SUMMARY
BALB/c mice, infected with human metapneumovirus (hMPV), were treated with NMSO3, one dose of 50 mg/kg given at the time of infection. NMSO3 significantly reduced viral replication in the lungs, as well as hMPV-induced body weight loss, pulmonary inflammation and cytokine production, suggesting that antiviral treatment initiated at the beginning of viral infection can modify hMPV-induced disease.
Human metapneumovirus (hMPV) is a recently identified human respiratory viral pathogen belonging to the Paramyxoviridae family (van den Hoogen et al., 2001). HMPV is associated with both upper and lower respiratory tract infections in children and adults, as reported in studies worldwide. According to the different reports, between 5 and 12% of all respiratory tract infections in younger infants are caused by hMPV, second only to respiratory syncytial virus (RSV), and it may account for 10% of all hospitalizations of elderly patients with respiratory tract infections [Reviewed in (Hamelin & Boivin, 2005)]. No specific antivirals or other therapeutic agents are licensed to treat hMPV infection. Similarly, no vaccine is currently available. We and others have recently identified BALB/c mice as an effective murine model to investigate hMPV-induced airway disease (Alvarez et al., 2004;Guerrero-Plata et al., 2005;Hamelin et al., 2005). Ribavirin, a nucleoside analog of guanosine used in RSV infection, has been recently shown to affect hMPV replication in vitro and in vivo (Hamelin et al., 2006;Wyde et al., 2003). Similarly, NMSO3, a sulfated sialyl lipid with potent antiviral activity against RSV, has been recently shown to inhibit hMPV replication in vitro (Wyde et al., 2004). In this study, we assessed the effect of NMSO3 treatment on hMPV replication and pulmonary inflammation in vivo, using a BALB/c model of infection.
Animal studies
Six to seven wk old female BALB/c mice (Harlan, Houston, TX) were housed in pathogen-free conditions. A total of four experimental groups consisting of two treatment groups, vehicle and NMSO3, for each infection group, sham and hMPV, were used for all experiments. For each experiment, we used four to five mice/group. Experiments were repeated two to three times. There was no effect of the vehicle by itself, therefore the groups sham + vehicle and hMPV + vehicle are identified in the text and figures as sham and hMPV. Under light anesthesia, mice were inoculated intranasally (i.n.) with 1×107 TCID50 hMPV, in a total volume of 50 μl. This inoculum has been shown to deliver the virus directly to the lungs. Thirty to sixty minutes after viral inoculation, mice were administered NMSO3 i.n. at a concentration of 10, 50, or 100 mg/kg in a total volume of 50 μl. NMSO3 was prepared in water at a stock concentration of 100 mg/ml and then diluted in PBS at the final desired concentration right before administration. Mice were weighed daily from day 0 to day 10 post-infection (p.i.).At day 1 and 4 p.i., mice were sacrificed to determine lung viral titer, cellular inflammation, and to measure cytokine and chemokine production, as previously described (Guerrero-Plata et al., 2005;Guerrero-Plata et al., 2006;Haeberle et al., 2001).
Effects of NMSO3 on hMPV infection
We initially examined whether NMSO3 altered hMPV replication in vivo. We tested 3 different concentrations of NMSO3, given on the first day of hMPV infection, based on a previous study that used NMSO3 in a rat model of RSV infection, showing amelioration of RSV-induced clinical illness (Kimura et al., 2000). In our mouse model of hMPV infection, increased viral titer can be detected as early as day 3 p.i., with peak viral titer occurring at day 5 p.i. and viral clearance by day 7 p.i. (data not shown). NMSO3 administration of 10 mg/kg only marginally reduced hMPV replication from 4.5+/-0.1 to 4.1+/- 0.05 log10, while treatment with 50 mg/kg significantly decreased peak viral load of about one log, from 5.12+/-0.31 to 4.1+/- 0.32 log10. Administration of 100 mg/kg resulted in further reduction of viral replication (5.2+/-0.1 to 3.9+/- 0.2 log10), however it was also associated with toxicity, as indicated by increased body weight loss, an important parameter of hMPV-induced clinical illness (see below), in the animals infected and treated with NMSO3 compared to the animals infected only.
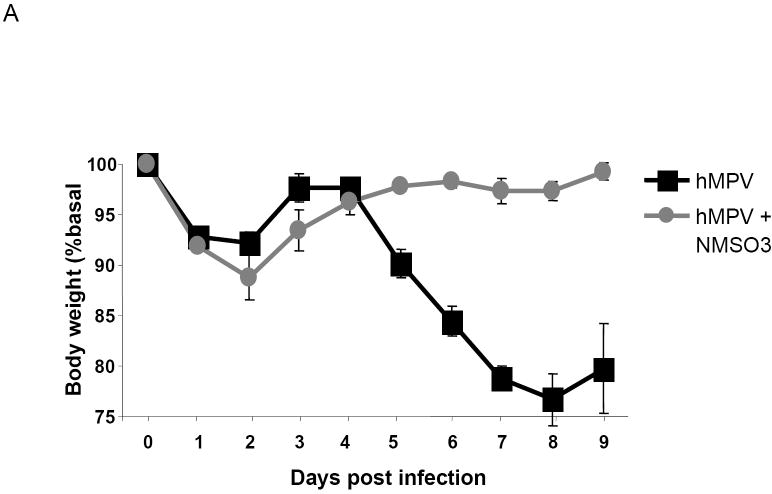
Mice infected with 1×107 TCID50 of hMPV lost between 5 to 10% of original body weight, with a peak that occurred between day 1 and 2 p.i. They gradually returned to their original weight, and then started to lose weight again for an additional four to five days, before returning to baseline. Sham-infected mice did not lose weight (data not shown). HMPV-infected mice treated with NMSO3 showed a similar body weight loss in the first three days of infection, however they never lost additional weight after returning to their baseline, as shown in Fig.1A.
Fig. 1. Effect of NMSO3 administration on hMPV-induced body weight loss.
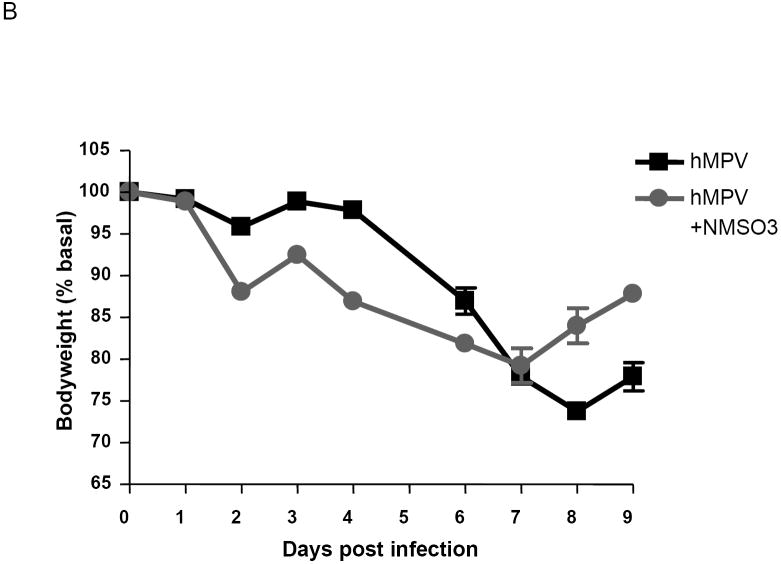
Mice were treated with 50 mg/kg of NMSO3 at the time of hMPV infection (1A) or 24 h post-infection (1B). The following is a representative diagram of three different experiments. All data points represent the mean of four to five animals.
We then tested the efficacy of NMSO3 50mg/kg given one day p.i. NMSO3 treatment was still effective in reducing viral replication, although not as well as administration right after infection (reduction of about 2/3 of a log versus a log in viral titers). Mice infected with hMPV and treated with NMSO3 the day after infection showed a body weight loss similar to the untreated infected mice, however, they seemed to recover faster than the untreated mice, as shown in Fig.1B. As administering 50 mg/kg at the time of infection seemed to be the most effective treatment in modulating hMPV-induced clinical disease, all subsequent experiments were performed using this treatment protocol.
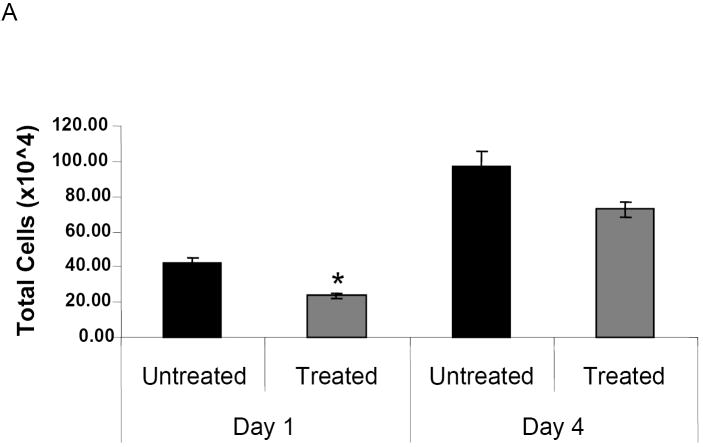
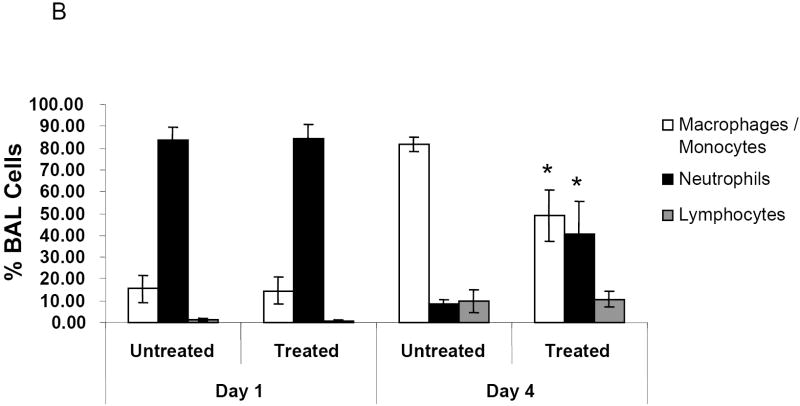
To determine whether NMSO3 altered hMPV-induced lung inflammation, total and differential cell counts in bronchoalveolar lavages (BALs) were measured. In sham-infected mice, total lung cell count was between 1 and 1.5×104, with macrophages representing the majority of the cell population. We observed a significant attenuation of total cellular influx by NMSO3 treatment in HMPV infected mice at day 1 p.i., with a similar trend at day 4 p.i. (p 0.056) (Fig. 2A). HMPV infection induces a significant lung recruitment of neutrophils, within the first three days of infection, while mononuclear cells, including macrophages/monocytes and lymphocytes, start to increase at day 3 p.i., representing the majority of the lung inflammatory cells from day 5 p.i. (Kolli D et al., 2008). NMSO3 administration did not cause significant changes in the distribution of inflammatory cell population at day 1 p.i., while there was a significant increase in neutrophil recruitment in the airways by day 4 p.i., between treated and untreated groups (Fig. 2B).
Fig. 2. Effect of NMSO3 on airway inflammation.
Panel A. Total number of cells was measured in BAL of hMPV-infected mice, either untreated or treated with NMSO3 at day 1 and 4 p.i. Data are expressed as mean ± standard deviation and representative of three different experiments. *, P< 0.01 relative to HMPV-infected mice. Panel B. Differential cell count was measured in BAL of hMPV-infected, either untreated or treated with NMSO3, at day 1 and 4 .i. *, P< 0.01 relative to hMPV-infected mice.
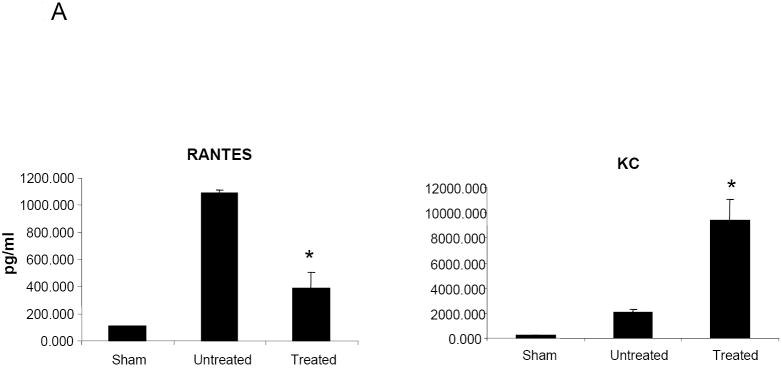
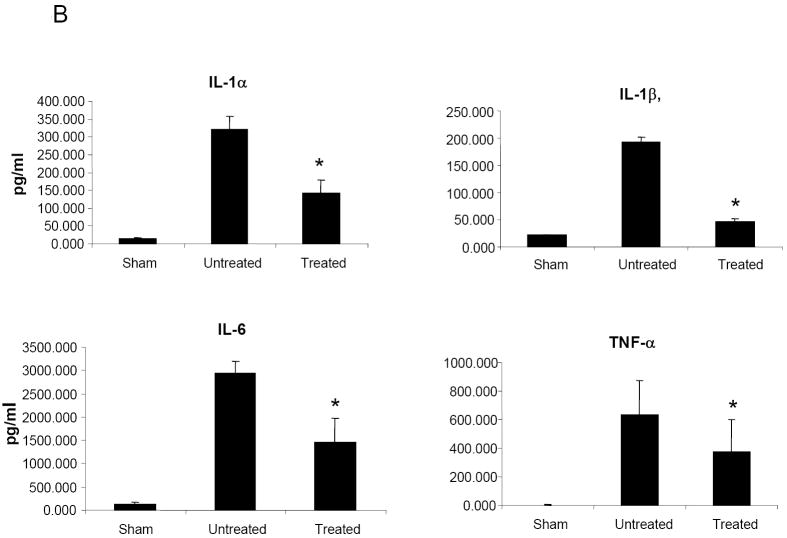
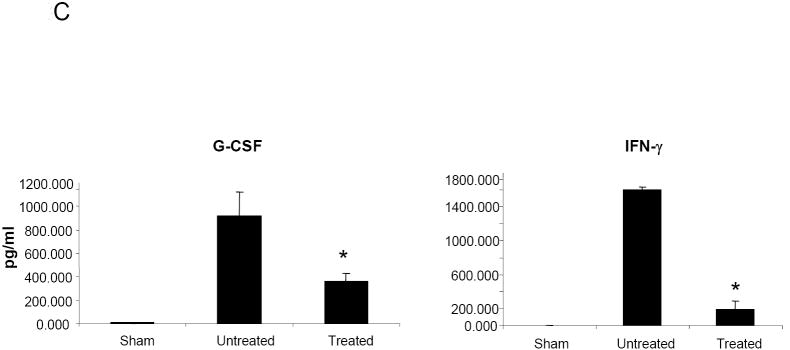
HMPV infection of BALB/c mice results in significant production of cytokines and chemokines (Alvarez et al., 2004;Guerrero-Plata et al., 2005;Hamelin et al., 2005), which are likely to play a major role in pulmonary inflammation. Therefore, we determined whether NMSO3 treatment could modulate hMPV-induced cytokine and chemokine secretion by analyzing their levels in BAL samples collected at day 1 and 4 p.i. As shown in Fig.3A, NMSO3 treatment significantly reduced the level of the chemokine RANTES, a potent chemoattractant of mononuclear cells, while increasing the amount of KC, which is important for neutrophil recruitment. The proinflammatory cytokines IL-1α, IL-1β, IL-6 and TNF-α were strongly upregulated in the lungs of infected animals at day 1 p.i. and NMSO3 treatment significantly reduced all four cytokine protein levels (Fig.3B). These cytokines were no longer detectable in BALs of infected mice at day 4 p.i. On the other hand, G-CSF and IFN-γ were secreted in significant amounts at day 4 p.i., and were significantly reduced in hMPV-infected animals treated with NMSO3 (Fig.3C).
Fig. 3. Effect of NMSO3 on cytokine and chemokine release in the BAL.
Cytokine and chemokine production was measured by Bioplex array in the BAL of sham and hMPV-infected mice, either untreated or treated with NMSO3 at day 1 (panel A and B) and 4 (panel C) post-infection. Data are expressed as mean ± standard deviation and representative of three different experiments. *, P< 0.01 relative to hMPV-infected mice.
Our study demonstrates that a single administration of NMSO3, at the time of hMPV infection, is effective in reducing viral replication, clinical illness, pulmonary inflammation and chemokine/cytokine production. The initial in vitro study suggested that NMSO3 was most effective when present at the beginning of hMPV replication, possibly affecting viral attachment and internalization (Wyde et al., 2004). For this reason, we decided to treat the mice concurrently with hMPV infection. A single dose of 50 mg/kg of NMSO3 was very effective in preventing significant body weight loss, suggesting that antiviral treatment can have a significant impact on hMPV-induced disease, as recently shown also by the use of ribavirin in a similar model of hMPV infection (Hamelin et al., 2006), in particular when administered at the beginning of infection. NMSO3 given 24 h after infection showed only a marginal improvement in hMPV-induced illness, assessed by body weight loss. This result was similar to what we have previously shown with antioxidant treatment in the context of RSV infection, which was effective if given before or at the moment of infection, but did not result in significant improvement of clinical disease administered one day after infection, when clinical disease and lung inflammation are already present (Castro et al., 2006). This finding suggests that early therapeutic intervention may be necessary to improve clinical outcome in both hMPV and RSV infection. In future studies, it would be important to assess other important parameters of clinical illness such as airway hyperresponsiveness, which has been reported in BALB/c mice infected with hMPV or with other paramyxoviruses such as RSV. Similarly, alternative ways of administration, for example via aerosolization, could be tested for efficacy. HMPV infection of BALB/c mice induces a significant lung inflammatory response, with recruitment of phagocytes and then mononuclear cells to the airways, concomitantly with the induction of chemokines and cytokines. The role of the inflammatory response in hMPV-induced clinical illness has not yet been investigated. However, the recent finding of a significant reduction of body weight loss, following glucocorticoids treatment (Hamelin et al., 2006), in a similar mouse model of hMPV infection, suggests that modulating pulmonary inflammation would be beneficial in hMPV-induced disease. A recent in vitro study has shown that NMSO3 reduces RSV-induced chemokine production (Miller et al., 2004). Similarly, our results show that NMSO3 treatment significantly reduces hMPV-induced chemokine/cytokine production, paralleled by a reduction of pulmonary inflammation. The only exception was secretion of KC, a neutrophil chemoattractant, which explains the relatively higher proportion of these phagocytic cells at day 4 p.i. in the NMSO3-treated group. Although this beneficial effect is likely due to the reduction in hMPV replication, it is possible that, in addition to its antiviral activity, NMSO3 might also possess general anti-inflammatory properties, as recently shown in vitro by inhibition of adhesion-dependent leukocyte activation (Shodai et al., 2003). We have recently shown that CD4+ T-lymphocytes play an important role in modulating hMPV-induced clinical illness and pulmonary inflammation (Kolli D et al., 2008). It is possible that NMSO3 treatment modulation of the innate response modified the adaptive immune response too, leading to a beneficial effect on disease not only due to the antiviral mechanism. Future studies should address whether NMSO3 modifies recruitment/maturation of dendritic cells and whether there are changes in the phenotype of T-cells.
In summary, NMSO3 treatment improved several aspects of hMPV-induced disease and should be considered for further evaluation in other animal models.
Acknowledgments
This work was supported in part by a grant from the Sealy Center for Vaccine Development, UTMB (A.C.) and NIH-NIAID AI060202 (T.L.B). The authors would like to thank Cynthia Tribble for her assistance in the manuscript editing and submission.
References
- Alvarez R, Harrod KS, Shieh WJ, Zaki S, Tripp RA. Human metapneumovirus persists in BALB/c mice despite the presence of neutralizing antibodies. J Virol. 2004;78:14003–14011. doi: 10.1128/JVI.78.24.14003-14011.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro SM, Guerrero-Plata A, Suarez-Real G, Adegboyega PA, Colasurdo GN, Khan AM, Garofalo RP, Casola A. Antioxidant Treatment Ameliorates Respiratory Syncytial Virus-induced Disease and Lung Inflammation. Am J Respir Crit Care Med. 2006;174:1361–1369. doi: 10.1164/rccm.200603-319OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero-Plata A, Casola A, Garofalo RP. Human metapneumovirus induces a profile of lung cytokines distinct from that of respiratory syncytial virus. J Virol. 2005;79:14992–14997. doi: 10.1128/JVI.79.23.14992-14997.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero-Plata A, Casola A, Suarez G, Yu X, Spetch L, Peeples ME, Garofalo RP. Differential response of dendritic cells to human metapneumovirus and respiratory syncytial virus. Am J Respir Cell Mol Biol. 2006;34:320–329. doi: 10.1165/rcmb.2005-0287OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeberle HA, Kuziel WA, Dieterich HJ, Casola A, Gatalica Z, Garofalo RP. Inducible expression of inflammatory chemokines in respiratory syncytial virus-infected mice: role of MIP-1alpha in lung pathology. J Virol. 2001;75:878–890. doi: 10.1128/JVI.75.2.878-890.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamelin ME, Boivin G. Human metapneumovirus: a ubiquitous and long-standing respiratory pathogen. Pediatr Infect Dis J. 2005;24:S203–S207. doi: 10.1097/01.inf.0000188158.27840.7c. [DOI] [PubMed] [Google Scholar]
- Hamelin ME, Prince GA, Boivin G. Effect of ribavirin and glucocorticoid treatment in a mouse model of human metapneumovirus infection. Antimicrob Agents Chemother. 2006;50:774–777. doi: 10.1128/AAC.50.2.774-777.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamelin ME, Yim K, Kuhn KH, Cragin RP, Boukhvalova M, Blanco JC, Prince GA, Boivin G. Pathogenesis of human metapneumovirus lung infection in BALB/c mice and cotton rats. J Virol. 2005;79:8894–8903. doi: 10.1128/JVI.79.14.8894-8903.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura K, Mori S, Tomita K, Ohno K, Takahashi K, Shigeta S, Terada M. Antiviral activity of NMSO3 against respiratory syncytial virus infection in vitro and in vivo. Antiviral Res. 2000;47:41–51. doi: 10.1016/s0166-3542(00)00091-7. [DOI] [PubMed] [Google Scholar]
- Kolli D, Bataki E, Spetch L, Guerrero-Plata A, Milligan GN, Garofalo RP, Casola A. T lymphocytes contribute to antiviral immunity and pathogenesis in experimental human metapneumovirus infection. J of Virol. 2008 doi: 10.1128/JVI.00699-08. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AL, Bowlin TL, Lukacs NW. Respiratory syncytial virus-induced chemokine production: linking viral replication to chemokine production in vitro and in vivo. J Infect Dis. 2004;189:1419–1430. doi: 10.1086/382958. [DOI] [PubMed] [Google Scholar]
- Shodai T, Suzuki J, Kudo S, Itoh S, Terada M, Fujita S, Shimazu H, Tsuji T. Inhibition of P-selectin-mediated cell adhesion by a sulfated derivative of sialic acid. Biochem Biophys Res Commun. 2003;312:787–793. doi: 10.1016/j.bbrc.2003.10.188. [DOI] [PubMed] [Google Scholar]
- van den Hoogen BG, de Jong JC, Groen J, Kuiken T, de Groot R, Fouchier RA, Osterhaus AD. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat Med. 2001;7:719–724. doi: 10.1038/89098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyde PR, Chetty SN, Jewell AM, Boivin G, Piedra PA. Comparison of the inhibition of human metapneumovirus and respiratory syncytial virus by ribavirin and immune serum globulin in vitro. Antiviral Res. 2003;60:51–59. doi: 10.1016/s0166-3542(03)00153-0. [DOI] [PubMed] [Google Scholar]
- Wyde PR, Moylett EH, Chetty SN, Jewell A, Bowlin TL, Piedra PA. Comparison of the inhibition of human metapneumovirus and respiratory syncytial virus by NMSO3 in tissue culture assays. Antiviral Res. 2004;63:51–59. doi: 10.1016/j.antiviral.2004.02.006. [DOI] [PubMed] [Google Scholar]