Nghiem T, Hagadorn JI, Terrin N, Syke S, MacKinnon B, Cole CH. Nurse Opinions and Pulse Oximeter Saturation Target Limits for Preterm Infants. Pediatrics 2008;121(5):e1046. [DOI] [PubMed] [Google Scholar]
- 8.Das A, Mhanna M, Sears J, et al. Effect of fluctuation of oxygenation and time spent in the target range on retinopathy of prematurity in extremely low birth weight infants. Journal of neonatal-perinatal medicine 2018;11(3):257–263 [DOI] [PubMed] [Google Scholar]
- 9.Amin S, Burnell E. Monitoring Apnea of Prematurity: Validity of Nursing Documentation and Bedside Cardiorespiratory Monitor. Amer J Perinatol 2013;30(8):643–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muttitt SC, Finer NN, Tierney AJ, Rossmann J. Neonatal apnea: diagnosis by nurse versus computer. Pediatrics 1988;82(5):713. [PubMed] [Google Scholar]
- 11.Brockmann PE, Wiechers C, Pantalitschka T, Diebold J, Vagedes J, Poets CF. Under-recognition of alarms in a neonatal intensive care unit. Archives of disease in childhood. Fetal and neonatal edition 2013;98(6):F527. [DOI] [PubMed] [Google Scholar]
- 12.Das A, Mhanna M, Teleron-Khorshad A, et al. A comparison of manual versus automated saturation of peripheral oxygenation in the neonatal intensive care unit. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstetricians 2016;29(10):1631–1635 [DOI] [PubMed] [Google Scholar]
- 13.Hay William W, Rodden Donna J, Collins Shannon M, Melara Diane L, Hale Kathy A, Fashaw Lucy M. Reliability of Conventional and New Pulse Oximetry in Neonatal Patients. Journal of Perinatology 2002;22(5):360–366 [DOI] [PubMed] [Google Scholar]
- 14.Alonzo Corrie J, Nagraj Vijay P, Zschaebitz Jenna V, Lake Douglas E, Moorman J Randall, Spaeder Michael C. Heart rate ranges in premature neonates using high resolution physiologic data. Journal of Perinatology 2018;38(9):1242–1245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shaffer F, Ginsberg JP. An Overview of Heart Rate Variability Metrics and Norms. Frontiers in public health 2017;5:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hon EH, Lee ST. Electronic evaluation of the fetal heart rate. Viii. Patterns preceding fetal death, further observations. American journal of obstetrics and gynecology 1963;87:814. [PubMed] [Google Scholar]
- 17.Herry Christophe L, Cortes Marina, Wu Hau-Tieng, et al. Temporal Patterns in Sheep Fetal Heart Rate Variability Correlate to Systemic Cytokine Inflammatory Response: A Methodological Exploration of Monitoring Potential Using Complex Signals Bioinformatics. PLoS One 2016;11(4):e0153515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Durosier LD, Herry CL, Cortes M, et al. Does heart rate variability reflect the systemic inflammatory response in a fetal sheep model of lipopolysaccharide-induced sepsis?. Physiological measurement 2015;36(10):2089–2102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Griffin MP, O’Shea TM, Bissonette EA, Harrell J, Frank E, Lake DE, Moorman JR. Abnormal Heart Rate Characteristics Preceding Neonatal Sepsis and Sepsis-Like Illness. Pediatric research 2003;53(6):920–926 [DOI] [PubMed] [Google Scholar]
- 20.Fairchild Karen D., Srinivasan Varadamurthy, Randall Moorman J, Gaykema Ronald P. A., Goehler Lisa E. Pathogen-induced heart rate changes associated with cholinergic nervous system activation. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology 2011;300(2):330–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fairchild Karen D., Saucerman Jeffrey J., Raynor Laura L., et al. Endotoxin depresses heart rate variability in mice: cytokine and steroid effects. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology 2009;297(4):1019–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huston JM, Tracey KJ. The pulse of inflammation: heart rate variability, the cholinergic anti‐inflammatory pathway and implications for therapy. Journal of Internal Medicine 2011;269(1):45–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greenberg RG, Kandefer S, Do BT, et al. Late-onset Sepsis in Extremely Premature Infants: 2000–2011. The Pediatric infectious disease journal 2017;36(8):774–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yee Wendy H, Soraisham Amuchou Singh, Shah Vibhuti S, Aziz Khalid, Yoon Woojin, Lee Shoo K. Incidence and Timing of Presentation of Necrotizing Enterocolitis in Preterm Infants. Pediatrics 2012;129(2):e304. [DOI] [PubMed] [Google Scholar]
- 25.Adams-Chapman I, Stoll B. Neonatal infection and long-term neurodevelopmental outcome in the preterm infant. Current Opinion in Infectious Diseases 2006;19(3):290–297 [DOI] [PubMed] [Google Scholar]
- 26.Griffin MP, Lake DE, Bissonette EA, Harrell FE Jr, O’Shea TM, Moorman JR. Heart Rate Characteristics: Novel Physiomarkers to Predict Neonatal Infection and Death. Pediatrics 2005;116(5):1070–1074 [DOI] [PubMed] [Google Scholar]
- 27.Lake Douglas E., Richman Joshua S., Griffin M. Pamela, Moorman J. Randall. Sample entropy analysis of neonatal heart rate variability. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology 2002;283(3):789–797 [DOI] [PubMed] [Google Scholar]
- 28.Kovatchev BP, Farhy LS, Cao H, Griffin MP, Lake DE, Moorman JR. Sample Asymmetry Analysis of Heart Rate Characteristics with Application to Neonatal Sepsis and Systemic Inflammatory Response Syndrome. Pediatric research 2003;54(6):892–898 [DOI] [PubMed] [Google Scholar]
- 29.Moorman JR, Carlo WA, Kattwinkel J, et al. Mortality Reduction by Heart Rate Characteristic Monitoring in Very Low Birth Weight Neonates: A Randomized Trial. Journal of Pediatrics, The 2011;159(6):906.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fairchild KD, Schelonka RL, Kaufman DA, et al. Septicemia mortality reduction in neonates in a heart rate characteristics monitoring trial. Pediatric research 2013;74(5):570–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stone ML, Tatum PM, Weitkamp J-h, et al. Abnormal heart rate characteristics before clinical diagnosis of necrotizing enterocolitis. Journal of Perinatology 2013;33(11):847–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Doheny KK, Palmer C, Browning KN, et al. Diminished vagal tone is a predictive biomarker of necrotizing enterocolitis‐risk in preterm infants. Neurogastroenterology & Motility 2014;26(6):832–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Addison K, Griffin MP, Moorman JR, Lake DE, O’shea TM. Heart rate characteristics and neurodevelopmental outcome in very low birth weight infants. Journal of Perinatology 2009;29(11):750–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sullivan BA, McClure C, Hicks J, Lake DE, Moorman JR, Fairchild KD. Early Heart Rate Characteristics Predict Death and Morbidities in Preterm Infants. Journal of Pediatrics, The 2016;174:57–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sullivan BA, Grice SM, Lake DE, Moorman JR, Fairchild KD. Infection and Other Clinical Correlates of Abnormal Heart Rate Characteristics in Preterm Infants. Journal of Pediatrics, The 2014;164(4):775–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clark MT, Vergales BD, Paget-Brown AO, et al. Predictive monitoring for respiratory decompensation leading to urgent unplanned intubation in the neonatal intensive care unit. Pediatric research 2013;73(1):104–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goel N, Chakraborty M, Watkins WJ, Banerjee S. Predicting Extubation Outcomes—A Model Incorporating Heart Rate Characteristics Index. The Journal of Pediatrics 2018;195:58.e1. [DOI] [PubMed] [Google Scholar]
- 38.Fairchild KD, Sinkin RA, Davalian F, et al. Abnormal heart rate characteristics are associated with abnormal neuroimaging and outcomes in extremely low birth weight infants. Journal of Perinatology 2014;34(5):375–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vergales B, Zanelli S, Matsumoto J, et al. Depressed Heart Rate Variability is Associated with Abnormal EEG, MRI, and Death in Neonates with Hypoxic Ischemic Encephalopathy. Amer J Perinatol 2014;31(10):855–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tuzcu V, Nas S, Ulusar U, Ugur A, Kaiser JR. Altered Heart Rhythm Dynamics in Very Low Birth Weight Infants With Impending Intraventricular Hemorrhage. Pediatrics 2009;123(3):810–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sortica da Costa C, Placek MM, Czosnyka M, et al. Complexity of brain signals is associated with outcome in preterm infants. Journal of Cerebral Blood Flow & Metabolism 2017;37(10):3368–3379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Griffin MP, Lake DE, O’Shea TM, Moorman JR. Heart Rate Characteristics and Clinical Signs in Neonatal Sepsis. Pediatric research 2007;61(2):222–227 [DOI] [PubMed] [Google Scholar]
- 43.Fairchild K, Mohr M, Paget-Brown A, et al. Clinical associations of immature breathing in preterm infants: part 1—central apnea. Pediatric research 2016;80(1):21–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Patel M, Mohr M, Lake D, et al. Clinical associations with immature breathing in preterm infants: part 2—periodic breathing. Pediatric research 2016;80(1):28–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Siljehav, Veronica, Hofstetter Annika M., Leifsdottir K, Herlenius Eric. Prostaglandin E2 Mediates Cardiorespiratory Disturbances during Infection in Neonates. Journal of Pediatrics, The 2015;167(6):1213.e3. [DOI] [PubMed] [Google Scholar]
- 46.Fairchild KD, Lake DE. Cross-Correlation of Heart Rate and Oxygen Saturation in Very Low Birthweight Infants: Association with Apnea and Adverse Events. American journal of perinatology 2018;35(5):463–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fairchild KD, Lake DE, Kattwinkel J, et al. Vital signs and their cross-correlation in sepsis and NEC: a study of 1,065 very-low-birth-weight infants in two NICUs. Pediatric research 2017;81(2):315–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saria S, Rajani AK, Gould J, Koller D, Penn AA. Integration of Early Physiological Responses Predicts Later Illness Severity in Preterm Infants. Science translational medicine 2010;2(48):48ra65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gur I, Riskin A, Markel G, et al. Pilot Study of a New Mathematical Algorithm for Early Detection of Late-Onset Sepsis in Very Low-Birth-Weight Infants. Amer J Perinatol 2015;32(4):321–330 [DOI] [PubMed] [Google Scholar]
- 50.Wukitsch MW, Petterson MT, Tobler DR, Pologe JA. Pulse oximetry: Analysis of theory, technology, and practice. Journal of clinical monitoring 1988;4(4):290–301 [DOI] [PubMed] [Google Scholar]
- 51.Avidan A, Levin PD. Pulse oximetry. The New England journal of medicine 2011;365(2):184; author reply 185 [DOI] [PubMed] [Google Scholar]
- 52.Jubran A Pulse oximetry. Critical care (London, England) 2015;19(1):272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Buonocore, Perrone Giuseppe, Tataranno Serafina, Luisa Maria. Oxygen toxicity: chemistry and biology of reactive oxygen species. Seminars in Fetal and Neonatal Medicine 2010;15(4):186–190 [DOI] [PubMed] [Google Scholar]
- 54.Davis, Auten Jonathan M., Richard L. Maturation of the antioxidant system and the effects on preterm birth. Seminars in Fetal and Neonatal Medicine 2010;15(4):191–195 [DOI] [PubMed] [Google Scholar]
- 55.Saugstad OD, Aune D. Optimal Oxygenation of Extremely Low Birth Weight Infants: A Meta-Analysis and Systematic Review of the Oxygen Saturation Target Studies. Neonatology 2013;105(1):55–63 [DOI] [PubMed] [Google Scholar]
- 56.Askie Lisa M, Darlow Brian A, Finer Neil, et al. Association Between Oxygen Saturation Targeting and Death or Disability in Extremely Preterm Infants in the Neonatal Oxygenation Prospective Meta-analysis Collaboration. JAMA 2018;319(21):2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Poets CF, Roberts RS, Schmidt B, et al. Association Between Intermittent Hypoxemia or Bradycardia and Late Death or Disability in Extremely Preterm Infants. JAMA 2015;314(6):595–603 [DOI] [PubMed] [Google Scholar]
- 58.Fiore Di, Juliann M, Martin RJ, Li H, et al. Patterns of Oxygenation, Mortality, and Growth Status in the Surfactant Positive Pressure and Oxygen Trial Cohort. Journal of Pediatrics, The 2017;186:56.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hagadorn JI, Furey AM, Nghiem T, et al. Achieved Versus Intended Pulse Oximeter Saturation in Infants Born Less Than 28 Weeks’ Gestation: The AVIOx Study. Pediatrics 2006;118(4):1574–1582 [DOI] [PubMed] [Google Scholar]
- 60.Lim K, Wheeler, Kevin I, Gale TJ, et al. Oxygen Saturation Targeting in Preterm Infants Receiving Continuous Positive Airway Pressure. Journal of Pediatrics, The 2014;164(4):736.e1. [DOI] [PubMed] [Google Scholar]
- 61.APGAR V. A proposal for a new method of evaluation of the newborn infant. Current researches in anesthesia & analgesia 1953;32(4):260. [PubMed] [Google Scholar]
- 62.Richardson DK, Corcoran JD, Escobar GJ, Lee SK. SNAP-II and SNAPPE-II: Simplified newborn illness severity and mortality risk scores. The Journal of Pediatrics 2001;138(1):92–100 [DOI] [PubMed] [Google Scholar]
- 63.Parry G, Tucker J, Tarnow-Mordi W. CRIB II: an update of the clinical risk index for babies score. The Lancet 2003;361(9371):1789–1791 [DOI] [PubMed] [Google Scholar]
- 64.Sullivan BA, Wallman-Stokes A, Isler J, et al. Early Pulse Oximetry Data Improves Prediction of Death and Adverse Outcomes in a Two-Center Cohort of Very Low Birth Weight Infants. American journal of perinatology 2018;35(13):1331–1338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Raffay TM, Dylag AM, Sattar A, et al. Neonatal intermittent hypoxemia events are associated with diagnosis of bronchopulmonary dysplasia at 36 weeks postmenstrual age. Pediatric research 2019;85(3):318–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vesoulis Zachary A, Bank Rachel L, Lake Doug, et al. Early hypoxemia burden is strongly associated with severe intracranial hemorrhage in preterm infants. Journal of Perinatology 2019;39(1):48–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Di Fiore JM, Kaffashi F, Loparo K, et al. The relationship between patterns of intermittent hypoxia and retinopathy of prematurity in preterm infants. Pediatric research 2012;72(6):606–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fairchild KD, Lake DE, Kattwinkel J, et al. Vital signs and their cross-correlation in sepsis and NEC: a study of 1,065 very-low-birth-weight infants in two NICUs. Pediatric research 2017;81(2):315–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mascoll-Robertson KK, Viscardi RM, Woo HC. The Objective Use of Pulse Oximetry to Predict Respiratory Support Transition in Preterm Infants: An Observational Pilot Study. Respiratory care 2016;61(4):416–422 [DOI] [PubMed] [Google Scholar]
- 70.Pozo ME, Cave A, Köroğlu ÖA, et al. Effect of Postnatal Intermittent Hypoxia on Growth and Cardiovascular Regulation of Rat Pups. Neonatology 2012;102(2):107–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bizzarro MJ, Li FY, Katz K, Shabanova V, Ehrenkranz RA, Bhandari V. Temporal quantification of oxygen saturation ranges: an effort to reduce hyperoxia in the neonatal intensive care unit. Journal of Perinatology 2014;34(1):33–38 [DOI] [PubMed] [Google Scholar]
- 72.Claure Nelson, Bancalari Eduardo, D’Ugard Carmen, et al. Multicenter Crossover Study of Automated Control of Inspired Oxygen in Ventilated Preterm Infants. Pediatrics 2011;127(1):e83. [DOI] [PubMed] [Google Scholar]
- 73.van Kaam AH, te Pas AB, Bachman TE, et al. Automated versus Manual Oxygen Control with Different Saturation Targets and Modes of Respiratory Support in Preterm Infants. Journal of Pediatrics, The 2015;167(3):550.e2. [DOI] [PubMed] [Google Scholar]
- 74.Zapata J, Gómez JJ, Araque Campo R, Matiz Rubio A, Sola A. A randomised controlled trial of an automated oxygen delivery algorithm for preterm neonates receiving supplemental oxygen without mechanical ventilation. Acta Paediatrica 2014;103(9):928–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Saihi K, Richard JM, Gonin X, Krüger T, Dojat M, Brochard L. Feasibility and reliability of an automated controller of inspired oxygen concentration during mechanical ventilation. Critical care (London, England) 2014;18(2):R35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hallenberger Antonietta, Christian Friedrich Poets Werner Horn, Seyfang Andreas, Michael Siegfried Urschitz. Closed-Loop Automatic Oxygen Control (CLAC) in Preterm Infants: A Randomized Controlled Trial. Pediatrics 2014;133(2):e385. [DOI] [PubMed] [Google Scholar]
- 77.Eduardo Claure N. Closed-loop control of inspired oxygen in premature infants. Seminars in Fetal and Neonatal Medicine 2015;20(3):198–204 [DOI] [PubMed] [Google Scholar]
- 78.Claure N, Bancalari E. Automated closed loop control of inspired oxygen concentration. Respiratory care 2013;58(1):151–161 [DOI] [PubMed] [Google Scholar]
- 79.Di Fiore JM. Neonatal cardiorespiratory monitoring techniques. Seminars in Neonatology 2004;9(3):195–203 [DOI] [PubMed] [Google Scholar]
- 80.Folke M, Cernerud L, Ekström M, Hök B. Critical review of non-invasive respiratory monitoring in medical care. Med Biol Eng Comput 2003;41(4):377–383 [DOI] [PubMed] [Google Scholar]
- 81.Lee H, Rusin CG, Lake DE, et al. A new algorithm for detecting central apnea in neonates. Physiological measurement 2012;33(1):1–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Weese-Mayer DE, Corwin MJ, Peucker MR, et al. Comparison of apnea identified by respiratory inductance plethysmography with that detected by end-tidal CO(2) or thermistor. The CHIME Study Group. American journal of respiratory and critical care medicine 2000;162(2 Pt 1):471–480 [DOI] [PubMed] [Google Scholar]
- 83.Massaroni C, Nicolò A, Lo Presti D, Sacchetti M, Silvestri S, Schena E. Contact-Based Methods for Measuring Respiratory Rate. Sensors (Basel, Switzerland) 2019;19(4):908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Charlton PH, Birrenkott DA, Bonnici T, et al. Breathing Rate Estimation From the Electrocardiogram and Photoplethysmogram: A Review. RBME 2018;11:2–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gerhardt T, Bancalari E. Apnea of prematurity: I. Lung function and regulation of breathing. Pediatrics 1984;74(1):58. [PubMed] [Google Scholar]
- 86.Martin RJ, DiFiore JM, Korenke CB, Randal H, Miller MJ, Brooks LJ. Vulnerability of respiratory control in healthy preterm infants placed supine. The Journal of Pediatrics 1995;127(4):609–614 [DOI] [PubMed] [Google Scholar]
- 87.Katz-Salamon M Delayed chemoreceptor responses in infants with apnoea. Archives of disease in childhood 2004;89(3):261–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gauda EB, McLemore GL, Tolosa J, Marston-Nelson J, Kwak D. Maturation of peripheral arterial chemoreceptors in relation to neonatal apnoea. Seminars in Neonatology 2004;9(3):181–194 [DOI] [PubMed] [Google Scholar]
- 89.Abu‐Shaweesh JM, Martin RJ. Neonatal Apnea: What’s New?. Pediatric Pulmonology 2008;43(10):937–944 [DOI] [PubMed] [Google Scholar]
- 90.Darnall RA. The role of CO2 and central chemoreception in the control of breathing in the fetus and the neonate. Respiratory Physiology & Neurobiology 2010;173(3):201–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Balan KV, Kc P, Hoxha Z, Mayer CA, Wilson CG, Martin RJ. Vagal afferents modulate cytokine-mediated respiratory control at the neonatal medulla oblongata. Respiratory Physiology & Neurobiology 2011;178(3):458–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Balan KV, Kc P, Mayer CA, Wilson CG, Belkadi A, Martin RJ. Intrapulmonary lipopolysaccharide exposure upregulates cytokine expression in the neonatal brainstem. Acta Paediatrica 2012;101(5):466–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Vergales B, Paget-Brown A, Lee H, et al. Accurate Automated Apnea Analysis in Preterm Infants. Amer J Perinatol 2014;31(2):157–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Southall DP, Levitt GA, Richards JM, et al. Undetected episodes of prolonged apnea and severe bradycardia in preterm infants. Pediatrics 1983;72(4):541. [PubMed] [Google Scholar]
- 95.Mohr MA, Vergales BD, Lee H, et al. Very long apnea events in preterm infants. Journal of applied physiology (Bethesda, Md. : 1985) 2015;118(5):558–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zagol K, Lake DE, Vergales B, et al. Anemia, Apnea of Prematurity, and Blood Transfusions. Journal of Pediatrics, The 2012;161(3):421.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mohr MA, Fairchild KD, Patel M, et al. Quantification of periodic breathing in premature infants. Physiological measurement 2015;36(7):1415–1427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hofstetter AO, Legnevall L, Herlenius E, Katz-Salamon M. Cardiorespiratory development in extremely preterm infants: vulnerability to infection and persistence of events beyond term-equivalent age. Acta Pædiatrica 2008;97(3):285–292 [DOI] [PubMed] [Google Scholar]
- 99.Shannon DC, Carley DW, Kelly DH. Periodic breathing: Quantitative analysis and clinical description. Pediatric pulmonology 1988;4(2):98–102 [DOI] [PubMed] [Google Scholar]
- 100.Barrington KJ, Finer NN. Periodic Breathing and Apnea in Preterm Infants. Pediatric research 1990;27(2):118–121 [DOI] [PubMed] [Google Scholar]
- 101.Tabacaru CR, Jang SY, Patel M, Davalian F, Zanelli S, Fairchild KD. Impact of Caffeine Boluses and Caffeine Discontinuation on Apnea and Hypoxemia in Preterm Infants. Journal of Caffeine Research 2017;7(3):13–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cunningham S, Symon AG, Elton RA, Zhu C, McIntosh N. Intra-arterial blood pressure reference ranges, death and morbidity in very low birthweight infants during the first seven days of life. Early Human Development 1999;56(2–3):151–165 [DOI] [PubMed] [Google Scholar]
- 103.Jones JE, Jose PA. Neonatal blood pressure regulation. Seminars in Perinatology 2004;28(2):141–148 [DOI] [PubMed] [Google Scholar]
- 104.Toyono M, Harada K, Takahashi Y, Takada G. Maturational changes in left ventricular contractile state. International Journal of Cardiology 1998;64(3):247–252 [DOI] [PubMed] [Google Scholar]
- 105.Takahashi Y, Harada K, Kishkurno S, Arai H, Ishida A, Takada G. Postnatal Left Ventricular Contractility in Very Low Birth Weight Infants. Pediatr Cardiol 1997;18(2):112–117 [DOI] [PubMed] [Google Scholar]
- 106.Alan Finnemore A. Physiology of the fetal and transitional circulation. Seminars in Fetal and Neonatal Medicine 2015;20(4):210–216 [DOI] [PubMed] [Google Scholar]
- 107.Ng PC, Lee CH, Lam CWK, et al. Transient adrenocortical insufficiency of prematurity and systemic hypotension in very low birthweight infants. Archives of disease in childhood. Fetal and neonatal edition 2004;89(2):119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Laughon MM, Simmons MA, Bose CL. Patency of the ductus arteriosus in the premature infant: is it pathologic? Should it be treated?. Current opinion in pediatrics 2004;16(2):146–151 [DOI] [PubMed] [Google Scholar]
- 109.Kluckow M Low systemic blood flow and pathophysiology of the preterm transitional circulation. Early Human Development 2005;81(5):429–437 [DOI] [PubMed] [Google Scholar]
- 110.Fernandez EF, Watterberg KL. Relative adrenal insufficiency in the preterm and term infant. Journal of Perinatology 2009;29(S2):S49. [DOI] [PubMed] [Google Scholar]
- 111.Vesoulis ZA, El Ters NM, Wallendorf M, Mathur AM. Empirical estimation of the normative blood pressure in infants [DOI] [PMC free article] [PubMed]
- 112.Pladys P, Wodey E, Beuchée A, Branger B, Bétrémieux P. Left ventricle output and mean arterial blood pressure in preterm infants during the 1st day of life. Eur J Pediatr 1999;158(10):817–824 [DOI] [PubMed] [Google Scholar]
- 113.Kluckow M, Evans N. Relationship between blood pressure and cardiac output in preterm infants requiring mechanical ventilation. The Journal of Pediatrics 1996;129(4):506–512 [DOI] [PubMed] [Google Scholar]
- 114.Weindling AM. Blood pressure monitoring in the newborn. Archives of disease in childhood 1989;64(4 Spec No):444–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gevers M, van Genderingen HR, Lafeber HN, Hack WW. Accuracy of oscillometric blood pressure measurement in critically ill neonates with reference to the arterial pressure wave shape. Intensive care medicine 1996;22(3):242–248 [DOI] [PubMed] [Google Scholar]
- 116.Takci S, Yigit S, Korkmaz A, Yurdakök M. Comparison between oscillometric and invasive blood pressure measurements in critically ill premature infants. Acta Paediatrica 2012;101(2):132–135 [DOI] [PubMed] [Google Scholar]
- 117.Dannevig I, Dale HC, Liestol K, Lindemann R. Blood pressure in the neonate: Three non-invasive oscillometric pressure monitors compared with invasively measured blood pressure. Acta Paediatrica 2005;94(2):191–196 [DOI] [PubMed] [Google Scholar]
- 118.Werther Tobias, Aichhorn Lukas, Baumgartner Sigrid, Berger Angelika, Klebermass-Schrehof Katrin, Salzer-Muhar Ulrike. Discrepancy between invasive and non-invasive blood pressure readings in extremely preterm infants in the first four weeks of life. PLoS One 2018;13(12):e0209831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Goldstein RF, Thompson RJJ, Oehler JM, Brazy JE. Influence of acidosis, hypoxemia, and hypotension on neurodevelopmental outcome in very low birth weight infants. Pediatrics 1995;95(2):238. [PubMed] [Google Scholar]
- 120.Fanaroff JM, Wilson-Costello DE, Newman NS, Montpetite MM, Fanaroff AA. Treated Hypotension Is Associated With Neonatal Morbidity and Hearing Loss in Extremely Low Birth Weight Infants. Pediatrics 2006;117(4):1131–1135 [DOI] [PubMed] [Google Scholar]
- 121.Miall-Allen VM, de Vries LS, Whitelaw AG. Mean arterial blood pressure and neonatal cerebral lesions. Archives of disease in childhood 1987;62(10):1068–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bada HS, Korones SB, Perry EH, et al. Mean arterial blood pressure changes in premature infants and those at risk for intraventricular hemorrhage. The Journal of Pediatrics 1990;117(4):607–614 [DOI] [PubMed] [Google Scholar]
- 123.Fok TF, Davies DP, Ng HK. A study of periventricular haemorrhage, post-haemorrhagic ventricular dilatation and periventricular leucomalacia in Chinese preterm infants. Journal of paediatrics and child health 1990;26(5):271–275 [DOI] [PubMed] [Google Scholar]
- 124.Low JA, Froese AB, Galbraith RS, Smith JT, Sauerbrei EE, Derrick EJ. The association between preterm newborn hypotension and hypoxemia and outcome during the first year. Acta paediatrica (Oslo, Norway : 1992) 1993;82(5):433–437 [DOI] [PubMed] [Google Scholar]
- 125.Versmold HT, Kitterman JA, Phibbs RH, Gregory GA, Tooley WH. Aortic blood pressure during the first 12 hours of life in infants with birth weight 610 to 4,220 grams. Pediatrics 1981;67(5):607. [PubMed] [Google Scholar]
- 126.Lee J, Rajadurai VS, Tan KW. Blood pressure standards for very low birthweight infants during the first day of life. Archives of disease in childhood. Fetal and neonatal edition 1999;81(3):F170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Batton B, Li L, Newman NS, et al. Evolving blood pressure dynamics for extremely preterm infants. Journal of Perinatology 2014;34(4):301–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Trounce JQ, Shaw DE, Levene MI, Rutter N. Clinical risk factors and periventricular leucomalacia. Archives of disease in childhood 1988;63(1):17–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Grönlund JU, Korvenranta H, Kero P, Jalonen J, Välimäki IA. Elevated arterial blood pressure is associated with peri-intraventricular haemorrhage. European journal of pediatrics 1994;153(11):836–841 [DOI] [PubMed] [Google Scholar]
- 130.D’Souza SW, Janakova H, Minors D, et al. Blood pressure, heart rate, and skin temperature in preterm infants: associations with periventricular haemorrhage. Archives of disease in childhood. Fetal and neonatal edition 1995;72(3):F167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Perlman Jeffrey M, Risser Rick, Sue Broyles R. Bilateral cystic periventricular leukomalacia in the premature infant: Associated risk factors. Pediatrics 1996;97(6):822. [PubMed] [Google Scholar]
- 132.Wiswell TE, Graziani LJ, Kornhuaser MS, Stanley C. Effects of hypocarbia on the development of cystic periventricular leukomalacia in premature infants treated with high-frequency jet ventilation. Pediatrics 1996;98(5):918. [PubMed] [Google Scholar]
- 133.Dammann O, Allred EN, Kuban KCK, et al. Systemic hypotension and white-matter damage in preterm infants. Developmental Medicine and Child Neurology 2002;44(2):82–90 [DOI] [PubMed] [Google Scholar]
- 134.Limperopoulos C, Bassan H, Kalish LA, et al. Current Definitions of Hypotension Do Not Predict Abnormal Cranial Ultrasound Findings in Preterm Infants. Pediatrics 2007;120(5):966–977 [DOI] [PubMed] [Google Scholar]
- 135.Batton B, Zhu MD, Xiobei MD, Fanaroff MS, Jonathan MD,JD, et al. Blood Pressure, Anti-Hypotensive Therapy, and Neurodevelopment in Extremely Preterm Infants. Journal of Pediatrics, The 2009;154(3):357.e1 [DOI] [PubMed] [Google Scholar]
- 136.Logan JW, O’Shea TM, Allred EN, et al. Early postnatal hypotension and developmental delay at 24 months of age among extremely low gestational age newborns. Archives of disease in childhood. Fetal and neonatal edition 2011;96(5):F328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Logan JW, O’shea TM, Allred EN, et al. Early postnatal hypotension is not associated with indicators of white matter damage or cerebral palsy in extremely low gestational age newborns. Journal of Perinatology 2011;31(8):524–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hoffman SB, Cheng Y, Magder LS, Shet N, Viscardi RM. Cerebral autoregulation in premature infants during the first 96 hours of life and relationship to adverse outcomes. Archives of disease in childhood. Fetal and neonatal edition 2018:315725 [DOI] [PubMed] [Google Scholar]
- 139.Lou HC, Lassen NA, Friis-Hansen B. Impaired autoregulation of cerebral blood flow in the distressed newborn infant. The Journal of Pediatrics 1979;94(1):118–121 [DOI] [PubMed] [Google Scholar]
- 140.Tsuji M, Saul JP, du Plessis A, et al. Cerebral Intravascular Oxygenation Correlates With Mean Arterial Pressure in Critically Ill Premature Infants. Pediatrics 2000;106(4):625–632 [DOI] [PubMed] [Google Scholar]
- 141.Miall-Allen VM, de Vries LS, Dubowitz LM, Whitelaw AG. Blood pressure fluctuation and intraventricular hemorrhage in the preterm infant of less than 31 weeks’ gestation. Pediatrics 1989;83(5):657. [PubMed] [Google Scholar]
- 142.Perlman JM, McMenamin JB, Volpe JJ. Fluctuating Cerebral Blood-Flow Velocity in Respiratory-Distress Syndrome. The New England Journal of Medicine 1983;309(4):204–209 [DOI] [PubMed] [Google Scholar]
- 143.Soul JS, Hammer PE, Tsuji M, et al. Fluctuating Pressure-Passivity Is Common in the Cerebral Circulation of Sick Premature Infants. Pediatric research 2007;61(4):467–473 [DOI] [PubMed] [Google Scholar]
- 144.da Costa CS, Czosnyka M, Smielewski P, Austin T. Optimal Mean Arterial Blood Pressure in Extremely Preterm Infants within the First 24 Hours of Life. The Journal of Pediatrics 2018;203:242–248 [DOI] [PubMed] [Google Scholar]
- 145.Semenova Oksana, Lightbody Gordon, M O’Toole John, Boylan Geraldine, Dempsey Eugene, Temko Andriy. Coupling between mean blood pressure and EEG in preterm neonates is associated with reduced illness severity scores. PLoS One 2018;13(6):e0199587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kharrat Ashraf, Rios Daniel Ibarra, Weisz Dany E, et al. The Relationship between blood pressure parameters and left ventricular output in neonates. Journal of Perinatology 2019;39(5):619–625 [DOI] [PubMed] [Google Scholar]
- 147.Groves AM, Kuschel CA, Knight DB, Skinner JR. Relationship between blood pressure and blood flow in newborn preterm infants. Archives of disease in childhood. Fetal and neonatal edition 2008;93(1):F32. [DOI] [PubMed] [Google Scholar]
- 148.Miletin J, Dempsey EM. Low superior vena cava flow on day 1 and adverse outcome in the very low birthweight infant. Archives of disease in childhood. Fetal and neonatal edition 2008;93(5):F371. [DOI] [PubMed] [Google Scholar]
- 149.Miletin J, Pichova K, Dempsey E. Bedside detection of low systemic flow in the very low birth weight infant on day 1 of life. Eur J Pediatr 2009;168(7):809–813 [DOI] [PubMed] [Google Scholar]
- 150.Kissack CM, Garr R, Wardle SP, Weindling AM. Cerebral Fractional Oxygen Extraction in Very Low Birth Weight Infants Is High When There Is Low Left Ventricular Output and Hypocarbia but Is Unaffected by Hypotension. Pediatric research 2004;55(3):400–405 [DOI] [PubMed] [Google Scholar]