Understanding the intersection of the biology of pain, opioid therapy, and disease progression and survival is complicated, and potentially of great importance in cancer care. Pain occurs in two-thirds of patients with advanced malignancy, and continues to impact a third of cancer patients even after completion of curative treatment [61]. Pain and other symptoms affect quality of life (QoL) during and after treatment [5,43,48,59]. In addition, pain itself has been associated with shorter survival in diverse malignancies [72]. As long as the benefits clearly outweigh risks, and opioids are safe and effective for cancer-related pain, this therapy is an important option [9,46]. Like all medical drugs, though, opioids have unavoidable adverse effects. These include constipation, nausea, sedation, cardiovascular instability, respiratory depression, central nervous system toxicity, hyperalgesia, tolerance, and opioid use disorder [46]. Such risks are well understood and typically discussed with patients. On the other hand, there is less awareness about the potential adverse effects of pain and opioids on the growth of certain cancers. Here we review the evidence regarding opioid therapy, disease progression, and survival to raise awareness and stimulate greater dialogue regarding these issues among stakeholders in the cancer and pain communities. By doing so, our goal is that cancer patients will be better positioned to address their co-existing priorities of pain relief and survival.
The analgesic activity of opioids is mediated via mu opioid receptors (MORs) in the central nervous system. MORs are also present on endothelial cells [33] and in human tumors (peripheral MORs), including prostate and lung cancer [22,39,40,52]. Pre-clinical studies show that opioids promote angiogenesis by stimulating endothelial proliferation and migration and by activating survival- and growth-promoting signaling via protein kinase B (Akt) and mitogen-activated protein kinase (MAPK) pathways, respectively in the endothelium (Fig. 1) [16,24,36]. In addition to promoting tumor angiogenesis, chronic morphine treatment also stimulates lymphangiogenesis, activates mast cells, promotes tumor growth and metastasis, impairs survival in mouse models of breast cancer [2,33,45], and is immunosuppressive [47,62]. Further, MORs and receptor tyrosine kinases (RTKs) are expressed and co-localized in advanced lung cancer, which may play a role in cancer growth and spread [39]. Preclinical studies provide strong evidence that in animal models of several different malignancies, activation of peripheral MORs (on endothelial cells and tumors) by clinically used opioid medications promotes tumor progression via several different mechanisms. These involve signal transducers and activators of transcription 3 (STAT3), MAPK/extracellular signal-regulated kinase (ERK) and Akt signaling pathways, nitric oxide synthesis, cyclo-oxygenase (COX)-2 activation, prostaglandin E2 production, cross-activation of epidermal growth factor receptor and vascular endothelial growth factor receptor 2 (VEGFR2), release of substance P, and mast cell activation [8,13,24,27,33,36,40]. Our recent findings on morphine-induced retinal neovascularization in mice with sickle cell disease further validate the role of morphine in promoting angiogenesis via co-activation of VEGFR2 and the contribution of inflammatory cytokines and the STAT3 pathway in stimulating expression of endothelial MOR [24]. Heightened inflammation as well as activation of VEGFR2 and STAT3 signaling are the rule, not the exception, in most cancers. Importantly, opioids via MORs contribute to epithelial mesenchymal transformation in lung cancer, a process critical for progression of this cancer [35]. Recent clinical studies raise the possibility that these mechanisms may play a role in both cancer progression and nociception in patients. These mechanistic insights also provide targets for intervention to ameliorate the inadvertent effect of opioids on cancer progression and QoL (Fig. 1).
Figure 1. Mechanisms of opioid activity in cancer.
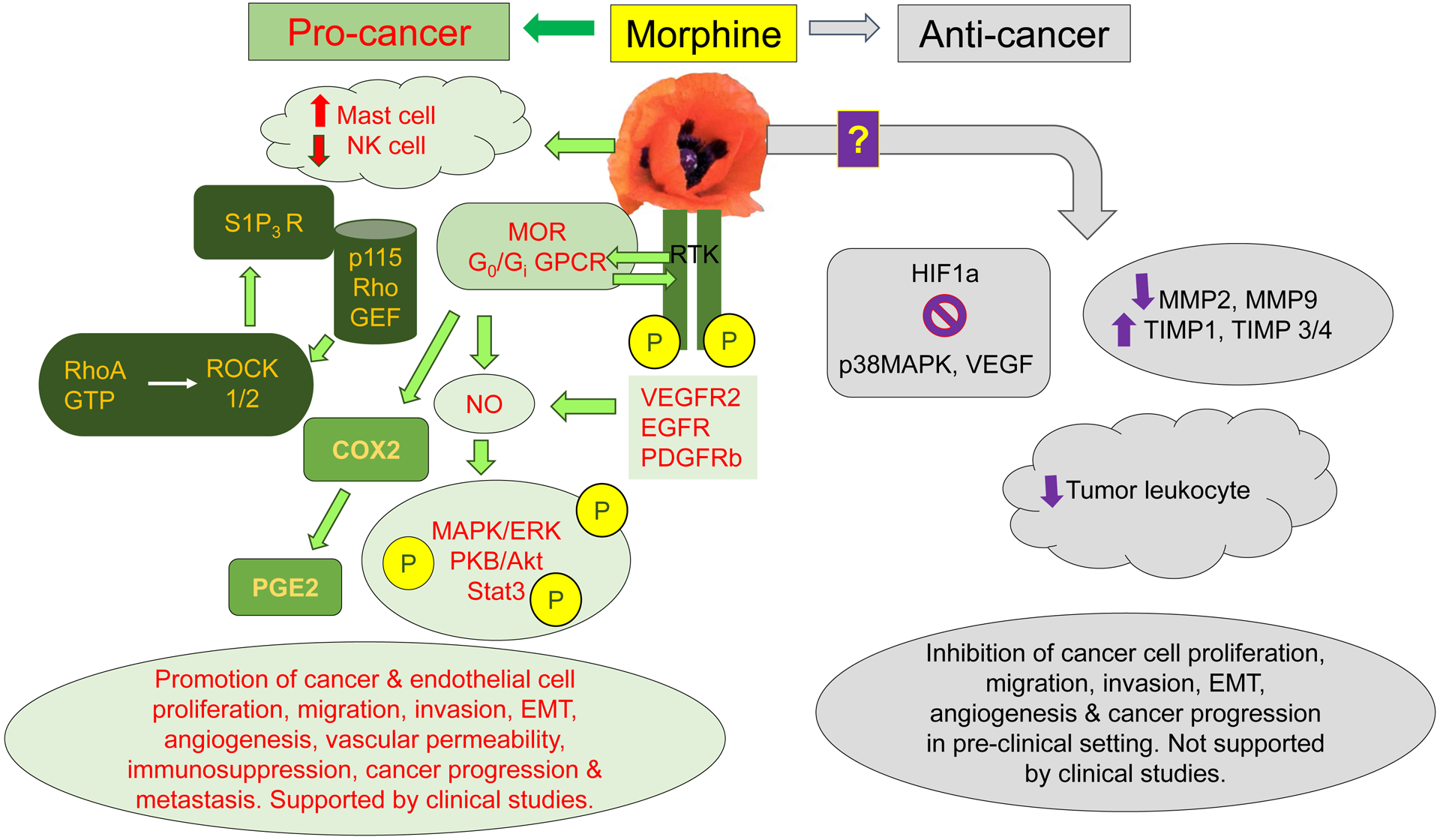
Pre-clinical and clinical studies demonstrate multiple signaling pathways and cellular effects stimulated by morphine and/or MOR leading to progression of cancer and metastasis (left side). MOR directly and via co-activation of RTKs for growth factors, VEGFR2, EGFR, and PDGFRβ, stimulates mitogenic and survival-promoting signaling via MAPK/ERK, Stat3, and PKB/Akt in endothelial and/or tumor cells. Simultaneously, morphine activates S1P3R via Rho/ROCK pathway following the recruitment of p115 Rho GEF by MOR, leading to increased vascular permeability. Inhibition of NK cells and activation of mast cells by morphine further abrogates protective anti-tumor effects and simultaneous release of pro-cancer cytokines and neuropeptides such as substance P, respectively. Additionally, stimulation of COX2 leads to formation of PGE2, which has pro-angiogenic and pro-nociceptive activity, and thus may even increase pain. Together, morphine/opioid induced cellular effects and signaling pathways lead to endothelial and tumor cell proliferation, migration, invasion and EMT, immunosuppression and increased vascular permeability which is critical to tumor cell infiltration and metastasis, thus promoting cancer progression and metastasis. While most of the strong evidence is from human tumor and endothelial cells and mouse models of cancer and metastasis, there is emerging data from clinical studies (mostly retrospective) showing the association of MOR with these signaling pathways and/or cellular activation in lung, prostate, and pancreatic cancer, leading to cancer progression and shorter survival. Conversely, anti-tumor effects of morphine/opioids via modulation of HIF1a, p38 MAPK, VEGF, MMPs, and TIMPs in endothelial and/or tumor cells lead to inhibition of cancer progression in mice. However, the only study in a clinical setting failed to replicate the pre-clinical observations on MMPs.
Abbreviations: COX2, cyclooxygenase 2; EGFR, epidermal growth factor receptor; ERK, extracellular signal regulated kinase; GEF, guanine nucleotide exchange factor; GPCR, G-protein coupled receptor; HIF1α, hypoxia inducible factor 1 α; MAPK, mitogen activated protein kinase; MOR, mu opioid receptor; NK cell, natural killer cell; NO, nitric oxide; PDGFR, platelet-derived growth factor; PGE2, prostaglandin E2; ROCK, rho-associated protein kinase; RTK, receptor tyrosine kinase; S1P3R, sphingosine 1 phosphate receptor 3; Stat3, signal transducer and activator of transcription 3; TIMP, tissue inhibitor of metalloprotease; VEGF, vascular endothelial growth factor; VEGFR2, vascular endothelial growth factor receptor 2.
Pain also increases circulating endogenous opioid (e.g., endorphin) levels and causes significant immunosuppression [47,62]. Therefore, it is possible that even in patients who are not receiving opioid medications, elevated levels of endogenous opioids may activate peripheral MORs and cross-activate signaling pathways that influence tumor progression. Patients with inadequate pain control after major surgery also develop significant immunosuppression [62]. These mechanisms may contribute to the known adverse prognostic effect of pain in malignancies [43,50,59,72].
Several lines of evidence suggest that pain, systemic opioid exposure, and opioid receptor activity may be associated with adverse cancer outcomes in humans. In early stage cancers (primarily those treated with potentially curative surgery), some retrospective studies reported that exposure to systemic opioids during anesthesia for cancer surgery was associated with a higher incidence of recurrence and metastases [14], but other studies noted no such association [6]. Opioid reduction strategies with multimodal analgesia have been investigated as a potential means to reduce cancer recurrence. These strategies included administration of non-steroidal anti-inflammatory drugs or COX-2 inhibitors (e.g. celecoxib), which are known to have significant anticancer effects [17,18]. Celecoxib slightly reduced perioperative cyclooxygenase activity during cancer surgery and lower postoperative pain scores [29]. Complementary to these observations, celecoxib ameliorated the proangiogenic effect of morphine in a breast cancer model while reducing hyperalgesia compared to morphine [16]. Dexmedetomidine has been successfully included in protocols designed to reduce perioperatiave opioid comsumption. Two recent investigations in animals and humans have indicated a potential negative impact of dexmedetomidine in the context of cancer [7,34]. Yet, this drug has been shown to induce apoptosis in oral squamous carcinoma and glioblastoma cells and to have significant anti-inflammatory effects when given in cancer surgery, which could potentially offest the negative effects of inflammation in cancer progression [13,60]. Ketamine and gabapentinoids can affect survival, proliferation and metastatic activities in cancer cells, and modulation of the immune system. While there is no evidence to recommend the use of any of these adjuvant analgesics to reduce cancer following oncologic surgery or any other clinical setting [42], further research has been recommended [29]. In addition, it has been hypothesized that reduction of opioid consumption by implementation of regional anesthesia during oncologic surgery would be associated with lower rates of cancer recurrence or longer recurrence-free survival [42]. Following an initial report in 2006 that use of regional anesthesia was associated with a significant decrease in breast cancer recurrence after mastectomy [15], more than 20 retrospective studies have reported conflicting results. Accordingly, a recent consensus article by the American and European Societies of Regional Anesthesia concluded that there is insufficient evidence to recommend regional anesthesia to reduce cancer recurrence [42].
In patients with advanced, metastatic cancers, retrospective studies reported that higher MOR expression is associated with poorer clinical outcomes in advanced cancers of the prostate [70], lung [39], stomach [67], and esophagus [69], and that impaired opioid receptor activity is associated with longer survival in breast cancer [4]. In the first study to simultaneously examine the association of quantitative long-term opioid use and level of MOR expression with cancer outcomes, we reported that higher MOR expression and greater opioid requirement are independently associated with shorter progression-free and overall survival in patients with metastatic prostate cancer receiving first-line androgen deprivation therapy [70]. We also found that chronic pain and greater opioid requirement early after diagnosis are independently associated with shorter survival in advanced non-small cell lung cancer [71]. However, the retrospective clinical studies had limitations, including some that used analgesic intake as an indicator for cancer pain; thus making it difficult to determine whether pain or opioid therapy was more relevant in impacting survival. Some prospective studies indicate that reducing systemic opioid exposure may improve survival in advanced cancer; for example, by celiac plexus block in pancreatic cancer [38] and administration of opioids via intrathecal pump in various malignancies [55]. Retrospective studies also reported that pain is independently associated with shorter survival in metastatic prostate [1,10,11,25,56], lung [28], and various other malignancies [72].
In contrast, other basic and pre-clinical studies suggest a potentially protective effect of certain opioids in cancers. For example, methadone increases sensitivity of some cancer cell lines to chemotherapy. Methadone is a racemic mixture of the two enantiomeric forms, d-methadone and l-methadone, with the l form having 10 times more affinity to MOR than the d form. It is a unique opioid with agonist activity at the mu and delta opioid receptors, and antagonist activity at the N-methyl-D-aspartate receptor [58]. Methadone appears to have an anti-cancer effect by activating apoptosis pathways by caspase activation and downregulation of X-linked apoptosis protein and B-cell lymphoma-extra-large [19]. Methadone also overcomes apoptosis resistance and chemotherapy resistance in leukemic cells by activating apoptosis pathways [20], and may sensitize glioma cells to doxorubicin [19]. Other in vitro experiments showed that high concentrations of MOR agonists such as morphine (1–10 μM) and D,L-methadone (1–10 μg/mL) had direct pro-apoptotic effects on gastric cancer, leukemia, and glioblastoma cells [19,21,49,57]. Similar effects were observed in animals: chronic treatment with fentanyl (>14 days, 0.4 mg/kg, twice the analgesic dose) suppressed tumor growth in mice implanted with a gastric cancer cell line [27]. In addition, very high doses of morphine (30 mg/kg vs 0.5–5 mg/kg) induced tumor shrinkage via anti-angiogenesis in a mouse model of lung cancer [32]. Also, in vitro and in vivo studies have shown that MOR agonists inhibited metastatic mechanisms in breast and colon cancer cells. Some anti-metastatic effects were linked to inhibition in production and release of matrix metalloproteinase (MMP)-2 and −9 and adhesion molecules including intercellular adhesion molecule-1,vascular cell adhesion molecule-1, and E-selectin) [23,26,27,41,65]. However, in a clinical setting, there was no correlation between perioperative morphine intake and MOR activation or MMPs [66]. Further, although many patients with advanced cancers receive high doses of opioids for long periods of time, opioids have not been reported to be associated with tumor regression or inhibition of metastasis.
Another line of research has targeted MORs for anticancer therapy. Preclinical studies demonstrated that MOR antagonists inhibited tumorigenic and metastatic behaviors in colorectal and lung cancer cells, and synergistically potentiated the anti-angiogenic effects of chemotherapeutic agents such as 5-fluorouracil, bevacizumab, and mammalian target of rapamycin inhibitors via protein tyrosine phosphatase receptor signaling [37,51,52,64]. In animals, low doses of naltrexone enhanced the anti-cancer effects of cisplatin in a mouse model of ovarian cancer [12]. In vivo studies are in agreement with the antitumoral effects of MOR antagonists not only in cancer cells but also in cells of the tumor microenvironment. As an example, the MOR antagonist CTOP (D-Phe-Cys-Tyr-D-Trp-Orn-Thr-Pen-Thr-NH2) reverses the immune-suppressive effects of opioids [3]. One clinical study has reported (in a post-hoc analysis) the association between the administration of a MOR antagonist, methylnaltrexone, and overall survival of patients with advanced malignancies. This study included patients from two randomized controlled trials designed to assess the efficacy of methylnaltrexone vs placebo for treatment of opioid-induced constipation. Methylnaltrexone administration was associated with significantly longer overall survival in cancer patients, but not in non-cancer patients. This finding suggests that the beneficial effect of opioids on overall survival may be specific to cancer pathogenesis [30].
In summary, although preclinical evidence linking opioids to cancer growth appears robust, whether and how this phenomenon may be occurring in humans requires further investigation. What may be true in theory or in laboratory and animal studies may be quite different from phenomena occurring in humans. Further, most of the preclinical data are for morphine, whereas other opioids such as hydromorphone, methadone, or fentanyl, which are often used to treat pain in advanced malignancies, have not been investigated as extensively. Taken as a whole, the current status of understanding of adverse effects of pain and opioid therapy on tumor progression and overall survival is complicated and not definitive. In fact, we do not even have the first series of clinical trials on opioids and disease progression to guide us. In existing studies, it is difficult to determine the specific contribution of opioids to overall results and outcomes. This is an important area for future research.
Current awareness of the opioid epidemic has led to initiatives aimed at discovering non-opioid analgesics. However, many recent non-pharmacologic strategies such as deep brain stimulation and spinal cord stimulation function by releasing endogenous opioids. Therefore, the roles of pharmacologic as well as endogenous opioids need to be investigated in prospective clinical studies, to identify their inadvertent effects and develop specific preventive strategies (Fig. 2).
Figure 2.
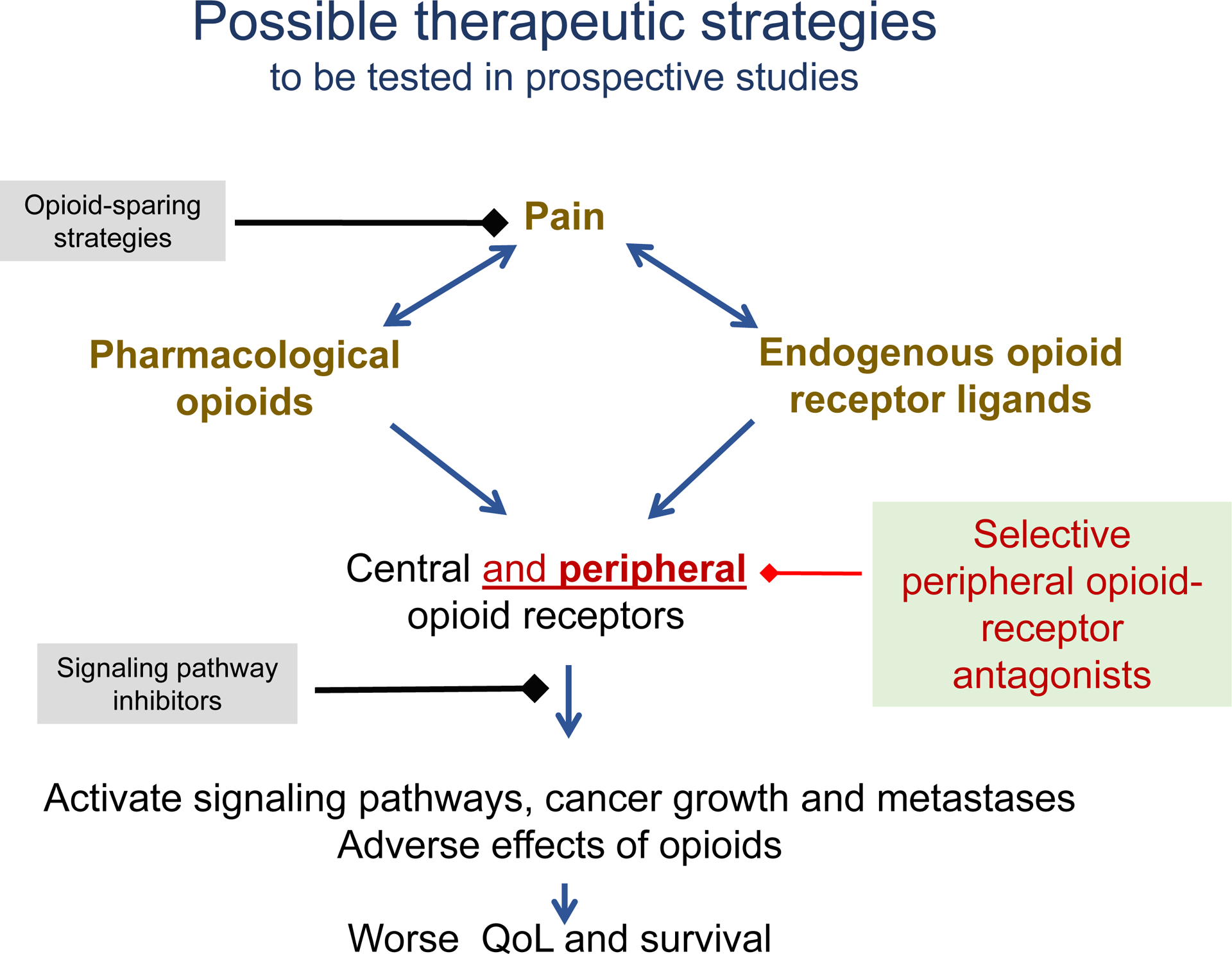
Proposed mechanism-based interventions to ameliorate opioid-induced cancer progression.
Taken together, these findings lead potentially to both a practical and ethical predicament. It is not conscionable to undertreat pain or to not consider existing research on potential adverse effects of opioid therapy on cancer progression and overall survival. A delicate balance needs to be accomplished between treating pain with opioids to improve functional status and tolerability of cancer treatment while preventing over-prescribing of opioids which may cause undesirable side effects and potentially affect cancer outcomes. Providers will need to remain updated about the rapidly evolving evidence base regarding the potential adverse effects of pain or opioid therapy in patients with advanced malignancies.
It is understandable that the lack of definitive findings about the adverse roles of pain and opioid therapy may be challenging for patients and physicians when deciding on pain management strategies. Given the primarily preclinical and emerging clinical evidence base regarding opioids, pain, and survival, it may be premature to discuss such information in great detail when obtaining initial informed consent for pain treatment. Reduction of patient suffering remains the foremost treatment goal, together with the ethical obligation to provide sufficiently-informed, patient-centered care. When safe and appropriate, providers can recommend the use of non-opioid therapies and other pain management interventions, and utilize opioids sparingly and for a short period, in early stage curable cancer where pain is usually due to cancer treatment or related to non-cancer conditions [46,48]. On the other hand, patients with advanced cancer, who experience pain related to progressive cancer, may be enrolled in studies testing strategies to reduce the potential adverse impact of long-term opioids on tumor progression. One such ongoing study (ClinicalTrials.gov #03087708) is the first prospective, multi-center, randomized, placebo controlled, double blind study testing whether a peripheral opioid receptor antagonist improves QOL and cancer outcomes in patients with advanced non-small cell lung cancer.
It is our hope that the relevant safety and ethical obligations of pain management and opioid therapy among cancer patients will be openly and transparently discussed among the diverse stakeholders, including patients, health-care providers, regulatory and governmental agencies, pharmaceutic industry and other interest groups. It is also our hope that prospective studies will investigate the efficacy of opioid-sparing strategies and novel non-opioid analgesics. We recommend that opioid use be recorded for all cancer patients, including (a) the etiology of pain for which opioids are required (i.e., cancer vs. non-cancer pain [46,48], (b) the specific opioids and doses taken, (c) whether opioids were taken prior to a diagnosis of malignancy and, if so, for how long and for what condition, and (d) the doses, effectiveness, and tolerability of opioids taken on an ongoing basis after diagnosis of cancer. Such a registry would contain data generated during the course of active cancer treatment and survivorship and could provide information about opioid use and tumor growth. Registry data have informed acute [68] and chronic fields of pain medicine [44,54,63]. By doing the same in cancer pain, we hope there will be improved understanding of the roles of pain and opioid therapy on tumor progression and overall survival. Until such prospective data are available, opioids should continue to be used as needed to control cancer pain adequately.
Funding sources:
No funding sources for Drs. Novy, Nelson, Koyyalagunta, and Cata; Dr. Pankaj Gupta received support from the Veterans Health Administration; Dr. Kalpna Gupta is thankful to NIH/NHLBI 1U01 HL117664-01 for funding.
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of Interest: No conflict of interest for Drs. Novy, Nelson, Koyyalagunta, Cata, and Pankaj Gupta; Dr. Kalpna Gupta is a consultant for Novartis and Tautona group and has received funding for drug research from 1910 Genetics and Grifols.
References
- [1].Armstrong AJ, Garrett-Mayer E, Ou Yang YC, Carducci MA, Tannock I, de Wit R, Eisenberger M. Prostate-specific antigen and pain surrogacy analysis in metastatic hormone-refractory prostate cancer. J Clin Oncol 2007; 25:3965–70. [DOI] [PubMed] [Google Scholar]
- [2].Bimonte S, Barbieri A, Rea D, Palma G, Luciano A, Cuomo A, Arra C, Izzo F. Morphine promotes tumor angiogenesis and increases breast cancer progression. BioMed Res Int 2015:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Börner C, Warnick B, Smida M, Hartig R, Lindquist JA, Schraven B, Höllt V, Kraus J. Mechanisms of opioid-mediated inhibition of human T cell receptor signaling. J Immunol 2009;183:882–9. [DOI] [PubMed] [Google Scholar]
- [4].Bortsov AV, Millikan RC, Belfer I, Boortz-Marx RL, Arora H, McLean SA. Mu-opioid receptor gene A118G polymorphism predicts survival in patients with breast cancer. Anesthesiol 2012;116:896–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Breivik H, Cherny B, Collett F, de Conno F, Filbet M, Foubert AJ, Cohen R, Dow L. Cancer-related pain: a pan-European survey of prevalence, treatment, and patient attitudes. Ann Oncol 2009;20:1420–33. [DOI] [PubMed] [Google Scholar]
- [6].Cata JP, Gottumukkala V, Thakar D, Keerty D, Gebhardt R, Liu DD. Effects of postoperative epidural analgesia on recurrence-free and overall survival in patients with nonsmall cell lung cancer. J Clin Anesth. 2014;26(1):317–23. [DOI] [PubMed] [Google Scholar]
- [7].Cata JP, Singh V, Lee BM, Villarreal J, Mehran JR, Yu J, Gottumukkala V, Lavon H, Ben-Eliyahu S. Intraoperative use of dexmedetomidine is associated with decreased overall survival after lung cancer surgery. J Anaesthesiol Clin Pharmacol 2017;33:317–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Chen C, Farooqui M, Gupta K. Morphine stimulates vascular endothelial growth factor-like signaling in mouse retinal endothelial cells. Curr Neurovasc 2006; Res 3:171–80. [DOI] [PubMed] [Google Scholar]
- [9].Connor SR, Pyenson B, Fitch K, Spence C, Iwasaki K. Comparing hospice and non-hospice patient survival among patients who die within a three-year window. J Pain Symptom Manage 2007;33(3):238–46. [DOI] [PubMed] [Google Scholar]
- [10].de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, Chi KN, Jones RJ, Goodman OB Jr, Saad F, Staffurth JN, Mainwaring P, Harland S, Flaig TW, Hutson TE, Cheng T, Patterson H, Hainsworth JD, Ryan CJ, Sternberg CN, Ellard SL, Fléchon A, Saleh M, Scholz M, Efstathiou E, Zivi A, Bianchini D, Loriot Y, Chieffo N, Kheoh T, Haqq CM, Scher HI. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med 2011;364:1995–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].de Bono JS, Oudard S, Ozguroglu M, Hansen S, Machiels JP, Kocak I, Gravis G, Bodrogi I, Mackenzie MJ, Shen L, Roessner M, Gupta S, Sartor AO; TROPIC Investigators. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomized open-label trial. Lancet 2010;376:1147–54. [DOI] [PubMed] [Google Scholar]
- [12].Donahue RN, McLaughlin PJ, Zagon IS. Low-dose naltrexone suppresses ovarian cancer and exhibits enhanced inhibition in combination with cisplatin. Exp Bio Med 2011;236:883–95. [DOI] [PubMed] [Google Scholar]
- [13].Dong W, Chen MH, Yang YH, Zhang X, Huang MJ, Yang XJ, Wang HZ. The effect of dexmedetomidine on expressions of inflammatory factors in patients with radical resection of gastric cancer. Eur Rev Med Pharmacol Sci 2017;21(15):3510–5. [PubMed] [Google Scholar]
- [14].Du KN, Feng L, Newhouse A, Mehta J, Lasala J, Mena GE, Hofstetter WL, Cata JP. Effects of intraoperative opioid use on recurrence-free and overall survival in patients with esophageal adenocarcinoma and squamous cell carcinoma. Anesth Analg. 2018;127(1):210–16. [DOI] [PubMed] [Google Scholar]
- [15].Exadaktylos AK, Buggy DJ, Moriarty DC, Mascha E, Sessler DI. Can anesthetic technique for primary breast cancer surgery affect recurrence or metastasis? Anesthesiol 2006;105:660–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Farooqui M1, Li Y, Rogers T, Poonawala T, Griffin RJ, Song CW, Gupta K. COX-2 inhibitor celecoxib prevents chronic morphine-induced promotion of angiogenesis, tumor growth, metastasis and mortality, without compromising analgesia. Br J Cancer 2007;97:1523–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Forget P, Machiels JP, Coulie PG, Berliere M, Poncelet AJ, Tombal B, Stainier A, Legrand C, Canon JL, Kremer Y, De Kock M. Neutrophil: lymphocyte ratio and intraoperative use of ketorolac or diclofenac are prognostic factors in different cohorts of patients undergoing breast, lung, and kidney cancer surgery. Ann Surg Oncol 2013;20(S3),650–60. [DOI] [PubMed] [Google Scholar]
- [18].Forget P, Vandenhende J, Berliere M, Machiels JP, Nussbaum B, Legrand C, De Kock M. Do intraoperative analgesics influence breast cancer recurrence after mastectomy? a retrospective analysis. Anesth Analg 2010;110:1630–5. [DOI] [PubMed] [Google Scholar]
- [19].Friesen C, Hormann I, Roscher M, Fichtner I, Alt A, Hilger R, Debatin KM, Miltner E. Opioid receptor activation triggering downregulation of cAMP improves effectiveness of anti-cancer drugs in treatment of glioblastoma. Cell Cycle 2014;13:1560–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Friesen C, Roscher M, Alt A, Miltner E. Methadone, commonly used as maintenance medication for outpatient treatment of opioid dependence, kills leukemia cells and overcomes chemoresistance. Cancer Res 2008;68:6059–64. [DOI] [PubMed] [Google Scholar]
- [21].Friesen C, Roscher M, Hormann I, Fichtner I, Alt A, Hilger RA, Debatin KM, Miltner E. Cell death sensitization of leukemia cells by opioid receptor activation. Oncotarget 2013;4(5):677–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Fujioka N, Nguyen J, Chen C, Li Y, Pasrija T, Niehans G, Johnson KN, Gupta V, Kratzke RA, Gupta K. Morphine-induced epidermal growth factor pathway activation in non-small cell lung cancer. Anesth Analg 2011;113:1353–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Gach K, Szemraj J, Wyrębska A, Janecka A. The influence of opioids on matrix metalloproteinase-2 and −9 secretion and mRNA levels in MCF-7 breast cancer cell line. Mol Biol Rep 2010;38(2):1231–6. [DOI] [PubMed] [Google Scholar]
- [24].Gupta K, Chen C, Lutty G, Hebbel RP. Morphine promotes neovascularizing retinopathy in transgenic sickle mice. Blood Advances (In Press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Halabi S, Vogelzang NJ, Kornblith AB, Ou SS, Kantoff PW, Dawson NA, Small EJ. Pain predicts overall survival in men with metastatic castration-refractory prostate cancer. J Clin Oncol 2008; 26:2544- [DOI] [PubMed] [Google Scholar]
- [26].Harimaya Y, Koizumi K, Andoh T, Nojima H, Kuraishi Y, Saiki I. Potential ability of morphine to inhibit the adhesion, invasion and metastasis of metastatic colon 26-L5 carcinoma cells. Cancer Lett 2002;187(1–2):121–7. [DOI] [PubMed] [Google Scholar]
- [27].He G, Li LI, Guan E, Chen J, Qin YI, Xie Y. Fentanyl inhibits the progression of human gastric carcinoma MGC-803 cells by modulating NF-kappaB-dependent gene expression in vivo. Onc Lett 2016;12(1):563–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Herndon JE 2nd, Fleishman S, Kornblith AB, Kosty M, Green MR, Holland J. Is quality of life predictive of the survival of patients with advanced non-small cell lung carcinoma? Cancer 1999; 85:333- [DOI] [PubMed] [Google Scholar]
- [29].Hiller JG, Sampurno S, Millen R, Kuruvilla N, Ho KM, Ramsay R, Riedel B. Impact of celecoxib on inflammation during cancer surgery: a randomized clinical trial. Can J Anaesth 2017;64(5):497–505. [DOI] [PubMed] [Google Scholar]
- [30].Janku F, Johnson LK, Karp DD, Atkins JT, Singleton PA, Moss J. Treatment with methylnaltrexone is associated with increased survival in patients with advanced cancer. Ann Oncol 2016;27:2032–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Jarvik JG, Comstock BA, Heagerty PJ, Turner JA, Sullivan SD, Shi X, Nerenz DR, Nedeljkovic SS, Kessler L, James K, Friedly JL, Bresnahan BW, Bauer Z, Avins AL, Deyo RA. Back pain in seniors: the Back pain Outcomes using Longitudinal Data (BOLD) cohort baseline data. BMC Musculoskelet Disord 2014;23;15:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Koodie L, Ramakrishnan S, Roy S. Morphine suppresses tumor angiogenesis through a HIF-1alpha/p38MAPK pathway. Am J Pathol 2010;177(2):984–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Kshirsagar S, Gupta K, Chang L, Schwartz R, Law PY, Yee D, Hebbel RP. Morphine stimulates angiogenesis by activating proangiogenic and survival-promotion signaling and promotes breast tumor growth. Cancer Res 2002;62:4491–8. [PubMed] [Google Scholar]
- [34].Lavon H, Matzner P, Benbenishty A, Sorski L, Rossene E, Haldar R, Elbaz E, Cata JP, Gottumukkala V, Ben-Eliyahu S. Dexmedetomidine promotes metastasis in rodent models of breast, lung, and colon cancers. Br J Anaesth 2018;120:188–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Lennon FE, Mirzapoiazova T, Mambetsariev B, Poroyko VA, Salgia R, Moss J, Singleton PA.: The mu opioid receptor promotes opioid and growth factor-induced proliferation, migration and epithelial mesenchymal transition (EMT) in human lung cancer. PLoS One 2014;9:e91577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Lennon FE, Mirzapoiazova T, Mambetsariev B, Salgia R, Moss J, Singleton PA. Overexpression of the μ-opioid receptor in human non-small cell lung cancer promotes Akt and mTOR activation, tumor growth, and metastasis. Anesthesiol 2012;116:857–67. [DOI] [PubMed] [Google Scholar]
- [37].Lennon FE, Moss J, Singleton PA.: The μ-opioid receptor in cancer progression: is there a direct effect? Anesthesiol 2012;116:940–5. [DOI] [PubMed] [Google Scholar]
- [38].Lillemoe KD, Cameron JL, Kaufman HS, Yeo CJ, Pitt HA, Sauter PK. Chemical splanchnicectomy in patients with unresectable pancreatic cancer. a prospective randomized trial. Ann Surg 1993;217:447- [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Madar I, Bencherif B, Lever J, Heitmiller RF, Yang SC, Brock M, Brahmer J, Ravert H, Dannals R, Frost JJ. Imaging delta- and mu-opioid receptors by PET in lung carcinoma patients. J Nucl Med 2007;48:207–13. [PubMed] [Google Scholar]
- [40].Mathew B, Lennon FE, Siegler J, Mirzapoiazova T, Mambetsariev N, Sammani S, Gerhold LM, LaRiviere PJ, Chen CT, Garcia JG, Salgia R, Moss J, Singleton PA. The novel role of the mu opioid receptor in lung cancer progression: a laboratory investigation. Anes Analg 2011;112:558–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Min TJ, Park S-H, Ji Y-H, Lee Y-S, Kim TW, Kim JH, Kim W-Y, Park Y-C. Morphine attenuates endothelial cell adhesion molecules induced by the supernatant of LPS-stimulated colon cancer cells. J Korean Med Sci 2011;26(6):747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Missair A, Cata JP, Votta-Velis G, Johnson M, Borgeat A, Tiouririne M, Gottumukkala V, Buggy D, Vallejo R, Marrero EB, Sessler D, Huntoon MA, Andres J, Casasola OL. Impact of perioperative pain management on cancer recurrence: an ASRA/ESRA special article. Reg Anesth Pain Med 2019;44:13–28. [DOI] [PubMed] [Google Scholar]
- [43].Montazeri A Quality of life data as prognostic indicators of survival in cancer patients: an overview of the literature from 1982 to 2008. Health Qual Life Outcomes 2008;7:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Moulin DE, Clark AJ, Gordon A, Lynch M, Morley-Forster PK, Nathan H, Smyth C, Toth C, VanDenKerkhof E, Gilani A, Ware MA. Long-term outcome of the management of chronic neuropathic pain: a prospective observational study. J Pain 2015;16(9):852–61. [DOI] [PubMed] [Google Scholar]
- [45].Nguyen J, Luk K, Vang D, Soto W, Vincent L, Robiner S, Saavedra R, Li Y, Gupta P, Gupta K. Morphine stimulates cancer progression and mast cell activation and impairs survival in transgenic mice with breast cancer. Br J Anaesth 2014;113(S1):i4–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Paice JA, Portenoy R, Lacchetti C, Campbell T, Cheville A, Citron M, Constine LS, Cooper A, Glare P, Keefe F, Koyyalagunta L, Levy M, Miaskowski C, Otis-Green S, Sloan P, Bruera E. Management of chronic pain in survivors of adult cancers: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2016;34(27):3325–45. [DOI] [PubMed] [Google Scholar]
- [47].Plein LM, Rittner HL. Opioids and the immune system-friend or foe. British J Pharmacol 2017:175(14)271725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Posternak V, Dunn LB, Dhruva A, Paul SM, Luce J, Mastick J, Levine JD, Aouizerat BE, Hammer M, Wright F, Miaskowski C. Differences in demographic, clinical, and symptom characteristics and quality of life outcomes among oncology patients with different types of pain. Pain 2016;157(4):892–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Qin Y, Chen J, Li L, Liao CJ, Liang YB, Guan EJ, Xie YB. Exogenous morphine inhibits human gastric cancer MGC- 803 cell growth by cell cycle arrest and apoptosis induction. Asian Pac J Cancer P 2012;13(4):1377–82. [DOI] [PubMed] [Google Scholar]
- [50].Quinten C, Coens C, Mauer M, Comte S, Sprangers MA, Cleeland C, Osoba D, Bjordal K, Bottomley A. Baseline quality of life as a prognostic indicator of survival: a meta-analysis of individual patient data from EORTC clinical trials. Lancet Oncol 2009;10:865–71. [DOI] [PubMed] [Google Scholar]
- [51].Singleton P, Garcia J, Moss J. Synergistic effects of methylnaltrexone with 5-fluorouracil and bevacizumab on inhibition of vascular endothelial growth factor-induced angiogenesis. Mol Cancer Ther 2008;7:1669–1679. [DOI] [PubMed] [Google Scholar]
- [52].Singleton PA, Lingen MW, Fekete MJ, Garcia JG, Moss J. Methylnaltrexone inhibits opiate and VEGF-induced angiogenesis: role of receptor transactivation. Microvasc Res 2006;72:3–11. [DOI] [PubMed] [Google Scholar]
- [53].Singleton PA, Mirzapoiazova T, Hasina R, Salgia R, Moss J. Increased mu-opioid receptor expression in metastatic lung cancer. Br J Anaesth 2014;113(S1)103–08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Smith MY, Sobel RE, Wallace CA. Monitoring the long-term safety of therapies for children with juvenile idiopathic arthritis: time for a consolidated patient registry. Arthritis Care Res 2010;62(6):800–4. [DOI] [PubMed] [Google Scholar]
- [55].Smith TJ, Staats PS, Deer T, Stearns LJ, Rauck RL, Boortz-Marx RL, Buchser E, Català E, Bryce DA, Coyne PJ, Pool GE. Randomized clinical trial of an implantable drug delivery system compared with comprehensive medical management for refractory cancer pain: impact on pain, drug-related toxicity, and survival. J Clin Oncol 2002;20:4040–9. [DOI] [PubMed] [Google Scholar]
- [56].Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, Oudard S, Théodore C, James ND, Turesson I, Rosenthal MA, Eisenberger MA. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 2004;351:1502–12. [DOI] [PubMed] [Google Scholar]
- [57].Tegeder I, Grosch S, Schmidtko A, Haussler A, Schmidt H, Niederberger E, Scholich K, Geisslinger G. G protein-independent G1 cell cycle block and apoptosis with morphine in adenocarcinoma cells: involvement of p53 phosphorylation. Cancer Res 2003;63(8):1846–52. [PubMed] [Google Scholar]
- [58].Trafton JA, Ramani A. Methadone: a new old drug with promises and pitfalls. Curr Pain Headache Rep 2009;13:24–30. [DOI] [PubMed] [Google Scholar]
- [59].Trajkovic-Vidakovic M, de Graeff A, Voest EE, Teunissen SC. Symptoms tell it all: a systematic review of the value of symptom assessment to predict survival in advanced cancer patients. Crit Rev Oncol Hematol 2012; 84(1):130–148. [DOI] [PubMed] [Google Scholar]
- [60].Uchida S, Kobayashi K, Ohno S, Sakagami H, Kohase H, Nagasaka H. Induction of non-apoptotic cell death by adrenergic agonists in human oral squamous cell carcinoma cell lines. Anticancer Res 2019;39(7):3519–29. [DOI] [PubMed] [Google Scholar]
- [61].van den Beuken-van Everdingen MH, Hochstenbach LM, Joosten EA, Tjan-Heijnen VC, Janssen DJ. Update on prevalence of pain in patients with cancer: systematic review and meta-analysis. J Pain Symptom Manage 2016;51 (6):1070–90. [DOI] [PubMed] [Google Scholar]
- [62].Wang RD, Zhu Y, Ge YS, Xu GL, Jia WD. Perioperative analgesia with parecoxib sodium improves postoperative pain and immune function in patients undergoing hepatectomy for hepatocellular carcinoma. J Eval Clin Prac 2019. [DOI] [PubMed] [Google Scholar]
- [63].Wolfe F, Michaud K. The National Data Bank for rheumatic diseases: a multi-registry rheumatic disease data bank. Rheumatol 2011;50(1):16–24. [DOI] [PubMed] [Google Scholar]
- [64].Wu Q, Chen X, Wang J, Sun P, Weng M, Chen W, Sun Z, Zhu M, Miao C. Nalmefene attenuates malignant potential in colorectal cancer cell via inhibition of opioid receptor. Acta Biochim Biophys Sin (Shanghai) 2018;50:156–163. [DOI] [PubMed] [Google Scholar]
- [65].Xie N, Khabbazi S, Nassar ZD, Gregory K, Vithanage T, Anand-Apte B, Cabot PJ, Sturgess D, Shaw PN, Parat MO. Morphine alters the circulating proteolytic profile in mice: functional consequences on cellular migration and invasion. FASEB J 2017;31(12):5208–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Xie N , Matigian N , Vithanage T , Gregory K , Nassar ZD , Cabot PJ , Shaw PN , Kikpatrick CM, Le Cao KA, Sturgess D, Parat M. Effect of perioperative opioids on cancer relevant circulating parameters: mu opioid receptor and toll-like receptor 4 activation potential, and proteolytic profile. Clin Cancer Res. 2018;24(10):2319–27. [DOI] [PubMed] [Google Scholar]
- [67].Yao YS, Yao RY, Zhuang LK, Qi WW, Lv J, Zhou F, Qiu WS, Yue L. MOR1 expression in gastric cancer: a biomarker associated with poor outcome. Clin Transl Sci 2015; 8:137–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Zaslansky R, Rothaug J, Chapman RC, Backström R, Brill S, Engel C, Fletcher D, Fodor L, Funk P, Gordon D, Komann M, Konrad C, Kopf A, Leykin Y, Pogatzki-Zahn E, Puig M, Rawal N, Schwenkglenks M, Taylor RS, Ullrich K, Volk T, Yahiaoui-Doktor M, Meissner W. PAIN OUT: an international acute pain registry supporting clinicians in decision making and in quality improvement activities. J Eval Clin Pract 2014;20(6):1090–8. [DOI] [PubMed] [Google Scholar]
- [69].Zhang YF, Xu QX, Liao LD, Xu XE, Wu JY, Wu ZY, Shen JH, Li EM, Xu LY. Association of mu-opioid receptor expression with lymph node metastasis in esophageal squamous cell carcinoma. Dis Esophagus 2014;28:196–203. [DOI] [PubMed] [Google Scholar]
- [70].Zylla D, Gourley BL, Vang D, Jackson S, Boatman S, Lindgren B, Kuskowski MA, Le C, Gupta K, Gupta P. Opioid requirement, opioid receptor expression, and clinical outcomes in patients with advanced prostate cancer. Cancer 2013;119:4103–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Zylla D, Kuskowski MA, Gupta K, Gupta P. Association of opioid requirement and cancer pain with survival in advanced non-small cell lung cancer. Br J Anaesth 2014;113(S1)109–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Zylla D, Steele G, Gupta P. A systematic review of the impact of pain on overall survival in patients with cancer. Support Care Cancer 2017;25:1687–98. [DOI] [PubMed] [Google Scholar]


