Abstract
How parents manifest symptoms of anxiety or depression may affect how children learn to modulate their own distress, thereby influencing the children’s risk for developing an anxiety or mood disorder. Conversely, children’s mental health symptoms may impact parents’ experiences of negative emotions. Therefore, mental health symptoms can have bidirectional effects in parent-child relationships, particularly during moments of distress or frustration (e.g., when a parent or child makes a costly mistake). The present study used simultaneous functional magnetic resonance imaging (fMRI) of parent-adolescent dyads to examine how brain activity when responding to each other’s costly errors (i.e., dyadic error processing) may be associated with symptoms of anxiety and depression. While undergoing simultaneous fMRI scans, healthy dyads completed a task involving feigned errors that indicated their family member made a costly mistake. Inter-brain, random-effects multivariate modeling revealed that parents who exhibited decreased medial prefrontal cortex and posterior cingulate cortex activation when viewing their child’s costly error response had children with more symptoms of depression and anxiety. Adolescents with increased anterior insula activation when viewing a costly error made by their parent had more anxious parents. These results reveal cross-brain associations between mental health symptomatology and brain activity during parent-child dyadic error processing.
Keywords: fMRI, Parent-child interactions, Error processing, Adolescence, Anxiety, Depression
1. Introduction
Mood and anxiety disorders are highly prevalent in adolescent populations (Merikangas et al., 2010). An adolescent’s risk for developing these disorders increases substantially if they have a parent with anxiety or depression (Spence et al., 2002). Indeed, symptoms of anxiety and depression are influenced by both genetic and environmental factors (e.g., parenting practices and family conflict; Fisak and Grills-Taquechel, 2007; Petersen et al., 1993). The vast prevalence of these disorders is alarming, as anxiety and depression are some of the most disabling and costly public health problems (Hoffman et al., 2008; Olesen et al., 2012; Reddy, 2010). Therefore, research on risk factors for anxiety and depression in adolescence is needed.
Parents provide children with emotion-related knowledge on how to experience and respond to negative affectivity through direct conversation (i.e., emotion coaching) and behaviors (i.e., modeling; Morris et al., 2007, 2018). Thus, parents play a pivotal role in supporting children’s emotional development, even throughout adolescence (Thompson and Goodman, 2010; Waller et al., 2014). For example, adolescents with more supportive mothers exhibit decreased amygdala activation when viewing negative faces, which may represent decreased reactivity to negative affect (Romund et al., 2016). When parents experience mental health symptoms, it may negatively impact the process by which children learn how to respond to distress (Silk et al., 2006). Importantly, emotional interactions in the parent-child relationship can also influence a parent’s functioning. For instance, emotionally reactive children tend to evoke more criticism and harsh discipline from parents (Kochanska et al., 2004; Scaramella and Leve, 2004). Therefore, mental health problems can have bidirectional effects within parent-child dyads, particularly during instances of negative affect (e.g., distress and frustration).
The experience of errors is one specific situation known to elicit negative affect and for which the neural circuitry has been delineated in previous research. In the context of a parent-child relationship, experiencing errors can be a dyadic process. For instance, a parent or child might make a costly error or mistake that impacts the other person. Most error processing research has focused on participants’ responses to their own errors (rather than someone else’s; i.e., dyadic error processing). Error processing tasks elicit activation in brain regions including the insula (Klein et al., 2007; Menon et al., 2001), amygdala (McCormick and Telzer, 2018), and medial prefrontal cortex (mPFC)/dorsal anterior cingulate cortex (dACC; McCormick and Telzer, 2018; Menon et al., 2001). Notably, these brain regions are also thought to play a role in regulating negative affect (Murphy et al., 2003; Ochsner et al., 2004; Phan and Sripada, 2013) as well as in anxiety and depression (Drevets, 2001; Shin and Liberzon, 2010). In studies that have examined participants’ reactions to others making an error (i.e., one that does not affect the participant), researchers have found that this also elicits mPFC/dACC activity, especially when the participant is close with the other person (Kang et al., 2010). It has been hypothesized that the similarity in neural response to one’s own error and the error of someone else represents empathy, or a shared emotional response (Thoma and Bellebaum, 2012).
A question that remains unanswered in the literature is how dyadic error processing relates to neural activation in real-time interactions between parents and their children. Furthermore, the role of mental health symptoms in these interactions has not been explored. Research on this topic is needed, as it may provide insight into how parenting practices relate to adolescent development of depression and anxiety. Not only does previous evidence suggest that mental health symptoms have bidirectional effects in emotional parent-child interactions, but these symptoms have also been shown to affect neural processes underlying error processing. In fact, both adolescents and adults with anxiety and depression exhibit altered neural responses to their own errors (Chiu and Deldin, 2007; Ladouceur et al., 2006, 2012; Weinberg et al., 2010). Therefore, it is likely that the neural mechanisms underlying dyadic error processing are impacted by mental health symptoms in parent-child dyads. However, empirical research on this idea is lacking.
We addressed the aforementioned gaps in the literature by employing simultaneous fMRI scanning to study parent-adolescent dyads. We utilized simultaneous fMRI (as opposed to other imaging modalities such as electroencephalography [EEG] or functional near-infrared spectroscopy [fNIRS]) because it has the unique capacity to measure activity in deep brain structures involved in social processes and emotion (e.g., amygdala; Koike et al., 2015), which makes it particularly useful for studying social interactions. During simultaneous fMRI scanning, dyads completed the Testing Emotional Attunement and Mutuality (TEAM) task. The TEAM task contains trials in which participants are given feedback that their partner made a costly error. The goal of ‘costly error’ trials is to invoke negative affectivity (e.g., disappointment and frustration), which is a common response to monetary loss (Abler et al., 2005; Angus and Harmon-Jones, 2019). These trials thereby provided a naturalistic opportunity to observe dyadic error processing, or how participants’ brains respond to their partner’s mistake. We chose to study dyadic error processing in psychiatrically healthy dyads in order to examine possible risk factors in the development of adolescent internalizing disorders.
Given the potential links between error processing, anxiety, and depression, we explored how mental health symptoms relate to participants’ brain responses to their partner’s costly errors in the amygdala, anterior insula, and mPFC/dACC. Specifically, we hypothesized that during dyadic error processing, participants’ symptoms of anxiety and depression would relate to increased activity in the brain regions involved in error processing. This would suggest that increased mental health symptoms relate to stronger reactions to a partner’s costly error. Because we were assessing real-time dyadic interactions, we hypothesized that these effects would be observed in participants’ own brains (i. e., intra-brain analyses) and their partners’ (i.e., inter-brain analyses).
2. Methods
2.1. Participants
The research protocol of the present study was approved by Oklahoma State University Center for Health Sciences Institutional Review Board (IRB) prior to data collection (IRB # 2017011). All participants provided written informed consent (assent for adolescents) to participate and were compensated for their time and effort. Participants were recruited as parent-adolescent dyads. The adolescents were between 14 and 16 years of age. Adolescents completed the study with one of their biological parents with whom they resided at least four days a week. Because the sample was comprised of healthy controls, all participants were excluded for current psychiatric disorder, and adolescents were additionally excluded for previous psychiatric disorder (excluding attention-deficit/hyperactivity disorder and learning disorders). The Mini International Neuropsychiatric Interview (MINI 7.0.1 or MINI-KID 7.0.1) was used to assess for psychiatric disorder in parents (i.e., depression and anxiety disorders, suicidality, bipolar disorder, obsessive compulsive disorder, posttraumatic stress disorder, substance use disorders, psychotic disorders, eating disorders, and antisocial personality disorders; Sheehan et al., 1997) and in adolescents (i.e., depression and anxiety disorders, suicidality, bipolar disorder, obsessive compulsive disorders, trauma- and stressor-related disorders, substance use disorders, neurodevelopmental disorders, conduct disorders, psychotic disorders, and eating disorders; Sheehan et al., 2010). Adolescent participants were excluded for developmental delays that would interfere with completion of study procedures. Additional exclusion criteria for all participants included left-handedness, primary language other than English, current pregnancy (based on urine pregnancy test), alcohol or drug intoxication (based on breathalyzer and saliva drug test), body mass index (BMI) larger than 40, and medical conditions or concomitant medications likely to influence cerebral blood flow or neurological function. All psychotropic medications were exclusionary, and participants could not have taken any psychotropic medications for three weeks (six weeks for fluoxetine). Participants could not take stimulant medications for at least 36 h before their scanning visit.
Forty dyads (i.e., 80 total participants) met criteria for study inclusion and completed the study protocol described below. For the present analyses, individual participants’ fMRI data were excluded for artifacts and poor quality (n = 6), excessive head motion (i.e., average motion > 0.15 mm across all runs; n = 10), and incidental MRI findings (e.g., significant structural abnormalities; n = 3). Imaging data were also excluded for issues related to the TEAM task including: poor performance on the error condition of interest (i.e., committing errors themselves on at least half of the costly error trials; n = 6) and technical difficulties with the task (e.g., problems with the response box; n = 2). One participant was excluded for disclosing suspicions that the task was pre-programmed during debriefing. The final sample used for imaging analyses consisted of 25 parents (M age = 43 years, SD = 6 years; female n = 23; Caucasian n = 22) and 27 adolescents (M age = 15 years, SD =1 year; female n = 15; Caucasian n = 20). The parent sample was predominantly comprised of mothers. See Table 1 for additional participant characteristics. Because participants’ imaging data were excluded on an individual rather than dyadic level, some participants were included in the sample even if their partner was not. This allowed for the maximization of sample size in order to maintain power. The number of dyads in which either the parent or adolescent had usable fMRI data was 35 (i.e., 70 total participants) and the number of dyads in which both the parent and adolescent had usable fMRI data was 17 (i.e., 34 total participants). The analyses presented in this manuscript were also conducted in this subsample of 17 dyads, and the results can be found in Supplementary Materials.
Table 1.
Demographic and mental health characteristics of the parent and adolescent samples.
| Adolescents (n = 27) | Parents (n = 25) | |||
|---|---|---|---|---|
| Gender (% Female) | 56 | 92 | ||
| Race (% Caucasian) | 74 | 88 | ||
| M (SD) | Range Within Sample | M (SD) | Range Within Sample | |
| Age in years | 15 (1) | 14 – 16 | 43 (6) | 30 – 53 |
| Depression | ||||
| Mood and Feelings Questionnaire (MFQ) | 5.44 (4.09) | 0 – 14 | – | – |
| Quick Inventory of Depressive Symptomatology (QIDS) | – | – | 3.36 (3.59) | 0 – 14 |
| Anxiety | ||||
| Screen for Child Anxiety Related Disorders (SCARED) | 13.00 (8.66) | 0 – 39 | – | – |
| Hamilton Anxiety Rating Scale (HAM-A) | – | – | 3.00 (2.94) | 0 – 9 |
2.2. Study design
All participants completed two data collection visits: a screening session and an fMRI session. The average duration between these visits was 15 days. The screening session was conducted with parents and adolescents concurrently, but separately. During this visit, participants provided demographic and MRI safety information, were administered the MINI 7.0.1 or MINI-KID 7.0.1 by trained research assistants, and responded to several questionnaires regarding mental health, family environment, and behavior. To assess symptoms of depression, parents completed the Quick Inventory of Depressive Symptomatology Self-Report (QIDS; Rush et al., 2003) and adolescents completed the Mood and Feelings Questionnaire (MFQ; Angold and Costello, 1987). Symptoms of anxiety were measured using the Hamilton Anxiety Rating Scale (HAM-A; Hamilton, 1959) for parents and the Screen for Child Anxiety Related Disorders (SCARED; Birmaher et al., 1997) for adolescents. Regardless of their scores on these measures, all participants were given a list of local mental health resources. After completing the screening visit, eligible parent-adolescent dyads were invited back for the simultaneous fMRI scanning session.
At the beginning of the scanning session, participants completed alcohol, drug, and pregnancy tests. They were also provided instructions for the fMRI task described in Section 2.3. Then, participants were placed in a mock MRI scanner in order to acclimate to the scanning environment and to practice staying still during scans. After being in the mock scanner, participants practiced the fMRI task on a computer. Next, parents and their adolescent children were positioned in two identical 3-Tesla, whole-body MRI scanners. The scanners are in close proximity to each other but are located in separate rooms. While in the scanners, dyads completed the TEAM task, which was developed for this study (Burrows et al., 2018; Kerr et al., 2019a.(under review).
2.3. TEAM task
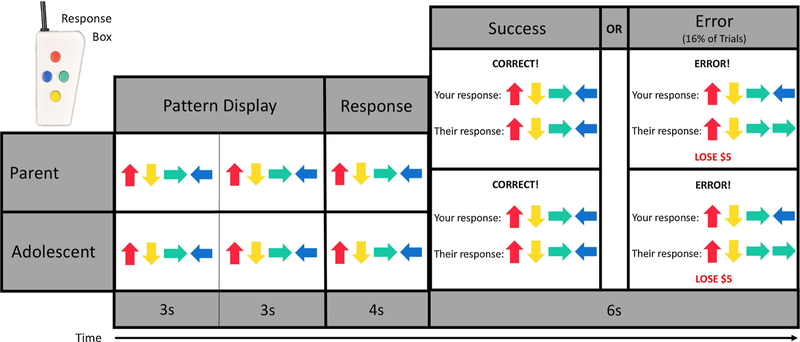
The TEAM task was designed by the study investigators to assess dyadic error processing (see Fig. 1). The task is presented as a cooperative game that dyads complete together. Before the task begins, dyads are told that they can collectively win up to $50 from the game. During the task, both participants are shown a pattern of four colored arrows twice, lasting three seconds each time. Then, the pattern disappears, and participants are given four seconds to recreate it using an MRI-compatible handheld response device. Participants are told that both members of the dyad must recreate the pattern correctly or the dyad will lose $5 from the total shared prize of $50. The TEAM task, however, is preprogrammed to show participants that their partner responded incorrectly during 16 percent of the trials. The remaining trials are programmed to show feedback that the other person responded correctly. The feedback for all trials of the TEAM task was presented for six seconds. The task was programmed to ensure that participants would be shown a standardized number of correct and incorrect responses by their partner. The use of simultaneous fMRI scanning enhanced the believability of the TEAM task because participants were aware that their partner was also being scanned and completing the task.
Fig. 1.
Example pattern trial of the TEAM task. Partners (e.g., parents and their adolescent children) complete this task while being simultaneously scanned and are told that they are completing the task cooperatively. In each pattern trial, participants are shown a pattern of four arrows twice. Then, they have four seconds to recreate this pattern from memory using a handheld response box. Finally, they are given feedback on their own performance (i.e., whether they entered the pattern correctly) as well as their partner’s. However, the feedback about their partner’s performance is preprogrammed, and in 16% of the trials, participants are told their partner made an error that cost the dyad five dollars.
For the present study, dyads completed two runs of the TEAM task. Parents and their adolescent children were shown the same set of trials and were always shown accurate feedback of their own performance. In addition to the pattern trials, the TEAM task also involves an active baseline condition of 14 ‘s-detection’ trials. During these trials, participants are asked to press a button if they see the letter ‘s’ in a random string of 20 letters. Participants do not lose money or receive performance feedback during the s-detection trials. Overall, the intent of the TEAM task is to assess participant brain activation when they perceive that their partner (in this case, their parent or adolescent child) has made a costly error (i.e., dyadic error processing). After the completion of the task, participants were debriefed and told that they would receive the full $50 prize (see Kerr et al., under review for a thorough description of the TEAM task design).
2.4. MRI and fMRI data acquisition and preprocessing
In each MRI scanner, we used an eight-channel phased array head coil for signal reception. Blood oxygenation level-dependent (BOLD) fMRI was conducted with a single-shot, Sensitivity Encoded (SENSE) gradient-recalled echo-planar imaging (EPI) sequence. The following EPI parameters were used: FOV/slice/gap = 240/2.9/0 mm, axial slices per volume = 41, acquisition matrix = 96 × 96, flip angle = 78°, SENSE acceleration factor R = 2, TR/TE = 2000/25 ms, sampling bandwidth = 250 kHz, 235 volumes per run (scan duration = 7 min 50 s). A 128 × 128 matrix was used to reconstruct the EPI images resulting in voxel volume of 1.9 × 1.9 × 2.9 mm3. The EPI images for each participant were aligned to an anatomical scan created by a T1-weighted magnetization-prepared rapid gradient-echo (MPRAGE) sequence accelerated with SENSE. The parameters for the MPRAGE sequence were as follows: FOV = 240 mm, axial slices per volume = 128, slice thickness = 0.9 mm, image matrix = 256 × 256, voxel volume = 0.9 × 0.9 × 0.9 mm3, flip angle = 8°, SENSE acceleration factor R = 2, =TR/TE = 5/2.0 ms, inversion/delay time TI/TD = 725/1400 ms, sampling bandwidth = 31.3 kHz, scan time = 6 min and 13 s.
Each participant’s fMRI data were preprocessed using Analysis of Functional Neuroimaging (AFNI) package (Cox, 1996). First, participants’ anatomical scans were aligned to the first volume of their EPI timecourse. Then, anatomical scans were spatially transformed to Talairach space (Talairach and Tournoux, 1988). The first four volumes from the timecourse of each voxel were removed to allow the fMRI signal to reach steady state. After correcting for motion using 3dvolreg and volumes censoring in the AFNI pipeline, the EPI data were resampled to 1.75 × 1.75 × 1.75 mm3 voxels. Lastly, EPI data were smoothed using a 6-mm full-width, half-maximum Gaussian kernel and normalized to percent signal change using the mean signal from each voxel. Single subject analyses were then conducted with multiple linear regression in order to prepare participant data for group analyses.
The task regressors used for single subject analyses were created by convolving a stimulus reference time series with hemodynamic response function (gamma-variate function). The regressor model included four feedback conditions: 1) the participant and their partner re-created the stimulus pattern correctly (‘both correct’); 2) the participant responded correctly but was shown their partner did not (‘costly error’); 3) the participant re-created the pattern incorrectly and was shown their partner re-created it correctly; and 4) the participant and their partner responded incorrectly. As these regressors were dependent upon one’s unique performance during the task, some participants may not have had all four of these regressors (e.g., if they never re-created the pattern incorrectly). Other task regressors included in the model were s-detection trials, pattern display periods, and response periods. Regressors of non-interest included in the model were each run’s signal mean, linear, quadratic, and cubic signal trends and six motion parameters (i.e., rotation in the roll, pitch, and yaw axes and displacement in the superior, left, and posterior directions).
2.5. Statistical analyses
Correlational analyses between mental health symptoms reported by parents and adolescents were conducted using R statistical package (R; R Core Team, 2017). Separate analyses were conducted to look at the association between parent-child anxiety and depression in all participants with usable fMRI data (as this maximized statistical power; N = 35 dyads) and in only the dyads in which both participants had usable fMRI data (n = 17 dyads).
Group analyses of the TEAM task were conducted using voxel-wise, random-effects multivariate modeling (AFNI’s 3dMVM) in the parent (n = 25) and adolescent (n = 27) samples separately. For results of the 3dMVM analyses in the 17 complete dyads, see Supplementary Materials Section 3.0. The contrast used in the 3dMVM analyses was between the ‘costly error’ and ‘both correct’ conditions. We focused on this contrast because the TEAM task was designed to capture dyadic error processing. We therefore examined participants’ brain activation in response to their partner’s error (when they themselves responded correctly) compared to when both they and their partner responded correctly. After collecting our findings, we determined the ‘costly error’ condition was driving the results using post-hoc correlations (see Supplementary Materials Section 5.1). In order to examine how dyadic error processing relates to mental health symptoms, anxiety and depression scores were included as predictors in the model. Because of the dyadic nature of error processing in this task, separate analyses were conducted to assess the association between a participant’s BOLD activation and their partner’s anxiety and depression scores (i.e., inter-brain analyses) as well as their own mental health symptom scores (i.e., intra-brain analyses). Age was included as a covariate in all analyses, and gender was included as a covariate for analyses involving adolescent BOLD activation. In the inter-brain analyses, we included one’s own mental health symptoms as covariates.
Group analyses focused on voxel-wise results identified for whole-brain analyses, as well as analyses constricted to five a priori regions of interest (ROIs). These ROIs included regions implicated in error processing, anxiety, and depression (i.e., bilateral anterior insula, bilateral amygdala, medial PFC [combined mask encompassing mPFC, dmPFC, and dACC], ventromedial PFC [vmPFC], and bilateral dorsolateral PFC [dlPFC]). For information of the creation of the ROI masks, see Supplementary Materials Section 1.0. Separate masks for whole brain analyses were created for parents and adolescents, and these masks were comprised of all voxels in which at least 70% of the participants had EPI data.
We employed a traditionally conservative multiple comparisons correction approach to control for the likelihood of false positive inferences (Eklund et al., 2016) by utilizing the spatial autocorrelation function (acf) option for AFNI’s 3dFWHMx and 3dClustSim (Cox et al., 2017). Results were considered significant using a conservative voxel-wise threshold of p < 0.005 and a cluster-size multiple comparisons correction threshold of p < 0.05. For whole brain results, minimum cluster thresholds were 1764 mm3 for parents and 2008 mm3 for adolescents. Parent ROI cluster thresholds were 164 mm3 for anterior insula, 59 mm3 for amygdala, 369 mm3 for mPFC, 177 mm3 for vmPFC, and 417 mm3 for dlPFC. Adolescent ROI cluster thresholds were 175 mm3 for anterior insula, 60 mm3 for amygdala, 409 mm3 for mPFC, 173 mm3 for vmPFC, and 520 mm3 for dlPFC.
3. Results
3.1. Mental health assessments
The present study explored dyadic error processing in healthy parents and adolescents. Therefore, participants did not meet criteria for any current (or past for adolescents) mental health diagnoses according to the MINI 7.0.1 or MINI-KID 7.0.1. Despite this, there was variability in the severity of mental health symptoms reported by participants. This is expected, as natural variation in mental health symptoms occurs in the general population at sub-syndromal levels. On the QIDS, 21 parents’ scores suggested no depression, two parents scored in the mild range, and two parents scored in the moderate range. All parents were in the mild range for the HAM-A. One adolescent exceeded the clinical cutoff score for the SCARED (i.e., ≥25), and three adolescents exceeded the cutoff score for the MFQ (i.e., ≥12). Table 1 exhibits the average scores on these measures as well as the range of scores within the sample. There were no significant correlations between parents’ and adolescents’ scores on measures of anxiety or depression (N = 35 dyads, Parent QIDS and Adolescent MFQ: r = 0.01, p = 0.95, Parent HAM-A and Adolescent SCARED: r = −0.10, p = 0.56; n = 17 dyads, Parent HAM-A and Adolescent SCARED: r = −0.33, p = 0.19, Parent QIDS and Adolescent MFQ: r = −0.13, p = 0.62).
3.2. TEAM task
3.2.1. Task effects
A paper focused on the design and general effects of the TEAM task is currently under review (Kerr et al., under review). Therefore, the task effects are not a focus of the present paper, which concentrates on the TEAM task effects related to dyadic symptoms of anxiety and depression. To summarize the general task results, parents and adolescents demonstrated increased BOLD activation in regions including the dlPFC, anterior insula, fusiform gyrus, thalamus, caudate, precuneus, and superior parietal lobule when seeing that their partner made a costly error. Additionally, adolescents exhibited greater left amygdala activation than parents. See Supplementary Materials Section 2.0 for a summary of Kerr et al., 2019b (under review) findings on the TEAM task effects.
3.2.2. Parent results
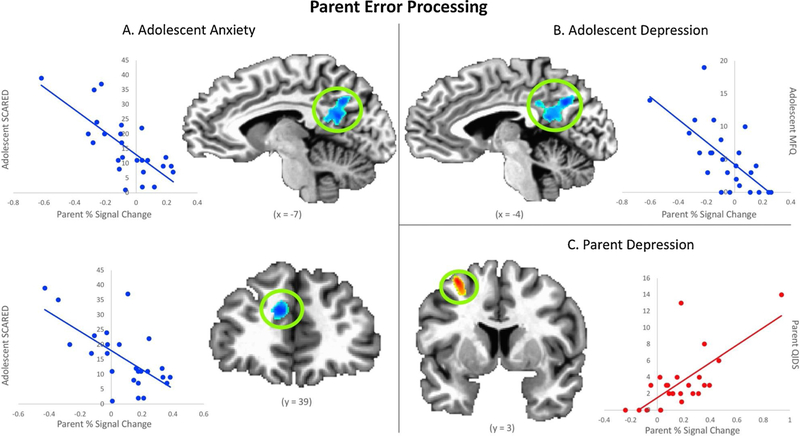
In whole-brain analyses, parents of children with greater anxiety and depression (Adolescent SCARED and MFQ scores, respectively) exhibited decreased BOLD activation in the posterior cingulate cortex (PCC) and precuneus when viewing their child’s costly error (controlling for parents’ own anxiety and depression). No significant whole-brain results were found in analyses examining the association between parents’ own mental health symptoms and BOLD activation when viewing their child’s error. Within the ROIs, decreased BOLD activation in parent mPFC when viewing their child’s costly error was associated with greater adolescent anxiety (Adolescent SCARED score; controlling for parents’ own anxiety). Increased BOLD activation in parent left dlPFC when viewing their child’s costly error was associated with greater parent depression (Parent QIDS score; see Table 2 and Fig. 2). If a more stringent cluster-wise threshold was used (i.e., p < 0.01 rather than p < 0.05 to account for the number of ROIs), these ROI-based results would not remain statistically significant. Despite this, these results appear meaningful based on their respective effect size estimates (although it should be noted that effect sizes in fMRI should be interpreted with caution; Yarkoni et al., 2009). While we did not have a large enough sample of fathers to determine between-group gender differences in the parent sample, we assessed how the removal of the fathers impacted the findings within the clusters presented above (see Supplemental Materials Section 4.0). Overall, the effects were similar.
Table 2.
Clusters demonstrating a significant relationship between mental health symptoms and BOLD activation of parent or adolescent while processing a costly error committed by their dyadic partner (voxel-wise p < 0.005).
| Symptom Measure | Regions in Cluster | L/R | Vol (mm3) | x | y | z | t-value | η2G | p-value | BA |
|---|---|---|---|---|---|---|---|---|---|---|
| Parent BOLD Activation | ||||||||||
| Adolescent Depression (MFQ) | Posterior Cingulate, Precuneus, Cuneus | Bilateral | 2936 | −6 | −61 | 32 | −5.61 | 0.60 | < 0.01 | 7, 23, 29, 30, 31 |
| Adolescent Anxiety (SCARED) | Posterior Cingulate, Precuneus, Cuneus | Bilateral | 2213 | −10 | −55 | 21 | −5.30 | 0.58 | 0.02 | 7, 23, 29, 30, 31 |
| Adolescent Anxiety (SCARED) | Medial Frontal Gyrus, Anterior Cingulate | L | 488 | −8 | 39 | 23 | −5.34 | 0.59 | 0.03 | 9, 32 |
| Parent Depression (QIDS) | Dorsolateral Prefrontal Cortex | L | 595 | −29 | 3 | 51 | 5.11 | 0.54 | 0.02 | 6 |
| Adolescent BOLD Activation | ||||||||||
| Parent Anxiety (HAM-A) | Anterior Insula | L | 188 | −45 | 11 | 4 | 4.53 | 0.48 | 0.05 | 13, 44 |
Note. MFQ = Mood and Feelings Questionnaire. SCARED = Screen for Child Anxiety Related Disorders. QIDS = Quick Inventory of Depressive Symptomatology. HAM-A = Hamilton Anxiety Rating Scale. L = left hemisphere. Vol = Volume in cubic millimeters. Talairach coordinates and t-value reflect the area of peak activation in the cluster. η2G = Generalized Eta-Squared, which should be interpreted with caution due to possible inflation (Yarkoni, 2009). p-value = Minimum cluster-wise threshold. BA = Brodmann Area gathered from the ’whereami’ function in AFNI.
Fig. 2.
Results from analyses examining parents’ BOLD activation when processing a costly error committed by their child (voxel-wise p < 0.005). A) Adolescent Anxiety - Parents with more anxious children exhibit decreased activation in posterior cingulate cortex, precuneus, and medial prefrontal cortex. B) Adolescent Depression - Parents with more depressed children exhibit decreased activation in posterior cingulate cortex and precuneus. C) Parent Depression - Parents who were more depressed themselves demonstrated increased activation in left dorsolateral prefrontal cortex. Coordinates are in Talairach space.
3.2.3. Adolescent results
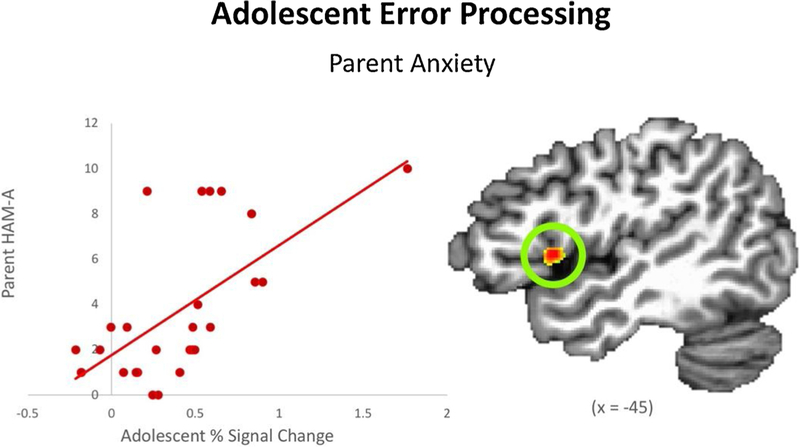
Increased BOLD activation in adolescent left anterior insula when viewing their parent’s costly error was associated with greater parent anxiety (Parent HAM-A score; when controlling for adolescents’ own anxiety; see Table 2 and Fig. 3). Similar to the parent ROI-based results, this finding would not survive a more stringent cluster-wise threshold of p < 0.01. There were no significant clusters identified in whole-brain analyses or in any of the other ROIs among the adolescent sample.
Fig. 3.
Result from analyses examining adolescents’ BOLD activation when processing a costly error committed by their parent (voxel-wise p < 0.005). Adolescents with more anxious parents exhibit increased activation in left anterior insula. Coordinates are in Talairach space.
4. Discussion
The present study utilized simultaneous fMRI scanning to explore associations between symptoms of anxiety and depression and brain activation during dyadic error processing. Results from the fMRI task designed for this study (i.e., TEAM task) demonstrated that when witnessing their partner’s costly error, both parent and adolescent participants exhibited activation in regions previously implicated in responding to one’s own error (Klein et al., 2007; McCormick and Telzer, 2018; Menon et al., 2001). Our analyses revealed three primary findings. First, parents with more depressive symptoms exhibited increased activation in dlPFC when reacting to a costly error made by their child. Second, parents who had more anxious children exhibited less activation in mPFC, PCC, and precuneus when reacting to a costly error made by their child. Similarly, parents of children with more depressive symptoms exhibited decreased activation in PCC and precuneus. Finally, adolescents with more anxious parents exhibited increased activation in anterior insula when reacting to a costly error made by their parent. These findings appear to be driven by the ‘costly error’ condition of the task and are unrelated to parent education level or error rate on the TEAM task (see Supplemental Materials Section 5.0).
Our results suggest that parents with greater depressive symptomatology exhibit increased activation in dlPFC when viewing their child’s error. The dlPFC is a region recruited during cognitive control (Brosnan and Wiegand, 2017; Weissman et al., 2008), including cognitive control of emotion (i.e., emotional reappraisal and suppression; Eippert et al., 2007; Goldin et al., 2008; Golkar et al., 2012). Individuals with major depressive disorder demonstrate decreased dlPFC activation during emotion regulation tasks as compared to healthy controls (Davidson et al., 2002; Erk et al., 2010; Taylor and Liberzon, 2007), which may indicate a dysfunction in emotion regulation abilities (Koenigs and Grafman, 2009). However, our results suggest that in the dyadic parent-child context, parents with more depressive symptoms demonstrate increased dlPFC activation when their child commits a costly error. Although this appears contrary to previous literature, the present sample was comprised of psychiatrically healthy individuals. Therefore, symptoms experienced within this sample are sub-syndromal and may not be severe enough to be associated with underlying aberrant hypoactive dlPFC activity. Interpreted within this context, the current findings may indicate that healthy parents with more depressive symptoms need to regulate their emotional responses to their child’s errors more than parents who have fewer or no depressive symptoms. This may reveal an adaptive response in which parents with mild depressive symptomology work harder to regulate their emotions and to prevent poor reactions to their children’s mistakes. Relatedly, parents with sub-syndromal depressive symptoms who are able to engage the dlPFC in emotion regulation may be less likely to develop major depressive disorder. These interpretations align with previous research that suggests adaptive emotion regulation is a protective factor against the development of major depressive disorder (Southwick and Charney, 2012; Troy and Mauss, 2011).
Beyond the association between parent depression and activity in their own dlPFC when seeing their child make a costly error, we did not find any other significant intra-brain results (i.e., the association between one’s own mental health symptoms and brain activation during dyadic error processing). This was surprising due to the established relationship between mental illness and maladaptive error processing (Chiu and Deldin, 2007; Ladouceur et al., 2006, 2012; Weinberg et al., 2010). However, previous studies have focused on participant reactions to their own errors rather than a partner’s. Therefore, we propose that this lack of intra-brain findings may be attributable to the dyadic nature of the TEAM task and our focus on instances in which participants received feedback that they responded correctly, and their partner made a costly error. As a result, participants likely may have interpreted these errors within the context of their partner’s reactions rather than solely focusing on how it impacted themselves.
Next, we examined the association between parents’ neural responses to their adolescents’ errors and adolescent symptoms of anxiety and depression. We found that parents who exhibited lower activation in the PCC and precuneus had children with more symptoms of depression and anxiety. Additionally, parents’ mPFC activity was negatively associated with adolescent anxiety. As mentioned previously, the mPFC has been implicated in error processing. However, mPFC along with the PCC and precuneus are all primary foci within the default mode network (Raichle, 2015). Regions in the default mode network (DMN) are activated during self-referential thought, introspection, and reflecting on the past or present (Andrews-Hanna, 2012; Buckner et al., 2008; Raichle, 2015). When applied to the current findings, one possible interpretation is that parents with more distressed children may exhibit less self-referential thought and are more engaged in the task when their child makes an error. Future research could further examine the association between activity in DMN regions and the use of specific strategies (i.e., reappraisal) to regulate responses to one’s own or others’ errors, and whether changes in DMN activity could be a marker of successful implementation of these strategies in parents to help ameliorate anxiety and depression symptoms in their children.
When considering adolescents’ neural responses to costly errors made by their parents, we found that adolescents’ anterior insula activation was positively associated with their parents’ anxiety symptoms. The anterior insula is implicated in emotional reactivity and awareness (Gu et al., 2013; Murphy et al., 2003). In fact, real-time fMRI neuro-feedback studies with both adult and adolescent samples have revealed that participants are able to increase or decrease salience of responses to emotional stimuli by regulating their anterior insula activity (Caria et al., 2010; Cohen Kadosh et al., 2016). Our finding that adolescents with anxious parents respond with greater anterior insula activation could be reflective of greater emotional reactivity to their parents’ mistakes. More specifically, activity in the anterior insula has been found to represent social emotions such as empathy and compassion (Kanske et al., 2015; Lamm and Singer, 2010). For instance, adolescents who exhibit greater anterior insula activity while witnessing social exclusion have been found to engage in more prosocial behaviors to the excluded victims (Masten et al., 2011). Thus, the anterior insula activity of adolescents in our sample may be reflective of more empathic responses to their parents’ errors, if their parent was high on anxiety. When taken together, our anterior insula finding may indicate that children of parents with greater anxiety find their parents’ mistakes to be more emotionally salient (i.e., because they believe their parent may be more upset by the error) and may therefore respond more empathically to this mistake.
Our results reveal an interesting contrast between the parent and adolescent samples. The brain regions in parents associated with mental health symptoms were all regions commonly implicated in higher-order cognitive processing (e.g., emotion regulation and self-referential thought). Conversely, adolescents exhibited associations in the anterior insula, which is involved in emotional reactivity and salience processing. This is likely indicative of the development of emotion-related neurocircuitry over time. The PFC matures with age (Guyer et al., 2016) and develops increased functional connectivity with emotion reactivity regions over time. Therefore, as children advance through adolescence, the PFC begins to downregulate emotional reactivity regions (Gee et al., 2013b; Wu et al., 2016). This neurodevelopmental process is believed to represent enhanced emotion regulation abilities. Parenting practices as well as mental health symptoms can impact this process (Aupperle et al., 2016; Gee et al., 2014, 2013a; Silk et al., 2017). Our results support this idea, as mental health symptoms appear to have cross-brain associations within parent-adolescent dyads.
The present study is not without limitations. First, the dyadic nature of the experiment (i.e., simultaneous fMRI of two participants) resulted in increased likelihood for data and participant exclusion. Although we began with 40 dyads (i.e., 80 participants), our imaging analyses incorporated only 52 participants. The number of dyads in which both participants had usable imaging data was even lower (n = 17). Future studies involving simultaneous fMRI scanning can accommodate for this limitation and improve experimental robustness through anticipatory recruitment of larger samples. In the present study, we maximized usable data and statistical power by including all participants in our primary analyses, regardless of whether their dyadic partner had usable data. Second, this study examined the effects of mental health symptoms on dyadic error processing within a psychiatrically healthy population. Results may differ for participants who meet criteria for mental health disorders (e.g., generalized anxiety disorder and major depressive disorder). For instance, we would expect correlations between parent and adolescent mental health symptoms to be significant in clinical populations; however, we did not observe this relationship in the present sample. The reasoning behind this is unknown; however, it is possible that our findings could be influenced by range restriction or by the exclusion criteria used in the study. Our results remain important, however, as subthreshold symptoms of anxiety and depression are common, especially in adolescent populations (Burstein et al., 2014). Moreover, sub-clinical presentations of mood and anxiety disorders in adolescence are associated with functional impairment and suicidality (Balázs et al., 2013) as well as the subsequent diagnosis of mood and anxiety disorders in adulthood (Klein et al., 2009). Third, because participants were shown different stimuli during the TEAM task (i.e., their own responses and pre-programmed partner responses), our analyses focused on regional activation patterns within each group (i.e., parents and adolescents) rather than trial-by-trial synchrony or cross-brain connectivity (i.e., concurrent and time-lagged connectivity between parent and adolescent brain regions). Physiological synchrony between dyads is thought to be beneficial for healthy parent-child interactions and later child outcomes (Feldman, 2007; Kudinova et al., 2019). Therefore, future research with more continuous interaction tasks (i.e., parent-adolescent conflict tasks) would be ideal for assessing how neural synchrony between parents and adolescents may relate to mental health. Fourth, only two fathers were included in the present sample, so we were unable to examine gender differences in the parent sample due to insufficient power. In addition, the current analytic approach focused on small volume corrected analyses within a priori ROIs, rather than correcting for whole-brain voxel-wise analyses. Further, the ROI-based findings would not survive a cluster-wise threshold corrected for the number of ROIs, yet the effect size estimates provide some evidence for the significance of these results. Future, larger studies are needed to determine whether fathers’ and mothers’ dyadic brain activation differentially relates to adolescent mental health, and to allow for a well-powered investigation of relationships across the entire brain. Lastly, the data were collected cross-sectionally, so interpretations of causation between mental health symptoms and neural activation pat- terns cannot be made.
Acknowledging these limitations, the present study also possesses many strengths. The use of simultaneous fMRI scanning provided a novel opportunity for examining, characterizing, and understanding parent-child relationships and cross-brain patterns of activation within a social context. Simultaneous scanning not only allowed us to examine both participants in a parent-adolescent dyad but also enhanced the believability of the TEAM task. Our findings support the use of simultaneous fMRI scanning in future studies of dyadic social interaction, as examining these processes in isolation (e.g., dealing with one’s own costly error) might result in vastly different activation patterns. Moreover, the TEAM task, developed specifically for the present study, allowed for the evaluation of dyadic error processing through an ecologically valid paradigm. Results from the TEAM task suggest that the task is effective in activating brain regions involved in error processing. The TEAM task may therefore be an effective way to study dyadic error processing in other important social relationships (e.g., teacher-student, romantic couples, and siblings), which are rarely studied with fMRI.
Overall, the findings in the present study extend the current literature by suggesting the neurobiological bases by which parents’ and their adolescent children’s dyadic error processing may be associated with mental health symptoms. This supports the idea that, within the context of dyadic error processing in the parent-child relationship, mental health symptoms can have bidirectional effects. Parents play an important role in their children’s emotional development, and this likely includes modeling and providing instruction on how to respond when mistakes are made. Our results suggest that parents’ mental health symptoms may impact how children learn to respond to emotional situations. Future longitudinal research on the role of cross-brain associations of mental health symptoms in predicting adolescent development of anxiety and depressive disorders is warranted.
Supplementary Material
Acknowledgements
The authors would like to thank Dr. Jennifer Stewart for her thoughtful feedback on the manuscript. We would also like to thank Katherine Thompson, Maggie Johnson, Jillian Bailey, Shea McClellan, Maddy Koehn, and Sylvia Zhu for their assistance in recruitment and data collection.
Funding
This research was supported by a Centers of Biomedical Research Excellence grant (P20GM199097, PD: Jennifer Hays-Grudo; funded by the National Institutes of Health) and The William K. Warren Foundation. The funding sources were not involved in study design; in the collection, analysis, and interpretation of data; in the writing of the article; or in the decision to submit the article for publication.
RLA receives funding from the National Institute of Mental Health (K23MH108707) and (P20GM121312). JB receives funding from the National Institute of General Medical Sciences (P20GM121312). WKS is an employee of Janssen Research & Development, LLC., of Johnson & Johnson, and holds equity in Johnson & Johnson. WKS is an inventor of a patent regarding appetite change in depression.
Footnotes
Declaration of Competing Interest
The remaining authors have no declarations of interest.
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.dcn.2019.100729.
References
- Abler B, Walter H, Erk S, 2005. Neural correlates of frustration. Neuroreport 16 (7), 669–672. [DOI] [PubMed] [Google Scholar]
- Andrews-Hanna JR, 2012. The brain’s default network and its adaptive role in internal mentation. Neuroscientist 18 (3), 251–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angold A, Costello E, 1987. Mood and Feelings Questionnaire (MFQ). Developmental Epidemiology Program, Duke University, Durham. [Google Scholar]
- Angus DJ, Harmon-Jones E, 2019. The anger incentive delay task: a novel method for studying anger in neuroscience research. Psychophysiology 56 (2), e13290. [DOI] [PubMed] [Google Scholar]
- Aupperle RL, Morris AS, Silk JS, Criss MM, Judah MR, Eagleton SG, et al. , 2016. Neural responses to maternal praise and criticism: relationship to depression and anxiety symptoms in high-risk adolescent girls. Neuroimage Clin. 11, 548–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balázs J, MikIósi M, Keresztény Á, Hoven CW, Carli V, Wasserman C, et al. , 2013. Adolescent subthreshold-depression and anxiety: psychopathology, functional impairment and increased suicide risk. J. Child Psychol. Psychiatry 54 (6), 670–677. [DOI] [PubMed] [Google Scholar]
- Birmaher B, Khetarpal S, Brent D, Cully M, Balach L, Kaufman J, Neer SM, 1997. The screen for child anxiety related emotional disorders (SCARED): scale construction and psychometric characteristics. J. Am. Acad. Child Adolesc. Psychiatry 36 (4), 545–553. [DOI] [PubMed] [Google Scholar]
- Brosnan MB, Wiegand I, 2017. The dorsolateral prefrontal cortex, a dynamic cortical area to enhance top-down attentional control. J. Neurosci. 37 (13), 3445–3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL, 2008. The brain’s default network. Ann. N. Y. Acad. Sci. U. S. A. 1124 (1), 1–38. [DOI] [PubMed] [Google Scholar]
- Burrows K, Kerr KL, DeVille DC, Ratliff EL, Cosgrove KT, Bodurka J, Paulus M, Morris AS, Simmons WK, 2018. Testing emotional attachment and mutuality (TEAM) fMRI task for studying dyadic interactions. In: Poster Presented at the Society for Neuroscience Conference San Diego, CA. [Google Scholar]
- Burstein M, Beesdo-Baum K, He J-P, Merikangas K, 2014. Threshold and subthreshold generalized anxiety disorder among US adolescents: prevalence, sociodemographic, and clinical characteristics. Psychol. Med. 44 (11), 2351–2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caria A, Sitaram R, Veit R, Begliomini C, Birbaumer N, 2010. Volitional control of anterior insula activity modulates the response to aversive stimuli. A real-time functional magnetic resonance imaging study. Biol. Psychiatry 68 (5), 425–432. [DOI] [PubMed] [Google Scholar]
- Chiu PH, Deldin PJ, 2007. Neural evidence for enhanced error detection in major depressive disorder. Am. J. Psychiatry 164 (4), 608–616. [DOI] [PubMed] [Google Scholar]
- Cohen Kadosh K, Luo Q, de Burca C, Sokunbi MO, Feng J, Linden D, Lau J, 2016. Using real-time fMRI to influence effective connectivity in the developing emotion regulation network. NeuroImage 125, 616–626. 10.1016/j.neuroimage.2015.09.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW, 1996. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 29 (3), 162–173. [DOI] [PubMed] [Google Scholar]
- Cox RW, Chen G, Glen DR, Reynolds RC, Taylor PA, 2017. FMRI clustering in AFNI: false-positive rates redux. Brain Connect. 7 (3), 152–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson RJ, Pizzagalli D, Nitschke JB, Putnam K, 2002. Depression: perspectives from affective neuroscience. Annu. Rev. Psychol. 53 (1), 545–574. [DOI] [PubMed] [Google Scholar]
- Drevets WC, 2001. Neuroimaging and neuropathological studies of depression: implications for the cognitive-emotional features of mood disorders. Curr. Opin. Neurobiol. 11 (2), 240–249. [DOI] [PubMed] [Google Scholar]
- Eippert F, Veit R, Weiskopf N, Erb M, Birbaumer N, Anders S, 2007. Regulation of emotional responses elicited by threat-related stimuli. Hum. Brain Mapp. 28 (5), 409–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund A, Nichols TE, Knutsson H, 2016. Cluster failure: why fMRI inferences for spatial extent have inflated false-positive rates. Proc. Natl. Acad. Sci. U. S. A. 201602413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erk S, Mikschl A, Stier S, Ciaramidaro A, Gapp V, Weber B, Walter H, 2010. Acute and sustained effects of cognitive emotion regulation in major depression. J. Neurosci. 30 (47), 15726–15734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman R, 2007. Parent–infant synchrony: biological foundations and developmental outcomes. Curr. Dir. Psychol. Sci. 16 (6), 340–345. [Google Scholar]
- Fisak B, Grills-Taquechel AE, 2007. Parental modeling, reinforcement, and information transfer: risk factors in the development of child anxiety? Clin. Child Fam. Psychol. Rev 10 (3), 213–231. 10.1007/s10567-007-0020-x. [DOI] [PubMed] [Google Scholar]
- Gee DG, Gabard-Durnam L, Telzer EH, Humphreys KL, Goff B, Shapiro M, et al. , 2014. Maternal buffering of human amygdala-prefrontal circuitry during childhood but not during adolescence. Psychol. Sci. 25 (11), 2067–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee DG, Gabard-Durnam LJ, Flannery J, Goff B, Humphreys KL, Telzer EH, et al. , 2013. Early developmental emergence of human amygdala–prefrontal connectivity after maternal deprivation. Proc. Natl. Acad. Sci. U. S. A. 110 (39), 15638–15643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee DG, Humphreys KL, Flannery J, Goff B, Telzer EH, Shapiro M, et al. , 2013. A developmental shift from positive to negative connectivity in human amygdala–prefrontal circuitry. J. Neurosci. 33 (10), 4584–4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin PR, McRae K, Ramel W, Gross JJ, 2008. The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biol. Psychiatry 63 (6), 577–586. 10.1016/j.biopsych.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golkar A, Lonsdorf TB, Olsson A, Lindstrom KM, Berrebi J, Fransson P, et al. , 2012. Distinct contributions of the dorsolateral prefrontal and orbitofrontal cortex during emotion regulation. PLoS One 7 (11), e48107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X, Hof PR, Friston KJ, Fan J, 2013. Anterior insular cortex and emotional awareness. J. Comp. Neurol. 521 (15), 3371–3388. 10.1002/cne.23368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, Silk JS, Nelson EE, 2016. The neurobiology of the emotional adolescent: from the inside out. Neurosci. Biobehav. Rev. 70, 74–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M, 1959. The assessment of anxiety states by rating. Br. J. Med. Psychol. 32 (1), 50–55. [DOI] [PubMed] [Google Scholar]
- Hoffman DL, Dukes EM, Wittchen HU, 2008. Human and economic burden of generalized anxiety disorder. Depress. Anxiety 25 (1), 72–90. [DOI] [PubMed] [Google Scholar]
- Kang SK, Hirsh JB, Chasteen AL, 2010. Your mistakes are mine: self-other overlap predicts neural response to observed errors. J. Exp. Soc. Psychol. 46 (1), 229–232. [Google Scholar]
- Kanske P, Böckler A, Trautwein FM, Singer T, 2015. Dissecting the social brain: introducing the EmpaToM to reveal distinct neural networks and brain–behavior relations for empathy and Theory of Mind. Neuroimage 122, 6–19. [DOI] [PubMed] [Google Scholar]
- Kerr KL, Cosgrove KT, Ratliff EL, Burrows K, Moore AJ, Deville DC, Bodurka J, Simmons WK, Morris AS, 2019. Studying neural mechanisms of parenting influence on adolescent emotion regulation with an fMRI dyadic interaction. In: Symposium Presented at the Society for Research in Child Development Biennial Meeting Baltimore, MD. [Google Scholar]
- Kerr KL, Cosgrove KT, Ratliff EL, Burrows K, Moore AJ, DeVille DC, Silk JS, Tapert SF, Bodurka J, Simmons WK, Morris AS (under review), TEAM work: Testing emotional attunement and mutuality during concurrent parent-adolescent fMRI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein DN, Shankman SA, Lewinsohn PM, Seeley JR, 2009. Subthreshold depressive disorder in adolescents: predictors of escalation to full-syndrome depressive disorders. J. Am. Acad. Child Adolesc. Psychiatry 48 (7), 703–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein TA, Endrass T, Kathmann N, Neumann J, von Cramon DY, Ullsperger M, 2007. Neural correlates of error awareness. NeuroImage 34 (4), 1774–1781. [DOI] [PubMed] [Google Scholar]
- Kochanska G, Friesenborg AE, Lange LA, Martel MM, 2004. Parents’ personality and infants’ temperament as contributors to their emerging relationship. J. Pers. Soc. Psychol. 86 (5), 744. [DOI] [PubMed] [Google Scholar]
- Koenigs M, Grafman J, 2009. The functional neuroanatomy of depression: distinct roles for ventromedial and dorsolateral prefrontal cortex. Behav. Brain Res. 201 (2), 239–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike T, Tanabe HC, Sadato N, 2015. Hyperscanning neuroimaging technique to reveal the “two-in-one” system in social interactions. Neurosci. Res. 90, 25–32. [DOI] [PubMed] [Google Scholar]
- Kudinova AY, Woody ML, James KM, Burkhouse KL, Feurer C, Foster CE, Gibb BE, 2019. Maternal major depression and synchrony of facial affect during mother-child interactions. J. Abnorm. Psychol. 128 (4), 284–294 https://0-doi-org.library.utulsa.edu/10.1037/abn0000411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladouceur CD, Dahl RE, Birmaher B, Axelson DA, Ryan ND, 2006. Increased error-related negativity (ERN) in childhood anxiety disorders: ERP and source localization. J. Child Psychol. Psychiatry 47 (10), 1073–1082. [DOI] [PubMed] [Google Scholar]
- Ladouceur CD, Slifka JS, Dahl RE, Birmaher B, Axelson DA, Ryan ND, 2012. Altered error-related brain activity in youth with major depression. Dev. Cogn. Neurosci. 2 (3), 351–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm C, Singer T, 2010. The role of anterior insular cortex in social emotions. Brain Struct. Funct. 214 (5–6), 579–591. [DOI] [PubMed] [Google Scholar]
- Masten CL, Morelli SA, Eisenberger NI, 2011. An fMRI investigation of empathy for ‘social pain’and subsequent prosocial behavior. Neuroimage 55 (1), 381–388. [DOI] [PubMed] [Google Scholar]
- McCormick EM, Telzer EH, 2018. Not doomed to repeat: enhanced medial prefrontal cortex tracking of errors promotes adaptive behavior during adolescence. J. Cogn. Neurosci. 30 (3), 281–289. 10.1162/jocn_a_01206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V, Adleman NE, White CD, Glover GH, Reiss AL, 2001. Error-related brain activation during a Go/NoGo response inhibition task. Hum. Brain Mapp. 12 (3), 131–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merikangas KR, He J-P, Burstein M, Swanson SA, Avenevoli S, Cui L, et al. , 2010. Lifetime prevalence of mental disorders in U.S. adolescents: results from the National Comorbidity Survey Replication–adolescent Supplement (NCS-A). J. Am. Acad. Child Adolesc. Psychiatry 49 (10), 980–989. 10.1016/j.jaac.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris AS, Cui L, Criss MM, Simmons WK, 2018. Emotion Regulation Dynamics during Parent–Child Interactions: Implications for Research and Practice In Emotion Regulation. Routledge, pp. 88–108. [Google Scholar]
- Morris AS, Silk JS, Steinberg L, Myers SS, Robinson LR, 2007. The role of the family context in the development of emotion regulation. Soc. Dev. 16 (2), 361–388. 10.1111/j.1467-9507.2007.00389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy FC, Nimmo-Smith I, Lawrence AD, 2003. Functional neuroanatomy of emotions: a meta-analysis. Cogn. Affect. Behav. Neurosci. 3 (3), 207–233. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JD, Gross JJ, 2004. For better or for worse: neural systems supporting the cognitive down-and up-regulation of negative emotion. NeuroImage 23 (2), 483–499. [DOI] [PubMed] [Google Scholar]
- Olesen J, Gustavsson A, Svensson M, Wittchen HU, Jönsson B, Group CS, Council EB, 2012. The economic cost of brain disorders in Europe. Eur. J. Neurol. 19 (1), 155–162. [DOI] [PubMed] [Google Scholar]
- Petersen AC, Compas BE, Brooks-Gunn J, Stemmler M, Ey S, Grant KE, 1993. Depression in adolescence. Am. Psychol. 48 (2), 155. [DOI] [PubMed] [Google Scholar]
- Phan KL, Sripada CS, 2013. Emotion regulation In: Armony J, Vuilleumier P (Eds.), The Cambridge Handbook of Human Affective Neuroscience. Cambridge University Press, New York, NY. [Google Scholar]
- R Core Team, 2017. R: a Language and Environment for Statistical Computing. www.R-project.org. [Google Scholar]
- Raichle ME, 2015. The brain’s default mode network. Annu. Rev. Neurosci. 38, 433–447. [DOI] [PubMed] [Google Scholar]
- Reddy MS, 2010. Depression: the disorder and the burden. Indian J. Psychol. Med. 32 (1), 1–2. 10.4103/0253-7176.70510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romund L, Raufelder D, Flemming E, Lorenz RC, Pelz P, Gleich T, et al. , 2016. Maternal parenting behavior and emotion processing in adolescents—an fMRI study. Biol. Psychol. 120, 120–125. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN, et al. , 2003. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol. Psychiatry 54 (5), 573–583. [DOI] [PubMed] [Google Scholar]
- Scaramella LV, Leve LD, 2004. Clarifying parent–child reciprocities during early childhood: the early childhood coercion model. Clin. Child Fam. Psychol. Rev. 7 (2), 89–107. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Janavs J, Weiller E, Keskiner A, et al. , 1997. The validity of the Mini International Neuropsychiatric Interview (MINI) according to the SCID-P and its reliability. Eur. Psychiatry 12 (5), 232–241. [Google Scholar]
- Sheehan DV, Sheehan KH, Shytle RD, Janavs J, Bannon Y, Rogers JE, et al. , 2010. Reliability and validity of the mini international neuropsychiatric interview for children and adolescents (MINI-KID). J. Clin. Psychiatry. [DOI] [PubMed] [Google Scholar]
- Shin LM, Liberzon I, 2010. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology 35 (1), 169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk JS, Lee KH, Elliott RD, Hooley JM, Dahl RE, Barber A, Siegle GJ, 2017. ‘Mom—I don’t want to hear it’: brain response to maternal praise and criticism in adolescents with major depressive disorder. Soc. Cogn. Affect. Neurosci. 12 (5), 729–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk JS, Shaw DS, Skuban EM, Oland AA, Kovacs M, 2006. Emotion regulation strategies in offspring of childhood-onset depressed mothers. J. Child Psychol. Psychiatry 47 (1), 69–78. [DOI] [PubMed] [Google Scholar]
- Southwick SM, Charney DS, 2012. The science of resilience: implications for the prevention and treatment of depression. Science 338 (6103), 79–82. [DOI] [PubMed] [Google Scholar]
- Spence SH, Najman JM, Bor W, O’Callaghan MJ, Williams GM, 2002. Maternal anxiety and depression, poverty and marital relationship factors during early childhood as predictors of anxiety and depressive symptoms in adolescence. J. Child Psychol. Psychiatry 43 (4), 457–469. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P, 1988. Co-planar Stereotaxic Atlas of the Human Brain: 3-dimensional Proportional System: an Approach to Cerebral Imaging. [Google Scholar]
- Taylor SF, Liberzon I, 2007. Neural correlates of emotion regulation in psychopathology. Trends Cogn. Sci. 11 (10), 413–418. [DOI] [PubMed] [Google Scholar]
- Thoma P, Bellebaum C, 2012. Your error’s got me feeling–how empathy relates to the electrophysiological correlates of performance monitoring. Front. Hum. Neurosci. 6, 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RA, Goodman M, 2010. Development of emotion regulation In: Kring AM, Sloan DM (Eds.), Emotion Regulation and Psychopathology: A Transdiagnostic Approach to Etiology and Treatment. The Guilford Press, New York, NY, pp. 38–58. [Google Scholar]
- Troy AS, Mauss IB, 2011. Resilience in the face of stress: emotion regulation as a protective factor. Resilience Mental Health: Challenges 1 (2), 30–44. [Google Scholar]
- Waller JM, Silk JS, Stone LB, Dahl RE, 2014. Co-rumination and co-problem-solving in the daily lives of adolescents with major depressive disorder. J. Am. Acad. Child Adolesc. Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg A, Olvet DM, Hajcak G, 2010. Increased error-related brain activity in generalized anxiety disorder. Biol. Psychol. 85 (3), 472–480. [DOI] [PubMed] [Google Scholar]
- Weissman DH, Perkins A, Woldorff MG, 2008. Cognitive control in social situations: a role for the dorsolateral prefrontal cortex. NeuroImage 40 (2), 955–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M, Kujawa A, Lu LH, Fitzgerald DA, Klumpp H, Fitzgerald KD, et al. , 2016. Age-related changes in amygdala-frontal connectivity during emotional face processing from childhood into young adulthood. Hum. Brain Mapp. 37 (5), 1684–1695. 10.1002/hbm.2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarkoni T, 2009. Big correlations in little studies: inflated fMRI correlations reflect low statistical power—commentary on Vul et al. (2009). Perspect. Psychol. Sci. 4 (3), 294–298. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.