Abstract
Effective cancer immunotherapy depends on the body’s ability to generate tumor antigen-presenting cells and tumor-reactive effector lymphocytes. As the most potent antigen presenting cells (APCs), dendritic cells (DCs) are capable of sensitizing T cells to new and recall antigens. Clinical trials of antigen-pulsed autologous DCs have been conducted in patients with a number of hematological and solid cancers, including malignant melanoma, lymphoma, myeloma, and non-small cell lung cancer. These studies suggest that antigen-loaded DC vaccination is a potentially safe and effective cancer therapy. However, the clinical results have been variable. Since the elderly are preferentially affected by diseases targeted by DC-directed immunotherapy, it is quite striking that few studies to date have focused on the effect of aging on DC function, a key aspect of optimal immunotherapy design in an aging population. In the present paper, we will discuss the consequences of aging on murine bone marrow-derived DC function and their use in cancer immunotherapy.
Keywords: Aging, Dendritic cells, Immunotherapy
Introduction
Dendritic cells (DCs) are specialized and potent antigen presenting cells (APCs) that are critical for the initiation of in vivo T cell responses, including sensitization of MHC-restricted T cells, development of T-cell-dependent antibody production and induction of immunological tolerance [27]. They are derived from pluripotent hematopoietic progenitor cells in the bone marrow (BM) and comprise a system of leukocytes widely distributed in all tissues, particularly in those that provide an environmental interface. Both myeloid and lymphoid precursors can give rise to DCs. The heterogeneity of these cells was emphasized by the identification of several different subtypes, including Langerhans cells, interstitial DCs, interdigitating DCs, follicular DCs, plasmacytoid DCs, and veiled DCs from blood and lymph nodes. Although the ability to stimulate naïve T cell proliferation appears to be shared between these various DC subsets, their diverse anatomic locations indicate that they have distinct strategies for differentiation and regulation of function [28] and represent a control point for the onset of immunity.
Dendritic Cells exist primarily in two distinct phenotypic forms. Immature DCs (iDCs) are able to circulate through peripheral tissues and sample soluble antigen (Ag) and possibly necrotic or apoptotic cells. These DCs express few co-stimulatory molecules and are unable to prime T cell responses. Once DCs begin to process and present antigen they “mature” into a more stellate shape characterized by reduced ability to ingest Ag and enhance T cell costimulatory capacity. Activation and subsequent migration of DCs from non-lymphoid tissue to regional lymph nodes has been demonstrated to be early steps occurring during inflammatory reactions and an important step in the development of a cell-mediated immune response against a number of pathogens. Mature DCs (tDCs) achieve migratory potential by upregulating chemokine receptors CCR7 and CD62L, and downregulating chemokines and chemokine receptors associated with tissue retention. This allows DC translocation via lymphatic vessels to the regional lymph node where the Ag-pulsed DCs interact with their cognate T lymphocytes. Since naïve T cells also express CCR7, the nodal chemokines orchestrate the co-localization of Ag-presenting DCs with T cells, increasing the likelihood of DC-T cell interaction and thus T cell priming [14, 23]. Activation of naïve T cells requires the cooperation of DCs expressing surface MHC molecules and costimulatory molecules. Accessory molecules on DCs are required to ensure that T cells will differentiate into effector cells. Thus, DC function involves three components that occur in sequence: an acquisition step during which Ag is internalized and undergoes proteolytic cleavage; a sensitization step during which DCs develop the capacity to induce a response in T lymphocytes and migrate to regional draining lymph nodes; and an interaction step during which DCs activate Ag-specific cytotoxic T cells (CTLs), which have the capacity to eliminate tumor cells.
The incidence of cancer increases exponentially with aging [4]. It is well established that the immune system undergoes characteristic changes, termed immunosenescence, in both human and animal models with advancing age. Age-dependent modulation of immunological mechanisms and functions involves both the adaptive and innate immune system [17, 29]. In particular, aging may affect antitumor immune response. Several mechanisms involved in the decline of immune functions have been elucidated. It has been shown that the proliferation and the cytotoxicity of T cells, the cytokine secretion, and the capacity to mount humoral or cell-mediated responses against novel antigens, are all impaired in old age [3, 6, 17, 21]. Attempts to correlate these defects to disease process or clinical outcome have largely been unsuccessful, suggesting that other supporting members of the immune system or the coordinated effect of immune cell deregulation may be responsible for the age-associated defect in antitumor immune responsiveness.
The unique ability of DCs to induce and sustain primary immune responses makes them prime candidates in vaccination protocols in cancer therapy [13]. Despite the critical role of DCs in immune reactions, surprisingly little is known about the effect of aging on these powerful APCs. Although there are many reports demonstrating that vaccination with DCs loaded with the appropriate tumor-associated Ags can induce protective/rejection-immune responses in animal models [5, 20, 22], the results of human clinical trials involving tumor-Ag-pulsed ex vivo derived-DC vaccination for the treatment of advanced malignancies have been variable. Indeed, the successful eradication of established tumors through DC immunotherapy remains a rarity and continues to pose an urgent challenge [16, 19]. Based on the fact that most preclinical models test young animals, whereas most common cancers develop in older patients, we and a limited number of investigators have considered the potential explanation that an intrinsic functional defect(s) exists in aged DCs.
Dendritic cells and antitumor immunity
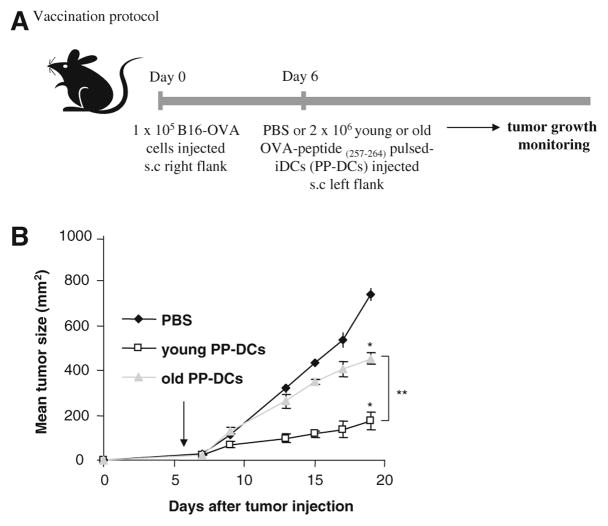
There have been relatively few reports examining the effect of aging on DC antitumor activity (reviewed in [2, 26]). We recently analyzed the ability of bone-marrow derived CD11c + CD4− CD8− DCs, obtained from young (3–6 months) and old (21–24 months) C57BL/6 mice, to induce regression of preestablished tumors in vivo [11]. Using the B16-OVA melanoma model, where the B16 melanoma cells have been made to express the chicken Ag ovalbumin (OVA) [8], we established subcutaneous (s.c.) B16-OVA tumors in the right flanks of young C57BL/6 mice. After six days, the mice were injected in their left flanks with either saline buffer or 2 × 106 OVA-peptide(257–264) (SIINF-ELK) pulsed DCs (PP-DCs) and their tumor growth monitored over time (Fig. 1a). We found that the Ag-pulsed old DCs have impaired in vivo antitumor activity. Thus, the mean tumor size (at day 19) in mice receiving the young DCs (173.3 ± 39.2 mm2) is significantly smaller than those receiving old PP-DCs (compared to 454.2 ± 27.5, Fig. 1b).
Fig. 1.
Treatment of established tumor. a Vaccination protocol. B6 mice were given 1 × 105 viable B16-OVA melanoma cells s.c. in the right flank. Six days later, when the tumor size reaches 3–4 mm in diameter, young and old OVA-peptide(257–264) pulsed-DCs (PP-DCs), or PBS were injected once s.c. in the left flank. iDCs purified from recombinant mouse GM-CSF and IL-4 stimulated old and young bone marrow cells were pulsed with OVA-peptide at 10 μg/ml for 6 h. b Graph showing tumor growth over time. These data are representative of three experiments with similar results, and are reported as the mean tumor area ±SEM of five or six mice per group. *P < 0.001: young PP-DCs versus PBS control, old PP-DCs versus PBS control; ** P < 0.0005: young PP-DCs versus old PP-DCs. Copyright (c) The Gerontological Society of America. Reproduced by permission of the publisher [11]
Recent studies have demonstrated that aging may also indirectly affect DCs through the aging microenvironment. Shi et al. [25] reported that old mice immunized with bone marrow-derived young DCs pulsed with OVA lived significantly shorter lifespan after challenge with MO4 tumor cells as compared to equally treated young mice. Another report by Sharma et al. [24] demonstrated that the efficacy of autologous DC vaccines from aged mice in inducing in vivo anti-tumor immune response in a prostate tumor model of mice was impaired. To study the influence of the aged microenvironment in our tumor model, young-derived DC vaccines were injected into young and old tumor-bearing hosts. We found that the efficacy of young PP-DCs in old mice was reduced by two fold in comparison to young mice receiving the same DCs (data not shown). These results clearly indicate that young DC-based vaccination in aged mice was undermined, suggesting that indeed the aging microenvironment affects the DC anti-tumor response as well. Further studies will be needed to determine the exact contribution of the aged environment on DC reduced immunosurveillance. Interestingly, in the same paper Sharma et al. demonstrated that alterations in the co-stimulatory environment in aged mice could play a role in this phenomenon by describing that the in vivo co-administration of anti-OX-40 or anti-4-1-BB monoclonal antibodies resulted in normalization of antitumor response by aged DC.
Dendritic cell and T cell interaction
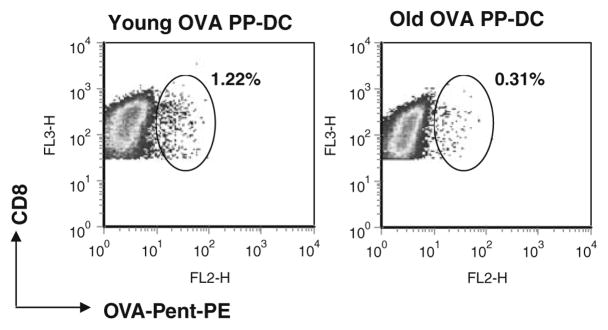
To generate cytotoxic killer cells, DCs have to present antigenic peptides-MHC class I molecule complex to CD8-expressing T cells. To investigate whether impaired OVA-peptide presentation to T cells by old DCs could account for the observed reduction in antitumor activity in our melanoma model, we compared in vitro the ability of young and old DCs to stimulate the proliferation of CD8+ T cells isolated from OVA TCR transgenic mice (OT-I). Day 5 purified CD11c+ iDCs from young and old mice were pulsed with OVA-peptide(257–264), and co-cultured with syngeneic OVA-specific microbead-purified T cells. We found that old iDCs were less effective than young DCs at stimulating syngeneic T cell proliferation [12]. Using fluorescent-labeled OVA-peptide(257–264), we demonstrated that this was not due to impaired uptake and presentation into MHC Class I molecules [12]. It has been demonstrated that the generation and activation of antitumor long term memory CD8+ T cells need the presence of Ag-specific CD4+ T-helper cells [7, 15]. We have shown that induction of Ag-specific CD4+ T cell in vitro proliferation was also impaired with aging [11]. Importantly, using the pentamer H2-Kb-SIINFELK technology, we reported that the frequency of splenic Ag-specific CD8+ T cells isolated from B16-OVA tumor-bearing mice decreased by more than 2.5-fold in mice that received the old PP-DC vaccine, compared to mice receiving the young PP-DC vaccine (0.39 ± 0.1 vs. 1.04 ± 0.22; P < 0.025) (Fig. 2). Interestingly, we found that the effector functions on a per cell basis of those T cells, after old DC stimulation were largely intact [12]. In addition, the influx of CD8+ T cells into s.c. tumors of the old-derived DC immunized group decreased significantly by 20% [12]. This suggests that impaired in vivo Ag-specific T cell induction per se by aged DCs could explain the reduced inhibition of tumor growth observed in mice vaccinated with old PP-DCs.
Fig. 2.
In vivo detection of OVA-specific CD8+ T cells by MHC pentamer staining. Day 7 tumor-bearing B6 mice were vaccinated with young or old OVA PP-DCs. Seven days later, total splenocytes were stained for CD8-RPE Alexa Fluor 647 and OVA-Pent-PE. A representative figure of FACScan plots depicting the percentage of OVA-specific CD8+ T cells is shown. Reprint with permission [12]
In recent years, DC-specific/intracellular adhesion molecule type 3-grabbing, non integrin (DC-SIGN), a member of the type II C-type lectin family exclusively expressed on APCs, has been identified as having a co-stimulatory function in T-cell activation. DC-SIGN mediates transient adhesion with T cells by binding with high affinity to inter-cellular adhesion molecule-3 (ICAM-3) [9, 10], enabling DCs to interact with a large number of resting T cells. When we compared young and old iDCs, before and after OVA peptide pulsing, we did not find any significant age-related differences in the expression of MHC class I or II molecules and selected co-stimulatory molecules, including CD80, CD86, CD40 and CD54. However, we observed a significant decrease in the expression of DC-SIGN in aged DCs [11, 12]. A similar age-associated reduction in the expression of DC-SIGN was also observed on freshly isolated splenic CD11c + DCs (unpublished data). Because antibodies against DC-SIGN have been shown to reduce human T-cell proliferation [9, 18], it is reasonable to speculate that efficient priming of T cells could be affected by the lower expression of DC-SIGN observed in aged DCs. We are currently investigating the significance of DC-SIGN in the regulation of T cell activation, as well as the possible involvement of other molecules in this process.
Dendritic cell migration and trafficking
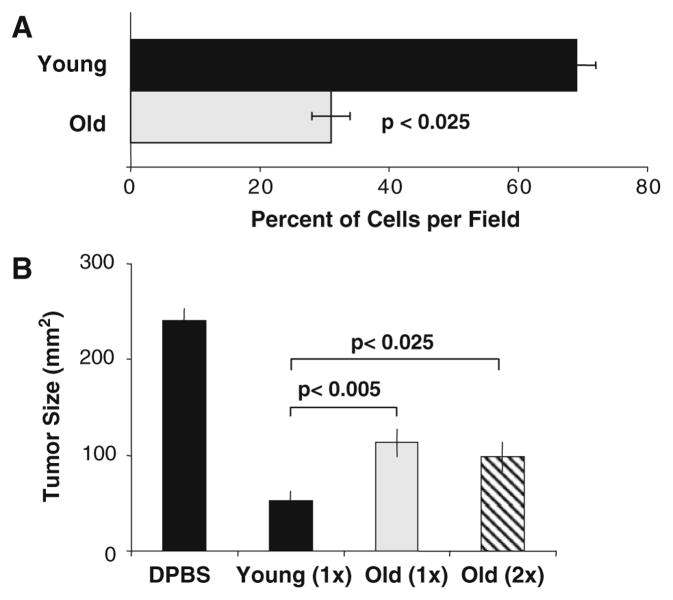
Migration of DCs to the secondary lymphoid organs is essential for the cells to exert T cell regulatory function. It is therefore plausible that decreased DC migratory function may play a role in the age-dependent decline in DC anti-tumor function. in vivo DC trafficking was examined by labeling young and old tDCs with CFSE and CMPTX dyes, respectively, and an equal ratio of labeled young and old DCs were injected into the footpad of young mice. We reported that the vigor of DC migration and subsequent influx of bone marrow derived-DC into draining lymph nodes is also impacted by aging [12]. We also showed that aged DCs have impaired capacity to migrate in vitro in response to the chemokine SLC. This was not due to a defect in CCR7 surface protein expression, but rather by a defect in signal transduction as demonstrated by comparison with the pattern of tyrosine phosphorylation from SLC-stimulated young and old DCs obtained under identical conditions [12]. This result is consistent with the recent publication by Agrawal et al. [1], in which the authors reported that despite similar levels of expression of CCR7, monocyte-derived myeloid LPS-stimulated DCs obtained from elderly subjects have impaired in the in vitro migration towards MIP-3β, another CCR7 ligand. They attributed their results to defects in the downstream signaling pathway, possibly in the PI3K pathway. However, the exact mechanism is unknown and further studies will be needed to unravel it. We found that although the impaired in vivo migration of aged DCs can be restored to a level comparable to that seen in young DCs by increasing the quantity of cells injected, the age-associated impairment in tumor surveillance as defined by tumor growth (Fig. 3b) and Ag-specific T cell induction [12] remains, suggesting that DC migration through CCR7-CCL21 interaction is not the primary mechanism for the observed aging defects.
Fig. 3.
In vivo lymph node DC migration in aging. a Groups of young mice (n = 3) received 50 μl of equal numbers (2 × 106) of CMPTX-labeled young DC and CFSE-labeled old LPS-stimulated DCs into their footpad hind. Popliteal draining LNs were excised 24 h later and the frequency of DC/node was assessed by fluorescent microscopy. b Effect of increasing number of old DCs on tumor growth. Day-6 tumor-bearing mice were treated with 2 × 106 young PP-DCs (1×), 2 × 106 old PP-DCs (1×) or 4 × 106 old PP-DCs (2×) or saline (DPBS), and tumor size measured 7 days later (n = 6 tumors per treatment group). Adapted from [12]
Conclusions
Among the inherent changes of aging that may favor cancer development, impairment of antitumor immune mechanisms may represent a crucial event. The number of studies utilizing dendritic cell based therapies for cancer is expanding exponentially. With a powerful antigen-presentation capability, DCs have the potential to overcome tumor tolerance and promote a vigorous antitumor immune response. In order to optimize this approach, multiple strategies have been devised to enhance immunopotency of therapeutic DCs. Although, DCs have been extensively characterized on a molecular and cellular level, few studies have specifically addressed the topic of DCs and aging anti-tumor response. Our research findings may thus provide a valid explanation for the discordance between the promising pre-clinical data in young animals and the relatively poor efficacy of DC cancer immunotherapy in human clinical trials involving older cancer patients. The improved characterization of aging DC defects in tumor immunotherapy should allow us to devise means to optimize the antitumor response in older cancer patients.
Footnotes
This article is part of the Symposium in Writing on “Impact of Ageing on Cancer Immunity and Immunotherapy”.
Contributor Information
Annabelle Grolleau-Julius, Email: grolleau@umich.edu.
Raymond L. Yung, Email: ryung@umich.edu.
References
- 1.Agrawal A, Agrawal S, Cao JN, Su H, Osann K, Gupta S. Altered innate immune functioning of dendritic cells in elderly humans: a role of phosphoinositide 3-kinase-signaling pathway. J Immunol. 2007;178:6912–6922. doi: 10.4049/jimmunol.178.11.6912. [DOI] [PubMed] [Google Scholar]
- 2.Agrawal A, Agrawal S, Tay J, Gupta S. Biology of dendritic cells in aging. J Clin Immunol. 2008;28:14–20. doi: 10.1007/s10875-007-9127-6. [DOI] [PubMed] [Google Scholar]
- 3.Chen J, Mo R, Lescure PA, Misek DE, Hanash S, Rochford R, Hobbs M, Yung RL. Aging is associated with increased T-cell chemokine expression in C57BL/6 mice. J Gerontol A Biol Sci Med Sci. 2003;58:975–983. doi: 10.1093/gerona/58.11.b975. [DOI] [PubMed] [Google Scholar]
- 4.DePinho RA. The age of cancer. Nature. 2000;408:248–254. doi: 10.1038/35041694. [DOI] [PubMed] [Google Scholar]
- 5.Flamand V, Sornasse T, Thielemans K, Demanet C, Bakkus M, Bazin H, Tielemans F, Leo O, Urbain J, Moser M. Murine dendritic cells pulsed in vitro with tumor antigen induce tumor resistance in vivo. Eur J Immunol. 1994;24:605–610. doi: 10.1002/eji.1830240317. [DOI] [PubMed] [Google Scholar]
- 6.Fulop T, Larbi A, Wikby A, Mocchegiani E, Hirokawa K, Pawelec G. Dysregulation of T-cell function in the elderly: scientific basis and clinical implications. Drugs Aging. 2005;22:589–603. doi: 10.2165/00002512-200522070-00005. [DOI] [PubMed] [Google Scholar]
- 7.Gao FG, Khammanivong V, Liu WJ, Leggatt GR, Frazer IH, Fernando GJ. Antigen-specific CD4+ T-cell help is required to activate a memory CD8+ T cell to a fully functional tumor killer cell. Cancer Res. 2002;62:6438–6441. [PubMed] [Google Scholar]
- 8.Geiger JD, Wagner PD, Shu S, Chang AE. A novel role for autologous tumour cell vaccination in the immunotherapy of the poorly immunogenic B16-BL6 melanoma. Surg Oncol. 1992;1:199–208. doi: 10.1016/0960-7404(92)90065-s. [DOI] [PubMed] [Google Scholar]
- 9.Geijtenbeek TB, Torensma R, van Vliet SJ, van Duijnhoven GC, Adema GJ, van Kooyk Y, Figdor CG. Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell. 2000;100:575–585. doi: 10.1016/s0092-8674(00)80693-5. [DOI] [PubMed] [Google Scholar]
- 10.Gijzen K, Tacken PJ, Zimmerman A, Joosten B, de Vries IJ, Figdor CG, Torensma R. Relevance of DC-SIGN in DC-induced T cell proliferation. J Leukoc Biol. 2007;81:729–740. doi: 10.1189/jlb.0606414. [DOI] [PubMed] [Google Scholar]
- 11.Grolleau-Julius A, Garg MR, Mo R, Stoolman LL, Yung RL. Effect of aging on bone marrow-derived murine CD11c+ CD4-CD8alpha-dendritic cell function. J Gerontol A Biol Sci Med Sci. 2006;61:1039–1047. doi: 10.1093/gerona/61.10.1039. [DOI] [PubMed] [Google Scholar]
- 12.Grolleau-Julius A, Harning EK, Abernathy LM, Yung LR. Impaired dendritic cell function in aging leads to defective antitumor immunity. Cancer Res. 2008 doi: 10.1158/0008-5472.CAN-07-5769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grolleau A, Sloan A, Mule JJ. Dendritic cell-based vaccines for cancer therapy. Cancer Treat Res. 2005;123:181–205. doi: 10.1007/0-387-27545-2_8. [DOI] [PubMed] [Google Scholar]
- 14.Gunn MD, Kyuwa S, Tam C, Kakiuchi T, Matsuzawa A, Williams LT, Nakano H. Mice lacking expression of secondary lymphoid organ chemokine have defects in lymphocyte homing and dendritic cell localization. J Exp Med. 1999;189:451–460. doi: 10.1084/jem.189.3.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krishnan L, Sad S, Patel GB, Sprott GD. Archaeosomes induce long-term CD8 + cytotoxic T cell response to entrapped soluble protein by the exogenous cytosolic pathway, in the absence of CD4+ T cell help. J Immunol. 2000;165:5177–5185. doi: 10.4049/jimmunol.165.9.5177. [DOI] [PubMed] [Google Scholar]
- 16.Lesterhuis WJ, Aarntzen EH, De Vries IJ, Schuurhuis DH, Figdor CG, Adema GJ, Punt CJ. Dendritic cell vaccines in melanoma: from promise to proof? Crit Rev Oncol Hematol. 2008;66:118–134. doi: 10.1016/j.critrevonc.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 17.Miller RA. The aging immune system: primer and prospectus. Science. 1996;273:70–74. doi: 10.1126/science.273.5271.70. [DOI] [PubMed] [Google Scholar]
- 18.Montoya MC, Sancho D, Bonello G, Collette Y, Langlet C, He HT, Aparicio P, Alcover A, Olive D, Sanchez-Madrid F. Role of ICAM-3 in the initial interaction of T lymphocytes and APCs. Nat Immunol. 2002;3:159–168. doi: 10.1038/ni753. [DOI] [PubMed] [Google Scholar]
- 19.Nencioni A, Grunebach F, Schmidt SM, Muller MR, Boy D, Patrone F, Ballestrero A, Brossart P. The use of dendritic cells in cancer immunotherapy. Crit Rev Oncol Hematol. 2008;65:191–199. doi: 10.1016/j.critrevonc.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 20.Paglia P, Chiodoni C, Rodolfo M, Colombo MP. Murine dendritic cells loaded in vitro with soluble protein prime cytotoxic T lymphocytes against tumor antigen in vivo. J Exp Med. 1996;183:317–322. doi: 10.1084/jem.183.1.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pawelec G, Solana R. Immunosenescence. Immunol Today. 1997;18:514–516. doi: 10.1016/s0167-5699(97)01145-6. [DOI] [PubMed] [Google Scholar]
- 22.Porgador A, Gilboa E. Bone marrow-generated dendritic cells pulsed with a class I-restricted peptide are potent inducers of cytotoxic T lymphocytes. J Exp Med. 1995;182:255–260. doi: 10.1084/jem.182.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Randolph GJ, Angeli V, Swartz MA. Dendritic-cell trafficking to lymph nodes through lymphatic vessels. Nat Rev Immunol. 2005;5:617–628. doi: 10.1038/nri1670. [DOI] [PubMed] [Google Scholar]
- 24.Sharma S, Dominguez AL, Lustgarten J. Aging affect the anti-tumor potential of dendritic cell vaccination, but it can be overcome by co-stimulation with anti-OX40 or anti-4-1BB. Exp Gerontol. 2006;41:78–84. doi: 10.1016/j.exger.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 25.Shi M, Bi X, Xu S, He Y, Guo X, Xiang J. Increased susceptibility of tumorigenicity and decreased anti-tumor effect of DC vaccination in aged mice are potentially associated with increased number of NK1.1+ CD3+ NKT cells. Exp Oncol. 2005;27:125–129. [PubMed] [Google Scholar]
- 26.Shurin MR, Shurin GV, Chatta GS. Aging and the dendritic cell system: implications for cancer. Crit Rev Oncol Hematol. 2007;64:90–105. doi: 10.1016/j.critrevonc.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 28.Villadangos JA, Schnorrer P. Intrinsic and cooperative antigen-presenting functions of dendritic-cell subsets in vivo. Nat Rev Immunol. 2007;7:543–555. doi: 10.1038/nri2103. [DOI] [PubMed] [Google Scholar]
- 29.Wick G, Grubeck-Loebenstein B. The aging immune system: primary and secondary alterations of immune reactivity in the elderly. Exp Gerontol. 1997;32:401–413. doi: 10.1016/s0531-5565(96)00152-0. [DOI] [PubMed] [Google Scholar]