Abstract
This review on the mechanisms of neuroinflammation following subarachnoid hemorrhage will focus mainly on Toll like Receptor 4 (TLR4), Heme Oxygenase-1 (HO-1), and the role of microglia and macrophages in this process. Vasospasm has long been the focus of research in SAH, however clinical trials have shown that amelioration of vasospasm does not lead to an improved clinical outcome. This necessitates the need for novel avenues of research. Our work has demonstrated that microglial TLR4 and microglial HO-1, not only affects cognitive dysfunction, but also circadian dysrhythmia in a mouse model of SAH. To attempt to translate these findings, we have also begun investigating macrophages in the cerebrospinal fluid of SAH patients. The goal of this review is to provide an update on the role of TLR4, HO-1, and other signal transduction pathways in SAH-induced neuroinflammation.
Introduction
28,000 Americans will fall victim to an aneurysmal subarachnoid hemorrhage (SAH) this year, and one third of the survivors will have a poor cognitive outcome1–2. While this is a small number compared to the total number that will have a stroke, SAH accounts for a disproportionately large health care cost because many of the patients are relatively young compared to other kinds of stroke3–5. Furthermore, 65% of SAH patients will have severe constriction of their cerebral vasculature, otherwise known as vasospasm6–9. In these studies, vasospasm has been found to be independently associated with mortality and poor neurological outcome. Remarkably, clinical trials that succeeded in reducing vasospasm showed no improvement in mortality or neurological outcome of SAH patients10–13. Perhaps this is not surprising when one realizes that vasospasm could be an epiphenomenon of disease severity, and that treatments aimed solely at this sequalae of SAH do not address the underlying red blood cell (RBC)-induced cerebral inflammation that persists.
The neuronal damage seen after SAH could be indirectly caused by an immune response initiated by danger proteins from lysed red blood cells. Several pro-inflammatory molecules such as heme, methemoglobin, and high mobility group box 1 bind to TLR4 and induce an inflammatory response14–16. On the other hand, the presence of heme oxygenase-1 (HO-1) in microglia reduces inflammation by degrading heme and producing carbon monoxide (CO). CO has been found to play an important neuroprotective role by clearing subarachnoid blood17.
Innate Immune Responses to SAH
Macrophages are a member of the innate immune system, and one of the professional antigen presenting cells (APCs). Until recently, it was thought that all macrophages were derived from circulating monocytes, which in turn were generated by the bone marrow. Elegant experiments now reveal that tissue resident macrophages, such as Kupffer cells, Langerhans cells, and microglia are derived from the embryonic yolk sac; whereas, circulating monocytes which after invasion of tissues become macrophages, are derived from the bone marrow18. Our work has shown that in a mouse model of SAH, microglia seem to have both protective and deleterious roles, depending on the time frame. Early in SAH, microglia seem to have a more deleterious role and eliminating them decreased neuronal apoptosis. Later on, neuronal apoptosis seemed to be independent of the existence of microglia19. Furthermore, at least with respect to microglial heme oxygenase, a protective role was observed, which will be described in detail in the following section17. Using mouse chimeras, where only peripheral marrow was ablated and reconstituted with green fluorescent protein-tagged leukocytes, our group found no significant GFP infiltrate 7 days after SAH induction17. This would suggest that microglia, at least at 7 days, are the only critical mediators of neuroinflammation in SAH.
On the other hand, others have shown an important role for neutrophils in SAH. Early in SAH, neutrophil depletion via Ly-6G demonstrated normal cortical perfusion compared to neutrophil-intact, SAH mice20. This neutrophil-induced cortical hypoperfusion is thought to be mediated by prostaglandin F2α21. Other studies have shown that the myeloperoxidase produced by neutrophils is found in human cerebral aneurysms and facilitates rupture of cerebral aneurysms in a mouse model. Other groups have found macrophages to be critical in aneurysm formation and rupture. None of these observations are mutually exclusive. Likely, many, if not all, components of the innate immune system have a part in neuroinflammation and possibly even neuroprotection, at different times, after SAH22–25.
Adaptive Immune Responses to SAH
In contrast to innate immune responses, the adaptive immune system is based on antigen specific receptors such as T cell receptors and immunoglobulins. Therefore, several days are required to allow for memory of specific antigen and antigen-driven clonal cell expansion26. In ischemic stroke, adaptive immune responses can be activated by multiple mediators generated by the innate immune system, leading to autoimmunity. With respect to hemorrhagic stroke, little has been done with respect to the adaptive immune system. Only one preclinical study has shown neuroprotective effects of statins via upregulation of regulatory T lymphocytes in rodent SAH models27. Furthermore, only two clinical studies have shown proliferation of CD4+ and CD8+ T cells in CSF and peripheral blood of SAH patients28,29. Due to the paucity of research, the clinical implications of the adaptive immune system in SAH remain unexplored.
Delayed Neurological Deficits
About 30% of surviving SAH patients will have delayed neurological deficits (DND)30. DND generally occurs 3–14 days after aneurysm rupture and carries a high morbidity and mortality 2,31. While DND was thought to be a direct result of large vessel vasospasm, evidence now exists that vasospasm can occur independently of DND10–13, 32. Likewise, DND can occur in the absence of vasospasm; this is where RBC-induced cerebral inflammation, as well as cortical spreading depression and microcirculatory dysfunction could be culprits in DND, based on both clinical and pre-clinical studies10–13, 19, 30, 33, 34.
Toll-Like Receptor 4 Pathway
Toll-like receptors (TLRs) are membrane-bound proteins that belong to the pattern recognition receptor (PRR) family, are ubiquitously expressed, and trigger an innate immune response when bound to their respective ligands35. Toll-like Receptor 4 (TLR4), in an SAH mouse model, is predominantly expressed on antigen presenting cells (APC), such as microglia and macrophages, although it is also expressed to a lesser extent on astrocytes and neurons19.
TLR4 recognizes a wide range of pathogenic components known as DAMPs (Damage Associated Molecular Patterns), with lipopolysaccharide (LPS) being the canonical agonist, as well as endogenous molecules such as heme and fibrinogen which are released during SAH35. The activation of TLR4 leads to the synthesis of pro-inflammatory cytokines, chemokines, and the expression of co-stimulatory molecules35. Thus, since neuroinflammation is a consequence of SAH, the study of TLR4-mediated inflammation has drawn interest. Of note, heme has been shown to be a specific agonist of TLR4 expressed on APCs, stressing the need for further understanding of microglia in the heme-induced cerebral inflammatory response (CIR) after SAH 25. Heme also has TLR4-independent effects that could contribute to the CIR after SAH such as an oxidative burst, increased neutrophil recruitment, and increased HO-1 expression25. Our lab has shown that heme induces a significant amount of neuronal apoptosis in a mouse model of SAH, compared to LPS stimulation. These findings are not entirely surprising given that the toll receptor associated activator of interferon (TRIF) pathway, via interferon expression, does exert anti-apoptotic effects19,36,37.
Among all the TLRs, TLR4 is unique in the sense that it can signal through both the myeloid differentiation primary-response protein 88 (MyD88) and the TRIF pathways to induce inflammatory responses38. Using an SAH mouse model, it was shown that in the early phase of SAH, neuronal apoptosis was mostly TLR4-MyD88-dependent and microglial-dependent, whereas, during late phase of SAH, neuronal apoptosis was largely TRIF-dependent and microglia-independent. This bimodal pattern of cerebral injury is important because it demonstrates that delayed neurological injury can occur in a mouse model of SAH as well13,19.
Both MyD88 and TRIF pathways trigger the expression of nuclear factor-κB (NF-κB), a key transcriptional regulator of inflammatory-related genes38. However, unlike MyD88, TRIF also has the ability to induce interferon response elements, thereby producing anti-apoptotic interferons. This anti-apoptotic effect of TRIF ensures that inflammation from NF-kB activation will be long lasting38. NF-κB, in turn, triggers the transcription of pro-inflammatory genes such as tumor necrosis factor (TNF-α), interleukin-1β (IL-1β), and intercellular adhesion molecule-1 (ICAM-1). TNF-α can induce (RAS-related C3 Botulinum Toxin Substrate-1) Rac-1-mediated oxidative stress and vasoconstriction39. Moreover, increased levels of TNF-α in brain interstitial fluid were found to correlate with worsened cerebral vasospasm40. IL-1β can also induce apoptosis and cyclooxygenase-2-facilitated inflammation. Finally, increased ICAM-1 is but one of a multitude of endothelial proteins that can be upregulated in response to inflammation and is thought to have a critical role in microcirculatory dysfunction41.
Mitogen-activated protein kinases (MAPKs)
The MyD88-dependent pathway also has effects on cell survival via the activation of mitogen-activated protein kinases (MAPKs), such as the signal regulated kinase (ERK), p38, and c-Jun N-terminal kinase (JNK), which in turn leads to stimulation of the transcription factor activator protein-1 (AP-1)42. MAPKs are directly involved in many cellular responses to a vast range of stimuli such as mitogens, heat shock, and inflammation43. Furthermore, the MAPK pathway seems to play a crucial role in the CIR. In a rat model of SAH, the MAPK pathway was critical to the regulation of cerebral blood flow44,45. Conversely, both the p38 and JNK MAPK pathways were also found to induce post-SAH neuronal and endothelial cell apoptosis, inflammatory cytokine expression, and facilitate the CIR along with delayed neuronal injury44,46,47. To elucidate the role of MAPK pathways in post-SAH injury, recombinant osteopontin (r-OPN) was used in a rodent model. r-OPN enhances the endogenous MAPK inhibitor, MKP-1, which suppresses the phosphorylation of MAPKs, caldesmon, and heat shock protein 27 in spastic cerebral arteries of a rat model at 24 hours post-SAH 48. Interestingly, it was shown that administration of r-OPN prior to SAH prevents vasospasm and neurological impairments at 24–72 hours post-SAH, in a rat model 48.
High mobility group box 1
HMGB1 is a DNA binding protein that regulates gene expression. It is passively released during necrosis by cells in order to alert neighboring cells of cellular damage49. Some immune cells such as monocytes, macrophages, and dendritic cells secrete HMGB1 in an active manner, in response to various cellular stresses50–52. Important receptors such as the receptor for advanced glycation end products (RAGE), TLR2, TLR4, and TLR9 have been found to participate in HMGB1 signaling. RAGE is a receptor found at low levels in normal tissues, but upregulated at sites where its ligands concentrate53. HMGB1 signaling through RAGE upregulates the production of chemotaxins and cytokines via NF-kB54,55. The activation of TLR2 and TLR4 by HMGB1 leads to the upregulation of NF-kB56–58; hence, HMGB1 likely leads to the release of pro-inflammatory cytokines through these pathways. Furthermore, the interaction of IL-1β, IFNγ, and TNFα with HMGB1 leads to an amplified inflammatory response compared with HMGB1 stimulation alone59. In addition, HMGB1 stimulates the release of reactive oxygen species by neutrophils via a TLR4-dependent activation of (Nicotinamide adenine dinucleotide phosphate) NADPH oxidase60 which results in further release of cytokines51,54. HMGB1 also mediates the adhesion of inflammatory cells to the endothelial lumen by increasing the expression of ICAM-1 and (Vascular Cell Adhesion Molecule ) VCAM-161,62.
It has been shown that HMGB1 is released from the nucleus of neuronal cells to the extracellular space during ischemic and traumatic brain injuries, and that the targeting of HMGB1 with monoclonal antibodies (mAb) reduces brain injury by preventing the breakdown of the blood-brain barrier (BBB) and reducing the inflammatory response63–65 In addition, data from several clinical studies indicate that HMGB1 could play a critical role in CIR and DND after SAH due to the high levels of HMGB1 found in plasma during the post-SAH period66–68.
Finally, in a rat SAH model, administration of anti-HMGB1 antibodies decreased vasospasm by inhibiting HMGB1 translocation into arterial smooth muscle cells, thereby suppressing vasoconstriction and vascular inflammatory responses69.
Heme Oxygenase (HO)
HO is an enzyme that catalyzes the degradation of heme. There are two isoforms of heme oxygenase (HO): HO-1 and HO-2. HO-1, the inducible form, is found in neuronal cells, glial cells, and macrophages such as microglia; whereas, HO-2 is constitutively expressed in neuronal cells and vascular endothelial cells70. Our lab demonstrated that microglial HO-1 is necessary to alleviate neuronal cell death and cognitive dysfunction, as well as facilitate erythrophagocytosis17. Free heme released into the subarachnoid space during SAH is metabolized by HO-1, releasing iron (Fe2+), biliverdin, and carbon monoxide (CO)71.Free iron is thought to cause cell membrane damage via free radicals and the Fenton reaction72. Previous studies have shown a causal relationship between free iron and brain injury following SAH73. Moreover, it has been shown that treatment with the iron-chelating agent, deferoxamine (DFX), decreases brain edema, oxidative stress, and neuronal apoptosis;74,75 further corroborating the damaging role of free iron72,76. Our lab has shown that intrathecal administration of DFX may mediate some of its neuroprotective effects by increasing the expression of microglial HO-1, as well as reducing neuronal apoptosis, reactive mitochondrial species, and improving cognitive function34.
To further elucidate the neuroprotective role of microglial HO-1 after SAH, we investigated one of the byproducts of heme metabolism: CO. Despite the nefarious reputation of CO, we found it to be the neuroprotective byproduct of heme catabolism by microglial HO-117. This was elucidated by exposing mice lacking microglial HO-1 to gaseous CO, after SAH, which resulted in reduced injury, and improved cognitive function. This could be due to increasing erythrophagocytosis, although CO’s effects are pleiotropic due to its gaseous nature. To this end we found that administration of gaseous CO aids in normalizing circadian dysrhythmia after SAH77. We found that SAH induced at dawn compared to sunset resulted in worse cognitive function, more neuronal apoptosis, and an increased inflammatory milieu; all this correlated with reduced microglial HO-1 expression at dawn and was rescued with exogenous CO administration77.
Additionally, CO seems to function similarly to nitric oxide (NO) as a vasodilator, neurotransmitter, and platelet aggregation inhibitor, as well as serving other anti-inflammatory roles78. It is thought to act via soluble guanylyl cyclase (sGC), as well as cyclic GMP (cGMP), and BKca channels leading to vasodilation in the vascular smooth muscle cells,79–83 and thus the reduction of vasoconstriction. In addition, CO seems to inhibit TLR 2, 4, 5, and 9 signaling pathways in macrophages by interrupting their recruitment to membrane rafts84. These rafts, are specialized lipid domains that contribute to immune signal transduction. CO was shown to inhibit TLR trafficking to lipid rafts by suppressing NADPH oxidase-dependent ROS generation84.
CD163
Haptoglobin is a protein found in the plasma that binds free hemoglobin (Hb) released from red blood cells forming the hemoglobin-haptoglobin complex85. Cluster of differentiation 163 (CD163) was found to be a specific receptor of the hemoglobin-haptoglobin complex and is exclusively expressed on monocytes and macrophages. CD163 is involved in the clearance and endocytosis of hemoglobin-haptoglobin complexes, and thus it may protect tissues from hemoglobin-mediated oxidative damage, serving as an alternative to the heme-TLR4/HO-1 pathway. To determine the potential role of CD163 in SAH patients, our lab performed flow cytometry on the cerebrospinal fluid (CSF) from SAH patients and found increased expression of CD163 on macrophages from SAH patients compared to unruptured aneurysm controls. To verify these findings, we then performed immunohistochemistry on the CSF macrophages from SAH patients with increasing modified Fisher scales, where the Fisher scale refers to the red blood cell burden of an SAH patient noted on CT scan. As expected, we found increased CD163 expression on macrophages which had phagocytosed more blood. Surprisingly, we found an inverse correlation between CSF macrophage CD163 expression measured on day 1 after SAH, and 90 day outcome of these patients as measured by the modified Rankin Scale (mRS). That is, increasing CD163 expression seemed to correlate with improved neurological outcome, or a lower mRS. With further study, CSF macrophage CD163 expression may prove to be an important biomarker for SAH prognostication86. Understanding why this is so might lead to novel immunotherapies.
Anti-inflammatory treatments in SAH patients
There is great interest in identifying an inflammatory biomarker that is associated with delayed neurological deficits. Despite the fact that no biomarker has been validated to this end, a number of small scale clinical trials have attempted to use various anti-inflammatory agents in SAH, but to no avail. Acetylsalicylic acid87, steroids88–90, various non-steroid anti-inflammatory agents91, immunosuppressants92,93, and IL-1 receptor antagonists94 have all been failures. There are many potential explanations for these failures, but perhaps a more directed immune based approach might be necessary, mirroring novel therapies in the oncology world like chimeric antigen receptor T cells, but for the innate immune system.
Conclusion
The mechanisms behind the adverse sequalae of SAH are still poorly understood; although a summary of the cerebral inflammatory signal transduction pathways highlighted in this review are presented in Figure 1. While neuroinflammation itself is well known to cause cognitive dysfunction in diseases such as multiple sclerosis, post-stroke recrudescence, and even systemic bacteremia; an exact mechanism behind the cognitive dysfunction in SAH has yet to be elucidated. Moreover, the high mortality rate of SAH patients makes it imperative to find new and better therapeutic treatments. SAH neuroinflammation seems to be caused primarily by the breakdown of hemoglobin in the subarachnoid space, which leads to the release of heme. Heme works as a potent TLR4 activator, and also activates the MyD88 and TRIF cascades. Microglia, macrophages, and neutrophils likely all have roles in potentiating heme-mediated inflammation. While the involvement of the adaptive immune system in hemorrhagic stroke is not well-understood, it could be important as well. RAGE, MAPK, and HMGB1 are involved in the initiation and propagation of inflammation, while CD163 and CO quell the inflammatory response. Despite the recent discovery of the neuroprotective effects presented by DFX and CO, there is still a clear need to further understand the neuroinflammation in SAH.
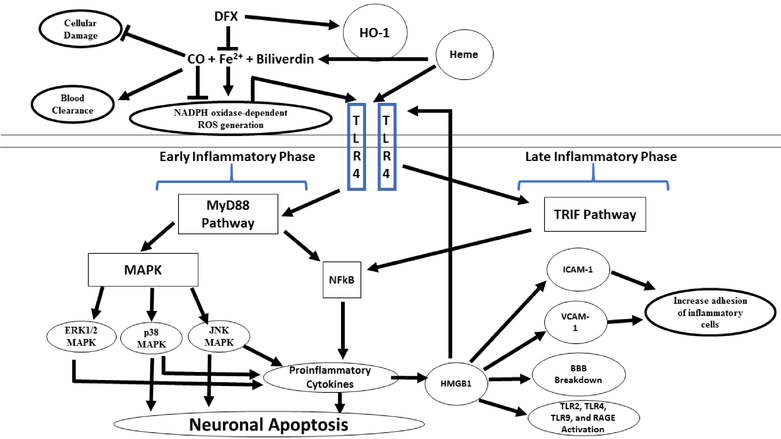
Figure 1. Heme metabolism and the microglial toll-like receptor 4 (TLR4) signaling pathway following SAH:
In the subarachnoid space, free heme is metabolized by heme oxygenase (HO)-1, releasing iron (Fe2+), biliverdin, and carbon monoxide (CO). Deferoxamine (DFX), an iron-chelating agent, decreases the oxidative toxicity of free iron and increase the HO-1 mediated neuroprotective effect. Low dose CO also has neuroprotective effect by increasing erythrophagocytosis. Heme initiates microglial TLR4 signaling and activates the myeloid differentiation primary-response protein 88-dependent (MyD88) in early phase of SAH and the toll receptor associated activator of interferon-dependent (TRIF) cascade in late phase of SAH. MyD88 triggers the expression of nuclear factor-κB (NF-κB) and mitogen-activate protein kinase (MAPK), resulting in apoptosis and pro-inflammatory gene expression. BBB; brain blood barrier, ICAM-1; intercellular adhesion molecule 1 and, VCAM-1; vascular cell adhesion molecule 1
Acknowledgments
Funding: Dr. Hanafy is funded by the NINDS (R21 NS099606-02 and 1R01NS109174-01) and the American Heart Association Grant-in-Aid Award #17GRNT33670058.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest
Disclosures: The authors have nothing to disclose.
References
- 1.Etminan N, Chang HS, Hackenberg K, de Rooij NK, Vergouwen MDI, Rinkel GJE, Algra A. Worldwide Incidence of Aneurysmal Subarachnoid Hemorrhage According to Region, Time Period, Blood Pressure, and Smoking Prevalence in the Population: A Systematic Review and Meta-analysis. JAMA Neurol (2019)doi: 10.1001/jamaneurol.2019.0006. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bederson JB, Connolly ES, Batjer HH, Dacey RG, Dion JE, Diringer MN, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke. 2009;40:994–1025. [DOI] [PubMed] [Google Scholar]
- 3.Taylor TN, Davis PH, Torner JC, Holmes J, Meyer JW, Jacobson MF. Lifetime cost of stroke in the United States. Stroke. 1996;27:1459–1466. [DOI] [PubMed] [Google Scholar]
- 4.Reaven NL, Lovett JE, Funk SE. Brain injury and fever: hospital length of stay and cost outcomes. J Intensive Care Med. 2009;24:131–139. [DOI] [PubMed] [Google Scholar]
- 5.Bosetti F, Koenig JI, Ayata C, Back SA, Becker K, Broderick JP, et al. Translational Stroke Research: Vision and Opportunities. Stroke. 2017;48:2632–2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dankbaar JW, Rijsdijk M, van der Schaaf IC, Velthuis BK, Wermer MJH, Rinkel GJE. Relationship between vasospasm, cerebral perfusion, and delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. Neuroradiology. 2009;51:813–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dankbaar JW, de Rooij NK, Velthuis BK, Frijns CJM, Rinkel GJE, van der Schaaf IC. Diagnosing delayed cerebral ischemia with different CT modalities in patients with subarachnoid hemorrhage with clinical deterioration. Stroke. 2009;40:3493–3498. [DOI] [PubMed] [Google Scholar]
- 8.de Rooij NK, Rinkel GJE, Dankbaar JW, Frijns CJM. Delayed cerebral ischemia after subarachnoid hemorrhage: a systematic review of clinical, laboratory, and radiological predictors. Stroke. 2013;44:43–54. [DOI] [PubMed] [Google Scholar]
- 9.Vergouwen MDI, Participants in the International Multi-Disciplinary Consensus Conference on the Critical Care Management of Subarachnoid Hemorrhage. Vasospasm versus delayed cerebral ischemia as an outcome event in clinical trials and observational studies. Neurocrit Care. 2011;15:308–311. [DOI] [PubMed] [Google Scholar]
- 10.Macdonald RL, Higashida RT, Keller E, Mayer SA, Molyneux A, Raabe A, et al. Clazosentan, an endothelin receptor antagonist, in patients with aneurysmal subarachnoid haemorrhage undergoing surgical clipping: a randomised, double-blind, placebo-controlled phase 3 trial (CONSCIOUS-2). Lancet Neurol. 2011;10:618–625. [DOI] [PubMed] [Google Scholar]
- 11.Macdonald RL, Higashida RT, Keller E, Mayer SA, Molyneux A, Raabe A, et al. Randomized trial of clazosentan in patients with aneurysmal subarachnoid hemorrhage undergoing endovascular coiling. Stroke. 2012;43:1463–1469. [DOI] [PubMed] [Google Scholar]
- 12.Macdonald RL, Kassell NF, Mayer S, Ruefenacht D, Schmiedek P, Weidauer S, et al. Clazosentan to overcome neurological ischemia and infarction occurring after subarachnoid hemorrhage (CONSCIOUS-1): randomized, double-blind, placebo-controlled phase 2 dose-finding trial. Stroke. 2008;39:3015–3021. [DOI] [PubMed] [Google Scholar]
- 13.Vergouwen MDI, Ilodigwe D, Macdonald RL. Cerebral infarction after subarachnoid hemorrhage contributes to poor outcome by vasospasm-dependent and -independent effects. Stroke. 2011;42:924–929. [DOI] [PubMed] [Google Scholar]
- 14.Figueiredo RT, Fernandez PL, Mourao-Sa DS, Porto BN, Dutra FF, Alves LS, et al. Characterization of heme as activator of Toll-like receptor 4. J. Biol. Chem 2007;282:20221–20229. [DOI] [PubMed] [Google Scholar]
- 15.Kwon MS, Woo SK, Kurland DB, Yoon SH, Palmer AF, Banerjee U, et al. Methemoglobin is an endogenous toll-like receptor 4 ligand-relevance to subarachnoid hemorrhage. Int J Mol Sci 2015;16:5028–5046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takizawa T, Shibata M, Kayama Y, Shimizu T, Toriumi H, Ebine T, et al. High-mobility group box 1 is an important mediator of microglial activation induced by cortical spreading depression. J. Cereb. Blood Flow Metab 2017;37:890–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schallner N, Pandit R, LeBlanc R, Thomas AJ, Ogilvy CS, Zuckerbraun BS, et al. Microglia regulate blood clearance in subarachnoid hemorrhage by heme oxygenase-1. J. Clin. Invest 2015;125:2609–2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gomez Perdiguero E, Klapproth K, Schulz C, Busch K, Azzoni E, Crozet L, et al. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature. 2015;518:547–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanafy KA. The role of microglia and the TLR4 pathway in neuronal apoptosis and vasospasm after subarachnoid hemorrhage. J Neuroinflammation. 2013;10:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Provencio JJ, Altay T, Smithason S, Moore SK, Ransohoff RM. Depletion of Ly6G/C(+) cells ameliorates delayed cerebral vasospasm in subarachnoid hemorrhage. J Neuroimmunol. 2011;232:94–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neulen A, Pantel T, Kosterhon M, Kramer A, Kunath S, Petermeyer M, Moosmann B, Lotz J, Kantelhardt SR, Ringel F, Thal SC. Neutrophils mediate early cerebral cortical hypoperfusion in a murine model of subarachnoid haemorrhage. Sci Rep. 2019;11:8460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jassam YN, Izzy S, Whalen M, McGavern DB, El Khoury J. Neuroimmunology of Traumatic Brain Injury: Time for a Paradigm Shift. Neuron. 2017;95:1246–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kanazawa M, Ninomiya I, Hatakeyama M, Takahashi T, Shimohata T. Microglia and Monocytes/Macrophages Polarization Reveal Novel Therapeutic Mechanism against Stroke. Int J Mol Sci. 2017;18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qin C, Fan W-H, Liu Q, Shang K, Murugan M, Wu L-J, et al. Fingolimod Protects Against Ischemic White Matter Damage by Modulating Microglia Toward M2 Polarization via STAT3 Pathway. Stroke. 2017;48:3336–3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yao X, Liu S, Ding W, Yue P, Jiang Q, Zhao M, et al. TLR4 signal ablation attenuated neurological deficits by regulating microglial M1/M2 phenotype after traumatic brain injury in mice. J. Neuroimmunol 2017;310:38–45. [DOI] [PubMed] [Google Scholar]
- 26.Abbas AK Basic Immunology Updated Edition: Functions and Disorders of the Immune System Ch. 5 (Saunders, 2010). [Google Scholar]
- 27.Ayer RE, Ostrowski RP, Sugawara T, Ma Q, Jafarian N, Tang J, Zhang JH. Statin-induced T-lymphocyte modulation and neuroprotection following experimental subarachnoid hemorrhage. Acta Neurochir Suppl. 2013;115:259–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mathiesen T, Andersson B, Loftenius A, von Holst H. Increased interleukin-6 levels in cerebrospinal fluid following subarachnoid hemorrhage. J Neurosurg 78:562–567 (1993). [DOI] [PubMed] [Google Scholar]
- 29.Moraes L, Grille S, Morelli P, Mila R, Trias N, Brugnini A, LLuberas N, Biestro A, Lens D. Immune cells subpopulations in cerebrospinal fluid and peripheral blood of patients with Aneurysmal Subarachnoid Hemorrhage. Springerplus 23:195 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vergouwen MDI & Participants in the International Multi-Disciplinary Consensus Conference on the Critical Care Management of Subarachnoid Hemorrhage. Vasospasm versus delayed cerebral ischemia as an outcome event in clinical trials and observational studies. Neurocrit Care 15, 308–311 (2011). [DOI] [PubMed] [Google Scholar]
- 31.Diringer MN et al. Critical care management of patients following aneurysmal subarachnoid hemorrhage: recommendations from the Neurocritical Care Society’s Multidisciplinary Consensus Conference. Neurocrit Care 15, 211–240 (2011). [DOI] [PubMed] [Google Scholar]
- 32.Pegoli M, Mandrekar J, Rabinstein AA & Lanzino G Predictors of excellent functional outcome in aneurysmal subarachnoid hemorrhage. J. Neurosurg 122, 414–418 (2015). [DOI] [PubMed] [Google Scholar]
- 33.Schneider UC et al. Microglia inflict delayed brain injury after subarachnoid hemorrhage. Acta Neuropathol. 130, 215–231 (2015). [DOI] [PubMed] [Google Scholar]
- 34.LeBlanc RH, Chen R, Selim MH, Hanafy KA. Heme oxygenase-1-mediated neuroprotection in subarachnoid hemorrhage via intracerebroventricular deferoxamine. J Neuroinflammation. 2016;13:244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vaure C, Liu Y A Comparative Review of Toll-Like Receptor 4 Expression and Functionality in Different Animal Species. Front Immunol 5, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shim J-H, Xiao C, Paschal AE, Bailey ST, Rao P, Hayden MS, et al. TAK1, but not TAB1 or TAB2, plays an essential role in multiple signaling pathways in vivo. Genes Dev. 2005;19:2668–2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blander JM. A long-awaited merger of the pathways mediating host defence and programmed cell death. Nature Reviews Immunology. 2014;14:601–618. [DOI] [PubMed] [Google Scholar]
- 38.O’Neill LAJ, Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat. Rev. Immunol 2007;7:353–364. [DOI] [PubMed] [Google Scholar]
- 39.Vecchione C, Frati A, Di Pardo A, Cifelli G, Carnevale D, Gentile MT, et al. Tumor necrosis factor-alpha mediates hemolysis-induced vasoconstriction and the cerebral vasospasm evoked by subarachnoid hemorrhage. Hypertension. 2009;54:150–156. [DOI] [PubMed] [Google Scholar]
- 40.Hanafy KA, Stuart RM, Khandji AG, Connolly ES, Badjatia N, Mayer SA, et al. Relationship between brain interstitial fluid tumor necrosis factor-α and cerebral vasospasm after aneurysmal subarachnoid hemorrhage. J Clin Neurosci. 2010;17:853–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kong D-H, Kim YK, Kim MR, Jang JH & Lee S Emerging Roles of Vascular Cell Adhesion Molecule-1 (VCAM-1) in Immunological Disorders and Cancer. Int J Mol Sci 19, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fang H, Wang P-F, Zhou Y, Wang Y-C, Yang Q-W. Toll-like receptor 4 signaling in intracerebral hemorrhage-induced inflammation and injury. J Neuroinflammation. 2013;10:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pearson G et al. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr. Rev 22, 153–183 (2001). [DOI] [PubMed] [Google Scholar]
- 44.Sun J & Nan G The Mitogen-Activated Protein Kinase (MAPK) Signaling Pathway as a Discovery Target in Stroke. J. Mol. Neurosci 59, 90–98 (2016). [DOI] [PubMed] [Google Scholar]
- 45.Maddahi A, Ansar S, Chen Q & Edvinsson L Blockade of the MEK/ERK pathway with a raf inhibitor prevents activation of pro-inflammatory mediators in cerebral arteries and reduction in cerebral blood flow after subarachnoid hemorrhage in a rat model. J. Cereb. Blood Flow Metab 31, 144–154 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang X, Zhao XD, Shi JX & Yin HX Inhibition of the p38 mitogen-activated protein kinase (MAPK) pathway attenuates cerebral vasospasm following experimental subarachnoid hemorrhage in rabbits. Ann. Clin. Lab. Sci 41, 244–250 (2011). [PubMed] [Google Scholar]
- 47.Huang L et al. Inhibitory effects of p38 inhibitor against mitochondrial dysfunction in the early brain injury after subarachnoid hemorrhage in mice. Brain Res. 1517, 133–140 (2013). [DOI] [PubMed] [Google Scholar]
- 48.Suzuki H, Hasegawa Y, Chen W, Kanamaru K & Zhang JH Recombinant osteopontin in cerebral vasospasm after subarachnoid hemorrhage. Ann. Neurol 68, 650–660 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scaffidi P, Misteli T & Bianchi ME Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 418, 191–195 (2002). [DOI] [PubMed] [Google Scholar]
- 50.Wang H et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science 285, 248–251 (1999). [DOI] [PubMed] [Google Scholar]
- 51.Lotze MT & Tracey KJ High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat. Rev. Immunol 5, 331–342 (2005). [DOI] [PubMed] [Google Scholar]
- 52.Abraham E, Arcaroli J, Carmody A, Wang H & Tracey KJ HMG-1 as a mediator of acute lung inflammation. J. Immunol 165, 2950–2954 (2000). [DOI] [PubMed] [Google Scholar]
- 53.Chavakis T, Bierhaus A & Nawroth PP RAGE (receptor for advanced glycation end products): a central player in the inflammatory response. Microbes Infect. 6, 1219–1225 (2004). [DOI] [PubMed] [Google Scholar]
- 54.Palumbo R et al. Cells migrating to sites of tissue damage in response to the danger signal HMGB1 require NF-κB activation. J Cell Biol 179, 33–40 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Park JS et al. Activation of gene expression in human neutrophils by high mobility group box 1 protein. Am. J. Physiol., Cell Physiol 284, C870–879 (2003). [DOI] [PubMed] [Google Scholar]
- 56.Park JS et al. High mobility group box 1 protein interacts with multiple Toll-like receptors. Am. J. Physiol., Cell Physiol. 290, C917–924 (2006). [DOI] [PubMed] [Google Scholar]
- 57.Kokkola R et al. RAGE is the major receptor for the proinflammatory activity of HMGB1 in rodent macrophages. Scand. J. Immunol 61, 1–9 (2005). [DOI] [PubMed] [Google Scholar]
- 58.Park JS et al. Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J. Biol. Chem 279, 7370–7377 (2004). [DOI] [PubMed] [Google Scholar]
- 59.Sha Y, Zmijewski J, Xu Z & Abraham E HMGB1 develops enhanced proinflammatory activity by binding to cytokines. J. Immunol 180, 2531–2537 (2008). [DOI] [PubMed] [Google Scholar]
- 60.Fan J, Li Y, Levy RM, Fan JJ, Hackam DJ, Vodovotz Y, et al. Hemorrhagic shock induces NAD(P)H oxidase activation in neutrophils: role of HMGB1-TLR4 signaling. J. Immunol 2007;178:6573–6580. [DOI] [PubMed] [Google Scholar]
- 61.Fiuza C, Bustin M, Talwar S, Tropea M, Gerstenberger E, Shelhamer JH, et al. Inflammation-promoting activity of HMGB1 on human microvascular endothelial cells. Blood. 2003;101:2652–2660. [DOI] [PubMed] [Google Scholar]
- 62.Treutiger CJ, Mullins GE, Johansson A-SM, Rouhiainen A, Rauvala HME, Erlandsson-Harris H, et al. High mobility group 1 B-box mediates activation of human endothelium. J. Intern. Med 2003;254:375–385. [DOI] [PubMed] [Google Scholar]
- 63.Zhang J, Takahashi HK, Liu K, Wake H, Liu R, Maruo T, et al. Anti-high mobility group box-1 monoclonal antibody protects the blood-brain barrier from ischemia-induced disruption in rats. Stroke. 2011;42:1420–1428. [DOI] [PubMed] [Google Scholar]
- 64.Okuma Y, Liu K, Wake H, Zhang J, Maruo T, Date I, et al. Anti-high mobility group box-1 antibody therapy for traumatic brain injury. Ann. Neurol 2012;72:373–384. [DOI] [PubMed] [Google Scholar]
- 65.Liu K, Mori S, Takahashi HK, Tomono Y, Wake H, Kanke T, et al. Anti-high mobility group box 1 monoclonal antibody ameliorates brain infarction induced by transient ischemia in rats. FASEB J. 2007;21:3904–3916. [DOI] [PubMed] [Google Scholar]
- 66.Zhu X-D, Chen J-S, Zhou F, Liu Q-C, Chen G, Zhang J-M. Relationship between plasma high mobility group box-1 protein levels and clinical outcomes of aneurysmal subarachnoid hemorrhage. J Neuroinflammation. 2012;9:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nakahara T, Tsuruta R, Kaneko T, Yamashita S, Fujita M, Kasaoka S, et al. High-Mobility Group Box 1 Protein in CSF of Patients with Subarachnoid Hemorrhage. Neurocrit Care. 2009;11:362. [DOI] [PubMed] [Google Scholar]
- 68.Murakami K, Koide M, Dumont TM, Russell SR, Tranmer BI, Wellman GC. Subarachnoid Hemorrhage Induces Gliosis and Increased Expression of the Pro-inflammatory Cytokine High Mobility Group Box 1 Protein. Transl Stroke Res. 2011;2:72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Haruma J, Teshigawara K, Hishikawa T, Wang D, Liu K, Wake H, et al. Anti-high mobility group box-1 (HMGB1) antibody attenuates delayed cerebral vasospasm and brain injury after subarachnoid hemorrhage in rats. Scientific Reports. 2016;6:37755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sutherland BA et al. Cerebral heme oxygenase 1 and 2 spatial distribution is modulated following injury from hypoxia-ischemia and middle cerebral artery occlusion in rats. Neurosci. Res 65, 326–334 (2009). [DOI] [PubMed] [Google Scholar]
- 71.Kikuchi G, Yoshida T & Noguchi M Heme oxygenase and heme degradation. Biochem. Biophys. Res. Commun 338, 558–567 (2005). [DOI] [PubMed] [Google Scholar]
- 72.Loftspring MC Iron and early brain injury after subarachnoid hemorrhage. J. Cereb. Blood Flow Metab 30, 1791–1792 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gomes JA et al. Brain iron metabolism and brain injury following subarachnoid hemorrhage: iCeFISH-pilot (CSF iron in SAH). Neurocrit Care 21, 285–293 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yu Z-Q, Jia Y & Chen G Possible involvement of cathepsin B/D and caspase-3 in deferoxamine-related neuroprotection of early brain injury after subarachnoid haemorrhage in rats. Neuropathol. Appl. Neurobiol 40, 270–283 (2014). [DOI] [PubMed] [Google Scholar]
- 75.Lee J-Y et al. Hemoglobin and iron handling in brain after subarachnoid hemorrhage and the effect of deferoxamine on early brain injury. J. Cereb. Blood Flow Metab 30, 1793–1803 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Selim M Deferoxamine mesylate: a new hope for intracerebral hemorrhage: from bench to clinical trials. Stroke. 2009;40:S90–91. [DOI] [PubMed] [Google Scholar]
- 77.Schallner N, Lieberum JL, Gallo D, LeBlanc RH 3rd, Fuller PM, Hanafy KA, Otterbein LE. Carbon Monoxide Preserves Circadian Rhythm to Reduce the Severity of Subarachnoid Hemorrhage in Mice. Stroke. 2017. 48:2565–2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hanafy KA, Oh J, Otterbein LE. Carbon Monoxide and the brain: time to rethink the dogma. Curr. Pharm. Des 2013;19:2771–2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hou S, Xu R, Heinemann SH, Hoshi T. The RCK1 high-affinity Ca2+ sensor confers carbon monoxide sensitivity to Slo1 BK channels. Proc. Natl. Acad. Sci. U.S.A 2008;105:4039–4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jaggar JH, Li A, Parfenova H, Liu J, Umstot ES, Dopico AM, et al. Heme is a carbon monoxide receptor for large-conductance Ca2+-activated K+ channels. Circ. Res 2005;97:805–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kaide JI, Zhang F, Wei Y, Jiang H, Yu C, Wang WH, et al. Carbon monoxide of vascular origin attenuates the sensitivity of renal arterial vessels to vasoconstrictors. J. Clin. Invest 2001;107:1163–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang R, Wu L, Wang Z. The direct effect of carbon monoxide on KCa channels in vascular smooth muscle cells. Pflugers Arch. 1997;434:285–291. [DOI] [PubMed] [Google Scholar]
- 83.Wu L, Cao K, Lu Y, Wang R. Different mechanisms underlying the stimulation of K(Ca) channels by nitric oxide and carbon monoxide. J. Clin. Invest 2002;110:691–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nakahira K, Kim HP, Geng XH, Nakao A, Wang X, Murase N, et al. Carbon monoxide differentially inhibits TLR signaling pathways by regulating ROS-induced trafficking of TLRs to lipid rafts. J. Exp. Med 2006;203:2377–2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kristiansen M, Graversen JH, Jacobsen C, Sonne O, Hoffman HJ, Law SK, et al. : Identification of the haemoglobin scavenger receptor. Nature 409:198–201 (2001). [DOI] [PubMed] [Google Scholar]
- 86.Thomas A, Ogilvy CS, Griessenauer CJ, Hanafy KA. Macrophage CD163 expression in cerebrospinal fluid: association with subarachnoid hemorrhage outcome. J Neurosurg 1:1–7 (2018). [DOI] [PubMed] [Google Scholar]
- 87.Dorhout Mees SM, van den Bergh WM, Algra A, Rinkel GJ. Antiplatelet therapy for aneurysmal subarachnoid haemorrhage. Cochrane Database Syst Rev (4):CD006184 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chyatte D, Fode NC, Nichols DA, Sundt TM. Preliminary report: effects of high dose methylprednisolone on delayed cerebral ischemia in patients at high risk for vasospasm after aneurysmal subarachnoid hemorrhage. Neurosurgery 21:157–60 (1987). [DOI] [PubMed] [Google Scholar]
- 89.Gomis P, Graftieaux JP, Sercombe R, Hettler D, Scherpereel B, Rousseaux P. Randomized, double-blind, placebo-controlled, pilot trial of high-dose methylprednisolone in aneurysmal subarachnoid hemorrhage. J Neurosurg 112:681–8 (2010). [DOI] [PubMed] [Google Scholar]
- 90.Mohney N, Williamson CA, Rothman E, Ball R, Sheehan KM, Pandey AS, et al. A propensity score analysis of the impact of dexamethasone use on delayed cerebral ischemia and poor functional outcomes after subarachnoid hemorrhage. World Neurosurg 109:e655–61 (2018). [DOI] [PubMed] [Google Scholar]
- 91.Nassiri F, Ibrahim GM, Badhiwala JH, Witiw CD, Mansouri A, Alotaibi NM, et al. Propensity score-matched study of the use of non-steroidal anti-inflammatory agents following aneurysmal subarachnoid hemorrhage. Neurocrit Care 25:351–358 (2016). [DOI] [PubMed] [Google Scholar]
- 92.Manno EM, Gress DR, Ogilvy CS, Stone CM, Zervas NT. The safety and efficacy of cyclosporine A in the prevention of vasospasm in patients with Fisher grade 3 subarachnoid hemorrhages: a pilot study. Neurosurgery 40:289–93 (1997). [DOI] [PubMed] [Google Scholar]
- 93.Ryba M, Pastuszko M, Iwanska K, Bidzinski J, Dziewiecki C. Cyclosporine A prevents neurological deterioration of patients with SAH – a preliminary report. Acta Neurochir 112:25–27 (1991). [DOI] [PubMed] [Google Scholar]
- 94.Singh N, Hopkins SJ, Hulme S, Galea JP, Hoadley M, Vail A, et al. The effect intravenous interleukin-1 receptor antagonist on inflammatory mediators in cerebrospinal fluid after subarachnoid haemorrhage: a phase II randomised controlled trial. J Neuroinflammation 11:1 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]