Abstract
The generation of tissue-resident memory T cells (TRM) is an essential aspect of immunity at mucosal surfaces, and it has been suggested that preferential generation of TRM is one of the principal advantages of mucosally administered vaccines. We have previously shown that antigen-specific, IL-17-producing CD4+ T cells can provide capsular antibody-independent protection against nasal carriage of Streptococcus pneumoniae; but whether pneumococcus-responsive TRM are localized within the nasal mucosa and are sufficient for protection from carriage has not been determined. Here we show that intranasal administration of live or killed pneumococci to mice generates pneumococcus-responsive IL-17A-producing CD4+ mucosal TRM. Furthermore, we show that these cells are sufficient to mediate long-lived, neutrophil-dependent protection against subsequent pneumococcal nasal challenge. Unexpectedly, and in contrast with the prevailing paradigm, we found that parenteral administration of killed pneumococci also generates protective IL-17A+CD4+ TRM in the nasal mucosa. These results demonstrate a critical and sufficient role of TRM in prevention of pneumococcal colonization, and further that these cells can be generated by parenteral immunization. Our findings therefore have important implications regarding the generation of immune protection at mucosal surfaces by vaccination.
Introduction
Parenteral immunization with purified antigens has resulted in remarkable protection against a number of mucosal pathogens including Haemophilus influenzae type b, Streptococcus pneumoniae, and Neisseria meningitidis.These vaccines generate immunity by inducing the production of highly neutralizing antibodies directed against the capsule, which inhibit attachment to mucosal surfaces and abrogate initial colonization events. However, for a number of other mucosal pathogens such as Staphylococcus aureus, HIV-1, herpes simplex virus or Mycobacterium tuberculosis, this approach has not been successful. Alternative vaccination strategies that are directed at generating more robust mucosal immunity, including cellular responses1, have been proposed.
In this vein, a promising approach has been the focus on mucosal immunization strategies2–5. Following mucosal immunization, antigen-responsive T cells are activated in local draining lymph nodes. It is thought that activation in mucosa-associated lymph nodes leads to the expression of specific chemokine and adhesion receptors that facilitate selective access of effector T cells to mucosal tissue6, a process which may be enhanced by persistence of local antigen7. A subset of these recruited effector T cells can remain in the tissue and further differentiate into tissue-resident memory T cells (TRM) that exhibit limited migratory potential8–10. These cells have the ability to respond rapidly to antigen re-challenge, thereby providing frontline immunity upon subsequent pathogen exposure11–14. Based on these observations, the development of vaccines that can elicit TRM at mucosal surfaces has become an area of active investigation. One of the mucosal surfaces that is first encountered by many pathogens is the nasopharynx. For instance, colonization of the nasal pharynx with pneumococcus is an obligate first step for invasive pathogenesis, but the role played by TRM in protecting the nasopharynx from colonization remains incompletely elucidated. Specifically, it has been shown that prior infection of the lung with an avirulent pneumococcal strain results in the formation of protective tissue-resident CD4+ T cells within the lung, but whether prior colonization of the nose with pneumococci induces the formation of protective anti-pneumococcal CD4+ TRM has not been previously determined.
If generation of nasal TRM is a key mechanism for protection of the nasopharynx from subsequent pneumococcal challenge, then this may be an important immunological marker for effective vaccination. We have studied a killed pneumococcal whole cell preparation that elicits protection against subsequent pneumococcal nasopharyngeal challenge15–23. When delivered either intranasally (I.N.) with cholera toxin (CT) as an adjuvant, or subcutaneously (S.C.) with alum, killed pneumococci can elicit a multi-pronged immune response. This includes protein antigen-specific antibodies that protect against invasive disease, as well as IL-17A-producing CD4+ T cells that protect against nasal colonization19–22, 24, 25. However, whether this vaccine induces CD4+ TRM in the nose and if so whether these are essential for protection of the nose from pneumococcal challenge remain to be determined. Further, it has been hypothesized that a significant advantage of mucosal immunization compared to parenteral administration might be the ability to generate mucosal TRM. However, the observation that the administration of killed pneumococci by S.C. injection mediates protection from nasal colonization raises the question of whether protection induced by this route of administration is also based on generation of mucosal TRM in the nasopharynx, potentially challenging the paradigm that generation of mucosal TRM requires mucosal vaccination.
Here, we show that intranasal exposure of mice to a live encapsulated strain of pneumococcus induces the accumulation of pneumococcal-responsive IL-17A+CD4+ TRM cells in the nose, and futher that these cells are sufficient to mediate nasal clearance upon subsequent intranasal rechallenge of previously exposed mice. Further, we demonstrate that protective pneumococcal-responsive IL-17A+CD4+ TRM cells accumulate in the nose following either I.N. and S.C. administration of killed pneumococci. Finally, we show that nasal CD4+ TRM are essential for the recruitment of neutrophils necessary to limit colonization following nasal challenge of immunized mice. These data demonstrate that nasal CD4+ TRM can provide protection against nasal colonization with pneumococcus based on their ability to recruit neutrophils to the nasal mucosa. Potentially of greatest interest, these findings indicate that highly protective, long lasting mucosal TRM can be generated following parenteral vaccination, and thus have important implications for vaccine development.
Results
Intranasal infection with pneumococcus induces IL-17-producing CD4+ TRM in the nasopharynx
IL-17A+CD4+ memory T cells mediate adaptive immunity towards nasal colonization with S. pneumoniae in mice18, 21, 22, 26. However the phenotype and location of the protective CD4+ T cell has not been determined. A protective role for CD4+ TRM at mucosal surfaces has recently been described for a number of bacterial pathogens of public health significance1, 2, 27. Exposure to live pneumococcus in the nasopharynx protects mice against subsequent homologous or heterologous pneumococcal challenge20, 21. Hence, we hypothesized that I.N. exposure to a pneumococcal strain (followed by clearance) would generate antigen-specific nasal CD4+ TRM that protect against subsequent pneumococcal colonization. To test this hypothesis, mice were exposed intranasally to live strain 0603 or PBS. One to two months later mice received APC-labeled anti-Thy 1.2 antibody by intravenous injection to discriminate between lymphocytes that were located intravascularly and those in the tissue parenchyma, and were then euthanized 5 minutes later. Single-cell suspensions were prepared from the nasal mucosa (NM), blood and spleen and evaluated by flow cytometry (gating strategies for the NM are shown in Supplementary Figure 1a).
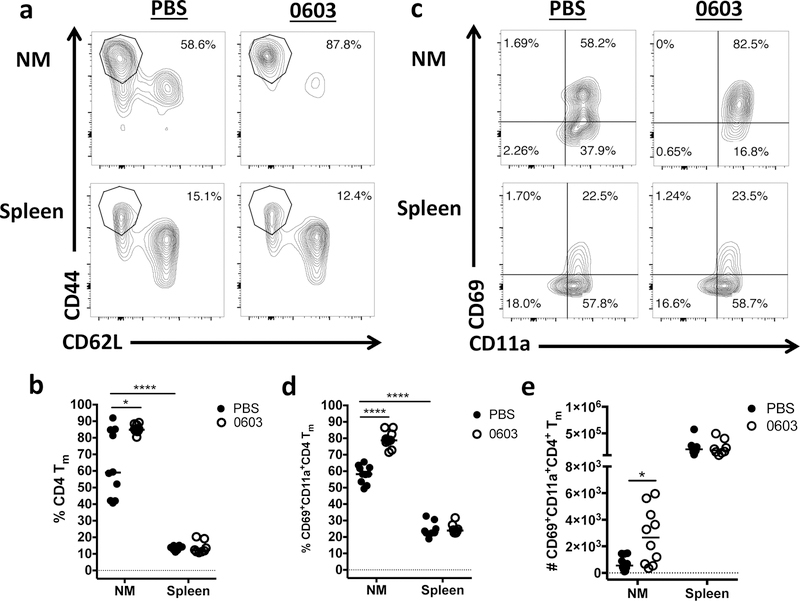
As expected, the majority of CD4+ T cells within the blood were labeled by the Thy1.2 antibody, while the majority of the CD4+ T cells in the spleen were protected from Thy1.2 labeling (Supplementary Figure 1b), demonstrating that the Thy1.2 antibody accurately discriminated between the vascular and parenchymal compartments. The majority of CD4+ T cells within the NM were also protected from labeling with the Thy1.2 antibody, demonstrating their location in an extravascular compartment. Interestingly, in mice exposed to PBS alone, the percent of protected CD4+ T cells that expressed markers associated with a memory phenotype (Tm, CD62LloCD44high) was considerably higher in the NM than the spleen (Fig. 1a and b), and further, the percent of CD4+ Tm that expressed markers associated with tissue residency (CD69 and CD11a) was significantly higher in the NM than the spleen (Fig. 1c and d). Remarkably, both the percent of nasal CD4+ Tm (Fig. 1a and b) and the proportion of these cells that expressed CD69 and CD11a was significantly higher in mice previously exposed to 0603 compared to mice exposed to PBS alone (Fig. 1c and d). Consistent with these observations, the absolute number of CD69+CD11a+ CD4+ Tm cells in the NM was significantly higher in 0603-exposed mice compared to naïve mice (Fig. 1e) These results indicate that the majority of CD4+ Tm in the nose of naïve mice are in fact TRM, and further that the density of these cells increases following pneumococcal exposure.
Fig. 1.
Memory CD4 T cells that demonstrate characteristics associated with tissue residence are present in the nose, and are enriched after intranasal exposure to pneumococcus. Nasal mucosa (NM) and spleen were harvested from mice 1–2 months after intranasal (I.N.) inoculation with serotype 6B pneumococcal strain 0603 or sham infection with PBS. Representative contour plots of “protected” CD4 Tm cells (CD44HiCD62L-) is shown in (a) and quantitated in (b). Representative contour plots of parenchymal CD4 Tm cells stained for CD69 and CD11a in (c). Quantitation of frequency (d) and absolute number (e) of CD69+CD11a+CD4+ Tm cells. Results represent a pool of 3 experiments with 2–5 mice/group/experiment. Horizontal bars indicate the median value. * = P < 0.05, **** = P < 0.0001
To determine if pneumococcal exposure induces pneumococcus-responsive IL-17A-producing CD4+ T cells in the NM, single cell suspension isolated from both the NM and spleen of the mice described above were stimulated with PMA/Ionomycin (P/I) or pneumococcal whole cell antigen (WCA, a killed preparation of pneumococcal strain RM200 as described in the methods). While the frequency of IL-17A+CD4+ T cells was low in the NM or spleen of naïve control mice, the frequency of IL-17A+CD4+ T cells was significantly increased in both the nose and spleen of mice previously exposed to pneumococcus following stimulation with either P/I or WCA (Fig. 2a, b, c, and Supplemental Figure 2). Consistent with these observations, the absolute number of IL-17A+CD4+ T cells in NM and spleen was significantly higher in mice previously exposed to pneumococcus than in naïve control mice (Fig. 2d), although as expected given the relative size of the immune cell compartment in the spleen compared to the NM, the absolute number of IL-17A+CD4+ T cells was considerably higher in the spleen than in the NM. These data strongly suggest that intranasal exposure to live pneumococcus induces the accumulation of pneumococcus-responsive IL-17A+CD4 TRM in the nasal mucosa.
Fig. 2.
Pneumococcal-responsive IL-17A+CD4+ nasal TRM are detected after I.N. exposure. Nasal mucosa (NM) and spleen were harvested from mice 1–2 months after I.N. inoculation with serotype 6B pneumococcal strain 0603 or sham infection with PBS. (a) Representative dot plot showing intracellular staining for IL-17A in CD4+ T cells following ex vivo stimulation of NM and splenic cells with PMA/ionomycin (P/I) or pneumococal whole cell antigen (WCA). Quantitation of frequency of IL-17A+CD4+ T cells after (b) P/I or (c) WCA stimulation. (d) Absolute number of IL-17A+CD4+ T cells in nose and spleen after stimulation with WCA. Results represent a pool of 3 experiments, n = 2–5 mice/group/experiment. Horizontal lines indicate the median value. * = P < 0.05, ** = P < 0.01. *** = P < 0.001, **** = P < 0.0001
TRM interfere with nasal colonization in previously exposed mice.
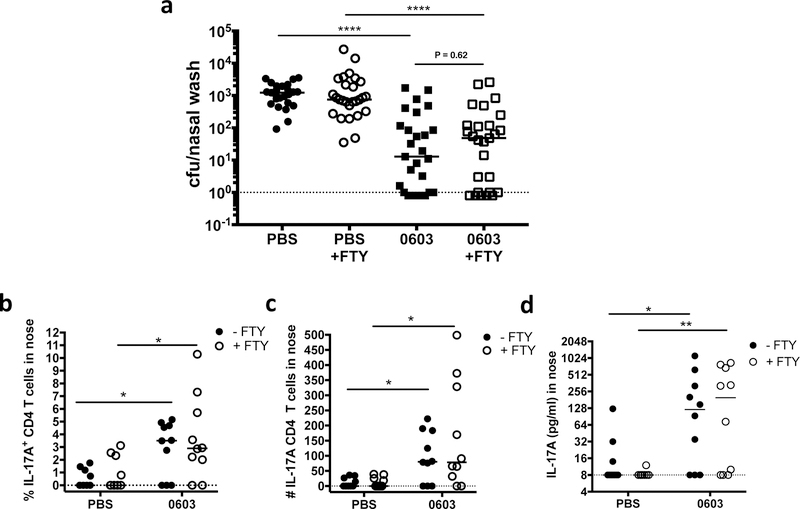
FTY720 inhibits recruitment of circulating lymphocytes into tissue, and is a well-established tool to discriminate the contribution of tissue-resident lymphocytes from recently recruited effector T cells28. To determine if TRM generated in response to prior exposure to strain 0603 were sufficient to interfere with nasal colonization following rechallenge with live pneumococcus, naïve mice or those infected with strain 0603 intranasally one month earlier were treated with FTY720 or left untreated. Mice were then challenged intranasally with pneumococcus and the number of pneumococci in the nasopharynx was enumerated 7 days later. As expected, nasopharyngeal aspirates from naïve mice had high concentrations of pneumococcus following challenge, while the nasopharynx of mice previously exposed to pneumococcus were significantly protected (Fig. 3a and Supplemental Figure 3a). Remarkably, treatment of WCV-immunized mice with FTY720 did not interfere with protection (P=0.62 when compared to WCV-immunized mice that did not receive FTY) suggesting that TRM that accumulate within the nasal mucosa following colonization with live pneumococcus are sufficient to mediate protection from a secondary challenge.
Fig. 3.
Nasal TRM generated after exposure to live pneumococcus are sufficient to mediate protection from secondary challenge. Mice were exposed to live pneumococcus (0603) or not (PBS), one month later treated with FTY720 (+FTY) or left untreated, then challenged I.N. with 0603. Nasal washes and tissue harvest were performed 7 days post-challenge. (a) CFU in nasal washes. (b) Frequency (c) and absolute number of IL-17A+CD4+ T cells in nose after ex vivo stimulation with WCA for 24 hours. (d) Concentration of IL-17A in supernatants from nasal cells after ex vivo stimulation with WCA for 7 days. (a) represents a pool of four experiments, n = 5–10 mice/group/experiment. (b) to (d) represent a pool of two experiments, n = 5 mice/group/experiment. Horizontal lines indicate the median value. * = P < 0.05, ** = P < 0.01, *** = P < 0.001, **** = P < 0.0001
To confirm that mice previously exposed to pneumococcus had higher IL-17A responses in the nose compared to naïve mice following challenge, cell suspensions prepared from the NM of the mice described in Figure 3a were stimulated ex vivo overnight with WCA and the frequency and absolute number of IL-17A+CD4+ T cells were determined. As expected, mice previously exposed to pneumococcus had significantly higher percentages and numbers of IL-17A+CD4+ T cells in the nose compared to naïve mice (Fig. 3b, c and Supplemental Figure 3b), and notably the density of IL-17A+CD4+ remained significantly higher in previously exposed mice in the presence of FTY720, strongly suggesting that these cells are TRM. Similarly, levels of IL-17A secreted by nasal cells after ex vivo stimulation with WCA for 7 days were significantly higher in previously exposed mice regardless of treatment with FTY720 (Fig. 3d). Together these data indicate that pneumococcus-responsive IL-17 secreting CD4+ TRM are generated in the nose following exposure to live pneumococcus and interfere with colonization following secondary challenge.
Intranasal immunization with pneumococcal whole cell vaccine (WCV) induces protective CD4+ TRM
We have previously shown that intranasal administration of the WCV (WCA and CT) provides excellent protection against subsequent nasal challenge with live pneumococcus20. Thus, we wondered whether, similar to nasal exposure to strain 0603, intranasal administration of WCV would induce protective TRM. To test this hypothesis, we vaccinated mice with WCV or CT intranasally and repeated one week later. One month after the final vaccination, mice were left untreated, treated with FTY720, or treated with a CD4-depleting antibody and then challenged intranasally with pneumococcus. Seven days later nasopharyngeal aspirates from mice vaccinated with CT alone had high concentrations of pneumococcus, while the concentration of pneumococcus in the nose of mice immunized with WCV was significantly reduced. Importantly, treatment with FTY720 did not interfere with protection (Fig. 4a), although as we have previously shown21, protection was completely abrogated following treatment with an anti-CD4 antibody.
Fig. 4.
CD4+ TRM generated after mucosal immunization with WCV are sufficient to provide protection following nasal challenge with pneumococcus. Nasal washes and nasal tissue harvest were performed 4–7 days after challenge of mice immunized with CT or WCV and then left untreated, treated with FTY720, or anti-CD4 antibody (as indicated). (a) Levels of nasal colonization with pneumococcus. Results are representative of 2 experiments, n= 10 mice / group. (b) Absolute number of CD69+CD11a+CD4+ Tm cells in nose. Frequency (c) and absolute number (d) of IL-17A+CD4+ T cells in nose after ex vivo stimulation with WCA for 24 hours. (e) Concentration of IL-17A in supernatants from nasal cells after ex vivo stimulation with WCA for 7 days. Results represent a pool of two experiments, n = 4–5 mice/group/experiment. Horizontal bars indicate the median value. * = P ≤ 0.05, ** = P < 0.01, *** = P < 0.001, **** = P < 0.0001
To determine if protection induced by intranasal vaccination with WCV was associated with increased numbers of TRM and induction of pneumococcal specific IL-17 responses in the nose, we enumerated the number of CD69+CD11a+CD4+ Tm in the nose of mice immunized intranasally with WCV following challenge with pneumococcus, and evaluated both bulk and antigen-specific IL-17 responses. Consistent with the hypothesis that WCV induces protective TRM, we observed significantly higher numbers of CD69+CD11a+CD4+ Tm in the nose of mice immunized with WCV (Fig. 4b and Supplemental Figure 4a), as well as increased frequency and higher absolute numbers of IL-17A+CD4+ T cells (Fig. 4c, d, and Supplemental Figure 4b) compared to those immunized with CT alone. Further, we found that total IL-17A secreted by nasal cells after ex vivo stimulation with WCA for 7 days was significantly higher in mice immunized with WCA compared to those immunized with CT alone (Fig. 4e). Finally, mice immunized I.N. with WCV and then treated with FTY720 prior to pneumococcal challenge also exhibited significantly higher density of both CD69+CD11a+ and IL-17A+CD4+ T cells and increased secretion of IL-17A in the nose compared to mice immunized with CT alone (and treated with FTY720). As expected treatment with the anti-CD4 antibody significantly depleted CD4+ T cells and interfered with IL-17A secretion following ex vivo stimulation (Fig. 4b–e). Taken together these observations strongly suggest that WCV induces protective pneumococcus-specific IL-17A-producing CD4+ TRM within the nose following I.N. immunization with WCV.
S.C. immunization generates antigen-specific IL-17A-producing TRM in the mucosa.
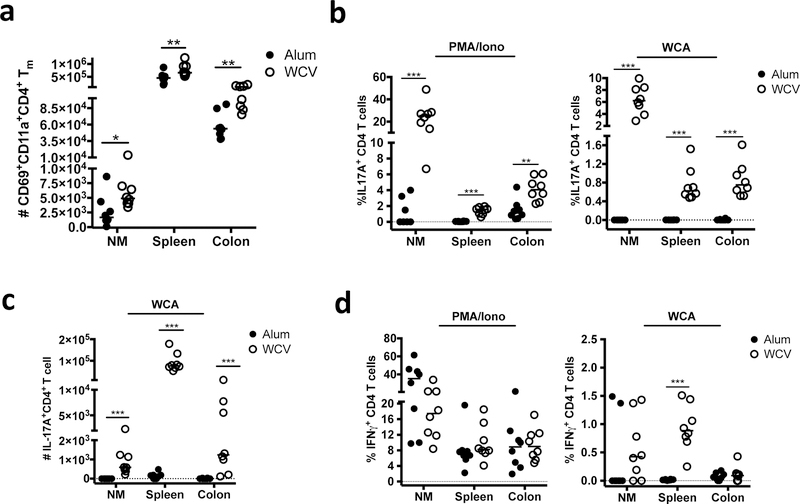
We have previously shown that parenteral immunization with WCV (WCA and aluminum hydroxide) protects mice from nasal colonization with pneumococcus for up to 12 weeks post-immunization19. Based on prior evidence that generation of tissue-resident memory responses following S.C. immunization is inefficient3–5, 29, 30, we had presumed that WCV-induced immunity in the nose was based on recruitment of circulating effector CD4+ T cells following pneumococcal challenge. However, having demonstrated that I.N.-administered WCV induces the accumulation of protective CD4+ TRM within the NM, we wondered whether parenteral immunization might do the same. To address this question, mice were immunized three times at bi-weekly intervals S.C. with alum alone or WCV, and 4 weeks later single cell suspensions were prepared from mucosal sites (the NM and colonic lamina propria), as well as the spleen. Interestingly, in addition to inducing increased numbers of CD69+CD11a+CD4+ Tm in the spleen, WCV induced significantly higher absolute numbers of CD69+CD11a+CD4+ Tm cells in both the colon and nasal mucosa compared to alum alone (Fig. 5a, and Supplemental Figure 5a), indicating that, unexpectedly, parenteral immunization with WCV induces the accumulation of CD4+ TRM at mucosal sites.
Fig. 5.
Pneumococcus-responsive CD4+ TRM are detected in the nose and colon after parenteral immunization. Cells isolated from the NM, spleen or colon from groups of mice immunized with alum alone or WCV were analyzed by flow cytometry, either after staining with antibodies to identify CD4+ TRM, or 5 hours after stimulation with PMA/Ionomycin (P/I) or 24 hours after stimulation with WCA. (a) Absolute number of CD69+CD11a+CD4+ Tm cells in each tissue.(b) Frequency of CD4+ T cells staining with IL-17A following stimulation with PMA/ionomycin or WCA. (c) Absolute numbers of IL-17A+CD4+ T cells after stimulation with WCA. (d) Frequency of CD4+ T cells staining with IFNγ following stimulation with PMA/ionomycin or WCA. Results represent a pool of 2 experiments, n = 3–5 mice / group / experiment. Horizontal bars indicate the median value. * = P < 0.05, ** = P < 0.01, *** = P < 0.001
To determine if S.C. administration of WCV induced pneumococcus-responsive CD4+ T cells in these mucosal compartments, cells suspensions from the NM, spleen and colon were stimulated with P/I or WCA. While there were few IL-17A-producing CD4+ cells identified in mice immunized with alum alone, the percentage and absolute numbers of CD4+ T cells producing IL-17A following stimulation with either P/I or WCA were significantly higher in all tissues following immunization with WCV (Fig. 5b and c and Supplemental Figure 5b). These observations indicate that S.C. vaccination with WCV induces the accumulation of IL-17A producing antigen-specific CD4+ TM at mucosal surfaces. In contrast, while IFN-Y-producing CD4+ T cells could be easily detected following P/I stimulation of cells isolated from both the NM and colon of either alum- or WCV-immunized mice, the frequency of these IFN-γ secreting cells detected within the nasal mucosa following stimulation with WCA was quite low (Fig. 5d), suggesting that within the nasal mucosa, pneumococcus-specific T cell responses induced by WCV are strongly biased towards IL-17A (Fig. 5d).
Nasal CD4+ TRM induced by parenteral immunization with WCV are sufficient for long-lived protection and are generated early in life.
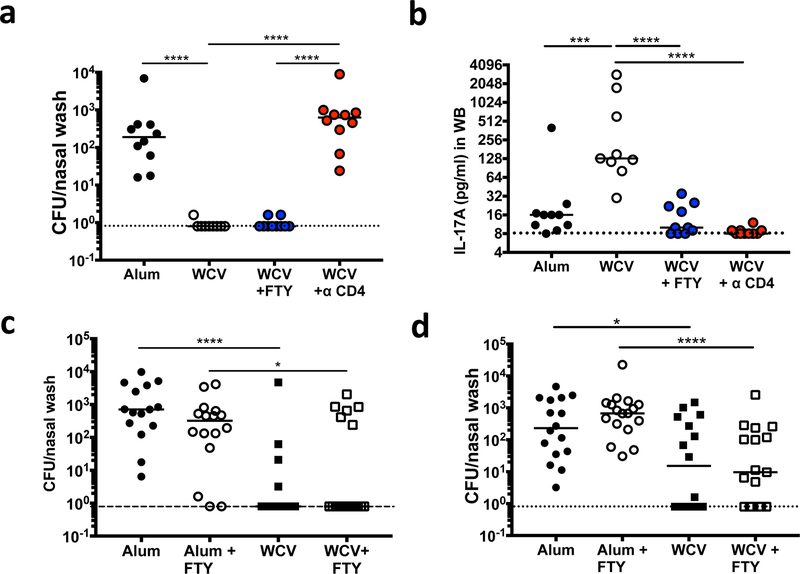
Next, to confirm that nasal CD4+ TRM induced by parenteral immunization with WCV are sufficient to prevent nasal colonization with pneumococcus, immunized mice were left untreated, treated with FTY720, or treated with a CD4-depleting antibody and then challenged. Similar to what we observed following I.N. immunization, WCV-immunized mice treated with FTY720 (but not anti-CD4 antibody) remained significantly protected from nasal colonization (Fig. 6a), despite the observation that FTY720 virtually eliminated IL-17A production from peripheral blood following ex vivo stimulation, indicating successful inhibition of circulating T cells responses (Fig. 6b).
Fig. 6.
WCV-induced CD4+ TRM generated after parenteral immunization provide long-lived protection and are generated early in life after a single dose. Mice immunized with alum alone or WCV were left untreated, treated with FTY720, or treated with anti-CD4 antibody as indicated, challenged I.N. with pneumococcus, and analyzed 10 days later. (a) Levels of nasal colonization with pneumococcus. (b) Concentration of IL-17A in supernatants from whole blood samples 6 days post ex vivo stimulation with WCA. (c) Five-week-old female mice were immunized three times S.C. with alum or WCV, 9 – 22 months later mice were left untreated, or treated with FTY720, challenged I.N. with 0603 and levels of colonization were measured 10 days later. (d) Mice were immunized once S.C. at 6 days of life (DOL 6) with Alum or WCV. Eight weeks later mice were left untreated, or treated with FTY720 as indicated, challenged I.N. with 0603 and levels of colonization were measured 10 days later. Results in (a) and (b) are a representative experiment from 2 experiments, n = 8–10 mice / group. Results in (c) and (d) represent a pool of two experiments, n= 7–10 mice / group /experiment. Horizontal bars indicate the median value. * = P < 0.05, ** = P < 0.01, *** = P < 0.001, **** = P < 0.0001
To assess the duration of protection, mice immunized parenterally with WCV or alum were left untreated, or were treated with FTY720 9 to 22 months after their last immunization. WCV-immunized mice were significantly protected from colonization compared to adjuvant alone immunized mice, regardless of FTY720 treatment (Fig. 6c).
Many vaccines are ineffective if given to infants. The data above suggesting that TRM are critical to vaccine-mediated protection following immunization of adult animals raise the question of whether TRM could be generated following immunization of neonates. To evaluate this issue, we immunized neonatal mice (day of life, DOL, 6) with a single dose of WCV S.C.. One month later mice were treated with FTY720 or left untreated, and intranasally exposed to pneumococcus. Ten days later, mice immunized with WCV had significantly lower levels of colonization compared to mice vaccinated with adjuvant alone, whether or not they had received FTY720 (Fig. 6d). These data suggest immunization of neonates induces protective nasal CD4 TRM.
Overall our results support the hypothesis that protective pneumococcal vaccine-induced nasal CD4+ TRM can be generated early in life after a single dose of WCV, and are long-lived.
Vaccine-induced CD4+ TRM are sufficient to mediate neutrophil recruitment to the nasal mucosa during colonization with pneumoccocus
We have previously demonstrated that neutrophils are required for WCV-mediated protection from nasal colonization18. Having shown that WCV-induced nasal CD4+ TRM provide protection against mucosal colonization, we wondered whether these TRM were sufficient to mediate neutrophil recruitment. To address this, histological sections prepared from the nasal cavity of mice from the groups described above in Figure 6a were stained with anti-CD4 and antimyeloperoxidase (MPO) antibodies, and the absolute number of CD4+ T cells and neutrophils were enumerated. Mice that had been previously immunized with WCV demonstrated significantly higher numbers of both CD4+ T cells and neutrophils in the nasal mucosa following pneumococcal challenge than mice immunized with alum alone, and these differences in cell numbers remained in the presence of FTY720. In contrast, treatment with anti-CD4 antibody significantly reduced the number of both CD4+ T cell and neutrophil accumulation in mice immunized S.C. with WCV (Fig. 7a–c). A potential caveat for this experiment is the limited sample size for the group treated with anti-CD4 antibody (n=5), although a small pilot experiment using mice immunized intranasally with WCV also demonstrated reduced neutrophil recruitment following treatment with anti-CD4 antibody (data not shown). Nontheless, these results indicate that WCV-induced neutrophil accumulation remains intact when recruitment of circulating effector T cells is blocked by FTY720, strongly suggesting nasal CD4+ TRM are sufficient to mediate both neutrophil recruitment and protection from nasal challenge.
Fig. 7.
WCV-induced CD4+ TRM generated after parenteral immunization are sufficient to mediate neutrophil recruitment to the NM. Mice immunized with alum alone or WCV were left untreated, treated with FTY720, or treated with anti-CD4 antibody as indicated, challenged I.N. with pneumococcus, and nasal tissue was harvested 10 days later for analysis by immunofluorescent confocal microscopy. (a) Example of CD4+ (red) and MPO+ (green) immunofluorescent staining of cells in the nasal passages. Image = 40X zoom. Quantitation of (b) CD4+ cells, and (c) MPO+ cells in the nasal passage. Results in (b) and (c) are a pool of 2 experiments, n = 3–5 mice for Alum, WCV and WCV+FTY groups. Single experiment, n = 5 mice for WCV+ anti-CD4 antibody group. Horizontal bars indicate the median value. * = P < 0.05, ** = P < 0.01, *** = P < 0.001
Discussion
Serotype-specific prevention of pneumococcal colonization by conjugate vaccine has resulted in a dramatic reduction in the incidence of disease due to serotypes included in the vaccine. The mechanism of this protection is entirely dependent on the generation of anticapsular antibodies, which are thought to prevent acquisition of the organism in the nasopharynx. Because conjugate vaccines only provide protection against the 10–13 serotypes that are included, these are subject to limited coverage and the phenomenon of serotype replacement.
In contrast to immunity induced by conjugate vaccines, naturally acquired immunity to pneumococcus is unlikely to be primarily mediated by the development of serotype-specific antibodies31; in fact, T- and B-cell responses to pneumococcal protein components that are conserved across all strains have been hypothesized to play a major role in naturally-acquired prevention of disease24, 32. Using either live exposure to pneumococcus or immunization with WCV, we have previously shown that CD4+ Th17 responses to a number of antigens provide protection against colonization in mice18 and can be detected in the peripheral blood mononuclear cells of human adults and children33–35. However, the nature and localization of these protective Tm cells have not been previously examined.
Here we demonstrate that pathogen-specific CD4+ TRM in the NM of mice generated by exposure to live or killed pneumococci are sufficient for long-lasting T-cell mediated protection against nasal colonization. While our previous results had suggested that protection may be mediated by activation of pneumococcal-specific circulating Tm cells, we were surprised to find an abundance of antigen-specific memory T cells within the nose. Furthermore, our data employing FTY720 as well as staining for markers associated with tissue-residency, strongly suggest that antigen-specific resident (rather than recruited) T cells are sufficient for protection. Although it is theoretically possible that FTY720 could be trapping circulating effector cells within the tissues and it is these trapped cells that mediate protection, our observation that the majority of antigen-experienced CD4+ T cells isolated from the NM exhibit markers associated with TRM make this possibility unlikely. Future experiments applying alternative approaches such as parabiosis will help further delineate these issues.
A second major finding of our work is that antigen-specific, mucosal tissue-resident memory T cells can be elicited by parenteral immunization. Prior data has suggested that parenteral vaccination is an ineffective approach for the generation of antigen-specific CD4+ TRM-mediated protective responses within the respiratory tract1, 29, 36. For example, Zens and colleagues demonstrated that while FluMist administered to mice mucosally generated lung CD4+ TRM that provided heterosubtypic protection to influenza infection, such cells were not generated following parenteral administration of Fluzone, although in this study TRM generation was not evaluated in the nasopharynx30. In contrast, it has been suggested that the gut is permissive for T cell recruitment following parenteral immunization even in the absence of local stimulation6. Given that parenteral immunization with WCV was sufficient to generate antigen-specific TRM in the NM as well as the gut, we propose that the upper respiratory tract (URT) may also be permissive for T cell recruitment following parenteral immunization.
How TRM are generated in the NM or at any mucosal site in the absence of local cognate antigen is an area of active investigation37. CD8+ TRM can be generated in multiple tissues including the intestine, kidney and brain by antigen-independent mechanisms6, 10, 38, 39, and this process does not depend on commensal flora, dietary or cross-reactive antigens6, 40. Masopust and others have demonstrated that T cell migratory patterns can be dynamic during the early effector phase, allowing for seeding of effector cells in tissues beyond the original site of challenge10, 41–44 More recently Frederick and colleagues also implicated adjuvant choice as important for regulating homing of antigen specific CD4+ T cells to the gut after parenteral immunization45. Adjuvant selection may also be important for generating TRM in the nasopharynx. In support of this notion, Allen et al. recently demonstrated that the choice of adjuvant influenced the generation of CD4+ TRM in the lung following parenteral immunization with an acellular protein-based pertussis vaccine, although whether these lung CD4+ TRM were truly antigen-specific was not evaluated46. Hence, it could be postulated that there are unique properties of WCV combined with alum that lead to priming of CD4+ T cells within skin draining lymph nodes, which subsequently exhibit the ability to home broadly to mucosal sites. This is supported by our observation that S.C. administration of WCV with alum as an adjuvant induced antigen-responsive T cells in the colon, as well as in the nasopharynx. This finding, which could have been anticipated with CT (a well known potent driver of mucosal Th17 responses47), is more surprising for alum, which has not been previously associated with generation of TRM.
An alternative possibility to explain the generation of antigen-specific mucosal TRM following S.C. immunization with WCV is that priming is able to occur in mucosal draining lymph nodes, such as the cervical lymph node (CLN). This could be the result of either antigen alone or antigen-carrying antigen-presenting cells (APCs) migrating to the CLN. It has previously been suggested that CLN rather than nasal-associated lymphoid tissue (NALT) or spleen is the site of priming for antigen-specific Th17 cells generated following I.N. inoculation with Streptococcus pyogenes48. Furthermore, I.N. immunization with a pneumococcal conjugate vaccine can provide protection against nasal colonization in infant mice in the absence of NALT but not in the absence of the CLN49, but whether there is priming of antigen-specific T cells within the CLN following S.C. immunization with WCV remains to be determined. The specific APC population involved in the uptake and presentation of antigen to cognate T cells at inductive sites can also be important for optimal generation of TRM50, and thus identifying APC’s involved in generating TRM within the nasopharynx should be an important goal of future studies.
Taken together our results demonstrate that antigen-specific protective IL-17A producing CD4+ TRM can be generated within the NM following S.C. vaccination with aluminum hydroxide as an adjuvant. While the mechanisms that allow the pneumococcal WCV to direct the generation of mucosal TRM remain to be determined, these findings will have important implications for the design of vaccines that effectively protect from mucosal pathogens.
Methods
Mice
Four- to eight-week old C57BL/6J mice were purchased from the Jackson Laboratory (Bar Harbor, ME) or Taconic Farms (Germantown, NY). WT C57BL/6J neonatal mice were delivered from Jackson labs with mothers at day of life (DOL) 5. Mice were housed under ABSL2 conditions, and all experiments were performed under Boston Children’s Hospital (BCH) IACUC-approved animal use and care protocol 16-11-3323R.
Nasal colonization with pneumococcus
Nasopharyngeal colonization was performed as described previously 20.Mice were nasally exposed to 1 ×106 −1 ×107 CFU of S. pneumoniae serotype 6B (strain 0603) once or twice under isoflurane anesthesia and live sampling was performed 1 month later to document nasal clearance. For immunophenotyping experiments, mice were sacrificed one month after the initial colonization, and nasal tissue was harvested. For rechallenge experiments, previously colonized mice were challenged intranasally with 107 CFU of strain 0603. Four to ten days later mice were euthanized by CO2 asphyxiation, and sterile PBS was instilled through the transected trachea in a retrograde fashion. The first six drops (about 0.1 ml) of fluid that extruded from the nostrils were collected into 100 μl of PBS, and 100 μl undiluted fluid and 3 serial 5-fold dilutions were plated onto blood agar containing gentamicin (2.5 μg/ml) trimethoprim (3.2 μg/ml), and sulfamethoxazole (16 μg/ml). CFUs were enumerated after incubation at 37°C for 18–24 hours.
Animals with no detectable colonies were assigned a value of 0.8 CFU/nasal wash (one-half the detectable lower limit), which is represented by the dotted line in the nasal colonization graphs. For immunophenotyping experiments post challenge, nasal tissue was harvested after exsanguination and nasal washes were performed.
FTY720 and anti-CD4 antibody treatment
FTY720 (Selleckchem, Houston, TX) was administered either intraperitoneally by injection in 500 μl saline (1.25 mg/kg) daily, or added to the drinking water (diluted to 1.85 mg/L in saline) as previously described51 beginning three days before intranasal challenge, and continuing until the end of the experiment (4–10 days following challenge). For CD4+ T cell depletion, mice were administered anti-CD4 antibody (clone GK1.5, BioXcell, Lebanon, NH) via intraperitoneal (I.P.) injection one-day prior (500 μg), and 2 (500 μg), 5 (500 μg) (for I.N.and S.C. immunized mice), and 8 (250 μg) days post-challenge (for S.C. immunized mice only) post intranasal challenge with pneumococci.
Vaccine preparation and immunization of mice
Pneumococcal whole cell vaccine strain designated RM200 (Rx1E PdT ΔlytA), a capsule- and autolysin-negative mutant, in which the pneumolysin gene was replaced with detoxified mutant PdT, was prepared and inactivated with chloroform as described previously, and designated WCA20, 52. For I.N. immunizations, WCA (100 μg) was diluted in saline and combined with CT (List Biological Laboratories, Campbell, CA) (0.1 μg / 10 μl) and administered to the nares of mice twice at weekly intervals. For S.C. immunizations, WCA (100 μg) was combined with aluminum hydroxide (alum) (Brenntag, Essen, Germany) (0.25 mg / 200 μl) for adult mice as previously described19, or 100 μl per dose for neonatal mice. Mice were immunized three time S.C. with WCV at two week intervals, unless otherwise indicated.
In vivo labeling with Thy1.2 or CD45 antibody and processing of blood
Mice were anesthetized with isoflurane, injected retro-orbitally with 3 ug APC-Thy1.2 antibody or 4 ug APC-CD45 (Biolegend, Dedham, MA) and euthanized 5 minutes later by CO2 asphyxiation53, 54. Blood was obtained by cardiac puncture and placed into heparin-coated microtainers (BD, Franklin Lakes, NJ). Red blood cells (RBC) were lysed with ACK lysis buffer (Lonza Biologics, Porthsmouth,NH) (1ml/100 ul blood) for 4 minutes on ice, then washed and kept on ice until ready for analysis.
Isolation and processing of nasal tissue, colon, and spleen by flow cytometry.
Following sacrifice by CO2 asphyxiation, mice were exsanguinated by cardiac puncture. The lower jaw was removed, followed by the palate as previously described 55 to remove the NALT. Next, the skin of the head was removed starting caudally at the base of the neck and moving rostral towards the mouse nares, with minimal manipulation of the rostral end of the head to avoid damaging the nasal tissue. The nasal passage was separated from the rest of the head by cutting coronally behind the eyes and before the ear canal. Using forceps and gauze, the eyes and any excess cheek and olfactory bulb/brain tissue were removed, and the remaining part of the head containing the nose was placed into ice cold Hanks Balanced Salt Solution (HBSS) (Cellgro, Manassas, VA). The nasal tissue was disrupted in a petri dish, and bones were fragmented to release contaminating bone marrow cells. The disrupted tissue was vigorously washed in HBSS to remove any remaining blood and marrow contaminants, then placed in a 50 ml conical tube with 25 ml of collagenase solution per animal (collagenase type IV (Thermo Fisher Scientific, Waltham, MA); 225U/ml, 20% Fetal Bovine Serum (FBS) (Hyclone, Novato, CA), 1.5 mM CaCl2 in HBSS). Tissue was digested at 37°C for 45 minutes with shaking at 220 rpm. The tissue was further dissociated by drawing the cells through a 5 or 10 ml syringe plunging the cell solution 15–20 times. Dissociated tissue was then passed through a 70 μm strainer and washed twice in PBS (Lonza Biologics) to neutralize the collagenase activity, and stored on ice until analyzed by flow cytometry.
The colon tissue was harvested, and lamina propria (LP) immune cells were prepared as described previously56, with minor modifications. Briefly, colons were stripped of epithelial cells by performing agitation in 10 mM EDTA (Thermo Fisher Scientific) for 30 min at 37°C before digestion in collagenase buffer (Sigma-Aldrich; collagenase type VIII; 200U/ml, 20% FBS, 1.5 mM CaCl2 in HBSS) for 30–45 min at 37°C with shaking at 220 rpm. Undigested tissue was disrupted by repeated flushing through a 10 ml syringe without a needle and then subjected to an additional 10 min incubation with shaking at 220 rpm. Single cell suspensions were filtered as for nasal cells above and kept on ice until ready for FACS analysis.
Preparation of single cell suspensions from spleen were achieved by using the base of a plunger of a 3 ml syringe, and passing them through a 70 μm cell strainer into wash buffer (HBSS + 2% FBS). RBC’s in spleen samples were lysed with 1 ml of ACK lysis buffer per spleen, followed by incubation for 5 minutes at RT. Cells were washed and kept on ice until ready for analysis by flow cytometry.
Cell surface immunophenotyping and intracellular cytokine staining by flow cytometry
Single cell suspensions were treated with Zombie violet as per the manufacturers insert instructions (Biolegend). Following treatment with Fc block (1 μg/ml) (Biolegend), cells were stained with combinations of the following antibodies: CD69 (clone H1.2F3), CD44 (clone IM7), CD8α (clone 53–6.7), CD3e (clone 145– 2C11), CD3 (clone 17A2), CD11a (clone I21/7), CD45 (clone 30-F11), Thy1.2 (clone 53–2.1), CD4 (clone GK1.5), CD4 (clone RM 4–4), CD4 (clone RM 4–5), CD62L (clone MEL-14) (BioLegend). Intracellular cytokine staining was performed with anti-IFN-γ (clone XMG1.2) and anti-IL17A (clone TC11–18H10.1) antibodies (BioLegend). Data was acquired using a FACS Canto II flow cytometer (Becton Dickinson (BD)) or Attune NxT flow cytometer (ThermoFischer Scientific) and analyzed using FlowJo software (TreeStar, version 10). All gating stragies used are described in the supplementary data.
Measurement of cytokine responses
Cells derived from the nasal mucosa, spleen, or colon were stimulated for 4–5 hours with PMA (50 ng/ml), lonomycin (500 μg/ml) (Sigma Aldrich), and golgi stop (BD) (1 μg/ml), or stimulated overnight with WCA (10 μg/ml) followed by Golgistop (1 μg/ml) for 4–5 hours. Following these incubations, cells were harvested and prepared for surface and intracellular cytokine staining as described above. Nasal cell suspensions were aliquoted into 24-well TC plates in complete tissue culture media (TCM) and stimulated with WCA (10 μg/ml ) for 7 days. Supernatants were harvested and IL-17A was detected in the supernatants by ELISA as per the mouse IL-17A ELISA DUO set kit instructions (reference DY421; R&D Systems). IL-17A was detected in samples of WCA-stimulated whole blood as previously described18. A value of 8 pg/ml was set as the lower limit of detection of IL-17A cytokine protein by ELISA and is represented by the dotted line in IL-17A cytokine detection graphs.
Immunofluorescence
Heads of mice were processed as described above, except that prior to removing the palate the entire head (without the lower jaw and with skin removed) was detached and submerged in 30 ml of 10% neutral buffered formalin (VWR, Radnor, PA) or Zinc formal-fixx (Thermo Scientific) and incubated on a rocker at RT for 16–24 hrs. Heads were then decalcified in 30 ml of 0.12 M EDTA pH 7 by gentle mixing on a rocker for 2 weeks with daily solution changes. Coronal sections were cut and slides containing tissue were baked at 60°C for 3 hrs, followed by xylene paraffin clearing and rehydration of the tissue with decreasing amounts of ethanol (100% to 50%). Antigen retrieval was completed in a Tris-EDTA buffer pH 9 at 85°C for 20 minutes. Tissue was washed in PBS, blocked with 5% goat serum, 5%BSA, 0.05% Triton-X in PBS for 2 hrs, then incubated overnight at 4°C with rat anti-mouse CD4 monoclonal antibody (1/250 dilution) (clone 4SM95) (Thermo Fisher Scientific) and rabbit anti-mouse MPO polyclonal antibody (1/50) (clone ab9535) (Abcam) followed by secondary antibodies (goat anti-rat IgG –Alexa fluor 555; clone ab 150158, and goat anti-rabbit IgG –Alexa fluor 647; clone ab 150079, Abcam) for 45 min at RT in a humidified chamber, and then generously washed with PBS-tween (0.05%). Tissue was then counterstained with Trueblack according to instructions (Biotium, Fremont, CA), and nuclei stained with Hoescht 33342 solution (Thermo Fisher Scientific). Slides were mounted with prolong gold anti-fade mounting medium (Molecular Probes) and coverslips. Slides were imaged using a Zeiss LSM 880 upright confocal microscope with a 20X objective lens with numerical aperture (N.A) 0.8, or a 40X objective lens with N.A 1.3. Tiled images of 35 fields per animal were acquired and cells were counted using the cell counter option in Fiji imaging software by two individuals (J.M.O and N.G.R) in a blinded manner and averaged 57.
Statistical analysis
All statistical analyses were done using GraphPad Prism (version 7 for OSX for Macs, GraphPad Software, Inc). Cytokine production in tissues and whole blood, cell counts in histology samples, and CFUs in nasal washes were compared between groups using the two-tailed Mann-Whitney U test. Horizontal bars in each graph indicate the median value. * = P < 0.05, ** = P < 0.01, *** = P < 0.001, **** = P < 0.0001.
Supplementary Material
Acknowledgments
We thank Olivia Ledue, Raecliffe Daly, Tracy Yeung and Christina Merakou for technical assistance. We acknowledge Dr. Roderick Bronson and Li Zhang from the Rodent Histopathology Core at Harvard Medical School for advice on preparing nasal tissue for histological analysis as well performing paraffin embedding and sectioning nasal tissue. We are grateful to Dr. Lay-Hong Ang and Aniket Gad from the Confocal Microscopy Core at Beth Israel Deaconess Medical Center for help with nasal tissue imaging. We appreciate the helpful discussions from the Malley and Horwitz labs. NSR is supported by Crohn’s and Colitis Foundation grant RFA381023. This work was supported by NIH/NIAID grant R01 AI100114 (RM and BHH) and AI100119 (DLF). RM gratefully acknowledges support from the Translational Research Program at Boston Children’s Hospital and Program 1for Appropriate Technology in Health (PATH).
Footnotes
Additional information
Supplementary Material is linked to the online version of the paper at http://www.nature.com/mi”.
Disclosure
The authors declare no competing financial interests.
References
- 1.Belyakov IM, Ahlers JD. What role does the route of immunization play in the generation of protective immunity against mucosal pathogens? J Immunol 2009; 183(11): 6883–6892. [DOI] [PubMed] [Google Scholar]
- 2.Neutra MR, Kozlowski PA. Mucosal vaccines: the promise and the challenge. Nat Rev Immunol 2006; 6(2): 148–158. [DOI] [PubMed] [Google Scholar]
- 3.Belyakov IM, Isakov D, Zhu Q, Dzutsev A, Berzofsky JA. A novel functional CTL avidity/activity compartmentalization to the site of mucosal immunization contributes to protection of macaques against simian/human immunodeficiency viral depletion of mucosal CD4+ T cells. J Immunol 2007; 178(11): 7211–7221. [DOI] [PubMed] [Google Scholar]
- 4.Li Z, Zhang M, Zhou C, Zhao X, Iijima N, Frankel FR. Novel vaccination protocol with two live mucosal vectors elicits strong cell-mediated immunity in the vagina and protects against vaginal virus challenge. J Immunol 2008; 180(4): 2504–2513. [DOI] [PubMed] [Google Scholar]
- 5.Amorij JP, Saluja V, Petersen AH, Hinrichs WL, Huckriede A, Frijlink HW. Pulmonary delivery of an inulin-stabilized influenza subunit vaccine prepared by spray-freeze drying induces systemic, mucosal humoral as well as cell-mediated immune responses in BALB/c mice. Vaccine 2007; 25(52): 8707–8717. [DOI] [PubMed] [Google Scholar]
- 6.Shin H, Iwasaki A. Tissue-resident memory T cells. Immunol Rev 2013; 255(1): 165–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beura LK, Anderson KG, Schenkel JM, Locquiao JJ, Fraser KA, Vezys V et al. Lymphocytic choriomeningitis virus persistence promotes effectorlike memory differentiation and enhances mucosal T cell distribution. J Leukoc Biol 2015; 97(2): 217–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gebhardt T, Wakim LM, Eidsmo L, Reading PC, Heath WR, Carbone FR. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat Immunol 2009; 10(5): 524–530. [DOI] [PubMed] [Google Scholar]
- 9.Wakim LM, Waithman J, van Rooijen N, Heath WR, Carbone FR. Dendritic cell-induced memory T cell activation in nonlymphoid tissues. Science (New York, NY) 2008; 319(5860): 198–202. [DOI] [PubMed] [Google Scholar]
- 10.Masopust D, Choo D, Vezys V, Wherry EJ, Duraiswamy J, Akondy R et al. Dynamic T cell migration program provides resident memory within intestinal epithelium. J Exp Med 2010; 207(3): 553–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mueller SN, Mackay LK. Tissue-resident memory T cells: local specialists in immune defence. Nat Rev Immunol 2016; 16(2): 79–89. [DOI] [PubMed] [Google Scholar]
- 12.Carbone FR. Tissue-Resident Memory T Cells and Fixed Immune Surveillance in Nonlymphoid Organs. J Immunol 2015; 195(1): 17–22. [DOI] [PubMed] [Google Scholar]
- 13.Turner DL, Farber DL. Mucosal resident memory CD4 T cells in protection and immunopathology. Front Immunol 2014; 5: 331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosato PC, Beura LK, Masopust D. Tissue resident memory T cells and viral immunity. Curr Opin Virol 2017; 22: 44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Campos IB, Herd M, Moffitt KL, Lu YJ, Darrieux M, Malley R et al. IL-17A and complement contribute to killing of pneumococci following immunization with a pneumococcal whole cell vaccine. Vaccine 2017; 35(9): 1306–1315. [DOI] [PubMed] [Google Scholar]
- 16.Moffitt K, Cheung E, Manis J, Malley R. Evaluation of the Role of stat3 in Antibody and TH17-Mediated Responses to Pneumococcal Immunization and Infection by Use of a Mouse Model of Autosomal Dominant Hyper-IgE Syndrome. Infect Immun 2018; 86(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moffitt KL, Malley R, Lu YJ. Identification of protective pneumococcal t(h)17 antigens from the soluble fraction of a killed whole cell vaccine. PLoS ONE 2012; 7(8): e43445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu YJ, Gross J, Bogaert D, Finn A, Bagrade L, Zhang Q et al. Interleukin-17A mediates acquired immunity to pneumococcal colonization. PLoS Pathog 2008; 4(9): e1000159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu YJ, Leite L, Goncalves VM, Dias Wde O, Liberman C, Fratelli F et al. GMP-grade pneumococcal whole-cell vaccine injected subcutaneously protects mice from nasopharyngeal colonization and fatal aspirationsepsis. Vaccine 2010; 28(47): 7468–7475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malley R, Lipsitch M, Stack A, Saladino R, Fleisher G, Pelton S et al. Intranasal immunization with killed unencapsulated whole cells prevents colonization and invasive disease by capsulated pneumococci. Infect Immun 2001; 69(8): 4870–4873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malley R, Trzcinski K, Srivastava A, Thompson CM, Anderson PW, Lipsitch M. CD4+ T cells mediate antibody-independent acquired immunity to pneumococcal colonization. Proc Natl Acad Sci U S A 2005; 102(13): 4848–4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trzcinski K, Thompson CM, Srivastava A, Basset A, Malley R, Lipsitch M. Protection against nasopharyngeal colonization by Streptococcus pneumoniae is mediated by antigen-specific CD4+ T cells. Infect Immun 2008; 76(6): 2678–2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moffitt KL, Yadav P, Weinberger DM, Anderson PW, Malley R. Broad antibody and T cell reactivity induced by a pneumococcal whole-cell vaccine. Vaccine 2012; 30(29): 4316–4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Antibody Malley R. and cell-mediated immunity to Streptococcus pneumoniae: implications for vaccine development. J Mol Med (Berl) 2010; 88(2): 135–142. [DOI] [PubMed] [Google Scholar]
- 25.Malley R, Anderson PW. Serotype-independent pneumococcal experimental vaccines that induce cellular as well as humoral immunity. Proc Natl Acad Sci U S A 2012; 109(10): 3623–3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Z, Clarke TB, Weiser JN. Cellular effectors mediating Th17-dependent clearance of pneumococcal colonization in mice. J Clin Invest 2009; 119(7): 1899–1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilk MM, Misiak A, McManus RM, Allen AC, Lynch MA, Mills KHG. Lung CD4 Tissue-Resident Memory T Cells Mediate Adaptive Immunity Induced by Previous Infection of Mice with Bordetella pertussis. J Immunol 2017; 199(1): 233–243. [DOI] [PubMed] [Google Scholar]
- 28.Schwab SR, Cyster JG. Finding a way out: lymphocyte egress from lymphoid organs. Nat Immunol 2007; 8(12): 1295–1301. [DOI] [PubMed] [Google Scholar]
- 29.Aguilo N, Alvarez-Arguedas S, Uranga S, Marinova D, Monzon M, Badiola J et al. Pulmonary but Not Subcutaneous Delivery of BCG Vaccine Confers Protection to Tuberculosis-Susceptible Mice by an Interleukin 17-Dependent Mechanism. The Journal of infectious diseases 2016; 213(5): 831–839. [DOI] [PubMed] [Google Scholar]
- 30.Zens KD, Chen JK, Farber DL. Vaccine-generated lung tissue-resident memory T cells provide heterosubtypic protection to influenza infection. JCI Insight 2016; 1(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lipsitch M, Whitney CG, Zell E, Kaijalainen T, Dagan R, Malley R. Are anticapsular antibodies the primary mechanism of protection against invasive pneumococcal disease? PLoS medicine 2005; 2(1): e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jochems SP, Weiser JN, Malley R, Ferreira DM. The immunological mechanisms that control pneumococcal carriage. PLoS Pathog 2017; 13(12): e1006665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lundgren A, Bhuiyan TR, Novak D, Kaim J, Reske A, Lu YJ et al. Characterization of Th17 responses to Streptococcus pneumoniae in humans: comparisons between adults and children in a developed and a developing country. Vaccine 2012; 30(26): 3897–3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu YJ, Oliver E, Zhang F, Pope C, Finn A, Malley R. Screening for Th17- dependent pneumococcal vaccine antigens: comparison of murine and human cellular immune responses. Infect Immun 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oliver E, Pope C, Clarke E, Langton Hewer C, Ogunniyi AD, Paton JC et al. Th17 responses to pneumococcus in blood and adenoidal cells in children. Clinical and experimental immunology 2019; 195(2): 213–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holmgren J, Svennerholm AM. Vaccines against mucosal infections. Curr Opin Immunol 2012; 24(3): 343–353. [DOI] [PubMed] [Google Scholar]
- 37.Pizzolla A, Nguyen THO, Smith JM, Brooks AG, Kedzieska K, Heath WR et al. Resident memory CD8(+) T cells in the upper respiratory tract prevent pulmonary influenza virus infection. Sci Immunol 2017; 2(12). [DOI] [PubMed] [Google Scholar]
- 38.Klonowski KD, Williams KJ, Marzo AL, Blair DA, Lingenheld EG, Lefrancois L. Dynamics of blood-borne CD8 memory T cell migration in vivo. Immunity 2004; 20(5): 551–562. [DOI] [PubMed] [Google Scholar]
- 39.Wakim LM, Woodward-Davis A, Bevan MJ. Memory T cells persisting within the brain after local infection show functional adaptations to their tissue of residence. Proc Natl Acad Sci U S A 2010; 107(42): 1787217879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Casey KA, Fraser KA, Schenkel JM, Moran A, Abt MC, Beura LK et al. Antigen-independent differentiation and maintenance of effector-like resident memory T cells in tissues. J Immunol 2012; 188(10): 4866–4875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stevceva L, Alvarez X, Lackner AA, Tryniszewska E, Kelsall B, Nacsa J et al. Both mucosal and systemic routes of immunization with the live, attenuated NYVAC/simian immunodeficiency virus SIV(gpe) recombinant vaccine result in gag-specific CD8(+) T-cell responses in mucosal tissues of macaques. Journal of virology 2002; 76(22): 11659–11676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Masopust D, Vezys V, Usherwood EJ, Cauley LS, Olson S, Marzo AL et al. Activated Primary and Memory CD8 T Cells Migrate to Nonlymphoid Tissues Regardless of Site of Activation or Tissue of Origin. The Journal of Immunology 2004; 172(8): 4875–4882. [DOI] [PubMed] [Google Scholar]
- 43.Liu L, Fuhlbrigge RC, Karibian K, Tian T, Kupper TS. Dynamic programming of CD8+ T cell trafficking after live viral immunization. Immunity 2006; 25(3): 511–520. [DOI] [PubMed] [Google Scholar]
- 44.Agrewala JN, Brown DM, Lepak NM, Duso D, Huston G, Swain SL. Unique ability of activated CD4+ T cells but not rested effectors to migrate to non-lymphoid sites in the absence of inflammation. J Biol Chem 2007; 282(9): 6106–6115. [DOI] [PubMed] [Google Scholar]
- 45.Frederick DR, Goggins JA, Sabbagh LM, Freytag LC, Clements JD, McLachlan JB. Adjuvant selection regulates gut migration and phenotypic diversity of antigen-specific CD4(+) T cells following parenteral immunization. Mucosal Immunol 2018; 11(2): 549–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Allen AC, Wilk MM, Misiak A, Borkner L, Murphy D, Mills KHG. Sustained protective immunity against Bordetella pertussis nasal colonization by intranasal immunization with a vaccine-adjuvant combination that induces IL-17-secreting TRM cells. Mucosal Immunol 2018; 11(6): 1763–1776. [DOI] [PubMed] [Google Scholar]
- 47.Lee JB, Jang JE, Song MK, Chang J. Intranasal delivery of cholera toxin induces th17-dominated T-cell response to bystander antigens. PLoS ONE 2009; 4(4): e5190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Linehan JL, Dileepan T, Kashem SW, Kaplan DH, Cleary P, Jenkins MK. Generation of Th17 cells in response to intranasal infection requires TGF-betal from dendritic cells and IL-6 from CD301b+ dendritic cells. Proc Natl Acad Sci U S A 2015; 112(41): 12782–12787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sabirov A, Metzger DW. Intranasal vaccination of infant mice induces protective immunity in the absence of nasal-associated lymphoid tissue. Vaccine 2008; 26(12): 1566–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Iborra S, Martinez-Lopez M, Khouili SC, Enamorado M, Cueto FJ, Conde- Garrosa R et al. Optimal Generation of Tissue-Resident but Not Circulating Memory T Cells during Viral Infection Requires Crosspriming by DNGR-1(+) Dendritic Cells. Immunity 2016; 45(4): 847–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Morris MA, Gibb DR, Picard F, Brinkmann V, Straume M, Ley K. Transient T cell accumulation in lymph nodes and sustained lymphopenia in mice treated with FTY720. Eur J Immunol 2005; 35(12): 3570–3580. [DOI] [PubMed] [Google Scholar]
- 52.Lu YJ, Yadav P, Clements JD, Forte S, Srivastava A, Thompson CM et al. Options for inactivation, adjuvant, and route of topical administration of a killed, unencapsulated pneumococcal whole-cell vaccine. Clin Vaccine Immunol 2010; 17(6): 1005–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Turner DL, Bickham KL, Thome JJ, Kim CY, D’Ovidio F, Wherry EJ et al. Lung niches for the generation and maintenance of tissue-resident memory T cells. Mucosal Immunol 2014; 7(3): 501–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Anderson KG, Mayer-Barber K, Sung H, Beura L, James BR, Taylor JJ et al. Intravascular staining for discrimination of vascular and tissue leukocytes. Nat Protoc 2014; 9(1): 209–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rodriguez-Monroy MA, Rojas-Hernandez S, Moreno-Fierros L. Phenotypic and functional differences between lymphocytes from NALT and nasal passages of mice. Scandinavian journal of immunology 2007; 65(3): 276–288. [DOI] [PubMed] [Google Scholar]
- 56.Redhu NS, Bakthavatchalu V, Conaway EA, Shouval DS, Tsou A, Goettel JA et al. Macrophage dysfunction initiates colitis during weaning of infant mice lacking the interleukin-10 receptor. eLife 2017; 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T et al. Fiji: an open-source platform for biological-image analysis. Nature methods 2012; 9(7): 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.