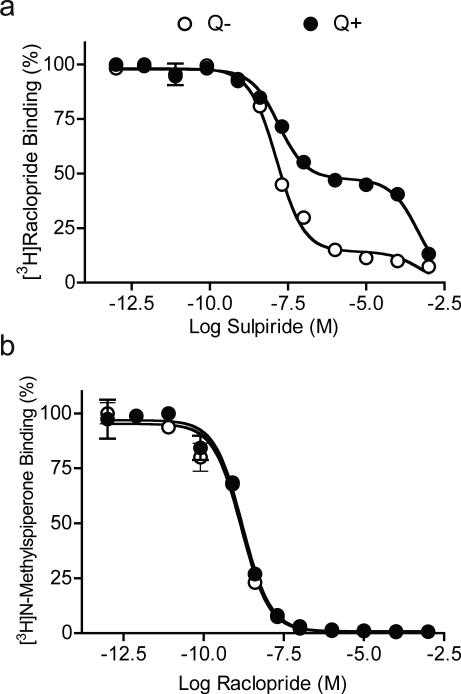
Figure 4. Competition binding of [3H]raclopride or [3H]NMSP under control and internalization conditions.
A. [3H]Raclopride binding in competition with sulpiride. A two-site binding curve was observed in control cells ( , Q−). Most D2 receptors (85.8%) were on the surface and showed high-affinity (Ki(s)= 7.7 ± 2.3 nM). A small proportion (14.2%) were internalized and showed low affinity binding (Ki(i)= 141.8 ± 45.2 μM). Following quinpirole treatment ( , Q+), 43.1% of D2 receptors remained on the surface (Ki(s)= 7.9 ± 3.0 nM) and 56.9% were internalized (Ki(i)= 120.7 ± 35.8 μM). B. [3H]NMSP binding in competition with raclopride. Competition experiments showed indistinguishable one-site binding curves between control (
, Q−). Most D2 receptors (85.8%) were on the surface and showed high-affinity (Ki(s)= 7.7 ± 2.3 nM). A small proportion (14.2%) were internalized and showed low affinity binding (Ki(i)= 141.8 ± 45.2 μM). Following quinpirole treatment ( , Q+), 43.1% of D2 receptors remained on the surface (Ki(s)= 7.9 ± 3.0 nM) and 56.9% were internalized (Ki(i)= 120.7 ± 35.8 μM). B. [3H]NMSP binding in competition with raclopride. Competition experiments showed indistinguishable one-site binding curves between control ( , Q−; Ki= 0.26 ± 0.09 nM) and quinpirole treated cells ( , Q+; Ki= 0.28 ± 0.05 nM). Thus, like NMSP, raclopride readily accesses internalized D2 receptors.
, Q−; Ki= 0.26 ± 0.09 nM) and quinpirole treated cells ( , Q+; Ki= 0.28 ± 0.05 nM). Thus, like NMSP, raclopride readily accesses internalized D2 receptors.