Abstract
Background:
Over the last quarter century, the number of publications using vessel wall MR imaging (VWI) has increased. Though many narrative reviews offer insight into technique and diagnostic applications, a systematic review of publication trends and reporting quality has not been conducted to identify unmet needs and future directions.
Purpose:
We aimed to identify which intracranial vasculopathies need more data and highlight areas of strengths and weaknesses in reporting.
Data Sources:
PubMed, EMBASE and MEDLINE databases were searched up to September 2018 in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.
Data Analysis:
Two independent reviewers screened and extracted data from 128 articles. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guideline was used to assess reporting quality of analytic observational studies.
Data Synthesis:
There is an exponentially increasing trend in the number of VWI publications over the last 24 years (p<0.0001). Intracranial atherosclerosis is the most commonly studied intracranial vasculopathy (49%), followed by dissections (13%), aneurysms (8%), and vasculitis (5%). Analytic observational study designs comprised 48% of the studies. Trans-continental collaborations showed non-significantly higher reporting quality compared to work originating from single continents (p=0.20).
Limitations:
Heterogeneity in study designs.
Conclusions:
More efforts are warranted to further understand the utility of VWI in less commonly studied intracranial vasculopathies such as dissections, aneurysms, and vasculitis. More consistent adherence to STROBE guidelines should improve transparency and maximize effective synthesis for clinical translation. Diverse, collaborative teams are encouraged to advance the understanding of intracranial vasculopathies using VWI.
Introduction
Vessel wall MR Imaging (VWI) is increasingly being used world-wide to evaluate intracranial vasculopathies.1 This adoption has paralleled a rise in the number of publications using VWI. Many narrative reviews report on the applications and utility of VWI for different types of vasculopathies.2, 3 However, no study has systematically assessed the frequency or trends of VWI publications for all intracranial vasculopathies or the reporting quality.
Interpretation of data and secondary analyses from observational studies is often limited by the methodology and completeness of reporting. Reporting quality is important to critically assess a study’s strengths, weaknesses and generalizability as well as for investigators who desire to assess a study’s reproducibility. The clinical and scientific utility of research data may be lost in poorly reported studies. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines were developed to improve the quality of reporting of observational studies in medical research.4, 5 We set out to systematically survey VWI publications by examining the types of vasculopathies studied as well as assess the quality of reporting of analytic observational studies using the STROBE checklist. The aims of this study were to identify which intracranial vasculopathies may need more data as well as highlight areas of reporting that could be improved.
Materials and Methods
Search Strategy
The systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. PubMed, EMBASE, and Medline were searched on September 12, 2018. To identify eligible studies, keywords were searched using the Boolean operators “OR” and “AND.” Keywords covered imaging, vessel wall imaging, intracranial circulation, vasculopathy, and vascular disease terms (Supplemental Table 1). A manual review of the citations of each included article was also performed. All foreign language articles were translated.
Study selection
Two researchers independently reviewed all publications for inclusion. Inclusion criteria were: (1) case series or observational studies; (2) imaging humans; (3) intracranial arteries; (4) intracranial vasculopathies; (5) imaging arterial wall; and (6) magnetic resonance imaging. Single case reports, conference abstracts, animal studies and studies of the pediatric population were excluded.
Data extraction
Two reviewers independently screened and extracted data from each study that fulfilled the inclusion and exclusion criteria. Disagreements were resolved by consensus. The following data were collected: publication characteristics (year of publication, country(ies) of publication, funding sources), type of vasculopathy studied, study design (case series or analytic observational study), and subject enrollment design (prospective or retrospective). Analytic observational studies were identified using a published study design classification algorithm6 and included studies with comparator groups or designed as prevalence/cross-sectional or diagnostic accuracy studies.7–9 Studies that reported obtaining informed consent or explicitly reported prospective enrollment by the authors were categorized as being prospective. Trans-continental collaborative publications were identified by author affiliations. Reporting quality was assessed using the STROBE checklist.4 Each STROBE item was assessed as 1=reported, 0.5=partly reported, or 0=not reported. A complete reporting score (CRS) per publication was calculated by summing the total number of items divided by 22 (the total number of STROBE criteria). Scores by manuscript section (introduction, methods, results, and conclusions) were also calculated.
Statistical and Sensitivity Analysis
Categorical variables are expressed in counts and percentages. Distributions of continuous variables are summarized with means and standard deviations or medians and inter-quartile ranges. Agreement was calculated with an unweighted Cohen’s kappa. Based on goodness of fit, exponential and linear regression analyses were fit to test trends over time in the total number of VWI studies and analytic observational studies by year, respectively. Shapiro-Wilks test was used to test normal distributions of CRS measures. CRS measures were calculated by a summary statistic (mean or median) based on the test for a normal distribution. Kruskal-Wallis test assessed differences among CRS scores by continent. Two sensitivity analyses assessed the robustness of the results. The first sensitivity analysis considered fulfilment of STROBE items reported in footnotes, bylines, and in different sections of the manuscript. A second analysis was conducted by considering fulfilment of STROBE items that were “partly reported” in any part of the manuscript, including footnotes and bylines. Publication bias was assessed using the likelihood ratio Chi-square test to compare the distributions of the intracranial vasculopathies of the included studies versus the excluded conference abstracts. SPSS v19 (Chicago, IL) was used for statistical analysis.
Results
Search
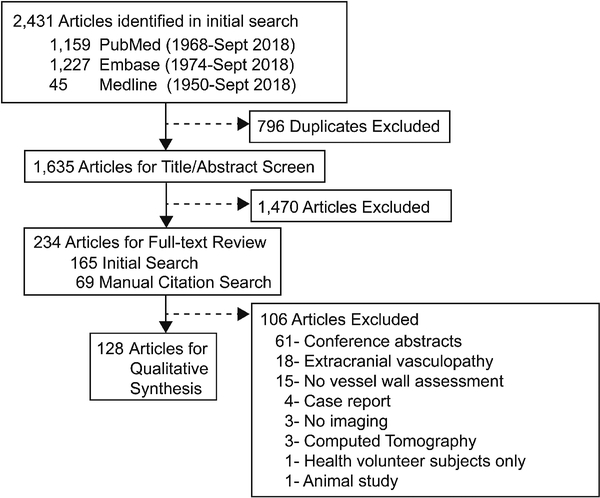
The search strategy identified 2,431 publications among which 1,635 were screened by title/abstract (κ=0.77, 95% CI 0.71–0.83, p<0.01). Of those, 234 articles were selected for full-text review (κ=0.89, 95% CI 0.82–0.96, p<0.01). Manual review of the citations of the included articles yielded 807 citations, which were further screened by title/abstract (κ=0.73, 95% CI 0.52–0.93, p<0.01). Full data extraction for qualitative synthesis was performed on 128 articles identified from the initial and manual citation review (Figure 1; Supplemental References).
Figure 1:
Systematic Review of the Databases
From the initial database search, 165 articles were identified for full-text review. Manual review of the citations of those 165 articles identified 807 citations, which were further screened by title and abstract. This resulted in 69 articles for full-text review from the manual citation search. A total of 234 articles underwent full-text review, from which 128 articles met the pre-determined inclusion and exclusion criteria.
Publication Trends
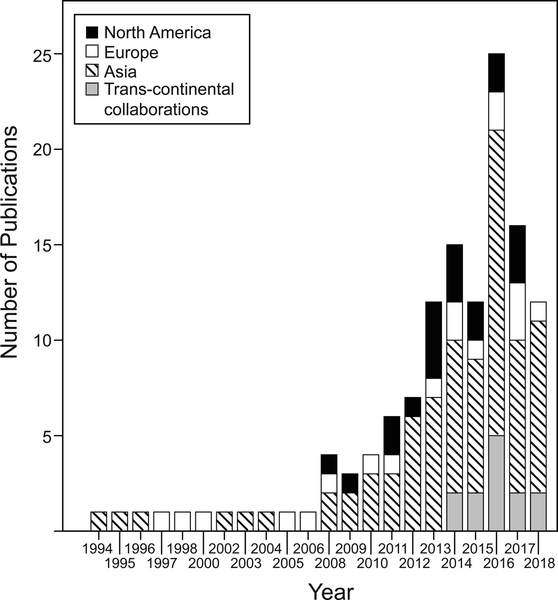
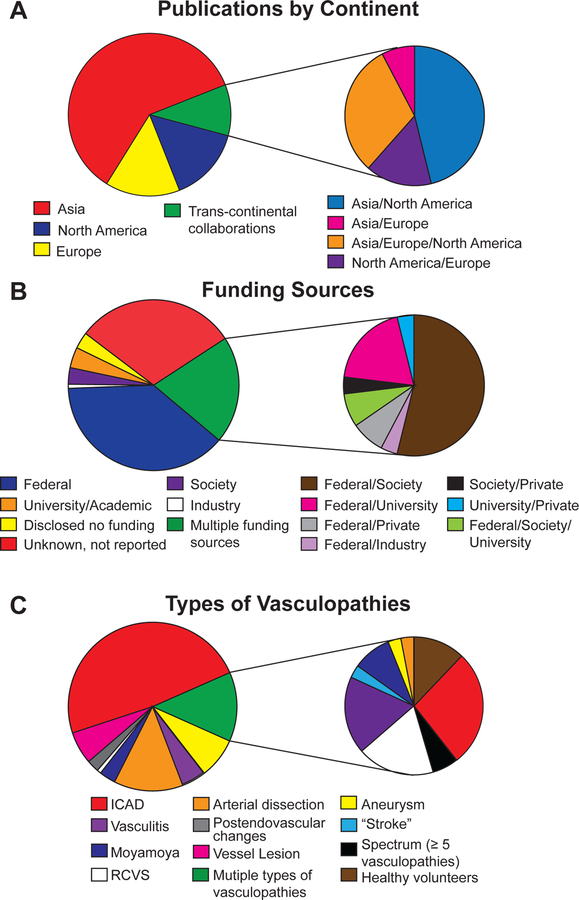
The first article evaluating intracranial vessel wall characteristics from this systematic review was reported in 1994 evaluating intramural hematomas in dissections.10 The second publication was in 1995 and evaluated vessel walls for cerebral atherosclerosis.11 An exponentially increasing trend in the number of VWI publications over the last 24 years was seen (Figure 2) (β=0.14, 95% CI 0.11–0.17, p<0.0001). Asia published the highest number of publications (61%) over 24 years, followed by North America (n=19) and Europe (n=18). In 2014, trans-continental collaborative publications emerged, comprising 10% of the included publications. Asia was part of 92% of the trans-continental collaborations (Figure 3A) and 46% of the trans-continental collaborations were between Asia and North America.
Figure 2:
Annual Number of Publications using Vessel Wall Imaging
Figure 3:
- Distribution of publications by continent is shown. Distribution of trans-continental collaborations is further elaborated to show four different groups of collaborating partners.
- Distribution of different funding sources as well as the combination of funding sources are shown.
- Distribution of studied intracranial vasculopathies are shown.
Abbreviations: ICAD, intracranial atherosclerotic disease, RCVS, reversible cerebral vasoconstriction syndrome
Most investigations were solely federally funded (39%). Publications with mixed types of funding sources accounted for 21% with the most common combination being federal and medical society sources (54%). No funding source was reported for 30% of all publications (Figure 3B).
Survey of studies focused on 1 vasculopathy revealed intracranial atherosclerotic disease (ICAD) to be the most commonly studied vasculopathy (49%, n=62, Supplemental References), followed by arterial dissection (13%, n=16)10, 12–26, aneurysm (8%, n= 10)27–36, vasculitis (5%, n=6)37–42, moyamoya disease (3%, n=4)43–46, post-endovascular changes (2%, n=3)47–49, and reversible cerebral vasoconstriction syndrome (1%, n=1)50 (Figure 3C). Among the 16 publications investigating arterial dissections, 11 used VWI12, 14–18, 22–26 and the other 5 publications10, 13, 19–21 assessed intramural hematoma signal characteristics on conventional MRI. Note 21% of the studies examined 2 or more types of vasculopathies; a breakdown of the types of vasculopathies studied among these publications are further illustrated by the pie-chart inset.
Publication bias was assessed by comparing the number of intracranial vasculopathy types that were included in this study versus excluded conference abstracts, which showed no significant difference (Supplemental Table 2, p=0.95).
Study Designs
Case series comprised 52% of the publications and 48% were analytic observational study designs. A significant yearly increase in analytic observational studies emerged since the year 2000 (β= 0.39, 95% CI 0.26–0.51, p<0.0001). Most studies were conducted with prospective subject enrollment (50%) as compared to retrospective subject identification (44%). Examples of prospective case series include studies that obtained written informed consent to study circle of Willis cadaveric specimens and characterize intracranial atherosclerotic plaque components,51 recruited 3 subjects to describe atherosclerosis enhancement characteristics by VWI,52 and methodological papers reporting inter-rater/intra-rater reliability53 and scan-rescan reproducibility of the imaging technique.54
STROBE Reporting Assessment
The 62 analytic observational studies were also evaluated for reporting quality using the STROBE checklist (Table 1) (κ=0.76, 95% CI 0.72–0.79, p<0.001). The average CRS for all studies was 0.64±0.10. The introduction section had the highest and the methods had the lowest scores. Trans-continental collaborative publications showed higher scores (CRSall=0.67±0.05) compared to single-continent studies (Table 1) and multi-site/single-continent collaborations (CRSall=0.63±0.12), although these results did not reach statistical significance (p=0.20).
Table 1.
STROBE Complete Reporting Scores
| CRSAll | CRSIntroduction | CRSMethods | CRSResults | CRSDiscussion | |
|---|---|---|---|---|---|
| All (n=62) |
0.64 (0.10) | 1.00 (0) | 0.56 (0.22) | 0.60 (0.20) | 0.63 (0.25) |
| North America (n=11) |
0.62 (0.10) | 1.00 (0) | 0.52 (0.11) | 0.56 (0.22) | 0.69 (0.28) |
| Asia (n=40) |
0.65 (0.11) | 1.00 (0) | 0.61 (0.17) | 0.65 (0.20) | 0.63 (0.25) |
| Europe (n=6) |
0.58 (0.14) | 1.00 (0) | 0.42 (0.15) | 0.60 (0.15) | 0.63 (0.19) |
| Trans-continental collaborations (n=5) |
0.67 (0.05) | 1.00 (0) | 0.60 (0.07) | 0.64 (0.11) | 0.73 (0.10) |
CRS measures reported in means (standard deviations) or medians (interquartile range). Summary statistic chosen based on test for normality.
Two sensitivity analyses were performed that showed the same direction of the results but notably did not reach statistical significance. First, a sensitivity analysis evaluating each study for items reported in any part of the manuscript, including footnotes and bylines, showed higher scores from trans-continental collaborations compared to single-continent studies (p=0.30). A second sensitivity analysis considered all partially reported criteria as fulfilling reporting and also showed higher CRS measures by trans-continental collaborations than North American and European studies. The studies from Asia had only marginally higher CRS measures (p=0.27) (Supplemental Table 3).
Supplemental Table 4 reports the mean score per STROBE item for the 62 articles and checklist descriptions. Items 1 and 22 were scored separately, as they reflect title/abstract and funding reporting, respectively. Most studies provided an informative abstract but did not indicate the study design in the title (item 1) to fully meet the STROBE criterion resulting in a score of 0.52±0.13. Also, 70% of the studies did not disclose whether or not there was a funding source (item 22) resulting in a score of 0.71±0.46. The two introduction section criteria, evaluating the reporting of the scientific background and rationale (item 2) and specific objectives or hypothesis (item 3), scored the highest among all sections.
The methods section included nine criteria. No publication reported a sample size determination (item 10). Explicitly presenting key elements of the study (item 4) also scored low (0.08±0.24). Although 73% of the studies reported a prospective or retrospective subject enrollment method, few studies named the study design type.5 Clearly defining outcomes, exposures, predictors, confounders, and diagnostic criteria (item 7) also had a low score (0.34±0.32); for example, only 14% of the 43 publications studying ICAD reported diagnostic criteria for cardiovascular risk factors such as hypertension. The importance of reporting diagnostic criteria for hypertension is emphasized in light of the new 2017 American Heart Association classifications of hypertension and evolving definitions.55
Five criteria were included in the results section. Reporting of the study participants and information on exposures and potential confounders (item 14) was suboptimal (0.45+0.19). Publications lacking information on confounders such as race/ethnicity were scored as partial reporting. Only 8 studies18, 56–62 reported race/ethnicity, among which 5 studies18, 56–59 reported the information in the discussion section as a limitation of generalizability. 24 publications originated from Asia, were single-center studies, and did not report a description of ethnicity; in these studies, one could assume that all enrolled subjects were Asian but clarity in reporting could be strengthened.
In the Discussion section, generalizability (item 21) scored the lowest (0.26±0.44). Most studies were single center studies (74.2%), but this was not commonly addressed as a limitation for external validity. A discussion on limitations and reporting direction/magnitude of potential bias (item 19) also scored poorly (0.55±0.24), with most studies partially fulfilling this item due to an absence of a discussion on direction and magnitude of the bias.
Discussion
The increasing trend in the number of VWI publications and more trans-continental collaborations over the past 24 years suggests the widespread interest in the diagnostic utility of VWI. Evaluation of the reporting quality of analytic observational studies using the STROBE checklist highlighted strengths as well as weaknesses in the current literature. Subgroup analyses showed that trans-continental collaborations yielded higher CRS measures compared to single-center/single-continent and multi-center/single-continent publications, suggesting an advantage for multi-cultural, diverse collaborative work. Notably, the results did not reach significance, potentially due to the relatively small number of trans-continental publications.
Survey of the literature to date shows Asia as the leading contributor of VWI publications and ICAD to be the most commonly studied vasculopathy. Fewer VWI studies were identified evaluating arterial dissections, aneurysms, vasculitis, and moyamoya disease. More efforts are warranted to further understand the utility of VWI in these less commonly studied intracranial vasculopathies.
Given stroke is one of the leading causes of morbidity and mortality world-wide and a wide-spread public health problem, it is not surprising to see federal funding supporting many of the VWI investigations across all continents. Medical societies such as the Dutch Heart Foundation, American Society of Neuroradiology, Radiologic Society of North America, and American Heart Association (AHA) also emerged as common funding sources, showing societal missions to improve stroke outcomes. Nearly one-third of the studies did not disclose a funding source, reflecting either no funding source or funding that was not disclosed.
The availability of the STROBE guidelines has encouraged many research domains and medical specialties to improve reporting quality63, 64 and ultimately improve research reproducibility. Assessment of reporting quality of VWI publications has not yet been evaluated. Our results show a CRS of 0.64 for the 62 analytic observational VWI studies. The results of this study highlight areas where reporting of analytic observational studies are good along with other areas where improvements are needed. In particular, there is a need for studies to improve the clear reporting of definitions of exposures, predictors and potential confounders (item 7). The lack of clearly defined diagnostic criteria becomes important when, for example, guidelines are updated; in 2017, the AHA changed the blood pressure guidelines with some secondary analyses suggesting the new classification correlates to different prevalence rates and outcomes.65, 66
Study size calculations (item 10) were not reported in any study. Sample size estimates are informative as they indicate the magnitude of the aimed effect and address whether there were challenges with recruitment due to drop out or attrition bias.5
Additional areas of weakness in reporting included the description of study design (item 4) and settings and locations (item 5). Reporting a prospective or retrospective enrollment is insufficient. For example, a cross-sectional study assessing imaging findings on a subject at one time-point may be prospectively enrolled or retrospectively collected from a registry.
In the Discussion, reporting the generalizability or external validity (item 21) of the results was also noted to be a weakness. Generalizability is evaluating the extent to which the results of the publication can be applied to other settings and populations and is important for the reader to see if the results are applicable to his/her own practice setting.
Analysis of reporting quality by continent showed trans-continental collaborations had higher CRS measures (Table 1). Collaborations are encouraged for the cross-pollination of ideas, to increase recruitment or enhance generalizability by coordinating a multi-site study, among other advantages.67 Our findings suggest an additional advantage of higher reporting quality. Multilingual authors and increased diversity within teams from different continents may be possible explanations for this finding.
This systematic review has some limitations. First, neuroradiology research often reports innovative techniques and drive cutting-edge methods that are not tested as part of larger epidemiologic studies. These technological advancement publications are typically smaller scale and “proof of concept” studies that are designed differently but may have larger impact. The STROBE guidelines do not always meet the radiology research framework. However, as an assessment for completeness of reporting with consistency in evaluation by 2 independent raters, the results provide at least a basis of reporting quality among VWI publications. Second, classifying studies as case series or analytic observational studies were often challenging due to the heterogeneity in study designs. Many of the included studies did not fit traditional epidemiologic study designs. This challenge has been addressed in other systematic review methodologies and an algorithm has been tested to appropriately classify study designs for systematic reviews. We based our classification as descriptive (case series) or analytic observational studies following a simplified version of this algorithm.6 Third, methodologic quality was not assessed, as the aim was to identify trends in VWI publications rather than conduct a quantitative meta-analysis. Instead, an assessment of reporting quality was conducted to highlight areas for improvement. Fourth, use of the STROBE checklist is inconsistently recommended in author guidelines among journals, which could be a confounder in our analysis. Notably, some journals have their own required checklists requiring a statement on data sharing and open source availability, which also improve transparency of the conducted research. As a future direction, an assessment of the journals can be conducted to assess CRS measures by journal impact factor as well as explicit author instructions to follow the STROBE checklist. Finally, the CRS was calculated using the STROBE guidelines and items were scored by section as determined by the STROBE checklist. Studies that did not report items in the correct section per the STROBE guidelines were not considered fulfilling the criteria. This method may underestimate CRS measures. To account for this, a sensitivity analysis was performed with items scored as reported if present in any part of the manuscript, including footnotes and author bylines. This sensitivity analysis yielded similar conclusions. A second sensitivity analysis considering partially reported items as completely reported showed a similar direction of the results with higher reporting quality CRS by trans-continental collaborative work compared to all, North American, and European studies, while Asia had a marginally higher CRS measure.
Conclusions
We systematically assessed VWI publications to identify trends and assess reporting quality. Our results show that ICAD is by far the most common intracranial vasculopathy studied. The utility of VWI for ICAD and importantly, for other types of intracranial vasculopathies, will likely benefit from additional rigorous studies. We also highlight deficiencies in the reporting of analytic observational VWI studies. Trans-continental collaborative efforts yielded a higher reporting quality, although this result did not reach statistical significance. Nonetheless, there may be advantages to diverse and multi-lingual cross-cultural teams. More consistent adherence to STROBE guidelines should improve transparency and maximize effective synthesis and clinical translation of findings for future studies.
Supplementary Material
Funding support:
The project described was supported by RSNA Research & Education Foundation, through grant number RSCH1929. The content is solely the responsibility of the authors and does not necessarily represent the official views of the RSNA R&E Foundation. This was work was also supported by the Institute for Translational Medicine and Therapeutics/Thomas B. McCabe and Jeannette E. Laws McCabe Fund for Junior Faculty (JWS), and NIH National Heart, Lung, and Blood Institute R01 HL137984 (WRW).
References
- 1.Arenillas JF, Dieleman N, Bos D. Intracranial arterial wall imaging: Techniques, clinical applicability, and future perspectives. Int J Stroke doi: 10.1177/1747493019840942 [DOI] [PubMed] [Google Scholar]
- 2.Lindenholz A, van d K, Zwanenburg JJM, et al. The use and pitfalls of intracranial vessel wall imaging: How we do it. Radiology 2018;286:12–28. [DOI] [PubMed] [Google Scholar]
- 3.Mandell DM, Mossa-Basha M, Qiao Y, et al. Intracranial vessel wall MRI: Principles and expert consensus recommendations of the american society of neuroradiology. Am J Neuroradiol 2017;38:218–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 2007;370:1453–7. [DOI] [PubMed] [Google Scholar]
- 5.Vandenbroucke JP, von Elm E, Altman DG, et al. Strengthening the reporting of observational studies in epidemiology (STROBE): Explanation and elaboration. Epidemiology 2007;18:805–35. [DOI] [PubMed] [Google Scholar]
- 6.Seo HJ, Kim SY, Lee YJ, et al. A newly developed tool for classifying study designs in systematic reviews of interventions and exposures showed substantial reliability and validity. J Clin Epidemiol 2016;70:200–5. [DOI] [PubMed] [Google Scholar]
- 7.Song JW, Chung KC. Observational studies: Cohort and case-control studies. Plast Reconstr Surg 2010;126:2234–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grimes DA, Schulz KF. An overview of clinical research: The lay of the land. Lancet 2002;359:57–61. [DOI] [PubMed] [Google Scholar]
- 9.Rutjes AW, Reitsma JB, Vandenbroucke JP, et al. Case-control and two-gate designs in diagnostic accuracy studies. Clin Chem 2005;51:1335–41. [DOI] [PubMed] [Google Scholar]
- 10.Kitanaka C, Tanaka J-, Kuwahara M, et al. Magnetic resonance imaging study of intracranial vertebrobasilar artery dissections. Stroke 1994;25:571–5. [DOI] [PubMed] [Google Scholar]
- 11.Aoki S, Shirouzu I, Sasaki Y, et al. Enhancement of the intracranial arterial wall at MR imaging: Relationship to cerebral atherosclerosis. Radiology 1995;194:477–81. [DOI] [PubMed] [Google Scholar]
- 12.Natori T, Sasaki M, Miyoshi M, et al. Detection of vessel wall lesions in spontaneous symptomatic vertebrobasilar artery dissection using T1-weighted 3-dimensional imaging. Journal of Stroke and Cerebrovascular Diseases: The Official Journal of National Stroke Association 2014;23:2419–24. [DOI] [PubMed] [Google Scholar]
- 13.Urbach H, Meyer-Lindenberg A, Bendszus M, et al. Dissections of the basilar artery]. RoFo: Fortschritte Auf Dem Gebiete Der Rontgenstrahlen Und Der Nuklearmedizin 1998;169:170–4. [DOI] [PubMed] [Google Scholar]
- 14.Ishitsuka K, Sakaki Y, Sakai S, et al. Diagnosis and follow-up of posterior inferior cerebellar artery dissection complicated with ischemic stroke assisted by T1-VISTA: A report of two cases. BMC Neurology 2016;16:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sakurai K, Miura T, Sagisaka T, et al. Evaluation of luminal and vessel wall abnormalities in subacute and other stages of intracranial vertebrobasilar artery dissections using the volume isotropic turbo-spin-echo acquisition (VISTA) sequence: A preliminary study. Journal of Neuroradiology. Journal De Neuroradiologie 2013;40:19–28. [DOI] [PubMed] [Google Scholar]
- 16.Choi JW, Han M, Hong JM, et al. Feasibility of improved motion-sensitized driven-equilibrium (iMSDE) prepared 3D T1-weighted imaging in the diagnosis of vertebrobasilar artery dissection. Journal of Neuroradiology 2018;45:186–91. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y, Lou X, Li Y, et al. Imaging investigation of intracranial arterial dissecting aneurysms by using 3 T high-resolution MRI and DSA: From the interventional neuroradiologists’ view. Acta Neurochir 2014;156:515–25. [DOI] [PubMed] [Google Scholar]
- 18.Kwon JY, Kim N-, Suh DC, et al. Intracranial and extracranial arterial dissection presenting with ischemic stroke: Lesion location and stroke mechanism. J Neurol Sci 2015;358:371–6. [DOI] [PubMed] [Google Scholar]
- 19.Hirai T, Korogi Y, Murata Y, et al. Intracranial artery dissections: Serial evaluation with MR imaging, MR angiography, and source images of MR angiography. Radiation Medicine - Medical Imaging and Radiation Oncology 2003;21:86–93. [PubMed] [Google Scholar]
- 20.Kim T-, Choi HS, Koo J, et al. Intramural hematoma detection by susceptibility-weighted imaging in intracranial vertebral artery dissection. Cerebrovascular Diseases 2013;36:292–8. [DOI] [PubMed] [Google Scholar]
- 21.Mascalchi M, Bianchi MC, Mangiafico S, et al. MRI and MR angiography of vertebral artery dissection. Neuroradiology 1997;39:329–40. [DOI] [PubMed] [Google Scholar]
- 22.Takano K, Yamashita S, Takemoto K, et al. MRI of intracranial vertebral artery dissection: Evaluation of intramural haematoma using a black blood, variable-flip-angle 3D turbo spin-echo sequence. Neuroradiology 2013;55:845–51. [DOI] [PubMed] [Google Scholar]
- 23.Park KJ, Jung SC, Kim HS, et al. Multi-contrast high-resolution magnetic resonance findings of spontaneous and unruptured intracranial vertebral artery dissection: Qualitative and quantitative analysis according to stages. Cerebrovasc Dis 2016;42:23–31. [DOI] [PubMed] [Google Scholar]
- 24.Jung SC, Kim HS, Choi C-, et al. Spontaneous and unruptured chronic intracranial artery dissection: High-resolution magnetic resonance imaging findings. Clinical Neuroradiology 2018;28:171–81. [DOI] [PubMed] [Google Scholar]
- 25.Yun SY, Heo YJ, Jeong HW, et al. Spontaneous intracranial vertebral artery dissection with acute ischemic stroke: High-resolution magnetic resonance imaging findings. The Neuroradiology Journal 2018;31:262–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Madokoro Y, Sakurai K, Kato D, et al. Utility of T1- and T2-weighted high-resolution vessel wall imaging for the diagnosis and follow up of isolated posterior inferior cerebellar artery dissection with ischemic stroke: Report of 4 cases and review of the literature. Journal of Stroke and Cerebrovascular Diseases: The Official Journal of National Stroke Association 2017;26:2645–51. [DOI] [PubMed] [Google Scholar]
- 27.Mizutani T. A fatal, chronically growing basilar artery: A new type of dissecting aneurysm. J Neurosurg 1996;84:962–71. [DOI] [PubMed] [Google Scholar]
- 28.Edjlali M, Gentric J-, Régent-Rodriguez C, et al. Does aneurysmal wall enhancement on vessel wall MRI help to distinguish stable from unstable intracranial aneurysms? Stroke 2014;45:3704–6. [DOI] [PubMed] [Google Scholar]
- 29.Park JK, Lee CS, Sim KB, et al. Imaging of the walls of saccular cerebral aneurysms with double inversion recovery black-blood sequence. Journal of magnetic resonance imaging: JMRI 2009;30:1179–83. [DOI] [PubMed] [Google Scholar]
- 30.Boussel L, Wintermark M, Martin A, et al. Monitoring serial change in the lumen and outer wall of vertebrobasilar aneurysms. American journal of neuroradiology 2008;29:259–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martin AJ, Hetts SW, Dillon WP, et al. MR imaging of partially thrombosed cerebral aneurysms: Characteristics and evolution. American journal of neuroradiology 2011;32:346–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Omodaka S, Endo H, Niizuma K, et al. Quantitative assessment of circumferential enhancement along the wall of cerebral aneurysms using MR imaging. American journal of neuroradiology 2016;37:1262–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blankena R, Kleinloog R, Verweij BH, et al. Thinner regions of intracranial aneurysm wall correlate with regions of higher wall shear stress: A 7T MRI study. American journal of neuroradiology 2016;37:1310–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matouk CC, Mandell DM, Günel M, et al. Vessel wall magnetic resonance imaging identifies the site of rupture in patients with multiple intracranial aneurysms: Proof of principle. Neurosurgery 2013;72:492–6. [DOI] [PubMed] [Google Scholar]
- 35.Kleinloog R, Korkmaz E, Zwanenburg JJM, et al. Visualization of the aneurysm wall: A 7.0-tesla magnetic resonance imaging study. Neurosurgery 2014;75:614–22. [DOI] [PubMed] [Google Scholar]
- 36.Nagahata S, Nagahata M, Obara M, et al. Wall enhancement of the intracranial aneurysms revealed by magnetic resonance vessel wall imaging using three-dimensional turbo spin-echo sequence with motion-sensitized driven-equilibrium: A sign of ruptured aneurysm? Clinical Neuroradiology 2016;26:277–83. [DOI] [PubMed] [Google Scholar]
- 37.Cheng-Ching E, Jones S, Hui FK, et al. High-resolution MRI vessel wall imaging in varicella zoster virus vasculopathy. J Neurol Sci 2015;351:168–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ide S, Kakeda S, Miyata M, et al. Intracranial vessel wall lesions in patients with systematic lupus erythematosus: Vessel wall lesions in SLE patients. Journal of Magnetic Resonance Imaging 2018;48:1237–46. [DOI] [PubMed] [Google Scholar]
- 39.Berkefeld J, Enzensberger W, Lanfermann H. MRI in human immunodeficiency virus-associated cerebral vasculitis. Neuroradiology 2000;42:526–8. [DOI] [PubMed] [Google Scholar]
- 40.Thaler C, Kaufmann-Bühler A, Gansukh T, et al. Neuroradiologic characteristics of primary angiitis of the central nervous system according to the affected vessel size. Clinical Neuroradiology 2017;29:37–44. [DOI] [PubMed] [Google Scholar]
- 41.Schuster S, Bachmann H, Thom V, et al. Subtypes of primary angiitis of the CNS identified by MRI patterns reflect the size of affected vessels. J Neurol Neurosurg Psychiatr 2017;88:749–55. [DOI] [PubMed] [Google Scholar]
- 42.Küker W, Gaertner S, Nagele T, et al. Vessel wall contrast enhancement: A diagnostic sign of cerebral vasculitis. Cerebrovasc Dis 2008;26:23–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Han C, Li M-, Xu Y, et al. Adult moyamoya-atherosclerosis syndrome: Clinical and vessel wall imaging features. J Neurol Sci 2016;369:181–4. [DOI] [PubMed] [Google Scholar]
- 44.Muraoka S, Araki Y, Taoka T, et al. Prediction of intracranial arterial stenosis progression in patients with moyamoya vasculopathy: Contrast-enhanced high-resolution magnetic resonance vessel wall imaging. World Neurosurgery 2018;116:e1121. [DOI] [PubMed] [Google Scholar]
- 45.Yu L-, He H, Zhao J-, et al. More precise imaging analysis and diagnosis of moyamoya disease and moyamoya syndrome using high-resolution magnetic resonance imaging. World Neurosurgery 2016;96:252–60. [DOI] [PubMed] [Google Scholar]
- 46.Wang M, Yang Y, Zhou F, et al. The contrast enhancement of intracranial arterial wall on high-resolution MRI and its clinical relevance in patients with moyamoya vasculopathy. Scientific Reports 2017;7:44264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Seo W-, Oh K, Suh S-, et al. Clinical significance of wall changes after recanalization therapy in acute stroke: High-resolution vessel wall imaging. Stroke 2017;48:1077–80. [DOI] [PubMed] [Google Scholar]
- 48.Power S, Matouk C, Casaubon LK, et al. Vessel wall magnetic resonance imaging in acute ischemic stroke: Effects of embolism and mechanical thrombectomy on the arterial wall. Stroke 2014;45:2330–4. [DOI] [PubMed] [Google Scholar]
- 49.Abraham P, Scott Pannell J, Santiago-Dieppa D, et al. Vessel wall signal enhancement on 3-T MRI in acute stroke patients after stent retriever thrombectomy. Neurosurgical Focus 2017;42:E20. [DOI] [PubMed] [Google Scholar]
- 50.Chen C-, Chen S-, Fuh J-, et al. Vascular wall imaging in reversible cerebral vasoconstriction syndrome - A 3-T contrast-enhanced MRI study. Journal of Headache and Pain 2018;19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jiang Y, Zhu C, Peng W, et al. Ex-vivo imaging and plaque type classification of intracranial atherosclerotic plaque using high resolution MRI. Atherosclerosis 2016;249:10–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jiang W-, Yu W, Ma N, et al. High resolution MRI guided endovascular intervention of basilar artery disease. Journal of NeuroInterventional Surgery 2011;3:375–8. [DOI] [PubMed] [Google Scholar]
- 53.Ma N, Jiang WJ, Lou X, et al. Arterial remodeling of advanced basilar atherosclerosis: A 3-tesla MRI study. Neurology 2010;75:253–8. [DOI] [PubMed] [Google Scholar]
- 54.Zhang X, Zhu C, Peng W, et al. Scan-rescan reproducibility of high resolution magnetic resonance imaging of atherosclerotic plaque in the middle cerebral artery. PLoS ONE 2015;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: Executive summary: A report of the american college of cardiology/american heart association task force on clinical practice guidelines. Hypertension 2018;71:1269–324. [DOI] [PubMed] [Google Scholar]
- 56.Xu W-, Li M-, Niu J-, et al. Intracranial artery atherosclerosis and lumen dilation in cerebral small-vessel diseases: A high-resolution MRI study. CNS Neuroscience and Therapeutics 2014;20:364–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim J-, Jung K-, Sohn C-, et al. Intracranial plaque enhancement from high resolution vessel wall magnetic resonance imaging predicts stroke recurrence. International Journal of Stroke 2016;11:171–9. [DOI] [PubMed] [Google Scholar]
- 58.Kim YS, Lim S-, Oh K-, et al. The advantage of high-resolution MRI in evaluating basilar plaques: A comparison study with MRA. Atherosclerosis 2012;224:411–6. [DOI] [PubMed] [Google Scholar]
- 59.Ahn S-, Lee J, Kim Y-, et al. Isolated MCA disease in patients without significant atherosclerotic risk factors: A high-resolution magnetic resonance imaging study. Stroke 2015;46:697–703. [DOI] [PubMed] [Google Scholar]
- 60.Niu P-, Yu Y, Zhou H, et al. Vessel wall differences between middle cerebral artery and basilar artery plaques on magnetic resonance imaging. Scientific reports 2016;6:38534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dieleman N, Yang W, Abrigo JM, et al. Magnetic resonance imaging of plaque morphology, burden, and distribution in patients with symptomatic middle cerebral artery stenosis. Stroke 2016;47:1797–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Qiao Y, Anwar Z, Intrapiromkul J, et al. Patterns and implications of intracranial arterial remodeling in stroke patients. Stroke 2016;47:434–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Langan S, Schmitt J, Coenraads PJ, et al. The reporting of observational research studies in dermatology journals: A literature-based study. Arch Dermatol 2010;146:534–41. [DOI] [PubMed] [Google Scholar]
- 64.Fung AE, Palanki R, Bakri SJ, et al. Applying the CONSORT and STROBE statements to evaluate the reporting quality of neovascular age-related macular degeneration studies. Ophthalmology 2009;116:286–96. [DOI] [PubMed] [Google Scholar]
- 65.Papaioannou TG, Soulis D, Tousoulis D. Reversibility of hypertension-induced subclinical vascular changes: Do the new ACC/AHA 2017 blood pressure guidelines and heart rate changes make a difference? J Clin Hypertension doi: 10.1111/jch.13602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Muntner P, Carey RM, Gidding S, et al. Potential US population impact of the 2017 ACC/AHA high blood pressure guideline. Circulation 2018;137:109–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hall KL, Vogel AL, Huang GC, et al. The science of team science: A review of the empirical evidence and research gaps on collaboration in science. Am Psychol 2018;73:532–48. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.