Abstract
T-type Ca2+ currents have been detected in cells from the external muscular layers of gastrointestinal smooth muscles and appear to contribute to the generation of pacemaker potentials in interstitial cells of Cajal from those tissues. However, the Ca2+ channel α subunit responsible for these currents has not been determined. We established that the α subunit of the α1H Ca2+ channel is expressed in single myocytes and interstitial cells of Cajal using reverse transcription and polymerase chain reaction from whole tissue, laser capture microdissected tissue, and single cells isolated from the mouse jejunum. Whole cell voltage clamp recordings demonstrated that a nifedipine and Cd2+ resistant, mibefradil-sensitive current is present in myocytes dissociated from the jejunum. Electrical recordings from the circular muscle layer demonstrated that mibefradil reduced the frequency and initial rate of rise of the electrical slow wave. Gene targeted knockout of both alleles of the cacna1h gene, which encodes the α1H Ca2+ channel subunit, resulted in embryonic lethality due to death of the homozygous knockouts prior to E13.5 days in utero. We conclude that a channel with the pharmacological and molecular characteristics of theα1H Ca2+ channel subunit is expressed in interstitial cells of Cajal and myocytes from the mouse jejunum, and that ionic conductances through the α1H Ca2+ channel contribute to the upstroke of the pacemaker potential. Furthermore, the survival of mice that do not express the α1H Ca2+ channel protein is dependent on the genetic background and targeting approach used to generate the knockout mice.
Keywords: Gastrointestinal motility, Electrical slow wave, Ion channels, Transgenic Animals
INTRODUCTION
T-type or low voltage activated Ca2+ currents are detected in a variety of smooth muscle cells including gastrointestinal, vascular, myometrial and genito-urinary tract myocytes [1–4] where they are nearly always co-expressed with the L-type, high-voltage activated Ca2+ currents. Unlike L-type Ca2+ currents, the physiological roles of T-type Ca2+ currents are not clear although several studies have implicated these currents in regulation of rhythmic oscillations of membrane potential and control of myocyte proliferation during vascular development and remodeling [2].
The presence of low voltage-activated, Ca2+-permeable ionic conductances in cells from the external muscle layers of gastrointestinal smooth muscles has been reported in smooth muscle cells from the mouse colon [5], guinea pig taenia coli [6,7], rat colon [3] and human colon [4] as well as interstitial cells of Cajal (ICC) from dog colon [8] and mouse colon and small intestine [9]. Many of these studies have identified the conductance as a T-type Ca2+ current [3,4,6,7], and some of the other currents recorded in colonic myocytes have T-type properties [8,9]. The physiological role of T-type Ca2+ currents in gastrointestinal myocytes has not been determined.
Data that do not support the presence of T-type Ca2+ channels in myocytes come from studies showing that inward Ca2+ permeable conductances recorded in myocytes from mouse colon are not as selective for Ca2+ as expected for T-type Ca2+ channels or are impermeable to Ba2+. These data are consistent with the presence of an unclassified non-selective cation conductance in mouse colonic myocytes [5].
Together with enteric nerves and myocytes, interstitial cells of Cajal (ICC) are required for normal gastrointestinal motility [10]. ICC generate the electrical slow wave; an oscillation in membrane potential that is required for normal phasic contractions of gastro-intestinal smooth muscles [11,12] and a role for T-type Ca2+ currents in the generation of slow waves has been proposed. Normal slow wave activity results from Ca2+ influx through plasma membrane ion channels, Ca2+ release from inositol 1,4,5-trisphosphate sensitive Ca2+ stores, and re-polarization dependent on a variety of ion channel types including non-selective cation channels and/or Ca2+ activated Cl− channels [13,14]. The Ca2+ influx that contributes to the upstroke of the electrical slow wave is sensitive to block by agents that alter T-type Ca2+ channel activity. Intracellular recordings from the external muscle layers of mouse small intestine [15] indicate that a nifedipine-insensitive, Ca2+-permeable conductance is responsible for Ca2+ influx during the electrical slow wave. In submucosal ICC from mouse colon, Ni2+ (10–100 μM) and mibefradil (3 μM) application resulted in a reduced rate of rise of the upstroke of the electrical slow wave [16,17]. Detailed analysis of the pacemaker potentials and electrical slow waves recorded by impaling ICC and smooth muscle cells in mouse small intestine showed that mibefradil (≥ 10 μM) reduced the rate of rise of the upstroke depolarization due to failure to entrain unitary potentials recorded from ICC [18]. Experiments using imaging of intracellular Ca2+ transients to follow pacemaker activity in myenteric ICC of human [19] and mouse [20] small intestine, demonstrated that the upstroke phase of the transient depends on activation of dihydropyridine-resistant Ca2+ influx. In mouse ileum, this Ca2+ influx was blocked by low concentrations of mibefradil (0.1 μM) and Ni2+ (100μM) [20] whereas in human small intestine [19], higher concentrations of mibefradil (10 – 50 μM) were required to inhibit the Ca2+ influx. These observations indicate that the conductance responsible for the upstroke of the slow wave is probably a T-type Ca2+ current in mouse but could be due to a different conductance in ICC of the human small intestine [21].
The genes that encode three types of T-type Ca2+ channels have been cloned from mouse and human tissue. These proteins, Cav3.1, Cav3.2 and Cav3.3 (or α1G, α1H, and α1I respectively) have the properties of T-type Ca2+ channels in heterologous expression systems [22]. The messenger RNA (mRNA) for all three T-type Ca2+ channels have been identified in mouse small intestine by PCR but ICC of the deep muscular plexus (ICC-DMP) do not express any T-type Ca2+ channel mRNA [23]. In mouse colonic myocytes, α1G complementary DNA (cDNA) could not be amplified by reverse transcriptase polymerase chain reaction (RT-PCR) [5].
For this study we have further investigated whether T-type Ca2+ currents play a role in the electrical properties of the mouse jejunum based on the increased knowledge of the physiological and pharmacological properties of the T-type Ca2+ current. We have identified the T-type Ca2+ channel mRNA that is expressed in myocytes and in ICC from the external muscle layers as α1H and we attempted to study the effect of knocking out expression of this gene by gene-targeted mutagenesis.
MATERIALS AND METHODS
The Institutional Animal Care and Use Committee at Mayo Clinic, Rochester approved all animal handling procedures.
Harvest of Tissue
Adult mice were killed by CO2 inhalation followed by cervical dislocation. The small intestine was then rapidly removed and a 1–2 cm segment was rapidly frozen in liquid nitrogen to be cut into sections for laser capture microdissection. For RNA isolation, a longer 8–15 cm segment of jejunum was removed and placed in ice-cold, sterile Hanks balanced salt solution. The external muscle coat was rapidly peeled away from the mucosa and immediately placed in RNA later (Ambion, Austin, TX) for transfer to the molecular biology lab. For intracellular electrical recordings, a segment of small intestine wall, approximately 6 cm from the pylorus, was removed and placed in pre-oxygenated, normal Krebs solution at room temperature.
RNA isolation and Reverse Transcription and PCR Amplification of cDNA
RNA was isolated from the external muscle coat of the adult mouse jejunum immediately after dissection as described previously for isolation of RNA from human jejunum smooth muscle [24].
Reverse-transcription PCR (RT-PCR)
PCR amplifications were performed using GeneAmp 2400 PCR Systems (PE Biosystems, Foster City, CA) or icycler (Biorad, Hercules CA) using standard procedures as previously published [24]. Reverse transcription (RT) was performed using a mixture of random hexamer and oligo dT primers following the instructions of the manufacturer (PE Biosystems, Foster City, CA). The product of the RT reaction was then amplified for T-type Ca2+ channel α subunits using gene specific primers that were specifically designed to flank regions containing introns in the genomic sequence (see Table 1 for details). All PCR products were purified and sent to the Mayo Molecular Core Facility for automated DNA sequencing.
TABLE 1.
| Target (Primer Name) | Forward Primer | Reverse Primer | Expected size (bp) |
|---|---|---|---|
| PCR Primers | |||
| Alpha 1H (YP1) | GCATGGCCTTCCTCACGTTGTT | GTGTAGTCTGGGATGCCGTCTT | 697 |
| (YP2) | TGCCGGTGGTGCCAAGATCCTA | GGCGCGTGTGTGAATAGTCTGC | 628 |
| Alpha 1G | CTACGGTCCCTTCGGCTACATT | TCCACTCGTATCTTCCCGTTTG | 531 |
| Alpha 1I | CTGTTTAGTCTGCGTGGGCTG | CTAACCCAGACCCTCTCAGTC | 608 |
| Single Cell PCR Primers | |||
| Alpha 1H (Outer) | CTTCTTGCGGCCATACTACC | CTGGTTTTCCCTCTGCTTTG | 475 |
| Alpha 1H (Inner) | TCACAATGGTGCCATCAACT | CTGGTTTTCCCTCTGCTTTG | 232 |
| Kit (Outer) | ATTATGAACGCCAGGAGACG | GAATCCCTCTGCCACACACT | 497 |
| Kit (Inner) | TACGAGGCCTACCCCAAACC | CTCTGCCACACACTGGAGCA | 285 |
| Genotyping Primers | |||
| Wild Type | AGGAGAGGCACTTACTGG | TAGGTATCAAGGACTGTGAGG | 487 |
| Knockout | CGCAAGCCCGGTGCCTGA | CCCTGTCCTGAGTAGAACTG | 520 |
Laser Capture Microdissection (LCM)
Sections of mouse jejunum, 6 μm thick, were mounted on glass slides and fixed in ice-cold acetone according to the protocol described previously [24]. A number of spots of tissue containing about 1500 smooth muscle cells from the circular muscle layer or the longitudinal muscle layer were collected using the PIX II Cell LCM system (Arcturus Engineering Inc., Santa Clara, CA) with the 7.5 μm spot size. The caps with collected cells were then immediately placed into sterile 0.5 ml microcentrifuge tubes containing 300 μl RNA STAT-60 reagent (Tel-TEST Inc., Friendswood, TX) for isolation of total RNA. After washing with 75% ethanol, the RNA pellet was resuspended in nuclease-free water (Ambion Inc., Austin, TX) and used for the RT-PCR.
Single Cell PCR Amplification from Identified ICC
Single ICC from 3–5 day old mice were obtained as previously described [25] from a dissociation of jejunal smooth muscle that had been immunolabeled with an antibody directly conjugated to the fluorophore Alexa 546 (Invitrogen). 3–5 labeled cells and 3–5 un-labeled, spindle-shaped cells were collected using a glass pipette and placed into an RNAse free tube. A sample of bath solution was collected as a negative control against possible contamination. RNA was extracted then reverse transcribed using the ViLo Superscript III kit from Invitrogen. The cDNA was then probed for the presence of the α1H Ca2+ channel transcript and c-Kit by two-step nested PCR using the primers shown in Table 1.
Generation of Gene Targeted Knockout Mice
Mice targeted for the knockout of the gene for the α1H Ca2+ channel subunit by targeting the Cacna1h gene. The targeting vector was transfected into Lex-1 ES cells derived from 129SvEvBrd mice (Lexicon, The Woodlands TX) and the resulting clones were screened by PCR. Positively identified clones were injected into C57Bl/6 blastocysts and chimeric animals were bred to homozygosity.
Genotyping
Genotyping was done by Southern analysis using a probe (XP1) generated by PCR from genomic DNA. The genomic DNA was obtained from tails of the mice extracted using the Tissue Direct™ multiplex PCR system (GenScript, Piscataway, NJ). EcoRI was used to digest 10 μg of the genomic DNA for Southern blotting. The probe recognized a 11 kb fragment of wild-type DNA and a 9.5 kb fragment of DNA from the targeted allele.
PCR genotyping was also used to distinguish the wild-type and targeted alleles. The primers JLR5 and JLR6 were used to identify a 487 nucleotide band in the wild type alleles and primers Puro3a and KO37 identified a 520 nucleotide band in the knockout allele (see Table 1 for primer sequences).
Intracellular Electrical Recordings
The segments of small intestine were opened along the anti-mesenteric border and transferred to a Petri dish filled with fresh oxygenated normal Krebs solution. The mucosa was removed under direct vision by using a binocular microscope and muscle strips (5 × 8 mm) were cut with the long axis parallel to the longitudinal muscle layer. Muscle strips were placed in a recording chamber and pinned with the serosal side down to a Sylgard-coatedfloor. The recording chamber had a volume of 1 ml and was perfused continuously with oxygenized normal Krebs solution at 37°C at a rate of 2 ml/min. The composition of the solution was (in mM) 137.4 Na+, 5.9 K+, 2.5 Ca2+, 1.2 Mg2+, 124 Cl−, 15.5 HCO3−, 1.2 H2PO4−, and 11.5 glucose. It was continuously bubbled with 97% O2, 3% CO2, and maintained at pH 7.4.
Sharp glass microelectrodes filled with 3 M KCl (with input resistances ranging from 40–70 MΩ) were used to record intracellularly the membrane potential of smooth muscle cells. Approximately 30 min before recording, 1 μM nifedipine was added to reduce contractile activity of the muscle strip thereby facilitating long-term recordings from single cells. Recorded signals were amplified through an amplifier (Intra 767, WPI), digitized (Digidata 1322A, Axon Instruments), analyzed, and stored in a computer. When spontaneous electrical slow waves occurred, the membrane potential recorded between slow waves was considered the resting membrane potential (RMP). The time constant was measured as the time from the point at which the slow wave started to rise to the point the membrane potential reaches 63% of the maximum amplitude of the slow wave cycle. Values are reported as mean ± standard error (SEM). The student t-test was used to compare mean slow wave frequency in control conditions and during drug treatment. A P value ≤ 0.05 was considered a significant difference.
Whole Cell Voltage Clamp Recordings
Currents were recorded from voltage clamped cells at 22 °C using standard whole cell techniques [26,27]. Microelectrodes were pulled from Kimble KG-12 glass on a P-97 puller (Sutter Instruments, Novato, CA). Electrodes coated with R6101 (Dow Corning, Midland, MI) were fire-polished to a final resistance of 3 to 5 MΩ. Currents were amplified, digitized, and processed using an Axopatch 200B amplifier, Digidata 1322A, and pCLAMP 9.2 software (Axon Instruments). Whole cell records were sampled at 10 kHz and filtered at 4 kHz with an 8 pole Bessel filter. During analysis, the 0.1 ms (10 Hz) sampling interval of whole cell records was decimated 10-fold down to 1 ms (1 kHz). 70–85% series resistance compensation with a lag of 60 μs was applied during each recording. Freshly dissociated mouse intestinal smooth cells were held at a potential of −100 mV and stepped from −80 to 35 mV in 5 mV intervals for 400 ms. The start-to-start time was 516 ms.
Drug and solutions
The intracellular solution contained (in mM) 130 Cs+, 125 methanesulfonate, 20 Cl−, 5 Na+, 5 Mg2+, 5 HEPES, 2 EGTA, 2.5 ATP, and 0.1 GTP, equilibrated to pH 7.0 (CsOH) with an osmolality 300 mmol/kg. Barium currents were recorded in an extracellular solution containing (in mM) 80 Ba2+, 160 Cl−, and 5 HEPES, equilibrated to pH 7.35 (BaOH) and osmolality 297 mmol/kg (98.8 mM D-mannitol). To block L-type Ca2+ channels, extracellular solutions were replaced with the same solution additionally containing nifedipine (1 μM, 1:10,000 ethanol:water) and/or CdCl2 (30 μM). Mibefradil (2.7 μM) was used to block 50% of L-type Ca2+ and 90% of T-type Ca2+ channel current [28]. Chemicals and drugs were purchased from Sigma-Aldrich Co. (St. Louis, MO).
Data analysis
Patch clamp data were analyzed using Clampfit (MDS Inc, Union City, CA) and SigmaPlot (SPSS Inc, Chicago, IL).
RESULTS
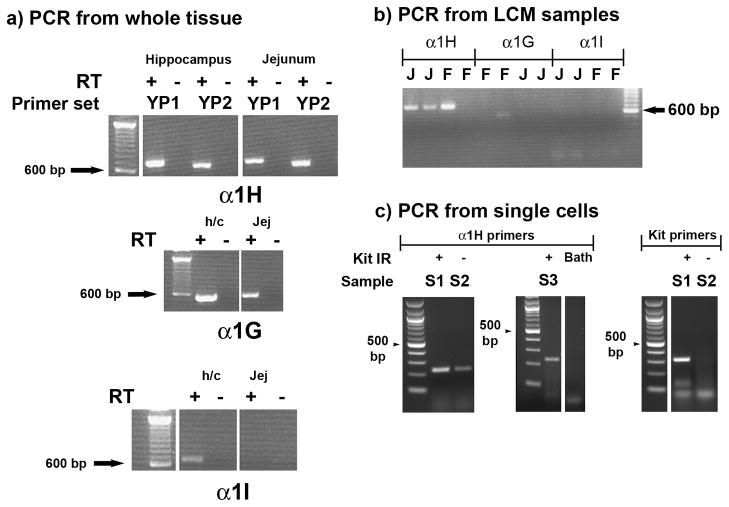
The presence of mRNA for the T-type Ca channel α subunits, α1G, α1H and α1I was investigated by reverse transcription and PCR of total RNA derived from the external muscle layers of adult mouse jejunum. Total RNA purified from mouse hippocampus was used to provide a positive control for the effectiveness of the primers. α1G, and α1H mRNA was amplified from the jejunum but although α1I transcripts were detected in the hippocampal samples, this was not detected in samples from jejunum (Fig 1a). The identity of the products was confirmed by sequencing of the excised, purified products. We used the same primers to test whether any of the transcripts were specifically expressed in cells from the circular smooth muscle layers of the gastric fundus and jejunum by isolating cells using laser capture micro-dissection. α1H mRNA was amplified from all of the samples containing cells collected from the jejunum but the primers for α1G and α1I did not amplify products of the expected size in any of the jejunal LCM samples (Fig 1b).α1H mRNA but not α1I mRNA was also detected in cells collected from the circular muscle layer of the gastric fundus. The presence of α1G mRNA in circular smooth muscle from the gastric fundus was indicated by a faint band in one of the four gastric fundus samples (Fig 1b). PCR amplification from single cells was used to further determine whether α1H mRNA could be detected in smooth muscle cells identified by their spindle shape and in ICC identified by Kit immunoreactivity. A product that was confirmed by sequencing to be amplified from α1H mRNA was detected in all of the tubes (n = 5 tubes) that contained either smooth muscle cells or Kit-positive ICC (examples shown in Fig 1c). c-Kit mRNA was amplified from tubes containing Kit immunoreactive cells but was not amplified from tubes containing Kit-negative, spindle shaped cells (Fig 1c) confirming the identity of the collected cells. Representative PCR results are shown for a sample containing Kit-positive cells (S1) and Kit-negative cells (S2) as well as results from a separate RT-PCR reaction using Kit-positive cells (S3) and bath solution. A product was not amplified from a tube containing the bath water.
Figure 1.
a) PCR amplification of T-type Ca2+ channel α subunit mRNA from the external muscle layers of adult mouse jejunum. Amplification from hippocampal mRNA is shown as a positive control. YP1 and YP2 represent two different primer sets (see Table 1 for details). b) PCR amplification of T-type Ca2+ channel α subunit mRNA from smooth muscle cells from wild type (WT) mouse jejunum and fundus that were collected by laser capture micro-dissection (LCM). C) Representative PCR amplifications of T-type Ca2+ channel α subunit mRNA from single tubes of Kit immunoreactive (Kit-IR, S1 and S3) and Kit-negative spindle-shaped cells (S2). A sample of the bath solution (BATH) was also collected and subjected to RT-PCR as a negative control. Primers were designed against the T-type Ca2+ channel subunit covering at least one intron to avoid genomic contamination. The specific products for α1H were amplified by reverse transcription PCR (RT-PCR). J = jejunum; F = fundus, RT –, control for genomic contamination i.e. underwent RT-PCR in the absence of reverse transcriptase. A 100bp DNA ladder was loaded and is shown to indicate product size.
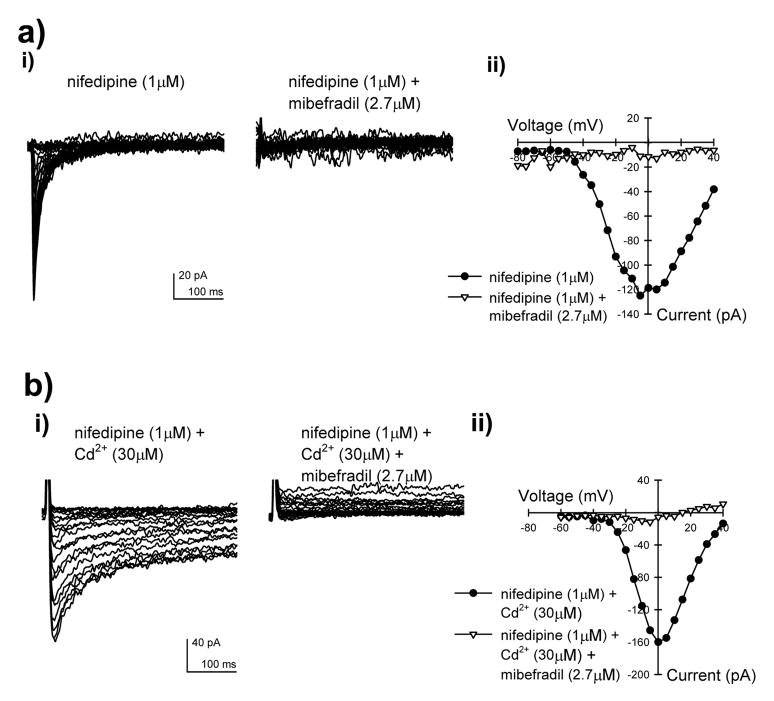
The presence of α1H transcripts in cells from mouse jejunum smooth muscle suggested that cells from this region may express mibefradil-sensitive, nifedipine and Cd2+- resistant T-type Ca2+ channels. To verify this, we did whole cell voltage clamp recordings on freshly dissociated myocytes from the mouse jejunum (Fig 2). In the presence of 1 μM nifedipine and 80 mM Ba2+, an inward current was recorded that was inhibited by >90% with 2.7 μM mibefradil (Fig 2a). This rapidly inactivating current activated at −50 mV with a peak inward current at −5 mV (Fig 2aii). Cadmium (30 μM), expected to block L-type but not T-type Ca2+ channels, did not block the current and this Cd2+-resistant current was also inhibited by more than 90% with 2.7 μM mibefradil (Fig 2b).
Figure 2.
Representative T-type whole cell currents recorded in myocytes from the outer muscle layers of the mouse jejunum. a) i) Nifedipine-resistant, mibefradil-sensitive Ba2+ currents, ii) Current-voltage relationship for the peak inward currents show in (a)i. b) i) Mibefradil-sensitive Ba2+ currents obtained in the presence of nifedipine and Cd2+, ii) Current-voltage relationship for the peak inward currents shown in (b)i. Currents were recorded by stepping the command voltage from −100 mV to between −80 to +35 mV in 5 mV steps.
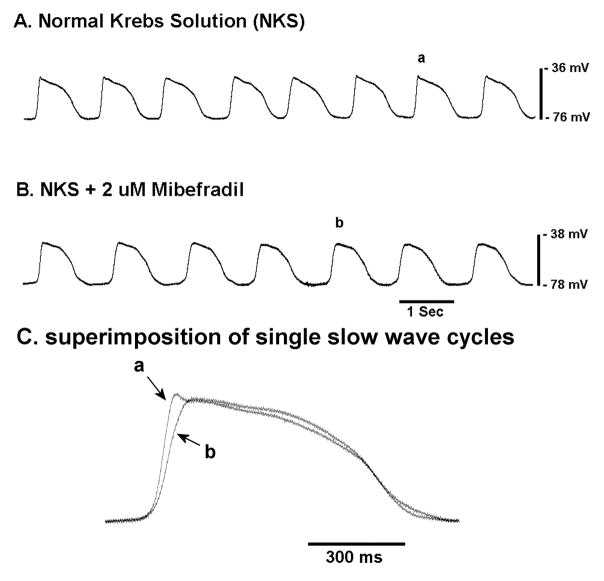
The molecular and functional evidence for T-type Ca2+ channels in the mouse jejunum prompted us to investigate whether inhibitors of T-type Ca2+ channels would affect the electrical slow wave in mouse jejunum. When studying balb/c wild type mice, mibefradil, at a concentration that will predominantly block T-type Ca2+ channels (2 μM, [28]), did not completely block the slow wave. However, the time constant for the rising phase of the electrical slow wave was increased from 0.093 ± 0.005 sec (n = 9 cells from 5 mice) to 0.171 ± 0.015 sec (n = 10 cells from 3 mice, p < 0.01) and the frequency of electrical slow waves was reduced from 0.67 ± 0.02 Hz to 0.59 ± 0.02 (p < 0.05). Mibefradil had no significant effect on the resting membrane potential of the impaled smooth muscle cells (−64.7 ± 2.9 mV in control, −69.2 ± 2.6 mV in mibefradil, P = 0.26) (Fig 3).
Figure 3.
Mibefradil reduces the initial rate of rise and frequency of the electrical slow wave in the circular smooth muscle layer of the mouse jejunum.
a) Control recording, b) recording in the presence of 2 μM mibefradil c) superimposition of single slow wave cycles to show the effect of mibefradil.
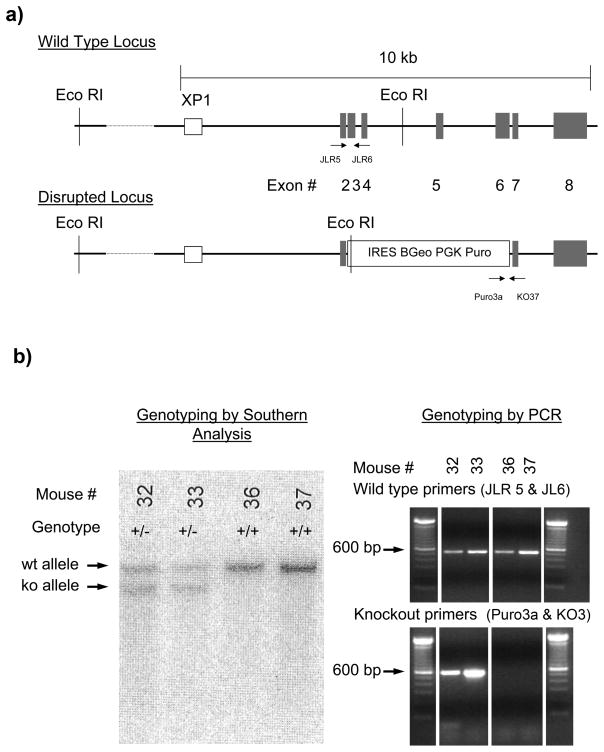
To further examine the contribution of α1H T-type Ca2+ channel subunits on gastrointestinal smooth muscle function, we attempted to knock out expression of the functional protein by targeting the gene for α1H, cacna1h. The targeting vector was designed to disrupt exons 3 to 6 of the genomic sequence as shown in Fig 4a. This region corresponds to amino acid residues 138 to 373 in the expressed protein. Southern analysis of genomic DNA obtained from tails of heterozygous animals and digested using EcoRI detected an 11 kb wildtype band and a 9.5 kb targeted band when using the external probe XP1, as shown in Fig 4b. Examples of Southern blots from wild type and heterozygous mice are shown in Fig 4b. Genotyping was also done by PCR using the primers JLR5 and JLR6 to identify the wild type allele and Puro3a and KO37 to identify the knockout allele. Mice heterozygous for the knockout allele survived, had no obvious phenotype and had no problems with breeding. However only one mouse homozygous for the knockout allele was identified by either PCR or Southern blot as shown in Table 1 (see also Fig 4b for examples).
Figure 4.
Gene targeted knockout of the α1H Ca2+ channel subunit in mice.
a) Schematic of the wild type and disrupted alleles showing the distribution of exons 2 to 8, the location of the key EcoR1 restriction sites for Southern blot analysis. The location of the binding sites for the Southern blot probe (XP1) and the oligonucleotide PCR primers for identifying the wild type (JLR5 and JLR6) and disrupted (Puro3a and KO37) alleles are also shown. A 100bp DNA ladder was loaded and is shown to indicate product size.
b) Example of Southern blot analysis of the alleles present in the genomes of 4 mice is shown together with PCR confirmation of the genotype using DNA samples from the same mice.
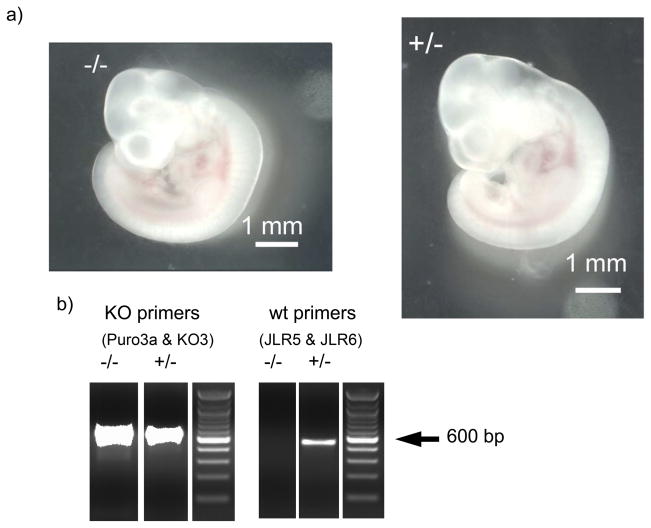
In total more than 200 adult mice were tested for genotype without identifying a homozygous knockout animal, so we examined the proportions of the alleles in mice generated from ten separate pairs of heterozygous animals. The proportions of the genotypes of the mice obtained from breeding heterozygotes (Table 1), significantly deviated from the proportions predicted by Hardy Weinberg principles (p < 0.0001, χ2 test) indicating that the homozygous knockout animals were dying prior to genotyping. We saw no evidence of pups dying shortly after birth. Therefore, we reasoned that the homozygous mutant animals were dying in utero. We tested whether the homozygous knockouts were dying before birth by mating 4 heterozygous pairs of mice. The females were killed at fixed times from the detection of vaginal plugs and the foetuses removed under sterile conditions. The foetuses were photographed and then tissue from the head was removed for DNA extraction and genotyping. The proportions of the genotypes deviated significantly from Hardy Weinberg proportions (p = 0.0015, χ2 test) and no foetuses homozygous for the mutated allele were detected in female mice more than 10.5 days after the plug was detected (see Table 1). However foetuses homozygous for the knockout allele were detected before embryonic day 10.5 in numbers that did not deviate significantly from Hardy Weinberg proportions (p >0.05, χ2 test, Table 1). The foetuses did not have anatomical abnormalities that allowed us to distinguish between knockout and heterozygous or wild type animals at embryonic age 9.5 to 10.5 (see Fig 5). The hearts were beating when the foetuses were removed and there were no clear neural tube defects. However, later during gestation (E17.5 to E19.5) reabsorbed foetuses were observed in the uteruses removed for genotyping of the foetuses.
Figure 5.
Foetuses homozygous or heterozygous for the knockout of the cacna1h gene exhibit no gross abnormalities. a) Images of foetuses obtained 9 days after detection of a vaginal plug (E9.5). b) Results of PCR genotyping of the foetuses shown. (KO – knockout, wt - wild type). A 100bp DNA ladder was loaded and is shown to indicate product size.
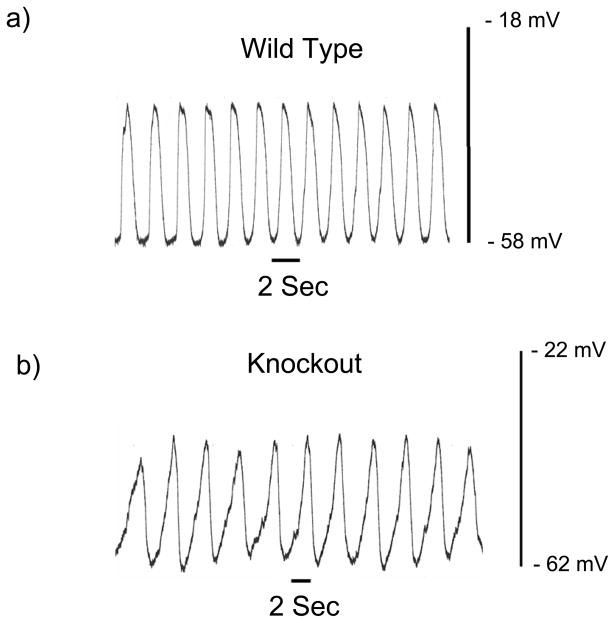
We were unable to obtain more than one mouse that was homozygous for the knockout allele so we compared the properties of the electrical slow wave in the circular smooth muscle layer from the jejunum of 4 mice heterozygous for the knockout with the properties in 4 wild type siblings. 41 cells from heterozygous mutant mice and 39 cells from wild type mice were studied and no differences were detected in the rate of rise of the electrical slow wave as (Time constant in heterozygous animals = 0.143 ± 0.007 sec, homozygous wild type = 0.139 ± 0.006 sec, P > 0.05). The electrical slow wave recorded from smooth muscle cells in the jejunum in the one mouse homozygous for knockout of the α1H gene appeared abnormal. The slope of the initial rising phase of the slow wave was lower than usual and the frequency of the slow waves was less than half the normal value (0.296 ± 0.009 Hz, n = 7 cells, Fig 6 vs 0.67 ± 0.02 Hz in 9 cells from 5 balb/c wild type mice, see above) with no clear difference in the resting membrane potential of the cells (−56.1 ± 2.3 mV, n = 7 cells vs −64.7 ± 2.9 mV in 9 cells from 5 balb/c wild type mice, see above).
Figure 6.
The initial rate of rise and frequency of the electrical slow wave recorded in the circular smooth muscle layer of the mouse jejunum are lower in a mouse homozygous for the α1H knockout.
a) Control recording from a wild type balb/c mouse, b) Recording from a homozygous knockout mouse.
DISCUSSION
Inward cationic conductances that are activated at comparatively negative membrane potentials (<−30 mV) and that are not selectively permeable, or are permeable to Ca2+ or Na+, have been demonstrated in gastrointestinal smooth muscle cells and interstitial cells of Cajal from many species [3–9,24,29]. Due to recent pharmacological and technical advances, the biophysical and molecular identification of these conductances should be possible but so far few have been definitively identified [24]. In this study, we have demonstrated the expression of the α1H Ca2+ channel subunit in the circular smooth muscle layer of the mouse jejunum and fundus, specifically in myocytes and ICC from the jejunum. We have also determined that inhibition of T-type Ca2+ currents affects the electrical slow wave in this tissue. We did not directly record from identified ICC in this manuscript and attempts to directly determine the contribution of the α1H subunit to gastrointestinal function were not successful due to the lethal effects of knocking out the cacna1h gene, which encodes this protein.
The problems with dissecting out Ca2+ currents in myocytes and ICC are that some of the T-type Ca2+ channels are quite resistant to the effect of Ni2+ (>200 μM for α1I and α1G, [30]) and Ni2+ has effects on other Ca2+ permeable channels or transporters in the plasma membrane [31–34]. When used at the correct concentration, mibefradil is a fairly selective inhibitor of T-type Ca2+ channels [35] with a 4–10 fold higher potency as an inhibitor of T-type Ca2+ channels when compared to its effects on L-type Ca2+ channels and the sodium channel, Nav1.5 [28] but it is often tested at concentrations that are not selective and its effects are diminished at positive membrane voltages [36]. Similarly, excluding the other Ca2+ permeable conductances in the cells can be difficult due to the lack of selective inhibitors of non-selective cation conductances. When L-type Ca2+ currents are present in the cells, these currents can be inhibited by dihydropyridine calcium channel blockers but the effectiveness of dihydropyridines is reduced at the more negative membrane voltages [37] where the low voltage activated current is observed, possibly leading to the erroneous identification of residual L-type current as a low voltage activated Ca2+ current. In addition dihydropyridines inhibit some T-type Ca2+ channels at concentrations greater than 5 μM [38].
The molecular identification of the α1H subunit in mouse jejunal smooth muscle is consistent with the biophysical and pharmacological properties of the low voltage activated Ca2+ selective conductance reported herein in mouse jejunal myocytes and recorded by several groups in smooth muscle cells from rat small intestine [3] as well as myocytes from other gastrointestinal tissue [3,6–9]. Specifically, the α1H Ca2+ channel subunit is resistant to inhibition by nifedipine and Cd2+ and is blocked by mibefradil at 2.7 μM [35]. Other distinguishing properties of the α1H Ca2+ channel subunit are activation starting at −60 mV and similar relative selectivity and permeability to Ca2+ and Ba2+ ions.
None of the previously published studies on the expression of T-type Ca2+ channel α subunits have tested for the presence of the α1H subunit and none have identified a candidate for the low voltage activated cation conductance in myocytes from rodent circular smooth muscle [5,23]. In one study, mRNA for the α1G subunit was not detected in myocytes from mouse colonic circular muscle [5]. In the other study, the presence was demonstrated of all three T-type Ca2+ channel α subunits in the external muscle layers of mouse small intestine but this study did not determine if myocytes expressed the mRNA for any of the subunits. We could not detect the α1I subunit mRNA in our samples even though the primers worked in our positive control experiment. Three different primer sets were tested for the α1I subunit and none amplified a product of the expected size (data not shown). One possible explanation for the amplification of α1H Ca2+ channel mRNA from the circular smooth muscle layer of the mouse jejunum is that the mRNA came from a cell type other than the myocytes or ICC. In human myometrium, α1H Ca2+ channel immunoreactivity was detected in lymphocytes but not smooth muscle cells [1]. This is consistent with a role for T-type Ca2+ channels in the migration of leucocytes [39]. However, the samples that we collected by laser capture microdissection were from histochemically stained sections and were taken from the circular smooth muscle layer in regions that did not show evidence of lymphocytic contamination. In addition, the single cells that we collected were either Kit-immunoreactive or had myocyte morphology, so we consider it unlikely that this could account for the results.
The reduction in the rate of rise of the electrical slow wave recorded in myocytes of mouse jejunum in response to mibefradil treatment is consistent with an effect of mibefradil on ICC as previously published in other tissues. The upstroke of electrical slow waves recorded in mouse small intestine involves Ca2+ influx through a non-L type Ca2+ channel [15], Ni2+ (1 – 100 μM) reduces the rate of rise of slow waves in guinea pig gastric antrum [40] and the upstroke of pacemaker potentials in mouse ICC recorded by Ca2+ imaging was inhibited by mibefradil (0.1 μM) and Ni2+ (100 μM) [20]. These experimental results are further supported by mathematical models of pacemaker potentials in ICC that show the necessity for a voltage activated Ca2+ current in order to accurately model the pacemaker potentials. [41]. Interestingly, slowing of the rise time for the electrical slow wave in mice in mibefradil and Ni2+ is similar to the effects of inhibiting the tetrodotoxin (TTX) -resistant Na+ channel, Nav1.5 on the electrical slow wave recorded in circular muscle of human jejunum [29]. Also, mibefradil and Ni2+ do not completely block the upstroke at concentrations where near complete inhibition of a T-type channel is expected [18] suggesting the possibility that more than one channel type may contribute to the upstroke. These pharmacological properties also raise the possibility that the observed current carried by non-L-type Ca2+ channels is carried by T-type-like channels and the possibility that the current is carried by a combination of alpha subunits or a novel T-type alpha subunit cannot be excluded.
The target for mibefradil is likely the α1H channel subunit identified in ICC by single cell PCR rather than in smooth muscle because the electrical slow wave is generated by ICC in the myenteric plexus region of the mouse jejunum, where the pacemaker potential is initiated. As the slow wave is generated by ICC, there should not be a need for a T-type Ca2+ channel to participate in the generation of the upstroke in smooth muscle cells. It may be that Ca2+ influx through T-type Ca2+ channels in myocytes is more important for functions of Ca2+ unrelated to contractility such as cellular proliferation or migration. In this respect, myocytes from mouse jejunum are similar to other smooth muscles [2,42] although there does appear to be role for α 1H Ca2+ channel subunits in controlling relaxation of coronary smooth muscle [43].
Studies in the mice heterozygous for the knockout of the α1H Ca2+ channel subunit did not provide any further information because when compared with wild type litter-mates, there were no differences in the properties of the electrical slow wave. This indicates that the partial knockout did not have a gene dosing effect.
The lethal effects of knocking out expression of the α1H Ca2+ channel subunit in all but one of the many hundreds of mice studied were surprising given that another group has successfully generated a strain of mice by deletion of exon 6 in the cacna1h gene [43]. The reported effects of this knockout were cardiac injury due to coronary artery constriction and reduced body mass compared to wild type litter mates. These are both phenotypes consistent with reduced survival but were clearly not sufficient to prevent survival to maturity of the knockout mice [43]. We were only able to obtain one adult mouse homozygous for the knockout allele over a period of 4 years studying these animals and we conclude that this very low survival rate reflects the genetic backgrounds of the strain of either embryonic stem cells or recipient blastocysts [44]. We could not identify the actual cause of death of the homozygous knockout foetuses but death of foetuses at ages around E10.5 is associated with cardiovascular abnormalities (e.g. [45] and this is consistent with both the phenotype of the published α1H knockout [43] and the known role of T-type Ca2+ channels in smooth muscle cell growth and proliferation [2]. Neural tube abnormalities, which also can cause embryonic lethality at around E10.5 [46], were not observed. Interestingly, the rate of rise and frequency of the electrical slow wave were lower than normal values in recordings from the jejunum of the one mouse that did survive.
In conclusion, the α1H Ca2+ channel subunit is expressed in myocytes and ICC from the circular muscle layer of mouse jejunum. A T-type Ca2+ current also appears to play a role in the generation of the upstroke of the electrical slow wave in mouse tissue and the pharmacological properties of this current are consistent with expression of the α1H Ca2+ channel subunit in ICC contributing to this current. The phenotypic effect of knocking out the gene for the α1H Ca2+ channel subunit is dependent the genetic background of the mouse strain used for the construct and can be embryonic lethal in some mouse strains.
TABLE 2.
Genotypes of mice resulting from mating of pairs of heterozygous animals
| Post-natal | Embryonic Age E17.5 to E19.5 | Embryonic Age E9.5 to E10.5 | |
|---|---|---|---|
| Wild Type | 26 (33%) | 3 (16%) | 2 (10%) |
| Heterozygous | 54 (66%) | 16 (84%) | 10 (47%) |
| Homozygous Knockout | 1 (1%) | 0 (0%) | 9 (43%) |
| No. Litters | 10 | 2 | 2 |
Acknowledgments
We thank Dr Stephen Thibodeau (and his lab) for his assistance with the Southern blots and Dr Jan Van Deursen (and his lab, especially Darren Baker) for their assistance with dissecting out the mouse embryos. This work was supported by the following grants from the NIH, DK 52766, DK 57061 and DK 68055.
References
- 1.Blanks AM, Zhao ZH, Shmygol A, Bru-Mercier G, Astle S, Thornton S. Characterization of the molecular and electrophysiological properties of the T-type calcium channel in human myometrium. J Physiol. 2007;581:915–26. doi: 10.1113/jphysiol.2007.132126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cribbs LL. T-type Ca2+ channels in vascular smooth muscle: multiple functions. Cell Calcium. 2006;40:221–30. doi: 10.1016/j.ceca.2006.04.026. [DOI] [PubMed] [Google Scholar]
- 3.Xiong Z, Sperelakis N, Noffsinger A, Fenoglio-Preiser C. Changes in calcium channel current densities in rat colonic smooth muscle cells during development and aging. Am J Physiol. 1993;265:C617–25. doi: 10.1152/ajpcell.1993.265.3.C617. [DOI] [PubMed] [Google Scholar]
- 4.Xiong Z, Sperelakis N, Noffsinger A, Fenoglio-Preiser C. Ca2+ currents in human colonic smooth muscle cells. Am J Physiol. 1995;269:G378–85. doi: 10.1152/ajpgi.1995.269.3.G378. [DOI] [PubMed] [Google Scholar]
- 5.Koh SD, Monaghan K, Ro S, Mason HS, Kenyon JL, Sanders KM. Novel voltage-dependent non-selective cation conductance in murine colonic myocytes. J Physiol. 2001;533:341–55. doi: 10.1111/j.1469-7793.2001.0341a.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamamoto Y, Hu SL, Kao CY. Inward current in single smooth muscle cells of the guinea pig taenia coli. J Gen Physiol. 1989;93:521–50. doi: 10.1085/jgp.93.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoshino M, Someya T, Nishio A, Yazawa K, Usuki T, Yabu H. Multiple types of voltage-dependent Ca channels in mammalian intestinal smooth muscle cells. Pflugers Arch. 1989;414:401–9. doi: 10.1007/BF00585049. [DOI] [PubMed] [Google Scholar]
- 8.Lee HK, Sanders KM. Comparison of ionic currents from interstitial cells and smooth muscle cells of canine colon. J Physiol. 1993;460:135–52. doi: 10.1113/jphysiol.1993.sp019463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim YC, Koh SD, Sanders KM. Voltage-dependent inward currents of interstitial cells of Cajal from murine colon and small intestine. J Physiol. 2002;541:797–810. doi: 10.1113/jphysiol.2002.018796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farrugia G. Interstitial cells of Cajal in health and disease. Neurogastroenterol Motil. 2008;20:54–63. doi: 10.1111/j.1365-2982.2008.01109.x. [DOI] [PubMed] [Google Scholar]
- 11.Huizinga JD, Thuneberg L, Kluppel M, Malysz J, Mikkelsen HB, Bernstein A. W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature. 1995;373:347–9. doi: 10.1038/373347a0. [DOI] [PubMed] [Google Scholar]
- 12.Ward SM, Burns AJ, Torihashi S, Sanders KM. Mutation of the proto-oncogene c-kit blocks development of interstitial cells and electrical rhythmicity in murine intestine. J Physiol. 1994;480:91–7. doi: 10.1113/jphysiol.1994.sp020343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huizinga JD, Zhu Y, Ye J, Molleman A. High-conductance chloride channels generate pacemaker currents in interstitial cells of Cajal. Gastroenterology. 2002;123:1627–36. doi: 10.1053/gast.2002.36549. [DOI] [PubMed] [Google Scholar]
- 14.Sanders KM, Koh SD, Ward SM. Interstitial cells of cajal as pacemakers in the gastrointestinal tract. Annu Rev Physiol. 2006;68:307–43. doi: 10.1146/annurev.physiol.68.040504.094718. [DOI] [PubMed] [Google Scholar]
- 15.Malysz J, Richardson D, Farraway L, Christen MO, Huizinga JD. Generation of slow wave type action potentials in the mouse small intestine involves a non-L-type calcium channel. Can J Physiol Pharmacol. 1995;73:1502–11. doi: 10.1139/y95-208. [DOI] [PubMed] [Google Scholar]
- 16.Hotta A, Okada N, Suzuki H. Mibefradil-sensitive component involved in the plateau potential in submucosal interstitial cells of the murine proximal colon. Biochem Biophys Res Commun. 2007;353:170–6. doi: 10.1016/j.bbrc.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 17.Yoneda S, Takano H, Takaki M, Suzuki H. Effects of nifedipine and nickel on plateau potentials generated in submucosal interstitial cells distributed in the mouse proximal colon. J Smooth Muscle Res. 2003;39:55–65. doi: 10.1540/jsmr.39.55. [DOI] [PubMed] [Google Scholar]
- 18.Kito Y, Ward SM, Sanders KM. Pacemaker potentials generated by interstitial cells of Cajal in the murine intestine. Am J Physiol Cell Physiol. 2005;288:C710–20. doi: 10.1152/ajpcell.00361.2004. [DOI] [PubMed] [Google Scholar]
- 19.Lee HT, Hennig GW, Fleming NW, Keef KD, Spencer NJ, Ward SM, Sanders KM, Smith TK. The mechanism and spread of pacemaker activity through myenteric interstitial cells of Cajal in human small intestine. Gastroenterology. 2007;132:1852–65. doi: 10.1053/j.gastro.2007.02.049. [DOI] [PubMed] [Google Scholar]
- 20.Park KJ, Hennig GW, Lee HT, Spencer NJ, Ward SM, Smith TK, Sanders KM. Spatial and temporal mapping of pacemaker activity in interstitial cells of Cajal in mouse ileum in situ. Am J Physiol Cell Physiol. 2006;290:C1411–27. doi: 10.1152/ajpcell.00447.2005. [DOI] [PubMed] [Google Scholar]
- 21.Farrugia G. Ca2+ handling in human interstitial cells of Cajal. Gastroenterology. 2007;132:2057–9. doi: 10.1053/j.gastro.2007.03.076. [DOI] [PubMed] [Google Scholar]
- 22.Perez-Reyes E. Molecular characterization of T-type calcium channels. Cell Calcium. 2006;40:89–96. doi: 10.1016/j.ceca.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 23.Chen H, Redelman D, Ro S, Ward SM, Ordog T, Sanders KM. Selective labeling and isolation of functional classes of interstitial cells of Cajal of human and murine small intestine. Am J Physiol Cell Physiol. 2007;292:C497–507. doi: 10.1152/ajpcell.00147.2006. [DOI] [PubMed] [Google Scholar]
- 24.Ou Y, Gibbons SJ, Miller SM, Strege PR, Rich A, Distad MA, Ackerman MJ, Rae JL, Szurszewski JH, Farrugia G. SCN5A is expressed in human jejunal circular smooth muscle cells. Neurogastroenterol Motil. 2002;14:477–86. doi: 10.1046/j.1365-2982.2002.00348.x. [DOI] [PubMed] [Google Scholar]
- 25.Wouters MM, Gibbons SJ, Roeder JL, Distad M, Ou Y, Strege PR, Szurszewski JH, Farrugia G. Exogenous serotonin regulates proliferation of interstitial cells of Cajal in mouse jejunum through 5-HT2B receptors. Gastroenterology. 2007;133:897–906. doi: 10.1053/j.gastro.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 26.Farrugia G, Irons WA, Rae JL, Sarr MG, Szurszewski JH. Activation of whole cell currents in isolated human jejunal circular smooth muscle cells by carbon monoxide. Am J Physiol. 1993;264:G1184–9. doi: 10.1152/ajpgi.1993.264.6.G1184. [DOI] [PubMed] [Google Scholar]
- 27.Farrugia G, Rae JL, Szurszewski JH. Characterization of an outward potassium current in canine jejunal circular smooth muscle and its activation by fenamates. J Physiol. 1993;468:297–310. doi: 10.1113/jphysiol.1993.sp019772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Strege PR, Bernard CE, Ou Y, Gibbons SJ, Farrugia G. Effect of mibefradil on sodium and calcium currents. Am J Physiol Gastrointest Liver Physiol. 2005;289:G249–53. doi: 10.1152/ajpgi.00022.2005. [DOI] [PubMed] [Google Scholar]
- 29.Strege PR, Ou Y, Sha L, Rich A, Gibbons SJ, Szurszewski JH, Sarr MG, Farrugia G. Sodium current in human intestinal interstitial cells of Cajal. Am J Physiol Gastrointest Liver Physiol. 2003;285:G1111–21. doi: 10.1152/ajpgi.00152.2003. [DOI] [PubMed] [Google Scholar]
- 30.Lee JH, Gomora JC, Cribbs LL, Perez-Reyes E. Nickel block of three cloned T-type calcium channels: low concentrations selectively block alpha1H. Biophys J. 1999;77:3034–42. doi: 10.1016/S0006-3495(99)77134-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chaib N, Kabre E, Metioui M, Alzola E, Dantinne C, Marino A, Dehaye JP. Differential sensitivity to nickel and SK&F96365 of second messenger-operated and receptor-operated calcium channels in rat submandibular ductal cells. Cell Calcium. 1998;23:395–404. doi: 10.1016/s0143-4160(98)90096-3. [DOI] [PubMed] [Google Scholar]
- 32.Hinde AK, Perchenet L, Hobai IA, Levi AJ, Hancox JC. Inhibition of Na/Ca exchange by external Ni in guinea-pig ventricular myocytes at 37 degrees C, dialysed internally with cAMP-free and cAMP-containing solutions. Cell Calcium. 1999;25:321–31. doi: 10.1054/ceca.1999.0035. [DOI] [PubMed] [Google Scholar]
- 33.Kimura J, Miyamae S, Noma A. Identification of sodium-calcium exchange current in single ventricular cells of guinea-pig. J Physiol. 1987;384:199–222. doi: 10.1113/jphysiol.1987.sp016450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ng LC, Gurney AM. Store-operated channels mediate Ca(2+) influx and contraction in rat pulmonary artery. Circ Res. 2001;89:923–9. doi: 10.1161/hh2201.100315. [DOI] [PubMed] [Google Scholar]
- 35.Martin RL, Lee JH, Cribbs LL, Perez-Reyes E, Hanck DA. Mibefradil block of cloned T-type calcium channels. J Pharmacol Exp Ther. 2000;295:302–8. [PubMed] [Google Scholar]
- 36.Bezprozvanny I, Tsien RW. Voltage-dependent blockade of diverse types of voltage-gated Ca2+ channels expressed in Xenopus oocytes by the Ca2+ channel antagonist mibefradil (Ro 40-5967) Mol Pharmacol. 1995;48:540–9. [PubMed] [Google Scholar]
- 37.Bean BP. Nitrendipine block of cardiac calcium channels: high-affinity binding to the inactivated state. Proc Natl Acad Sci U S A. 1984;81:6388–92. doi: 10.1073/pnas.81.20.6388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shcheglovitov A, Zhelay T, Vitko Y, Osipenko V, Perez-Reyes E, Kostyuk P, Shuba Y. Contrasting the effects of nifedipine on subtypes of endogenous and recombinant T-type Ca2+ channels. Biochem Pharmacol. 2005;69:841–54. doi: 10.1016/j.bcp.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 39.Nebe B, Holzhausen C, Rychly J, Urbaszek W. Impaired mechanisms of leukocyte adhesion in vitro by the calcium channel antagonist mibefradil. Cardiovasc Drugs Ther. 2002;16:183–93. doi: 10.1023/a:1020688019792. [DOI] [PubMed] [Google Scholar]
- 40.Tomita T, Pang YW, Ogino K. The effects of nickel and cobalt ions on the spontaneous electrical activity, slow wave, in the circular muscle of the guinea-pig gastric antrum. J Smooth Muscle Res. 1998;34:89–100. doi: 10.1540/jsmr.34.89. [DOI] [PubMed] [Google Scholar]
- 41.Faville RA, Pullan AJ, Sanders KM, Smith NP. A biophysically based mathematical model of unitary potential activity in interstitial cells of Cajal. Biophys J. 2008;95:88–104. doi: 10.1529/biophysj.107.122507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fry CH, Sui G, Wu C. T-type Ca2+ channels in non-vascular smooth muscles. Cell Calcium. 2006;40:231–9. doi: 10.1016/j.ceca.2006.04.027. [DOI] [PubMed] [Google Scholar]
- 43.Chen CC, Lamping KG, Nuno DW, Barresi R, Prouty SJ, Lavoie JL, Cribbs LL, England SK, Sigmund CD, Weiss RM, Williamson RA, Hill JA, Campbell KP. Abnormal coronary function in mice deficient in alpha1H T-type Ca2+ channels. Science. 2003;302:1416–8. doi: 10.1126/science.1089268. [DOI] [PubMed] [Google Scholar]
- 44.Gerlai R. Gene-targeting studies of mammalian behavior: is it the mutation or the background genotype? Trends Neurosci. 1996;19:177–81. doi: 10.1016/s0166-2236(96)20020-7. [DOI] [PubMed] [Google Scholar]
- 45.Takeshima H, Komazaki S, Hirose K, Nishi M, Noda T, Iino M. Embryonic lethality and abnormal cardiac myocytes in mice lacking ryanodine receptor type 2. Embo J. 1998;17:3309–16. doi: 10.1093/emboj/17.12.3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Harris MJ, Juriloff DM. Mini-review: toward understanding mechanisms of genetic neural tube defects in mice. Teratology. 1999;60:292–305. doi: 10.1002/(SICI)1096-9926(199911)60:5<292::AID-TERA10>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]