Abstract
Cancer patients and survivors of cancer have a greater burden of cardiovascular disease compared to the general population. Much of the elevated cardiovascular risk in these individuals is likely attributable to hypertension, as individuals with cancer have a particularly high incidence of hypertension following cancer diagnosis. Treatment with chemotherapy is an independent risk factor for hypertension due to direct effects of many agents on endothelial function, sympathetic activity, and renin-angiotensin system activity as well as nephrotoxicity. Diagnosis and management of hypertension in cancer patients requires accurate blood pressure measurement and consideration of potential confounding factors, such as adjuvant treatments and acute pain, that can temporarily elevate blood pressure readings. Home blood pressure monitoring can be a useful tool to facilitate longitudinal blood pressure monitoring for titration of antihypertensive medications. Selection of antihypertensive agents in cancer patients should account for treatment-specific morbidities and target organ injury.
Keywords: Hypertension, Cancer Survivorship, Outcomes, Pharmacotherapy
INTRODUCTION
Essential hypertension is a leading cause of cardiovascular and kidney morbidity and mortality in the United States. Based on data from the 2011-2014 National Health and Nutrition Examination Survey, 46% of adults in the United States have hypertension when defined as ≥130/80 mmHg or self-reported to be taking an anti-hypertensive agent, and 32% have hypertension using the older definition of ≥140/90 mmHg (1). Non-Hispanic Black individuals have a higher prevalence of hypertension compared to Hispanic, non-Hispanic white, and Asian individuals. The vast majority of people in the United States will develop hypertension during their lifetime, with lifetime prevalence estimates of >80% for white and Asian individuals, and >90% for Black and Hispanic individuals (2).
The prevalence of hypertension is greater in cancer patients and survivors of cancer compared to the general population (3). Accordingly, hypertension is the foremost modifiable risk factor of adverse cardiovascular outcomes among cancer patients (3). The relationship between hypertension, cancer, and cardiovascular risk is multidimensional (Central Illustration). Hypertension, chronic kidney disease, cardiovascular disease, and cancer have several common risk factors, including smoking, diabetes mellitus, and obesity (4,5). Several cancers and cancer-related treatments directly cause hypertension, or indirectly mediate the develop of hypertension through nephrotoxicity. Several factors related to cancer treatment can confound blood pressure measurements. It is important to carefully measure and closely monitor blood pressures in cancer patients due to their particularly high risk of developing new or worsening hypertension. Furthermore, selection of antihypertensive agents should account for cancer treatment-specific adverse effects and individual risk factors. The goal of this review is to provide an approach to the monitoring and management of hypertension in cancer patients and survivors accounting for patient-specific risk factors for the development and worsening of hypertension.
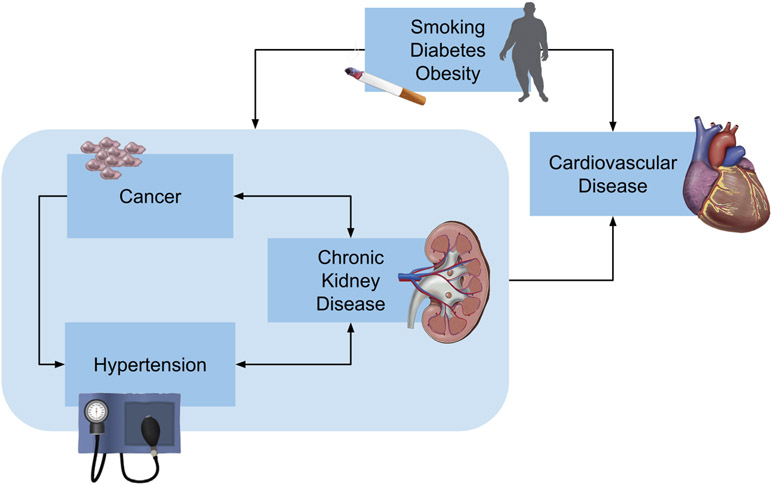
Central Illustration: Multidimensional relationship between cancer, hypertension, and cardiovascular disease.
Hypertension, chronic kidney disease, and cancer have a number of common risk factors, including smoking, diabetes, and obesity, which in turn are associated with increased risk of major adverse cardiovascular events. Cancer and cancer treatment are risk factors for hypertension and chronic kidney disease. Hypertension and chronic kidney disease have a bidirectional relationship. Chronic kidney disease is associated with an increased risk of several cancers, including urothelial cancer, skin cancer, and thyroid cancer.
Epidemiology and Etiology of Hypertension in Cancer Patients and Survivors
Burden of hypertension in patients with cancer
Limited data exist examining the prevalence of hypertension among patients with cancer prior to undergoing cancer treatment. Small studies have found a similar prevalence of hypertension in patients with solid and neuroendocrine tumors before sorafenib therapy compared to the general population (6,7). One exception is Wilms tumor in children, where hypertension is more prevalent than in the general population, and may be associated with poor prognosis and response to therapy (8).
Several cancer treatments are associated with the development or exacerbation of hypertension (Table 1). Hypertension is the most common severe adverse event in patients with cancer receiving chemotherapy (9). One retrospective study analyzed the incidence of new-onset hypertension in a population of 25,090 adults with solid malignancies in the United States, and found that approximately one-third developed hypertension during follow-up (10). Patients with renal cancer had the highest rates of moderate hypertension (150-160/100-110 mmHg), whereas patients with gastric and ovarian cancers had the highest rates of severe (160-180/110-120 mmHg) or crisis-level (≥180/120 mmHg) hypertension, respectively. The median time to first event of moderate hypertension was 96 days from the time of their initial diagnosis with cancer. Chemotherapy exposure was identified as an independent risk factor for the development of hypertension.
Table 1.
Cancer treatments associated with the development and exacerbation of hypertension
| Treatment | Mechanism(s) of blood pressure elevation |
|---|---|
| Chemotherapeutic agents | |
| Anti-VEGF therapy and tyrosine kinase inhibitors | Increased vascular resistance Reduced nitric oxide production (14) Reduced angiogenesis (15) Impaired natriuresis (16) Endothelin-1-mediated vasoconstriction (17) Thrombotic microangiopathy (18) |
| Alkylating and alkyl-like agents Cyclophosphamide Ifosphamide Cisplatin |
Vascular endothelial injury (24) Nephrotoxicity (31,32) Nephrotoxicity (33) and vascular endothelial injury (34) |
| Vinblastine | Vascular endothelial injury (in vitro) (35) |
| Gemcitabine | Thrombotic microangiopathy (37) Vascular endothelial injury (in vitro) (38) |
| Radiation | |
| Abdominal radiation | Renal artery stenosis (41) |
| Head and neck radiation | Baroreflex failure (42,43) |
| Adjuvant therapies | |
| Erythropoietin stimulating agents | Increased erythrocyte mass Altered response to endogenous vasodilators and vasopressors (44) |
| Non-steroidal anti-inflammatory drugs | Impaired natriuresis due to reduction in prostaglandin synthesis (45) |
| Corticosteroids | Sodium retention due to mineralocorticoid receptor stimulation (46) |
| Calcineurin Inhibitors | Systemic and renal vasoconstriction (47) |
VEGF = vascular endothelial growth factor
Burden of hypertension in cancer survivors
Patients who have a history of cancer have a high prevalence of hypertension compared to the general population. The Childhood Cancer Survivor Study found that hypertension was more common in >10,000 adults who had survived childhood cancer vs. >3,000 siblings, and that this difference persisted as both groups aged (prevalence of 40% vs. 25% at age 45) (3). Obesity is associated with a 4-fold increased risk of hypertension in childhood cancer survivors. Other potential risk factors include prior treatment with high-dose corticosteroids, cyclophosphamide, ifosfamide, cisplatin, or abdominal radiotherapy (4). The prevalence of hypertension in childhood cancer survivors increases sharply with age, exceeding 70% by age 50 (11); this prevalence is substantially higher than the general population after accounting for age-, sex-, race/ethnicity-, and BMI-specific population rates.
Hypertension due to cancer treatment
Anti-vascular endothelial growth factor (VEGF) therapy and tyrosine kinase inhibitors
Hypertension associated with anti-VEGF therapy and tyrosine kinase inhibitors is well-described. Hypertension has been reported in over half of patients treated with anti-VEGF therapy (12,13). The mechanism of anti-VEGF therapy related hypertension is due to disruption of vascular homeostasis related to normal VEGF activity. This inhibition of VEGF yields a reduction in nitric oxide production (14) and angiogenesis (15) that leads to increased vascular resistance. Anti-VEGF therapy can also lead to fluid retention due to impaired natriuresis (16), endothelin-1-mediated vasoconstriction (17), as well as systemic thrombotic microangiopathy (18), similar to what is seen in preeclampsia.
A recent meta-analysis (19) studied the risk of cardiovascular disease in tyrosine kinase inhibitors therapy versus standard chemotherapy, and included 71 randomized controlled trials comprising >29,000 patients. The relative risk of hypertension with tyrosine kinase inhibitor therapy was 3.78 (95% Confidence Interval (CI) 3.15-4.54). Treatment with tyrosine kinase inhibitors was also associated with a higher risk of cardiac ischemia (relative risk 1.69, 95% CI 1.12-2.57; in subgroup analyses, highest with sorafenib and in renal cancer) and left ventricular systolic dysfunction (relative risk 2.53, 95% CI 1.79-3.57). Another systematic review and meta-analysis (20) of 77 studies of angiogenesis inhibitors determined that the odds ratio for hypertension was 5.28 (95% CI 4.53-6.15) with angiogenesis inhibitors compared to routine care (number need to harm 6), and the odds ratio for severe (≥160/100 mmHg) hypertension was 5.59 (95% CI 4.67-6.69) (number needed to harm 17). The meta-analysis did not find risk differences in patients exposed to direct VEGF inhibitors compared to tyrosine kinase inhibitors.
Alkylating Agents
Alkylating agents have been important anti-neoplastic agents for decades. In current practice, alkylating agents are almost always used in combination with other agents, leading to the challenge of attributing specific adverse events to a liable agent. There are pre-clinical and clinical data indicating that some alkylating agents cause vascular toxicity and nephrotoxicity, which can indirectly result in hypertension. However, the causal link between alkylating agents and hypertension remains unclear.
Cyclophosphamide has been associated with multiple vascular complications such as veno-occlusive disease in the lung and liver after hematopoietic cell transplantation, thromboembolic disease, and myocardial ischemia (21-23). Preclinical evidence has demonstrated endothelial injury and abnormalities in the renin-angiotensin system in animals treated with cyclophosphamide (24). Therefore, there is biological plausibility for cyclophosphamide-associated hypertension to be due to vascular injury. However, cyclophosphamide has not been identified as an independent risk factor for hypertension in cancer survivors. Busulfan is an alkylating agent used in combination with oral cyclophosphamide as a conditioning regimen prior to allogeneic hematopoietic cell transplantation. This regimen has been used as an alternative, myeloablative strategy to oral cyclophosphamide plus total body irradiation. Hypertension was noted in 25-36% of adults who received busulfan, and in 58% of pediatric patients (25). Additional vascular toxicity has not been described, and no specific mechanism of action has been proposed (26,27). Correspondingly, bendamustine was reported to cause hypertensive emergency in 4 of 162 (2.4%) patients in a randomized controlled trial compared to chlorambucil for patients with previously untreated chronic lymphocytic leukemia (28,29). However, several patients in this study also experienced hypotension with bendamustine administration (6/162=3.7%).
Nephrotoxicity of certain alkylating and alkyl-like agents is a likely driver of hypertension. Ifosphamide is known to cause nephrotoxicity, particularly with high dose therapy in children (30). Hypertension has also been reported in cancer survivors who were previously treated with ifosphamide; it remains unclear if ifosphamide exposure is an independent risk factor for the development of hypertension, or if hypertension is entirely mediated by ifosphamide-associated nephrotoxicity (31,32). Similarly, cisplatin and other platinum-based compounds, which are alkyl-like agents, have also been associated with nephrotoxicity and hypertension. The etiology of hypertension in patients treated with these agents is thought to be due to underlying renal injury (33), though vascular endothelial damage may also play a role (34).
Anti-microtubule agents
Anti-microtubule agents affect mitosis by acting on tubulin to prevent microtubule polymerization. In vitro studies support an effect of vinblastine on endothelial cell gene expression, particularly genes involved in apoptosis, cytoskeletal structure, cell cycle, and protein destruction (35). Vinca alkaloids have been noted to cause hypertension (36). However, since they are typically used in combination with other chemotherapies, the independent contribution of vinca alkaloids to the development or exacerbation of hypertension is not clear.
Antimetabolite therapy
Gemcitabine has been associated with the development of hypertension in the setting of thrombotic microangiopathy (37), with some evidence of endothelial damage in pre-clinical models of rapidly dividing endothelial cells (38).
Proteasome inhibitors
The proteasome inhibitors bortezomib and carfilzomib are currently used mostly as anti-plasma cell therapies in multiple myeloma. They have been observed to cause cardiac toxicity, which has occurred most commonly in patients treated with carfilzomib (39). Severe hypertension (i.e. blood pressure ≥160/100 mmHg) is rare with proteasome inhibitors, and it is difficult to determine the relative contribution of proteasome inhibitors to hypertension in these cases since they are almost always used in combination with other therapies such as alkylating agents and corticosteroids. Cases of proteasome inhibitor-associated thrombotic microangiopathy have been reported (40), but the pathophysiologic mechanism is unclear.
Radiation
Abdominal radiation has resulted in hypertension due to renal artery stenosis in rare cases (41). Radiation to the head and neck has been associated with baroreflex failure (42,43), which can manifest as labile hypertension or hypertensive crisis.
Adjuvant therapies
Many patients with cancer receive adjuvant therapies that can cause or worsen hypertension. These include erythropoietin stimulating agents (44), non-steroidal anti-inflammatory drugs (45), and corticosteroids (46). Calcineurin inhibitors, which are often prescribed after hematopoietic cell transplantation to prevent or treat graft versus host disease, can incite or exacerbate existing hypertension (47).
Radical nephrectomy for kidney cancer is also associated with the development of hypertension (48), with partial nephrectomy (i.e. nephron sparing surgery) potentially attenuating this risk (49).
Hypertension due to cancer
Paraneoplastic hypertension
Hypertension can be a paraneoplastic feature of hepatocellular carcinoma, renal cell carcinoma, carcinoid, and several other cancers. In hepatocellular carcinoma, paraneoplastic hypertension is due to an excessive production of either renin, angiotensinogen, or angiotensin I by the carcinoma cells (50,51). Paraneoplastic hypertension secondary to excessive catecholamine urinary secretion has been described in some case reports of carcinoid tumors (52).
Among individuals with renal cell carcinoma, the prevalence of hypertension exceeds 75%. Hypertension in renal cell carcinoma has multiple contributing etiologies, particularly loss of nephron mass post-nephrectomy and treatment with VEGF inhibitors and tyrosine-kinase inhibitors (53). Renal cell carcinoma cells can also secrete vasoactive peptides, notably endothelin-1, leading to paraneoplastic hypertension (54). Paraneoplastic hypertension occurs in approximately 2% of patients diagnosed with renal cell carcinoma (55). The presence of paraneoplastic syndrome in renal cell carcinoma is a sign of aggressive disease, with worse prognosis.
Pheochromocytoma and paraganglioma
Pheochromocytoma and paraganglioma are neuroendocrine tumors arising from chromaffin cells in the adrenal medulla in the case of pheochromocytoma, and in the extra-adrenal autonomic paraganglia in the case of paraganglioma (56). Pheochromocytoma and paraganglioma are rare tumors, with an annual incidence of 0.8 per 100000 person-years (57). Approximately 10% of these tumors are malignant. Hypertension in pheochromocytoma and paraganglioma is caused by catecholamine hypersecretion (norepinephrine, epinephrine and dopamine), and can be associated with symptoms including headaches, palpitations, and diaphoresis. However, at the time of diagnosis with pheochromocytoma or paraganglioma, these adrenergic symptoms are only present in about half of patients. Dopamine hypersecretion, documented by high plasma and urinary levels of dihydroxyphenylalanine and dopamine, has been associated with a more aggressive course and worse prognosis (58). Treatment is surgical resection, adjuvant chemotherapy, and/or radiotherapy.
Adrenocortical carcinoma
Adrenocortical carcinoma is very rare tumor, with an incidence of 0.5 to 2 cases per one million person-years (59). These carcinomas most commonly present with Cushings syndrome, with features resulting from hypersecretion of glucocorticoid and/or androgens. Presentation with hyperaldosteronism is uncommon, and has only been reported in a few case reports (60). In either case, patients are likely to have hypertension as part of their presenting symptoms. Treatment is surgical resection, mitotane, adjuvant chemotherapy and/or radiotherapy.
Relationship between target organ damage and hypertension in cancer patients
Chronic kidney disease
The relationship between hypertension and chronic kidney disease is bidirectional. Hypertension can result in glomerulosclerosis and microangiopathy, resulting in chronic kidney disease (61). Alternatively, chronic kidney disease causes and exacerbates existing hypertension via several mechanisms, including impaired natriuresis, elevated renin-angiotensin system activity, heightened sympathetic activity, and vascular endothelial injury.
The relationship between chronic kidney disease and cancer is also bidirectional. Cancer survivors have higher rates of chronic kidney disease secondary to therapy-related toxicities including chemotherapy nephrotoxicity (ifosfamide, cisplatin, anti-VEGF), recurrent acute kidney injury, abdominal radiotherapy, loss of nephron mass following nephrectomy, and direct cancer nephrotoxicity due to paraproteins or cryogloblins (33,62). Individuals with chronic kidney disease are at a high risk of developing several cancers, including urothelial cancer, skin cancer, and thyroid cancer (63,64). An illustrative example of the bidirectional relationship between chronic kidney disease and cancer is that of end stage kidney disease and renal cell carcinoma. Individuals with end stage kidney disease have a 100-fold increased risk of developing renal cell carcinoma compared to the general population, whereas loss of nephron mass following nephrectomy for renal cell carcinoma leads to chronic kidney disease (65).
The association between chronic kidney disease and cancer is well-studied in childhood cancer survivors. In this population, the reported prevalence of chronic kidney disease ranges between 2.4% and 32%; this highly variable prevalence is related to differences in follow-up duration, chemotherapeutic regimens, and the definition of chronic kidney disease across different studies (11,33). Wilms tumor has a cumulative incidence of end stage kidney disease of 0.7% after 20 years of follow-up (4); this incidence increases to 4.0% at 3 years after diagnosis in patients with synchronous bilateral Wilms tumor, and 19.3% in those with metachronous bilateral Wilms’ tumor.
Cardiovascular disease
With the increase in cancer survivorship, late treatment-related complications, including cardiovascular disease, are the primary source of long-term morbidity and mortality in cancer survivors (66,67). Hypertension is a significant risk factor in cancer survivors for developing coronary artery disease, heart failure, valvular heart disease and arrhythmia. Hypertension has also been found to be more prevalent (66% vs. 60%), and was an independent risk factor for cardiovascular events, among adult cancer survivors compared to controls in a large study of the Kaiser Permanente Southern California-SEER cancer registry (68). Furthermore, hypertension increases the risk of cardiotoxicity due to chest radiotherapy and anthracycline (3). Data are lacking regarding whether treating hypertension reduces the risk of cardiovascular events in cancer survivors; nonetheless, hypertension is the leading potentially modifiable risk factor for cardiovascular disease in this patient population.
DIAGNOSIS AND MONITORING OF HYPERTENSION IN CANCER PATIENTS AND SURVIVORS
In-office blood pressure measurement
In the United States, the majority of blood pressure measurement for screening for hypertension and titration of antihypertensive therapy occurs in the clinic setting. Clinic blood pressure measurement can be performed using a manual aneroid manometer with auscultation of Korotkoff sounds or using an automated blood pressure monitor. Most blood pressure measurements in the office are performed by a medical assistant or nurse. These measurements may occur in the setting of time constraints or inadequate training, frequently resulting in inaccurate measurements (69). Consistent in-office measurements of blood pressure, using the appropriate approach to minimize confounders, is strongly recommended (69,70). This includes having the patient rest for 3-5 minutes prior to blood pressure measurement, with the measurement performed in a quiet room in the seated position, with the legs flat on the floor, the back supported (an examination table is typically not ideal), the arm supported at the level of the heart, the correct cuff size against a bare arm, an empty bladder, and no caffeine or cigarette smoking within 30 minutes prior to the measurement (71). Particularly in cancer patients and survivors, it is also important to assess for the presence of temporarily interfering substances (e.g., non-steroidal anti-inflammatory drugs, erythropoietin stimulating agents, and high-dose corticosteroids) and acute pain as potential confounders of blood pressure measurement during any given clinic visit (see below, Management of hypertension in cancer patients and survivors).
Individuals with an elevated clinic blood pressure reading should have at least two additional blood pressure measurements performed during that clinic visit, since blood pressure improves with successive measurements in many individuals and treatment recommendations are based on the average of three office readings (1,70). Automated office blood pressure measurement is a useful tool for achieving multiple blood pressure readings in a single visit. Automated office blood pressure measurement refers to the use of a fully automated device that has the ability to perform multiple consecutive blood pressure measurements with a single activation. Blood pressure measured using automated office blood pressures should be performed in a quiet room with or without the presence of a provider (72), and more closely resemble research-quality and daytime ambulatory blood pressure readings than typical clinic blood pressures (73).
Understanding the high risk of vascular toxicity and thromboembolic disease with many chemotherapies and cancers, patients should be assessed for interarm differences in blood pressure at least one time during the course of cancer treatment and again following treatment. If there is a reproducible, ≥10 mmHg difference in systolic or diastolic blood pressure between the arms, the arm with the higher blood pressure should be used for future measurements (70).
Out-of-office blood pressure measurement
White coat hypertension and masked hypertension
Out-of-office blood pressure measurement addresses many of the limitations of clinic blood pressure measurement (74). In particular, out-of-office blood pressure measurement facilitates identification of white coat hypertension (elevated office blood pressure with normal out-of-office blood pressure) and masked hypertension (normal office blood pressure with elevated out-of-office blood pressure). Untreated white coat hypertension is associated with an increased risk of transition to sustained hypertension and adverse cardiovascular outcomes, whereas treated white coat hypertension is not associated with increased risk (75). Both treated and untreated masked hypertension are associated with a similarly increased risk of adverse cardiovascular outcomes as sustained hypertension (76,77). Thus, ongoing out-of-office monitoring is recommended in individuals with both white coat and sustained hypertension. Current guidelines recommend out-of-office blood pressure measurement in individuals whose office blood pressure is ≥120/70 mmHg to screen for masked hypertension (1).
Evidence suggests that white coat hypertension and masked hypertension may be more common in individuals receiving cancer treatment compared to the general population (78,79). The increased prevalence of white coat hypertension is proposed to be due to heightened anxiety associated with a diagnosis of cancer and fears surrounding prognosis. The increased prevalence of masked hypertension is likely in part due to delayed adverse effects of cancer treatments.
Approach to out-of-office blood pressure monitoring
In patients undergoing active cancer treatment, blood pressure elevations can occur within a few hours, days, or may take up to a year to be evident (80). Given the rise in blood pressure following initiation of some chemotherapies, it is useful to supplement office blood pressures with out-of-office blood pressure monitoring. Options for out-of-office blood pressure measurement include ambulatory blood pressure monitoring and home blood pressure monitoring, also referred to as self-measured blood pressure at home (Table 2). Ambulatory blood pressure monitoring provides fully automated measurements over a 24-hour period, typically performed every 15-30 minutes during the day and every 30-60 minutes at night. Ambulatory blood pressure monitoring is the reference standard for blood pressure measurement due to a stronger association with cardiovascular outcomes than clinic blood pressure measurements (74). However, ambulatory blood pressure monitoring can be intrusive, and is difficult for patients to perform repeatedly in close succession for monitoring of changes in blood pressure (81).
Table 2.
Considerations for selection of out-of-office blood pressure monitoring modalities
| Ambulatory Blood Pressure Monitoring |
Home Blood Pressure Monitoring |
|
|---|---|---|
| Appropriate indications | Initial diagnosis and intermittent monitoring of masked hypertension, white coat hypertension, and nocturnal hypertension. | Long-term monitoring and medication titration. |
| Measurement frequency and duration | Every 15-30 minutes over a 24-hour period. | Two measurements at least one minute apart in the morning before antihypertensive medications and in the evening before bed. In unstable patients or patients on high risk cancer therapy, measurements should be performed twice daily at minimum once a week (consider daily). In stable patients, measurements should typically be performed for a minimum of 3 (ideally 5-7) consecutive days per month and beginning 7 days after any changes in medication. |
| Measurement setting | Performed during usual daily activities and while sleeping. | Performed after resting 3-5 minutes in a quiet room, sitting in a chair with feet flat on the floor and back supported, and with an empty bladder. Patients are asked to avoid caffeine, exercise, and smoking for the 30 minutes prior to measurement. Measure with a bare arm, elevated and supported at the level of the heart. |
| Patient engagement | Patient is unaware of and unable to see blood pressure readings. Monitoring may be perceived as intrusive. | Patient activates the device to perform measurements, and sees the blood pressure readings. |
| Accessibility | Often only available in hypertension specialty offices (e.g., cardiology, nephrology, hypertension centers) due to cost of monitors. | Low cost, readily accessible to most patients. |
| Quality and reliability of measurements | Highly reliable readings, strongly associated with prognostic outcomes. | Highly reproducible readings, require patient training and education to ensure adequate quality. |
Home blood pressure monitoring typically requires a patient to use a semi-automated blood pressure monitor to perform two measurements twice daily for a minimum of 3 (ideally 5-7) consecutive days. While home blood pressure monitoring is prone to some of the measurement inaccuracies of clinic blood pressure monitoring, these can be readily addressed with patient education on appropriate measurement technique (82). Home blood pressure monitoring is able to identify white coat hypertension and masked hypertension, and facilitates close blood pressure monitoring for titration of antihypertensive medications, (83) making it favorable for longitudinal blood pressure monitoring in cancer patients.
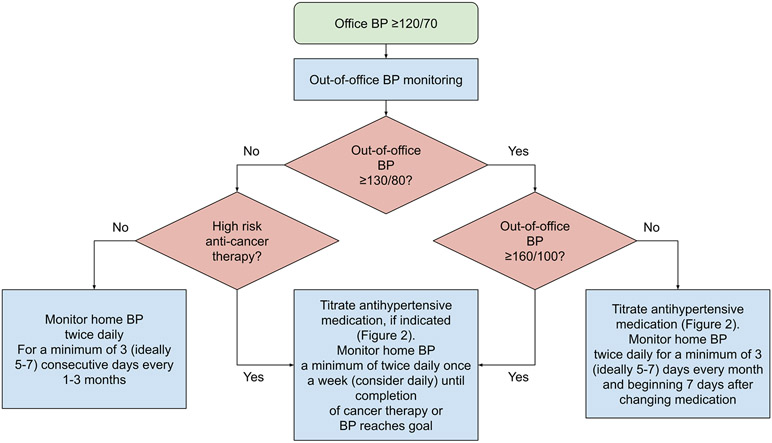
Based on recent guidelines, we recommend 24-hour ambulatory blood pressure monitoring for initial evaluation in all patients with an office blood pressure ≥120/70 mmHg (1). While ambulatory blood pressure monitoring provides the most accurate and prognostically useful assessment of blood pressure, it is typically not feasible to perform more frequently than every 6-12 months (81). Home blood pressure monitoring has greater reproducibility and tolerability than ambulatory blood pressure monitoring, and thus is preferable for more frequent, monthly monitoring and for titration of medications over prolonged periods of time (69,84). Thus, we recommend home, rather than ambulatory, blood pressure monitoring to monitor for sufficient blood pressure control. Based on the pharmacokinetics of most antihypertensive medications, we typically recommend that patients start to monitor their blood pressures at home for a minimum of 3 (ideally 5-7) days beginning 7 days after any changes to antihypertensive therapy, sooner if the individual is having severe or symptomatic hypertension. Specific cancer treatments may warrant more frequent monitoring, including anti-VEGF therapy, tyrosine kinase inhibitors, alkylating agents, and high-dose corticosteroids. Figure 1 presents an approach to out-of-office blood pressure monitoring in cancer patients and survivors, adapted from recommendations for home blood pressure monitoring in the general population to account for greater acuity in many patients on high risk cancer therapy (70,82,85).
Figure 1: Approach to home blood pressure monitoring in cancer patients and survivors.
Abbreviation: BP = blood pressure
High risk cancer therapies include anti-VEGF therapy, tyrosine kinase inhibitors, alkylating agents, and high-dose corticosteroids
Selection of an automated blood pressure monitor
Most automated office and home devices use proprietary algorithms to estimate the systolic and diastolic blood pressure. It is important to select a clinically validated blood pressure monitor (86,87). A listing of validated blood pressure devices available in the US will be available in the near future from the American Heart Association and American Medical Association (87). Current listings are also maintained by Hypertension Canada (88), the British and Irish Hypertension Society (89), and other international hypertension societies. Automated blood pressure monitors are prone to inaccuracies in certain clinical circumstances, such as arrhythmias and vascular disease. Given the elevated risk of these comorbidities in cancer patients and survivors, patient-specific validation of automated devices with a manual reading can be useful to ensure accuracy.
Due to the poor accuracy of most wrist, finger, and smartphone blood pressure devices, (90,91) upper arm devices are preferred. For individuals who have a contraindication to upper arm blood pressure measurement, such as those who have undergone bilateral lymph node dissection, there are currently three clinically validated wrist devices available in the United States (Omron BP4350, BP6100, and BP8000-M) (92,93).
MANAGEMENT OF HYPERTENSION IN CANCER PATIENTS AND SURVIVORS
Blood pressure thresholds to initiate treatment and treatment targets
For normotensive patients with additional cardiovascular risk factors such as diabetes, elevated cholesterol, prior coronary heart disease, or active treatment with cardiotoxic chemotherapeutic agents who experience an increase in blood pressure, but whose blood pressure does not exceed a threshold level of ≥130/80 mmHg or those with a blood pressure ≥140/90 mmHg and are without additional cardiovascular risk (1), lifestyle measures, especially sodium intake restriction, are a reasonable approach.
In previously normotensive patients who exceed the thresholds described above, or in hypertensive patients whose blood pressure becomes uncontrolled, adding therapy or titrating existing antihypertensive therapy is recommended. From a pragmatic standpoint, patients with active cancer have been excluded from standard hypertension trials in the past. Thus, there are little outcome data supporting antihypertensive therapy and blood pressure treatment thresholds. However, the increasing survival in cancer patients, and the cardiovascular toxicities of many cancer chemotherapeutic agents, predisposes these patients to cardiac death and future cardiovascular diseases (66,67), making antihypertensive therapy a rational and useful consideration.
Recent trials support intensive blood pressure lowering in individuals at high risk of cardiovascular disease (94-96), however these studies did not include cancer patients. Whether the goal should be <130/80 in those at higher cardiovascular risk is unknown in this patient population.
Selection of agents for the management of hypertension in patients on cancer therapy.
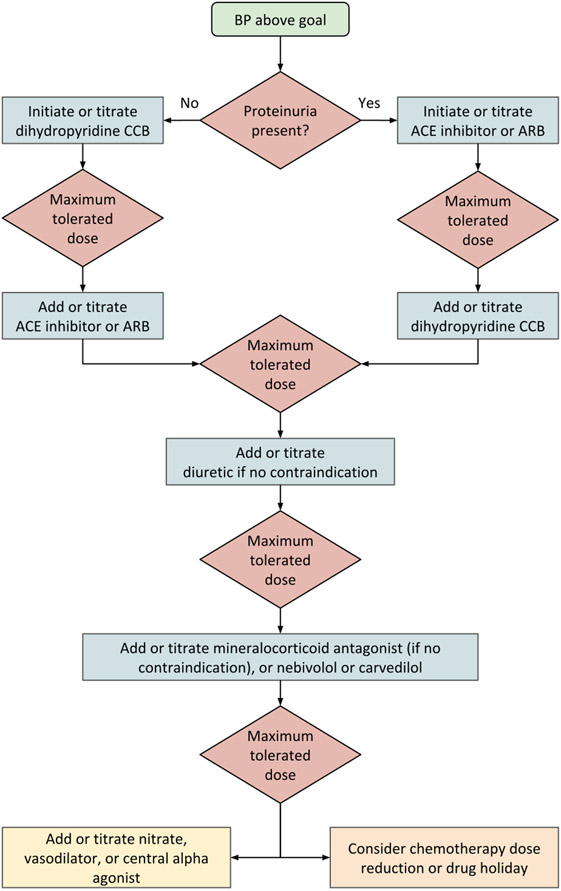
Figure 2 presents an approach to therapy in the cancer patient whose blood pressure warrants drug treatment. Currently, no one class of antihypertensive drug is preferred. Since hypertension results from nephrotoxicity in several cancers and cancer treatments, our approach is to first assess for the presence of proteinuria. If proteinuria is present (spot albuminuria-to-creatinine ratio of ≥300 mg/g, or spot protein-to-creatinine ratio of ≥500 mg/g), drugs which block the renin-angiotensin system are reasonable agents to initiate or titrate (1,97-99). Similarly, if left ventricular dysfunction is present, neurohormonal antagonists may be appropriate first-line drugs (1,100). Moreover, limited retrospective data suggest that the use of renin-angiotensin system blocking drugs may improve survival in cancer patients (101). Although there was initial concern that lowering blood pressure using medications like angiotensin converting enzyme inhibitors could, theoretically, offset the anti-tumor effect of VEGF inhibitors, this has not been observed in clinical practice, and antihypertensive therapy is recommended for these patients. In the absence of proteinuria, either a dihydropyridine calcium channel blocker or a renin-angiotensin system blocking drug can be initiated. In our experience, the efficacy of calcium channel blockers like amlodipine is reasonably high, particularly in African American patients (102,103), and their tendency to drug interactions and serious side effects are relatively low. Thus, we prefer adding, or titrating, amlodipine first when proteinuria is absent.
Figure 2: Approach to treating hypertension in patients receiving cancer therapy.
Abbreviations: ACE = angiotensin-converting enzyme; ARB = angiotensin receptor blocker; CCB = calcium channel blocker.
The blood pressure threshold for initiation and titration of treatment will vary depending on an individual’s risk factors and goals of care (1). It may be beneficial to defer ACE inhibitors, ARBs, diuretics, and mineralocorticoid antagonists in individuals at risk of volume depletion, or to employ sick-day protocols (104). The yellow box at the lower left indicates fourth-line agents; we recommend exhausting other options before using these agents. The orange box at the lower right indicates a possible choice of action when the blood pressure remains uncontrolled despite the addition or titration of multiple antihypertensive agents.
In individuals at high risk of volume depletion who also have proteinuria, it may be preferable to defer renin-angiotensin system blocking drugs or, in those with transient risk of volume loss, recommend a sick-day protocol (104) to temporarily withhold these medications on days in which they have symptoms. Correspondingly, diuretic and mineralocorticoid antagonist therapies are often added, or titrated, later in the cascade of antihypertensive therapy in patients on undergoing active cancer treatment, since these patients are at higher risk for volume depletion through reduced intake of nutrients and fluids, as well as increased volume losses from diarrhea or vomiting, predisposing them to electrolyte abnormalities and acute kidney injury. If there is no further individual-level contraindication, diuretic therapy (specifically thiazide and thiazide-like diuretics) should be considered first-line therapy in patients undergoing active surveillance and in cancer survivors (102). Similarly, if there is no contraindication, mineralocorticoid antagonist therapy should be used in individuals with resistant hypertension (105), with close monitoring for hyperkalemia.
Depending on the half-life and frequency of chemotherapy administration, some individuals may not be able to be treated with a fixed dose of antihypertensive medication. These individuals may particularly benefit from frequent home blood pressure monitoring (see above Approach to out-of-office blood pressure monitoring), including instructions on antihypertensive medication holding parameters and appropriate supplemental dosing of antihypertensive medications for fluctuations in blood pressures related to chemotherapy administration and side effects.
Consideration of medication interactions, interfering substances, and polypharmacy
Currently, non-dihydropyridine calcium channel blockers like verapamil and diltiazem are avoided since they utilize Cytochrome p450 3A4, a feature shared by many chemotherapy agents, risking potentiation of chemotherapy toxicity by inhibiting chemotherapy drug metabolism (80).
In some individuals undergoing active cancer treatment, the blood pressure cannot be controlled even with multiple antihypertensive agents. In this case, it is reasonable to discuss with the oncologist and the patient a trial of chemotherapy dose reduction, or a chemotherapeutic holiday period. It is also reasonable to consider dose reduction or temporary discontinuation of other therapeutic agents that may be contributing to high blood pressures, including non-steroidal anti-inflammatory drugs, erythropoietin stimulating agents, and high-dose corticosteroids.
Polypharmacy is common in cancer patients (106). In individuals who require >1 agent to achieve adequate blood pressure control, it is reasonable to use fixed-dose combinations of first-line agents to minimize pill burden and optimize adherence (107).
Approach to elevated blood pressure in the setting of pain and accounting for goals of care
The relationship between pain and blood pressure is complex, and the pathophysiology of this relationship seems to vary depending on the acuity of pain (108). Evidence suggests that greater intensity of chronic pain is associated with higher risk of hypertension (109). We recommend assessment of adequate pain control and titration of pain medications prior to initiating and uptitrating antihypertensive therapy in cancer patients. If chronic pain cannot be adequately controlled, there may be cardiovascular benefit to treatment with antihypertensive therapy to reduce blood pressure, especially if the blood pressure is persistently and/or severely elevated; however, there is a paucity of data to guide decision-making in this setting. In individuals with limited life expectancy, it is reasonable to liberalize the treatment goal to <160/100 mmHg (110). In this case, the risks and benefits of antihypertensive treatment should be discussed with the patient based on their individual comorbidities, prognosis, and goals of care.
SUMMARY AND CONCLUSIONS
The burden of hypertension is particularly high in cancer patients and survivors, likely contributing to increased cardiovascular morbidity and mortality in these patients compared to the general population. There is a paucity of data on the benefit of blood pressure treatment in cancer patients with regard to cardiovascular risk reduction. Future studies are needed to identify optimal treatment targets and therapies for the management of hypertension in this patient population.
In the absence of high-quality evidence, individualized monitoring and treatment of hypertension in cancer patients and survivors is paramount. It is especially important to consider active cancer treatment as well as the presence, intensity, and duration of adjuvant medications and pain when initiating and titrating antihypertensive medications. Proper blood pressure measurement technique and use of validated blood pressure devices is critical to obtaining accurate blood pressure measurements with which to make treatment decisions. Given improved survival among cancer patients in recent decades and the potential to reduce adverse long-term cardiovascular outcomes, it is important to engage cancer patients and survivors in the use of home blood pressure monitoring.
HIGHLIGHTS.
Cancer patients and survivors are at a high risk for hypertension.
Hypertension likely contributes to the high burden of cardiovascular disease in cancer patients and survivors.
Accurate in- and out-of-office blood pressure measurement is important in cancer patients and survivors.
Target organ damage and treatment-specific morbidities should be considered when selecting antihypertensives in cancer patients.
Acknowledgments
Financial support: NIH-NHBLI K23-HL133843 (PI: Cohen)
ABBREVIATIONS
- VEGF
vascular endothelial growth factor
Footnotes
Disclosures: None
REFERENCES
- 1.Whelton PK, Carey RM, Aronow WS et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018;71:e13–e115. [DOI] [PubMed] [Google Scholar]
- 2.Carson AP, Howard G, Burke GL, Shea S, Levitan EB, Muntner P. Ethnic differences in hypertension incidence among middle-aged and older adults: the multi-ethnic study of atherosclerosis. Hypertension 2011;57:1101–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armstrong GT, Oeffinger KC, Chen Y et al. Modifiable risk factors and major cardiac events among adult survivors of childhood cancer. J Clin Oncol 2013;31:3673–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kantor AF, Li FP, Janov AJ, Tarbell NJ, Sallan SE. Hypertension in long-term survivors of childhood renal cancers. J Clin Oncol 1989;7:912–5. [DOI] [PubMed] [Google Scholar]
- 5.Cohen JB. Hypertension in Obesity and the Impact of Weight Loss. Curr Cardiol Rep 2017;19:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chu TF, Rupnick MA, Kerkela R et al. Cardiotoxicity associated with tyrosine kinase inhibitor sunitinib. Lancet 2007;370:2011–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maitland ML, Kasza KE, Karrison T et al. Ambulatory monitoring detects sorafenib-induced blood pressure elevations on the first day of treatment. Clin Cancer Res 2009;15:6250–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hill RJ Jr. Manufacturer’s labeling for paclitaxel. Cancer Invest 1996;14:644. [DOI] [PubMed] [Google Scholar]
- 9.Izzedine H, Ederhy S, Goldwasser F et al. Management of hypertension in angiogenesis inhibitor-treated patients. AnnOncol 2009;20:807–815. [DOI] [PubMed] [Google Scholar]
- 10.Fraeman KH, Nordstrom BL, Luo W, Landis SH, Shantakumar S. Incidence of new-onset hypertension in cancer patients: a retrospective cohort study. Int J Hypertens 2013;2013:379252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gibson TM, Li Z, Green DM et al. Blood Pressure Status in Adult Survivors of Childhood Cancer: A Report from the St. Jude Lifetime Cohort Study. Cancer Epidemiol Biomarkers Prev 2017;26:1705–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li W, Croce K, Steensma DP, McDermott DF, Ben-Yehuda O, Moslehi J. Vascular and Metabolic Implications of Novel Targeted Cancer Therapies: Focus on Kinase Inhibitors. J Am Coll Cardiol 2015;66:1160–78. [DOI] [PubMed] [Google Scholar]
- 13.Pinkhas D, Ho T, Smith S. Assessment of pazopanib-related hypertension, cardiac dysfunction and identification of clinical risk factors for their development. Cardiooncology 2017;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hood JD, Meininger CJ, Ziche M, Granger HJ. VEGF upregulates ecNOS message, protein, and NO production in human endothelial cells. Am J Physiol 1998;274:H1054–8. [DOI] [PubMed] [Google Scholar]
- 15.Madeddu P Therapeutic angiogenesis and vasculogenesis for tissue regeneration. Exp Physiol 2005;90:315–26. [DOI] [PubMed] [Google Scholar]
- 16.Pandey AK, Singhi EK, Arroyo JP et al. Mechanisms of VEGF (Vascular Endothelial Growth Factor) Inhibitor-Associated Hypertension and Vascular Disease. Hypertension 2018;71:e1–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lankhorst S, Danser AH, van den Meiracker AH. Endothelin-1 and antiangiogenesis. Am J Physiol Regul Integr Comp Physiol 2016;310:R230–4. [DOI] [PubMed] [Google Scholar]
- 18.Vigneau C, Lorcy N, Dolley-Hitze T et al. All anti-vascular endothelial growth factor drugs can induce ‘pre-eclampsia-like syndrome’: a RARe study. Nephrol Dial Transplant 2014;29:325–32. [DOI] [PubMed] [Google Scholar]
- 19.Totzeck M, Mincu RI, Mrotzek S, Schadendorf D, Rassaf T. Cardiovascular diseases in patients receiving small molecules with anti-vascular endothelial growth factor activity: A meta-analysis of approximately 29,000 cancer patients. Eur J Prev Cardiol 2018;25:482–494. [DOI] [PubMed] [Google Scholar]
- 20.Abdel-Qadir H, Ethier JL, Lee DS, Thavendiranathan P, Amir E. Cardiovascular toxicity of angiogenesis inhibitors in treatment of malignancy: A systematic review and meta-analysis. Cancer Treat Rev 2017;53:120–127. [DOI] [PubMed] [Google Scholar]
- 21.Beschorner WE, Pino J, Boitnott JK, Tutschka PJ, Santos GW. Pathology of the liver with bone marrow transplantation. Effects of busulfan, carmustine, acute graft-versus-host disease, and cytomegalovirus infection. Am J Pathol 1980;99:369–86. [PMC free article] [PubMed] [Google Scholar]
- 22.Talcott JA, Herman TS. Acute ischemic vascular events and cisplatin. Ann Intern Med 1987;107:121–2. [DOI] [PubMed] [Google Scholar]
- 23.Braverman AC, Antin JH, Plappert MT, Cook EF, Lee RT. Cyclophosphamide cardiotoxicity in bone marrow transplantation: a prospective evaluation of new dosing regimens. J Clin Oncol 1991;9:1215–23. [DOI] [PubMed] [Google Scholar]
- 24.Al-Hashmi S, Boels PJ, Zadjali F et al. Busulphan-cyclophosphamide cause endothelial injury, remodeling of resistance arteries and enhanced expression of endothelial nitric oxide synthase. PLoS One 2012;7:e30897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ben Venue Laboratories. Busulfan [package insert]. U.S. Food and Drug Administration; website. https://www.accessdata.fda.gov/drugsatfda_docs/label/1999/20954lbl.pdf. Revised 10 February 1999. Accessed 15 Jul 2019. [Google Scholar]
- 26.Hartman AR, Williams SF, Dillon JJ. Survival, disease-free survival and adverse effects of conditioning for allogeneic bone marrow transplantation with busulfan/cyclophosphamide vs total body irradiation: a meta-analysis. Bone Marrow Transplant 1998;22:439–43. [DOI] [PubMed] [Google Scholar]
- 27.Socie G, Clift RA, Blaise D et al. Busulfan plus cyclophosphamide compared with total-body irradiation plus cyclophosphamide before marrow transplantation for myeloid leukemia: long-term follow-up of 4 randomized studies. Blood 2001;98:3569–74. [DOI] [PubMed] [Google Scholar]
- 28.Knauf WU, Lissichkov T, Aldaoud A et al. Phase III randomized study of bendamustine compared with chlorambucil in previously untreated patients with chronic lymphocytic leukemia. J Clin Oncol 2009;27:4378–84. [DOI] [PubMed] [Google Scholar]
- 29.Cephalon, Inc. TREANDA (bendamustine hydrochloride) [package insert]. U.S. Food and Drug Abdministration; website. https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/022303lbl.pdf. Revised October 2008. Accessed 15 Jul 2019. [Google Scholar]
- 30.Skinner R, Cotterill SJ, Stevens MC. Risk factors for nephrotoxicity after ifosfamide treatment in children: a UKCCSG Late Effects Group study. United Kingdom Children’s Cancer Study Group. Br J Cancer 2000;82:1636–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McMahon KR, Harel-Sterling M, Pizzi M, Huynh L, Hessey E, Zappitelli M. Long-term renal follow-up of children treated with cisplatin, carboplatin, or ifosfamide: a pilot study. Pediatr Nephrol 2018;33:2311–2320. [DOI] [PubMed] [Google Scholar]
- 32.Knijnenburg SL, Jaspers MW, van der Pal HJ et al. Renal dysfunction and elevated blood pressure in long-term childhood cancer survivors. Clin J Am Soc Nephrol 2012;7:1416–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kooijmans EC, Bokenkamp A, Tjahjadi NS et al. Early and late adverse renal effects after potentially nephrotoxic treatment for childhood cancer. Cochrane Database Syst Rev 2019;3:CD008944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dursun B, He Z, Somerset H, Oh DJ, Faubel S, Edelstein CL. Caspases and calpain are independent mediators of cisplatin-induced endothelial cell necrosis. Am J Physiol Renal Physiol 2006;291:F578–87. [DOI] [PubMed] [Google Scholar]
- 35.McLucas E, Gallagher H, Rochev Y, Carroll WM, Gorelov A, Smith TJ. Global gene expression analysis of the effects of vinblastine on endothelial cells, when eluted from a thermo-responsive polymer. J Biomed Mater Res A 2006;79:246–53. [DOI] [PubMed] [Google Scholar]
- 36.Stoter G, Koopman A, Vendrik CP et al. Ten-year survival and late sequelae in testicular cancer patients treated with cisplatin, vinblastine, and bleomycin. J Clin Oncol 1989;7:1099–104. [DOI] [PubMed] [Google Scholar]
- 37.Izzedine H, Isnard-Bagnis C, Launay-Vacher V et al. Gemcitabine-induced thrombotic microangiopathy: a systematic review. Nephrol Dial Transplant 2006;21:3038–45. [DOI] [PubMed] [Google Scholar]
- 38.van Hell AJ, Haimovitz-Friedman A, Fuks Z, Tap WD, Kolesnick R. Gemcitabine kills proliferating endothelial cells exclusively via acid sphingomyelinase activation. Cell Signal 2017;34:86–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shah C, Bishnoi R, Jain A et al. Cardiotoxicity associated with carfilzomib: systematic review and meta-analysis. Leuk Lymphoma 2018;59:2557–2569. [DOI] [PubMed] [Google Scholar]
- 40.Yui JC, Van Keer J, Weiss BM et al. Proteasome inhibitor associated thrombotic microangiopathy. Am J Hematol 2016;91:E348–52. [DOI] [PubMed] [Google Scholar]
- 41.Fakhouri F, La Batide Alanore A, Rerolle JP, Guery B, Raynaud A, Plouin PF. Presentation and revascularization outcomes in patients with radiation-induced renal artery stenosis. Am J Kidney Dis 2001;38:302–9. [DOI] [PubMed] [Google Scholar]
- 42.Timmers HJ, Karemaker JM, Lenders JW, Wieling W. Baroreflex failure following radiation therapy for nasopharyngeal carcinoma. Clin Auton Res 1999;9:317–24. [DOI] [PubMed] [Google Scholar]
- 43.Sharabi Y, Dendi R, Holmes C, Goldstein DS. Baroreflex failure as a late sequela of neck irradiation. Hypertension 2003;42:110–6. [DOI] [PubMed] [Google Scholar]
- 44.Tonia T, Mettler A, Robert N et al. Erythropoietin or darbepoetin for patients with cancer. Cochrane Database Syst Rev 2012;12:CD003407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fournier JP, Sommet A, Bourrel R et al. Non-steroidal anti-inflammatory drugs (NSAIDs) and hypertension treatment intensification: a population-based cohort study. Eur J Clin Pharmacol 2012;68:1533–40. [DOI] [PubMed] [Google Scholar]
- 46.Goodwin JE, Geller DS. Glucocorticoid-induced hypertension. Pediatr Nephrol 2012;27:1059–66. [DOI] [PubMed] [Google Scholar]
- 47.Hoskova L, Malek I, Kopkan L, Kautzner J. Pathophysiological mechanisms of calcineurin inhibitor-induced nephrotoxicity and arterial hypertension. Physiol Res 2017;66:167–180. [DOI] [PubMed] [Google Scholar]
- 48.Nestler S, Levien P, Neisius A et al. Incidence of Cardiovascular Events after Nephrectomy - A Single Centre, Matched Pair Analysis between Donor and Tumour Nephrectomy in a Long Term Follow-Up. Urol Int 2016;97:142–7. [DOI] [PubMed] [Google Scholar]
- 49.Capitanio U, Larcher A, Cianflone F et al. Hypertension and Cardiovascular Morbidity Following Surgery for Kidney Cancer. European Urology Oncology 2019. [DOI] [PubMed]
- 50.Arai H, Saitoh S, Matsumoto T et al. Hypertension as a paraneoplastic syndrome in hepatocellular carcinoma. J Gastroenterol 1999;34:530–534. [DOI] [PubMed] [Google Scholar]
- 51.Kew MC, Leckie BJ, Greeff MC. Arterial hypertension as a paraneoplastic phenomenon in hepatocellular carcinoma. Arch Intern Med 1989;149:2111–2113. [PubMed] [Google Scholar]
- 52.van der Horst-Schrivers AN, Wymenga AN, Links TP, Willemse PH, Kema IP, de Vries EG. Complications of midgut carcinoid tumors and carcinoid syndrome. Neuroendocrinology 2004;80 Suppl 1:28–32. [DOI] [PubMed] [Google Scholar]
- 53.Stojanovic M, Goldner B, Ivkovic D. Renal cell carcinoma and arterial hypertension. Clin Exp Nephrol 2009;13:295–299. [DOI] [PubMed] [Google Scholar]
- 54.Takahashi K, Totsune K, Kitamuro T, Sone M, Murakami O, Shibahara S. Three vasoactive peptides, endothelin-1, adrenomedullin and urotensin-II, in human tumour cell lines of different origin: expression and effects on proliferation. Clin Sci (Lond) 2002;103 Suppl 48:35S–38S. [DOI] [PubMed] [Google Scholar]
- 55.Moreira DM, Gershman B, Lohse CM et al. Paraneoplastic syndromes are associated with adverse prognosis among patients with renal cell carcinoma undergoing nephrectomy. World J Urol 2016;34:1465–72. [DOI] [PubMed] [Google Scholar]
- 56.Lenders JW, Duh QY, Eisenhofer G et al. Pheochromocytoma and paraganglioma: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 2014;99:1915–42. [DOI] [PubMed] [Google Scholar]
- 57.Beard CM, Sheps SG, Kurland LT, Carney JA, Lie JT. Occurrence of pheochromocytoma in Rochester, Minnesota, 1950 through 1979. Mayo Clin Proc 1983;58:802–4. [PubMed] [Google Scholar]
- 58.Hamidi O, Young WF Jr., Iniguez-Ariza NM et al. Malignant Pheochromocytoma and Paraganglioma: 272 Patients Over 55 Years. J Clin Endocrinol Metab 2017;102:3296–3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Luton JP, Cerdas S, Billaud L et al. Clinical features of adrenocortical carcinoma, prognostic factors, and the effect of mitotane therapy. N Engl J Med 1990;322:1195–201. [DOI] [PubMed] [Google Scholar]
- 60.Veron Esquivel D, Batiz F, Farias Vega A, Carrillo Gonzalez PA. Adrenocortical carcinoma, an unusual cause of secondary hypertension. BMJ Case Rep 2016;2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Townsend RR, Taler SJ. Management of hypertension in chronic kidney disease. Nat Rev Nephrol 2015;11:555–63. [DOI] [PubMed] [Google Scholar]
- 62.Hogan JJ, Alexander MP, Leung N. Dysproteinemia and the Kidney: Core Curriculum 2019. Am J Kidney Dis 2019. [DOI] [PubMed]
- 63.Lowrance WT, Ordonez J, Udaltsova N, Russo P, Go AS. CKD and the risk of incident cancer. J Am Soc Nephrol 2014;25:2327–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xu H, Matsushita K, Su G et al. Estimated Glomerular Filtration Rate and the Risk of Cancer. Clin J Am Soc Nephrol 2019;14:530–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Denton MD, Magee CC, Ovuworie C et al. Prevalence of renal cell carcinoma in patients with ESRD pre-transplantation: a pathologic analysis. Kidney Int 2002;61:2201–9. [DOI] [PubMed] [Google Scholar]
- 66.Mertens AC, Liu Q, Neglia JP et al. Cause-specific late mortality among 5-year survivors of childhood cancer: the Childhood Cancer Survivor Study. J Natl Cancer Inst 2008;100:1368–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Agmon Nardi I, Iakobishvili Z. Cardiovascular Risk in Cancer Survivors. Curr Treat Options Cardiovasc Med 2018;20:47. [DOI] [PubMed] [Google Scholar]
- 68.Armenian SH, Xu L, Ky B et al. Cardiovascular Disease Among Survivors of Adult-Onset Cancer: A Community-Based Retrospective Cohort Study. J Clin Oncol 2016;34:1122–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Muntner P, Einhorn PT, Cushman WC et al. Blood Pressure Assessment in Adults in Clinical Practice and Clinic-Based Research: JACC Scientific Expert Panel. J Am Coll Cardiol 2019;73:317–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Muntner P, Shimbo D, Carey RM et al. Measurement of Blood Pressure in Humans: A Scientific Statement From the American Heart Association. Hypertension 2019. [DOI] [PMC free article] [PubMed]
- 71.Kallioinen N, Hill A, Horswill MS, Ward HE, Watson MO. Sources of inaccuracy in the measurement of adult patients’ resting blood pressure in clinical settings: a systematic review. J Hypertens 2017;35:421–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Johnson KC, Whelton PK, Cushman WC et al. Blood Pressure Measurement in SPRINT (Systolic Blood Pressure Intervention Trial). Hypertension 2018;71:848–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Roerecke M, Kaczorowski J, Myers MG. Comparing Automated Office Blood Pressure Readings With Other Methods of Blood Pressure Measurement for Identifying Patients With Possible Hypertension: A Systematic Review and Meta-analysis. JAMA Intern Med 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cohen JB, Cohen DL. Integrating Out-of-Office Blood Pressure in the Diagnosis and Management of Hypertension. Curr Cardiol Rep 2016;18:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cohen JB, Lotito MJ, Trivedi UK, Denker MG, Cohen DL, Townsend RR. Cardiovascular Events and Mortality in White Coat Hypertension: A Systematic Review and Meta-analysis. Ann Intern Med 2019. [DOI] [PMC free article] [PubMed]
- 76.Pierdomenico SD, Pierdomenico AM, Coccina F et al. Prognostic Value of Masked Uncontrolled Hypertension. Hypertension 2018;72:862–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Banegas JR, Ruilope LM, de la Sierra A et al. Relationship between Clinic and Ambulatory Blood-Pressure Measurements and Mortality. N Engl J Med 2018;378:1509–1520. [DOI] [PubMed] [Google Scholar]
- 78.Costa LJ, Varella PC, Del Giglio A. White coat effect in breast cancer patients undergoing chemotherapy. Eur J Cancer Care (Engl) 2003;12:372–3. [DOI] [PubMed] [Google Scholar]
- 79.Bamias A, Manios E, Karadimou A et al. The use of 24-h ambulatory blood pressure monitoring (ABPM) during the first cycle of sunitinib improves the diagnostic accuracy and management of hypertension in patients with advanced renal cancer. Eur J Cancer 2011;47:1660–8. [DOI] [PubMed] [Google Scholar]
- 80.Zamorano JL, Lancellotti P, Rodriguez Munoz D et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur Heart J 2016;37:2768–2801. [DOI] [PubMed] [Google Scholar]
- 81.Kronish IM, Kent S, Moise N et al. Barriers to conducting ambulatory and home blood pressure monitoring during hypertension screening in the United States. J Am Soc Hypertens 2017;11:573–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.American Heart Association and American Medical Association. Target:BP (Tools & Downloads). https://targetbp.org/tools-downloads/?sort=topic&topics=Patient-Measured%20BP&. Revised 25 July 2019. Accessed 28 Jul 2019.
- 83.McManus RJ, Mant J, Haque MS et al. Effect of self-monitoring and medication self-titration on systolic blood pressure in hypertensive patients at high risk of cardiovascular disease: the TASMIN-SR randomized clinical trial. JAMA 2014;312:799–808. [DOI] [PubMed] [Google Scholar]
- 84.Piper MA, Evans CV, Burda BU, Margolis KL, O’Connor E, Whitlock EP. Diagnostic and predictive accuracy of blood pressure screening methods with consideration of rescreening intervals: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med 2015;162:192–204. [DOI] [PubMed] [Google Scholar]
- 85.Self-Measured Blood Pressure Monitoring: Actions Steps for Clinicians. Atlanta, GA: Centers for Disease Control and Prevention, US Dept of Health and Human Services, 2014. [Google Scholar]
- 86.Melville S, Byrd JB. Out-of-Office Blood Pressure Monitoring in 2018. JAMA 2018;320:1805–1806. [DOI] [PubMed] [Google Scholar]
- 87.Cohen JB, Padwal RS, Gutkin M et al. History and Justification of a National Blood Pressure Measurement Validated Device Listing. Hypertension 2019;73:258–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Blood Pressure Devices Recommended by Hypertension Canada. https://hypertension.ca/hypertension-and-you/managing-hypertension/measuring-blood-pressure/devices/. 2018. Accessed 20 Jun 2018.
- 89.British and Irish Hypertension Society. BP Monitors. https://bihsoc.org/bp-monitors/. 2018. Accessed 20 Jun 2018.
- 90.Casiglia E, Tikhonoff V, Albertini F, Palatini P. Poor Reliability of Wrist Blood Pressure Self-Measurement at Home: A Population-Based Study. Hypertension 2016;68:896–903. [DOI] [PubMed] [Google Scholar]
- 91.Plante TB, Urrea B, MacFarlane ZT et al. Validation of the Instant Blood Pressure Smartphone App. JAMA Intern Med 2016;176:700–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kuwabara M, Harada K, Hishiki Y, Kario K. Validation of two watch-type wearable blood pressure monitors according to the ANSI/AAMI/ISO81060-2:2013 guidelines: Omron HEM-6410T-ZM and HEM-6410T-ZL. J Clin Hypertens (Greenwich) 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Saito K, Hishiki Y, Takahashi H. Validation of two automatic devices, Omron HEM-6232T and HEM-6181, for self-measurement of blood pressure at the wrist according to the ANSI/AAMI/ISO 81060-2:2013 protocol and the European Society of Hypertension International Protocol revision 2010. Vasc Health Risk Manag 2019;15:47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Reboussin DM, Allen NB, Griswold ME et al. Systematic Review for the 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2018;71:2176–2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wright JT Jr., Williamson JD, Whelton PK et al. SPRINT Research Group. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med 2015;373:2103–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cushman WC, Evans GW, Byington RP et al. ACCORD Study Group. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010;362:1575–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Agarwal M, Thareja N, Benjamin M, Akhondi A, Mitchell GD. Tyrosine Kinase Inhibitor-Induced Hypertension. Curr Oncol Rep 2018;20:65. [DOI] [PubMed] [Google Scholar]
- 98.Wright JT Jr., Bakris G, Greene T et al. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA 2002;288:2421–31. [DOI] [PubMed] [Google Scholar]
- 99.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013;3:1–150. [Google Scholar]
- 100.Yancy CW, Jessup M, Bozkurt B et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 2017;136:e137–e161. [DOI] [PubMed] [Google Scholar]
- 101.Li XY, Sun JF, Hu SQ. The renin-angiotensin system blockers as adjunctive therapy for cancer: a meta-analysis of survival outcome. Eur Rev Med Pharmacol Sci 2017;21:1375–1383. [PubMed] [Google Scholar]
- 102.Wright JT Jr., Dunn JK, Cutler JA et al. Outcomes in hypertensive black and nonblack patients treated with chlorthalidone, amlodipine, and lisinopril. JAMA 2005;293:1595–608. [DOI] [PubMed] [Google Scholar]
- 103.Leenen FH, Nwachuku CE, Black HR et al. Clinical events in high-risk hypertensive patients randomly assigned to calcium channel blocker versus angiotensin-converting enzyme inhibitor in the antihypertensive and lipid-lowering treatment to prevent heart attack trial. Hypertension 2006;48:374–84. [DOI] [PubMed] [Google Scholar]
- 104.Doerfler RM, Diamantidis CJ, Wagner LA et al. Usability Testing of a Sick-Day Protocol in CKD. Clin J Am Soc Nephrol 2019;14:583–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Carey RM, Calhoun DA, Bakris GL et al. Resistant Hypertension: Detection, Evaluation, and Management: A Scientific Statement From the American Heart Association. Hypertension 2018;72:e53–e90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Goh I, Lai O, Chew L. Prevalence and Risk of Polypharmacy Among Elderly Cancer Patients Receiving Chemotherapy in Ambulatory Oncology Setting. Curr Oncol Rep 2018;20:38. [DOI] [PubMed] [Google Scholar]
- 107.Kishore SP, Salam A, Rodgers A, Jaffe MG, Frieden T. Fixed-dose combinations for hypertension. Lancet 2018;392:819–820. [DOI] [PubMed] [Google Scholar]
- 108.Sacco M, Meschi M, Regolisti G et al. The relationship between blood pressure and pain. J Clin Hypertens (Greenwich) 2013;15:600–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bruehl S, Chung OY, Jirjis JN, Biridepalli S. Prevalence of clinical hypertension in patients with chronic pain compared to nonpain general medical patients. Clin J Pain 2005;21:147–53. [DOI] [PubMed] [Google Scholar]
- 110.Mallery LH, Allen M, Fleming I et al. Promoting higher blood pressure targets for frail older adults: a consensus guideline from Canada. Cleve Clin J Med 2014;81:427–37. [DOI] [PubMed] [Google Scholar]