Abstract
Early-adulthood body size is strongly inversely associated with risk of premenopausal breast cancer. It is unclear whether subsequent changes in weight affect risk. We pooled individual-level data from 17 prospective studies to investigate the association of weight change with premenopausal breast cancer risk, considering strata of initial weight, timing of weight change, other breast cancer risk factors, and breast cancer subtype. Hazard ratios (HR) and 95% confidence intervals (CI) were obtained using Cox regression. Among 628,463 women, 10,886 were diagnosed with breast cancer before menopause. Models adjusted for initial weight at ages 18–24 years and other breast cancer risk factors showed that weight gain from ages 18–24 to 35–44 or to 45–54 years was inversely associated with breast cancer overall (e.g. HR per 5kg to ages 45–54: 0.96, 95% CI: 0.95–0.98) and with oestrogen-receptor(ER)-positive breast cancer (HR per 5kg to ages 45–54: 0.96, 95% CI: 0.94–0.98). Weight gain from ages 25–34 was inversely associated with ER-positive breast cancer only and weight gain from ages 35–44 was not associated with risk. None of these weight gains were associated with ER-negative breast cancer. Weight loss was not consistently associated with overall or ER-specific risk after adjusting for initial weight. Weight increase from early-adulthood to ages 45–54 years is associated with a reduced premenopausal breast cancer risk independently of early-adulthood weight. Biological explanations are needed to account for these two separate factors.
Keywords: breast neoplasms, premenopause, body weight changes, risk factors, cohort studies
Introduction
The influence of obesity on breast cancer risk varies by life-stage. Adiposity before menopause is inversely associated with risk, whereas adiposity during the postmenopausal years is positively associated with risk1–3. The inverse association with premenopausal adiposity is particularly strong for adiposity in early adulthood, i.e. ages 18–24 years4, and it is likely that the origin of this association lies in childhood5, 6.
It is not clear whether changes in weight after early adulthood further affect risk of premenopausal breast cancer. The role of weight gain in adulthood is of interest because increases in body weight during adulthood mostly reflect accumulation of adipose rather than lean tissue, and therefore any change might reflect body fatness better than adult weight itself, and because of its association with intra-abdominal fat deposition, which is more metabolically active than peripheral adiposity7. Timing of weight change might additionally be relevant in that weight change at different stages of life, e.g. during periods of hormonal change such as during pregnancy, might have different biological effects, and differentially affect breast cancer risk8.
Due to the relatively low incidence of premenopausal breast cancer, past studies have had limited numbers of cases to investigate the association of weight change with risk by timing of weight change, to examine the effect of weight loss or of weight gain, or to analyse these associations by participant or tumour characteristics. Additionally, past studies have often only used proxies for menopausal status, such as status at study entry or attained age, rather than time-updated menopausal status.
We pooled individual-level data from prospective studies to investigate the association of weight change and its timing with premenopausal breast cancer risk, overall and by breast cancer characteristics.
Materials and methods
We used data from 17 of the 22 cohort studies in the Premenopausal Breast Cancer Collaborative Group9 that had participants’ weight available at a minimum of two time points before women were age 55 years and had at least 100 breast cancer cases diagnosed before age 55 years. Individual-level data were pooled from cohorts in North America (n=9), Europe (n=6), Asia (n=1) and Australia (n=1), with participants recruited between 1963–2013. Data from 1–16 questionnaire rounds per study were harmonised to a common protocol. Women provided historical information on their weight prior to study entry on the baseline questionnaire and their current bodyweight was provided or measured at baseline and on follow-up questionnaires (if available). This work was approved by the relevant institutional review boards and women provided informed consent to partake.
Women were included in the analysis if they were breast cancer-free and premenopausal at study entry, and had premenopausal weight available for at least two age categories (defined below). Premenopausal follow-up time was determined from menopause information obtained from multiple questionnaire rounds, and, if missing, assumptions based on attained age using age 50 as cut-off (see supplement).
The main analytical endpoint was diagnosis with invasive or in-situ premenopausal breast cancer combined. We also conducted analyses of invasive and in-situ outcomes separately, as well outcomes by hormone-receptor status and a clinicopathological surrogate definition of intrinsic breast cancer subtypes (see supplement).
Analyses were conducted using STATA 14.210. Data on weight was available at 2–13 time points per study. We first investigated weight patterns across time with longitudinal trajectory models at a selected number of time points11 (Figure S1–S2). These models resulted in trajectories of weight gain delineated by initial weight, but did not delineate women with weight loss as a separate group. We therefore instead constructed variables for weight change between the age categories 18–24, 25–34, 35–44 and 45–54 years, to be able to use data from all the studies with varying numbers of time points and to examine the association of weight loss with risk. Weight at ages 18–24 was derived, for the great majority of subjects, from weight at age 18 or 20 (depending on the study) retrospectively reported on the baseline questionnaire. Weight at other ages was usually reported or measured at or after recruitment to the study.
Follow-up for breast cancer began at the second weight assessment, that was used to compute weight change, or at recruitment, whichever was later. Follow-up ended with the first of the following events: breast cancer diagnosis, menopause, last follow-up, death or age 55 years. Hazard ratios (HR) and 95% confidence intervals (CI) representing estimates of relative risk of breast cancer were derived from Cox proportional hazards models with attained age as the underlying time scale12. Models were adjusted for cohort; year of birth; age at menarche; parity; age at first birth; time since most recent birth; adult height at recruitment; and family history of breast cancer. Covariate information was updated over follow-up, where available. In the main analyses, we analysed weight change in categories of 5.0 kg increments and as linear trends in risk per 5.0 kg of weight gain. We also obtained results in finer strata of 2.5kg increments (supplement). We obtained HRs for weight change with and without adjustment for starting weight to investigate whether starting weight was a confounder, but presented results adjusted for starting weight unless otherwise stated.
Tests for effect modification of weight change by cohort, starting weight, other available breast cancer risk factors, and time since weight change were conducted using log-likelihood ratio tests13. We estimated separate risk-factor associations for breast cancer type-specific outcomes using an augmentation method14.
We conducted sensitivity analyses by (i) excluding the first two years of follow-up to reduce the chance of reverse causation by preclinical disease; (ii) restricting analyses to reported, rather than assumed, premenopausal follow-up time; (iii) repeating the analyses excluding one study at a time; (iv) additionally adjusting for the number of years between weight assessments; (v) in analyses restricted to subjects with weight at ages 18–24 available, adjusting for weight at ages 18–24 rather than weight at the start of the age category; (vi) restricting analyses to the 5 cohorts contributing to analyses of weight change at all of the six age groups (vii) repeating analyses for subjects with non-missing covariate information (viii) for subjects with available data, additionally adjusting one at a time for variables only available for some cohorts: race/ethnicity, cigarette smoking, alcohol consumption, physical activity level, polycystic ovary syndrome, childhood somatotype and mammographic screening history as at the most recent questionnaire round.
We additionally analysed the average annual weight change assuming a linear trajectory as a risk factor, rather than absolute increase in weight.
Data availability
Research data will be made available upon reasonable request due to privacy/ethical restrictions.
Results
The analyses included 628,463 women, whose median age at recruitment was 39.4 (interquartile range 33.8–44.0) years and who were followed for a median of 10.1 (interquartile range 5.9–15.5) years from recruitment during which 10,886 breast cancer cases (8,509 invasive) were diagnosed. Oestrogen-receptor (ER) status was known for 6,994 (72.5% of invasive and 43.5% of in-situ) breast cancer patients; ER, progesterone-receptor (PR) and HER2-status was available for 3,425 (37.2% invasive, 13.9% in-situ) breast cancer patients.
Most women were white (85.7%), from North America (56.6%), or Europe (41.1%) (Table 1). Women in the weight loss group were on average heavier at the onset than women who gained weight. Most women were parous at recruitment (80.7%) and 12.4% had a family history of breast cancer. The age-specific weight change variables were available for 5–14 cohorts per variable, representing weight change over median time intervals of 6.1–28.2 years and median follow-up for breast cancer of 4.2–17.2 years (Table S1 & S2). For all follow-up periods, the majority (80.3–90.4%) of women gained weight.
Table 1:
Characteristics of women included in the analyses, by degree of weight change between earliest available weight and weight at or close to recruitment to the study
| Weight change category (a) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Factor (b) | Loss ≥5kg | Stable (±4.9kg) | Gain 5–9.9kg | Gain 10–14.9kg | Gain 15–19.9kg | Gain ≥20kg | Overall | |
| Height (cms) | Mean | 165.0 | 164.4 | 164.9 | 165.0 | 165.2 | 165.6 | 164.8 |
| Age at first weight (c) | Mean | 19.9 | 22.1 | 20.1 | 19.5 | 19.3 | 19.1 | 20.6 |
| First weight (kg) | Mean | 71.4 | 57.5 | 56.4 | 56.7 | 57.8 | 60.5 | 58.2 |
| First BMI (kg/m2) | Mean | 26.2 | 21.3 | 20.7 | 20.8 | 21.2 | 22.0 | 21.4 |
| Age at recruitment (years) | Mean | 37.9 | 37.6 | 38.8 | 39.8 | 40.2 | 40.4 | 39.3 |
| Recruitment weight (kg) | Mean | 60.6 | 58.5 | 63.6 | 68.8 | 74.8 | 89.2 | 65.7 |
| Recruitment BMI (kg/m2) | Mean | 22.2 | 21.7 | 23.4 | 25.3 | 27.4 | 32.5 | 24.2 |
| Change in weight between starting age and recruitment (kg) | Mean | −10.8 | 1.0 | 7.2 | 12.1 | 17.0 | 28.7 | 7.5 |
| Median | −8.7 | 1.4 | 7.0 | 12.0 | 17.0 | 26.0 | 5.9 | |
| Rate of weight change between starting age and recruitment (kg/yr) | Mean | −0.8 | 0.1 | 0.5 | 0.7 | 0.9 | 1.5 | 0.4 |
| Median | −0.5 | 0.1 | 0.4 | 0.6 | 0.8 | 1.3 | 0.3 | |
| Race/ethnicity | ||||||||
| White | % | 90.0 | 90.4 | 87.7 | 84.4 | 79.6 | 70.5 | 85.7 |
| Black | % | 6.0 | 5.3 | 8.6 | 12.3 | 17.5 | 27.3 | 10.6 |
| Asian | % | 1.9 | 2.8 | 2.1 | 1.8 | 1.2 | 0.5 | 2.0 |
| Other | % | 2.1 | 1.6 | 1.6 | 1.6 | 1.7 | 1.7 | 1.6 |
| Continent of residence | ||||||||
| North America | % | 63.2 | 57.4 | 53.2 | 51.6 | 55.0 | 65.6 | 56.6 |
| Europe | % | 35.3 | 40.8 | 44.9 | 46.1 | 42.6 | 32.1 | 41.4 |
| Australia | % | 0.8 | 0.7 | 1.3 | 1.8 | 2.2 | 2.2 | 1.3 |
| Asia | % | 0.7 | 1.1 | 0.7 | 0.5 | 0.3 | 0.09 | 0.7 |
| All participants | Total | 32726 | 253164 | 140227 | 86632 | 48297 | 67417 | 628463 |
Weight change was computed between earliest available weight and first weight available at or after recruitment, with the exception of a small number of subjects for whom weight change was computed from two retrospectively assessed weights before recruitment because weight at or after recruitment was not available.
Frequency-distributions for non-missing values only
Weight was retrospectively assessed at age 18 or 20 for the majority of studies.
Figure 1 shows HRs in relation to weight change without (left) and with (right) adjustment for starting weight. There was an inverse U-shaped association with risk (Figure 1 & Figure S3, left), with women who lost weight and those who gained weight having lower HRs than women whose weight remained constant (within ±5.0 kg), in particular for weight change from ages 18–24 years. Women who lost ≥5 kg since ages 18–24 years had a statistically significantly lower breast cancer risk than those whose weight remained constant. However, after additionally adjusting for starting weight (Figure 1 & Figure S3, right), which was on average greater for women with weight loss than for those who gained weight, the inverse HRs for weight loss were attenuated and no longer associated with risk. Weight gain from ages 18–24 to 35–44 years or from ages 18–24 to 45–54 years was associated with lower breast cancer risk, and HRs were not appreciably attenuated with adjustment for starting weight. Linear inverse trends in risk per 5 kg gain over these time periods remained statistically significant (HRs: 0.97, 95% CI: 0.96–0.98 for ages 18–24 to 35–44 and 0.96, 95% CI: 0.95–0.98 for 18–24 to 45–54 years). The association of starting weight with risk remained statistically significant in these models.
Figure 1: Relative risk of premenopausal breast cancer in relation to weight change between various ages.
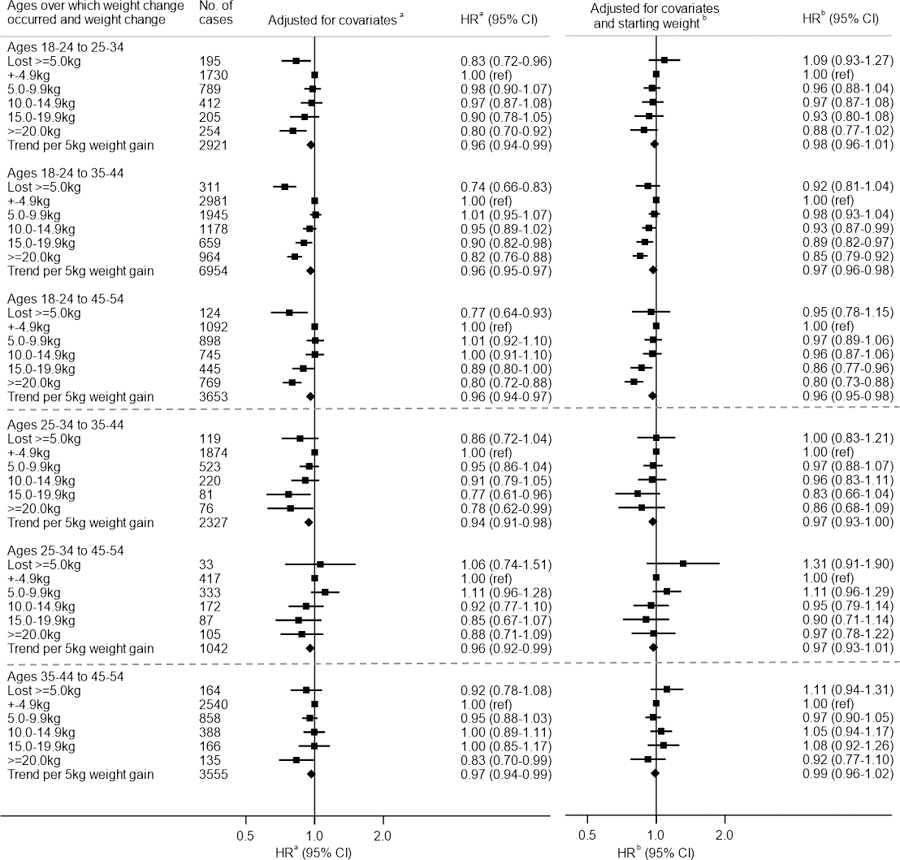
Footnotes:
Abbreviations: HR, hazard ratio; CI, confidence interval
(a) Adjusted for attained age, cohort, year of birth, adult height, age at menarche, age at first birth, number of births, time since last birth and family history of breast cancer
(b) Adjusted for covariates in (a) plus weight at start of age range
Patterns of risk with weight change from later ages (i.e. from 25–34 or 35–44 years) were less clear, with no association with weight loss and linear trends with weight gain not being statistically significantly associated with risk after adjustment for starting weight.
In analyses by breast cancer invasiveness, the inverse associations of weight gain with breast cancer risk tended to be stronger for in-situ than for invasive breast cancer, but only significantly so for weight gain between ages 18–24 and 25–34 years (p-interaction=0.007) (Table S3). Stronger associations for in-situ than invasive breast cancer were also observed when we repeated the analyses among subjects with a previous history of mammographic screening only.
Associations of weight gain from young ages tended to be stronger for ER-positive than ER-negative (Table 2) or for ER+/PR+ than ER− and PR− breast cancer (Table S4). The difference in hazard ratios between these subgroups were only strongly statistically significant for one weight change group, however.
Table 2:
Risk of premenopausal breast cancer in relation to weight change between various ages, by oestrogen-receptor status of breast cancer
| Weight gain category, kg |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Loss ≥5.0kg | Stable ± 4.9kg | Gain 5.0–9.9kg | Gain 10.0–14.9kg | Gain 15.0–19.9kg | Gain ≥20.0kg | Trend per 5kg gain (b) | ||||||
| Ages at weight change | Median weight change, kg (25–75th percentile) | ER status | No. of cases | HR (95% CI)(a) | HR (a) | HR (95% CI)(a) | HR (95% CI)(a) | HR (95% CI)(a) | HR (95% CI)(a) | P -int | HR (95% CI) (a) | P int- trend |
| Ages 18–24 to 25–34 | 4.5 (0.4–9.6) | ER+ | 1834 | 1.00 (0.80–1.25) | 1.00 (ref) | 0.89 (0.79–1.01) | 0.96 (0.83–1.12) | 0.87 (0.71–1.07) | 0.85 (0.70–1.02) | 0.98 (0.95–1.01) | ||
| ER− | 591 | 1.24 (0.86–1.79) | 1.00 (ref) | 1.10 (0.90–1.36) | 1.06 (0.82–1.38) | 0.85 (0.59–1.24) | 1.12 (0.83–1.50) | 0.39 | 1.01 (0.96–1.07) | 0.24 | ||
| Ages 18–24 to 35–44 | 6.9 (2.3–13.6) | ER+ | 3976 | 0.92 (0.78–1.09) | 1.00 (ref) | 1.00 (0.92–1.08) | 0.96 (0.87–1.05) | 0.88 (0.78–0.99) | 0.77 (0.69–0.86) | 0.95 (0.93–0.97) | ||
| ER− | 1268 | 0.85 (0.62–1.18) | 1.00 (ref) | 1.07 (0.93–1.24) | 1.11 (0.94–1.31) | 1.11 (0.90–1.35) | 1.01 (0.85–1.21) | 0.07 | 1.01 (0.98–1.04) | 0.0009 | ||
| Ages 18–24 to 45–54 | 10.0 (4.5–18.2) | ER+ | 2249 | 0.80 (0.60–1.05) | 1.00 (ref) | 0.98 (0.87–1.11) | 1.02 (0.90–1.15) | 0.89 (0.77–1.03) | 0.79 (0.70–0.90) | 0.96 (0.94–0.98) | ||
| ER− | 625 | 1.27 (0.81–2.00) | 1.00 (ref) | 1.26 (1.00–1.58) | 1.18 (0.93–1.52) | 0.97 (0.73–1.31) | 1.02 (0.80–1.30) | 0.27 | 0.99 (0.95–1.02) | 0.16 | ||
| Age 25–34 to 35–44 | 3.2 (0–7.3) | ER+ | 1547 | 0.99 (0.77–1.28) | 1.00 (ref) | 0.99 (0.86–1.13) | 0.99 (0.81–1.20) | 0.71 (0.50–0.99) | 0.72 (0.50–1.03) | 0.94 (0.89–0.99) | ||
| ER− | 466 | 0.74 (0.44–1.24) | 1.00 (ref) | 1.04 (0.82–1.33) | 1.13 (0.81–1.57) | 0.85 (0.49–1.49) | 1.08 (0.64–1.83) | 0.61 | 1.03 (0.96–1.10) | 0.03 | ||
| Age 25–34 to 45–54 | 7.3 (3.1–13.6) | ER+ | 726 | 1.45 (0.94–2.26) | 1.00 (ref) | 1.10 (0.92–1.32) | 0.91 (0.73–1.15) | 0.86 (0.64–1.16) | 0.90 (0.68–1.19) | 0.95 (0.90–1.00) | ||
| ER− | 169 | 0.25 (0.03–1.80) | 1.00 (ref) | 1.07 (0.74–1.54) | 0.91 (0.58–1.43) | 0.98 (0.56–1.71) | 0.69 (0.37–1.28) | 0.58 | 0.95 (0.86–1.04) | 1.00 | ||
| Age 35–44 to 45–54 | 3.2 (0–7.7) | ER+ | 2575 | 1.15 (0.94–1.41) | 1.00 (ref) | 0.95 (0.86–1.05) | 1.08 (0.94–1.24) | 1.16 (0.96–1.42) | 0.89 (0.71–1.12) | 1.00 (0.96–1.03) | ||
| ER− | 670 | 0.77 (0.48–1.22) | 1.00 (ref) | 1.04 (0.86–1.26) | 1.03 (0.79–1.35) | 0.71 (0.44–1.14) | 0.89 (0.58–1.37) | 0.19 | 0.98 (0.91–1.05) | 0.61 | ||
Abbreviations: CI, confidence interval; ER, oestrogen-receptor; HR, hazard ratio; P-int, p-value for interaction test
HRs are adjusted for attained age, cohort, year of birth, adult height, weight at start of age range, age at menarche, age at first birth, number of births, time since last birth and family history of breast cancer
Including subjects with weight gain only
In analysis by breast cancer intrinsic subtype, weight gain from ages 18–24 onwards was inversely associated with Luminal A-like (ER+PR+HER2−) breast cancer and weight gain from ages 18–24 to 35–44 and to 45–54 years additionally with luminal B-like (ER+PR− or ER−PR+) breast cancer risk (Figure S4). For some of the age groups, there was evidence of a positive association with non-luminal (ER−PR−) breast cancer risk, in particular HER2-enriched breast cancer, whereas there was no association with triple-negative breast cancer risk.
There was evidence for effect modification by starting weight for the linear effect of weight change at two age groups, ages 18–24 years to 45–54 years (p-interaction=0.02) and 25–34 to 35–44 years (p-interaction=0.006), but in opposite directions and some of the results were based on small numbers of cases (Table S5). We observed no statistically significant evidence for effect modification in the linear association of weight change with risk by other breast cancer risk factors considered (childhood body shape or weight, adult height, age at menarche, parity, age at first birth, number of births, family history of breast cancer, and race/ethnicity, see Table S6) or by time since weight change (Table S7).
The main findings did not materially differ in the sensitivity analyses conducted (see methods and Table S8 & Table S9 for selected results) except that the inverse associations with weight gain were somewhat stronger when a stricter definition of menopausal status was applied. When analysing risk in relation to average annual weight change, rather than absolute amount of weight change, conclusions were similar, with the strongest inverse association with risk observed for weight change over the longest interval, from ages 18–24 to 45–54 years (HR=0.82, 95% CI: 0.75–0.89 per kg/yr), reflecting the largest absolute weight gains (Figure S5).
Conclusions
We observed that both weight loss ≥5 kg and weight gain of ≥10–15 kg since early adulthood were inversely associated with premenopausal breast cancer risk, but that degree of weight change was associated with initial weight, and that only weight gain was associated with risk after controlling for early adult weight. Early adulthood weight remained significant in such models, indicating that both starting weight and weight gain are associated with risk. There was weak and inconsistent evidence that the effect of weight gain depended on starting weight, and no evidence that the association varied by other investigated breast cancer risk factors. We captured weight change between ages 18–24 and 35–44 years, when most parous women had their pregnancies, but did not find statistical evidence that the association of weight change with risk differed between parous and nulliparous women. Weight change from later ages, age 35 years onwards, was not associated with risk.
Our pooling project incorporates data from most15–22, although not all23–28, published prospective studies on long-term weight change and premenopausal breast cancer risk, and additionally includes previously unpublished data. It consequently had enhanced statistical power based on its large sample size. Few past studies reported on weight loss separately; those that did reported null associations or non-significant inverse associations with weight loss since age 18 or 20 years compared with women whose weight remained stable15, 21–23, 26, and not all adjusted for starting weight. In relation to weight gain, the majority of prospective studies have reported null or non-statistically significant inverse linear trends15, 18–28, except for two reporting positive associations, but with no clear dose-response relationship16, 20.
There was a tendency for inverse associations with risk to be somewhat stronger for in-situ than invasive breast cancer; this might reflect stage-specific aetiology or could be artefactual, e.g. a deficit of in-situ diagnoses could occur if increasing weight made women less likely to attend breast screening or if they presented later because breast self-examination and lump detection is more difficult29. Stronger associations for in-situ than invasive cancer were also observed among women who had previously had a screening mammogram, suggesting that it is not explained by past breast screening attendance, but unfortunately we did not have data on mode of detection of breast cancer.
We observed stronger inverse associations of weight gain with ER+ than ER− breast cancer, or with ER+/PR+ than ER−PR− breast cancer. This agrees with our previous finding that BMI at ages over 25 years is inversely associated with risk of hormone-receptor-positive breast cancer only4. In augmentation analyses by intrinsic subtype, however, we observed somewhat contradictory findings, with some weight change variables being positively associated with HER2-enriched breast cancer and nonluminal breast cancer overall. These analyses were conducted on somewhat different subsets of the data and some of them on small numbers. Whether there is an association of weight change with non-luminal subtypes remains therefore uncertain.
It is of interest that we observed the strongest inverse associations with risk for weight change from early adulthood and no significant association of risk with absolute or rate of weight gain from ages 35–44 years onwards. Weight gain soon after age 18, if not followed by later weight loss, would lead to the greatest cumulative exposure to adiposity. It is possible that it is cumulative exposure to excess weight that is inversely associated with risk or that late weight gain is outside the susceptibility window for premenopausal breast cancer e.g. because there is a lag time between weight gain and an effect on risk. Our analyses by time since weight since did not suggest the latter is the case. The lack of association with later (i.e. ≥35 years) weight gain appears discordant with the results from two previous studies. The EPIC-PANACEA study reported a positive association of rate of weight gain over four years with breast cancer diagnosed at age <50 years, based on 283 cases30. Women were premenopausal or perimenopausal at study entry (median age 40.7 years, M Emaus, personal communication) but no information on menopausal status at the second weight assessment was available; it is therefore possible that for some of the women postmenopausal weight gain was assessed. The Nurses’ Health Study reported a positive association of weight gain over four years among initially premenopausal women with breast cancer risk over the subsequent 2 years (HR: 1.38, 95% CI: 1.13–1.69 for ≥15 vs. <5 lbs, n=736 cases)31. In our study, weight change was assessed over longer periods but the reason for the disparity in results is unclear.
The strong inverse association of breast cancer risk with early adult body size4 may originate in early life, or in childhood/adolescence5, 6. It has been hypothesized to be due to greater differentiation of breast tissue during puberty2, 32, altered oestrogen metabolism33, lower adult mammographic density34, 35 and/or lower circulating IGF-1 levels36 in heavier girls. Additional weight gain is associated with a reduction in mammographic density37 and substantial weight gain leading to obesity suppresses ovarian function38, 39, with a consequent reduction in endogenous sex hormone, in particular, progesterone, exposure38. Weight gain might affect risk through changes in hormone profile because young women with high BMI have been reported to have lower levels of sex-hormone binding globulin (SHBG), oestradiol and progesterone, and higher levels of free testosterone than women with lower BMI40. Oestrogens and testosterone have been associated with premenopausal breast cancer risk41, although less clearly than for postmenopausal breast cancer, but the evidence for an association of risk with progesterone is inconsistent, however41. A recent study reported lower breast cell proliferation in heavier compared with leaner premenopausal women, and the reverse in postmenopausal women, which might be hormone-related42.
Strengths of our study include its prospective design, its large number of cases, and therefore its ability to investigate associations according to breast cancer characteristics, multiple time-points of weight assessments, and the use of time-updated covariates. Limitations include that weight at ages 18–24 years was ascertained by recall for most participants, but recalled weight at age 18 years has been shown to correlate well with measured weight43, and that we did not consider central adiposity measures. We studied weight change over six, some overlapping, age categories, using data of somewhat different populations, but a sensitivity analyses restricting to the five cohorts that contributed to all age categories showed similar results. There were too few women contributing to consecutive non-overlapping time periods of weight change to investigate the role timing of weight change in a single model. In analyses by breast cancer subtype, numbers of subtype-specific breast cancers were modest for some of the weight change variables. Furthermore, our data set was not well-suited to investigate Asian women. We did not observe effect modification by ethnicity/race, but the study included relatively few women of Asian descent. It has been suggested that among Asian women, there is a positive association between BMI and premenopausal breast cancer risk44, but prospective studies of weight gain in Asian women have, so far, shown an inverse or null association with premenopausal breast cancer risk overall24, 26, 45.
Our results may contribute to the understanding of breast cancer causation and aid in risk stratification. However, weight gain would not provide a strategy for long-term risk reduction because weight and weight gain are positively associated with risks of postmenopausal breast cancer, several other types of cancer, and other adverse health outcomes46, 47. Additionally, obese women diagnosed with breast cancer tend to have worse outcomes than leaner women, independent of their menopausal status48.
In conclusion, we have observed that both body size in early adulthood and subsequent weight gain are independently associated with reductions in premenopausal breast cancer risk. There is a need to understand mechanisms underlying this finding, which may provide a means for breast cancer prevention.
Supplementary Material
Novelty and impact:
Body weight in childhood and early adulthood is inversely associated with breast cancer risk diagnosed before the menopause. We investigated the role of subsequent changes in weight on breast cancer risk among 628 463 premenopausal women from 17 prospective studies. The results show, for the first time, that weight gain from early adulthood is inversely associated with risk of premenopausal breast cancer, providing further evidence of adiposity as a fundamental determinant of breast cancer risk.
Acknowledgements
We thank the National Cancer Institute Cohort Consortium for facilitating this collaboration. We thank Melissa House MS (WestStat Inc, MD, USA); Mustapha Abubakar MBBS PhD (National Cancer Institute, MD, USA); Niclas Håkansson PhD (Karolinska Institute, Stockholm, Sweden); Jane Sullivan-Halley BS (Beckman Research Institute of City of Hope, Duarte, CA, USA); Allison Iwan BS, Diane Kampa BS (University of Minnesota, MN, USA); Jerry Reid PHD (American Registry of Radiologic Technologists, MN, USA); Jeffrey Yu MPH (Slone Epidemiology Center at Boston University, Boston, MA, USA) for contributions to acquisition, analysis or interpretation of data. They received no compensation for their contributions other than their salaries.
We wish to acknowledge all study participants, staff, and participating cancer registries. The Nurses’ Health Study (NHS) and Nurses’ Health Study 2 (NHS2) would like to thank the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY. The study protocol was approved by the institutional review boards of the Brigham and Women’s Hospital and Harvard T.H. Chan School of Public Health, and those of participating registries as required.
Funding/support
Support for this research comes, in part, from Breast Cancer Now; The Institute of Cancer Research, London; the Avon Foundation (02-2014-080); the United States National Center for Advancing Translational Sciences (KL2-TR001109); Karolinska Institutet Distinguished Professor Award Dnr: 2368/10-221; the United States National Institutes of Health, National Institute of Environmental Health Sciences (Z01 ES044005, P30 ES000260, P30 CA016087), National Cancer Institute (UM1 CA176726, UM1 CA186107, UM1 CA182876, UM1 CA182934, UM1 CA164974, R01 CA058420, R01 CA092447, CA144034), the National Program of Cancer Registries of the Centers for Disease Control and Prevention, and the Department of Energy; The Dahod Breast Cancer Research Program at Boston University School of Medicine; the State of Maryland, the Maryland Cigarette Restitution Fund; VicHealth, Cancer Council Victoria and the Australian National Health and Medical Research Council (209057, 396414 and 1074383); the Breast Cancer Research Foundation (BCRF-17-138); the Swedish Research Council and Swedish Cancer Foundation; the Japanese Ministry of Health, Labor and Welfare; the Hellenic Health Foundation. The California Teachers Study is supported by R01 CA77398 and U01 CA199277. The coordination of the European Prospective Investigation in Cancer is financially supported by the European Commission (DG-SANCO) and the International Agency for Research on Cancer. The national cohorts are supported by German Cancer Aid, German Cancer Research Center (DKFZ), Federal Ministry of Education and Research (BMBF), Deutsche Krebshilfe, Deutsches Krebsforschungszentrum and Federal Ministry of Education and Research (Germany); the Hellenic Health Foundation (Greece); Associazione Italiana per la Ricerca sul Cancro-AIRC-Italy and National Research Council (Italy); Swedish Cancer Society, Swedish Research Council and County Councils of Skane and Västerbotten (Sweden); Cancer Research UK (14136 to EPIC-Norfolk; C570/A16491 and C8221/A19170 to EPIC-Oxford), Medical Research Council (1000143 to EPIC-Norfolk, MR/M012190/1 to EPIC-Oxford; United Kingdom). The Institute of Cancer Research, London, acknowledges National Health Service funding to the Royal Marsden/ICR NIHR Biomedical Research Centre.
List of abbreviations:
- BMI
body mass index
- CI
confidence interval
- ER
oestrogen-receptor
- HR
hazard ratio
- PR
progesterone-receptor
- SHBG
sex-hormone binding globulin
Footnotes
Author disclosure
Since 1 January 2019, Elisabete Weiderpass has been a staff member of the International Agency for Research on Cancer. Where authors are identified as personnel of the International Agency for Research on Cancer / World Health Organization (E Weiderpass, L Dossus), the authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy or views of the International Agency for Research on Cancer / World Health Organization.
Conflict of interest
None of the authors declared a conflict of interest with regard to this paper.
References
- 1.WCRF/AICR, Continuous Update Project Expert Report 2018. Body fatness and weight gain and the risk of cancer. World Cancer Research Fund/American Institute for Cancer Research, 2018.
- 2.Suzuki R, Saji S, Toi M. Impact of body mass index on breast cancer in accordance with the life-stage of women. Frontiers in oncology 2012;2:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Cancer Research Fund International Systematic Literature Review, The associations between Food, Nutrition and Physical Activity and the Risk of Breast Cancer: Continuous Update Project. World Cancer Research Fund, 2017. [Google Scholar]
- 4.Premenopausal Breast Cancer Collaborative G, Schoemaker MJ, Nichols HB, Wright LB, Brook MN, Jones ME, O’Brien KM, Adami HO, Baglietto L, Bernstein L, Bertrand KA, Boutron-Ruault MC, Braaten T, Chen Y, Connor AE, Dorronsoro M, Dossus L, Eliassen AH, Giles GG, Hankinson SE, Kaaks R, Key TJ, Kirsh VA, Kitahara CM, Koh WP, Larsson SC, Linet MS, Ma H, Masala G, Merritt MA, Milne RL, Overvad K, Ozasa K, Palmer JR, Peeters PH, Riboli E, Rohan TE, Sadakane A, Sund M, Tamimi RM, Trichopoulou A, Ursin G, Vatten L, Visvanathan K, Weiderpass E, Willett WC, Wolk A, Yuan JM, Zeleniuch-Jacquotte A, Sandler DP, Swerdlow AJ. Association of Body Mass Index and Age With Subsequent Breast Cancer Risk in Premenopausal Women. JAMA Oncol 2018;4:e181771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baer HJ, Tworoger SS, Hankinson SE, Willett WC. Body fatness at young ages and risk of breast cancer throughout life. Am J Epidemiol 2010;171:1183–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colditz GA, Bohlke K, Berkey CS. Breast cancer risk accumulation starts early: prevention must also. Breast cancer research and treatment 2014;145:567–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hunter GR, Gower BA, Kane BL. Age Related Shift in Visceral Fat. Int J Body Compos Res 2010;8:103–8. [PMC free article] [PubMed] [Google Scholar]
- 8.Ballard-Barbash R. Anthropometry and breast cancer. Body size--a moving target. Cancer 1994;74:1090–100. [DOI] [PubMed] [Google Scholar]
- 9.Nichols HB, Schoemaker MJ, Wright LB, McGowan C, Brook MN, McClain KM, Jones ME, Adami HO, Agnoli C, Baglietto L, Bernstein L, Bertrand KA, Blot WJ, Boutron-Ruault MC, Butler L, Chen Y, Doody MM, Dossus L, Eliassen AH, Giles GG, Gram IT, Hankinson SE, Hoffman-Bolton J, Kaaks R, Key TJ, Kirsh VA, Kitahara CM, Koh WP, Larsson SC, Lund E, Ma H, Merritt MA, Milne RL, Navarro C, Overvad K, Ozasa K, Palmer JR, Peeters PH, Riboli E, Rohan TE, Sadakane A, Sund M, Tamimi RM, Trichopoulou A, Vatten L, Visvanathan K, Weiderpass E, Willett WC, Wolk A, Zeleniuch-Jacquotte A, Zheng W, Sandler DP, Swerdlow AJ. The Premenopausal Breast Cancer Collaboration: A Pooling Project of Studies Participating in the National Cancer Institute Cohort Consortium. Cancer Epidemiol Biomarkers Prev 2017;26:1360–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.StataCorp, ed. Stata Statistical Software: Release 14ed. College Station, Texas: StataCorp LP, 2015. [Google Scholar]
- 11.Nagin DJ, BL; Passos LV, Tremblay RE;. Group-based multi-trajectory modeling. Statistics in Medical Research 2016. [DOI] [PubMed]
- 12.Cox DR. Regression Models and Life-Tables. Journal of the Royal Statistical Society 1972;34:187–220. [Google Scholar]
- 13.Buse A The Likelihood Ratio, Wald, and Lagrange Multiplier Tests - an Expository Note. Am Stat 1982;36:4. [Google Scholar]
- 14.Lunn M, McNeil D. Applying Cox regression to competing risks. Biometrics 1995;51:524–32. [PubMed] [Google Scholar]
- 15.Weiderpass E, Braaten T, Magnusson C, Kumle M, Vainio H, Lund E, Adami HO. A prospective study of body size in different periods of life and risk of premenopausal breast cancer. Cancer Epidemiol Biomarkers Prev 2004;13:1121–7. [PubMed] [Google Scholar]
- 16.Palmer JR, Adams-Campbell LL, Boggs DA, Wise LA, Rosenberg L. A prospective study of body size and breast cancer in black women. Cancer Epidemiol Biomarkers Prev 2007;16:1795–802. [DOI] [PubMed] [Google Scholar]
- 17.Horn-Ross PL, Canchola AJ, Bernstein L, Neuhausen SL, Nelson DO, Reynolds P. Lifetime body size and estrogen-receptor-positive breast cancer risk in the California Teachers Study cohort. Breast cancer research : BCR 2016;18:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lahmann PH, Schulz M, Hoffmann K, Boeing H, Tjonneland A, Olsen A, Overvad K, Key TJ, Allen NE, Khaw KT, Bingham S, Berglund G, Wirfalt E, Berrino F, Krogh V, Trichopoulou A, Lagiou P, Trichopoulos D, Kaaks R, Riboli E. Long-term weight change and breast cancer risk: the European prospective investigation into cancer and nutrition (EPIC). Br J Cancer 2005;93:582–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang Z, Hankinson SE, Colditz GA, Stampfer MJ, Hunter DJ, Manson JE, Hennekens CH, Rosner B, Speizer FE, Willett WC. Dual effects of weight and weight gain on breast cancer risk. JAMA 1997;278:1407–11. [PubMed] [Google Scholar]
- 20.Catsburg C, Kirsh VA, Soskolne CL, Kreiger N, Bruce E, Ho T, Leatherdale ST, Rohan TE. Associations between anthropometric characteristics, physical activity, and breast cancer risk in a Canadian cohort. Breast cancer research and treatment 2014;145:545–52. [DOI] [PubMed] [Google Scholar]
- 21.Rosner B, Eliassen AH, Toriola AT, Chen WY, Hankinson SE, Willett WC, Berkey CS, Colditz GA. Weight and weight changes in early adulthood and later breast cancer risk. Int J Cancer 2017;140:2003–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Michels KB, Terry KL, Eliassen AH, Hankinson SE, Willett WC. Adult weight change and incidence of premenopausal breast cancer. Int J Cancer 2012;130:902–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manders P, Pijpe A, Hooning MJ, Kluijt I, Vasen HF, Hoogerbrugge N, van Asperen CJ, Meijers-Heijboer H, Ausems MG, van Os TA, Gomez-Garcia EB, Brohet RM, Hebon, van Leeuwen FE, Rookus MA. Body weight and risk of breast cancer in BRCA1/2 mutation carriers. Breast cancer research and treatment 2011;126:193–202. [DOI] [PubMed] [Google Scholar]
- 24.Liu Y, Warren Andersen S, Wen W, Gao YT, Lan Q, Rothman N, Ji BT, Yang G, Xiang YB, Shu XO, Zheng W. Prospective cohort study of general and central obesity, weight change trajectory and risk of major cancers among Chinese women. Int J Cancer 2016;139:1461–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Breslow RA, Ballard-Barbash R, Munoz K, Graubard BI. Long-term recreational physical activity and breast cancer in the National Health and Nutrition Examination Survey I epidemiologic follow-up study. Cancer Epidemiol Biomarkers Prev 2001;10:805–8. [PubMed] [Google Scholar]
- 26.Suzuki R, Iwasaki M, Inoue M, Sasazuki S, Sawada N, Yamaji T, Shimazu T, Tsugane S, Japan Public Health Center-based Prospective Study G. Body weight at age 20 years, subsequent weight change and breast cancer risk defined by estrogen and progesterone receptor status--the Japan public health center-based prospective study. Int J Cancer 2011;129:1214–24. [DOI] [PubMed] [Google Scholar]
- 27.Nitta J, Nojima M, Ohnishi H, Mori M, Wakai K, Suzuki S, Fujino Y, Lin Y, Tamakoshi K, Tamakoshi A. Weight Gain and Alcohol Drinking Associations with Breast Cancer Risk in Japanese Postmenopausal Women - Results from the Japan Collaborative Cohort (JACC) Study. Asian Pac J Cancer Prev 2016;17:1437–43. [DOI] [PubMed] [Google Scholar]
- 28.Dite GS, MacInnis RJ, Bickerstaffe A, Dowty JG, Milne RL, Antoniou AC, Weideman P, Apicella C, Giles GG, Southey MC, Jenkins MA, Phillips KA, Win AK, Terry MB, Hopper JL. Testing for Gene-Environment Interactions Using a Prospective Family Cohort Design: Body Mass Index in Early and Later Adulthood and Risk of Breast Cancer. Am J Epidemiol 2017;185:487–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cui Y, Whiteman MK, Flaws JA, Langenberg P, Tkaczuk KH, Bush TL. Body mass and stage of breast cancer at diagnosis. Int J Cancer 2002;98:279–83. [DOI] [PubMed] [Google Scholar]
- 30.Emaus MJ, van Gils CH, Bakker MF, Bisschop CN, Monninkhof EM, Bueno-de-Mesquita HB, Travier N, Berentzen TL, Overvad K, Tjonneland A, Romieu I, Rinaldi S, Chajes V, Gunter MJ, Clavel-Chapelon F, Fagherazzi G, Mesrine S, Chang-Claude J, Kaaks R, Boeing H, Aleksandrova K, Trichopoulou A, Naska A, Orfanos P, Palli D, Agnoli C, Tumino R, Vineis P, Mattiello A, Braaten T, Borch KB, Lund E, Menendez V, Sanchez MJ, Navarro C, Barricarte A, Amiano P, Sund M, Andersson A, Borgquist S, Olsson A, Khaw KT, Wareham N, Travis RC, Riboli E, Peeters PH, May AM. Weight change in middle adulthood and breast cancer risk in the EPIC-PANACEA study. Int J Cancer 2014;135:2887–99. [DOI] [PubMed] [Google Scholar]
- 31.Rosner B, Eliassen AH, Toriola AT, Hankinson SE, Willett WC, Natarajan L, Colditz GA. Short-term weight gain and breast cancer risk by hormone receptor classification among pre- and postmenopausal women. Breast cancer research and treatment 2015;150:643–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hilakivi-Clarke L, Forsen T, Eriksson JG, Luoto R, Tuomilehto J, Osmond C, Barker DJ. Tallness and overweight during childhood have opposing effects on breast cancer risk. Br J Cancer 2001;85:1680–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Houghton LC, Sisti JS, Hankinson SE, Xie J, Xu X, Hoover RN, Eliassen AH, Ziegler RG. Estrogen Metabolism in Premenopausal Women Is Related to Early Life Body Fatness. Cancer Epidemiol Biomarkers Prev 2018. [DOI] [PMC free article] [PubMed]
- 34.Yochum L, Tamimi RM, Hankinson SE. Birthweight, early life body size and adult mammographic density: a review of epidemiologic studies. Cancer causes & control : CCC 2014;25:1247–59. [DOI] [PubMed] [Google Scholar]
- 35.Rice MS, Bertrand KA, VanderWeele TJ, Rosner BA, Liao X, Adami HO, Tamimi RM. Mammographic density and breast cancer risk: a mediation analysis. Breast cancer research : BCR 2016;18:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Poole EM, Tworoger SS, Hankinson SE, Schernhammer ES, Pollak MN, Baer HJ. Body size in early life and adult levels of insulin-like growth factor 1 and insulin-like growth factor binding protein 3. Am J Epidemiol 2011;174:642–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alimujiang A, Appleton C, Colditz GA, Toriola AT. Adiposity during early adulthood, changes in adiposity during adulthood, attained adiposity, and mammographic density among premenopausal women. Breast cancer research and treatment 2017;166:197–206. [DOI] [PubMed] [Google Scholar]
- 38.Dowsett M, Folkerd E. Reduced progesterone levels explain the reduced risk of breast cancer in obese premenopausal women: a new hypothesis. Breast cancer research and treatment 2015;149:1–4. [DOI] [PubMed] [Google Scholar]
- 39.Westhoff C, Gentile G, Lee J, Zacur H, Helbig D. Predictors of ovarian steroid secretion in reproductive-age women. Am J Epidemiol 1996;144:381–8. [DOI] [PubMed] [Google Scholar]
- 40.Tworoger SS, Eliassen AH, Missmer SA, Baer H, Rich-Edwards J, Michels KB, Barbieri RL, Dowsett M, Hankinson SE. Birthweight and body size throughout life in relation to sex hormones and prolactin concentrations in premenopausal women. Cancer EpidemiolBiomarkers Prev 2006;15:2494–501. [DOI] [PubMed] [Google Scholar]
- 41.Key TJ, Appleby PN, Reeves GK, Travis RC, Alberg AJ, Barricarte A, Berrino F, Krogh V, Sieri S, Brinton LA, Dorgan JF, Dossus L, Dowsett M, Eliassen AH, Fortner RT, Hankinson SE, Helzlsouer KJ, Hoff man-Bolton J, Comstock GW, Kaaks R, Kahle LL, Muti P, Overvad K, Peeters PH, Riboli E, Rinaldi S, Rollison DE, Stanczyk FZ, Trichopoulos D, Tworoger SS, Vineis P. Sex hormones and risk of breast cancer in premenopausal women: a collaborative reanalysis of individual participant data from seven prospective studies. Lancet Oncol 2013;14:1009–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao H, Wang J, Fang D, Lee O, Chatterton RT, Stearns V, Khan SA, Bulun SE. Adiposity Results in Metabolic and Inflammation Differences in Premenopausal and Postmenopausal Women Consistent with the Difference in Breast Cancer Risk. Horm Cancer 2018;9:229–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Troy LM, Hunter DJ, Manson JE, Colditz GA, Stampfer MJ, Willett WC. The validity of recalled weight among younger women. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity 1995;19:570–2. [PubMed] [Google Scholar]
- 44.Wada K, Nagata C, Tamakoshi A, Matsuo K, Oze I, Wakai K, Tsuji I, Sugawara Y, Mizoue T, Tanaka K, Iwasaki M, Inoue M, Tsugane S, Sasazuki S. Body mass index and breast cancer risk in Japan: a pooled analysis of eight population-based cohort studies. Ann Oncol 2014;25:519–24. [DOI] [PubMed] [Google Scholar]
- 45.Li HL, Gao YT, Li Q, Liu DK. [Anthropometry and female breast cancer: a prospective cohort study in urban Shanghai]. Zhonghua Liu Xing Bing Xue Za Zhi 2006;27:488–93. [PubMed] [Google Scholar]
- 46.Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, Lee A, Marczak L, Mokdad AH, Moradi-Lakeh M, Naghavi M, Salama JS, Vos T, Abate KH, Abbafati C, Ahmed MB, Al-Aly Z, Alkerwi A, Al-Raddadi R, Amare AT, Amberbir A, Amegah AK, Amini E, Amrock SM, Anjana RM, Arnlov J, Asayesh H, Banerjee A, Barac A, Baye E, Bennett DA, Beyene AS, Biadgilign S, Biryukov S, Bjertness E, Boneya DJ, Campos-Nonato I, Carrero JJ, Cecilio P, Cercy K, Ciobanu LG, Cornaby L, Damtew SA, Dandona L, Dandona R, Dharmaratne SD, Duncan BB, Eshrati B, Esteghamati A, Feigin VL, Fernandes JC, Furst T, Gebrehiwot TT, Gold A, Gona PN, Goto A, Habtewold TD, Hadush KT, Hafezi-Nejad N, Hay SI, Horino M, Islami F, Kamal R, Kasaeian A, Katikireddi SV, Kengne AP, Kesavachandran CN, Khader YS, Khang YH, Khubchandani J, Kim D, Kim YJ, Kinfu Y, Kosen S, Ku T, Defo BK, Kumar GA, Larson HJ, Leinsalu M, Liang X, Lim SS, Liu P, Lopez AD, Lozano R, Majeed A, Malekzadeh R, Malta DC, Mazidi M, McAlinden C, McGarvey ST, Mengistu DT, Mensah GA, Mensink GBM, Mezgebe HB, Mirrakhimov EM, Mueller UO, Noubiap JJ, Obermeyer CM, Ogbo FA, Owolabi MO, Patton GC, Pourmalek F, Qorbani M, Rafay A, Rai RK, Ranabhat CL, Reinig N, Safiri S, Salomon JA, Sanabria JR, Santos IS, Sartorius B, Sawhney M, Schmidhuber J, Schutte AE, Schmidt MI, Sepanlou SG, Shamsizadeh M, Sheikhbahaei S, Shin MJ, Shiri R, Shiue I, Roba HS, Silva DAS, Silverberg JI, Singh JA, Stranges S, Swaminathan S, Tabares-Seisdedos R, Tadese F, Tedla BA, Tegegne BS, Terkawi AS, Thakur JS, Tonelli M, Topor-Madry R, Tyrovolas S, Ukwaja KN, Uthman OA, Vaezghasemi M, Vasankari T, Vlassov VV, Vollset SE, Weiderpass E, Werdecker A, Wesana J, Westerman R, Yano Y, Yonemoto N, Yonga G, Zaidi Z, Zenebe ZM, Zipkin B, Murray CJL. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N Engl J Med 2017;377:13–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Keum N, Greenwood DC, Lee DH, Kim R, Aune D, Ju W, Hu FB, Giovannucci EL. Adult weight gain and adiposity-related cancers: a dose-response meta-analysis of prospective observational studies. J Natl Cancer Inst 2015;107. [DOI] [PubMed] [Google Scholar]
- 48.Chan DS, Vieira AR, Aune D, Bandera EV, Greenwood DC, McTiernan A, Navarro Rosenblatt D, Thune I, Vieira R, Norat T. Body mass index and survival in women with breast cancer-systematic literature review and meta-analysis of 82 follow-up studies. Ann Oncol 2014;25:1901–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Research data will be made available upon reasonable request due to privacy/ethical restrictions.


