Abstract
Breakthroughs in modern medicine have increased pediatric cancer survival rates throughout the last several decades. Despite enhanced cure rates, a subset of pediatric cancer survivors exhibit life-long psychological side effects. A large body of work has addressed potential mechanisms for secondary symptoms of anxiety, post-traumatic stress, impaired emotion regulation and cognitive deficits in adults. Yet, absent from many studies are the ways in which cancer treatment can impact the developing brain. Additionally, it remains less known whether typical neurobiological changes during adolescence and early adulthood may potentially buffer or exacerbate some of the known negative cancer survivorship outcomes. This review highlights genetic, animal, and human neuroimaging research across development. We focus on the neural circuitry associated with aversive learning, which matures throughout childhood, adolescence and early adulthood. We argue that along with other individual differences, the precise timing of oncological treatment insults on such neural circuitry may expose particular vulnerabilities for pediatric cancer patients. We also explore other moderators of treatment outcomes, including genetic polymorphisms and neural mechanisms underlying memory and cognitive control. We discuss how neural maturation extending into young adulthood may also provide a sensitive period for intervention to improve psychological and cognitive outcomes in pediatric cancer survivors.
1. Introduction
Adolescence and early adulthood are defined by physiological changes, social transitions, and increased independence (Foulkes and Blakemore, 2018; Fuhrmann et al., 2015; Smith et al., 2013; Vijayakumar et al., 2018). Findings from animal models and human imaging have provided insight about the neurobiological changes during the transitions into and out of adolescence (Crone and Dahl, 2012; Doremus-Fitzwater and Spear, 2016). Considerable work from our groups and colleagues, as well as many others, have demonstrated that adolescence is marked by increased affective lability relative to childhood and adulthood. Animal and human work has shown greater sensitivity to emotional cues during adolescence and accompanying dynamic changes in a neural circuit involving the prefrontal cortex (PFC) and connections with the amygdala and hippocampus (Casey, 2015; Cracco et al., 2017; Gee and Casey, 2015; Silvers et al., 2015; Tottenham and Galvan, 2016).
Advances in medicine, along with breakthroughs in scientific research, have enabled remarkable increases in overall survival for patients with a wide range of childhood and adolescent cancers. The average survival rate for pediatric cancer has gone from 50% in the 1970s to upwards of 80–85% in the past five years (Curtin et al., 2016; Gatta et al., 2009). It is estimated that there will be over 500,000 survivors of childhood and adolescent cancers living in the United States alone by 2020 (Armstrong et al., 2016). While cure is - and should unequivocally be - the number one priority of pediatric oncology, genetic, molecular, and developmental cognitive neuroscience research can provide critical frameworks for understanding the variability in psychological outcomes and how to improve the quality of life in survivors.
The purpose of this review is to discuss normative neural circuitry changes during development that likely interact with the onset, treatment, and psychological outcomes of pediatric cancer. Absent from many neuro-oncology studies are the ways in which developmental changes in emotional learning and cognitive control circuitry may contribute to psychological outcomes, and to the secondary effects that are associated with cancer survivorship. Further, we consider how genetic polymorphisms that directly impact the developing brain may further influence cancer outcomes. A recent review by Marusak and colleagues (Marusak et al., 2018) highlights the importance of considering neurodevelopment in pediatric cancer survivors while under-scoring that childhood cancer can be considered a form of early life adversity. Here we extend upon the research highlighted by Marusak et al. and suggest that recognizing the ongoing neural maturation during adolescence and young adulthood is critical for understanding, and optimizing, psychological outcomes. We largely focus on the affective circuitry changes that occur during adolescence and early adulthood. There is a large body of work suggesting that frontoamygdala circuitry, which supports aversive learning, undergoes significant structural and functional changes during adolescence and young adulthood. By focusing on the neurobiology of aversive learning, we aim to understand variation in pediatric cancer outcomes, including secondary symptoms of anxiety and post-traumatic stress (PTS). We suggest that adolescence and early adulthood may provide a sensitive period for intervention to improve psychological outcomes in pediatric cancer survivors as they mature into adulthood. Building upon a literature of clinical and neuroscientific research in pediatric cancer survivors (Marusak et al., 2018), we propose future translational research directions that bridge molecular neuroscience in animal models and human cognitive neuroimaging. The goal is to improve how we study, understand, and treat the potentially deleterious effects of pediatric cancer treatment on the developing brain to optimize capacity for learning, emotion regulation, and quality of life.
2. Background
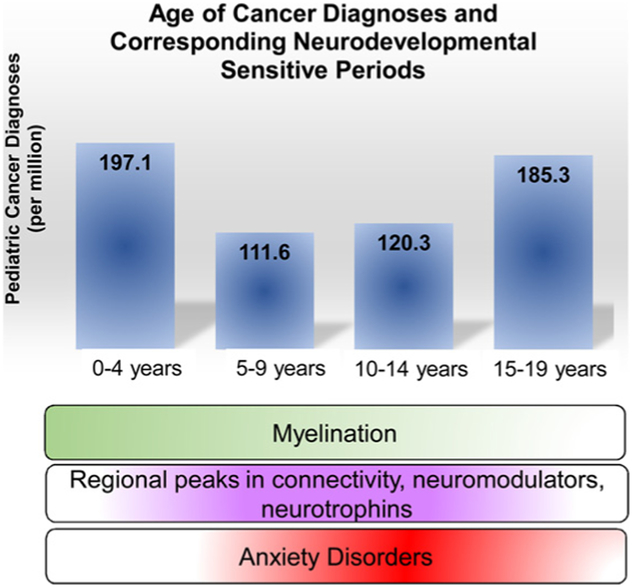
Recent age-specific incidence rates from 153 cancer registries world-wide (Steliarova-Foucher et al., 2017) show that for children ages 0–14 years, leukemias and tumors of the central nervous system (CNS) are most common. Following in prevalence are sympathetic nervous system tumors, retinoblastoma, renal tumors, and less frequent are hepatic, bone, soft tissue sarcomas, germ cell or epithelial tumors. Lymphomas and epithelial tumors were most prevalent in teens aged 15–19, followed by leukemias, CNS tumors, germ cell tumors, bone tumors, soft tissue sarcomas. Least frequent in this age group are renal and hepatic tumors or retinoblastoma. Unspecified or unclassified tumors make up a small percentage of tumors across all ages. High rates of leukemia, CNS tumors, and lymphomas across ages 0–19 are a consistent trend across most world regions, yet incidence rates vary between regions (Steliarova-Foucher et al., 2017). Of importance to this review is that various forms of cancer emerge consistently throughout infancy, childhood and into adolescence and early adulthood (see Fig. 1).
Fig. 1. Diagnoses and treatment for pediatric and adolescent cancer coincides with periods of massive neurodevelopmental change.
Blue bars represent the incidence of pediatric cancer across development (Steliarova-Foucher et al., 2017). During normal development, myelination in the brain peaks during early childhood, while synapses, neuromodulators and neurotrophins, as well as anxiety disorders, peak during early adolescence (Lee et al., 2014). How treatment regimens may shift these normal developmental patterns of myelination, neural connectivity, or emerging psychopathology is of interest to the developmental neuroscience community.
Unlike normal differentiated cells within the body, cancer cells are rapidly proliferating, undifferentiated cells that exhibit aberrant cellular signaling, immune evasion, and altered metabolism (Vander Heiden et al., 2009). Modern therapies designed to target cancer cells have included surgical resection for solid tumors, chemotherapeutic agents, ionizing radiation, and more recently immunotherapy, in order to alter the various features influencing a cancer cell’s replicative capacity. These treatments work via a variety of mechanisms by altering microtubule stability, DNA damage response, apoptotic machinery, and generalized proliferative capacity or cellular signaling pathways. While successful in targeting rapidly dividing cancer cells, such therapeutics do not fully spare healthy proliferating cells, which can lead to off-target and unwanted side effects.
One of the most significant psychological side effects for cancer treatment is “chemo brain,” a term popularized in the last decade to refer to alterations in cognitive functioning as a result of systemic chemotherapy (Simo et al., 2013). It is increasingly recognized that changes in cognitive functioning involving working and episodic memory, processing speed, executive functioning, and attention (Jansen et al., 2005; Simo et al., 2013) may not be solely the direct result of cancer treatment but also a result of the distress surrounding treatment (Andreotti et al., 2015; Scherling et al., 2011). In adult cancer survivors, these cognitive deficits often persist after treatment (Ahles et al., 2002),and up to 60% experience symptoms that progressively worsen over time (Janelsins et al., 2014; Ouimet et al., 2009; Palmer et al., 2007). Of note, at least one-third of all pediatric cancer survivors experience significant cognitive dysfunction (Duffner, 2010), which we describe in greater detail in subsequent sections. Relatedly, children and adolescents often have difficulties with academic performance and peer relationships (Bhat et al., 2005; Brinkman et al., 2016; Vannatta et al., 1998; Warner et al., 2016; Zebrack et al., 2004). The term ‘pediatric’ encompasses 18+ years of development, and combines infants, toddlers, children, adolescents, and late-adolescents into the same bracket for post-treatment analyses. Thus, while cognitive and emotional challenges following cancer treatment are well documented, how the developmental timing of cancer onset and treatment is associated with specific cognitive, emotional, and social outcomes is not as well delineated.
A significant body of research on child and adolescent cancer survivors has suggested that they experience higher rates of anxiety, PTS symptoms and emotional distress (Prasad et al., 2015; Seitz et al., 2010; Zeltzer et al., 2009), compared to both their siblings and to healthy peers. However, prevalence estimates vary widely across studies (Bruce, 2006; Stavinoha et al., 2018). Recent work (Phipps et al., 2009, 2014) has aimed to address whether some estimates may be inflated by a variety of factors, including: 1) attentional bias effects created by priming respondents to reflect on their experiences with cancer/treatment as traumatic; 2) attributing all distress measured to the cancer experience without modeling additional factors that may contribute more generally; or 3) lack of an appropriate comparison group that has experienced another type of traumatic event, such as in a study that found lower rates of PTS symptoms in children who had survived cancer compared to those who were bereaved by loss of a parent (Stoppelbein et al., 2006). When taking these factors into account, overall rates of PTS among this population may be lower than previously estimated. However, disparate findings across studies highlight the importance of understanding with greater nuance the developmental and individual differences that moderate the association between childhood cancer and psychological outcomes.
Despite findings that some pediatric survivors may be at increased risk for PTS and anxiety, these remain understudied psychosocial impacts of pediatric cancer survivorship (Marusak et al., 2018; McDonnell et al., 2017). Early life stress has been shown to increase the risk for later developing affective pathology, and a single stressor experienced during childhood increases the lifetime risk of anxiety or depressive disorders by approximately 30% (Anda et al., 2006). How pediatric cancer and its treatment may act as an early adverse experience for some survivors, with influences on cognitive, behavioral, and emotional outcomes, has been summarized in detail in a comprehensive review (Marusak et al., 2018). A recent literature review of over 24 studies exploring anxiety at both the clinical and subclinical level in adolescent cancer survivors concluded that aside from PTS symptoms and clinical levels of PTSD, there was little research on anxiety more broadly (McDonnell et al., 2017). It should be noted that the risk for affective pathology in the general population increases in late childhood and peaks during adolescence and early adulthood (Burt and Stein, 2002). Therefore, it is important to carefully examine the unique risk conferred by cancer diagnosis and treatment over and above the average levels expected across development. While these disorders are treatable in many individuals, they carry with them high rates of comorbidity and recurrence that have lasting effects on educational, social, and emotional outcomes (Burt and Stein, 2002).
3. Adolescent and young adult structural and functional brain development
In order to begin to disentangle the physiological and psychological impacts of cancers and their treatments on the developing brain, it is important to first appreciate the significant normative changes that occur across development in both structural and functional neural connections. Animal work (Hoops et al., 2018; Rosenberg and Lewis, 1995; Willing et al., 2017), human postmortem studies (Huttenlocher, 1979, 1990), and neuroimaging studies (Dennis et al., 2013; Giedd et al., 2015; Jernigan et al., 2016) have demonstrated dynamic changes in subcortical-cortical circuitry and connections throughout the adolescent and early adult years. In the sections below, we describe in greater detail the changes in these circuits gleaned from animal models. Broadly, maturation of subcortical regions and subcortical-subcortical connections (i.e., amygdala, ventral striatum and hippocampus) occur during childhood. Maturation of the prefrontal cortex and refinement of its connections with subcortical areas (ventral medial prefrontal cortex to both the amygdala and the ventral striatum) is protracted across development and extends into early adulthood (Asato et al., 2010; Gee, Humphreys, et al., 2013; Heller et al., 2016; Larsen et al., 2017; Perlman and Pelphrey, 2011). Collectively, this large body of work has provided robust evidence that the circuits supporting cognitive control, affective processing and psychosocial behavior are dynamically changing until early adulthood (Casey et al., 2016; Ernst, 2014; Steinberg, 2010).
Changes in the developing brain can be viewed in a hierarchical manner (Casey et al., 2017). The circuitry that supports successfully navigating the social and emotional environment is continually evolving and relies on sequential input throughout childhood and adolescence. It is thought that temporally defined cascading events can stimulate neurodevelopment and that each developmental phase is predicated on the prior phase. In the context of child and adolescent cancer, this means that significant events, such as cancer onset and treatment, can potentially disrupt the gradual emergence of functional neural connections, shift the developmental cascade, and have far-reaching downstream effects on subsequent developmental stages. Thus, the timing of cancer diagnosis and duration of treatment for the developing brain are important factors to consider when discussing neurocognitive changes associated with survivorship.
A number of studies have examined the impact of age at cancer diagnosis and time since treatment on neurocognitive and psychological outcomes, with some indication that younger patients suffer greater cognitive impacts (Ris et al., 2001), while adolescents and young adults may be at increased risk for psychological sequelae such as anxiety and PTS for reasons that are discussed in further detail below (Hobbie et al., 2000; Robison and Hudson, 2014; Schultz et al., 2007; Tai et al., 2012; Warner, Nam, et al., 2016). However, due to the cross-sectional designs of many studies, age at diagnosis and time since treatment are often confounded, making it difficult to interpret age-related differences (de Ruiter et al., 2013; Poggi et al., 2005). Research examining cognitive versus psychological outcomes in siloes may also underestimate the full extent of disease and treatment impacts on survivors, as cognitive dysfunction itself is linked to poorer social, emotional and academic functioning (Stavinoha et al., 2018; Ventura et al., 2018). Taken together, these studies suggest that there isn’t simply a linear, additive effect between age of cancer diagnosis, timing of treatment and outcomes, which highlights the need to consider the developing brain in order to better understand both the initial insult and subsequent outcomes.
4. Aversive learning – developmental influences to consider for adolescent cancer
Translational research offers important evidence about the development of fear-related disorders such as anxiety and PTS across development. Investigative studies exploring aversive learning (the acquisition of fear through classical conditioning) and the malleability of fear memories at different developmental stages are relevant to pediatric cancer survivorship for several reasons. First, aversive learning and fear extinction learning provide a simplified model for probing and understanding the neural circuitry associated with subsequent anxiety and PTS often observed in cancer survivors. Second, while fear extinction learning offers insight into treating anxiety and PTS, it also provides a robust and highly reproducible model for studying perturbations in general learning behaviors, through readily available assays for assessing memory acquisition, recall, extinction, and expression. Recent work highlights ‘fear of cancer recurrence’ in adolescent cancer survivors as one of many social and physical variables influencing learning achievement and overall quality of life (Molnar et al., 2019). This model system provides a framework for exploring chemotherapy or radiation-induced shifts in typical learning trajectories and can offer insight into both psychopathology and learning in general. Lastly, there has been a robust increase in aversive and fear learning studies focused on the developing brain, providing normative comparison data for these processes across developmental stages (Hartley and Lee, 2015).
Through associative learning techniques based on classical conditioning principles, long-lasting, aversive memories can be quickly formed in the rodent. Animal models of fear learning are frequently relied on and regarded highly for investigating learning properties, due to their ease and experimental control, along with well-defined behavioral assays. Thus, rodent studies of aversive memory allow for the study of precise, isolated fear-learning incidents and interventions without genetic or environmental confounds influencing results. Sensitive and critical periods of aversive learning have been the focus of infant and juvenile animal models (Kim et al., 2009, 2011; Moriceau and Sullivan, 2006; Rudy, 1993; Rudy and Morledge, 1994). Throughout the past decade, rodent models have begun incorporating more intermediate, adolescent ages (Colon et al., 2018; Hefner and Holmes, 2007; Heroux et al., 2018; Kim et al., 2011; McCallum et al., 2010; Pattwell et al., 2011, 2012; Pattwell et al., 2016; Schayek and Maroun, 2015; Shen et al., 2010), further highlighting unique sensitive periods for fear responsiveness. Recent studies have demonstrated that contextual fear is temporarily suppressed during adolescence (Pattwell et al., 2011). However, cued fear expression is enhanced, highly intact, and extinction-resistant in adolescent rodents, with parallel findings in humans (Drysdale et al., 2014; Johnson and Casey, 2015; McCallum et al., 2010; Pattwell et al., 2012). The dissociation between these two distinct forms of aversive learning has wide-ranging implications not only for understanding the adolescent brain, but also for uncovering glimpses into the unique etiology of adolescent anxiety. There are dynamic alterations between prefrontal areas and amygdala in both humans (Ganella et al., 2017a,b, Ganella et al., 2017a,b; Gee, Gabard-Durnam, et al., 2013; Park et al., 2017) and rodents (Cruz et al., 2015; Ganella et al., 2018; Kim et al., 2011; Pattwell et al., 2012) that underlie successful fear extinction. During the transition through adolescence, these amygdala-prefrontal connections can become susceptible to structural or functional modifications resulting from various psychological and physiological insults to the brain, such as those associated with both the stressors and treatments of cancer. There is also recent evidence for sex differences in fear extinction in rats (Colon et al., 2018), which may further interact with these other factors.
The enhanced capacity for cued fear expression and diminished cued extinction in adolescent rodents coincides with a sensitve period defined by a surge in connectivity between ventral CA1 and the prelimbic cortex (PL) of the ventral medial prefrontal cortex (vmPFC) (Pattwell et al., 2016), as well as enhanced connections between PL and amygdala. These enhanced connections suggest that a temporally-dependent surge in neural wiring during adolescence may be responsible for the unique behavioral properties associated with fear in rodents and adolescent anxiety. Optogenetic exploration also shows increases in feedforward inhibition with greater spontaneous inhibitory currents in excitatory neurons during adolescence, further highlighting the structural and functional changes associated with adolescent development in a prefrontal-amygdala circuit (Arruda-Carvalho et al., 2017). As these experiments demonstrate developmentally regulated shifts in neural connectivity that are both measurable and quanitifable in rodents, they provide an experimental baseline for exploring the effects of cancer treatment regimens on the developing brain. Questions such as “how does this treatment alter typically developing neural circuits?” or “how does behavior change as a result of treatment regimens and are there also changes in the underlying neural architecture?” can be examined.
It is well known from adult literature that the hippocampus is capable of modifying extinction learning via interactions with the amygdala and prefrontal cortex (PFC) (Li et al., 2018; Monfils et al., 2009; Orsini et al., 2011; Rao-Ruiz et al., 2011; Sotres-Bayon et al., 2012). Therefore, studies were designed to maximally target the adolescent surge in vCA1-PL connectivity by combining cued and contextual extinction into one session in order to take advantage of the brain’s unique heightened capacity for hippocampal-dependent extinction learning at this age (Pattwell et al., 2016). These experiments showed that extinction of adolescent-acquired fear memories could be enhanced when mice were exposed to a combined cue and contextual extinction session, which utilized the adolescent-specific surge in neural circuitry. These hippocampal-prefrontal connections are rapidly changing across adolescence and it is of interest to see how neurogenic waves and synaptic pruning associated with this stage of development may be altered by exposure to chemotherapy in children and adolescents. By utilizing what is already known about the temporally restricted increases in neural connectivity within this circuit and the simultaneous changes in aversive learning behaviors at this age, the hippocampal-prefrontal circuit provides a simplified model to further explore the effects of cancer treatment in a controlled, causal, and quantifiable way. By asking targeted questions such as “does X treatment inhibit, prolong, delay, enhance, stunt, hasten, or have no effect on this connectivity surge?” the field may be able to probe how cancer regimens impact the developing brain, first in an isolated incident-dependent way, and ultimately more broadly.
The hippocampus is involved in both cued and contextual fear processing and its developmental changes throughout adolescence are well documented across species (Benes et al., 1994; Kornack and Rakic, 1999). Shifts in neuronal or glial proliferation and apoptosis, synaptogenesis, synaptic pruning, dendritic spine remodeling, or myelination are likely contributors to the heterogeneous hippocampal development observed across childhood and adolescence. The changes correspond with volumetric increases in posterior regions and subsequent decreases in anterior regions (Gogtay et al., 2006). With connections to the PFC, amygdala, and hypothalamic pituitary axis, the anterior region of the hippocampus is a likely contributor to contextual fear alterations observed in adolescence. The changes within this region highlight an ever-changing dynamic circuit in which rodents do not exhibit contextual fear at adolescent ages, but express contextual fear to previously acquired memory later in life as adults (Bannerman et al., 2004; Cavada et al., 2000; Pattwell et al., 2011; Petrovich et al., 2001; Pitkanen et al., 2000).
How treatment-induced changes in neurogenesis may alter hippocampal development (specifically the anterior regions) and subsequent reciprocal connections in this circuitry will likely depend on patient age and genetics, as well as the particular treatment type and duration of treatment. Because neurogenesis in the subgranular zone of the dentate gyrus (DG) and subventricular zone (SVZ) of the lateral ventricle are key for learning and memory, rodent studies have sought to explore the role of cytotoxic agents on neurogenesis and decreases in neural proliferation and cell survival. Rodents demonstrated decreased neuronal proliferation and cell survival from cytotoxic agents as early as one day after treatment, and these anti-proliferative effects have been shown to last up to 6 months after treatment (Seigers et al., 2016). Even at subclinical dosages that have no effect on tumor cells in vitro, certain chemotherapeutic agents can cause cell death in normal CNS progenitors and oligodendrocytes, and lead to continued increases in cell death within the SVZ, DG, and corpus callosum of mice (Dietrich et al., 2006).
Systematic review of the chemotherapy-induced cognitive impairments in rodents shows decreases in hippocampal proliferation, neurogenesis, and cell survival. These changes in cell cycle behaviors likely result from the various chemotherapy agents, including anti-metabolites, DNA cross-linking agents, alkylating agents, mitotic inhibitors, microtubule destabilizers, and anti-hormonal agents. The majority of these rodent studies also reveal subsequent impairments on spatial and emotional memory tasks, yet the most of these studies were performed using adult animals (Dietrich et al., 2015). The impact of oxidative stress, myelin toxicity, neurovascular damage, inflammation, and direct cellular toxicity are identified as likely contributors to chemotherapeutic impairments on hippocampal neurogenesis and subsequent learning tasks (Dietrich et al., 2015). It is unknown how these mechanisms may differ in their insults on the developing brain, particularly on the adolescent and young adult brain, which has an increased capacity for plasticity, yet may also be more susceptible to toxicity.
Out of 22 rodent studies (Seigers et al., 2016) investigating the effects of chemotherapy on cognition, only one examined adolescent mice (Mondie et al., 2010), one examined late-adolescent/young adult mice (Gandal et al., 2008), and two employed juvenile (17-day-old) rats, yet did behavioral testing in adulthood (Stock et al., 1995; Yanovski et al., 1989). Interestingly, the two rodent studies using common chemotherapy agents in mid- and late-adolescent ages showed opposite results on the hippocampal-dependent task of novel object recognition (NOR). The five-week-old thioTEPA-treated (C57BL/6 J) mice showed impaired NOR and the 7-8-week-old methotrexate and 5-fluorouracil-treated (C57BL/6Hsd) mice showed no impairment in NOR. While these two adolescent rodent studies comprise less than 10% of the studies reviewed, the opposing results on the same task highlight the importance of the extremely sensitive time period surrounding adolescent prefrontal-cortex-amygdala-hippocampal circuit development. These disparate results are not surprising as the former agent (thioTEPA) is an alkylating antineoplastic agent and the latter agents (methotrexate and 5-FU) are antimetabolite neoplastic agents. Their opposing influences on an identical task (NOR) confirm the need for additional studies investigating how particular agents affect certain neural circuits and behaviors. With limited research to draw upon, making broad conclusions about the neurocognitive effects of chemotherapy across development will prove difficult until the literature embodies a wider array of studies investigating different drugs, ages, and behavioral tasks. Additionally, differences in aversive learning were observed in both cue- and context-dependent tasks in the rodent studies examined, but they did not always trend in the same way, suggesting drug, dose, and age-dependent differences interact in complicated ways (Seigers et al., 2016). Furthermore, cytostatic differences and minor genetic variation in mouse strains (C57BL/6J vs C57BL/6Hsd genetic background) in these groups add additional complexity – would thioTEPA-treated 8-week mice show NOR impairments, similar to their younger counterparts? Only by teasing apart the precise timing of distinct molecular and genetic events can we draw meaningful conclusions about the impact of chemotherapy on neurocognitive outcomes.
In the recent review by Marusak and colleagues, the authors aptly state that “concurrent evaluation of drug exposure and threat/stress may help to more fully explain neurodevelopmental changes, and ultimately, cognitive, behavioral, and emotional consequences” (Marusak et al., 2018). While the authors clearly outline the undeniable difficulties associated with measuring and defining adversity and a child’s level of distress, there are also many molecular challenges facing the field, and readers should be cautious not to infer that serum drug exposure is an adequate measure for quantitatively assessing brain exposure, especially in a temporally-dependent context. To do so would be to ignore the importance of the blood-brain-barrier (BBB) when estimating drug concentration within the brain. Furthermore, relying solely on serum drug concentration does not take into consideration how the BBB may break down or become leaky as a result of various diseases, a phenomenon commonly observed in brain cancer. Utilization of rodent models in combination with the review of patient charts, self-report psychological surveys, fMRI studies, etc., may offer great insight into the developmental effects of cancer treatment. The whole may be greater than the sum of its parts when the field is able to combine fine-tuned molecular, pharmacologic, behavioral, and genetic developmental rodent studies with those of patients, survivors, and age-matched healthy human subjects.
4.1. Environmental influences on aversive learning
Of particular importance for childhood and adolescent cancer survivors is their home, family environment, and support system (Whittle et al., 2017). A recent review compiled studies of psychological outcomes for parents of childhood cancer survivors (Ljungman et al., 2014). While mean levels of distress, coping, and family functioning were within typical ranges, there were clinical-level elevations among some subgroups in psychological distress, and as many as 44% of parents reported severe levels of PTS symptoms. It is not difficult to imagine the downstream effects of parental distress on the parent-child relationship and family functioning. A second environment that impacts child and adolescent cancer patients is the oncology setting itself, i.e., drugs, scans, needles, surgeries, and hospital stays. The ability to distinguish cues of threat versus safety may be compromised in the pediatric oncology setting, when the very treatments designed to save the patient actually appear as threatening, painful, or scary. One avenue for future research is to conceptualize the home and treatment environments in the context of safety and threat signals (Christianson et al., 2012; Kong et al., 2014). An important factor for whether an anxious child may grow up to have an adult life free from psychopathology may be whether they can reliably discriminate between the boundaries of safety and threat signals, which are distorted in individuals with anxiety disorders (Jovanovic et al., 2012).
While it is well known that early life stress can shift sensitive periods of neurodevelopment, and aversive or emotional learning in particular (Bath et al., 2016; Callaghan and Richardson, 2011; Dincheva et al., 2014; Jordan and Andersen, 2017; Pattwell and Bath, 2017; Silvers et al., 2016; Tottenham and Sheridan, 2009), it is not known how the psychological and physiological insults of cancer treatment may act as stressors to similarly shift these sensitive periods in children and teens. As adolescence is already a time associated with the emergence of psychopathologies and an increase in anxiety disorders, it should be of interest to understand how prior or active cancer treatment may create additional burden on an already challenging academic or social context (Ginsburg et al., 1998), and whether this may impact persistence of any such disorders into adulthood (Pine et al., 1998). While early studies investigating psychological functioning in adolescent survivors of cancer have yielded mixed results (Greenberg et al., 1989), others have found that despite increased PTS symptoms, survivors also exhibit increased posttraumatic growth that is dependent on age of diagnosis (Barakat et al., 2006). According to Barakat et al., posttraumatic growth is defined as “the cognitive process by which those who have experienced trauma apply positive interpretations to and find meaning in the traumatic event,” which can result in “restoration of pre-trauma schema and positive changes in one’s sense of self, relationships, and philosophy of life,” contributing to positive emotional outcomes (Barakat et al., 2006). The data on posttraumatic growth supports one potentially positive outcome associated with pediatric cancer survivorship, in which there is a newfound resiliency as a result of overcoming a traumatic experience. A recent review investigating posttraumatic growth in adolescent survivors of cancer found that younger age at diagnosis as well as lower use of avoidant coping strategies predicted lower levels of psychosocial stress, suggesting avoidant coping strategies correlated with higher posttraumatic growth (Turner-Sack et al., 2012).
5. Learning, memory, & cognitive control in adolescent cancer survivors
Human neuroimaging studies that include task-based, resting state and structural studies of pediatric cancer survivors are critical for translating work from animal models. Given concerns for anxiety and PTS in pediatric cancer survivors, it makes sense to examine underlying changes in amygdala-prefrontal-cortex activity associated with emotional processing and regulation. However, task-based human imaging studies in children and adolescents who have survived cancer have largely not focused on tapping into these processes (Marusak et al., 2018). Rather, the task-based studies have focused on primarily studying executive functioning, learning, and memory. The emphasis on these abilities is related to the significant side effects associated with cancer survivorship, such as challenges with working memory and declines in overall cognitive functioning. Future task-based neuroimaging research that explores emotional processing (Marusak et al., 2019) as well as the intersection between emotion and cognitive control as it relates to developmental timing (Ahmed et al., 2015) will provide novel and important contributions.
Cognitive control refers to a collection of abilities including working memory, flexibility, and inhibition, which allow us to adapt and control behavior in a top-down manner in order to achieve distinct goals. Research has demonstrated that cognitive control generally follows a protracted developmental time course into young adulthood (Casey, 2015; Huizinga et al., 2006; Luna et al., 2015; Prencipe et al., 2011; Vink et al., 2014). The ability to exert developmentally appropriate cognitive control is critical for successfully navigating social relationships (Holmes et al., 2016), academic performance (Alloway and Alloway, 2010; Blair and Razza, 2007), and psychological well-being. Aspects of cognitive control can be measured in a variety of ways, with tasks that target specific skills such as working memory (N-back tasks), cognitive flexibility (task-switching paradigms), and inhibition (go/no-go or stop-signal tasks). The pediatric cancer survivorship literature is mixed in terms of how cognitive control is measured and reported. Recent evidence in a sample of 130 acute lymphoblastic leukemia survivors demonstrated consistency between cognitive neuroscience behavior tasks, standardized cognitive testing, and caregiver report of executive functioning (Van Der Plas, Erdman et al., 2017). These findings support the fluidity in which the cancer literature uses these various paradigms to study cognitive deficits. However, the explicit developmental timing of the cancer onset and treatment (e.g. during adolescence) on these cognitive functions has not been explicitly tested.
The development of cognitive control is closely coupled with the development of the PFC. A review by Crone and Steinbeis suggests that different aspects of cognitive control rely on subregions of the PFC (Crone and Steinbeis, 2017). Specifically, stimulus-driven, deliberative cognitive control is dependent on the lateral PFC, whereas rule-based, internalized cognitive control, including emotion regulation, relies more on the medial PFC. Dividing cognitive control into sub-categories provides a context for the developmental changes that occur throughout childhood and adolescence in these abilities.
Cognitive problems, or declines in intellectual functioning including impairments in working memory and inhibition, are common for children who receive CNS-directed cancer therapies (Mulhern et al., 2004). Survivors of pediatric malignant brain tumors or acute lymphoblastic leukemia are at the greatest risk for cognitive difficulties (Mulhern and Butler, 2004; Palmer, 2008). Human neuroimaging studies have demonstrated structural, volumetric changes in survivors (Dellani et al., 2008; Marusak et al., 2018; Shan et al., 2006). As elegantly reviewed in Marusak et al., 2018, these are associated with challenges in response inhibition on a stop signal task (van der Plas, Schachar, et al., 2017), as well as with challenges in executive functioning as measured by self-report on the Behavior Rating Inventory of Executive Function (BRIEF) questionnaire (Tamnes et al., 2015). The underlying cellular and molecular mechanisms that drive these structural changes are not fully understood, although a likely candidate is toxicity of treatment and disturbance to normal cell function and neural connectivity. Recent work demonstrated that non-irradiated adult survivors of childhood leukemia had alterations in functional connectivity in the brain, as well as difficulties on a set-shifting task compared to controls, but no changes in white or gray matter volume (Billiet et al., 2018). These findings illustrate the complexities of cancer treatment and their downstream effects; they highlight how various treatments may have cumulative negative effects on brain volume and cognitive functioning. These cumulative effects may not simply be linear (Billiet et al., 2018), with earlier treatments corresponding to worse outcomes. Because chemotherapy dosage can vary at different points during development (Billiet et al., 2018), younger children may receive lower dosing, which can also significantly impact brain development trajectories differently.
fMRI task studies in pediatric cancer survivors have largely focused on working memory tasks (specifically, an N-back task that measures memory for sequences of stimuli). Increased activation patterns in the dorsal lateral prefrontal cortex, dorsal anterior cingulate (Robinson et al., 2010), and areas of the medial frontal gyrus (Robinson et al., 2014) have been found in pediatric cancer survivors, along with poorer accuracy on the task and parental ratings of executive functioning. These findings were similar to those reported by King et al., who also found that increased activation in superior and middle frontal gyrus in survivors of pediatric brain tumors during the N-back task was associated with poorer performance on the task (King et al., 2015). These results highlight neural hyperactivity, which may reflect compensatory mechanisms used for task completion. Of note, Robinson et al., tested children (average age 10 years) (Robinson et al., 2014) and teenagers (average age 14 years) (Robinson et al., 2010) whereas King et al. tested primarily adult survivors (17–35 years of age). Despite the similarities in results across studies, the age differences of the subjects were significant. Future studies that are powered to be able to tease apart how chemotherapy can impact developmentally typical, age-dependent changes in cognitive functioning will be informative for better understanding observed compensatory neural patterns.
Further evidence of compensatory activity patterns that support cognitive flexibility in pediatric cancer survivors comes from resting state studies showing increased strength in the default mode network, the salience network and executive control network (Chen et al., 2016). Hu et al. performed resting state scans in children with acute lymphoblastic leukemia before chemotherapy and compared their data to healthy children (Hu et al., 2017). There were differences in the neural patterns at rest of the default mode network, but no differences in cognitive testing that occurred outside of the scanner. The emergence of differences in neural connectivity prior to treatment may reflect the impact of the disease and treatment progression on the developing brain.
There is a growing body of work that has used cognitive training programs to increase attention, memory, and cognitive flexibility in pediatric cancer survivors. Individuals are often unable to use stimulant medications due to medical contraindication, side effects, or poor response. Thus, either therapist-delivered or computer-delivered cognitive training provides a viable alternative capable of yielding positive results. An extensive review of cognitive training in cancer survivors can be found elsewhere (Olson and Sands, 2016). Of note, Kesler et al. tested whether an 8-week cognitive training program would improve cognitive functioning and subsequently change brain activation (Kesler et al., 2011). 17 children and adolescents (ages 7–19 years) with a history of malignancy, radiation, and/or chemotherapy, completed the Wisconsin Sorting Task (testing flexible rule-following behavior) during fMRI before and after cognitive intervention. Post-intervention, participants demonstrated increased activation in the inferior frontal gyrus, medial frontal gyrus and superior frontal gyrus and had higher scores on standard cognitive testing. More recent work by Conklin et al. tested 8- to 16-year-old survivors of acute lymphoblastic leukemia or brain tumors who used a computerized cognitive training program (Cogmed) or were put on a waitlist for 5–9 weeks (Conklin et al., 2015). Children who received the intervention had improvements in working memory and processing speed. They also demonstrated decreases in activation during a spatial working memory task in the left lateral PFC and bilateral medial frontal cortex post-treatment. Interestingly, changes in neural activation were not associated with improvements in working memory scores as measured during cognitive testing. The decreased activation, in contrast to what was observed by Kesler et al., was thought to reflect increased neural efficiency, such that survivors who had developed compensatory mechanisms after training had a more efficient and normalized pattern of activation (Conklin et al., 2015). In part, the discrepancies in BOLD activation patterns (increases versus decreases) could be explained by the large age span of participants, as it is these neural circuits that are dynamically changing across childhood into adulthood. While these studies provide insight into the impact of cancer treatment on cognitive function, the age groups are sub-optimally defined. Future work in this area should consider the participant’s developmental stage as a moderating variable in order to help clarify discrepancies in findings.
6. Synthesis and next steps
A significant challenge with interpretation of the fMRI studies of pediatric cancer survivorship is that data from survivors are often collapsed across children and adolescents. While some studies examined age effects, none of the studies had the statistical power to separate children from adolescents into groups for analyses and/or study different developmental trajectories across age (e.g., continuous age-related increases or decreases in activation, non-linear changes that are adolescent-specific, etc.). Powering studies specifically to examine age in a more fine-grained way often yields critical differences missed by lumping all participants together across developmental stages (Jones et al., 2014; Schreuders et al., 2018; Somerville et al., 2013). Such analyses would be able to disentangle how developmental trajectories may be impacted by cancer onset and treatment, as well as tease apart which findings were a result of normal developmental differences across age groups. Many of the studies assessed whether age at diagnosis and/or years since diagnosis impacted the results, but it was not possible to further separate these findings into child versus adolescent cancer onset.
7. Genetic influences to consider for effects of cancer treatment on the developing brain
Individual differences in genetic makeup have also been examined to understand better why some individuals develop anxiety, PTS, and neurocognitive changes in response to particular life events like cancer, while others do not. While studies between twins and adopted siblings consistently demonstrate a role for genetics in behavior and neuropsychiatric disorders (Plomin et al., 1994), odds ratios for various risk alleles remain low due to contributing influences from many non-genetic factors. The delicate balance between genes and environmental factors (such as early life events or socioeconomic status) may tip differentially from one individual to the next, resulting in varying intermediate biological phenotypes, or endophenotypes (Gottesman and Gould, 2003). In the case of PTS, for example, trauma is necessary but not sufficient for the development of disorder. Some victims may develop subclinical symptomatology or PTSD, whereas others who have experienced a similar trauma might remain resilient (Milliken et al., 2007). Symptom severity for anxiety disorders and PTS also varies greatly between individuals, further suggesting an important role for other contributing factors, including environmental risks and genetic makeup (Mahan and Ressler, 2011). What might be the role of genes in resiliency to the combined physiological and psychological insults associated with child and adolescent cancer? And how might the inter-play between these insults, genetics, and the precise neural developmental stage of a patient further complicate who will be most or least susceptible to neurocognitive and emotional impairments? Are individuals with particular genetic polymorphisms prone to being more susceptible or more resilient to the neurocognitive effects of cancer treatment? Understanding how these genetic differences first affect individuals across development – without factoring cancer into the equation – will open new windows into the modern era of personalized psychiatric treatment. By exploring how various genetic differences or single nucleotide polymorphisms (SNPs) may alter neural pathways and molecular signaling cascades across development, the fields of cognitive, developmental and molecular neurosciences may collectively offer relevant findings to mental health providers regarding individualized treatment approaches. After first understanding how such genetic differences impact the developing brain in healthy individuals, the field can then explore how these genetic variants might influence outcomes during a pediatric cancer treatment regimen.
When looking at individual susceptibility to neurocognitive impairments associated with cancer regimens, polymorphisms in genes that regulate neural repair and are involved in plasticity and long-term potentiation may be particularly relevant. Genes known to be important for learning and memory formation, such as apoplipoprotein-E-4 (APOE4), brain-derived neurotrophic factor (BDNF), and catechol-O-methyltransferase (COMT) are important to consider. Additionally, individual differences in blood brain barrier (BBB) transporters (Simo et al., 2013) are likely also critical. While the BBB is capable of providing varying degrees of neuroprotection, treatment-induced changes in neurogenesis at the subcellular level or in resulting neural networks can occur, suggesting the importance of the aforementioned genes, and others, for modifying BBB permeability.
The SNP in the gene BDNF, BDNFVal66Met, is one example, among many, of an interesting candidate to explore for influencing chemotherapeutic impact and secondary effects across development. This SNP in the BDNF gene has been associated with altered susceptibility to a variety of neuropsychiatric disorders including anxiety and depression in adults (Frielingsdorf et al., 2010; Gatt et al., 2009; Gratacos et al., 2007; Jiang et al., 2005; Momose et al., 2002; Sen et al., 2003; Sklar et al., 2002; Ventriglia et al., 2002) and more recently has been shown to influence PTSD symptoms and cortical thickness in childhood trauma (Jin et al., 2019). This valine-to-methionine substitution at position 66 of the BDNF prodomain alters normal interactions with the sorting protein, sortilin, resulting in altered trafficking of BDNF into the regulated secretory pathway, which leads to an approximate 30% decrease in activity-dependent release (Chen et al., 2005, 2006; Chen et al., 2004; Egan et al., 2003). This decrease has vast implications, as BDNF is important for the regulation of neuronal development, differentiation, and survival (Huang and Reichardt, 2001), and has a critical role in activity-dependent processes implicated in learning and memory, such as long-term potentiation (LTP) (Chao, 2003; Korte et al., 1995). In addition to BDNF’s early role in assembly of the nervous system, postnatal BDNF levels in the brain remain dynamic, peaking during adolescence, in the rodent (Katoh-Semba et al., 1997). How chemotherapeutic agents may alter BDNF expression, trafficking, and signaling during these precise developmental peaks is not currently known.
This BDNF SNP has been genetically knocked into a mouse model, allowing for parallel rodent and human studies. BDNFMet mice recapitulate some of the hallmark phenotypes of human Met allele carriers, including decreased hippocampal volume, as well as impaired hippocampal-dependent contextual fear memory (Bueller et al., 2006; Chen et al., 2006; Egan et al., 2003; Hariri et al., 2003; Szeszko et al., 2005). Both human Met allele carriers and BDNFMet/Met mice show impairments in extinction of aversive memories compared to non-Met allele carriers (Soliman et al., 2010; Yu et al., 2009). BDNF expression in the hippocampus is induced during contextual learning (Hall et al., 2000) and plays a critical role in contextual fear conditioning (Liu et al., 2004). Deletion of BDNF from the hippocampus leads to impairments in spatial memory and extinction learning in adult rodents (Heldt et al., 2007). BDNF’s receptor, TrkB, has also been shown to be essential for hippocampal LTP required for learning (Minichiello et al., 1999; Patterson et al., 1996; Silhol et al., 2007). Consistent with the observed behavioral results, mice carrying the Met SNP (BDNFMet/Met) exhibit impairments in hippocampal NMDA-receptor dependent LTP (Ninan et al., 2010). Alterations in both Val66Met and TrkB receptor expression have been shown to have developmentally specific effects on learning (Dincheva et al., 2014). Recent work has begun to explore the role of activity-dependent regulation of neural development, plasticity, and cancer (Gillespie and Monje, 2018), highlighting the significant interdependence between normal neural signaling and the tumor microenvironment. This work suggests that mechanisms of neural regulation can have a strong impact on tumor growth and has implicated several candidates for this phenomenon, one of which, unsurprisingly, is BDNF (Gillespie and Monje, 2018; Venkatesh et al., 2015). How the oncological, neurophysiological, and psychological insults of pediatric and adolescent cancer and treatment may impact patients of different genotypes, and across different ages when BDNF levels are peaking developmentally, is of great interest.
Additionally, BDNF and the other aforementioned genes are among a subset of genes postulated to have dual roles in altering BBB integrity during chemotherapy and previously established roles in cognition, learning, and memory (Simo et al., 2013). It remains unknown how these well-characterized genetic variants – and many lesser characterized variants – may change neurocognitive susceptibility to chemotherapeutic treatments and subsequent development of anxiety and PTS, especially across development. Innovative animal models like the ones described above could be leveraged to address these questions.
8. Discussion
Even when disease remission or cure – the ultimate goal – has been achieved in pediatric cancer survivors, effects of the experience may reverberate across many areas of their functioning. Stepping back to consider age at diagnosis, duration and type of treatment, emotional resiliency of the patient, surrounding support networks, socioeconomic status and/or environmental factors, will be critical to inform scientific advances aimed at improving psychosocial treatment approaches and quality of life (Foulkes and Blakemore, 2018). There are various physiological aspects associated with survivorship, including effects on musculoskeletal and cardiopulmonary systems, alterations in endocrine and reproductive function, as well as risk of secondary disease or relapse (Oeffinger et al., 2006). But there is also a need to further understand the neurocognitive and psychological effects as they relate to the developing brain. In other words, examining pediatric cancer survivors through a neurodevelopmental lens will offer a perspective that will hopefully clarify secondary challenges associated with survivorship.
Comprehensive reviews of human imaging studies and rodent experiments have established an unambiguous link between cancer treatment early in life and various cognitive alterations in adulthood (Dietrich et al., 2015; Evenden, 2013; Marusak et al., 2018; Seigers and Fardell, 2011; Seigers et al., 2016), yet the field has not yet teased apart precisely when (infancy, childhood, adolescence, late adolescence) and which treatments (alkylating/DNA-damage agents, antimetabolites, topoisomerase agents, microtubule-destabilizing agents, ionizing radiation, surgery) have specific effects. It is expected, given the dynamic nature of the developing brain, that treatments will result in varying impairments at unique ages. Additional studies that address how the timing of physical and emotional stress due to pediatric cancer influences cognitive control will provide insight into how best to tailor remediation strategies such as cognitive training exercises. Through a combination of parallel rodent experiments and human imaging studies aimed at exploring genetic variants and precise developmental ages, further information can be gained into which treatments might be tailored, and when, to maximize efficacy and minimize unwanted side effects. Given the increasing evidence that cognitive training may be a good candidate for normalizing activity in cognitive control circuitry, it is possible that delivering such training preventively may be particularly efficacious at certain points during development. Further, as peers are particularly motivating for adolescents (Breiner et al., 2018; Jones et al., 2014; Somerville, 2013), work that targets whether adolescent cancer survivors may be more susceptible to both the positive and negative influences of peer feedback will help to further refine our understanding of psychosocial factors that have the potential to impact their outcomes.
Future translational rodent and human studies should use a developmental lens to examine the neural and molecular effects of oncological treatments. This will allow for a fuller understanding of the interaction of the developing brain and cancer treatments on outcomes such as emotional learning, memory, and cognitive control. These findings will provide deeper insight into how best to improve psychological and cognitive outcomes and improve the content of behavioral interventions in pediatric and adolescent cancer survivors. To move the field forward, we should harness what is already known about normative pediatric and adolescent neural circuitry in both mice and humans. By gaining a better understanding of how and when cancer treatments may impact the development of this circuitry, the field can uncover optimal treatments and therapeutic approaches for cancer survivors who may have a somewhat altered neurodevelopmental trajectory. By further exploring individual susceptibilities related to developmental and genetic differences, developmental neuroscientists, psychiatrists, and psychologists may discover new ways to enhance quality of life in pediatric and adolescent survivors.
Acknowledgements
National Institutes of Health, T32 CA9657-25 (S.S.P), U54 DK106829 (S.S.P), R21 CA223531 (S.S.P.); Jacobs Foundation Research Fellowship (S.S.P.). A generous gift from the Mortimer D. Sackler. Family (R.M.J) and the Leon Levy Foundation (R.M.J).
We thank Erika J. Ruberry for helpful discussions and feedback in preparing this review.
Footnotes
Author agreement/declaration
All authors have seen and approved the final version of this manuscript.
Conflict of interest
The authors have no competing interests and nothing to disclose.
References
- Ahles TA, Saykin AJ, Furstenberg CT, Cole B, Mott LA, Skalla K, et al. , 2002. Neuropsychologic impact of standard-dose systemic chemotherapy in long-term survivors of breast cancer and lymphoma. J. Clin. Oncol. 20 (2), 485–493. 10.1200/JCO.2002.20.2.485. [DOI] [PubMed] [Google Scholar]
- Ahmed SP, Bittencourt-Hewitt A, Sebastian CL, 2015. Neurocognitive bases of emotion regulation development in adolescence. Dev. Cogn. Neurosci. 15, 11–25. 10.1016/j.dcn.2015.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alloway TP, Alloway RG, 2010. Investigating the predictive roles of working memory and IQ in academic attainment. J. Exp. Child Psychol. 106 (1), 20–29. 10.1016/j.jecp.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Anda RF, Felitti VJ, Bremner JD, Walker JD, Whitfield C, Perry BD, et al. , 2006. The enduring effects of abuse and related adverse experiences in childhood. A convergence of evidence from neurobiology and epidemiology. Eur. Arch. Psychiatry Clin. Neurosci. 256 (3), 174–186. 10.1007/s00406-005-0624-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreotti C, Root JC, Ahles TA, McEwen BS, Compas BE, 2015. Cancer, coping, and cognition: a model for the role of stress reactivity in cancer-related cognitive decline. Psychooncology 24 (6), 617–623. 10.1002/pon.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong GT, Chen Y, Yasui Y, Leisenring W, Gibson TM, Mertens AC, et al. , 2016. Reduction in late mortality among 5-year survivors of childhood cancer. N. Engl. J. Med. 374 (9), 833–842. 10.1056/NEJMoa1510795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arruda-Carvalho M, Wu WC, Cummings KA, Clem RL, 2017. Optogenetic examination of prefrontal-amygdala synaptic development. J. Neurosci. 37 (11), 2976–2985. 10.1523/JNEUROSCI.3097-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asato MR, Terwilliger R, Woo J, Luna B, 2010. White matter development in adolescence: a DTI study. Cereb. Cortex 20 (9), 2122–2131. 10.1093/cercor/bhp282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannerman DM, Rawlins JN, McHugh SB, Deacon RM, Yee BK, Bast T, et al. , 2004. Regional dissociations within the hippocampus–memory and anxiety. Neurosci. Biobehav. Rev. 28 (3), 273–283. [DOI] [PubMed] [Google Scholar]
- Barakat LP, Alderfer MA, Kazak AE, 2006. Posttraumatic growth in adolescent survivors of cancer and their mothers and fathers. J. Pediatr. Psychol. 31 (4), 413–419. 10.1093/jpepsy/jsj058. [DOI] [PubMed] [Google Scholar]
- Bath KG, Manzano-Nieves G, Goodwill H, 2016. Early life stress accelerates behavioral and neural maturation of the hippocampus in male mice. Horm. Behav. 82, 64–71. 10.1016/j.yhbeh.2016.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benes FM, Turtle M, Khan Y, Farol P, 1994. Myelination of a key relay zone in the hippocampal formation occurs in the human brain during childhood, adolescence, and adulthood. Arch. Gen. Psychiatry 51 (6), 477–484. [DOI] [PubMed] [Google Scholar]
- Bhat SR, Goodwin TL, Burwinkle TM, Lansdale MF, Dahl GV, Huhn SL, et al. , 2005. Profile of daily life in children with brain tumors: an assessment of health-related quality of life. J. Clin. Oncol. 23 (24), 5493–5500. 10.1200/JCO.2005.10.190. [DOI] [PubMed] [Google Scholar]
- Billiet T, Elens I, Sleurs C, Uyttebroeck A, D’Hooge R, Lemiere J, Deprez S, 2018. Brain connectivity and cognitive flexibility in nonirradiated adult survivors of childhood leukemia. J. Natl. Cancer Inst. 10.1093/jnci/djy009. [DOI] [PubMed] [Google Scholar]
- Blair C, Razza RP, 2007. Relating effortful control, executive function, and false belief understanding to emerging math and literacy ability in kindergarten. Child Dev. 78 (2), 647–663. 10.1111/j.1467-8624.2007.01019.x. [DOI] [PubMed] [Google Scholar]
- Breiner K, Li A, Cohen AO, Steinberg L, Bonnie RJ, Scott ES, et al. , 2018. Combined effects of peer presence, social cues, and rewards on cognitive control in adolescents. Dev. Psychobiol. 60 (3), 292–302. 10.1002/dev.21599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkman TM, Li C, Vannatta K, Marchak JG, Lai JS, Prasad PK, et al. , 2016. Behavioral, social, and emotional symptom comorbidities and profiles in adolescent survivors of childhood cancer: a report from the childhood cancer survivor study. J. Clin. Oncol. 34 (28), 3417–3425. 10.1200/JCO.2016.66.4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce M, 2006. A systematic and conceptual review of posttraumatic stress in childhood cancer survivors and their parents. Clin. Psychol. Rev. 26 (3), 233–256. 10.1016/j.cpr.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Bueller JA, Aftab M, Sen S, Gomez-Hassan D, Burmeister M, Zubieta JK, 2006. BDNF Val66Met allele is associated with reduced hippocampal volume in healthy subjects. Biol. Psychiatry 59 (9), 812–815. [DOI] [PubMed] [Google Scholar]
- Burt VK, Stein K, 2002. Epidemiology of depression throughout the female life cycle. J. Clin. Psychiatry 63 (Suppl. 7), 9–15. [PubMed] [Google Scholar]
- Callaghan BL, Richardson R, 2011. Maternal separation results in early emergence of adult-like fear and extinction learning in infant rats. Behav. Neurosci. 125 (1), 20–28. 10.1037/a0022008. [DOI] [PubMed] [Google Scholar]
- Casey BJ, 2015. Beyond simple models of self-control to circuit-based accounts of adolescent behavior. Annu. Rev. Psychol. 66, 295–319. 10.1146/annurev-psych-010814-015156. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Galvan A, Somerville LH, 2016. Beyond simple models of adolescence to an integrated circuit-based account: a commentary. Dev. Cogn. Neurosci. 17, 128–130. 10.1016/j.dcn.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Heller AS, Gee DG, Cohen AO, 2017. Development of the emotional brain. Neurosci. Lett. 10.1016/j.neulet.2017.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavada C, Company T, Tejedor J, Cruz-Rizzolo RJ, Reinoso-Suarez F, 2000. The anatomical connections of the macaque monkey orbitofrontal cortex. A review. Cereb. Cortex 10 (3), 220–242. [DOI] [PubMed] [Google Scholar]
- Chao MV, 2003. Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat. Rev. Neurosci. 4 (4), 299–309. [DOI] [PubMed] [Google Scholar]
- Chen H, Wang L, King TZ, Mao H, 2016. Increased frontal functional networks in adult survivors of childhood brain tumors. Neuroimage Clin. 11, 339–346. 10.1016/j.nicl.2016.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZY, Ieraci A, Teng H, Dall H, Meng CX, Herrera DG, et al. , 2005. Sortilin controls intracellular sorting of brain-derived neurotrophic factor to the regulated secretory pathway. J. Neurosci. 25 (26), 6156–6166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZY, Jing D, Bath KG, Ieraci A, Khan T, Siao CJ, et al. , 2006. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science 314 (5796), 140–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZY, Patel PD, Sant G, Meng CX, Teng KK, Hempstead BL, Lee FS, 2004. Variant brain-derived neurotrophic factor (BDNF) (Met66) alters the intracellular trafficking and activity-dependent secretion of wild-type BDNF in neurosecretory cells and cortical neurons. J. Neurosci. 24 (18), 4401–4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson JP, Fernando AB, Kazama AM, Jovanovic T, Ostroff LE, Sangha S, 2012. Inhibition of fear by learned safety signals: a mini-symposium review. J. Neurosci. 32 (41), 14118–14124. 10.1523/JNEUROSCI.3340-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colon L, Odynocki N, Santarelli A, Poulos AM, 2018. Sexual differentiation of contextual fear responses. Learn. Mem. 25 (5), 230–240. 10.1101/lm.047159.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin HM, Ogg RJ, Ashford JM, Scoggins MA, Zou P, Clark KN, et al. , 2015. Computerized cognitive training for amelioration of cognitive late effects among childhood cancer survivors: a randomized controlled trial. J. Clin. Oncol. 33 (33), 3894–3902. 10.1200/JCO.2015.61.6672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cracco E, Goossens L, Braet C, 2017. Emotion regulation across childhood and adolescence: evidence for a maladaptive shift in adolescence. Eur. Child Adolesc. Psychiatry 26 (8), 909–921. 10.1007/s00787-017-0952-8. [DOI] [PubMed] [Google Scholar]
- Crone EA, Dahl RE, 2012. Understanding adolescence as a period of social-affective engagement and goal flexibility. Nat. Rev. Neurosci. 13 (9), 636–650. 10.1038/nrn3313. [DOI] [PubMed] [Google Scholar]
- Crone EA, Steinbeis N, 2017. Neural perspectives on cognitive control development during childhood and adolescence. Trends Cogn. Sci. 21 (3), 205–215. 10.1016/j.tics.2017.01.003. [DOI] [PubMed] [Google Scholar]
- Cruz E, Soler-Cedeno O, Negron G, Criado-Marrero M, Chompre G, Porter JT, 2015. Infralimbic EphB2 modulates fear extinction in adolescent rats. J. Neurosci. 35 (36), 12394–12403. 10.1523/JNEUROSCI.4254-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin SC, Minino AM, Anderson RN, 2016. Declines in cancer death rates among children and adolescents in the United States, 1999–2014. NCHS Data Brief (257), 1–8. [PubMed] [Google Scholar]
- de Ruiter MA, van Mourik R, Schouten-van Meeteren AY, Grootenhuis MA, Oosterlaan J, 2013. Neurocognitive consequences of a paediatric brain tumour and its treatment: a meta-analysis. Dev. Med. Child Neurol. 55 (5), 408–417. 10.1111/dmcn.12020. [DOI] [PubMed] [Google Scholar]
- Dellani PR, Eder S, Gawehn J, Vucurevic G, Fellgiebel A, Muller MJ, et al. , 2008. Late structural alterations of cerebral white matter in long-term survivors of childhood leukemia. J. Magn. Reson. Imaging 27 (6), 1250–1255. 10.1002/jmri.21364. [DOI] [PubMed] [Google Scholar]
- Dennis EL, Jahanshad N, McMahon KL, de Zubicaray GI, Martin NG, Hickie IB, et al. , 2013. Development of brain structural connectivity between ages 12 and 30: a 4-Tesla diffusion imaging study in 439 adolescents and adults. Neuroimage 64, 671–684. 10.1016/j.neuroimage.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich J, Han R, Yang Y, Mayer-Proschel M, Noble M, 2006. CNS progenitor cells and oligodendrocytes are targets of chemotherapeutic agents in vitro and in vivo. J. Biol. 5 (7), 22 10.1186/jbiol50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich J, Prust M, Kaiser J, 2015. Chemotherapy, cognitive impairment and hippocampal toxicity. Neuroscience 309, 224–232. 10.1016/j.neuroscience.2015.06.016. [DOI] [PubMed] [Google Scholar]
- Dincheva I, Pattwell SS, Tessarollo L, Bath KG, Lee FS, 2014. BDNF modulates contextual fear learning during adolescence. Dev. Neurosci. 36 (3–4), 269–276. 10.1159/000358824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Spear LP, 2016. Reward-centricity and attenuated aversions: an adolescent phenotype emerging from studies in laboratory animals. Neurosci. Biobehav. Rev. 70, 121–134. 10.1016/j.neubiorev.2016.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drysdale AT, Hartley CA, Pattwell SS, Ruberry EJ, Somerville LH, Compton SN, et al. , 2014. Fear and anxiety from principle to practice: implications for when to treat youth with anxiety disorders. Biol. Psychiatry 75 (11), e19–20. 10.1016/j.biopsych.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffner PK, 2010. Risk factors for cognitive decline in children treated for brain tumors. Eur. J. Paediatr. Neurol. 14 (2), 106–115. 10.1016/j.ejpn.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, et al. , 2003. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell 112 (2), 257–269. [DOI] [PubMed] [Google Scholar]
- Ernst M, 2014. The triadic model perspective for the study of adolescent motivated behavior. Brain Cogn. 89, 104–111. 10.1016/j.bandc.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evenden J, 2013. Cognitive impairments and cancer chemotherapy: translational research at a crossroads. Life Sci. 93 (17), 589–595. 10.1016/j.lfs.2013.03.020. [DOI] [PubMed] [Google Scholar]
- Foulkes L, Blakemore SJ, 2018. Studying individual differences in human adolescent brain development. Nat. Neurosci. 21 (3), 315–323. 10.1038/s41593-018-0078-4. [DOI] [PubMed] [Google Scholar]
- Frielingsdorf H, Bath KG, Soliman F, Difede J, Casey BJ, Lee FS, 2010. Variant brain-derived neurotrophic factor Val66Met endophenotypes: implications for post-traumatic stress disorder. Ann. N. Y. Acad. Sci. 1208, 150–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrmann D, Knoll LJ, Blakemore SJ, 2015. Adolescence as a sensitive period of brain development. Trends Cogn. Sci. 19 (10), 558–566. 10.1016/j.tics.2015.07.008. [DOI] [PubMed] [Google Scholar]
- Gandal MJ, Ehrlichman RS, Rudnick ND, Siegel SJ, 2008. A novel electro-physiological model of chemotherapy-induced cognitive impairments in mice. Neuroscience 157 (1), 95–104. 10.1016/j.neuroscience.2008.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganella DE, Barendse MEA, Kim JH, Whittle S, 2017a. Prefrontal-amygdala connectivity and state anxiety during fear extinction recall in adolescents. Front. Hum. Neurosci. 11, 587 10.3389/fnhum.2017.00587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganella DE, Drummond KD, Ganella EP, Whittle S, Kim JH, 2017b. Extinction of conditioned fear in adolescents and adults: a human fMRI study. Front. Hum. Neurosci. 11, 647 10.3389/fnhum.2017.00647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganella DE, Nguyen LD, Lee-Kardashyan L, Kim LE, Paolini AG, Kim JH, 2018. Neurocircuitry of fear extinction in adult and juvenile rats. Behav. Brain Res. 351, 161–167. 10.1016/j.bbr.2018.06.001. [DOI] [PubMed] [Google Scholar]
- Gatt JM, Nemeroff CB, Dobson-Stone C, Paul RH, Bryant RA, Schofield PR, et al. , 2009. Interactions between BDNF Val66Met polymorphism and early life stress predict brain and arousal pathways to syndromal depression and anxiety. Mol. Psychiatry 14 (7), 681–695. [DOI] [PubMed] [Google Scholar]
- Gatta G, Zigon G, Capocaccia R, Coebergh JW, Desandes E, Kaatsch P, et al. , 2009. Survival of European children and young adults with cancer diagnosed 1995-2002. Eur. J. Cancer 45 (6), 992–1005. 10.1016/j.ejca.2008.11.042. [DOI] [PubMed] [Google Scholar]
- Gee DG, Casey BJ, 2015. The impact of developmental timing for stress and recovery. Neurobiol. Stress 1, 184–194. 10.1016/j.ynstr.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee DG, Gabard-Durnam LJ, Flannery J, Goff B, Humphreys KL, Telzer EH, et al. , 2013. Early developmental emergence of human amygdala-prefrontal connectivity after maternal deprivation. Proc. Natl. Acad. Sci. U. S.A. 110 (39), 15638–15643. 10.1073/pnas.1307893110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee DG, Humphreys KL, Flannery J, Goff B, Telzer EH, Shapiro M, et al. , 2013. A developmental shift from positive to negative connectivity in human amygdala-pre-frontal circuitry. J. Neurosci. 33 (10), 4584–4593. 10.1523/JNEUROSCI.3446-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Raznahan A, Alexander-Bloch A, Schmitt E, Gogtay N, Rapoport JL, 2015. Child psychiatry branch of the National Institute of Mental Health longitudinal structural magnetic resonance imaging study of human brain development. Neuropsychopharmacology 40 (1), 43–49. 10.1038/npp.2014.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie S, Monje M, 2018. An active role for neurons in glioma progression: making sense of Scherer’s structures. Neuro Oncol. 20 (10), 1292–1299. 10.1093/neuonc/noy083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg GS, La Greca AM, Silverman WK, 1998. Social anxiety in children with anxiety disorders: relation with social and emotional functioning. J. Abnorm. Child Psychol. 26 (3), 175–185. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Nugent TF 3rd, Herman DH, Ordonez A, Greenstein D, Hayashi KM, et al. , 2006. Dynamic mapping of normal human hippocampal development. Hippocampus 16 (8), 664–672. 10.1002/hipo.20193. [DOI] [PubMed] [Google Scholar]
- Gottesman II, Gould TD, 2003. The endophenotype concept in psychiatry: etymology and strategic intentions. Am. J. Psychiatry 160 (4), 636–645. [DOI] [PubMed] [Google Scholar]
- Gratacos M, Gonzalez JR, Mercader JM, de Cid R, Urretavizcaya M, Estivill X, 2007. Brain-derived neurotrophic factor Val66Met and psychiatric disorders: metaanalysis of case-control studies confirm association to substance-related disorders, eating disorders, and schizophrenia. Biol. Psychiatry 61 (7), 911–922. [DOI] [PubMed] [Google Scholar]
- Greenberg HS, Kazak AE, Meadows AT, 1989. Psychologic functioning in 8- to 16-year-old cancer survivors and their parents. J. Pediatr. 114 (3), 488–493. [DOI] [PubMed] [Google Scholar]
- Hall J, Thomas KL, Everitt BJ, 2000. Rapid and selective induction of BDNF expression in the hippocampus during contextual learning. Nat. Neurosci. 3 (6), 533–535. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Goldberg TE, Mattay VS, Kolachana BS, Callicott JH, Egan MF, Weinberger DR, 2003. Brain-derived neurotrophic factor val66met polymorphism affects human memory-related hippocampal activity and predicts memory performance. J. Neurosci. 23 (17), 6690–6694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley CA, Lee FS, 2015. Sensitive periods in affective development: nonlinear maturation of fear learning. Neuropsychopharmacology 40 (1), 50–60. 10.1038/npp.2014.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefner K, Holmes A, 2007. Ontogeny of fear-, anxiety- and depression-related behavior across adolescence in C57BL/6J mice. Behav. Brain Res. 176 (2), 210–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldt SA, Stanek L, Chhatwal JP, Ressler KJ, 2007. Hippocampus-specific deletion of BDNF in adult mice impairs spatial memory and extinction of aversive memories. Mol. Psychiatry 12 (7), 656–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller AS, Cohen AO, Dreyfuss MF, Casey BJ, 2016. Changes in cortico-subcortical and subcortico-subcortical connectivity impact cognitive control to emotional cues across development. Soc. Cogn. Affect. Neurosci. 11 (12), 1910–1918. 10.1093/scan/nsw097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heroux NA, Osborne BF, Miller LA, Kawan M, Buban KN, Rosen JB, Stanton ME, 2018. Differential expression of the immediate early genes c-Fos, Arc, Egr-1, and Npas4 during long-term memory formation in the context preexposure facilitation effect (CPFE). Neurobiol. Learn. Mem. 147, 128–138. 10.1016/j.nlm.2017.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbie WL, Stuber M, Meeske K, Wissler K, Rourke MT, Ruccione K, et al. , 2000. Symptoms of posttraumatic stress in young adult survivors of childhood cancer. J. Clin. Oncol. 18 (24), 4060–4066. 10.1200/JCO.2000.18.24.4060. [DOI] [PubMed] [Google Scholar]
- Holmes CJ, Kim-Spoon J, Deater-Deckard K, 2016. Linking executive function and peer problems from early childhood through middle adolescence. J. Abnorm. Child Psychol. 44 (1), 31–42. 10.1007/s10802-015-0044-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoops D, Reynolds LM, Restrepo-Lozano JM, Flores C, 2018. Dopamine development in the mouse orbital prefrontal cortex is protracted and sensitive to amphetamine in adolescence. eNeuro 5 (1). 10.1523/ENEURO.0372-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z, Zou D, Mai H, Yuan X, Wang L, Li Y, et al. , 2017. Altered brain function in new onset childhood acute lymphoblastic leukemia before chemotherapy: a resting-state fMRI study. Brain Dev. 39 (9), 743–750. 10.1016/j.braindev.2017.04.014. [DOI] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF, 2001. Neurotrophins: roles in neuronal development and function. Annu. Rev. Neurosci. 24, 677–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizinga M, Dolan CV, van der Molen MW, 2006. Age-related change in executive function: developmental trends and a latent variable analysis. Neuropsychologia 44 (11), 2017–2036. 10.1016/j.neuropsychologia.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR, 1979. Synaptic density in human frontal cortex - developmental changes and effects of aging. Brain Res. 163, 195–205. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR, 1990. Morphometric study of human cerebral cortex development. Neuropsychologia 28 (6), 517–527. [DOI] [PubMed] [Google Scholar]
- Janelsins MC, Kesler SR, Ahles TA, Morrow GR, 2014. Prevalence, mechanisms, and management of cancer-related cognitive impairment. Int. Rev. Psychiatry 26 (1), 102–113. 10.3109/09540261.2013.864260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen CE, Miaskowski C, Dodd M, Dowling G, Kramer J, 2005. A metaanalysis of studies of the effects of cancer chemotherapy on various domains of cognitive function. Cancer 104 (10), 2222–2233. 10.1002/cncr.21469. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Brown TT, Bartsch H, Dale AM, 2016. Toward an integrative science of the developing human mind and brain: focus on the developing cortex. Dev. Cogn. Neurosci. 18, 2–11. 10.1016/j.dcn.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Xu K, Hoberman J, Tian F, Marko AJ, Waheed JF, et al. , 2005. BDNF variation and mood disorders: a novel functional promoter polymorphism and Val66Met are associated with anxiety but have opposing effects. Neuropsychopharmacology 30 (7), 1353–1361. [DOI] [PubMed] [Google Scholar]
- Jin MJ, Jeon H, Hyun MH, Lee SH, 2019. Influence of childhood trauma and brain-derived neurotrophic factor Val66Met polymorphism on posttraumatic stress symptoms and cortical thickness. Sci. Rep. 9 (1), 6028 10.1038/s41598-019-42563-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DC, Casey BJ, 2015. Extinction during memory reconsolidation blocks recovery of fear in adolescents. Sci. Rep. 5, 8863 10.1038/srep08863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RM, Somerville LH, Li J, Ruberry EJ, Powers A, Mehta N, et al. , 2014. Adolescent-specific patterns of behavior and neural activity during social reinforcement learning. Cogn. Affect. Behav. Neurosci. 14 (2), 683–697. 10.3758/s13415-014-0257-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan CJ, Andersen SL, 2017. Sensitive periods of substance abuse: early risk for the transition to dependence. Dev. Cogn. Neurosci. 25, 29–44. 10.1016/j.dcn.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Kazama A, Bachevalier J, Davis M, 2012. Impaired safety signal learning may be a biomarker of PTSD. Neuropharmacology 62 (2), 695–704. 10.1016/j.neuropharm.2011.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh-Semba R, Takeuchi IK, Semba R, Kato K, 1997. Distribution of brain-derived neurotrophic factor in rats and its changes with development in the brain. J. Neurochem. 69 (1), 34–42. [DOI] [PubMed] [Google Scholar]
- Kesler SR, Lacayo NJ, Jo B, 2011. A pilot study of an online cognitive rehabilitation program for executive function skills in children with cancer-related brain injury. Brain Inj. 25 (1), 101–112. 10.3109/02699052.2010.536194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Hamlin AS, Richardson R, 2009. Fear extinction across development: the involvement of the medial prefrontal cortex as assessed by temporary inactivation and immunohistochemistry. J. Neurosci. 29 (35), 10802–10808. 10.1523/JNEUROSCI.0596-09.2009.29/35/10802 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Li S, Richardson R, 2011. Immunohistochemical analyses of long-term extinction of conditioned fear in adolescent rats. Cereb. Cortex 21 (3), 530–538. [DOI] [PubMed] [Google Scholar]
- King TZ, Na S, Mao H, 2015. Neural underpinnings of working memory in adult survivors of childhood brain tumors. J. Int. Neuropsychol. Soc. 21 (7), 494–505. 10.1017/S135561771500051X. [DOI] [PubMed] [Google Scholar]
- Kong E, Monje FJ, Hirsch J, Pollak DD, 2014. Learning not to fear: neural correlates of learned safety. Neuropsychopharmacology 39 (3), 515–527. 10.1038/npp.2013.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornack DR, Rakic P, 1999. Continuation of neurogenesis in the hippocampus of the adult macaque monkey. Proc. Natl. Acad. Sci. U. S.A. 96 (10), 5768–5773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korte M, Carroll P, Wolf E, Brem G, Thoenen H, Bonhoeffer T, 1995. Hippocampal long-term potentiation is impaired in mice lacking brain-derived neurotrophic factor. Proc. Natl. Acad. Sci. U. S.A. 92 (19), 8856–8860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen B, Verstynen TD, Yeh FC, Luna B, 2017. Developmental changes in the integration of affective and cognitive corticostriatal pathways are associated with reward-driven behavior. Cereb. Cortex 1–12. 10.1093/cercor/bhx162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee FS, Heimer H, Giedd JN, Lein ES, Šestan N, Weinberger DR, Casey BJ, 2014. Mental health. Adolescent mental health-opportunity and obligation. Science. 346 (6209), 547–549. 10.1126/science.1260497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Gao X, Zhou Q, 2018. Absence of fear renewal and functional connections between prefrontal cortex and hippocampus in infant mice. Neurobiol. Learn. Mem. 152, 1–9. 10.1016/j.nlm.2018.04.011. [DOI] [PubMed] [Google Scholar]
- Liu IY, Lyons WE, Mamounas LA, Thompson RF, 2004. Brain-derived neurotrophic factor plays a critical role in contextual fear conditioning. J. Neurosci. 24 (36), 7958–7963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljungman L, Cernvall M, Gronqvist H, Ljotsson B, Ljungman G, von Essen L, 2014. Long-term positive and negative psychological late effects for parents of childhood cancer survivors: a systematic review. PLoS One 9 (7), e103340 10.1371/journal.pone.0103340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna B, Marek S, Larsen B, Tervo-Clemmens B, Chahal R, 2015. An integrative model of the maturation of cognitive control. Annu. Rev. Neurosci. 38, 151–170. 10.1146/annurev-neuro-071714-034054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahan AL, Ressler KJ, 2011. Fear conditioning, synaptic plasticity and the amygdala: implications for posttraumatic stress disorder. Trends Neurosci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusak HA, Iadipaolo AS, Harper FW, Elrahal F, Taub JW, Goldberg E, Rabinak CA, 2018. Neurodevelopmental consequences of pediatric cancer and its treatment: applying an early adversity framework to understanding cognitive, behavioral, and emotional outcomes. Neuropsychol. Rev 28 (2), 123–175. 10.1007/s11065-017-9365-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusak HA, Iadipaolo AS, Paulisin S, Harper FW, Taub JW, Dulay K, et al. , 2019. Emotion-related brain organization and behavioral responses to socio-emotional stimuli in pediatric cancer survivors with posttraumatic stress symptoms. Pediatr. Blood Cancer 66 (1), e27470 10.1002/pbc.27470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCallum J, Kim JH, Richardson R, 2010. Impaired extinction retention in adolescent rats: effects of D-cycloserine. Neuropsychopharmacology 35 (10), 2134–2142. 10.1038/npp.2010.92.npp201092 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnell GA, Salley CG, Barnett M, DeRosa AP, Werk RS, Hourani A, et al. , 2017. Anxiety among adolescent survivors of pediatric Cancer. J. Adolesc. Health 61 (4), 409–423. 10.1016/j.jadohealth.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milliken CS, Auchterlonie JL, Hoge CW, 2007. Longitudinal assessment of mental health problems among active and reserve component soldiers returning from the Iraq war. JAMA 298 (18), 2141–2148. [DOI] [PubMed] [Google Scholar]
- Minichiello L, Korte M, Wolfer D, Kuhn R, Unsicker K, Cestari V, et al. , 1999. Essential role for TrkB receptors in hippocampus-mediated learning. Neuron 24 (2), 401–414. [DOI] [PubMed] [Google Scholar]
- Molnar ED, Kovacs D, Bartyik K, 2019. Comparison of quality of life and learning success of adolescents surviving cancer and their classmates. J. Cancer Educ 10.1007/s13187-019-1472-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momose Y, Murata M, Kobayashi K, Tachikawa M, Nakabayashi Y, Kanazawa I, Toda T, 2002. Association studies of multiple candidate genes for Parkinson’s disease using single nucleotide polymorphisms. Ann. Neurol. 51 (1), 133–136. [DOI] [PubMed] [Google Scholar]
- Mondie CM, Vandergrift KA, Wilson CL, Gulinello ME, Weber ET, 2010. The chemotherapy agent, thioTEPA, yields long-term impairment of hippocampal cell proliferation and memory deficits but not depression-related behaviors in mice. Behav. Brain Res. 209 (1), 66–72. 10.1016/j.bbr.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monfils MH, Cowansage KK, Klann E, LeDoux JE, 2009. Extinction-reconsolidation boundaries: key to persistent attenuation of fear memories. Science 324 (5929), 951–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S, Sullivan RM, 2006. Maternal presence serves as a switch between learning fear and attraction in infancy. Nat. Neurosci. 9 (8), 1004–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulhern RK, Butler RW, 2004. Neurocognitive sequelae of childhood cancers and their treatment. Pediatr. Rehabil. 7 (1), 1–14. 10.1080/13638490310001655528. discussion 15–16. [DOI] [PubMed] [Google Scholar]
- Mulhern RK, Merchant TE, Gajjar A, Reddick WE, Kun LE, 2004. Late neurocognitive sequelae in survivors of brain tumours in childhood. Lancet Oncol. 5 (7), 399–408. 10.1016/S1470-2045(04)01507-4. [DOI] [PubMed] [Google Scholar]
- Ninan I, Bath KG, Dagar K, Perez-Castro R, Plummer MR, Lee FS, Chao MV, 2010. The BDNF Val66Met polymorphism impairs NMDA receptor-dependent synaptic plasticity in the hippocampus. J. Neurosci. 30 (26), 8866–8870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oeffinger KC, Mertens AC, Sklar CA, Kawashima T, Hudson MM, Meadows AT, et al. , 2006. Chronic health conditions in adult survivors of childhood cancer. N. Engl. J. Med. 355 (15), 1572–1582. 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- Olson K, Sands SA, 2016. Cognitive training programs for childhood cancer patients and survivors: a critical review and future directions. Child Neuropsychol. 22 (5), 509–536. 10.1080/09297049.2015.1049941. [DOI] [PubMed] [Google Scholar]
- Orsini CA, Kim JH, Knapska E, Maren S, 2011. Hippocampal and prefrontal projections to the basal amygdala mediate contextual regulation of fear after extinction. J. Neurosci. 31 (47), 17269–17277. 10.1523/JNEUROSCI.4095-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouimet LA, Stewart A, Collins B, Schindler D, Bielajew C, 2009. Measuring neuropsychological change following breast cancer treatment: an analysis of statistical models. J. Clin. Exp. Neuropsychol. 31 (1), 73–89. 10.1080/13803390801992725. [DOI] [PubMed] [Google Scholar]
- Palmer SL, 2008. Neurodevelopmental impact on children treated for medulloblastoma: a review and proposed conceptual model. Dev. Disabil. Res. Rev. 14 (3), 203–210. 10.1002/ddrr.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer SL, Reddick WE, Gajjar A, 2007. Understanding the cognitive impact on children who are treated for medulloblastoma. J. Pediatr. Psychol. 32 (9), 1040–1049. 10.1093/jpepsy/jsl056. [DOI] [PubMed] [Google Scholar]
- Park CHJ, Ganella DE, Kim JH, 2017. Juvenile female rats, but not male rats, show renewal, reinstatement, and spontaneous recovery following extinction of conditioned fear. Learn. Mem. 24 (12), 630–636. 10.1101/lm.045831.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson SL, Abel T, Deuel TA, Martin KC, Rose JC, Kandel ER, 1996. Recombinant BDNF rescues deficits in basal synaptic transmission and hippocampal LTP in BDNF knockout mice. Neuron 16 (6), 1137–1145. [DOI] [PubMed] [Google Scholar]
- Pattwell SS, Bath KG, 2017. Emotional learning, stress, and development: an ever-changing landscape shaped by early-life experience. Neurobiol. Learn. Mem. 143, 36–48. 10.1016/j.nlm.2017.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattwell SS, Bath KG, Casey BJ, Ninan I, Lee FS, 2011. Selective early-acquired fear memories undergo temporary suppression during adolescence. Proc. Natl. Acad. Sci. U. S.A. 108 (3), 1182–1187. 10.1073/pnas.1012975108.1012975108 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattwell SS, Duhoux S, Hartley CA, Johnson DC, Jing D, Elliott MD, et al. , 2012. Altered fear learning across development in both mouse and human. Proc. Natl. Acad. Sci. U. S.A. 109 (40), 16318–16323. 10.1073/pnas.1206834109.1206834109 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattwell SS, Liston C, Jing D, Ninan I, Yang RR, Witztum J, et al. , 2016. Dynamic changes in neural circuitry during adolescence are associated with persistent attenuation of fear memories. Nat. Commun. 7, 11475 10.1038/ncomms11475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman SB, Pelphrey KA, 2011. Developing connections for affective regulation: age-related changes in emotional brain connectivity. J. Exp. Child Psychol. 108 (3), 607–620. 10.1016/j.jecp.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovich GD, Canteras NS, Swanson LW, 2001. Combinatorial amygdalar inputs to hippocampal domains and hypothalamic behavior systems. Brain Res. Brain Res. Rev. 38 (1–2), 247–289. [DOI] [PubMed] [Google Scholar]
- Phipps S, Jurbergs N, Long A, 2009. Symptoms of post-traumatic stress in children with cancer: does personality trump health status? Psychooncology 18 (9), 992–1002. 10.1002/pon.1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phipps S, Klosky JL, Long A, Hudson MM, Huang Q, Zhang H, Noll RB, 2014. Posttraumatic stress and psychological growth in children with cancer: has the traumatic impact of cancer been overestimated? J. Clin. Oncol 32 (7), 641–646. 10.1200/JCO.2013.49.8212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine DS, Cohen P, Gurley D, Brook J, Ma Y, 1998. The risk for early-adulthood anxiety and depressive disorders in adolescents with anxiety and depressive disorders. Arch. Gen. Psychiatry 55 (1), 56–64. [DOI] [PubMed] [Google Scholar]
- Pitkanen A, Pikkarainen M, Nurminen N, Ylinen A, 2000. Reciprocal connections between the amygdala and the hippocampal formation, perirhinal cortex, and post-rhinal cortex in rat. A review. Ann. N. Y. Acad. Sci. 911, 369–391. [DOI] [PubMed] [Google Scholar]
- Plomin R, Owen MJ, McGuffin P, 1994. The genetic basis of complex human behaviors. Science 264 (5166), 1733–1739. [DOI] [PubMed] [Google Scholar]
- Poggi G, Liscio M, Galbiati S, Adduci A, Massimino M, Gandola L, et al. , 2005. Brain tumors in children and adolescents: cognitive and psychological disorders at different ages. Psychooncology 14 (5), 386–395. 10.1002/pon.855. [DOI] [PubMed] [Google Scholar]
- Prasad PK, Hardy KK, Zhang N, Edelstein K, Srivastava D, Zeltzer L, et al. , 2015. Psychosocial and neurocognitive outcomes in adult survivors of adolescent and early young adult cancer: a report from the childhood cancer survivor study. J. Clin. Oncol. 33 (23), 2545–2552. 10.1200/JCO.2014.57.7528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prencipe A, Kesek A, Cohen J, Lamm C, Lewis MD, Zelazo PD, 2011. Development of hot and cool executive function during the transition to adolescence. J. Exp. Child Psychol. 108 (3), 621–637. 10.1016/j.jecp.2010.09.008. [DOI] [PubMed] [Google Scholar]
- Rao-Ruiz P, Rotaru DC, van der Loo RJ, Mansvelder HD, Stiedl O, Smit AB, Spijker S, 2011. Retrieval-specific endocytosis of GluA2-AMPARs underlies adaptive reconsolidation of contextual fear. Nat. Neurosci. 14 (10), 1302–1308. 10.1038/nn.2907. [DOI] [PubMed] [Google Scholar]
- Ris MD, Packer R, Goldwein J, Jones-Wallace D, Boyett JM, 2001. Intellectual outcome after reduced-dose radiation therapy plus adjuvant chemotherapy for medulloblastoma: a children’s cancer group study. J. Clin. Oncol. 19 (15), 3470–3476. 10.1200/JCO.2001.19.15.3470. [DOI] [PubMed] [Google Scholar]
- Robinson KE, Livesay KL, Campbell LK, Scaduto M, Cannistraci CJ, Anderson AW, et al. , 2010. Working memory in survivors of childhood acute lymphocytic leukemia: functional neuroimaging analyses. Pediatr. Blood Cancer 54 (4), 585–590. 10.1002/pbc.22362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson KE, Pearson MM, Cannistraci CJ, Anderson AW, Kuttesch JF, Wymer K, et al. , 2014. Neuroimaging of executive function in survivors of pediatric brain tumors and healthy controls. Neuropsychology 28 (5), 791–800. 10.1037/neu0000077. [DOI] [PubMed] [Google Scholar]
- Robison LL, Hudson MM, 2014. Survivors of childhood and adolescent cancer: lifelong risks and responsibilities. Nat. Rev. Cancer 14 (1), 61–70. 10.1038/nrc3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg DR, Lewis DA, 1995. Postnatal maturation of the dopaminergic innervation of monkey prefrontal and motor cortices: a tyrosine hydroxylase immunohistochemical analysis. J. Comp. Neurol. 358 (3), 383–400. 10.1002/cne.903580306. [DOI] [PubMed] [Google Scholar]
- Rudy JW, 1993. Contextual conditioning and auditory cue conditioning dissociate during development. Behav. Neurosci. 107 (5), 887–891. [DOI] [PubMed] [Google Scholar]
- Rudy JW, Morledge P, 1994. Ontogeny of contextual fear conditioning in rats: implications for consolidation, infantile amnesia, and hippocampal system function. Behav. Neurosci. 108 (2), 227–234. [DOI] [PubMed] [Google Scholar]
- Schayek R, Maroun M, 2015. Differences in stress-induced changes in extinction and prefrontal plasticity in postweanling and adult animals. Biol. Psychiatry 78 (3), 159–166. 10.1016/j.biopsych.2014.10.004. [DOI] [PubMed] [Google Scholar]
- Scherling C, Collins B, Mackenzie J, Bielajew C, Smith A, 2011. Pre-chemotherapy differences in visuospatial working memory in breast cancer patients compared to controls: an FMRI study. Front. Hum. Neurosci. 5, 122 10.3389/fnhum.2011.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreuders E, Braams BR, Blankenstein NE, Peper JS, Guroglu B, Crone EA, 2018. Contributions of reward sensitivity to ventral striatum activity across adolescence and early adulthood. Child Dev. 89 (3), 797–810. 10.1111/cdev.13056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz KA, Ness KK, Whitton J, Recklitis C, Zebrack B, Robison LL, et al. , 2007. Behavioral and social outcomes in adolescent survivors of childhood cancer: a report from the childhood cancer survivor study. J. Clin. Oncol. 25 (24), 3649–3656. 10.1200/JCO.2006.09.2486. [DOI] [PubMed] [Google Scholar]
- Seigers R, Fardell JE, 2011. Neurobiological basis of chemotherapy-induced cognitive impairment: a review of rodent research. Neurosci. Biobehav. Rev. 35 (3), 729–741. 10.1016/j.neubiorev.2010.09.006. [DOI] [PubMed] [Google Scholar]
- Seigers R, Loos M, Van Tellingen O, Boogerd W, Smit AB, Schagen SB, 2016. Neurobiological changes by cytotoxic agents in mice. Behav. Brain Res. 299, 19–26. 10.1016/j.bbr.2015.10.057. [DOI] [PubMed] [Google Scholar]
- Seitz DC, Besier T, Debatin KM, Grabow D, Dieluweit U, Hinz A, et al. , 2010. Posttraumatic stress, depression and anxiety among adult long-term survivors of cancer in adolescence. Eur. J. Cancer 46 (9), 1596–1606. 10.1016/j.ejca.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Sen S, Nesse RM, Stoltenberg SF, Li S, Gleiberman L, Chakravarti A, et al. , 2003. A BDNF coding variant is associated with the NEO personality inventory domain neuroticism, a risk factor for depression. Neuropsychopharmacology 28 (2), 397–401. [DOI] [PubMed] [Google Scholar]
- Shan ZY, Liu JZ, Glass JO, Gajjar A, Li CS, Reddick WE, 2006. Quantitative morphologic evaluation of white matter in survivors of childhood medulloblastoma. Magn. Reson. Imaging 24 (8), 1015–1022. 10.1016/j.mri.2006.04.015. [DOI] [PubMed] [Google Scholar]
- Shen H, Sabaliauskas N, Sherpa A, Fenton AA, Stelzer A, Aoki C, Smith SS, 2010. A critical role for alpha4betadelta GABAA receptors in shaping learning deficits at puberty in mice. Science 327 (5972), 1515–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silhol M, Arancibia S, Maurice T, Tapia-Arancibia L, 2007. Spatial memory training modifies the expression of brain-derived neurotrophic factor tyrosine kinase receptors in young and aged rats. Neuroscience 146 (3), 962–973. [DOI] [PubMed] [Google Scholar]
- Silvers JA, Lumian DS, Gabard-Durnam L, Gee DG, Goff B, Fareri DS, et al. , 2016. Previous institutionalization is followed by broader Amygdala-Hippocampal-PFC network connectivity during aversive learning in human development. J. Neurosci. 36 (24), 6420–6430. 10.1523/JNEUROSCI.0038-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvers JA, Shu J, Hubbard AD, Weber J, Ochsner KN, 2015. Concurrent and lasting effects of emotion regulation on amygdala response in adolescence and young adulthood. Dev. Sci. 18 (5), 771–784. 10.1111/desc.12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simo M, Rifa-Ros X, Rodriguez-Fornells A, Bruna J, 2013. Chemobrain: a systematic review of structural and functional neuroimaging studies. Neurosci. Biobehav. Rev. 37 (8), 1311–1321. 10.1016/j.neubiorev.2013.04.015. [DOI] [PubMed] [Google Scholar]
- Sklar P, Gabriel SB, McInnis MG, Bennett P, Lim YM, Tsan G, et al. , 2002. Family-based association study of 76 candidate genes in bipolar disorder: BDNF is a potential risk locus. Brain-derived neutrophic factor. Mol. Psychiatry 7 (6), 579–593. [DOI] [PubMed] [Google Scholar]
- Smith AR, Chein J, Steinberg L, 2013. Impact of socio-emotional context, brain development, and pubertal maturation on adolescent risk-taking. Horm. Behav 64 (2), 323–332. 10.1016/j.yhbeh.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliman F, Glatt CE, Bath KG, Levita L, Jones RM, Pattwell SS, et al. , 2010. A genetic variant BDNF polymorphism alters extinction learning in both mouse and human. Science 327 (5967), 863–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, 2013. Special issue on the teenage brain: sensitivity to social evaluation. Curr. Dir. Psychol. Sci. 22 (2), 121–127. 10.1177/0963721413476512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Jones RM, Ruberry EJ, Dyke JP, Glover G, Casey BJ, 2013. The medial prefrontal cortex and the emergence of self-conscious emotion in adolescence. Psychol. Sci. 24 (8), 1554–1562. 10.1177/0956797613475633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotres-Bayon F, Sierra-Mercado D, Pardilla-Delgado E, Quirk GJ, 2012. Gating of fear in prelimbic cortex by hippocampal and amygdala inputs. Neuron 76 (4), 804–812. 10.1016/j.neuron.2012.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavinoha PL, Askins MA, Powell SK, Pillay Smiley N, Robert RS, 2018. Neurocognitive and psychosocial outcomes in pediatric brain tumor survivors. Bioengineering (Basel) 5 (3). 10.3390/bioengineering5030073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L, 2010. A dual systems model of adolescent risk-taking. Dev. Psychobiol. 52 (3), 216–224. 10.1002/dev.20445. [DOI] [PubMed] [Google Scholar]
- Steliarova-Foucher E, Colombet M, Ries LAG, Moreno F, Dolya A, Bray F, et al. , 2017. International incidence of childhood cancer, 2001-10: a population-based registry study. Lancet Oncol 18 (6), 719–731. 10.1016/S1470-2045(17)30186-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock HS, Rosellini RA, Abrahamsen GC, McCaffrey RJ, Ruckdeschel JC, 1995. Methotrexate does not interfere with an appetitive Pavlovian conditioning task in Sprague-Dawley rats. Physiol. Behav. 58 (5), 969–973. [DOI] [PubMed] [Google Scholar]
- Stoppelbein LA, Greening L, Elkin TD, 2006. Risk of posttraumatic stress symptoms: a comparison of child survivors of pediatric cancer and parental bereavement. J. Pediatr. Psychol. 31 (4), 367–376. 10.1093/jpepsy/jsj055. [DOI] [PubMed] [Google Scholar]
- Szeszko PR, Lipsky R, Mentschel C, Robinson D, Gunduz-Bruce H, Sevy S, et al. , 2005. Brain-derived neurotrophic factor val66met polymorphism and volume of the hippocampal formation. Mol. Psychiatry 10 (7), 631–636. [DOI] [PubMed] [Google Scholar]
- Tai E, Buchanan N, Townsend J, Fairley T, Moore A, Richardson LC, 2012. Health status of adolescent and young adult cancer survivors. Cancer 118 (19), 4884–4891. 10.1002/cncr.27445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamnes CK, Zeller B, Amlien IK, Kanellopoulos A, Andersson S, Due-Tonnessen P, et al. , 2015. Cortical surface area and thickness in adult survivors of pediatric acute lymphoblastic leukemia. Pediatr. Blood Cancer 62 (6), 1027–1034. 10.1002/pbc.25386. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Galvan A, 2016. Stress and the adolescent brain: amygdala-prefrontal cortex circuitry and ventral striatum as developmental targets. Neurosci. Biobehav. Rev. 70, 217–227. 10.1016/j.neubiorev.2016.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Sheridan MA, 2009. A review of adversity, the amygdala and the hippocampus: a consideration of developmental timing. Front. Hum. Neurosci. 3, 68 10.3389/neuro.09.068.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner-Sack AM, Menna R, Setchell SR, Maan C, Cataudella D, 2012. Posttraumatic growth, coping strategies, and psychological distress in adolescent survivors of cancer. J. Pediatr. Oncol. Nurs. 29 (2), 70–79. 10.1177/1043454212439472. [DOI] [PubMed] [Google Scholar]
- Van Der Plas E, Erdman L, Nieman BJ, Weksberg R, Butcher DT, O’Connor DL, et al. , 2017. Characterizing neurocognitivelate effects in childhoodleukemia survivors using a combination of neuropsychological and cognitive neuroscience measures. Child Neuropsychol. 1–16. 10.1080/09297049.2017.1386170. [DOI] [PubMed] [Google Scholar]
- van der Plas E, Schachar RJ, Hitzler J, Crosbie J, Guger SL, Spiegler BJ, et al. , 2017. Brain structure, working memory and response inhibition in childhood leukemia survivors. Brain Behav. 7 (2), e00621 10.1002/brb3.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Heiden MG, Cantley LC, Thompson CB, 2009. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324 (5930), 1029–1033. 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannatta K, Gartstein MA, Short A, Noll RB, 1998. A controlled study of peer relationships of children surviving brain tumors: teacher, peer, and self ratings. J. Pediatr. Psychol. 23 (5), 279–287. [DOI] [PubMed] [Google Scholar]
- Venkatesh HS, Johung TB, Caretti V, Noll A, Tang Y, Nagaraja S, et al. , 2015. Neuronal activity promotes glioma growth through neuroligin-3 secretion. Cell 161 (4), 803–816. 10.1016/j.cell.2015.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventriglia M, Bocchio Chiavetto L, Benussi L, Binetti G, Zanetti O, Riva MA, Gennarelli M, 2002. Association between the BDNF 196 A/G polymorphism and sporadic Alzheimer’s disease. Mol. Psychiatry 7 (2), 136–137. [DOI] [PubMed] [Google Scholar]
- Ventura LM, Grieco JA, Evans CL, Kuhlthau KA, MacDonald SM, Tarbell NJ, et al. , 2018. Executive functioning, academic skills, and quality of life in pediatric patients with brain tumors post-proton radiation therapy. J. Neurooncol. 137 (1), 119–126. 10.1007/s11060-017-2703-6. [DOI] [PubMed] [Google Scholar]
- Vijayakumar N, Op de Macks Z, Shirtcliff EA, Pfeifer JH, 2018. Puberty and the human brain: insights into adolescent development. Neurosci. Biobehav. Rev. 92, 417–436. 10.1016/j.neubiorev.2018.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vink M, Zandbelt BB, Gladwin T, Hillegers M, Hoogendam JM, van den Wildenberg WP, et al. , 2014. Frontostriatal activity and connectivity increase during proactive inhibition across adolescence and early adulthood. Hum. Brain Mapp. 35 (9), 4415–4427. 10.1002/hbm.22483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner EL, Kent EE, Trevino KM, Parsons HM, Zebrack BJ, Kirchhoff AC, 2016. Social well-being among adolescents and young adults with cancer: a systematic review. Cancer 122 (7), 1029–1037. 10.1002/cncr.29866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner EL, Nam GE, Zhang Y, McFadden M, Wright J, Spraker-Perlman H, et al. , 2016. Health behaviors, quality of life, and psychosocial health among survivors of adolescent and young adult cancers. J. Cancer Surviv. 10 (2), 280–290. 10.1007/s11764-015-0474-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle S, Vijayakumar N, Simmons JG, Dennison M, Schwartz O, Pantelis C, et al. , 2017. Role of positive parenting in the association between neighborhood social disadvantage and brain development across adolescence. JAMA Psychiatry 74 (8), 824–832. 10.1001/jamapsychiatry.2017.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willing J, Cortes LR, Brodsky JM, Kim T, Juraska JM, 2017. Innervation of the medial prefrontal cortex by tyrosine hydroxylase immunoreactive fibers during adolescence in male and female rats. Dev. Psychobiol. 59 (5), 583–589. 10.1002/dev.21525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanovski JA, Packer RJ, Levine JD, Davidson TL, Micalizzi M, D’Angio G, 1989. An animal model to detect the neuropsychological toxicity of anticancer agents. Med. Pediatr. Oncol. 17 (3), 216–221. [DOI] [PubMed] [Google Scholar]
- Yu H, Wang Y, Pattwell S, Jing D, Liu T, Zhang Y, et al. , 2009. Variant BDNF Val66Met polymorphism affects extinction of conditioned aversive memory. J. Neurosci. 29 (13), 4056–4064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zebrack BJ, Gurney JG, Oeffinger K, Whitton J, Packer RJ, Mertens A, et al. , 2004. Psychological outcomes in long-term survivors of childhood brain cancer: a report from the childhood cancer survivor study. J. Clin. Oncol. 22 (6), 999–1006. 10.1200/JCO.2004.06.148. [DOI] [PubMed] [Google Scholar]
- Zeltzer LK, Recklitis C, Buchbinder D, Zebrack B, Casillas J, Tsao JC, et al. , 2009. Psychological status in childhood cancer survivors: a report from the childhood cancer survivor study. J. Clin. Oncol. 27 (14), 2396–2404. 10.1200/JCO.2008.21.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]