Abstract
Background:
In SAH patients with multiple intracranial aneurysms (IAs), often the hemorrhage pattern does not indicate the rupture source. Angiographic findings (IA size and shape) could help but may not be reliable.
Objective:
To test if existing parameters could identify the ruptured IA in patients with multiple IAs and if composite predictive models could improve the identification.
Methods:
We retrospectively collected angiographic and medical records of 93 SAH patients with at least 2 IAs (total 206 saccular IAs; 93 ruptured), where the ruptured IA was confirmed through surgery or definitive hemorrhage patterns. We calculated 13 morphologic and 10 hemodynamic parameters along with location and type (sidewall/bifurcation) and tested their ability in identifying rupture in the 93 patients. To build predictive models, we randomly assigned 70 patients to training and 23 to hold-out testing cohorts. Using a linear regression model with a customized cost function and 10-fold cross-validation, we trained 2 rupture identification models: RIMC using all parameters and RIMM excluding hemodynamics.
Results:
The 25 study parameters had vastly different positive predictive values (PPVs, 31–87%) for identifying rupture, the highest being size ratio (SR) at 87%. RIMC incorporated SR, undulation index (UI), relative residence time, and Type; RIMM had only SR, UI, and type. During cross-validation, PPV for SR, RIMM, and RIMC was 86±4%, 90±4%, and 93±4%, respectively. In testing, SR and RIMM had PPV of 85%, while RIMC had 92%.
Conclusions:
SR was the best individual factor for identifying the ruptured aneurysm; however, RIMC, followed by RIMM, outperformed existing parameters.
Keywords: Aneurysm, Ruptured, Hemodynamics, Intracranial Aneurysm, Multiple Aneurysms, Subarachnoid Hemorrhage
Introduction
Approximately 30% of patients with intracranial aneurysms (IAs) present with multiple aneurysms.1 Correct identification of the ruptured IA in a patient with SAH is critical for treatment planning.2 Identifying the ruptured IA is increasingly important in an era of increased endovascular treatment because the source of hemorrhage cannot be confirmed visually and aneurysms are usually treated one at a time. Hemorrhage pattern on initial CT scans is the primary indicator of the bleeding source, as demonstrated by Orning et al. 3, who reported that a definitive hemorrhage pattern (localized to 1 IA) could accurately delineate the ruptured IAs. However, in approximately half of the patients with multiple aneurysms, hemorrhage pattern cannot delineate the ruptured IA.3 In such cases, rupture identification relies on angiographic findings, such as IA size, shape, and location.2, 3 Several studies have reported misidentification of the ruptured aneurysm, which has been attributed to the small size or benign shape of the ruptured IA.2–8 Therefore, developing more reliable means of identifying the ruptured IA has clinical value in patients with multiple IAs, especially when hemorrhage pattern cannot delineate the ruptured IA.
Several morphologic and hemodynamic parameters have been found to differ significantly between ruptured and unruptured aneurysm cohorts in patients with multiple IAs, albeit with conflicting findings.9–15 These studies have generally relied on univariate and multivariate regression analysis of pooled data of ruptured IAs and unruptured IAs. Although such methods model the general probability of aneurysm rupture, they are not tailored for identifying which aneurysm ruptured in SAH patients with multiple aneurysms. To that end, the ability of aneurysmal metrics for identification of the ruptured aneurysm should be evaluated by comparing all IAs within each patient, and models trained by associating the coexisting IAs within each patient should be developed.
In this study, we retrospectively analyzed IAs in SAH patients presenting with multiple aneurysms to test the performance of existing morphologic and hemodynamic parameters and to build new composite models for identifying the ruptured aneurysm in patients with multiple aneurysms. The performance of the new models was compared against existing morphologic and hemodynamic parameters along with aneurysm location and type (sidewall or bifurcation) and a previously developed rupture classification model, rupture resemblance score (RRS).16 Our findings may assist clinicians to better identify the ruptured IA in SAH patients presenting with multiple aneurysms.
Materials and Methods
Data Collection
We retrospectively collected cerebrovascular images and medical records from consecutive series of patients with aneurysmal SAH treated at 3 stroke centers located in China (image acquisition between 2011 and 2014), Japan (between 2014 and 2016), and the USA (between 2010 and 2017). Inclusion criteria mandated that patients had a ruptured IA and at least 1 unruptured aneurysm and that all aneurysms were saccular. For the patients who underwent craniotomy for aneurysm clipping, the ruptured aneurysm was confirmed through microscopic visual assessment. For patients who underwent endovascular or no treatment, we only included those with a definitive hemorrhage pattern on CT (localized to 1 IA). Examples of definitive and nondefinitive hemorrhage patterns are provided in Figure 1A and B, respectively. All patients included in the current study underwent 3D rotational DSA preoperatively, which was used for aneurysm geometry reconstruction. In general, the voxel sizes of images obtained at the 3 centers were similar, with little variation during the acquisition period: Chinese center, 0.280mm; Japanese center, 0.227–0.226mm; and USA center, 0.256–0.223mm. The images and data were anonymized, and the study received institutional review board approval. The operators who conducted image segmentation and morphologic and hemodynamic evaluations were blinded to aneurysm rupture status.
Figure 1:
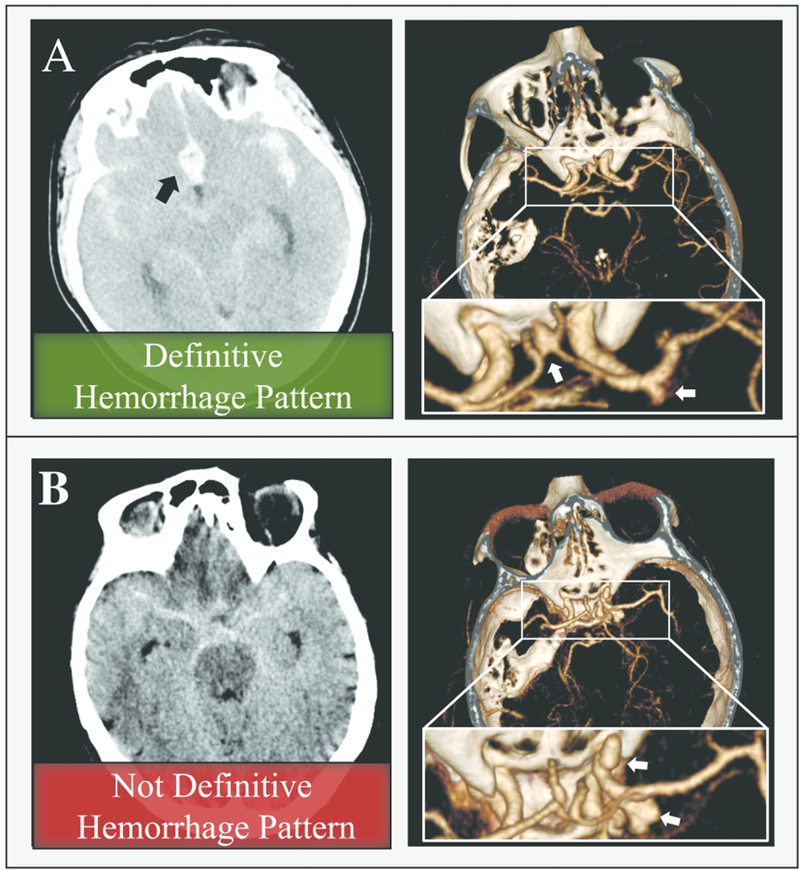
A) A 62-year-old woman who presented with SAH (left image, non-contrast CT scan) was found to have anterior communicating artery and MCA aneurysms (white arrows, right image, CTA). A focal hematoma in the anterior circulation (black arrow, left image) delineated the anterior communicating artery aneurysm as the source of bleeding, which was confirmed at the time of surgery. B) A 59-year-old woman presented with SAH (left image, non-contrast CT scan). She was found to have 2 large lobulated right ICA aneurysms (white arrows, right image, CTA). The source of the rupture could not be identified from the hemorrhage pattern (this patient was excluded from our study). Both aneurysms received endovascular treatment.
Image Segmentation and Computational Fluid Dynamics
The 3D DSA images of all patients were segmented using open-source Vascular Modeling Toolkit (VMTK, http://www.vmtk.org). VMTK is a semiautomated tool that uses a level-set method to place lumen contour at regions with maximum gradient intensity.17 After segmentation, a surface mesh for IAs with surrounding parent vessels was generated using the threshold-based marching cubes algorithm.17
CFD simulations were performed at 2 different centers (China and USA). For the Chinese database, CFD was performed using ICEM-CFD software (ANSYS, Inc., USA) for mesh generation and ANSYS-CFX 14.0 software (ANSYS, Inc) for CFD simulation. For the USA and Japanese databases, CFD was performed using STAR-CCM+ (Siemens, Inc., Germany) for both mesh generation and CFD simulations. All geometries were converted to computational domains using the same meshing setup: tetrahedral volumetric mesh, minimum element size of 0.1mm, and 3 refined prism layers. All CFD simulations were performed using the same numerical setup, assumptions, and boundary conditions, including rigid wall, Newtonian behavior of blood flow, location-based inflow rate,18 and distribution of outlet flow based on Murray’s Law.19 Complete details of CFD simulations, including the sensitivity of the hemodynamic results to the CFD solvers, are given in the Supplemental Text, Supplemental Figures I and II.
Morphologic Parameters
Aneurysmal morphology was calculated using AView,20, 21 a computational workflow for morphologic and hemodynamic assessment of IAs. The definitions of the 13 morphologic indices are illustrated in Supplemental Figure III. Maximum diameter (D) is the maximum distance between any two points on the aneurysm sac. Maximum height (Hmax) is the maximum distance between the sac and the center of the neck plane. Height (H) is the maximum perpendicular distance from the neck plane to the sac. Size ratio (SR) has 2 definitions in the literature,22, 23 so does aspect ratio (AR),22, 24 and we applied both. SRD is the ratio of D to the average parent vessel diameter, and SRHmax is the ratio of Hmax to the average parent vessel diameter. ARHmax and ARH are the ratios of Hmax and H to the average neck diameter, respectively. Aneurysm Number25 is the neck ratio (the ratio of neck diameter to the average parent vessel diameter) multiplied by the pulsatility index based on parent-artery location obtained from the literature.26, 27 Undulation Index (UI) represents the degree of aneurysm surface irregularity. Ellipticity Index represents deviation of the IA from a perfect hemisphere. Nonsphericity Index represents deviation of the IA from a perfect hemisphere while also considering surface undulations.22 Surface area and Volume of the IAs were calculated as well. We also classified aneurysms as bifurcation or sidewall. Bifurcation IAs are those that arise at the apex of a split from a main (proximal) artery into two or more daughter (distal) arteries.
Hemodynamic Parameters
After obtaining the flow field in each IA, we calculated the aneurysm-averaged values for the following 10 hemodynamic parameters: time-averaged wall shear stress (WSS), which is the WSS magnitude averaged over the aneurysm sac; normalized WSS (NWSS), which is WSS further normalized by the spatiotemporal average wall shear stress of the parent vessel; maximum and minimum WSS and NWSS, which is the maximum or minimum value of WSS that occurred on the aneurysm sac; oscillatory shear index (OSI), which measures the directional change of the WSS through the cardiac cycle; relative residence time (RRT), which quantifies the stasis of blood near the aneurysm wall; low shear area (LSA), which is the percentage of the sac area exposed to low WSS (defined as <10% of the averaged parent vessel WSS); and finally, complex flow, which is defined as an aneurysmal flow structure that contains more than 1 separate vortex core line.18, 28 This parameter is binary: 1 for complex flow structure and 0 for simple flow structure. These parameters are defined in the Supplemental Table I and illustrated in Supplemental Figure IV.
Because multiple operators performed numerical analyses, we conducted a post-hoc study to quantify the intra-class correlation coefficient among operators using our protocol for calculating morphologic and hemodynamic parameters and found excellent agreement. Details are provided in the Supplemental Text, Supplemental Table II, and Supplemental Figure V.
Rupture Resemblance Score (RRS)
We also calculated the RRS, a rupture classification model that was trained based on a database of 204 ruptured and unruptured IAs.29 RRS can provide a rupture probability for an IA, based on IA morphology and hemodynamics, which can be used to gauge the similarity of an IA to a cohort of ruptured IAs. The equation for the calculation of RRS is provided in the Supplemental Text.
Description of Ruptured and Unruptured Aneurysms
For all IAs, we described location, type (bifurcation vs. sidewall), morphology, hemodynamics, and RRS. Continuous variables were presented as means with standard deviations and conditional logistic regression 30 was used to assess differences between ruptured and unruptured cohorts. Discrete variables were presented as numbers, and a chi-square test was used to determine significant differences between ruptured and unruptured cohorts. A p-value of <0.01 was considered significant.
Testing the Performance of Existing Rupture Predictive Parameters in Identifying the Ruptured IA
We further investigated the performance of each parameter for identifying each patient’s ruptured IA. For aneurysm location, rupture prediction was based on rupture site frequencies as reported by Nehls et al.2 (anterior communicating artery, 62% of IAs identified at this location ruptured; basilar artery, 50%; PICA, 50%; posterior communicating artery, 38%; posterior cerebral artery, 33%; anterior cerebral artery (ACA), 33%; ICA, 32%; and MCA, 27%), and the aneurysm with the highest rupture frequency was assumed to be ruptured. If the ruptured IA and a coexisting unruptured IA were located on the same artery or arteries with the same rupture frequency, we assumed that identifying the ruptured aneurysm was not possible, and this was considered a false prediction.
Aneurysm type was used as a predictor of ruptured aneurysm based on the assumption that bifurcation aneurysms rupture more frequently than sidewall aneurysms, especially small bifurcation aneurysms.31 Aneurysmal flow pattern was used as a predictor of a ruptured aneurysm based on the assumption that ruptured IAs more frequently contain a complex flow structure.28 If the ruptured IA and a coexisting unruptured IA were of the same type or had the same flow pattern, we assumed that the rupture site was unidentifiable, and this was considered a false prediction.
For quantitative predictive parameters, the patient’s IAs were compared and the IA with the highest value was assumed to be the ruptured IA. For parameters that are generally lower in ruptured aneurysms (e.g., NWSS), the aneurysm with the lowest value was assumed to be the ruptured one.
We compared rupture prediction results with actual (confirmed through surgery or definitive hemorrhage pattern) aneurysm rupture status. The performance of each metric was quantified as a positive predictive value (PPV), which is the number of the patients with correct identification of ruptured IA divided by the total number of patients.
Generating Rupture Identification Models
After testing the performance of individual rupture predictors, we generated composite models that were trained to provide a higher score for the ruptured IA in SAH patients with multiple IAs, which we called rupture identification model (RIM). First, we randomly assigned 70 patients to a training cohort and 23 patients to a hold-out testing cohort. To distribute equal weight, all variables were centered to 0 (subtraction of the mean) and scaled (division with standard deviation). All variables were then used to generate all possible linear combinations, and those with collinear variables (|Pearson correlation| >0.5, moderate correlation; Supplemental Table III) were removed. We used a linear regression model with a customized cost function, which maximizes PPV and the differences between ruptured and coexistent unruptured IAs within each patient, to fit the remaining models. The best performing model during 10-fold cross-validation was identified. The resulting model, which was trained by all variables, was termed RIMC; where the subscript “C” indicates the need for CFD to obtain hemodynamic parameters. We also trained a second model by excluding hemodynamic variables, termed RIMM, where the subscript “M” indicates that it is a morphological parameter testing cohort as well.
The workflow, access link to the model training code, and additional details of model generation are provided in the Supplemental Text and Supplemental Figure VI. Statistical analysis and model fitting were performed using an in-house code developed in R software (Version 1.0.44; https://www.r-project.org/).
Results
Clinical Information
Table 1 summarizes the clinical information of the 93 patients with 206 IAs included in this study: Chinese center (59 patients, 130 IAs), Japanese center (17 patients, 38 IAs), and USA center (17 patients, 38 IAs). The average patient age was 60±13 years; 74% of all patients were women. The ruptured aneurysm was identified through craniotomy in 35 patients (38%) and a definitive hemorrhage pattern in 58 patients (62%). Seventy-six patients (82%) had 2 IAs, 15(16%) had 3 IAs, 1(1%) had 4 IAs, and 1(1%) had 5 IAs.
Table 1:
Clinical information for SAH patients with multiple IAs
| Demographics & Comorbidities | n=93 |
|---|---|
| Age (years) | 60±13 |
| Female sex | 69(74%) |
| Hypertension | 59(63%) |
| Smoking history | 35 (38%) |
| Coronary artery disease | 7(8%) |
| Hyperlipidemia | 28(30%) |
| Diabetes mellitus | 13(14%) |
| Polycystic Kidney Disease | 1(1%) |
| Received Treatment | |
| Clipping | 35(38%) |
| Endovascular | 53(57%) |
| No Treatment | 5(5%) |
| Aneurysm Multiplicity | |
| 2 Aneurysms | 76(82%) |
| 3 Aneurysms | 15(16%) |
| 4 Aneurysms | 1(1%) |
| 5 Aneurysms | 1(1%) |
Description of Ruptured and Unruptured Aneurysms
Table 2 shows the number of IAs at each location, IA type (bifurcation or sidewall), morphology, hemodynamics, and RRS for all IAs in the ruptured and unruptured cohorts. Eighty-two (40%) of all IAs were located along the ICA, 57(70%) of which were unruptured. The anterior circulation was associated with the highest rupture frequency; 7 of 10(70%) IAs located on the ACA and 16 of 22(73%) IAs located at the anterior communicating artery were ruptured. Only 6 IAs were located in the posterior circulation. Most ruptured IAs were bifurcation aneurysms (71%).
Table 2:
Description of ruptured and unruptured aneurysms in our database of SAH patients with multiple IAs
| Total | Ruptured | Unruptured | P-Value* | |
|---|---|---|---|---|
| Location (%) | n=206 | n=93 | n=113 | <0.001 |
| ACA | 10(5%) | 7(8%) | 3(3%) | |
| AComA | 22(11%) | 16(17%) | 6(5%) | |
| ICA | 82(40%) | 25(27%) | 57(50%) | |
| MCA | 40(19%) | 14(15%) | 26(23%) | |
| PComA | 46(22%) | 29(31%) | 17(15%) | |
| Posterior Circulation | 6(3%) | 2(2%) | 4(4%) | |
| Type | 0.003 | |||
| Bifurcation:Sidewall | 122(59%):84(41%) | 66(71%):27(29%) | 56(50%):57(50%) | |
| Morphology | ||||
| D(mm) | 5.68±2.96 | 7.12±3.44 | 4.49±1.77 | <0.001 |
| Hmax(mm) | 4.14±2.56 | 5.51±2.88 | 3.00±1.52 | <0.001 |
| H(mm) | 3.64±2.28 | 4.85±2.47 | 2.64±1.51 | <0.001 |
| SRD | 2.28±1.32 | 2.95±1.44 | 1.73±0.89 | <0.001 |
| SRHmax | 1.66±1.08 | 2.28±1.17 | 1.15±0.65 | <0.001 |
| ARHmax | 1.00±0.54 | 1.27±0.62 | 0.77±0.34 | <0.001 |
| ARH | 0.89±0.52 | 1.13±0.59 | 0.68±0.34 | <0.001 |
| Aneurysm Number | 0.93±0.54 | 1.10±0.57 | 0.79±0.46 | <0.001 |
| Undulation Index | 0.073±0.070 | 0.108±0.078 | 0.044±0.045 | <0.001 |
| Nonsphericity Index | 0.325±0.053 | 0.325±0.054 | 0.326±0.052 | 0.992 |
| Ellipticity Index | 0.302±0.041 | 0.290±0.036 | 0.312±0.053 | 0.035 |
| Surface Area(mm2) | 74.91±100.88 | 111.77±134.07 | 44.57±42.50 | 0.002 |
| Volume(mm3) | 72.43±225.59 | 123.75±324.36 | 30.20±52.37 | 0.005 |
| Hemodynamics | ||||
| WSS (Pa) | 11.18±8.56 | 9.54±8.71 | 12.53±8.23 | 0.001 |
| Maximum WSS (Pa) | 58.38±44.31 | 62.29±45.50 | 55.17±43.24 | 0.773 |
| Minimum WSS (Pa) | 0.76±3.12 | 0.23±0.28 | 1.19±4.16 | <0.001 |
| NWSS | 0.70±0.38 | 0.59±0.37 | 0.79±0.36 | 0.008 |
| Maximum NWSS | 3.77±2.18 | 3.67±1.86 | 3.85±2.42 | 0.504 |
| Minimum NWSS | 0.06±0.41 | 0.02±0.02 | 0.10±0.56 | <0.001 |
| OSI | 0.014±0.021 | 0.018±0.021 | 0.010±0.020 | 0.129 |
| RRT | 1.96±1.28 | 2.45±1.57 | 1.56±0.80 | <0.001 |
| LSA | 9.56±16.78 | 14.67±20.08 | 5.36±12.02 | <0.001 |
| Complex:Simple flow | 80(39%):126(61%) | 58 (62%):35(38%) | 22(20%):91(80%) | <0.001 |
| Rupture Resemblance Score | ||||
| RRS[range:0–100] | 30.63±32.04 | 45.46±33.79 | 18.42±24.65 | <0.001 |
Values indicating statistical significance (P-Value<0.01) are underlined.
Except for Nonsphericity Index and Ellipticity Index, the morphologic indices of the ruptured and unruptured cohorts were significantly different. Aneurysmal hemodynamics also differed, with ruptured IAs being exposed to lower WSS, higher RRT, and larger LSA than the unruptured IAs. Complex flow patterns were present in 62% of ruptured IAs and 20% of unruptured IAs. The RRS was significantly higher in the ruptured cohort (P<0.001).
Performance of Existing Parameters in Identifying the Ruptured Aneurysm
All parameters were further analyzed to test their ability in identifying the ruptured aneurysm by calculating the percentage of correctly identified ruptured IAs. We found that the PPV considerably varied by individual parameters from 31–87%. SRHmax and Hmax had the highest performance, identifying the ruptured IA in 87% and 85% of patients, respectively (Table 3). RRT identified the ruptured IA in 76% of patients and exhibited the best hemodynamic parameter performance. Figure 2 shows SRHmax and time-averaged RRT distributions on the aneurysm sac for ruptured and unruptured IAs in 3 representative patients. RRS identified the ruptured IA in 80% of the patients.
Table 3:
Performance of existing aneurysmal parameters in the prediction of ruptured aneurysm in SAH patients with multiple IAs*
| Parameter | Positive Predictive Value | |
|---|---|---|
| Location | 52% | |
| Type | 31% | |
| Morphology | D | 82% |
| Hmax | 85% | |
| H | 81% | |
| SRD | 83% | |
| SRHmax | 87% | |
| ARHmax | 80% | |
| ARH | 74% | |
| Aneurysm Number | 63% | |
| Undulation Index | 80% | |
| Nonsphericity Index | 49% | |
| Ellipticity Index | 40% | |
| Surface area | 80% | |
| Volume | 80% | |
| Hemodynamics | WSS | 67% |
| Max WSS | 55% | |
| Min WSS | 75% | |
| NWSS | 71% | |
| Maxim NWSS | 51% | |
| Min NWSS | 74% | |
| OSI | 68% | |
| RRT | 76% | |
| LSA | 75% | |
| Complex flow | 50% | |
| RRS | 80% | |
For WSS, Minimum WSS, NWSS, Maximum NWSS, and Minimum NWSS we hypothesized that among IAs belong to each patient, the IA with the lowest value is the ruptured IA. For the rest of the variables, we hypothesized that the IA with the highest value is the ruptured IA.
Figure 2:
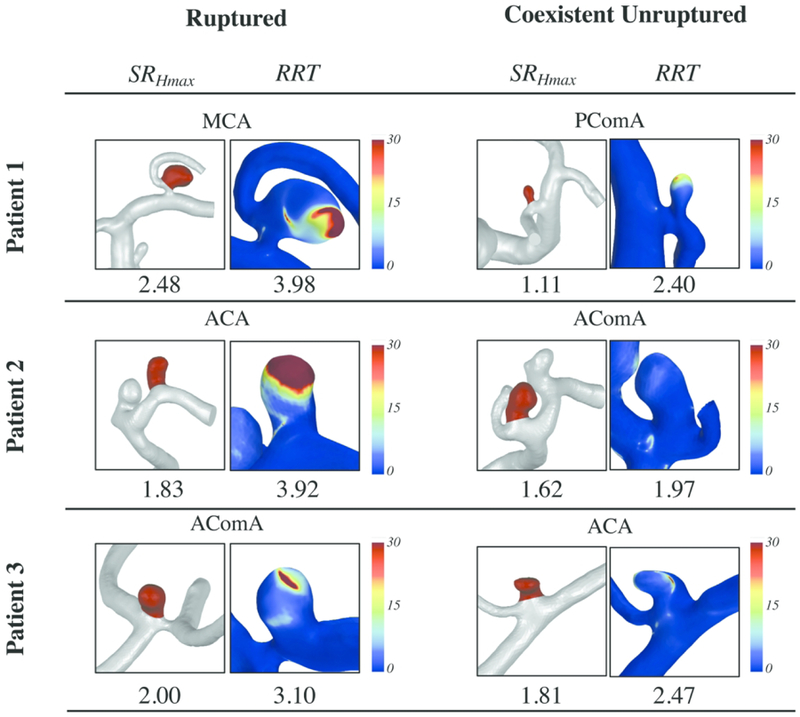
Size ratio (ratio of maximum height to vessel diameter; SRHmax) and relative residence time (RRT) for all IAs in 3 representative cases (each patient had 1 ruptured and 1 unruptured IA). The number for RRT is the spatiotemporal aneurysm-averaged over the aneurysm sac.
Rupture Identification Models
The two models, RIMC and RIMM, tailored to identify the ruptured aneurysm in SAH patients with multiple IAs, achieved PPV of 93±4% and 90±4%, respectively, during 10-fold cross-validation.
| (1) |
| (2) |
where Bifurcation equals 1 if an aneurysm is a bifurcation type and 0 if it is a sidewall type.
In the hold-out testing cohort, RIMC and RIMM could identify the ruptured IA in 92% and 85% of the 23 patients, respectively. Figure 3 shows the performance of RIMC and RIMM in the 10-fold cross-validation and hold-out testing cohorts.
Figure 3:
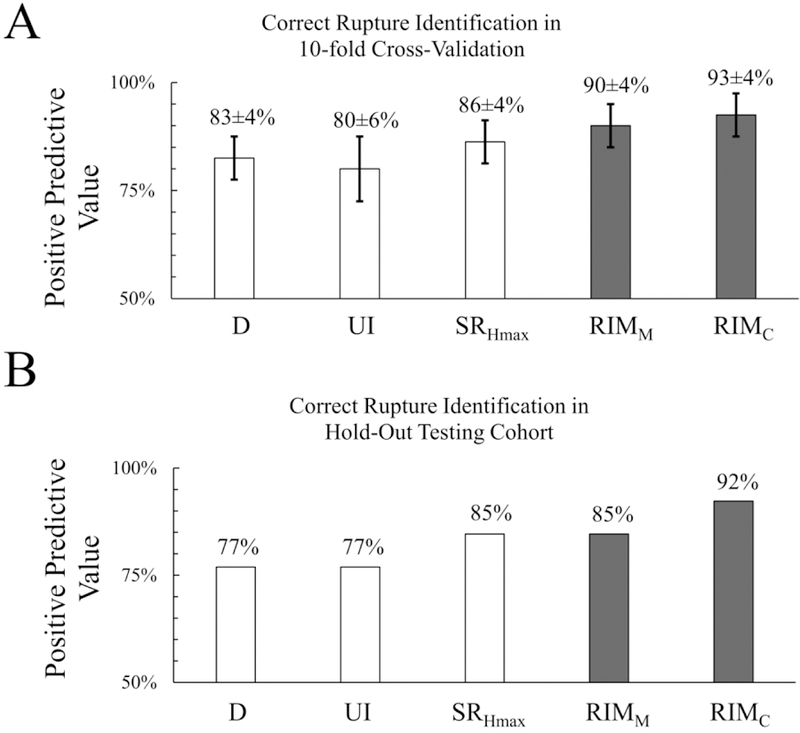
Comparison of rupture identification models (RIMC and RIMM) to the 3 existing rupture risk predictors in identifying the ruptured IA in SAH patients with multiple IAs in 10-fold cross-validation (A) and the hold-out testing cohort (B). D, maximum dimension; UI, undulation index; SRHmax, Size ratio.
Discussion
Identification of the bleeding site is essential to management of aneurysmal SAH because one of the early steps in clinical care is to secure the rupture site to prevent rebleeding. It is often unfeasible to secure all aneurysms discovered in the same setting, so clinicians prioritize treating the highest-risk aneurysm first. Misidentification of the ruptured IA may result in disastrous rebleeding of the ruptured lesion and mortality.2–8 In approximately 50% of patients, the hemorrhage pattern on CT images makes positive identification of the source of bleeding questionable, in which case clinicians often rely on angiographic findings.2, 3 In a study by Orning et al.,3 the ruptured aneurysm was misidentified in 16.2% of patients who had nondefinitive hemorrhage patterns. To create a more reliable aneurysmal metric for identifying the causative lesion, we generated 2 models (RIMC and RIMM) that identify the ruptured site in patients with multiple IAs better than existing aneurysmal parameters.
Recent studies have reported using high-resolution MRI of vessel wall for identifying the ruptured aneurysm among multiples.32, 33 However, the reliability of such an approach is not yet established, as 28.5% of stable unruptured IAs also demonstrated wall enhancement features.34 Moreover, such imaging requires long acquisition time, which limits its clinical application and requires cooperative or intubated patients.35 Consequently, the standard of care for identification of ruptured IAs in patients with multiples still relies on CT and angiographic findings.
Controversy exists in the literature regarding whether aneurysm size or shape is more reliable for identifying the ruptured IA in patients with multiple IAs. Nehls et al.2 noted that irregular shape, defined as “multilobulated, contained a nipple, or were markedly elongated,” is a better predictor of the ruptured aneurysm than aneurysm size, “greatest dimension.” Conversely, Shojima et al.36 reported that aneurysm size, “largest diameter,” predicts the ruptured aneurysm better than irregular shape, which was defined as an aneurysm with a daughter sac or “an irregular protrusion of the aneurysm wall.” We believe such opposing opinions exist because “irregular shape” was not clearly defined and is subject to inter-rater variations.21 To avoid such a problem, we quantified aneurysm shape by calculating 3 shape indices, UI, Nonsphericity Index, and Ellipticity Index.22 Among them, UI was the only parameter that was significantly different between ruptured and unruptured IAs and identified the ruptured IAs in 80% of patients. However, all definitions of aneurysm size, including Hmax and D, outperformed UI in identifying the ruptured IA, which indicates that aneurysm size is more reliable than aneurysm shape for ruptured IA identification.
Previous studies have investigated the morphologic and hemodynamic differences between ruptured and unruptured aneurysms in patients with multiple IAs mainly through regression analysis, which is more suitable for modeling event probabilities.9, 10, 12–14, 36 In the current study, the ruptured aneurysm was identified by directly comparing the IAs within each patient. We noted that morphology outperformed hemodynamics in identifying ruptured IAs. Among the morphologic parameters that we studied, SRHmax had the highest performance, better than traditional indices such as aneurysm size (D, Hmax) and ARHmax. SRHmax incorporates Hmax and vessel diameter, which is a surrogate for aneurysm location, another variable previously found to be well correlated with IA rupture.37
In a recent study, 17 research groups were asked to use their developed rupture classification models and identify the ruptured aneurysm in a single SAH patient with 5 IAs. However, only 4 groups could correctly identify the true ruptured aneurysm.38 In our study, we observed that some individual morphologic parameters, including SRHmax and Hmax, outperformed RRS, a previously developed rupture classification model.16 This indicates that existing rupture classification models, including RRS, may not be tailored for such a specific problem, namely, the identification of the ruptured IA in patients with multiple IAs. A plausible explanation is that those models were built based on pooled data of individuals with multiple and single IAs. A previous study indicated that the characteristics or natural history of IAs in patients with multiple aneurysms might be different from those with a single IA.39 For instance, it was reported that posterior circulation aneurysms were less frequent in patients with multiple IAs than in patients with a single aneurysm, which we also observed in our study (only 3% of IAs were located at the posterior circulation). Moreover, previous models were trained to discriminate ruptured cohorts from unruptured cohorts without associating the coexisting IAs within each patient. Such factors were considered when generating the RIMC and RIMM. The RIMC model incorporated aneurysm SRHmax, UI, RRT, and bifurcation type. SRHmax and UI represent aneurysm size and shape, respectively, which both are established morphological metrics for identification of ruptured IAs. RRT is a hemodynamic parameter that incorporates both WSS and OSI. IAs with high RRT are exposed to high near-wall flow stasis, which could promote inflammatory cell infiltration and aneurysm wall degradation, thereby increasing the risk of aneurysm rupture.40 Bifurcation type is a surrogate for locations with high risk of rupture, including the anterior communicating artery, PICA, and posterior communicating artery.2, 41
As an alternative model that does not require hemodynamic parameters, RIMM incorporates only morphologic factors, SRHmax, UI, and Bifurcation. Although RIMC slightly outperformed RIMM, we believe that RIMM will be more user-friendly in clinical settings by reducing the need for intensive CFD simulations.
Our novel predictive models (RIMM, RIMC) can be helpful in SAH patients with hemorrhage patterns that are not definitive. In such cases, instead of solely relying on aneurysm size, shape, or location to delineate the ruptured source, the composite RIM models may provide more reliable identification.
Limitations
First, our study had a small sample size and was limited to saccular aneurysms, which could be the reason for the low percentage of posterior circulation aneurysms (3%). Second, definitive hemorrhage patterns were used to identify the rupture source in some of our cases, but only craniotomy can reveal the true rupture status; therefore, there is a possibility of misidentification. Third, our cases come from 3 different centers and there could be a difference in their aneurysm rupture patterns due to genetics. Fourth, we utilized several widely used simplifications to perform CFD simulations such as a rigid wall, Newtonian blood, and generalized boundary conditions to make the computations tractable. Fifth, although we found excellent agreement among users in calculating morphologic and hemodynamic parameters, this was contingent upon users following the protocol described in this study. It is unclear how well the RIM models will hold if different imaging modalities, segmentation methods, or CFD settings are used. Finally, we used only linear regression models, because they were easier to customize for our specific problem. Also, it was easier to interpret the components (e.g., weights) of the final models. In future studies, the application of nonlinear models, such as artificial neural network and random forest, could be explored to account for possible nonlinear interaction among variables.
Conclusions
To identify ruptured IA in SAH patients with multiple IAs, SRHmax is the best predictor among individual morphologic parameters, including location and type, and hemodynamic parameters. However, composite models (RIMC and RIMM), specifically designed for identifying the ruptured IA in patients with multiple IAs, outperformed all individual parameters. Between the 2 models, RIMC, which incorporated aneurysm hemodynamics, had better positive predictive value in identifying ruptured IA, than RIMM. These findings may help to improve clinical identification of the ruptured IA in SAH patients presenting with multiple aneurysms.
Supplementary Material
Acknowledgments
We thank Paul H. Dressel BFA for figure preparation and W. Fawn Dorr BA and Debra J. Zimmer for editorial assistance.
Funding
Supported by NIH grants R01NS091075and R03NS090193 and Canon Medical Systems Corp. Davies was supported by the National Center for Advancing Translational Sciences of the NIH (award KL2TR001413 to University at Buffalo). This work is also supported by National Key Research and Development Plan of China (grant 2016YFC1300800), the National Natural Science Foundation of China (grants 81220108007, 81371315, 81471167, and 81671139) and the Special Research Project for Capital Health Development (grant 2018-4-1077).
Competing Interests
Rajazbadeh-Oghaz, Wang, Varble, Sugiyama, Shimizu, Jin, Liu, Yang: None Siddiqui: Co-investigator: NIH/NINDS 1R01NS091075 Virtual Intervention of Intracranial Aneurysms. Financial Interest/Investor/Stock Options/Ownership: Amnis Therapeutics, Apama Medical, BlinkTBI, Buffalo Technology Partners, Cardinal Health, Cerebrotech Medical Systems, Claret Medical, Cognition Medical, Endostream Medical, Imperative Care, International Medical Distribution Partners, Rebound Therapeutics, Silk Road Medical, StimMed, Synchron, Three Rivers Medical, Viseon Spine. Consultant/Advisory Board: Amnis Therapeutics, Boston Scientific, Canon Medical Systems USA, Cerebrotech Medical Systems, Cerenovus, Claret Medical, Corindus, Endostream Medical, Guidepoint Global Consulting, Imperative Care, Integra, Medtronic, MicroVention, Northwest University
Davies: Research grant: National Center for Advancing Translational Sciences of the NIH (award number KL2TR001413) to University at Buffalo. Speakers’ bureau: Penumbra;
Honoraria: Neurotrauma Science
Meng: Principal investigator of NIH grant 1R01NS091075, R03NS090193, and Canon Medical Systems Corporation grants [no grant number]. Co-founder, Neurovascular Diagnostics, Inc.
Abbreviations
- ACA
anterior cerebral artery
- AR
aspect ratio
- CFD
computational fluid dynamics
- D
maximum diameter
- H
height (perpendicular)
- Hmax
maximum height
- IA
intracranial aneurysm
- LSA
low shear area
- NWSS
normalized WSS
- OSI
Oscillatory shear index
- RIM
rupture identification model
- RRS
rupture resemblance score
- RRT
relative residence time
- SR
size ratio
- UI
undulation index
- WSS
wall shear stress
Footnotes
Ethics Approval
This study was approved by the institutional review board of the University at Buffalo (IRB: 30–510704: Virtual Intervention of Intracranial Aneurysms).
References
- 1.Juvela S. Risk factors for multiple intracranial aneurysms. Stroke 2000;31:392–97 [DOI] [PubMed] [Google Scholar]
- 2.Nehls DG, Flom RA, Carter LP, et al. Multiple intracranial aneurysms: determining the site of rupture. J Neurosurg 1985;63:342–8 [DOI] [PubMed] [Google Scholar]
- 3.Orning JL, Shakur SF, Alaraj A, et al. Accuracy in identifying the source of subarachnoid hemorrhage in the setting of multiple intracranial aneurysms. Neurosurgery 2017 [DOI] [PubMed]
- 4.Hino A, Fujimoto M, Iwamoto Y, et al. False localization of rupture site in patients with multiple cerebral aneurysms and subarachnoid hemorrhage. Neurosurgery 2000;46:825–30 [DOI] [PubMed] [Google Scholar]
- 5.Liu Z, Wei L-f, Wang R-m, et al. Misdiagnosis of rupture sites of multiple intracranial aneurysms: Special report of two cases. Sci Res Essays 2010;5:3794–98 [Google Scholar]
- 6.Lee KC, Joo JY, Lee KS. False localization of rupture by computed tomography in bilateral internal carotid artery aneurysms. Surg Neurol 1996;45:435–40; discussion 40–1 [DOI] [PubMed] [Google Scholar]
- 7.Zderkiewicz E, Pawlik Z, Czochra M, et al. Clinical signs pointing to the source of hemorrhage in multiple intracranial aneurysms. Med Sci Monit 2002;8:Cr83–6 [PubMed] [Google Scholar]
- 8.Koyama T, Tanaka Y, Kobayashi S, et al. Surgery of basilar aneurysms associated with unexpected rupture of an internal carotid artery aneurysm. Neurosurg Rev 2000;23:168–70 [DOI] [PubMed] [Google Scholar]
- 9.Bjorkman J, Frosen J, Tahtinen O, et al. Irregular shape identifies ruptured intracranial aneurysm in subarachnoid hemorrhage patients with multiple aneurysms. Stroke 2017;48:1986–89 [DOI] [PubMed] [Google Scholar]
- 10.Jing L, Fan J, Wang Y, et al. Morphologic and hemodynamic analysis in the patients with multiple intracranial aneurysms: ruptured versus unruptured. PLoS ONE 2015;10:e0132494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doddasomayajula R, Chung B, Mut F, et al. Hemodynamic characteristics of ruptured and unruptured multiple aneurysms at mirror and ipsilateral locations. AJNR Am J Neuroradiol 2017;38:2301–07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhogal P, AlMatter M, Hellstern V, et al. Difference in aneurysm characteristics between ruptured and unruptured aneurysms in patients with multiple intracranial aneurysms. Surg Neurol Int 2018;9:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Backes D, Vergouwen MD, Velthuis BK, et al. Difference in aneurysm characteristics between ruptured and unruptured aneurysms in patients with multiple intracranial aneurysms. Stroke 2014;45:1299–303 [DOI] [PubMed] [Google Scholar]
- 14.Jeon HJ, Lee JW, Kim SY, et al. Morphological parameters related to ruptured aneurysm in the patient with multiple cerebral aneurysms (clinical investigation). Neurol Res 2014;36:1056–62 [DOI] [PubMed] [Google Scholar]
- 15.Berg P, Beuing O. Multiple intracranial aneurysms: a direct hemodynamic comparison between ruptured and unruptured vessel malformations. Int J Comput Assist Radiol Surg 2018;13:83–93 [DOI] [PubMed] [Google Scholar]
- 16.Xiang J, Yu J, Choi H, et al. Rupture resemblance score (RRS): toward risk stratification of unruptured intracranial aneurysms using hemodynamic-morphological discriminants. J Neurointerv Surg 2015;7:490–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Antiga L, Piccinelli M, Botti L, et al. An image-based modeling framework for patient-specific computational hemodynamics. Med Biol Eng Comput 2008;46:1097–112 [DOI] [PubMed] [Google Scholar]
- 18.Xiang J, Natarajan SK, Tremmel M, et al. Hemodynamic–morphologic discriminants for intracranial aneurysm rupture. Stroke 2011;42:144–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murray CD. The physiological principle of minimum work I. The vascular system and the cost of blood volume. Proc Natl Acad Sci U S A. 1926;12:207–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rajabzadeh-Oghaz H, Varble N, Davies JM, et al. Computer-assisted adjuncts for aneurysmal morphologic assessment: toward more precise and accurate approaches. Proc SPIE Int Soc Opt Eng 2017;10134:101341C-41C-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rajabzadeh-Oghaz H, Varble N, Shallwani H, et al. Computer-assisted three-dimensional morphology evaluation of intracranial aneurysms. World Neurosurg 2018;119:e541–e50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dhar S, Tremmel M, Mocco J, et al. Morphology parameters for intracranial aneurysm rupture risk assessment. Neurosurgery 2008;63:185–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rahman M, Smietana J, Hauck E, et al. Size ratio correlates with intracranial aneurysm rupture status: a prospective study. Stroke 2010;41:916–20 [DOI] [PubMed] [Google Scholar]
- 24.Raghavan ML, Ma B, Harbaugh RE. Quantified aneurysm shape and rupture risk. J Neurosurg 2005;102:355–62 [DOI] [PubMed] [Google Scholar]
- 25.Asgharzadeh H, Borazjani I. Effects of Reynolds and Womersley numbers on the hemodynamics of intracranial aneurysms. Computational and mathematical methods in medicine 2016;2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsivgoulis G, Sharma VK, Hoover SL, et al. Applications and advantages of power motion-mode Doppler in acute posterior circulation cerebral ischemia. Stroke 2008;39:1197–204 [DOI] [PubMed] [Google Scholar]
- 27.Steinmeier R, Laumer R, Bondar I, et al. Cerebral hemodynamics in subarachnoid hemorrhage evaluated by transcranial Doppler sonography. Part 2. Pulsatility indices: normal reference values and characteristics in subarachnoid hemorrhage. Neurosurgery 1993;33:10–8; discussion 18–9 [DOI] [PubMed] [Google Scholar]
- 28.Byrne G, Mut F, Cebral J. Quantifying the large-scale hemodynamics of intracranial aneurysms. AJNR Am J Neuroradiol 2014;35:333–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xiang J, Yu J, Snyder KV, et al. Hemodynamic–morphological discriminant models for intracranial aneurysm rupture remain stable with increasing sample size. J Neurointerv Surg 2016;8:104–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gail MH, Lubin JH, Rubinstein LV. Likelihood calculations for matched case-control studies and survival studies with tied death times. Biometrika 1981;68:703–07 [Google Scholar]
- 31.Feng X, Ji W, Qian Z, et al. Bifurcation location is significantly associated with rupture of small intracranial aneurysms (< 5 mm). World neurosurgery 2017;98:538–45 [DOI] [PubMed] [Google Scholar]
- 32.Matouk CC, Mandell DM, Gunel M, et al. Vessel wall magnetic resonance imaging identifies the site of rupture in patients with multiple intracranial aneurysms: proof of principle. Neurosurgery 2013;72:492–6; discussion 96 [DOI] [PubMed] [Google Scholar]
- 33.Mandell DM, Mossa-Basha M, Qiao Y, et al. Intracranial Vessel Wall MRI: Principles and Expert Consensus Recommendations of the American Society of Neuroradiology. AJNR Am J Neuroradiol 2017;38:218–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Edjlali M, Gentric J-C, Régent-Rodriguez C, et al. Does Aneurysmal Wall Enhancement on Vessel Wall MRI Help to Distinguish Stable From Unstable Intracranial Aneurysms? Stroke 2014;45:3704–06 [DOI] [PubMed] [Google Scholar]
- 35.Lindenholz A, van der Kolk AG, Zwanenburg JJM, et al. The Use and Pitfalls of Intracranial Vessel Wall Imaging: How We Do It. Radiology 2017;286:12–28 [DOI] [PubMed] [Google Scholar]
- 36.Shojima M, Morita A, Nakatomi H, et al. Size is the most important predictor of aneurysm rupture among multiple cerebral aneurysms: post hoc subgroup analysis of unruptured cerebral aneurysm study Japan. Neurosurgery 2018;82:864–69 [DOI] [PubMed] [Google Scholar]
- 37.Ma D, Tremmel M, Paluch RA, et al. Size ratio for clinical assessment of intracranial aneurysm rupture risk. Neurol Res 2010;32:482–6 [DOI] [PubMed] [Google Scholar]
- 38.Berg P, Voß S, Janiga G, et al. Multiple Aneurysms AnaTomy CHallenge 2018 (MATCH)—phase II: rupture risk assessment. Int J Comput Assist Radiol Surg 10.1007/s11548-019-01986-2 (epub May 3, 2019). [DOI] [PubMed]
- 39.Lee YJ, Parreira T, Matouk CC, et al. Clinical characteristics and preferential location of intracranial mirror aneurysms: a comparison with non-mirror multiple and single aneurysms. Neuroradiology 2015;57:35–40 [DOI] [PubMed] [Google Scholar]
- 40.Meng H, Tutino VM, Xiang J, et al. High WSS or low WSS? Complex interactions of hemodynamics with intracranial aneurysm initiation, growth, and rupture: toward a unifying hypothesis. AJNR Am J Neuroradiol 2014;35:1254–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Varble N, Rajabzadeh-Oghaz H, Wang J, et al. Differences in Morphologic and Hemodynamic Characteristics for “PHASES-Based” Intracranial Aneurysm Locations. AJNR Am J Neuroradiol 2017;38:2105–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


