Abstract
Objectives
Given the importance of the dissemination of accurate family history to assess disease risk, we characterized the gatherers, disseminators, and blockers of health information within families at high genetic risk of cancer.
Methods
A total of 5466 personal network members of 183 female participants of the Breast Imaging Study from 124 families with known mutations in the BRCA1/2 genes (associated with high risk of breast, ovarian, and other types of cancer) were identified by using the Colored Eco-Genetic Relationship Map (CEGRM). Hierarchical nonlinear models were fitted to characterize information gatherers, disseminators, and blockers.
Results
Gatherers of information were more often female (P<.001), parents (P<.001), and emotional support providers (P<.001). Disseminators were more likely female first- and second-degree relatives (both P<.001), family members in the older or same generation as the participant (P<.001), those with a cancer history (P<.001), and providers of emotional (P<.001) or tangible support (P<.001). Blockers tended to be spouses or partners (P<.001) and male, first-degree relatives (P<.001).
Conclusions
Our results provide insight into which family members may, within a family-based intervention, effectively gather family risk information, disseminate information, and encourage discussions regarding shared family risk.
Acquiring accurate information regarding familial disease risk is a key component of a proactive approach to health care. This information is needed both to permit an accurate risk assessment and to develop appropriate, cost-effective prevention and risk-reducing management strategies.1-3 An increased disease risk based on family history has important implications for screening,4 and often leads to intervention at an earlier age than usual, increased screening frequency, modified surveillance recommendations, and the possibility of referral for genetic services. Both behavioral and genetic risk factors tend to cluster within families, which suggests that personalized risk information may have implications for the entire family.5,6 Consequently, family-based efforts to collect and disseminate accurate family health history and genetic risk information are warranted and require familial cooperation in the gathering and disseminating process, as well as in reducing barriers to information flow. Building upon the success of community-based interventions that utilize lay health advisors or peer leaders,7-10 we hypothesized that family-based interventions may be more effective if a family leader is integrated into the intervention efforts.
The challenge in developing these family-based approaches is identifying optimally positioned family members who can facilitate gathering and disseminating family health history and genetic risk information. Different family members may take on different roles related to gathering and disseminating information. For example, the risk dissemination literature suggests that women tend to take on the role of “kin-keepers,”11-16 who help to maintain communication among family members, monitor family relationships, and facilitate contact among family members.11,12 However, more specific information regarding the characteristics of these disseminators is warranted, because the current literature is sparse.
Individuals with deleterious BRCA1/2 mutations are effective in disseminating risk information to both close and more distant family members.14,17-19 There is evidence that genetic test results are disseminated to a large percentage (>75%) of at-risk family members.20-22 In contrast, the literature suggests that the effectiveness of dissemination efforts in the context of common disease risk23 or high-risk families with indeterminate genetic test results14,24 (Ersig et al, unpublished data, 2009) is more limited; efforts to expand this reach are vital to successful proactive health care. Because families with known BRCA1/2 mutations have been relatively effective in disseminating family health history and genetic risk information, they provide an ideal model for identifying the individual and relational characteristics of those persons within the family who play important roles in gathering and disseminating family health information.25
In the present study, we sought to identify the characteristics of (1) gatherers, (2) disseminators, and (3) blockers of health information flow within BRCA1/2 mutation-positive families. Knowledge regarding the characteristics of persons central to these processes should facilitate developing network-based interventions that use optimally positioned family lay health advisors. Additionally, characterizing the persons who impede the dissemination process will inform interventions that integrate blockers into the information transfer process.
METHODS
The Breast Imaging Study is a 4-year, prospective cohort study of women from BRCA1/2 mutation-positive families. Eligible women were aged 25 to 56 years and had a known deleterious BRCA1/2 mutation, were first- or second-degree relatives of carriers of a BRCA1/2 mutation, or were relatives of individuals with BRCA-associated cancers in mutation-positive families. Participants were recruited between 2001 and 2007 from families participating in a long-term prospective study of hereditary breast and ovarian cancer; from self-referrals in response to media advertising in the Washington, DC, area; and from nationwide referrals from physicians or genetic counselors. The current study considered the personal networks of 183 participants from 124 families seen at the National Institutes of Health. A study investigator obtained written and verbal consent for this protocol.
Procedures
Complete data were obtained from 183 of 200 participants who completed the Colored Eco-Genetic Relationship Map (CEGRM) at their baseline evaluation (1 participant refused to complete the CEGRM and 16 women could not be scheduled for baseline assessment). All participants had received prior genetic education and counseling, and nearly all had undergone clinical genetic testing, often several years before participating in the Breast Imaging Study. During the baseline evaluation, participants underwent extensive medical evaluations of the breasts and ovaries and completed the CEGRM.
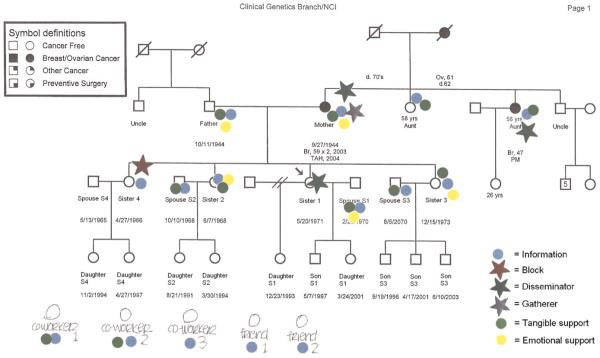
The CEGRM (Figure 1) is a visual research tool for assessing social interactions between study participants and their family members and friends.13 An investigator (J.A.P or L.M.H) administered a 20- to 60-minute semistructured interview with a genetic pedigree as a template.26,27 The participants added nonkin network members (nonbiological family, friends, and coworkers) to their pedigrees. Thus, personal network members, or “alters,” as they are referred to here, included biological family, non-biological family, and social ties. For each alter, the participants indicated exchange of social support related to informational, emotional, and tangible support domains by placing coded stickers onto the pedigree next to the relevant individuals. Additionally, persons who played specific roles in the risk communication process were further characterized by the placement of colored stars. The specific communication roles considered included gatherers, disseminators, and blockers of health information within the family. The CEGRM assessment process has been described in detail in previous publications.13,26,27
FIGURE 1. Colored Eco-Genetic Relationship Map (CEGRM) used in the Breast Imaging Study, United States, 2001-2007.
In addition to these social support and informational roles, the characteristics of each person represented on the CEGRM were also coded, including gender, pedigree generation relative to the participant, cancer history, kinship relation to the participant, and whether the individual was living or deceased. CEGRMs were scanned into digital images; social exchange data were coded into databases by 2 of 3 investigators (N. R. K, A. L. E, and L.M.H) for subsequent analyses (interrater reliability exceeded 0.96 for all coded variables). The investigators’ handwritten comments on the CEGRM scripts were used to clarify discrepancies in coding.
Measures
Three primary communication outcomes representing the health information roles of personal network members were analyzed. “Gatherers” represented persons who searched for new information about cancer or genetic testing; “disseminators” spread genetic and cancer risk information to other family members and encouraged cancer risk discussions with each other; and “blockers” indicated reluctance about learning or transmitting health information regarding cancer and genetic risk. Information-gathering was added to the CEGRM assessment as a separate category in February 2005.27
Both individual and relational characteristics of personal network members were considered as predictors. Individual characteristics of interest included gender and personal cancer history. Relational characteristics between the respondent and the alter included social support exchanges (e.g., tangible assistance and emotional support), kinship relation, and generation (younger, same, and older). Because information regarding the age of social ties (e.g., friends) was not collected, these personal network members were assumed to be in the same generation as the participant.
Covariates considered included the number of participants per family, whether the family was part of a historical cohort of BRCA1/2 families28 or was a newly recruited family from the breast imaging protocol,29 and whether the personal network member was deceased at the time of assessment.
Statistical Analyses
Descriptive statistics were constructed to characterize the study subjects and their personal network members. Separate hierarchical nonlinear models were fitted to identify those characteristics associated with the outcomes of health information gathering, disseminating, and blocking within the family. Each participant’s personal network members and the relationships involving these alters were analyzed.30 The alters included were first- and second-degree biological relatives, spouses and partners, nonbiological family (e.g., step-family, adopted family, and family through marriage), and social ties (e.g., friends, coworkers) identified during the CEGRM construction. A total of 5466 alters were included in the disseminator and blocker analyses; 4206 alters were considered in the gatherer analysis, reflecting the addition of gatherers to the CEGRM in 2005. In addition to the main effects of each predictor variable under consideration, interactions between gender, generation, and kinship relation were also investigated.
Models were fitted by using HLM version 6.06.31 Because several families with multiple participating family members were included in the analyses, we constructed and controlled for dependence structures defined from exponential random graph models. These dependence structures included the following: density (variability across families in the proportion of network members gathering, disseminating, or blocking information within a family); out-stars (variability across participants in the number of network members they report as gatherers, disseminators, or blockers of information); and in-stars (variability in the number of network members who are chosen as gatherers, disseminators, or blockers of information within a family).32,33 A Wald statistic was computed on the basis of robust standard errors and, given the large number of alters considered within these analyses, a type I error of 0.01 was used to establish statistical importance.34 Astep-up approach was used to fit the final models.35 Main effects and interactions that were not statistically significant were not included in the final model. If an interaction was found to be statistically significant, the component main effects were kept within the model even if they were not statistically significant.
RESULTS
All participants were white women; 30% had a personal history of cancer and 85% were carriers of a BRCA1/2 mutation. Seventy-three percent of the study participants were married or were in long-term relationships. The participants’ ages ranged from 22 to 57 years, with a mean of 40 years (SD=9 years). Most had completed high school (97%) and had pursued further education (91%).
Characteristics of Personal Network Members
Characteristics of the alters represented within the participants’ personal networks are summarized in Table 1. Examination of alters involved in communication ties provided by different respondents from the same families indicated a 33% overlap in communication ties. In other words, family members shared only a third of their communication ties with each other.
TABLE 1. Characteristics of Personal Network Members of Female Participants: Breast Imaging Study, United States, 2001–2007.
| Characteristic | No. (%) |
|---|---|
| Womena | 2717 (51.4) |
| Kinship to participant | |
| First-degree relative | 1064 (20.1) |
| Second-degree relative | 1448 (27.4) |
| Spouse or partnerb | 169 (3.2) |
| Nonbiological familyc | 1452 (27.5) |
| Social kin | 1111 (21.0) |
| Generation relative to participant | |
| Older | 1660 (31.4) |
| Same | 2571 (48.7) |
| Younger | 1052 (19.9) |
| Cancer history | 357 (6.8) |
| Provides emotional support | 1426 (27.0) |
| Provides tangible assistance | 1219 (23.1) |
Note. N = 5283. The participants (n = 183) are not included in this table.
Gender unknown or missing for 249 network members.
Includes ex-spouses and partners.
Excludes spouse or partner.
Information Gatherer, Disseminator, and Blocker Characteristics
On average, each participant named 1 information gatherer (range: 0–9), 2 disseminators (range: 0–12), and 1 information blocker (range: 0–5) within the family. Sixty-one percent of the disseminators of health information were also labeled as information gatherers (χ2(1)=176.00; P<.001). There were no significant associations between blockers and gatherers (χ2(1)=2.33; P=.13) or between blockers and disseminators (χ2(1)=1.07; P=.30). Theodds ratios and 99% confidence intervals for the final multilevel models are provided in Table 2. Information gatherers tended to be females, study participants, providers of emotional support, and parents (i.e., older first-degree relatives). Disseminators of health information tended to be those who had a personal history of cancer, study participants, providers of tangible assistance and emotional support, and female first- and second-degree relatives. Blockers of information tended to be spouses or partners and male first-degree relatives of study participants (adjusted odds ratio [AOR]=1.79; P<.01). Emotional support providers and younger relatives, particularly young first-degree relatives, were unlikely to be blockers of health information within the family system.
TABLE 2. Final Models Identifying Characteristics of Health Information Gatherers, Disseminators, and Blockers: Breast Imaging Study, United States, 2001–2007.
| Variable | Gatherers, OR (99% CI) | Disseminators, OR (99% CI) | Blockers, OR (99% CI) |
|---|---|---|---|
| Women | 1.74** (1.33, 2.26) | 1.18 (0.82, 1.69) | 1.65** (1.21, 2.25) |
| Kinship | |||
| Participant | 5.34** (3.23, 8.82) | 14.27** (8.65, 23.56) | 1.15 (0.60, 2.21) |
| First-degree relative | 1.58** (1.06, 2.36) | 1.24 (0.83, 1.85) | 10.69** (6.64, 17.22) |
| Second-degree relative | 1.27 (0.93, 1.73) | 0.69 (0.44, 1.06) | 2.00** (1.33, 3.00) |
| Spouse or partnera | 1.43 (0.72, 2.83) | 0.95 (0.48, 1.88) | 5.26** (2.60, 10.62) |
| Nonbiological familyb (Ref) | 1.00 | 1.00 | 1.00 |
| Social kin | 0.55** (0.36, 0.82) | 0.67 (0.38, 1.19) | 0.34** (0.21, 0.55) |
| Generation | |||
| Younger | 0.41** (0.25, 0.66) | 0.06** (0.04, 0.08) | 0.19** (0.11, 0.34) |
| Same (Ref) | 1.00 | 1.00 | 1.00 |
| Older | 0.33** (0.22, 0.48) | 1.23 (0.87, 1.75) | 0.71 (0.49, 1.03) |
| Cancer history | 2.03** (1.41, 2.93) | ||
| Provides tangible assistance | 1.66** (1.18, 2.35) | ||
| Provides emotional support | 2.28** (1.49, 3.49) | 1.57** (1.10, 2.24) | 0.39** (0.26, 0.58) |
| Female x first-degree relative | 3.60** (2.23, 5.80) | 0.47** (0.28, 0.80) | |
| Female x second-degree relative | 3.32** (1.82, 6.09) | ||
| Younger x first-degree relative | 0.36* (0.15, 0.84) | ||
| Older x first-degree relative | 4.11** (2.41, 7.03) |
Note. CI = confidence interval; OR = odds ratio. Sample size for gatherers was n = 4206; sample size for disseminators and for blockers was n = 5466. Analyses were controlled for the number of participants per family, newly recruited families, whether the alter was deceased, density, in-stars (variability in the number of network members who are chosen as gatherers, disseminators, or blockers of information within a family), and out-stars (variability across participants in the number of network members they report as gatherers, disseminators, or blockers of information).
Includes ex-spouses and partners.
Excludes spouse or partner.
P < .01
P < .001.
DISCUSSION
Our results define the important roles that family members play within the processes of gathering and disseminating health information. Prior research suggested that genetic risk information flows through first-degree relatives.16 Our current findings, however, refine and highlight the differential roles that family members assume in family health information gathering and dissemination. Parents were clearly of special importance in the gathering of health information, because they are often the gate-keepers to the health information of older and more distant family members for their children. Thus, interventions that focus on the gathering of accurate family health history information are likely to be more effective if they engage the older generation of the extended family. Reciprocally, the act of engaging older family members inherently enhances their health through improved social engagement within the family.36
This intergenerational communication pattern, with younger generations less likely to be engaged in gathering, disseminating, and blocking family risk information suggests that the family of origin, rather than the coresident or nuclear family, may be particularly important in health communication. The family of origin represents the family in which one is born and is characterized by extended multi-generational relationships.37 Coresident families are those living within the same household and is inclusive of diverse family structures,38 whereas nuclear families are defined by heterosexual parents and their children.37 Older generations play an important role in answering children’s questions regarding biology and genealogy.39 However, family-based interventions tend to focus on co-resident or nuclear families, rather than families of origin.40 Our results suggest that a shift in perspective may be warranted when the goal is to engage families, including children, in a dialogue regarding their family risk of disease, especially given evidence that health beliefs develop during childhood in the family of origin.41,42 Our data support a strategy that relies on older family members as a critical source of family health information.
Health information gatherers tended to be women, regardless of their biological relatedness to the participants, whereas information disseminators tended to be female first- and second-degree relatives. These findings suggest the importance of involving biological kin in disseminating health information to family members. The cancer risk associated with BRCA1/2 mutations is manifest disproportionately among female family members, which might explain why women play such an important role in intrafamilial communication. However, this pattern of women taking on the role of disseminating family health information has also been reported in familial syndromes in which men and women are at similar risk, such as hereditary colorectal cancer16 and familial melanoma.43
The central role played by cancer-affected family members in the dissemination process is consistent with using disease diagnosis as a teachable moment in a family health communication intervention.44-46 Being informed of their genetic risk from a family member who has been affected by a syndrome-associated cancer may carry particular salience for at-risk family members. In addition, it is important for affected family members to share their diagnosis with family members in an effort to update family health history.47
Family members associated with gathering and disseminating family cancer risk information were also involved in exchanges of tangible assistance and emotional support. Thus, these social support relationships permit identifying specific persons within the family system for recruitment and engagement in intervention efforts to enhance family health communication. The use of social-network approaches to identify peer leaders has been shown to be a reliable and valid method.48,49 However, the particular social relationships that are associated with identifying an effective family leader may differ depending upon the intervention context.9 Our results suggest that the gathering of information is a key component of the emotional support process, and that dissemination of information is associated with both emotional support and tangible assistance. In the aggregate, our data indicate that the ideal family leader would be a female family member who already provides both emotional and tangible support to others within the extended family system. Follow-up studies are needed to investigate whether such individuals are well-received in this role within families and to evaluate how such individuals can most effectively exert a positive influence on others in their families.
Importantly, blockers of information exchange tended to be spouses or partners and male first-degree relatives. This role of blocking may be reflective of a selective pattern of communication, in which women are more likely to communicate with their female family members than with male family members.50 Additionally, the family of origin has established routines for communicating about the genetic risk of cancer within the family,51 and families define their own mini-culture based on the values, rules, and rituals surrounding the exchange of resources and information.52 Spouses, as newcomers to the family, may have more difficulty in becoming a part of the communication routines regarding hereditary risk, because they have not been socialized within the culture that has developed through generations of cancer diagnoses and the family’s understanding of its genetic risk.53 However, spouses and partners are potentially key persons in gathering and disseminating information, particularly to their at-risk children, and in promoting appropriate screening behaviors to those at risk. Engagement of all family members in the education process, not just those at increased risk of disease because of genetics or strong family history, is an essential component in fostering a unified, cooperative approach to addressing a family’s shared health threat.
Male first-degree relatives within hereditary breast and ovarian cancer families have a 50% probability of carrying their family’s BRCA1/2 mutation, placing both themselves and potentially their children at risk as well. Their reluctance to discuss health information may compromise the care and support that they provide to members of their family of origin and to their own nuclear family. This behavior may be the result of gender role acculturation, grieving actual or anticipated loss of relatives, or their failure to appreciate that, despite hereditary breast and ovarian cancer being generally seen as a woman’s disease, male mutation carriers are at risk for specific BRCA1/2-associated cancers and at risk for transmitting the mutation to their children.54 Family scripts depicting roles and norms are passed through generations as part of the family narrative.55 Differences in the way males and females are socialized in understanding and coping with their genetic risk may be an intrinsic part of the family script.56 Thus, our results point to the need to identify these scripts within the family and to tailor intervention components specific to the family’s needs. It is important to note, however, that the current results represent the perceptions of female family members exclusively. Future research that captures the perspectives of men within these at-risk families would further enhance our understanding of the gathering, disseminating, and blocking of health history and genetic risk information within the family.
Although the results herein focus on the gathering and dissemination of health risk information as it relates to highly penetrant genetic mutations, they represent a model that informs family-based interventions aimed at facilitating family health information gathering and dissemination in general. The rapidly growing field of research in genomics has identified genetic variants that contribute to complex disease risk. Currently, these research advances are being marketed directly to consumers,57 and these new genetic tools are increasingly being used clinically.58,59 It is hoped that the personalized nature of genomic information may eventually facilitate health decision-making, motivate healthy lifestyles, and increase screening adherence, although we have a long way to go before the benefits of such interventions are proven. Because behavioral and genetic risk of disease also clusters within families, personalized proactive health care based on genomic risk information will have implications not only for individuals, but also their relatives. Development of tailored family health advisor interventions that facilitate the process of gathering and disseminating family risk information is likely to be vital as we move forward into this new health care arena.
The findings from this study may have limited generalizability because of the sample’s racial and educational homogeneity; future efforts should aim to assess whether similar patterns of gathering and disseminating health risk information are observed within families of varied cultural and socioeconomic back-grounds. Family risk education interventions that capitalize on known characteristics of the family structure, engaging older generations and families of origin in the process, may be particularly effective in informing individuals of their hereditary disease risk, as well as in helping family members adopt healthier lifestyles and adhere to screening recommendations. The success of personalized disease prevention is likely to require health care consumers to assume significant responsibility for the care of themselves and their loved ones.
Acknowledgments
This research was supported, in part, by funding from the Intramural Research Program of the National Human Genome Research Institute (HG200335-01), by funding from the Intramural Research Program of the National Cancer Institute to NCI’s Clinical Genetics Branch, and by support services contracts NO2-CP-11019 and NO2-CP-65504 with Westat, Inc. A. L. Ersig was supported by a Graduate Partnerships Program fellowship from the National Institute of Nursing Research.
We are very grateful to the families who participated in NCI protocol #01-C-0009 (NCT00012415); Breast Imaging Screening Studies in Women at High Genetic Risk of Breast Cancer; Annual Follow-up Study and NCI protocol #78-C-0039 (NCT00004007); and Clinical, Laboratory, and Epidemiologic Characterization of Individuals and Families at High Risk of Cancer. We also thank Thomas Valente, Keshia Pollack, Kimberly Kaphingst, and the 3 anonymous reviewers for providing comments on an earlier version of the article.
Human Participant Protection
This study was approved by the institutional review board of the National Institutes of Health Clinical Center. Written and verbal consent were obtained before study participation.
Footnotes
The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of Health and Human Services or the US government.
References
- 1.Yoediono Z, Snyderman R. Proposal for a new health record to support personalized, predictive, preventative, and participatory medicine. Personalized Med. 2008;5(1):47–54. doi: 10.2217/17410541.5.1.47. [DOI] [PubMed] [Google Scholar]
- 2.Ramsey SD, Burke W, Pinsky L, Clarke L, Newcomb P, Khoury MJ. Family history assessment to detect increased risk for colorectal cancer: conceptual considerations and a preliminary economic analysis. Cancer Epidemiol Biomarkers Prev. 2005;14(11):2494–2500. doi: 10.1158/1055-9965.EPI-05-0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramsey SD, Clarke L, Etzioni R, Higashi M, Berry K, Urban N. Cost-effectiveness of microsatellite instability screening as a method for detecting hereditary non-polyposis colorectal cancer. Ann Intern Med. 2001;135(8):577–588. doi: 10.7326/0003-4819-135-8_part_1-200110160-00008. [DOI] [PubMed] [Google Scholar]
- 4.McCusker ME, Yoon PW, Gwinn M, Malacher AM, Neff L, Khoury MJ. Family history of heart disease and cardiovascular disease risk-reducing behaviors. Genet Med. 2004;6(3):153–158. doi: 10.1097/01.gim.0000127271.60548.89. [DOI] [PubMed] [Google Scholar]
- 5.Burke V. Obesity in childhood and cardiovascular disease. Clin Exp Pharmacol Physiol. 2006;33(9):831–837. doi: 10.1111/j.1440-1681.2006.04449.x. [DOI] [PubMed] [Google Scholar]
- 6.Mitchell BD, Rainwater DL, Hsueh WC, Kennedy AJ, Stern MP, MacCluer JW. Familial aggregation of nutrient intake and physical activity: results from the San Antonio Family Heart Study. Ann Epidemiol. 2003;13(2):128–135. doi: 10.1016/s1047-2797(02)00255-7. [DOI] [PubMed] [Google Scholar]
- 7.Kelly JA, Amirkhanian YA, Kabakchieva E, et al. Prevention of HIV and sexually transmitted diseases in high risk social networks of young Roma (Gypsy) men in Bulgaria: randomized controlled trial. BMJ. 2006;333:1098–1101. doi: 10.1136/bmj.38992.478299.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hinton A, Downey J, Lisovicz N, Mayfield-Johnson S, White-Johnson F. The Community Health Advisor program and the Deep South Network for Cancer Control: health promotion programs for volunteer community health advisors. Fam Community Health. 2005;28(1):20–27. doi: 10.1097/00003727-200501000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Valente TW, Hoffman BR, Ritt-Olson A, Lichtman K, Johnson CA. Effects of a social-network method for group assignment strategies on peer-led tobacco prevention programs in schools. Am J Public Health. 2003;93(11):1837–1843. doi: 10.2105/ajph.93.11.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Valente TW, Ritt-Olson A, Stacy A, Unger JB, Okamoto J, Sussman S. Peer acceleration: effects of a social network tailored substance abuse prevention program among high-risk adolescents. Addiction. 2007;102(11):1804–1815. doi: 10.1111/j.1360-0443.2007.01992.x. [DOI] [PubMed] [Google Scholar]
- 11.Dubas JS. How gender moderates the grandparent-grandchild relationship: a comparison of kin-keeper and kin-selector theories. J Fam Issues. 2001;22(4):478–492. [Google Scholar]
- 12.Hagestad GO. The aging society as a context for family life. In: Jecker NAS, editor. Aging and Ethics: Philosophical Problems in Gerontology. Humana Press; Totowa, NJ: 1991. pp. 123–146. [Google Scholar]
- 13.Kenen R, Peters JA. The Colored, Eco-Genetic Relationship Map (CEGRM): a conceptual approach and tool for genetic counseling research. J Genet Couns. 2001;10(4):289–309. doi: 10.1023/A:1016627426430. [DOI] [PubMed] [Google Scholar]
- 14.Patenaude AF, Dorval M, DiGianni LS, Schneider KA, Chittenden A, Garber JE. Sharing BRCA1/2 test results with first-degree relatives: factors predicting who women tell. J Clin Oncol. 2006;24(4):700–706. doi: 10.1200/JCO.2005.01.7541. [DOI] [PubMed] [Google Scholar]
- 15.Wilson BJ, Forrest K, van Teijlingen ER, et al. Family communication about genetic risk: the little that is known. Community Genet. 2004;7:15–54. doi: 10.1159/000080300. [DOI] [PubMed] [Google Scholar]
- 16.Koehly LM, Peterson SK, Watts BG, Kempf KG, Vernon SW, Gritz ER. A social network analysis of communication about hereditary nonpolyposis colorectal cancer genetic testing and family functioning. Cancer Epidemiol Biomarkers Prev. 2003;12:304–313. [PubMed] [Google Scholar]
- 17.Gadzicki D, Wingen LU, Teige B, et al. Communicating BRCA1 and BRCA2 genetic test results. J Clin Oncol. 2006;24(18):2969–2970. doi: 10.1200/JCO.2006.06.3750. [DOI] [PubMed] [Google Scholar]
- 18.MacDonald DJ, Sarna L, van Servellen G, Bastani R, Giger JN, Weitzel JN. Selection of family members for communication of cancer risk and barriers to this communication before and after genetic cancer risk assessment. Genet Med. 2007;9(5):275–282. doi: 10.1097/gim.0b013e31804ec075. [DOI] [PubMed] [Google Scholar]
- 19.McGivern B, Everett J, Yager GG, Baumiller RC, Hafertepen A, Saal HM. Family communication about positive BRCA1 and BRCA2 genetic test results. Genet Med. 2004;6(6):503–509. doi: 10.1097/01.gim.0000144014.91237.a1. [DOI] [PubMed] [Google Scholar]
- 20.Blandy C, Chabal F, Stoppa-Lyonnet D, Julian-Reynier C. Testing participation in BRCA1/2-positive families: initiator role of index cases. Genet Test. 2003;7:225–233. doi: 10.1089/109065703322537241. [DOI] [PubMed] [Google Scholar]
- 21.Hughes C, Lerman C, Schwartz M, et al. All in the family: evaluation of the process and content of sisters’ communication about BRCA1 and BRCA2 genetic test results. Am J Med Genet. 2002;107(2):143–150. doi: 10.1002/ajmg.10110. [DOI] [PubMed] [Google Scholar]
- 22.Wagner Costalas J, Itzen M, Malick J, et al. Communication of BRCA1 and BRCA2 results to at-risk relatives: a cancer risk assessment program’s experience. Am J Med Genet C Semin Med Genet. 2003;119C(1):11–18. doi: 10.1002/ajmg.c.10003. [DOI] [PubMed] [Google Scholar]
- 23.Ashida S, Koehly LM, Roberts JS, Chen CA, Hiraki S, Green RC. Disclosing the disclosure: factors associated with communicating the results of susceptibility genetic testing for Alzheimer’s disease. J Health Commun. doi: 10.1080/10810730903295518. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stoffel EM, Ford B, Mercado RC, et al. Sharing genetic test results in Lynch Syndrome: communication with close and distant relatives. Clin Gastroenterol Hepatol. 2008;6(3):333–338. doi: 10.1016/j.cgh.2007.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scheuner MT, Yoon P, Khoury MJ. Contribution of Mendelian disorders to common chronic disease: opportunities for recognition, intervention, and prevention. Am J Med Genet C Semin Med Genet. 2004;125C(1):50–65. doi: 10.1002/ajmg.c.30008. [DOI] [PubMed] [Google Scholar]
- 26.Peters JA, Kenen R, Giusti R, Loud JT, Weissman N, Greene MH. Exploratory study of the feasibility and utility of the colored eco-genetic relationship map (CEGRM) in women at high genetic risk of developing breast cancer. Am J Med Genet A. 2004;130A:258–264. doi: 10.1002/ajmg.a.30271. [DOI] [PubMed] [Google Scholar]
- 27.Peters JA, Hoskins L, Prindiville S, Kenen R, Greene MH. Evolution of the colored eco-genetic relationship map (CEGRM) for assessing social functioning in women in hereditary breast-ovarian (HBOC) families. J Genet Couns. 2006;15(6):477–489. doi: 10.1007/s10897-006-9042-7. [DOI] [PubMed] [Google Scholar]
- 28.Kramer JL, Velasquez IA, Chen BE, Rosenberg PS, Struewing JP, Greene MH. Prophylactic oophorectomy reduces breast cancer penetrance during prospective, long-term follow-up of BRCA1 mutation carriers. J Clin Oncol. 2005;23:8629–8635. doi: 10.1200/JCO.2005.02.9199. [DOI] [PubMed] [Google Scholar]
- 29.Mueller C, Mai P, Bucher J, Peters JA, Loud JT, Greene MH. Patterns of complementary and alternative medication use in women at increased genetic risk of breast and ovarian cancer. BMC Complement Altern Med. 2008;8:17. doi: 10.1186/1472-6882-8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Snijders TAB, Spreen M, Zwaagstra R. The use of multilevel modelling for analysing personal networks (networks of cocaine users in an urban area) J Quant Anthropol. 1995;5(2):85–105. [Google Scholar]
- 31.Raudenbush S, Bryk A, Congdon R. HLM 6.06. 2006. (for Windows) [computer program] [Google Scholar]
- 32.Anderson CJ, Wasserman S, Crouch B. A p* primer: logit models for social networks. Soc Networks. 1999;21(1):37–66. [Google Scholar]
- 33.Wasserman S, Pattison P. Logit models and logistic regressions for social networks: I. An introduction to Markov random graphs and p*. Psychometrika. 1996;61:401–425. [Google Scholar]
- 34.Snijders TAB, Bosker RJ. Multilevel Analysis: An Introduction to Basic and Advanced Multilevel Modeling. Sage Publications; Thousand Oaks, CA: 1999. [Google Scholar]
- 35.Raudenbush SW, Bryk AS. Hierarchical Linear Models: Applications and Data Analysis Methods. 2nd ed. Sage Publications; Thousand Oaks, CA: 2002. [Google Scholar]
- 36.Lowenstein A, Katz R, Gur-yaish N. Reciprocity in parent-child exchange and life satisfaction among the elderly: A cross-national perspective. J Soc Issues. 2007;63(4):865–883. [Google Scholar]
- 37.Segrin C, Flora J. Family Communication. Lawrence Erlbaum; Mahwah, NJ: 2005. [Google Scholar]
- 38.Socha TJ. Communication in family units. In: Frey LR, Gouran DS, Poole MS, editors. The Handbook of Group Communication Theory and Research. Sage; Thousand Oaks, CA: 1999. pp. 475–492. [Google Scholar]
- 39.Driessnack M. Using the Colored Eco-Genetic Relationship with children. Nurs Res. 2009;58(5):304–311. doi: 10.1097/NNR.0b013e3181b49928. [DOI] [PubMed] [Google Scholar]
- 40.Williams RR, Hunt SC, Barlow GK, et al. Health family trees: a tool for finding and helping young family members of coronary and cancer prone pedigrees in Texas and Utah. Am J Public Health. 1988;78(10):1283–1286. doi: 10.2105/ajph.78.10.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bush PJ, Iannotti RJ. Origins and stability of children’s health beliefs relative to medicine use. Soc Sci Med. 1988;27(4):345–352. doi: 10.1016/0277-9536(88)90268-7. [DOI] [PubMed] [Google Scholar]
- 42.Lewis CE, Lewis MA. Determinants of children’s health-related beliefs and behavior. Fam Community Health. 1982;4(4):85–07. doi: 10.1097/00003727-198202000-00010. [DOI] [PubMed] [Google Scholar]
- 43.Hay J, Shuck E, Brady MS, Berwick M, Ostroff J, Halpern A. Family communication after melanoma diagnosis. Arch Dermatol. 2008;144(4):553–554. doi: 10.1001/archderm.144.4.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hochbaum GM. Public Participation In Medical Screening Programs: A Sociopsychological Study. US Government Printing Office; Washington, DC: 1958. [Google Scholar]
- 45.Smith ACM. Patient education. In: Baker DL, Schuette JL, Uhlmann WR, editors. A Guide to Genetic Counseling. J Wiley and Sons; New York, NY: 1998. pp. 99–126. [Google Scholar]
- 46.McBride CM, Emmons KM, Lipkus IM. Understanding the potential of teachable moments: the case of smoking cessation. Health Educ Res. 2003;18(2):156–170. doi: 10.1093/her/18.2.156. [DOI] [PubMed] [Google Scholar]
- 47.American Society of Clinical Oncology policy statement update: genetic testing for cancer susceptibility. J Clin Oncol. 2003;21:2397–2406. doi: 10.1200/JCO.2003.03.189. [DOI] [PubMed] [Google Scholar]
- 48.Valente TW, Pumpuang P. Identifying opinion leaders to promote behavior change. Health Educ Behav. 2007;34(6):881–896. doi: 10.1177/1090198106297855. [DOI] [PubMed] [Google Scholar]
- 49.Wolff M, Young S, Beck B, et al. Leadership in a public housing community. J Health Commun. 2004;9(2):119–126. doi: 10.1080/10810730490425286. [DOI] [PubMed] [Google Scholar]
- 50.Bowen DJ, Bourcier E, Press N, Lewis FM, Burke W. Effects of individual and family functioning on interest in genetic testing. Community Genet. 2004;7:25–31. doi: 10.1159/000080301. [DOI] [PubMed] [Google Scholar]
- 51.Socha TJ. Group communication across the life span. In: Frey LR, Barge JK, editors. Managing Group Life: Communicating in Decision-Making Groups. Houghton Mifflin; Boston, MA: 1997. pp. 3–28. [Google Scholar]
- 52.Fitzpatrick MA, Ritchie LD. Communication theory and the family. In: Boss P, Doherty WJ, LaRossa R, Schumm WR, Steinmetz SK, editors. Sourcebook of Family Theories and Methods: A Contextual Approach. Plenum; New York, NY: 1993. pp. 565–589. [Google Scholar]
- 53.Prentice CM. The assimilation of in-laws: the impact of newcomers on the communication routines of families. J Appl Commun Res. 2008;36(1):74–97. [Google Scholar]
- 54.Kenen R, Arden-Jones A, Eeles R. Healthy women from suspected hereditary breast and ovarian cancer (HBOC) families: the significant others in their lives. Eur J Cancer Care (Engl) 2004;13:169–179. doi: 10.1111/j.1365-2354.2004.00460.x. [DOI] [PubMed] [Google Scholar]
- 55.Kenen R, Arden-Jones A, Eeles R. We are talking, but are they listening? Communication patterns in families with a history of breast/ovarian cancer (HBOC) Psychooncology. 2004;13(5):335–345. doi: 10.1002/pon.745. [DOI] [PubMed] [Google Scholar]
- 56.Daly MB. The impact of social roles on the experience of men in BRCA1/2 families: implications for counseling. J Genet Couns. 2009;18:42–48. doi: 10.1007/s10897-008-9183-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hunter DJ, Khoury MJ, Drazen JM. Letting the genome out of the bottle—will we get our wish? N Engl J Med. 2008;358:105–107. doi: 10.1056/NEJMp0708162. [DOI] [PubMed] [Google Scholar]
- 58.Feero WG, Guttmacher AE, Collins FS. The genome gets personal—almost. JAMA. 2008;299(11):1351–1352. doi: 10.1001/jama.299.11.1351. [DOI] [PubMed] [Google Scholar]
- 59.McBride CM, Alford SH, Reid RJ, Larson EB, Baxevanis AD, Brody L. Putting science over supposition in the arena of personalized genomics. Nat Genet. 2008;40(8):939–942. doi: 10.1038/ng0808-939. [DOI] [PMC free article] [PubMed] [Google Scholar]