Abstract
Objective
To examine the association between long-term intake of total and the six classes of dietary flavonoids and decline in cognitive function over a follow-up period of up to 15 years.
Design
In this longitudinal study, we evaluated change in eight cognitive domain scores (verbal and visual memory, verbal learning, attention and concentration, abstract reasoning, language, visuoperceptual organization, and the global function) based on three neuropsychological exams and characterized the annualized change between consecutive exams. Long-term intakes of total and six flavonoid classes were assessed up to four times by a validated food frequency questionnaire. Repeated-measures regression models were used to examine the longitudinal association between total and six flavonoid classes and annualized change in the eight cognitive domains.
Setting
The Framingham Heart Study (FHS), a prospective cohort study.
Participants
One thousand seven hundred and seventy-nine subjects who were free of dementia, aged ≥45 years, and had attended at least two of the last three FHS Offspring cohort study exams.
Results
Over a median follow-up of 11.8 years among 1779 participants, nominally significant trends toward a slower decline in cognitive function were observed among those with higher flavonol and flavan-3-ol intakes for global function, verbal and visual memory; higher total flavonoids and flavonoid polymers for visual memory; and higher flavonols for verbal learning.
Conclusions
In spite of modest nominal trends, overall our findings do not support a clear association between higher long-term flavonoid intake and slowed age-related cognitive decline.
Keywords: Age-related cognitive decline, diet, flavonoid intake, observational study, longitudinal study
Introduction
Age-related cognitive decline is regarded as one of the most important public health challenges in the U.S. because of the rapidly growing population of older adults (1, 2). Diminished cognitive function compromises the quality of life and independence of later life and presents large societal and economic burdens (3–5).
To date, there are no effective means to alter the progression of age-related cognitive decline (6).Therefore, the identification of strategies through which it can be prevented, minimized, or delayed, may help immensely in maintaining cognitive health across the adult life-span (6, 7). There is limited evidence that certain dietary patterns, including the Mediterranean (8, 9) and Dietary Approaches to Stop Hypertension (DASH) (9, 10) patterns, and a pattern combining aspects of both of these, the Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) (11) dietary pattern, are associated with a slower age-related cognitive decline. These dietary patterns stress the importance of consuming plant-based foods such as fruits and vegetables. Bioactive components of these foods have also been widely explored for their potential neurocognitive benefits, particularly polyphenols such as the flavonoids (12–16).
Evidence from acute and short-term intervention studies on possible neurocognitive effects of flavonoid rich foods is promising but limited by the brief duration of exposure (17–24), and thus offer little understanding of the long-term benefits of flavonoids on age-related changes in cognitive health. On the other hand, available observational studies investigating the relation between flavonoid or flavonoid-rich food intake and changes in cognitive function have revealed inconclusive results. Yet, these existing observational studies have many notable limitations. These include the use of cross-sectional design, which precludes the establishment of a causal relationship between flavonoid intake and cognition (25–29). Longitudinal studies of the relation between flavonoid intake and age-related cognitive changes also have limitations such as the lack of repeated assessments of flavonoid intake during follow-up (30–32), the utilization of incomplete flavonoid databases (33), and the use of insensitive cognitive assessment tools such as the Mini Mental State Examination (MMSE) (31, 32, 34). Therefore, the existing evidence for the long-term association between flavonoid intake and age-related changes in cognitive function is similarly limited.
To address the shortcomings in our current understanding of the relationship between flavonoid intake and cognitive health, we undertook this study to examine the association between long-term intake of total and six classes of dietary flavonoids and decline in cognitive function using an extensive battery of neuropsychological (NP) tests based on repeated dietary and cognitive health assessments spanning up to 15 years.
Methods
Study population
The Framingham Heart Study (FHS), a prospective cohort study designed to explore risk factors associated with heart disease, started in 1948 with a total of 5,209 individuals aged 28–62 years old who were residents of Framingham, Massachusetts, U.S.A (35). In 1970, the Framingham Offspring Study cohort was established consisting of the 5,124 men and women who were offspring of the original cohort and their spouses (36). About every four years, this Offspring cohort completes a series of questionnaires, laboratory and cardiovascular tests, and undergoes a physical examination. Additionally, the cohort is closely monitored for various incident outcomes including, but not limited to, cardiovascular disease, stroke, diabetes, hypertension, and dementia.
The present study utilized data derived from the Offspring cohort 7th (1998–2001), 8th (2005–2008), and 9th (2011–2014) study examinations. NP testing was initiated in the Offspring cohort as separately funded ancillary studies, and was performed in conjunction with the 7th, 8th, and 9th Offspring cohort exams. As the parent and ancillary study examinations were not conducted concurrently, it was possible for protracted time differences between theses exams. Therefore, we assigned a six month lower cutoff and a three year upper cutoff for the length of time between any exam cycle and the NP testing.
Figure 1 displays the flow diagram of our study participants. Our eligibility criteria included age ≥45 years at baseline, absence of dementia, and attendance of at least two of the three study exams as a minimum of two exams were needed to establish change in NP tests. For participants free of dementia and aged ≥45 years at the 7th exam and attended the 8th and/or 9th exam, we used the 7th exam as their baseline exam. For those who did not attend the 7th exam, but did attend the 8th and 9th exams, we used the 8th exam as their baseline if they were aged ≥45 years and were free of dementia at this exam. Based on these criteria, there were 1918 eligible participants who contributed a total of 2868 observations. Of these observations, 196 were excluded because of an inability to impute body mass index (BMI, n=2); having fewer than two valid dietary data points (n=136; as described below); or having mild cognitive impairment (MCI) at exams 5 or 6 (n=58; as described below). Our final sample was comprised of 2672 observations from 1779 eligible participants: 1527 observations in the 7th–8th exam interval, 1054 observations in the 8th–9th exam interval (893 individuals had observations included in both the 7th–8th and 8th–9th exam intervals), and 91 observations in the 7th–9th exam interval. Participants who attended all three exams had two observations whereas participants who attended two exams had only one observation. We considered the rate of change in NP test scores between exams as the study unit of observation. This approach simplified the interpretation as there was large variability between participants for time between NP exams.
Figure 1.
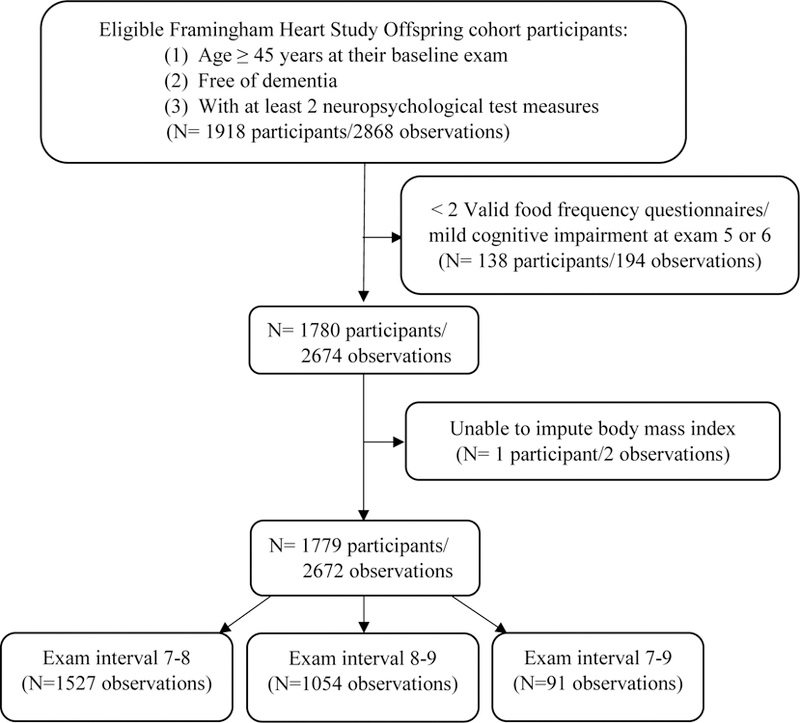
Flow diagram: Eligible Framingham Heart Offspring Cohort Study participants and observations, which represent the annualized change in neuropsychological tests between study exams.
Flavonoid Exposures
Dietary assessment
Dietary intakes of the participants were assessed at the 5th through the 8th Offspring cohort exam cycles using a validated semi-quantitative food frequency questionnaire (FFQ) developed by Willett et al. (Harvard FFQ) (37). The FFQs were mailed to the participants before the examination and the participants were asked to bring the completed questionnaire with them to their appointment. The FFQ consists of a list of 126 foods with a standard serving size and a selection of nine frequency categories ranging from “never or <one serving/month” to “≥ six servings/day.” Participants were asked to report their frequency of consumption of each food item during the last year. Participants could also add up to four additional foods that were important components of their diets but were not listed on the questionnaire. The FFQ also includes separate questions about use of vitamin and mineral supplements and type of breakfast cereal most commonly consumed. Intakes of food components, including both nutrients and non-nutrients, were computed by multiplying the frequency of consumption of each food item by the nutrient content of the specified portions.
The validity of flavonoid intake from the Harvard FFQ has not been directly evaluated, but the validity of food intake measurements based on a comparison between the FFQ and two 7-day diet records collected during the year time interval covered by the FFQ has been previously reported (38). This comparison revealed relatively high correlation coefficients between intakes from the FFQ and 7-day diet records for the major dietary sources of flavonoids in the Framingham Offspring cohort. These foods included red wine (0.83), orange juice (0.78), tea (0.77), oranges (0.76), apples/pears (0.70), and strawberries (0.38).
Validity of FFQ
An FFQ was judged as invalid if (1) reported energy intakes were <2510 kJ/d (<600 kcal/d) or >16736 kJ/d (>4000 kcal/d) for women, and >17573 kJ/d (>4200 kcal/d) for men, respectively; (2) more than 12 food items were left blank; or (3) participants had signs of significant cognitive decline (MCI) due to the concern of the accuracy of dietary reporting by individuals with evidence of cognitive impairment short of mild dementia. For this purpose, we defined MCI as performance falling below MMSE education-adjusted cutoff scores (score <22 for fewer than seven years of education, score <24 for 8–11 years of education, score <25 for high school graduate, and score <26 for any education beyond high school) (39). Any participant who had MCI at either exam 5 or 6 was excluded.
Characterizing Flavonoid intakes
The exposure of interest was the habitual intake of total and six principal flavonoid classes commonly consumed in the U.S. diet including flavonols, flavones, flavanones, flavan-3-ols, anthocyanins, and flavonoid polymers, which were derived using the USDA flavonoid content of foods and the proanthocyanidin databases (40, 41). Intakes of individual flavonoid compounds were calculated as the sum of the consumption frequency of each food multiplied by the content of the specific flavonoid for the specified portion size. To define the six flavonoid classes, we used the flavonoid classification of Cassidy et al. (42). Total flavonoid intakes were calculated by the addition of intakes of all six flavonoid classes. Isoflavone intakes were not evaluated, as their habitual consumption in the U.S. diet are very low (43–45). We categorized our exposure into quartile categories of intake. Given the available evidence on the potential neurocognitive benefit of total flavonoids (31, 46–48) and the four flavonoid classes: flavonols (15, 29, 46), flavanones (17, 20), flavan-3-ols (19, 21), and anthocyanins (18, 22, 23, 48), we considered these flavonoid classes as our primary exposures. As fewer data are available on the possible protective cognitive effects of flavones and flavonoid polymers, they were considered as secondary exposures.
NP Outcomes
The outcome of this study was the annualized change in cognitive function as assessed by a battery of NP tests that target various cognitive domains. Table 1 displays the NP test battery administered to the Framingham Offspring cohort along with their associated cognitive domains (49). Our analyses were comprised of 13 commonly used NP tests to assess cognitive decline in the following eight cognitive domains: (1) verbal memory, (2) visual memory, (3) verbal learning, (4) attention and concentration, (5) abstract reasoning, (6) language, (7) visuoperceptual organization, and the (8) global cognitive function. For cognitive domains represented by more than one NP test (verbal and visual memory, verbal learning, and attention and concentration), overall domain scores were calculated by averaging all of their associated NP test scores. The remaining NP tests (abstract reasoning, language, and visuoperceptual organization) each represented a unique cognitive domain. We also calculated a global cognitive function domain score by averaging all the individual scores from the aforementioned cognitive domains. As higher scores for the attention and concertation domain reflected poorer performance, we subtracted this domain score from the sum of the other domains in creating the average. As each NP test score was scaled differently, we used z-scores to create standardized cognitive domain scores, which were calculated by subtracting each participant’s score on a given NP test from the overall mean score of our sample for that NP test divided by the sample standard deviation.
Table 1.
Components of the neuropsychological test battery
| Cognitive Domain | NP test Measure | Range (min-max) | Number of observations | Number of participants | Higher score indicates |
|---|---|---|---|---|---|
| Verbal memory | -WMS-III Logical Memory-Immediate Recall | (0–23) | 2641 | 1769 | Better performance |
| -WMS-III Logical Memory-Delayed Recall | (0–23) | 2630 | 1760 | ||
| -WMS-III Logical Memory-Delayed Recognition | (0–11) | 2635 | 1760 | ||
| Visual Memory | -WMS-III Visual Reproductions-Immediate Recall | (0–14) | 2636 | 1763 | Better performance |
| -WMS-III Visual Reproductions-Delayed Recall | (0–140) | 2623 | 1756 | ||
| -WMS-III Visual Reproductions-Delayed Recognition | (0–4) | 2619 | 1754 | ||
| Verbal Learning | -WMS-III Paired Associates-Immediate Recall | (0–21) | 2598 | 1746 | Better performance |
| -WMS-III Paired Associates-Delayed Recall | (0–10) | 2609 | 1742 | ||
| Attention and Concentration | -Trail-making Test A (Trails A) | (0.10–7.0) | 2582 | 1727 | Poorer performance |
| -Trail-making Test B (Trails B) | (0.32–10.0) | 2506 | 1682 | ||
| Abstract Reasoning | WAIS-III Similarities subtest | (0–26) | 2648 | 1767 | Better performance |
| Language | Boston Naming Test (30-item version) | (0–30) | 2602 | 1747 | Better performance |
| Visuoperceptual Organization | Hooper Visual Organization Test | (0–30) | 2524 | 1701 | Better performance |
| Global Function | An average of the individual cognitive domain scores | ||||
WMS, Wechsler Memory Scale; WAIS, Wechsler Adult Intelligence Scale.
Covariates
We considered the following covariates as potential confounders in our analyses: age, sex, education (≤high school graduate, some or college graduate, >college graduate), prevalent stroke, hypercholesterolemia, hypertension, and diabetes, having at least one Apolipoprotein E ε4 allele (ApoE ε4), BMI (kg/m2), current smoking status (smoking within the past year: yes/no), physical activity index (PAI) expressed in metabolic equivalents (Mets) (50), total energy intake (TEI, kJ/d), overall diet quality (as assessed by the 2010 Dietary Guidelines Adherence Index, DGAI) (51), vitamin and mineral supplement use, and dietary intakes of non-nutritive sweetened beverages (52) (servings/week), caffeine (mg/d), alcohol (g/d), omega-3 fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) (g/d), and lutein and zeaxanthin (mcg/d).
We defined stroke as having any of the following: atherothrombotic infarction of brain, cerebral embolism, intracerebral hemorrhage, and subarachnoid hemorrhage (53). Hypercholesterolemia was determined by either taking cholesterol lowering medication or having total cholesterol levels ≥200 mg/dL. Hypertension was defined as either taking blood pressure lowering medication or having systolic or diastolic blood pressure of ≥130 mmHg and/or ≥80 mmHg respectively (54). Diabetes was assessed by any of the following criteria: (1) taking oral hypoglycemic medication; (2) insulin use; (3) fasting blood glucose levels ≥126 mg/dL; (4) non-fasting blood glucose levels ≥200 mg/dL.
In attempts to minimize missing covariate data in our analyses, we imputed the following continuous covariates: height, BMI, and PAI. Height was imputed by bringing forward height data from the previous exam. BMI was imputed by using the available weight and imputed height data. PAI was imputed by determining the median PAI score of the sample who had available PAI data, stratified by gender, BMI, age, and perceived health status, and applying those medians to participants with missing PAI data.
Statistical analyses
Main analyses
All data analyses were performed using SAS software (version 9.4; SAS Institute, Cary, NC). Our analyses focused on the standardized annualized change in the following eight cognitive domain scores: verbal and visual memory, verbal learning, attention and concentration, abstract reasoning, language, visuoperceptual organization, and the global function. The change in each NP test score was calculated for each participant as the difference between NP tests at two exam cycles with valid data. Further, we divided the change by the exam interval length (in years) in order to create the standardized annualized change. As the standardized annualized change was small across all cognitive domains, we multiplied it by 1000 in order to reduce the number of significant decimal points for the purpose of display in the tables. We used the standardized annualized change as our outcome in order to correct for the unequal time intervals between the exam cycles and to allow for missing the intermediate exam. In order to assess long-term flavonoid intake at each exam interval, we used the cumulative average of flavonoid intakes from Offspring exams 5 through 7/8. That is, for exam intervals 7–8 and 7–9, we used the cumulative average of flavonoid intakes from Offspring exams 5 through 7, and for exam interval 8–9, we used the cumulative average of flavonoid intakes from Offspring exams 5 through 8. Participants were required to have at least two valid FFQs in order to establish long-term/cumulative flavonoid intake. We categorized our exposures of interest, which included the cumulative average of total and six classes of flavonoids (flavonols, flavones, flavanones, flavan-3-ols, anthocyanins, flavonoid polymers) by quartiles of intake based on cumulative intake through each participant’s baseline.
Repeated-measures regression model (SAS PROC MIXED) was used to examine the longitudinal association between long-term total and each of the classes of dietary flavonoids and the standardized annualized change in the eight cognitive domain scores within exam intervals across the follow-up. Linear trend assessments across quartile categories of intake were performed by using the median value of intake within each category treated as a continuous variable. We used baseline data for the non-dietary covariates and cumulative average data for the dietary covariates for each exam interval. In order to minimize missing categorical covariate data, ApoE ε4, and prevalent diabetes and hypercholesterolemia included a third category for missing data. We used a nominal P value of 0.05 to present our results, which was not adjusted for the number of associations that were tested. Dunnett’s correction for multiple comparisons was used to assess any significant differences between means of cognitive scores in the three higher quartile categories relative to those in the lowest intake category for the eight cognitive domains.
Our main analyses consisted of the following four models:
Model 1 (basic model): sex, age, education, TEI, ApoE ε4, and baseline measure of the cognitive domain score (i.e., the score at the beginning of the exam interval).
Model 2 (clinical model): Model 1 covariates + BMI, prevalent stroke, diabetes, hypertension, and hypercholesterolemia.
Model 3 (lifestyle model): Model 1 covariates + PAI and smoking status.
Model 4 (dietary model): Model 1 covariates + overall dietary quality, vitamin and mineral supplement use, and dietary intakes of non-nutritive sweetened beverages, caffeine, alcohol, omega-3 fatty acids-EPA and DHA, and lutein and zeaxanthin.
Sensitivity analyses
In addition to our main analyses, we tested the potential effect modification of sex, age, ApoE ε4, and lag time between the NP ancillary exams at which NP testing was performed and their corresponding clinical exams at which dietary information was collected.
Results
The cumulative flavonoid intake quartile category description (median (minimum, maximum)) of our sample (n=1779) at baseline across total flavonoids and six flavonoid classes is presented in Table 2. Table 3 displays the baseline characteristics of participants (n =1779) based on total and extreme quartiles of cumulative total flavonoid intake. Overall, the mean age (95% confidence interval, CI) of the participants was 60.8 (60.4, 61.2) years and 55.9% were female. Participants were on average overweight, highly educated, moderately physically active, and had a low prevalence of smoking, diabetes and stroke. However, they had a high prevalence of hypertension and hypercholesterolemia and had on average moderate 2010 DGAI scores, a measure of overall diet quality. Relative to participants with lower intakes of total flavonoids, those with higher intakes were more likely to be women, highly educated, and physically active; and to consume more calories and a higher quality diet. They also smoked less and had a lower BMI.
Table 2.
Cumulative flavonoid intake quartile category description for members of the Framingham Study Offspring Cohort at baseline
| Flavonoid quartiles | ||||||||
|---|---|---|---|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | |||||
| Flavonoid class (mg/d) | Median | Min, Max | Median | Min, Max | Median | Min, Max | Median | Min, Max |
| Total flavonoids | 114.71 | 19.07, 155.25 | 199.39 | 155.66, 245.75 | 301.76 | 246.01, 389.68 | 532.06 | 389.99, 2021.20 |
| Flavonols | 6.053 | 1.347, 7.920 | 9.905 | 7.923, 11.733 | 13.963 | 11.735, 16.883 | 21.423 | 16.900, 78.015 |
| Flavones | 0.700 | 0.025, 1.100 | 1.482 | 1.103, 1.870 | 2.266 | 1.880, 2.693 | 3.465 | 2.700, 10.978 |
| Flavanones | 9.27 | 0.00, 18.85 | 29.50 | 18.86, 39.56 | 52.78 | 39.86, 61.91 | 79.44 | 61.95, 310.05 |
| Flavan-3-ols | 10.27 | 0.05, 15.30 | 20.83 | 15.35, 28.00 | 37.43 | 28.02, 53.63 | 89.67 | 53.92, 411.11 |
| Anthocyanins | 3.48 | 0.00, 6.03 | 8.88 | 6.04, 11.66 | 15.42 | 11.70, 19.61 | 27.34 | 19.63, 168.55 |
| Flavonoid polymers | 55.14 | 0.57, 79.63 | 106.80 | 79.80, 138.09 | 177.89 | 138.15, 241.47 | 351.16 | 241.78, 1530.24 |
Table 3.
Age- and sex-adjusted baseline characteristics of participants based on total sample and extreme quartiles of cumulative total flavonoid intake
| Characteristic | Total sample (n=1779)* | Total flavonoid intake quartile category | |||||
|---|---|---|---|---|---|---|---|
| Quartile 1 (n=445) | Quartile 4 (n=445) | P-trend† | |||||
| Total flavonoid intake (mg/d)‡ | 246 | (19, 2021) | 115 | (19, 155) | 532 | (390, 2021) | |
| Age (years)§ | 60.8 | (60.4, 61.2) | 60.3 | (59.5, 61.1) | 60.9 | (60.1, 61.7) | 0.35 |
| Female (%)‖ | 55.9 | (53.6, 58.2) | 50.7 | (46.1, 55.3) | 69.0 | (64.4, 73.6) | <0.0001 |
| BMI (kg/m2)§ | 28.0 | (27.8, 28.2) | 28.6 | (28.2, 29.1) | 27.1 | (26.6, 27.6) | <0.0001 |
| College degree + (%)‖ | 74.4 | (72.4, 76.4) | 66.1 | (62.1, 70.0) | 77.9 | (73.9, 82.0) | 0.0006 |
| ApoE ε4 (%)*,‖ | 23.1 | (21.1, 25.1) | 23.3 | (19.3, 27.2) | 25.5 | (21.5, 29.6) | 0.24 |
| Current smoker (%)‖ | 10.0 | (8.6, 11.4) | 15.3 | (12.5, 18.0) | 8.3 | (5.5, 11.1) | 0.003 |
| Physical activity index (Mets)§ | 37.4 | (37.2, 37.7) | 36.7 | (36.2, 37.3) | 38.0 | (37.4, 38.6) | 0.0005 |
| Diabetes (%)*,‖ | 9.8 | (8.5, 11.2) | 11.6 | (8.9, 14.3) | 7.3 | (4.6, 10.0) | 0.07 |
| Hypertension (%)‖ | 58.9 | (56.7, 61.2) | 60.1 | (55.6, 64.5) | 56.0 | (51.5, 60.6) | 0.14 |
| Stroke (%)‖ | 1.4 | (0.8, 1.9) | 0.5 | (0.0, 1.6) | 1.4 | (0.3, 2.5) | 0.42 |
| Hypercholesterolemia (%)*,‖ | 63.2 | (61.0, 65.5) | 64.6 | (60.2, 69.1) | 61.2 | (56.7, 65.8) | 0.26 |
| Oral hypoglycemic medication use (%)‖ | 5.3 | (4.3, 6.3) | 5.1 | (3.1, 7.1) | 4.0 | (1.9, 6.0) | 0.35 |
| Cholesterol lowering medication use (%)‖ | 22.0 | (20.1, 23.9) | 24.5 | (20.7, 28.3) | 17.9 | (14.1, 21.8) | 0.06 |
| Hypertension medication use (%)‖ | 31.8 | (29.8, 33.9) | 32.3 | (28.2, 36.4) | 28.7 | (24.6, 32.9) | 0.20 |
| Total energy intake (kJ/d)§ | 7905 | (7805, 8005) | 6760 | (6573, 6948) | 8673 | (8483, 8864) | <0.0001 |
| DGAI§ | 60.5 | (60.0, 60.9) | 54.5 | (53.6, 55.4) | 63.2 | (62.3, 64.1) | <0.0001 |
BMI, body mass index; ApoE ɛ4, apolipoprotein E ɛ4; DGAI, dietary guideline adherence index.
37 missing ApoE ε4, 11 missing diabetes, 11 missing hypercholesterolemia, 24 missing DGAI.
P values for the test of linear trend across extreme quartile categories of total flavonoid intake were based on linear regression models with the median intake of each extreme quartile category assigned to individuals with intake in that category, and this quartile median variable was used as a continuous measure in regression models.
Values are medians; minimum and maximums in parentheses.
All values are age- and sex-adjusted (least-squares) means; 95% confidence intervals (CIs) in parentheses.
All values are age- and sex-adjusted (least-squares) percentages; 95% CIs in parentheses.
Findings of the repeated-measures regression analyses relating long-term intake of total and six flavonoid classes to standardized annualized change in eight cognitive domains were largely null, even at a nominal P value of 0.05 (Tables 4a–h and Supplemental Tables 1–8). Nominally significant trends toward a slower decline in cognitive function among those with the highest flavonol intakes were seen in at least three of the four statistical models of the following four cognitive domains: global function (Table 4a and Supplemental Table 1), verbal memory (Table 4b and Supplemental Table 2), visual memory (Table 4c and Supplemental Table 3), and verbal learning (Table 4d and supplemental Table 4). However, after Dunnett’s correction for multiple comparisons, the mean scores in the highest quartile category of flavonols was only significantly different from those in the lowest quartile category for the verbal memory (Table 4b and Supplemental Table 2; models 1–3) and visual memory (Table 4c and Supplemental Table 3; models 1 and 2) cognitive domains. The same nominal significant trends were seen for flavan-3-ols in at least three of the four statistical models of the global, verbal and visual memory cognitive domains. Yet, after Dunnett’s correction, relative to those in the lowest quartile category of flavan-3-ol intakes, the mean scores of those in the highest quartile category was significantly different only for the verbal memory (models 1–3), and visual memory (model 2) cognitive domains. As well, similar trends were observed for flavonoid polymers in all the four statistical models, and for total flavonoids in two of the four statistical models of the visual memory cognitive domain. Yet, none of the visual memory scores in the upper quartile category means of these two flavonoid classes were significantly different from the means on the lowest intake quartile after Dunnett’s correction except for flavonoid polymers in model 2. None of the other flavonoid classes showed similar inverse trends for any of the eight cognitive domains.
Table 4a.
Mean annualized change in the standardized rate x1000* of cognitive decline over a median follow-up of 11.8 years, across quartiles of total and 6 classes of flavonoid intake: Global function cognitive domain†
| Flavonoid quartiles | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | ||||||
| Flavonoid class | Mean | (95% CIs) | Mean | (95% CIs) | Mean | (95% CIs) | Mean | (95% CIs) | P-trend‡ |
| Total flavonoids§ | −18.5 | (−22.8,−14.1) | −18.3 | (−22.3,−14.3) | −14.8 | (−18.9,−10.6) | −14.9 | (−19.1,−10.7) | 0.19 |
| Flavonols | −20.1 | (−24.4,−15.9) | −17.8 | (−21.9,−13.7) | −14.8 | (−18.9,−10.7) | −13.9 | (−18.0,−9.7) | 0.04 |
| Flavones | −16.0 | (−20.2,−11.9) | −16.1 | (−20.1,−12.0) | −16.8 | (−20.9,−12.7) | −17.6 | (−21.8,−13.4) | 0.58 |
| Flavanones | −15.4 | (−19.5,−11.3) | −15.7 | (−19.7,−11.6) | −17.9 | (−21.9,−13.8) | −17.6 | (−22.0,−13.3) | 0.36 |
| Flavan-3-ols | −20.6 | (−24.8,−16.3) | −17.0 | (−21.0,−12.9) | −15.3 | (−19.4,−11.2) | −13.7 | (−17.8,−9.6) | 0.04 |
| Anthocyanins | −15.9 | (−20.3,−11.5) | −16.3 | (−20.4,−12.2) | −17.3 | (−21.3,−13.3) | −16.9 | (−20.9,−12.9) | 0.77 |
| Flavonoid polymers | −18.4 | (−22.8,−14.0) | −20.0 | (−24.0,−16.0) | −13.5 | (−17.6,−9.5) | −14.6 | (−18.7,−10.5) | 0.10 |
All values are mean least squares annualized change in the global function cognitive domain score multiplied by 1000; 95% confidence intervals (CIs) in parentheses.
Of the total 2672 observations, there was 2255 complete data for the global function cognitive domain.
P values for the test of linear trend across quartile categories were based on linear regression models with the median intake of each quartile category assigned to individuals with intake in that category, and this quartile median variable was used as a continuous measure in regression models.
Adjusted for age, sex, education, total energy intake, Apolipoprotein E ε4, and global function score at the beginning of the exam interval (model 1).
Table 4h.
Mean annualized change in the standardized rate x1000* of cognitive decline over a median follow-up of 11.8 years, across quartiles of total and 6 classes of flavonoid intake: Visuoperceptual organization cognitive domain†
| Flavonoid quartiles | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | ||||||
| Flavonoid class | Mean | (95% CIs) | Mean | (95% CIs) | Mean | (95% CIs) | Mean | (95% CIs) | P-trend‡ |
| Total flavonoids§ | −23.5 | (−32.1,−15.0) | −20.6 | (−28.5,−12.7) | −24.2 | (−32.3,−16.2) | −16.7 | (−24.9,−8.5) | 0.30 |
| Flavonols | −22.7 | (−31.1,−14.3) | −22.2 | (−30.2,−14.2) | −22.4 | (−30.5,−14.3) | −17.6 | (−25.7,−9.5) | 0.37 |
| Flavones | −23.7 | (−32.0,−15.4) | −18.5 | (−26.4,−10.5) | −21.9 | (−29.9,−13.8) | −21.0 | (−29.3,−12.8) | 0.85 |
| Flavanones | −20.9 | (−29.1,−12.7) | −22.2 | (−30.2,−14.2) | −21.1 | (−29.0,−13.2) | −20.6 | (−29.1,−12.2) | 0.93 |
| Flavan-3-ols | −21.3 | (−29.7,−13.0) | −20.9 | (−28.9,−12.9) | −25.4 | (−33.5,−17.3) | −17.4 | (−25.5,−9.3) | 0.42 |
| Anthocyanins | −16.2 | (−24.8,−7.6) | −21.9 | (−30.1,−13.8) | −25.3 | (−33.2,−17.3) | −21.1 | (−28.9,−13.2) | 0.67 |
| Flavonoid polymers | −22.2 | (−30.9,−13.6) | −24.8 | (−32.7,−16.8) | −19.0 | (−26.9,−11.0) | −19.0 | (−27.1,−10.9) | 0.44 |
All values are mean least squares annualized change in the visuoperceptual organization cognitive domain score multiplied by 1000; 95% confidence intervals (CIs) in parentheses.
Of the total 2672 observations, there was 2524 complete data for the visuoperceptual organization cognitive domain.
P values for the test of linear trend across quartile categories were based on linear regression models with the median intake of each quartile category assigned to individuals with intake in that category, and this quartile median variable was used as a continuous measure in regression models.
Adjusted for age, sex, education, total energy intake, Apolipoprotein E ε4, and visuoperceptual organization score at the beginning of the exam interval (model 1).
Table 4e.
Mean annualized change in the standardized rate x1000* of cognitive decline over a median follow-up of 11.8 years, across quartiles of total and 6 classes of flavonoid intake: Attention and concentration cognitive domain†
| Flavonoid quartiles | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | ||||||
| Flavonoid class | Mean | (95% CIs) | Mean | (95% CIs) | Mean | (95% CIs) | Mean | (95% CIs) | P-trend‡ |
| Total flavonoids§ | 27.6 | (19.8,35.4) | 25.8 | (18.6,33.1) | 27.1 | (19.7,34.4) | 34.0 | (26.7,41.4) | 0.12 |
| Flavonols | 28.7 | (21.1,36.4) | 29.6 | (22.3,36.9) | 25.0 | (17.7,32.3) | 31.3 | (23.9,38.6) | 0.69 |
| Flavones | 29.1 | (21.5,36.7) | 23.9 | (16.8,31.1) | 27.4 | (20.1,34.7) | 34.4 | (27.0,41.9) | 0.20 |
| Flavanones | 28.8 | (21.4,36.3) | 25.2 | (18.0,32.4) | 29.0 | (21.8,36.2) | 31.9 | (24.2,39.6) | 0.40 |
| Flavan-3-ols | 28.1 | (20.5,35.7) | 28.1 | (20.8,35.4) | 27.9 | (20.6,35.3) | 30.5 | (23.2,37.8) | 0.58 |
| Anthocyanins | 27.7 | (19.9,35.6) | 30.6 | (23.2,38.1) | 31.4 | (24.2,38.6) | 25.2 | (18.0,32.3) | 0.41 |
| Flavonoid polymers | 25.0 | (17.1,32.9) | 31.0 | (23.8,38.3) | 25.3 | (18.0,32.5) | 33.0 | (25.7,40.3) | 0.21 |
All values are mean least squares annualized change in the attention and concentration cognitive domain score multiplied by 1000; 95% confidence intervals (CIs) in parentheses.
Of the total 2672 observations, there was 2491 complete data for the attention and concentration cognitive domain.
P values for the test of linear trend across quartile categories were based on linear regression models with the median intake of each quartile category assigned to individuals with intake in that category, and this quartile median variable was used as a continuous measure in regression models.
Adjusted for age, sex, education, total energy intake, Apolipoprotein E ε4, and attention and concentration score at the beginning of the exam interval (model 1).
Table 4f.
Mean annualized change in the standardized rate x1000* of cognitive decline over a median follow-up of 11.8 years, across quartiles of total and 6 classes of flavonoid intake: Abstract reasoning cognitive domain†
| Flavonoid quartiles | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | ||||||
| Flavonoid class | Mean | (95% CIs) | Mean | (95% CIs) | Mean | (95% CIs) | Mean | (95% CIs) | P-trend‡ |
| Total flavonoids§ | −25.2 | (−34.7,−15.8) | −14.7 | (−23.4,−5.9) | −10.5 | (−19.3,−1.6) | −21.1 | (−30.0,−12.2) | 0.97 |
| Flavonols | −26.6 | (−35.9,−17.3) | −14.1 | (−22.9,−5.3) | −10.8 | (−19.7,−2.0) | −19.7 | (−28.6,−10.8) | 0.56 |
| Flavones | −21.2 | (−30.4,−12.0) | −15.1 | (−23.8,−6.4) | −21.5 | (−30.3,−12.6) | −13.7 | (−22.7,−4.6) | 0.40 |
| Flavanones | −13.8 | (−22.9,−4.8) | −16.5 | (−25.3,−7.8) | −21.9 | (−30.6,−13.2) | −18.7 | (−27.9,−9.6) | 0.36 |
| Flavan-3-ols | −25.9 | (−35.1,−16.7) | −13.9 | (−22.7,−5.0) | −11.7 | (−20.5,−2.9) | −19.9 | (−28.8,−11.0) | 0.95 |
| Anthocyanins | −20.7 | (−30.2,−11.1) | −20.0 | (−28.9,−11.0) | −14.9 | (−23.6,−6.1) | −16.2 | (−24.8,−7.6) | 0.49 |
| Flavonoid polymers | −23.3 | (−32.9,−13.8) | −16.0 | (−24.8,−7.3) | −10.2 | (−19.0,−1.5) | −21.9 | (−30.7,−13.0) | 0.75 |
All values are mean least squares annualized change in the abstract reasoning cognitive domain score multiplied by 1000; 95% confidence intervals (CIs) in parentheses.
Of the total 2672 observations, there was 2648 complete data for the abstract reasoning cognitive domain.
P values for the test of linear trend across quartile categories were based on linear regression models with the median intake of each quartile category assigned to individuals with intake in that category, and this quartile median variable was used as a continuous measure in regression models.
Adjusted for age, sex, education, total energy intake, Apolipoprotein E ε4, and abstract reasoning score at the beginning of the exam interval (model 1).
Table 4g.
Mean annualized change in the standardized rate x1000* of cognitive decline over a median follow-up of 11.8 years, across quartiles of total and 6 classes of flavonoid intake: Language domain†
| Flavonoid quartiles | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | ||||||
| Flavonoid class | Mean | (95% CIs) | Mean | (95% CIs) | Mean | (95% CIs) | Mean | (95% CIs) | P-trend‡ |
| Total flavonoids§ | −20.7 | (−28.6,−12.8) | −18.0 | (−25.3,−10.7) | −10.7 | (−18.2,−3.3) | −21.4 | (−28.9,−14.0) | 0.72 |
| Flavonols | −23.8 | (−31.6,−16.1) | −15.0 | (−22.3,−7.6) | −15.0 | (−22.4,−7.6) | −17.2 | (−24.7,−9.8) | 0.42 |
| Flavones | −15.5 | (−23.2,−7.9) | −18.2 | (−25.5,−11.0) | −18.0 | (−25.4,−10.6) | −19.0 | (−26.6,−11.5) | 0.57 |
| Flavanones | −13.3 | (−20.9,−5.8) | −16.4 | (−23.7,−9.1) | −21.3 | (−28.6,−14.0) | −19.8 | (−27.5,−12.1) | 0.18 |
| Flavan-3-ols | −20.4 | (−28.0,−12.7) | −16.9 | (−24.3,−9.5) | −16.9 | (−24.3,−9.4) | −16.8 | (−24.2,−9.4) | 0.65 |
| Anthocyanins | −16.3 | (−24.2,−8.3) | −13.7 | (−21.2,−6.1) | −21.4 | (−28.6,−14.1) | −19.0 | (−26.2,−11.8) | 0.47 |
| Flavonoid polymers | −22.0 | (−29.9,−14.1) | −19.3 | (−26.6,−12.0) | −8.3 | (−15.6,−0.9) | −21.6 | (−29.0,−14.2) | 0.84 |
All values are mean least squares annualized change in the language cognitive domain score multiplied by 1000; 95% confidence intervals (CIs) in parentheses.
Of the total 2672 observations, there was 2602 complete data for the language cognitive domain.
P values for the test of linear trend across quartile categories were based on linear regression models with the median intake of each quartile category assigned to individuals with intake in that category, and this quartile median variable was used as a continuous measure in regression models.
Adjusted for age, sex, education, total energy intake, Apolipoprotein E ε4, and language score at the beginning of the exam interval (model 1).
Table 4b.
Mean annualized change in the standardized rate x1000* of cognitive decline over a median follow-up of 11.8 years, across quartiles of total and 6 classes of flavonoid intake: Verbal memory cognitive domain†
| Flavonoid quartiles | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | ||||||
| Flavonoid class | Mean | (95% CIs) | Mean | (95% CIs) | Mean | (95% CIs) | Mean | (95% CIs) | P-trend‡ |
| Total flavonoids§ | −9.6 | (−19.0,−0.3) | −9.6 | (−18.3,−0.8) | 0.0 | (−8.9,8.8) | −2.1 | (−11.0,6.8) | 0.19 |
| Flavonols | −16.6 | (−25.9,−7.4) | −3.2 | (−11.9,5.6) | −3.2 | (−12.0,5.5) | 1.6‖ | (−7.3,10.5) | 0.02 |
| Flavones | −9.1 | (−18.2,0.0) | −2.2 | (−10.9,6.5) | −6.6 | (−15.3,2.2) | −3.5 | (−12.6,5.5) | 0.57 |
| Flavanones | −6.1 | (−15.1,2.9) | 1.4 | (−7.3,10.1) | −11.0 | (−19.7,−2.3) | −5.5 | (−14.7,3.6) | 0.59 |
| Flavan-3-ols | −13.2 | (−22.4,−4.0) | −4.6 | (−13.4,4.2) | −6.3 | (−15.1,2.5) | 2.6‖ | (−6.2,11.5) | 0.03 |
| Anthocyanins | −8.8 | (−18.3,0.7) | −5.4 | (−14.3,3.5) | −2.0 | (−10.7,6.7) | −5.4 | (−13.9,3.2) | 0.67 |
| Flavonoid polymers | −11.9 | (−21.3,−2.4) | −8.6 | (−17.3,0.1) | 0.9 | (−7.8,9.7) | −2.2 | (−11.0,6.7) | 0.16 |
All values are mean least squares annualized change in the verbal memory cognitive domain score multiplied by 1000; 95% confidence intervals (CIs) in parentheses.
Of the total 2672 observations, there was 2603 complete data for the verbal memory cognitive domain.
P values for the test of linear trend across quartile categories were based on linear regression models with the median intake of each quartile category assigned to individuals with intake in that category, and this quartile median variable was used as a continuous measure in regression models.
Adjusted for age, sex, education, total energy intake, Apolipoprotein E ε4, and verbal memory score at the beginning of the exam interval (model 1).
Significantly different from Quartile 1 by Dunnett’s test (P<0.05).
Table 4c.
Mean annualized change in the standardized rate x1000* of cognitive decline over a median follow-up of 11.8 years, across quartiles of total and 6 classes of flavonoid intake: Visual memory cognitive domain†
| Flavonoid quartiles | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | ||||||
| Flavonoid class | Mean | (95% CIs) | Mean | (95% CIs) | Mean | (95% CIs) | Mean | (95% CIs) | P-trend‡ |
| Total flavonoids§ | −43.9 | (−51.5,−36.3) | −41.7 | (−48.7,−34.6) | −35.9 | (−43.1,−28.8) | −33.8 | (−41.0,−26.6) | 0.04 |
| Flavonols | −44.7 | (−52.2,−37.3) | −41.1 | (−48.2,−34.0) | −37.9 | (−45.0,−30.8) | −31.6‖ | (−38.8,−24.4) | 0.01 |
| Flavones | −41.0 | (−48.4,−33.6) | −36.3 | (−43.3,−29.3) | −38.2 | (−45.3,−31.1) | −39.7 | (−47.0,−32.4) | 0.95 |
| Flavanones | −38.6 | (−45.8,−31.3) | −36.8 | (−43.8,−29.7) | −41.5 | (−48.6,−34.5) | −38.2 | (−45.6,−30.8) | 0.84 |
| Flavan-3-ols | −44.4 | (−51.8,−37.0) | −38.6 | (−45.6,−31.5) | −40.4 | (−47.6,−33.2) | −31.9 | (−39.1,−24.8) | 0.02 |
| Anthocyanins | −37.9 | (−45.6,−30.1) | −46.2 | (−53.4,−38.9) | −33.0 | (−40.0,−26.0) | −38.4 | (−45.3,−31.5) | 0.59 |
| Flavonoid polymers | −43.8 | (−51.4,−36.1) | −44.3 | (−51.3,−37.2) | −35.6 | (−42.7,−28.5) | −32.0 | (−39.1,−24.8) | 0.01 |
All values are mean least squares annualized change in the visual memory cognitive domain score multiplied by 1000; 95% confidence intervals (CIs) in parentheses.
Of the total 2672 observations, there was 2609 complete data for the visual memory cognitive domain.
P values for the test of linear trend across quartile categories were based on linear regression models with the median intake of each quartile category assigned to individuals with intake in that category, and this quartile median variable was used as a continuous measure in regression models.
Adjusted for age, sex, education, total energy intake, Apolipoprotein E ε4, and visual memory score at the beginning of the exam interval (model 1).
Significantly different from Quartile 1 by Dunnett’s test (P<0.05).
Table 4d.
Mean annualized change in the standardized rate x1000* of cognitive decline over a median follow-up of 11.8 years, across quartiles of total and 6 classes of flavonoid intake: Verbal learning cognitive domain†
| Flavonoid quartiles | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | ||||||
| Flavonoid class | Mean | (95% CIs) | Mean | (95% CIs) | Mean | (95% CIs) | Mean | (95% CIs) | P-trend‡ |
| Total flavonoids§ | −11.3 | (−20.5,−2.1) | −18.5 | (−26.9,−10.0) | −12.7 | (−21.3,−4.0) | −7.3 | (−15.9,1.3) | 0.23 |
| Flavonols | −18.7 | (−27.7,−9.7) | −15.2 | (−23.7,−6.7) | −10.1 | (−18.7,−1.5) | −6.1 | (−14.7,2.5) | 0.04 |
| Flavones | −11.8 | (−20.7,−2.9) | −13.5 | (−21.9,−5.1) | −13.5 | (−22.1,−5.0) | −10.8 | (−19.6,−2.0) | 0.84 |
| Flavanones | −11.8 | (−20.6,−3.1) | −12.8 | (−21.3,−4.4) | −12.5 | (−20.9,−4.0) | −12.6 | (−21.6,−3.6) | 0.93 |
| Flavan-3-ols | −17.2 | (−26.1,−8.2) | −12.1 | (−20.6,−3.6) | −12.7 | (−21.3,−4.2) | −8.0 | (−16.6,0.6) | 0.21 |
| Anthocyanins | −10.5 | (−19.8,−1.2) | −15.1 | (−23.8,−6.5) | −9.5 | (−18.0,−1.0) | −14.3 | (−22.6,−6.0) | 0.73 |
| Flavonoid polymers | −10.6 | (−19.8,−1.3) | −19.2 | (−27.6,−10.7) | −11.5 | (−20.0,−3.0) | −8.3 | (−16.9,0.2) | 0.32 |
All values are mean least squares annualized change in the verbal learning cognitive domain score multiplied by 1000; 95% confidence intervals (CIs) in parentheses.
Of the total 2672 observations, there was 2571 complete data for the verbal learning cognitive domain.
P values for the test of linear trend across quartile categories were based on linear regression models with the median intake of each quartile category assigned to individuals with intake in that category, and this quartile median variable was used as a continuous measure in regression models.
Adjusted for age, sex, education, total energy intake, Apolipoprotein E ε4, and verbal learning score at the beginning of the exam interval (model 1).
Assessment of effect modification (interaction tests) of total and six classes of flavonoids by age and cognitive function based on all eight cognitive domains indicated only seven nominally significant interactions between (1) flavones, flavanones, and flavan-3-ols and age on the global function cognitive domain, (2) flavones and age on the verbal learning cognitive domain, (3) both total flavonoids and flavonoid polymers and age on the abstract reasoning cognitive domain, and (4) flavonoid polymers and age on the language cognitive domain (Supplemental Table 9). However, further examination with age-stratified analyses (three age categories (45–59, 60–69, 70+) showed no meaningful differences between the three age groups (data not shown). We observed no nominally significant interactions with sex (Supplemental Table 9). Additionally, we only observed modest nominal significant interactions of ApoE ε4 with flavones on the verbal memory (P<0.01), abstract reasoning (P<0.01), and language cognitive domains (P<0.05) (data not shown). Likewise, nominal significant interactions of lag time were seen with total flavonoids (P<0.04), flavan-3-ols (P<0.01), and flavonoid polymers (P<0.03) only on the visuoperceptual organization cognitive domain (data not shown). Given the large number of interactions considered in our analyses (7 flavonoids x 8 cognitive outcomes x 4 interactions=224 tests), we did not consider any of these interactions to be informative.
Discussion
To the best of our knowledge, this is the most comprehensive and longest longitudinal study to date to examine the long-term relationship between flavonoid intake and age-related cognitive decline. We hypothesized that greater intakes of total flavonoids and four flavonoid classes (flavonols, flavanones, flavan-3-ols, and anthocyanins) would be associated with improved maintenance of cognitive health with age based on prior observations. However, we observed only limited evidence among our 1779 participants of any beneficial associations between flavonoid intake, regardless of its class, and decline in cognitive function. Although we observed nominally significant inverse trends between higher intakes of flavonols and flavan-3-ols and cognitive decline across multiple cognitive domains (global function, verbal and visual memory), as well as between higher intakes of total flavonoids and flavonoid polymers on the visual memory cognitive domain, and flavonols on the verbal learning cognitive domain, none of these trends remained statistically significant after adjustment for the number of flavonoid classes and cognitive domains considered in these analyses. Some previous observational studies have demonstrated associations between higher flavonol and flavan-3-ol intake and slower cognitive decline (29, 30, 32), but flavonoid polymers have not previously been reported to be associated with changes in neurocognitive function.
Our null findings are in agreement with two prior longitudinal studies (33, 34), but are in contrast to four existing longitudinal studies that demonstrate protective cognitive effects of flavonoids and/or flavonoid rich foods (30–32, 48). With reference to the two null studies, Nooyens et al. (33) explored the relationship between dietary intake of flavonoids, as assessed by two FFQ assessments, and cognitive decline in healthy middle-aged (mean age 55 years) adults of the Doetinchem Cohort Study over a follow-up period of five years based on change in four cognitive domain scores (global function, memory function, speed of cognitive processing, and cognitive flexibility). No associations were observed between extreme quintiles of flavonoid intake and cognitive domains of global function, memory function, and speed of cognitive processing; unexpectedly, higher flavonoid intake was associated with a greater decline in cognitive flexibility. Similarly, Kalmijn et al. (34) did not support a role for flavonoid intake, as measured by the cross-check dietary history method at two time points, in the prevention of cognitive decline, as assessed by change in the MMSE score in participants of the Zutphen Elderly Study over three years of follow-up. As for the positive studies, Root et al. (30) found that across quintiles of intake, increased total flavonols was associated with less decline in total cognitive function (a summed score of three cognitive tests), over six years of follow-up in middle-aged U.S. individuals (mean age 54 years) of the Atherosclerosis Risk in Communities study population. In contrast to our approach, their dietary intake data was measured at only one time point that preceded the first cognitive function testing by three years. Additionally, they had minimal adjustment for overall diet quality. Another longitudinal study examining the relation between flavonoid intake and cognitive function among dementia-free French subjects of the Personnes Aǵees QUID cohort aged 65 year or older revealed that after 10 years of follow-up, individuals in the two highest quartiles of flavonoid intake experienced less cognitive decline, as assessed by change in the MMSE score, relative to those in the lowest quartile of flavonoid intake (31). This study also utilized dietary data from one time point (at baseline), used a less sensitive test of cognitive function (MMSE), and had no adjustment for diet quality. Moreira et al. examined the association between the consumption of chocolate, a flavan-3-ol-rich food, and cognitive decline in cognitively healthy Portuguese participants aged 65 or older over a median follow-up of four years. They found that dietary chocolate intake (yes/no), assessed at baseline, was associated with a lower risk of cognitive decline, as measured by change in the MMSE score, but only among subjects with low daily consumption of caffeine (average caffeine intake of <75 mg/day) (32). Overall diet quality was not accounted for in their analyses. Devore et al. prospectively evaluated the effects of long-term intakes of berries (strawberries and blueberries), and total and class-specific flavonoids on cognitive function, as assessed by two repeated assessments of three cognitive outcomes, in older women (mean age 74 years) of the Nurse’s Health Study over a period of four years (48). The study demonstrated that when comparing extreme categories of intake, higher consumption of berries, anthocyanins, and total flavonoids were associated with slower rates of cognitive decline in the global composite score. In addition, higher intakes of blueberries and total flavonoids were also associated with slower rates of cognitive decline in both the verbal memory composite score and the telephone interview of cognitive status.
Although some of these studies might have been limited by the short follow-up period, use of a single dietary assessment measure, and/or a less sensitive cognitive assessment test, these limitations would tend to underestimate the association between flavonoid intake and cognitive function. Therefore, it is unlikely that they are responsible for the discordant findings seen herein. Three out of the four studies did not account for overall diet quality, a major confounder of the relationship between flavonoid intake and cognitive health, which might have positively biased their results; however, this is unlikely the cause of the discrepancy with our findings as we did not observe any statistically significant associations prior to adjustment for diet quality. An important consideration in the failure to observe a relationship between flavonoids and cognitive health is that the effect of isolated flavonoid compounds may be relatively small and may be difficult to detect given the measurement error in the dietary and NP assessment tools, except in very large population samples. Therefore, a holistic dietary approach might be required to show any protective cognitive effects of flavonoids, as seen in studies exploring the neuroprotective effects of different dietary patterns that contain flavonoid-rich foods such as the Mediterranean (8, 9), DASH (9, 10), and the MIND (11), all of which have revealed promising results.
We sought to address many earlier limitations of studies assessing the long-term relationship between flavonoids and cognitive function through the use of a more inclusive approach. The strengths of our study include the prospective design, use of up to four dietary assessments of total and all six classes of flavonoids, utilization of three repeated assessments of a sensitive battery of NP tests that target eight cognitive domains, incorporation of more complete flavonoid databases, adjustment of overall diet quality and intake of key dietary components that have been established to be associated with cognitive function, exclusion of subjects with MCI because of possible effects on dietary reporting, and the relatively long follow-up time (median follow-up of 11.8 years). However, despite these strengths, we were not able to confirm the positive associations between flavonoid intake and cognitive decline seen in earlier longitudinal studies. Our study was limited in that we did not use the most recent 2018 version of the USDA flavonoid database, as it had not been incorporated into the Harvard FFQ database at the time the analyses were performed. Thus, the flavonoid content of some of the newer added foods were not captured. However, given that this represents only a small number of foods (including different types of olives and olive oils, additional data on blueberry varieties, updated and new data for cranberry and raspberry products), it is unlikely that this influenced the assessment of habitual dietary flavonoid intake. Further, the 2007 version of the USDA flavonoid database that was used for the current flavonoid content of the FFQ database is still considered to be a considerably more complete flavonoid database than the databases used in many of the earlier observational studies on flavonoids and cognition. Another potential limitation is that we assessed the overall mean change in the cognitive domain scores which might not capture the effect of flavonoid intake on individuals with the most extreme change in cognitive function. However, we explored the effect of total and six flavonoid classes on the prevalence of extreme MMSE score changes with the criteria being 0.5 units loss in the MMSE score per year over a median of 11.8 years of follow-up and did not see any significant associations (data not shown). A possible reason for our inability to see any major effects with flavonoid intakes in those with extreme changes in cognitive function is the relatively young age of our study population at baseline (mean age 60.8 years) as only 19.3% were 70 years or older, and the exclusion of participants with dementia. Moreover, lag time between the NP ancillary study visits and their corresponding routine clinical exams at which flavonoid intake data was obtained could have impacted our results. However, sensitivity analyses assessing the influence of lag time on study results did not show any significant impact. A final limitation is the generalizability of our results as all participants of the FHS Offspring cohort were white and of European descent.
In summary, the notion that flavonoids may exert long-term protective neurocognitive benefits remains unclear. Based on up to four repeated dietary and three repeated NP testing assessments over a median follow-up of 11.8 years, our results failed to assert any protective cognitive associations between total flavonoids and any flavonoid class in eight cognitive domains, apart from nominally significant trends observed for total flavonoids, flavonols, flavan-3-ols and flavonoid polymers. Our findings do not support an association between long-term higher flavonoid intakes and improved cognitive performance, although we cannot rule out such associations based on the nominally significant observations in this study. Future research in larger and more racially and ethnically diverse subjects with wide ranges of flavonoid intake is warranted. There is also a need for more studies assessing the cognitive effect of flavonoids as part of a whole diet, taking into consideration various dietary components concurrently as an overall dietary pattern.
Supplementary Material
Acknowledgements
We would like to thank Elizabeth Mahon and Ting Fang Alvin Ang from the Brain Health Research Lab/neuropsychology Group at the Framingham Heart Study for their assistance on the project.
Financial support
This research was supported in part by the U.S. Department of Agriculture-Agricultural Research Service (ARS), Agreement No. #58–1950-4–003, the National Heart Lung and Blood Institute by contract No. HHSN2682015000011, the National Institute on Aging by AG-008122, AG-16495 and AG062109, the National Institute of Neurological Disorders and Stroke, NS-17950, and the Embassy of the State of Kuwait (E.S.). The views expressed in this article are of those of the authors and do not necessarily represent the views of the funding organization.
Footnotes
Conflict of interest
None.
Ethical Standards disclosure
This study was conducted according to the guidelines laid down in the declaration of Helisinki. The original data collection protocols and procedures were approved by the Institutional Review Board at Boston University Medical Center, and written informed consent was obtained from all participants. The present study protocol was reviewed by Tufts University Health Sciences Institutional Review Board.
References
- 1.Harada CN, Natelson Love MC Triebel KL (2013) Normal cognitive aging. Clinics in geriatric medicine 29, 737–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson LA, Goodman RA, Holtzman D et al. (2012) Aging in the United States: opportunities and challenges for public health. American journal of public health 102, 393–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wiener JM & Tilly J (2002) Population ageing in the United States of America: implications for public programmes. Int J Epidemiol 31, 776–781. [DOI] [PubMed] [Google Scholar]
- 4.Harper S (2014) Economic and social implications of aging societies. Science 346, 587–591. [DOI] [PubMed] [Google Scholar]
- 5.Ahlqvist A, Nyfors H Suhonen R (2016) Factors associated with older people’s independent living from the viewpoint of health and functional capacity: a register-based study. Nursing open 3, 79–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shatenstein B, Barberger-Gateau P Mecocci P (2015) Prevention of Age-Related Cognitive Decline: Which Strategies, When, and for Whom? Journal of Alzheimer’s disease : JAD 48, 35–53. [DOI] [PubMed] [Google Scholar]
- 7.Dominguez LJ & Barbagallo M (2018) Nutritional prevention of cognitive decline and dementia. Acta bio-medica : Atenei Parmensis 89, 276–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martinez-Lapiscina EH, Clavero P, Toledo E et al. (2013) Mediterranean diet improves cognition: the PREDIMED-NAVARRA randomised trial. Journal of neurology, neurosurgery, and psychiatry 84, 1318–1325. [DOI] [PubMed] [Google Scholar]
- 9.Tangney CC, Li H, Wang Y et al. (2014) Relation of DASH- and Mediterranean-like dietary patterns to cognitive decline in older persons. Neurology 83, 1410–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith PJ, Blumenthal JA, Babyak MA et al. (2010) Effects of the dietary approaches to stop hypertension diet, exercise, and caloric restriction on neurocognition in overweight adults with high blood pressure. Hypertension (Dallas, Tex : 1979) 55, 1331–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morris MC, Tangney CC, Wang Y et al. (2015) MIND diet slows cognitive decline with aging. Alzheimer’s & dementia : the journal of the Alzheimer’s Association 11, 1015–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spencer JP, Vauzour D Rendeiro C (2009) Flavonoids and cognition: the molecular mechanisms underlying their behavioural effects. Arch Biochem Biophys 492, 1–9. [DOI] [PubMed] [Google Scholar]
- 13.Williams RJ & Spencer JP (2012) Flavonoids, cognition, and dementia: actions, mechanisms, and potential therapeutic utility for Alzheimer disease. Free Radic Biol Med 52, 35–45. [DOI] [PubMed] [Google Scholar]
- 14.Rendeiro C, Rhodes JS Spencer JP (2015) The mechanisms of action of flavonoids in the brain: Direct versus indirect effects. Neurochem Int 89, 126–139. [DOI] [PubMed] [Google Scholar]
- 15.Orhan IE, Daglia M, Nabavi SF et al. (2015) Flavonoids and dementia: an update. Current medicinal chemistry 22, 1004–1015. [DOI] [PubMed] [Google Scholar]
- 16.Beecher GR (2003) Overview of dietary flavonoids: nomenclature, occurrence and intake. The Journal of nutrition 133, 3248s–3254s. [DOI] [PubMed] [Google Scholar]
- 17.Alharbi MH, Lamport DJ, Dodd GF et al. (2016) Flavonoid-rich orange juice is associated with acute improvements in cognitive function in healthy middle-aged males. European journal of nutrition 55, 2021–2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dodd GF (2012) The Acute Effects of Flavonoid-rich Blueberries on Cognitive Function in Healthy Younger and Older Adults: University of Reading. [Google Scholar]
- 19.Field DT, Williams CM Butler LT (2011) Consumption of cocoa flavanols results in an acute improvement in visual and cognitive functions. Physiol Behav 103, 255–260. [DOI] [PubMed] [Google Scholar]
- 20.Lamport DJ, Pal D, Macready AL et al. (2016) The effects of flavanone-rich citrus juice on cognitive function and cerebral blood flow: an acute, randomised, placebo-controlled cross-over trial in healthy, young adults. The British journal of nutrition 116, 2160–2168. [DOI] [PubMed] [Google Scholar]
- 21.Scholey AB, French SJ, Morris PJ et al. (2010) Consumption of cocoa flavanols results in acute improvements in mood and cognitive performance during sustained mental effort. Journal of psychopharmacology (Oxford, England) 24, 1505–1514. [DOI] [PubMed] [Google Scholar]
- 22.Whyte AR, Schafer G Williams CM (2016) Cognitive effects following acute wild blueberry supplementation in 7- to 10-year-old children. European journal of nutrition 55, 2151–2162. [DOI] [PubMed] [Google Scholar]
- 23.Whyte AR & Williams CM (2015) Effects of a single dose of a flavonoid-rich blueberry drink on memory in 8 to 10 y old children. Nutrition 31, 531–534. [DOI] [PubMed] [Google Scholar]
- 24.McNamara RK, Kalt W, Shidler MD et al. (2018) Cognitive response to fish oil, blueberry, and combined supplementation in older adults with subjective cognitive impairment. Neurobiology of aging 64, 147–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Butchart C, Kyle J, McNeill G et al. (2011) Flavonoid intake in relation to cognitive function in later life in the Lothian Birth Cohort 1936. The British journal of nutrition 106, 141–148. [DOI] [PubMed] [Google Scholar]
- 26.Caldwell K, Roodenrys SJ, Charlton KE et al. (2016) Dietary flavonoid intake and cognitive performance in older adults with Alzheimer’s type dementia
- 27.Nurk E, Refsum H, Drevon CA et al. (2009) Intake of flavonoid-rich wine, tea, and chocolate by elderly men and women is associated with better cognitive test performance. The Journal of nutrition 139, 120–127. [DOI] [PubMed] [Google Scholar]
- 28.Crichton GE, Elias MF Alkerwi A (2016) Chocolate intake is associated with better cognitive function: The Maine-Syracuse Longitudinal Study. Appetite 100, 126–132. [DOI] [PubMed] [Google Scholar]
- 29.Kesse-Guyot E, Fezeu L, Andreeva VA et al. (2012) Total and specific polyphenol intakes in midlife are associated with cognitive function measured 13 years later. The Journal of nutrition 142, 76–83. [DOI] [PubMed] [Google Scholar]
- 30.Root M, Ravine E Harper A (2015) Flavonol Intake and Cognitive Decline in Middle-Aged Adults. Journal of medicinal food 18, 1327–1332. [DOI] [PubMed] [Google Scholar]
- 31.Letenneur L, Proust-Lima C, Le Gouge A et al. (2007) Flavonoid intake and cognitive decline over a 10-year period. American journal of epidemiology 165, 1364–1371. [DOI] [PubMed] [Google Scholar]
- 32.Moreira A, Diogenes MJ, de Mendonca A et al. (2016) Chocolate Consumption is Associated with a Lower Risk of Cognitive Decline. Journal of Alzheimer’s disease : JAD 53, 85–93. [DOI] [PubMed] [Google Scholar]
- 33.Nooyens AC, Milder IE, van Gelder BM et al. (2015) Diet and cognitive decline at middle age: the role of antioxidants. The British journal of nutrition 113, 1410–1417. [DOI] [PubMed] [Google Scholar]
- 34.Kalmijn S, Feskens EJ, Launer LJ et al. (1997) Polyunsaturated fatty acids, antioxidants, and cognitive function in very old men. American journal of epidemiology 145, 33–41. [DOI] [PubMed] [Google Scholar]
- 35.Dawber T, Meadors G Moore FJ (1951) Epidemiological approaches to heart disease: The Framingham Study. Am J Public Health 41, 279–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feinleib M, Kannel WB, Garrison RJ et al. (1975) The Framingham Offspring Study. Design and preliminary data. Preventive medicine 4, 518–525. [DOI] [PubMed] [Google Scholar]
- 37.Rimm EB, Giovannucci EL, Stampfer MJ et al. (1992) Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. American journal of epidemiology 135, 1114–1126; discussion 1127–1136. [DOI] [PubMed] [Google Scholar]
- 38.Feskanich D, Rimm EB, Giovannucci EL et al. (1993) Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J Am Diet Assoc 93, 790–796. [DOI] [PubMed] [Google Scholar]
- 39.Downer B, Fardo DW Schmitt FA (2015) A Summary Score for the Framingham Heart Study Neuropsychological Battery. Journal of aging and health 27, 1199–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.USDA (2004) USDA Database for the Proanthocyanidin Content of Selected Food Washington, DC: US Department of Agriculture. [Google Scholar]
- 41.USDA (2007) USDA Database for the Flavonoid Content of Selected Food, Release 2.1 ed. Washington, DC: US Department of Agriculture. [Google Scholar]
- 42.Cassidy A, O’Reilly EJ, Kay C et al. (2011) Habitual intake of flavonoid subclasses and incident hypertension in adults. Am J Clin Nutr 93, 338–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chun OK, Chung SJ Song WO (2009) Urinary isoflavones and their metabolites validate the dietary isoflavone intakes in US adults. J Am Diet Assoc 109, 245–254. [DOI] [PubMed] [Google Scholar]
- 44.de Kleijn MJ, van der Schouw YT, Wilson PW et al. (2001) Intake of dietary phytoestrogens is low in postmenopausal women in the United States: the Framingham study(1–4). J Nutr 131, 1826–1832. [DOI] [PubMed] [Google Scholar]
- 45.Nechuta SJ, Caan BJ, Chen WY et al. (2012) Soy food intake after diagnosis of breast cancer and survival: an in-depth analysis of combined evidence from cohort studies of US and Chinese women. Am J Clin Nutr 96, 123–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Beking K & Vieira A (2010) Flavonoid intake and disability-adjusted life years due to Alzheimer’s and related dementias: a population-based study involving twenty-three developed countries. Public Health Nutr 13, 1403–1409. [DOI] [PubMed] [Google Scholar]
- 47.Commenges D, Scotet V, Renaud S et al. (2000) Intake of flavonoids and risk of dementia. European journal of epidemiology 16, 357–363. [DOI] [PubMed] [Google Scholar]
- 48.Devore EE, Kang JH, Breteler MM et al. (2012) Dietary intakes of berries and flavonoids in relation to cognitive decline. Ann Neurol 72, 135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Au R, Seshadri S, Wolf PA et al. (2004) New norms for a new generation: cognitive performance in the framingham offspring cohort. Experimental aging research 30, 333–358. [DOI] [PubMed] [Google Scholar]
- 50.Kannel WB & Sorlie P (1979) Some health benefits of physical activity. The Framingham Study. Archives of internal medicine 139, 857–861. [PubMed] [Google Scholar]
- 51.Troy LM, Dwyer J Jacques P (2013) US adults and adherence to the Dietary Guidelines for Americans (DGA). The FASEB Journal 27, 124.127–124.127. [Google Scholar]
- 52.Pase MP, Himali JJ, Beiser AS et al. (2017) Sugar- and Artificially Sweetened Beverages and the Risks of Incident Stroke and Dementia: A Prospective Cohort Study. Stroke 48, 1139–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dufouil C, Beiser A, McLure LA et al. (2017) Revised Framingham Stroke Risk Profile to Reflect Temporal Trends. Circulation 135, 1145–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Muntner P, Carey RM, Gidding S et al. (2018) Potential U.S. Population Impact of the 2017 ACC/AHA High Blood Pressure Guideline. Journal of the American College of Cardiology 71, 109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


