Vaccine development and licensure for new viral diseases is a complex enterprise. In the past, pathogenic viruses might have been simply attenuated or inactivated to generate an effective vaccine. Such vaccines had an enormous impact on the spread of common viral diseases and have resulted in extraordinary improvements in personal and public health. However, as the frequency of epidemic disease has receded, public tolerance for adverse effects has diminished. Today, the perceived risk-to-benefit ratio for an individual must be lower than ever if a new vaccine is to be acceptable to the public. Even when objective data indicate that adverse events are rare and efficacy is nearly 100%, some vaccines have been anecdotally linked to prevalent disease conditions in popular opinion, creating a perception of harm and evading scientific rebuttal.
Ironically, the concept that vaccines may do more harm than good is a consequence of their success. Common viral diseases that once caused a great deal of of misery and mortality have now been controlled to the point that they are rare, unexpected, and no longer feared. Because vaccines are given to otherwise healthy people and can be given frequently, it is not surprising that nearly any health issue that arises in an individual might be temporally associated with, but not causally related to, vaccination. The increasingly large burden of proof for safety and efficacy, the trend for popular opinion to be influenced by rhetoric rather than scientific data, the risk of liability, and the crowded schedule of recommended vaccines are serious considerations in the decision to initiate new vaccine development. This article provides a perspective on how approaches to vaccine development should adapt to these modern circumstances to effectively prepare for and respond to emerging infections, biodefense threats, and other viral challenges.
The incidence of serious adverse responses to vaccines is low, especially when one considers the large number of vaccinations given worldwide every day. Despite overwhelming evidence that licensed vaccines are safe and prevent disease, adverse events have surfaced during vaccination campaigns, and there are known risks. For example, shortly after the licensure of Jonas Salk’s formalin-inactivated polio vaccine in the 1950s, paralysis occurred in some vaccinated individuals. The infections were quickly associated with vaccine produced by Cutter Laboratories, and some lots of inactivated polio vaccine were found to be contaminated with live poliovirus. In the Cutter incident, inactivation of the poliovirus was compromised by inadequate separation of the viral particles from cell culture debris before formalin treatment. In another event, simian polyomavirus SV-40-contaminated cell cultures were inadvertently used to produce inactivated polio vaccine.
These two incidents demonstrate the potential complications of manufacturing biological products, and they led to more stringent manufacturing requirements. But it is fortunate that poliovirus vaccine development was continued because of the extraordinary benefit to public health ultimately achieved through vaccination. There have since been tremendous advances in our knowledge of biology, particularly the nuances of cell substrates, gene regulation, and immune modulation. These advances have opened possibilities for scientific discovery, novel vaccine platforms, and safer and more efficient manufacturing techniques. At the same time, they have increased the rigor with which candidate vaccines are evaluated at each stage of development. The converging realities of high regulatory standards, increased knowledge, and a wealth of new analytical tools have increased the cost-to-benefit ratio calculation. Some estimates of the total cost of developing a new vaccine through licensure exceed US$1 billion.
The viruses for which new vaccines are now in development have also become more challenging. In part, this challenge is a consequence of success in developing vaccines for many important human viral pathogens, particularly those for which vaccine-induced antibodies have provided solid protection. Viral vaccines for several common diseases have been developed, but there remain serious viral diseases for which vaccines would provide significant public health benefit. Examples include HIV-1; her-pesviruses such as herpes simplex virus, cytomegalovirus, and Epstein–Barr virus; paramyxoviruses such as respiratory syncytial virus and parainfluenza virus; and flaviviruses such as dengue, West Nile virus, and hepatitis C. These viruses have biological properties that make vaccine development difficult. Several have been the target of unsuccessful vaccine development efforts over recent decades that were complicated by failure to achieve efficacy, the rare occurrence of vaccine-enhanced illness, or both.
Other important viral vaccine targets include new emerging viruses that have the potential for pandemic spread, including H5N1 avian influenza and the severe acute respiratory syndrome coronavirus, and viruses that cause sporadic epidemics, such as filoviruses (e.g., Mar-burg and Ebola), or epidemics that are widespread but with low incidence of severe disease expression, such as West Nile virus. These viruses pose a dilemma because the virus may never evolve to spread widely in humans, making it difficult to evaluate efficacy. Moreover, the value of such a vaccine is uncertain—market size may be small, and the medical need may be sporadic. Other viral targets are those that emerge and spread in developing countries in areas of poverty or in certain vulnerable populations. A vaccine for these viruses might have a large market, but the infrastructure for vaccine development and evaluation and the funding to support vaccine delivery may be lacking. Such viral targets pose regulatory, scientific, logistical, and ethical questions that militate against investments in vaccine development.
In considering the best options for managing difficult and emerging viruses that have resisted control by vaccination, the risk–benefit and cost–benefit analyses should take into account the primary constituencies. Private industry has been the primary driving force behind vaccine development because of the risk of failure or liability, and the cost of development was favorable relative to the potential benefits of distributing the product to a large market. This has resulted in great benefit to the public health, and because most of the viral targets thus far have been universal pathogens, cost sharing between developed and developing countries has been feasible. Many remaining infectious-disease targets are less attractive to private investors for the following reasons: (i) fundamental scientific discoveries are required to enable vaccine development, (ii) complicated biology incurs a high risk of failure, (iii) prior vaccine development failure and even remote safety issues raise liability concerns, and (iv) emerging or re-emerging viruses do not have a sufficient epidemiological history by which to judge the market or public health need. Therefore, traditional pathways for vaccine development need to be re-examined.
From an individual’s perspective, the risk–benefit calculation related to vaccine use is based on personal health and is independent of cost–benefit considerations related to vaccine development. For vaccine developers, however, the importance of the cost–benefit analysis generally outweighs risk–benefit considerations pertaining to the individual. Depending on whether the vaccine developer is in private industry or government, the basis and importance of each term in the formula may vary. For industry, although projects are initiated based on unmet medical needs, the motivation is primarily financial and the benefit related to corporate profitability. For government, the benefit relates to public health outcomes, which may have significant economic implications but not for direct revenue to the government. Effective vaccines will typically result in savings in overall health-care costs, and, because improved public health will translate into a more productive workforce, it is much easier to justify the cost of vaccine development in government than it would be for industry, for which the economic end point is corporate profit. Therefore, for the difficult and emerging virus diseases, the incentives for individuals and government in personal and public health are much more closely aligned with each other than with the incentives for industry. Government vaccine developers are in a better position to focus on biological and health-related risk–benefit considerations.
Vaccine development for pathogens with high human health impact but low commercial interest requires alternative development approaches. Government agencies will probably need to play a progressively larger role in advanced development, including manufacturing (Figure 1). Private-sector interest could be stimulated either by direct financial incentives or by providing candidate vaccines in late stages of development to diminish both risk and remaining research expenses. Public–private partnerships among government, industry, foundations, and nonprofit organizations have particular importance for difficult vaccine targets. Organizations focused on specific pathogens—for example, the Bill and Melinda Gates Foundation and the International AIDS Vaccine Initiative—have been able to continue advancing their agenda even as industry has played an ever-decreasing role in vaccines for HIV. Organizations such as the Malaria Vaccine Initiative and Aeras have shown sustained commitment to vaccine development for malaria and tuberculosis, respectively. These initiatives have been supported through collaborations with government agencies and federal funding as well as through philanthropic organizations such as the Wellcome Trust and cooperative consortia such as the Global HIV Vaccine Enterprise. The historic role played by the March of Dimes Foundation in advancing the poliovirus vaccine exemplifies the value of such public–private partnerships in vaccine development. When safe and effective products are identified in these programs, new approaches and partnerships for managing licensure, cost sharing, and product distribution will need to be pioneered. It is likely that public–private partnerships focused on specific vaccine targets will be required for other difficult viruses in the future. The transition from the public to private sectors will be dynamic and depend on whether thresholds of risk–benefit and cost–benefit are met by public health demands.
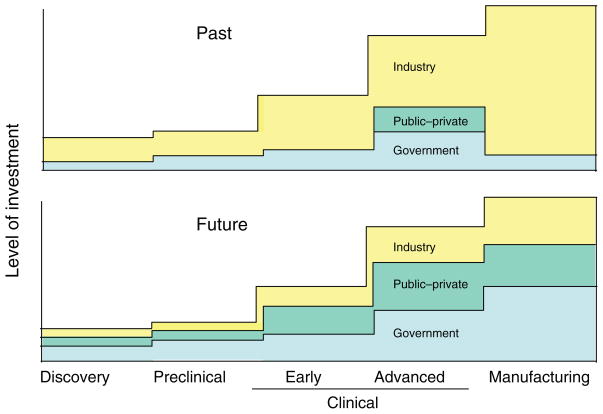
Figure 1.
Investment during vaccine development increases substantially when a decision is made to advance into efficacy trials and scale up manufacturing. In the past, industry has had the dominant role in preparing products for advanced testing and licensure. The graph depicts the concept that, to develop vaccines for difficult viral pathogens and emerging virus diseases in the future, the balance of investment may need to shift more to government and public–private partnerships. The y-axis indicates the relative level of investment required for successive stages of vaccine development.
For emerging viral pathogens with potential for pandemic spread, including pathogens relevant to biodefense, the need for a vaccine is either unforeseeable or difficult to estimate in advance. The infrastructure and product development pathways for new vaccines to prevent these types of pathogens are either nonexistent or too slow to be effectively implemented in a crisis following the identification of a new pathogen. The more feasible options for immediate deployment in the event of a widespread outbreak or attack are nonmedical countermeasures and, if available, preexisting antivirals. Several options should be considered if we want the capacity to produce deployable vaccines against new viral pathogens, including (i) global surveillance infrastructure development with a focus on new virus discovery, particularly looking for viruses with zoonotic or vector-borne transmission potential, (ii) establishment of platform technologies for vaccines against each family of viral pathogens, (iii) maintenance of publicly funded manufacturing capacity with the ability to rapidly produce gene-based vaccines, (iv) maintenance of healthy volunteer cohorts to allow rapid clinical evaluation, (v) new regulatory pathways for vaccine products consisting of a novel vaccine antigen expressed in the context of a gene-based vector with a well-characterized safety and manufacturing profile, and (vi) new business models that maintain a manufacturing capacity that is rapidly scalable.
It is certain that new viral pathogens will be identified in the future, and there are many difficult viral vaccines yet to develop. While we should adopt conservative ecological and cultural approaches to reduce the frequency of emerging viruses, as well as develop new classes of antivirals to cover a broader spectrum of potential pathogens, new paradigms for vaccine development against new and difficult viruses are needed to optimally protect the public health.
Footnotes
CONFLICT OF INTEREST
The authors declared no conflict of interest.