Abstract
Introduction:
Tuberculous meningitis accounts for 1-5% of tuberculosis (TB) cases. Diagnostic delay contributes to poor outcomes. We evaluated the performance of the new Xpert MTB/Rif Ultra (Ultra) for TB meningitis diagnosis.
Methods:
In a prospective diagnostic accuracy study, we tested the cerebrospinal fluid (CSF) of adults presenting with suspected meningitis to Mulago and Mbarara Hospitals, Uganda. We centrifuged the CSF, resuspended the cell pellet in 2mL CSF and tested 0.5ml aliquots with Ultra, Xpert, and mycobacterial growth indicator tube (MGIT) culture. We quantified diagnostic performance against 1) the uniform case definition of probable or definite tuberculous meningitis and 2) a composite microbiological reference standard.
Findings:
From November 2016 to January 2019, we screened 466 adults with suspected meningitis and tested 204 for tuberculous meningitis. Uniform clinical case definition classified 51 participants as probable/definite tuberculous meningitis. Against this uniform case definition, Ultra had 76% sensitivity (39/51) and 93% (153/165) negative predictive value, compared with 56% (25/45; P=0·001) and 86% (121/141) for Xpert or 61% (27/44; P=0·02) and 85% (98/115) for culture. Against the composite reference standard, Ultra had sensitivity of 93% (39/42), higher than Xpert at 66% (25/38; p=0·006) or culture at 72% (27/37; p=0·09). Ultra detected nine tuberculous meningitis cases missed by Xpert and culture.
Interpretation:
Ultra detects tuberculous meningitis with higher sensitivity than Xpert and culture in this HIV-positive population. However, with a negative predictive value of 93% Ultra cannot be used as a ‘rule out’ test. Clinical judgement and novel highly sensitive point of care tests are still required.
Funding:
Investigators are supported by Wellcome, National Institute of Health, National Institute of Neurologic Diseases and Stroke, Fogarty International Center, and National Institute of Allergy and Infectious Diseases. The funders had no role in study design, data collection, data analysis, interpretation or writing.
Keywords: Xpert MTB/Rif Ultra, Tuberculous meningitis, TBM, diagnostic tests, extrapulmonary tuberculosis
RESEARCH IN CONTEXT
Evidence before this study:
In vitro studies of the Xpert MTB/RIF (Xpert) and Xpert MTB/Rif Ultra (Ultra) assays were performed on sputum samples spiked with M. tuberculosis H37Rv colony forming units (CFU), which found the limit of detection (LOD) to be 16 CFU/ml for the Ultra versus 113 CFU/ml for Xpert; an eight-fold reduction in LOD. This signified a promising step in the right direction for the diagnosis of paucibacillary TB. Since then a handful of prospective and retrospective studies have evaluated Ultra in adult pulmonary TB (n=4), paediatric TB (n=2), extrapulmonary TB (n=2), paucibacillary TB (n=1) and TB meningitis (n=1), all of which have found Ultra to have a greater sensitivity than Xpert to varying degrees. The largest prospective diagnostic accuracy to date, including 1753 participants with suspected pulmonary TB (PTB), found Ultra to be +17% more sensitive than Xpert (63% versus 46%) in smear-negative PTB and +13% (90% versus 77%) more sensitive in HIV-associated PTB, albeit with a more modest difference of +5.4% in the general PTB population. To date, only Bahr et al. have specifically focussed on tuberculous meningitis (TBM). Bahr tested 129 cryopreserved CSF samples from HIV-positive Ugandan adults and found the sensitivity of Ultra and Xpert to be 70% (16/23) and 43% (10/23) respectively against a uniform reference standard of probable/definite TBM. The sensitivity against a composite microbiological reference standard was 95% for Ultra and 45% for Xpert (p=0.001).
Added value of this study:
This prospective study of CSF tested in real-time by Ultra for tuberculous meningitis is the largest to date. It corroborates the findings from cryopreserved CSF samples and adds to the data on use of Ultra in an HIV-positive population and in a paucibacillary forms of TB. We tested each sample with Xpert and Ultra to allow direct head-to-head comparison of the performance of the two assays. To optimise the uniform case definition reference standard we endeavoured to exclude other non-TB aetiologies of meningitis by testing CSF with a meningoencephalitis PCR panel (testing for 14 viral, bacterial and fungal pathogens) and offering post-mortem examinations where participants died without a microbiologically-confirmed diagnosis.
Implications of all the available evidence:
Our results support the WHO recommendations to use Ultra as the initial test in TBM. With its run-time of 84 minutes, Ultra, in the context of appropriate infrastructure, holds potential to provide same-day results and facilitate prompt TBM treatment. That being said, Ultra’s imperfect negative predictive value means that clinical judgement must override a negative Ultra result and empiric TB treatment is still warranted in cases where there is a high index of suspicion.
INTRODUCTION
Worldwide, Mycobacterium tuberculosis (MTB) affected >10 million people in 2017 with devastating consequences, including 1.7 million deaths.1 Tuberculous meningitis (TBM), the most serious form of TB infection, accounts for between 1% to 5% of new cases of TB, and results in death or significant disability in over half of those affected.2 Outcomes are particularly dire in HIV co-infection, which confers a two to three-fold increased risk of death from TBM, approximately 50%.3 Another major driver of poor outcomes is diagnostic delay.4
Cerebrospinal fluid (CSF) smear microscopy with Ziehl-Neelsen staining for acid fast bacilli (AFB) is the cheapest and most widely available test for TBM diagnosis but is insensitive in most settings without expert microscopists.5,6,7 Culture takes a minimum of two weeks to provide results (too slow for clinical utility), has only moderate sensitivity (30-60%), and is not readily available in most settings within low and middle income TB endemic countries.8,6 Xpert MTB/Rif (Xpert, Cepheid), a rapid, automated, cartridge-based molecular test was endorsed by the World Health Organisation (WHO) in 2015 as the best initial test for tuberculous meningitis.9 Further, Xpert has been deployed in 130 of 145 of the countries eligible for concessional pricing as of 2016.10 Xpert provides 45-67% sensitivity to detect microbiologically proven TBM, meaning a negative result does not provide adequate confidence that TBM is not present.8,11 Thus, empirical anti-tuberculous therapy for TBM with its associated drug toxicities, drug-drug interactions, pill burden, and cost is still used commonly, often unnecessarily.
Subsequently, Xpert has been re-engineered as Xpert MTB/Rif Ultra (Ultra), which has a larger chamber (50μl) for DNA amplification, allowing double the volume of sample to reach the PCR reaction. Ultra also has two new multicopy DNA targets (IS6110, IS1081), incorporates fully-nested nucleic acid amplification and uses melting temperature-based analysis instead of real-time PCR to improve the accuracy of rifampicin resistance detection. In vitro, these changes have lowered the limit of detection of MTB eight-fold (16 vs 113 colony forming units/mL).12,13 Another important benefit of Ultra is the shortened run time of 84 minutes (compared to 112 minutes for Xpert), enabling an extra five tests to be run on each module a working day, equating to an extra 40 tests per day on an eight-module unit. In order to upgrade from Xpert, only new software and the Ultra cartridges are required – the same instrument may be used. Initial studies have shown that Ultra’s sensitivity is markedly improved over Xpert in smear-negative pulmonary TB (63% versus 46%),14 HIV-associated pulmonary TB (90% versus 77%)14 and paediatric TB (74% versus 63%).15 The largest published study to date of Ultra in TBM used cryopreserved CSF samples and found sensitivities of 95% (95%CI 77-99%) for Ultra and 45% (95%CI 24-68%) for each of Xpert and mycobacterial growth indicator tube (MGIT) culture against a composite microbiological reference standard.8 Against the uniform clinical case definition of probable/definite TBM,16 sensitivity was 70% (16/23, 95%CI 47-87%) for Ultra and 43% for both culture and Xpert.8 Four additional small studies on CSF (containing a minimum of 4 and a maximum of 43 samples) have corroborated a higher sensitivity of Ultra.17,18,19,20 Additional larger studies using real-time CSF samples and pre-specified reference standards are needed to better inform our current understanding of Ultra’s performance in tuberculous meningitis.
The aim of this study was to conduct a robust prospective diagnostic accuracy study of Ultra for the diagnosis of tuberculous meningitis using fresh CSF specimens from an HIV-positive population.
METHODS
Setting and study population
We prospectively evaluated consecutive adults presenting to Mulago National Referral Hospital, Kampala and Mbarara Regional Referral Hospital, Mbarara, Uganda with suspected meningitis (headache >3 days or altered mental status with clinical signs of meningism i.e. neck stiffness or Kernig’s sign) . Clinical history, physical examination and detailed neurological assessment findings were recorded as recommended by the TB meningitis international research consortium.21 Other diagnostic tests including urine TB- lipoarabinomannan (LAM) (Alere Determine), chest radiograph, abdominal ultrasonography, brain imaging and sputum Xpert MTB/Rif were performed as clinically indicated and locally available. The period of enrolment ran from 26th November 2016 to 24th January 2019 at Mulago National Referral Hospital and from 25th November 2016 to 13th June 2017 at Mbarara Regional Referral Hospital. Institutional review board and Uganda National Council of Science and Technology approvals were obtained and informed consent was obtained from participants or their surrogate (in patients with altered mental status). The study was conducted in line with the Standards for Reporting Diagnostic Accuracy Studies.22
Study procedures
Given the high prevalence of Cryptococcus and the similar initial presentations between cryptococcal meningitis and TBM, fingerstick cryptococcal antigen (CrAg) lateral flow assay (LFA) (Immy Inc, Norman, OK, USA) was performed at the participant’s bedside. Thereafter lumbar puncture was performed for all participants, CSF opening pressure was recorded, and CSF was collected into a sterile tube with a target volume of >6mL. At the bedside, CSF glucose and lactate were measured from a drop of CSF collected into an Eppendorf tube using a handheld One Touch glucometer (One Touch, Lifescan Inc, INV Scotland, UK) and point-of-care lactate meter (Nova Biomedical, MA, USA). Xpert and Ultra results were returned to the study team within 24 hours to guide treatment decisions.
Microbiological testing
Laboratory CSF testing included cell count and differential, Gram stain, aerobic bacterial culture, Ziehl-Neelsen AFB stain (Kampala only), total protein, CSF CrAg, Biofire FilmArray meningoencephalitis PCR panel (Biomerieux) for 14 meningoencephalitis pathogens (Streptococcus pneumoniae, Neisseria meningitidis, Listeria monocytogenes, Haemophilus influenzae, Streptococcus agalactiae, Escherichia coli, herpes simplex virus (HSV) types 1 and 2, cytomegalovirus (CMV), varicella zoster virus (VZV), human herpes virus 6, enterovirus, human parechovirus, and Cryptococcus). All samples with a negative CSF CrAg underwent comprehensive TB diagnostic studies. For TB analysis, the CSF was centrifuged at 3000g for 20 minutes to concentrate bacilli into the cell pellet. Supernatant was pipetted out to leave a residual volume of 2mL in which the cell pellet was re-suspended by vortexing. The resuspended cell pellet was then divided into four 0·5ml aliquots for: 1) Xpert; 2) Ultra; 3) MGIT culture; and 4) cryopreservation at −80¸C. The aliquots for Xpert and Ultra were mixed with 1.5ml of sample reagent, shaken vigorously, allowed to sit for 15 minutes and then run on the Cepheid platform. The 0·5ml aliquot for MGIT culture was added to the MGIT tube and incubated in a Bactec 960 instrument (Becton Dickinson, Franklin Lakes, NJ, USA). The algorithm for CSF diagnostic testing is illustrated in supplemental figure 1. Where the volume of CSF was below 6mL a step-wise approach was used to maximise the likelihood of yielding a diagnosis for the participant, as shown in supplemental table 1. At clinician discretion participants with a positive CSF CrAg were allowed to undergo TBM testing as cryptococcosis co-infection with TBM does rarely occur.23,24 In Mbarara the TB testing was done at the Médecins Sans Frontières Epicentre laboratory. In Kampala, testing was done at the Makerere University Microbiology laboratory, Makerere University TB laboratory (MGIT culture), and the Infectious Diseases Institute Translational laboratory (Xpert, Xpert Ultra). Clinical information was not available to the performers of the index or reference tests. The same laboratory technologist performed Xpert and Ultra but MGIT results were read and reported by a different technologist.
Where participants died during hospitalisation at Mulago Hospital and in the absence of a microbiologically confirmed diagnosis for their meningitis, the families of the deceased were invited to provide informed consent for post-mortem examination in the Makerere University mortuary by a trained pathologist. Post-mortem examinations were unavailable in Mbarara.
In addition, CSF samples collected from subjects with cryptococcal meningitis and who survived without TB therapy (negative controls) were run on Ultra from the same parent study ().
Statistical analysis
We assessed the diagnostic accuracy of Ultra against two different reference standards. First, we used the consensus uniform case definition of probable (≥10 points without brain imaging or ≥12 points with brain imaging) or definite TBM (microbiologically confirmed MTB).16 Second, we used a composite microbiological reference standard of any positive CSF test for MTB. Here we included the index test (Ultra) in the reference standard as the existing tests are imperfect and we judged the likelihood of detection of MTB DNA in the CSF of an HIV-infected person with aseptic meningitis in a TB endemic area representing a false positive result as extremely low.
Baseline clinical characteristics were compared between those with ‘definite TBM’ and ‘other meningitis’ via Wilcoxon rank-sum test for continuous variables and Pearson’s Chi squared or Fisher’s exact test for categorical variables. We directly compared the sensitivity of Ultra to Xpert or MGIT using McNemar’s test for paired categorical data. For comparing two diagnostic test performance against the uniform case definition, we used the Cochran–Mantel–Haenszel statistic for comparing matched categorical data across two strata of diagnostic tests versus probable/definite TBM uniform definition.16 Negative predicted value was calculated against the primary reference standard - the uniform case definition of probable/definite TBM. We counted invalid tests (e.g. culture contamination or Xpert error) as negative results. A sensitivity analysis was performed including only those participants who received all three tests. We performed univariate and multivariate logistic regression analysis to investigate parameters that correlated with microbiological confirmation of TBM. Variables which showed association in the crude analysis (likelihood ratio test P<0.1) were eligible to be included in the multivariate model. For paired analysis comparing the sensitivity of Ultra to Xpert and MGIT among those positive for TBM on the composite reference we assumed a third of pairs would give discordant results and that the sensitivity of Ultra would be 25% higher than Xpert and MGIT. Under these assumptions 39 TBM cases are required to give 80% power with alpha set to 5%. Assuming the prevalence of TBM will be 20% we aimed to recruit 200 participants. Stata version 13·1 was used for statistical analyses.
Role of the funding source
The funders had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
RESULTS
During the study period, 466 HIV-positive adults presented with suspected meningitis and consented to diagnostic lumbar puncture. Of these, 233 were diagnosed with cryptococcal meningitis, and 204 participants underwent TBM diagnostic testing. Of the 204 participants tested for TBM, 39 (19%) had a positive CSF CrAg but were still tested for TBM due to physician concern for TB co-infection and an additional 31 (15%) of those tested for TBM had a positive serum CrAg LFA but a negative CSF CrAg LFA. Biofire meningoencephalitis PCR was performed on 80 CSF samples. We collected a median of 11 mL [IQR 7-15mL] of CSF per participant, which left a median of 8 mL [IQR 5-11mL] of CSF to be spun down for TB testing after routine microbiology and chemistry testing had been performed. Collected CSF was clear in 186/204 (91%) of participants, with the remaining 18/204 (9%) having turbid CSF. Brain imaging was limited due to lack of a functioning CT scanner in the study sites (CT performed in 18 participants). No adverse events were attributable to the diagnostic testing.
Baseline characteristics of those tested for TBM, categorised by ‘definite TBM’ or ‘other meningitis’, are shown in table 1. Presence of CSF lymphocytes, CSF glucose, CSF protein, and CSF lactate differed significantly between those with and without microbiologically-confirmed TBM (P<0·05), table 1. The demographic, clinical and diagnostic details of the 42 participants with microbiologically-confirmed TBM is shown in supplemental table 2.
Table 1.
Baseline characteristics of subjects who underwent TBM testing
| CHARACTERISTICS | DEFINITE TB MENINGITISa N=42 | OTHER MENINGITISa N=162 | P VALUE |
|---|---|---|---|
| Age, years | 32 (29, 38) | 35 (28, 42) | 0.43 |
| Men, n, (%) | 23 (55%) | 94 (58%) | 0.73 |
| HIV positive, n (%)b | 41 (98%) | 154 (95%) | 0.47 |
| On antiretroviral therapy | 23 (55%) | 99 (62%) | 0.48 |
| Headache duration, days | 14 (7, 21) | 14 (5, 21) | 0.40 |
| Glasgow Coma Scale (GCS) | 13 (10, 14) | 14 (13, 15) | 0.001 |
| CD4 T cells/μLc | 57 (13, 108) | 46 (10, 188} | 0.83 |
| CSF opening pressure, cmH2O | 20 (10, 31.5) | 18 (13, 25.5) | 0.91 |
| Acellular CSF <5 cells/μL | 11 (26%) | 99 (61%) | <0.001 |
| CSF white cells/μL d | 170 (70, 283) | 100 (40, 275) | 0.09 |
| CSF lymphocytes | 100% (84%, 100%) | 100% (80%, 100%) | 0.61 |
| CSF total protein, g/L | 1.2 (0.9, 2.0) | 0.3 (0.2, 0.8) | <0.001 |
| CSF glucose, mmol/L | 1.2 (0.9, 2.0) | 2.9 (1.9, 4.4) | <0.001 |
| CSF lactate, mmol/mLe | 9.5 (4.6, 11) | 3.6 (2.4, 5.1) | <0.001 |
| Alive at hospital dischargef | 25 (58%) | 101 (71%) e | 0.2 |
Values are n (%) or median (IQR). P-values are from Wilcoxon rank-sum for continuous data and Fisher’s Exact test for categorical data.
‘Definite TB meningitis’ consists of 42 participants positive for TBM by the composite microbiologic reference standard. ‘Other meningitis’ is the remaining 162 participants which included n=112 unknown aetiologies and 50 confirmed aetiologies included 7 viral meningitis (HSV1 n=2, HSV2 n=2, VZV n=2, CMV n=1), 34 cryptococcal meningitis, 5 bacterial meningitis, 2 cerebral vascular accidents, 1 PML, 1 BIH.
In the ‘definite TBM’ group one person was HIV-negative. In the ‘other meningitis’ group six were HIV-negative and two had unknown HIV status.
n=63 with CD4 data (n=12 in definite TBM group, n=51 in other meningitis group)
median CSF WBC in those with CSF WBC >5 cells/μL
lactate available for n=18 in TBM definite and n=70 in ‘other meningitis’ group
Amongst the 143 with a known hospital outcome
Table 2.
Diagnostic performance of Xpert, Ultra and MGIT for the diagnosis of TBM
| CSF Test | N | Sensitivity vs. composite microbiologic endpoint a, b | P-value c | Sensitivity vs. case definition d,e | P-value d | NPV vs. case definition e | Specificity vs. case definition e |
|---|---|---|---|---|---|---|---|
| Xpert Ultra | 204 | 92.9% (39/42) [80.5 – 98.5%] | - | 76.5% (39/51) [62.5 – 87.2%] | - | 92.7% (153/165) [87.6 – 96.2%] | 100% (153/153) [97.6 – 100%] |
| Xpert MTB/Rif | 166 | 65.8% (25/38) [48.6 – 80.4%] | 0.006 | 55.6% (25/45) [44.0 – 70.4%] | 0.001 | 85.8% (121/141) [78.9 – 91.1%] | 100% (121/121) [97.0 – 100%] |
| MGIT Culture | 142 | 72.2% (27/37) [55.9 – 86.2%] | 0.09 | 61.4% (27/44) [45.5 – 75.6%] | 0.02 | 85.2% (98/115) [77.4 – 91.1%] | 100% (98/98) [96.3 – 100%] |
Values are percent (numerator/denominator) and [95% Confidence Interval]
Composite microbiological endpoint included any positive CSF test including ZN stain microscopy, Xpert, Xpert Ultra, or MGIT culture.
Specificity (and positive predictive value) versus the composite endpoint is by definition 100% as the index test is included in the reference standard of ‘definite’ TBM. If the Ultra result is excluded when assigning the case definition, the specificity of Ultra is 96% (153/160, 95% CI 91-98%) and the PPV is 82% (32/39, (5% CI 66-93%).
McNemar’s test comparing the sensitivity of Xpert or MGIT culture to Xpert Ultra.
Cochran–Mantel–Haenszel test comparing the distribution of Xpert or MGIT to Xpert Ultra results versus the uniform clinical standard of definite/probable TBM or not.
Composite case definition for probable or definite TBM is as per the published uniform definition
PPV: positive predictive value, NPV, negative predictive value
Of the 204 participants who underwent TBM diagnostic testing, Ultra was positive in 39, of which Xpert was also positive in 24 and MGIT culture in 24. Ultra was negative in 165 participants of which 162 were negative, and 3 were positive, by composite microbiological reference standard (3 MGIT culture positive, 1 Xpert positive), figure 1. Ultra detected nine cases of TBM that were not identified by Xpert or MGIT culture, figure 2. We diagnosed probable or definite tuberculous meningitis in 51/204 (25%) when including the Ultra result in assigning uniform case definition. If the Ultra result was excluded, only 44 participants would have been classified as probable/definite TBM. Of the 42 participants with definite TBM 5 (12%) had a positive CSF cryptococcal antigen, including 2 (5%) who had positive fungal cultures (quantitative cryptococcal culture (520000 and 20000 CFU/ml).
Figure 1: Flow diagram showing the diagnostic outcomes of the study population.
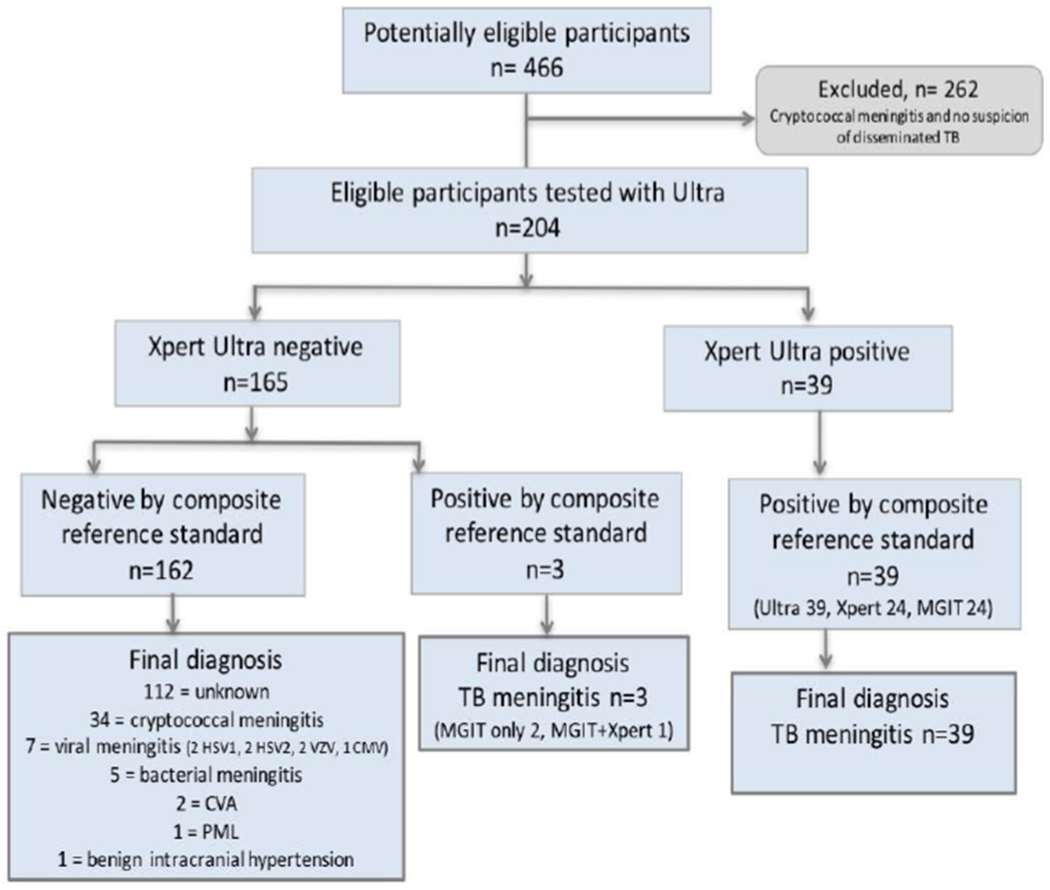
HSV = Herpes Simplex Meningitis, VZV = Varicella Zoster Meningitis, CMV = Cytomegalovirus, CVA = Cerebral Vascular Accident, PML = Progressive Multifocal Leukoencephalopathy
Figure 2: Venn diagram of positive diagnostic tests in the composite reference standard.
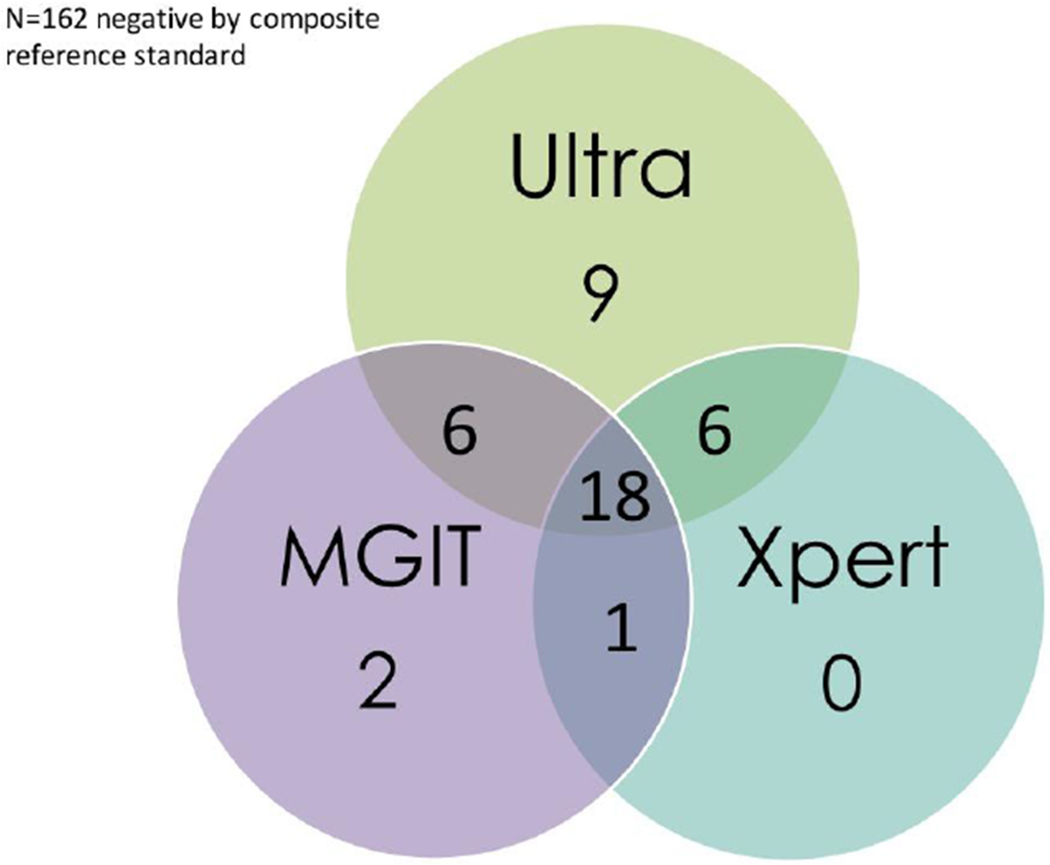
The Venn diagram displays 42 participants with confirmed TBM by either Xpert, Ultra, MGIT.
In the 162 participants without confirmed TBM we identified alternative aetiologies in 49 (30%): seven viral meningitis (HSV1 n=2, HSV2 n=2, VZV n=2, CMV n=1), 34 confirmed cryptococcal meningitis (culture or PCR positive), five bacterial meningitis (one confirmed S. pneumoniae on PCR, four clinical diagnosis based on CSF picture), two cerebral vascular accidents, one polymyeloleukoencephalopathy (PML), and one benign intracranial hypertension. In four cases without confirmed TBM post-mortem examinations were performed and attributed the causes of death to 1) HSV meningoencephalitis, PML, and disseminated TB without obvious central nervous system involvement; 2) PML; 3) pneumonia with no obvious CNS pathology; 4) meningoencephalitis with macroscopic appearance compatible with tuberculous meningitis, histopathological confirmation awaited.
Amongst the 42 participants with definite TBM 17 (41%) died in hospital with a median time to death of 4 days [IQR 2-6 days]. Amongst the other 162 meningitis cases hospital outcome was known in 143, of which 19 (13%) died in hospital.
Diagnostic performance
When compared to the uniform case definition (probable or definite TBM) Ultra, Xpert and MGIT displayed sensitivities of 76% (39/51), 56% (25/45) and 61% (27/44) respectively. Against the composite microbiologic reference standard, Ultra was 93% (39/42) sensitive, whilst Xpert was 66% (25/38) sensitive and MGIT culture was 73% (27/37) sensitive. Ultra was superior to Xpert at detecting TBM using either the composite microbiology reference (P=0·006) or uniform case definition (P=0·001). Ultra also appeared more sensitive than MGIT culture against either the composite microbiology reference (P=0·09) or uniform case definition (P=0·02). The negative predictive value of Ultra was 93% (153/165, 95% CI 88-96%) against the uniform case definition. The diagnostic accuracy results are summarised in table 2. On sensitivity analysis, whereby only participants who had received all 3 tests (n=117) were included, no major difference in performance of any of the tests was observed, see supplementary table 3.
Table 3.
Univariate and multivariate analyses of factors associated with microbiological confirmation of TBM
| Factor | Univariate model | Multivariate model | ||||||
|---|---|---|---|---|---|---|---|---|
| n | Odds Ratio | 95% CI | P value | n | Odds Ratio | 95% CI | P value | |
| Age, per year | 204 | 0·98 | 0·95 – 1·01 | 0·26 | - | - | - | |
| Male sex | 204 | 1·14 | 0·58 – 2·26 | 0·70 | - | - | - | |
| Days of headache | 168 | 1·00 | 0·98 – 1·01 | 0·95 | - | - | - | |
| GCS 15a | 72 | Reference | 35 | Reference | ||||
| GCS 11-14 | 97 | 3·62 | 1·39 – 9·39 | 0·008 | 46 | 3·71 | 0·84 – 16·48 | 0·08 |
| GCS≤10 | 33 | 5·5 | 1·8 – 16·6 | 0·003 | 21 | 4·71 | 0·93 – 23·82 | 0·06 |
| Log2 CD4 T cell count, cells/μl | 63 | 0·94 | 0·71 – 1·34 | 0·66 | - | - | - | |
| On antiretroviral therapy | 202 | 1·34 | 0·67 – 2·66 | 0·40 | - | - | - | |
| Log2 CSF volume, mL | 198 | 1·20 | 0·84 – 1·72 | 0·32 | - | - | - | |
| Log2 CSF volume spun down, ml | 96 | 1·40 | 0·84 – 2·35 | 0·20 | - | - | - | |
| CSF WBC pleocytosis | 189 | 4·94 | 2·28 – 10·72 | <0·001 | 101 | 2·04 | 0·33 – 12·74 | 0·44 |
| Log2 CSF glucose, mmol/L) | 122 | 0·31 | 0·18 – 0·54 | <0·001 | 101 | 0·33 | 0·17 – 0·65 | 0·001 |
| Log2 CSF lactate, mmol/Lb | 88 | 5·76 | 2·45 – 13·52 | <0·001 | - | - | - | |
| Log2 CSF protein, mg/dL | 166 | 2·10 | 1·52 – 2·89 | <0·001 | 101 | 1·02 | 0·56 – 1·87 | 0·25 |
Likelihood ratio test P value was 0·0001
CSF Lactate was excluded from the multivariate model due to the amount of missing data
No cases of rifampicin resistance were identified with either Xpert or Ultra. Ultra ‘trace’ category positive samples frequently have ‘indeterminate’ rifampicin resistance as >16 CFU/ml of MTB and amplification of the rpob DNA is required to generate a rifampicin susceptibility results. All 14 ‘trace’ positive results had indeterminate rifampicin resistance in our study.13 Culture-based drug susceptibility testing was not performed. MGIT culture reported ‘contaminated’ results in 13 (9%) of cultures performed.
Diagnostic performance by MRC disease grade
We analysed the sensitivity of all assays at MRC severity grade 1, 2 and 3 against both reference standards. Using the composite reference standard the sensitivity of Ultra was 100% in grade 1 disease, 96% in grade 2 and 82% in grade 3 disease. Using the uniform case definition the sensitivity of Ultra was 100% in grade 1 disease, 74% in grade 2 and 69% in grade 3 disease. The trend of decreasing sensitivity with advancing disease MRC severity grade held true for Xpert and MGIT, results are shown in supplementary table 5.
Negative controls
The CSF from 45 participants with confirmed cryptococcal meningitis and no clinical suspicion of TB co-infection were all negative on Ultra suggesting false positivity (e.g. laboratory contamination) is a rare occurrence (specificity 100%, 45/45, 95%CI, 92–100%).
Semi-quantitative categories
Of the 39 positive Ultra results, 14 were ‘trace’ category positive, 10 were ‘very low’, 7 were ‘low’, 5 were ‘medium’, none were ‘high’ and 3 were unknown. Of the 14 Ultra ‘trace’ positive samples only 4 were positive on Xpert (3 ‘very low’, 1 ‘low’) and 6 were positive on culture. Median time to CSF culture positivity was 14 days [IQR10,15]. Median time to positivity was 14·5, 15, 11·5, 8·5 days in trace, very low, low, medium categories respectively (supplemental figure 2 and supplemental table 4).
Predictors of microbiologically confirmed TBM
Univariate analysis found that Glasgow coma scale (≤10 and 11-14 compared with 15), >5 lymphocytes/μl CSF, low CSF glucose, higher CSF protein, and high CSF lactate were all strongly positively associated with microbiological confirmation of TBM (table 3). High log2 CSF lactate was strongly associated with definite TBM diagnosis (Odds Ratio (OR) = 5·76, 95%CI 2.45 – 13.52, P<0·001), however this was not taken through to the multivariate model due to the amount of missing data. In multivariate logistic regression model lower log2 CSF glucose (adjusted OR = 0·33 95%CI, 0·17 – 0.65; P=0·001) remained strongly positively associated with microbiological confirmation of TBM, though complete case analysis was restricted to 101 participants (table 3). The median volume of CSF spun down for TB testing was 8ml [IQR 5,11] and each doubling (log2 increase) of CSF volume spun down increased the odds of TB confirmation by 40%, although this did not achieve statistical significance (OR 1·4 95%CI 0·84 – 2.35, P=0·2).
Participants positive with Ultra in isolation
Of the 9 participants positive only by Ultra, 6 had been initiated on ART within the preceding 6 weeks (median duration 28 days [IQR 17,35 days]), a timing consistent with unmasking TBM.
DISCUSSION
In this HIV-positive population with suspected meningitis, Ultra demonstrated higher sensitivity to detect tuberculous meningitis (93%) than either Xpert (66%) or MGIT culture (72%) against a composite microbiologic reference standard and the uniform case definition. These findings on fresh CSF corroborate our earlier findings from cryopreserved CSF supporting the robustness of this data.8 Importantly, over a third (14/39) of positive Ultra results were in the ‘trace’ category (correlating to <100 CFU/ml), usually below the limit of detection of Xpert and potentially below the limit of detection of TB culture. Of these 14 Ultra ‘trace’ positive samples only 4 were positive by Xpert (3 ‘very low’, 1 ‘low’) and 6 by culture. The ability to detect non-viable organisms helps improve detection for Xpert and Ultra versus culture. We did not include participants with ‘possible’ TB meningitis (n=74) in our analysis due to the imprecise nature of this category in this study population with advanced HIV (median CD4 T cell count 46 cells/μL [IQR 11,130]).
With increasing market penetration of the Xpert platform in high TB burden countries 25 and a turnaround time of 84 minutes, Ultra is the best point of care test for TB meningitis currently available and has been endorsed by WHO as the best initial test for TBM.26 Yet, our study demonstrates a more modest 76% sensitivity of Ultra against a uniform case definition of probable/definite TBM giving a negative predictive value of 93%. In other words, nearly one fifth of patients (9 of 51) with clinical phenotypes and CSF profiles highly suggestive of TBM, and negative for all other tested pathogens, were negative by Ultra (and also Xpert and culture). Four of these participants died from their illness, one with macroscopic post-mortem findings compatible with TB meningitis. Our belief is that a proportion of these are false negative results but this remains to be determined in future studies. One might also consider testing CSF with Ultra on multiple days if safe to do so, we have employed this strategy with success in a number of patients (outside of this study). Markers of host response should also be considered as potential future adjunctive diagnostic tests, although to date, none have been successful.27 Currently, clinical judgement remains relevant when making treatment decisions regarding TBM.11
Multivariate analysis showed low CSF glucose to be strongly associated with microbiologically-confirmed TBM versus other forms of meningitis. CSF lactate could not be taken through to multivariate model due to the amount of missing data but was found to be strongly associated with microbiologically-confirmed TBM in the univariate model and warrants further investigation as a diagnostic marker. Handheld glucometers and lactate meters are true point of care tests – with results available at the bedside within a matter of seconds. A low CSF glucose (<2.2 mmol/L or <40 mg/dL or CSF:plasma glucose ratio <50%) in a patient with symptoms and signs suggestive of TBM, once bacterial meningitis or Cryptococcus have been excluded, is a good indication to consider antituberculous therapy regardless of TB-specific test results. The role of CSF glucose and lactate as components of a comprehensive diagnostic algorithm focused on probability of diagnosis, should be explored in the future.
We believe CSF Ultra to be highly specific for the diagnosis of tuberculous meningitis, though the lack of a perfect reference standard makes this difficult to prove. Further, the inclusion of Ultra in the composite reference standard does risk incorporation bias and is a limitation of this study. Yet, unlike the lungs where sputum can remain ‘trace’ category positive on Ultra even years after treatment of prior pulmonary TB,28 the CSF is a sterile body fluid that turns over 4-5 times daily.29 Presence of MTB in the CSF progresses to death if untreated. In this study of hospitalized patients with advanced HIV from a TB-endemic country, and symptoms of sub-acute meningitis with no other cause identified despite extensive testing, we feel that the probability of a false positive DNA-based test is negligible.
The median total volume of CSF spun down for TB testing was 8 mL; however, this was then divided up four ways (median of ~2.0 mL per diagnostic test). We used an algorithm for stepwise dropping of tests when the CSF volume collected was <6mL (supplemental table 1), such that the minimal input CSF volume always remained ≥2.0 mL per diagnostic test, to maximise the likelihood of obtaining an accurate result for the patient. Each doubling (log2 increase) of CSF volume spun down increased the odds of TB confirmation by 40%, although this did not achieve statistical significance (OR 1·4 95%CI 0·84 – 2.35, P=0·2). Xpert has been noted in some studies to have improved performance when higher CSF volumes are used, this study cannot inform whether the same is true for Ultra.5,30 The stepwise use of tests in low volume samples resulted in imperfect matching with 204 Ultra and 166 Xpert tests. There was also the real-world reality of the problems of culture, beyond diagnostic delay. Only 142 culture results were received (including 13 contaminated) due to a number being lost when sent to an external mycobacterial laboratory.
It is interesting to note that 11/42 (26%) with microbiologically-confirmed TBM had an acellular CSF (WBC <5 cells/μl). All those with acellular CSF also had CSF opening pressure and protein within the normal range, and lower median CSF lactate (CSF lactate 4.6, versus 9.5 in the definite TBM group). We have previously reported 1 in 3 of our definite TBM patients having an acellular CSF, a finding also noted in other HIV-positive cohorts outside of Asia.31 This observed absence of inflammatory response could be explained immuneparesis secondary to advanced HIV disease (CD4 T cell count known in 2/11 and was 3 cells/μl and 6 cells/μl), or by early presentation (3/11 had MRC grade 1 disease). Mortality was high in this sub-group despite the apparent absence of CSF inflammation, 6/11 (55%) died in hospital. Low CSF white cell count has been associated with death in a multivariate prognostic model in Vietnamese adults with TBM, and it is becoming increasingly clear that both absence inflammation, as well as excessive inflammation, are deleterious in TBM.2,32
Interestingly, the sensitivity of all assays was highest in early disease (MRC grade 1) and sensitivity decreased as disease severity progressed. Although the numbers are small and this analysis was post-hoc, this supports the notion that increasing immune response (i.e. inflammation) aimed to control bacillary load is the mediator of disease. Thereby, an inverse relationship may actually exist between bacillary load and disease severity in this population. This warrants further investigation with immunology studies and highlights the importance of finding the optimal host-directed therapy to control damaging inflammation in this population.
When translating these results to the field it is important to consider that in many hospital laboratory settings centrifuging the CSF may not be feasible, in which case we would advise loading 2mL of CSF directly into the Ultra cartridge to maximise the bacillary load, as dilution with sample reagent is not required for CSF. In addition, while our cohort cannot inform the diagnostic performance in an HIV-negative population, our paired analysis of specimens conclusively demonstrates that Xpert Ultra performs better than Xpert in an HIV-infected population. Whether Ultra can improve outcomes from TBM or lessen unnecessary exposures to TBM medications remains to be determined. In this prospective study, with real-time Ultra results available to the clinical team within 24 hours, in-hospital mortality was 40% (17/42) compared with 50% (11/22) in our prior study on cryopreserved CSF where results were not available to guide management decisions.8,33 We cannot say whether this observed reduction in mortality is attributable to the availability of real-time Ultra results as this study was not designed to do so. Additionally, this study included two clinical sites (Mulago and Mbarara Hospitals) whereas the prior study was conducted in Mbarara Hospital alone. Further, given the time between the current study and the prior study, additional potential confounding factors may be present which contribute towards the mortality difference. In addition to access to improved point of care diagnostics, earlier presentation to hospital, better supportive care and optimised antimicrobial and anti-inflammatory treatments are required to tackle this high case-fatality. Larger structural factors such as poverty and weak health systems also clearly continue to play a role in the high mortality of TB/HIV and need to be addressed. At present, regular use of empiric therapy continues to be required for tuberculous meningitis.
In conclusion, Ultra is a substantial improvement in rapid and accurate diagnosis of TB meningitis, and is superior to Xpert. However, Ultra does not represent a perfect ‘rule out’ test. Clinical judgement and empiric therapy remain important to improve outcomes for those with TBM. In this exciting era of rapidly evolving molecular diagnostics and biomarkers, discovery of a highly sensitive true point of care test which can facilitate rapid treatment and give the clinician confidence in rationalizing the use of empiric TB treatment, is a realistic goal.
Supplementary Material
Acknowledgements
This research was made possible through support from the National Institute of Neurologic Disorders and Stroke (R01NS086312, K23NS110470), the Fogarty International Center (K01TW010268, K43TW010718), and National Institute of Allergy and Infectious Diseases (T32AI055433). FVC is supported through a Wellcome Trust Clinical PhD Fellowship (Grant no. 210772/Z/18/Z). FVC is an honorary fellow of the Makerere University – Uganda Virus Research Institute Centre of Excellence for Infection and Immunity Research and Training (MUII-plus). MUII-plus is supported through the DELTAS Africa Initiative (Grant no. 107743). The DELTAS Africa Initiative is an independent funding scheme of the African Academy of Sciences (AAS), Alliance for Accelerating Excellence in Science in Africa (AESA) and supported by the New Partnership for Africa’s Development Planning and Coordinating Agency (NEPAD Agency) with funding from the Wellcome Trust (Grant no. 107743) and the UK Government. The MRC/UVRI and LSHTM Uganda Research Unit is jointly funded by the UK Medical Research Council (MRC) and the UK Department for International Development (DFID) under the MRC/DFID Concordat agreement and is also part of the EDCTP2 programme supported by the European Union.
We would like to acknowledge the technologists at Makerere University Microbiology Laboratory and MSF Epicentre Laboratory and the study participants and their caregivers. We would like to thank Mr Joshua Matovu for assisting with the running of some of the assays in the Infectious Diseases Institute Translational Laboratory.
Footnotes
Conflicts of Interest
The authors declare no competing interests.
REFERENCES
- 1.Organisation WH. Global Tuberculosis Report 2017. Geneva: World Health Organisation, 2017. [Google Scholar]
- 2.Thao LTP, Heemskerk AD, Geskus RB, et al. Prognostic Models for 9-Month Mortality in Tuberculous Meningitis. Clinical Infectious Diseases. 2018; 66(4): 523–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heemskerk AD, Bang ND, Thwaites GE. Therapy for Tuberculous Meningitis. The New England Journal of Medicine 2016; 374(22): 2188–9. [DOI] [PubMed] [Google Scholar]
- 4.Sheu JJ, Yuan RY, Yang CC. Predictors for outcome and treatment delay in patients with tuberculous meningitis. The American journal of the medical sciences 2009; 338(2): 134–9. [DOI] [PubMed] [Google Scholar]
- 5.Bahr NC, Tugume L, Rajasingham R, et al. Improved diagnostic sensitivity for tuberculous meningitis with Xpert((R)) MTB/RIF of centrifuged CSF. The international journal of tuberculosis and lung disease. 2015; 19(10): 1209–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heemskerk AD, Donovan J, Thu DDA, et al. Improving the microbiological diagnosis of tuberculous meningitis: A prospective, international, multicentre comparison of conventional and modified Ziehl-Neelsen stain, GeneXpert, and culture of cerebrospinal fluid. J Infect. 2018; 77(6): 509–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cresswell FV, Bangdiwala AS, Bahr NC, et al. Can improved diagnostics reduce mortality from Tuberculous meningitis? Findings from a 6.5-year cohort in Uganda. Wellcome open research 2018; 3: 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bahr NC, Nuwagira E, Evans EE, et al. Diagnostic accuracy of Xpert MTB/RIF Ultra for tuberculous meningitis in HIV-infected adults: a prospective cohort study. The Lancet Infectious Diseases 2018; 18(1): 68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organisation. Policy Update: Xpert MTB/RIF assay for the diagnosis of pulmonaru and extrapulmonary TB in adults and children. Geneva, 2015. [Google Scholar]
- 10.World Health Organisation. Xpert MTB/RIF roll out 2016. http://www.who.int/tb/areas-of-work/laboratory/mtb-rif-rollout/en/ (accessed 1st March 2019.
- 11.Bahr NC, Marais S, Caws M, et al. GeneXpert MTB/Rif to Diagnose Tuberculous Meningitis: Perhaps the First Test but not the Last. Clinical Infectious Diseases. 2016; 62(9): 1133–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organisation. Non-inferiority analysis of Xpert MTB/RIF Ultra compared to Xpert MTB/RIF. Geneva: World Health Organisation 2017. [Google Scholar]
- 13.Chakravorty S, Simmons AM, Rowneki M, et al. The New Xpert MTB/RIF Ultra: Improving Detection of Mycobacterium tuberculosis and Resistance to Rifampin in an Assay Suitable for Point-of-Care Testing. mBio 2017; 8(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dorman SE, Schumacher SG, Alland D, et al. Xpert MTB/RIF Ultra for detection of Mycobacterium tuberculosis and rifampicin resistance: a prospective multicentre diagnostic accuracy study. The Lancet Infectious diseases 2018; 18(1): 76–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nicol MP, Workman L, Prins M, et al. Accuracy of Xpert Mtb/Rif Ultra for the Diagnosis of Pulmonary Tuberculosis in Children. Pediatr Infect Dis J 2018; 37(10): e261–e3. [DOI] [PubMed] [Google Scholar]
- 16.Marais S, Thwaites G, Schoeman JF, et al. Tuberculous meningitis: a uniform case definition for use in clinical research. The Lancet Infectious Diseases 2010; 10(11): 803–12. [DOI] [PubMed] [Google Scholar]
- 17.Wu X, Tan G, Gao R, et al. Assessment of the Xpert MTB/RIF Ultra assay on rapid diagnosis of extrapulmonary tuberculosis. International journal of infectious diseases : IJID : official publication of the International Society for Infectious Diseases 2019; 81: 91–6. [DOI] [PubMed] [Google Scholar]
- 18.Perez-Risco D, Rodriguez-Temporal D, Valledor-Sanchez I, Alcaide F. Evaluation of the Xpert MTB/RIF Ultra Assay for Direct Detection of Mycobacterium tuberculosis Complex in Smear-Negative Extrapulmonary Samples. Journal of Clinical Microbiology 2018; 56(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chin JH, Musubire AK, Morgan N, et al. Xpert MTB/RIF Ultra for the Detection of Mycobacterium tuberculosis in Cerebrospinal Fluid. Journal of clinical microbiology 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang G WS, Jiang G, Yang X, Huang M, Huo F. Xpert MTB/RIF Ultra improved the diagnosis of paucibacillary tuberculosis: A prospective cohort study. J Infect 2019; 78(4): 311–6. [DOI] [PubMed] [Google Scholar]
- 21.Marais BJ, Heemskerk AD, Marais SS, et al. Standardized Methods for Enhanced Quality and Comparability of Tuberculous Meningitis Studies. Clinical Infectious Diseases. 2017; 64(4): 501–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cohen JF, Korevaar DA, Altman DG, et al. STARD 2015 guidelines for reporting diagnostic accuracy studies: explanation and elaboration. BMJ Open 2016; 6(11): e012799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ellis J, Cresswell FV, Joshua R, Kenneth S, Boulware DR. Cryptococcal Meningitis and Tuberculous Meningitis Co-infection in HIV-Infected Ugandan Adults. Open Forum Infectious Diseases 2018; 5(8): ofy193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ellis J, Cresswell FV, Rhein J, Ssebambulidde K, Boulware DR. Cryptococcal Meningitis and Tuberculous Meningitis Co-infection in HIV-Infected Ugandan Adults. Open Forum Infect Dis 2018; 5(8): ofy193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cazabon D, Alsdurf H, Satyanarayana S, et al. Quality of tuberculosis care in high burden countries: the urgent need to address gaps in the care cascade. International Journal of Infectious Diseases. 2017; 56: 111–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.World Health Organization. WHO Meeting Report of a Technical Expert Consultation: Non-inferiority analysis of Xpert MTB/RIF Ultra compared to Xpert MTB/RIF. 2017. http://www.who.int/tb/publications/2017/XpertUltra/en/ (accessed 24 March 2017.
- 27.Bahr NC, Meintjes G, Boulware DR. Inadequate diagnostics: the case to move beyond the bacilli for detection of meningitis due to Mycobacterium tuberculosis. Journal of medical microbiology 2019; 68(5): 755–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Theron G, Venter R, Calligaro G, et al. Xpert MTB/RIF Results in Patients With Previous Tuberculosis: Can We Distinguish True From False Positive Results? Clinical Infectious Diseases. 2016; 62(8): 995–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sakka L, Coll G, Chazal J. Anatomy and physiology of cerebrospinal fluid. Eur Ann Otorhinolaryngol Head Neck Dis 2011; 128(6): 309–16. [DOI] [PubMed] [Google Scholar]
- 30.Heemskerk AD, Donovan J, Thu DDA, et al. Improving the microbiological diagnosis of tuberculous meningitis: A prospective, international, multicentre comparison of conventional and modified Ziehl-Neelsen stain, GeneXpert, and culture of cerebrospinal fluid. The Journal of Infection 2018; 19(6): 1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cresswell FV, Bangdiwala AS, Meya DB, et al. Absence of cerebrospinal fluid pleocytosis in tuberculous meningitis is a common occurrence in HIV co-infection and a predictor of poor outcomes. International Journal of Infectious Diseases. 2018; 68: 77–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thuong NTT, Heemskerk D, Tram TTB, et al. Leukotriene A4 Hydrolase Genotype and HIV Infection Influence Intracerebral Inflammation and Survival From Tuberculous Meningitis. The Journal of Infectious Diseases 2017; 215(7): 1020–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cresswell FV, Bangdiwala AS, Bahr NC, et al. Tuberculous meningitis diagnosis and outcomes during the Xpert MTB/Rif era: a 6.5-year cohort study in Uganda. Wellcome Open Research 2018; 3(64). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


