Abstract
PURPOSE
The 21-gene recurrence score (RS) is used to identify patients with hormone receptor–positive early-stage breast cancer who may benefit from the addition of chemotherapy to endocrine therapy. We hypothesized that many women with poor prognostic histopathologic grade 3 disease may be offered chemotherapy irrespective of RS results, of whom a subset may not benefit from adjuvant chemotherapy.
PATIENTS AND METHODS
A total of 30,864 women in the National Cancer Database were diagnosed with pT1c to pT2, pN0 to pN1, grade 3 estrogen receptor–positive, human epidermal growth factor receptor 2–negative invasive breast carcinoma from 2010 to 2015. RS was stratified as low (less than 18), intermediate (18 to 30), and high (31 or more). Overall survival by RS was evaluated by Kaplan-Meier, log-rank, and multivariable proportional hazards, with adjustment for relevant clinical and demographic variables.
RESULTS
RS testing in grade 3 cancers increased between 2010 and 2015 (pN0, 53% to 72%; pN1, 16% to 36%). Among the 13,558 women with pN0 and the 2,840 with pN1 disease with RS testing, 27.1% and 30.0%, respectively, had low scores (less than 18). The 5-year overall survival rate for patients with a high RS, but not low RS, was significantly higher with chemotherapy (v no chemotherapy; absolute differences: high RS pN0 = 12.2% and pN1 = 25.5%, both P < .001; low RS pN0 = 2.5%, P = .07; and pN1 = 1.0%, P = .27), findings that were reinforced in multivariable analyses risk adjusted by clinicopathologic characteristics.
CONCLUSION
Increased use of RS may help to better tailor treatment recommendations by stratifying patients with grade 3 disease into those who will and will not derive survival benefit and should be considered in all patients with estrogen receptor–positive/human epidermal growth factor receptor 2–negative T1c to T2, N0 to N1 disease.
INTRODUCTION
The 21-gene Oncotype DX Breast Recurrence Score (RS; Genomic Health, Redwood City, CA) is a validated, predictive biomarker that helps to identify patients with breast cancer who benefit from the addition of adjuvant chemotherapy to endocrine therapy for estrogen receptor (ER)–positive, human epidermal growth factor receptor 2 (HER2)–negative early-stage breast cancer.1–11 The RS has been incorporated into clinical guidelines, including the National Comprehensive Cancer Network (version 1.2018; to be considered in patients with pN0 and pN1 status) and ASCO (to be considered in patients with pN0 status) guidelines.5,6 The American Joint Committee on Cancer (AJCC) eighth edition cancer staging system also includes RS testing to downstage disease as part of the new pathologic prognostic staging system.7–9 Specifically, patients with pT1 to 2 node-negative, ER-positive, HER2-negative breast cancer of any grade and a low RS (less than 11) are reduced to prognostic stage group IA as a result of their favorable prognoses, which reflects the utility of a low RS to identify luminal A–type invasive breast cancers (ie, those with a low proliferation rate and high levels of ER and progesterone receptor [PR] expression) that do not benefit from chemotherapy.
The Trial Assigning Individualized Options for Treatment (TAILORx) (ClinicalTrials.gov identifier: NCT00310180) and Rx for Positive-Node, Endocrine-Responsive Breast Cancer (RxPONDER) (ClinicalTrials.gov identifier: NCT01272037) trials were designed to validate the RS prospectively and to determine the benefit of adjuvant chemotherapy for tumors with intermediate-range RS (ie, 11 to 25, not 18 to 30) in patients with pN0 and pN1 status, respectively.10,11 TAILORx results have shown that the majority of tumors with low and intermediate RS do not benefit from chemotherapy,12 and the results from RxPONDER are forthcoming.
Tumor grade is prognostic and independently associated with risk for recurrence; however, histopathologic high grade may not correlate well with the risk provided by the RS. In the PlanB trial (ClinicalTrials.gov identifier: NCT01049425), approximately 50% of grade 3 tumors had an RS less than 31.13 The correlation of histologic grade with the predictive benefit of RS has not been assessed comprehensively. The proportion of grade 3 tumors in the TAILORx trial, although low, reflects the typical grade distribution, which accounts for 17.8% of all included tumors across scores, and it was 22% in the original cohort for the development of the RS.1 As a result, the predictive benefit of RS and its potential for preventing overtreatment in grade 3 invasive breast carcinomas may be underappreciated. This study was undertaken to determine the national practices for ordering RS, treatment choices, and survival outcomes in patients with grade 3 breast cancer in a large, national data set.
PATIENTS AND METHODS
Data Source and Cohort Selection
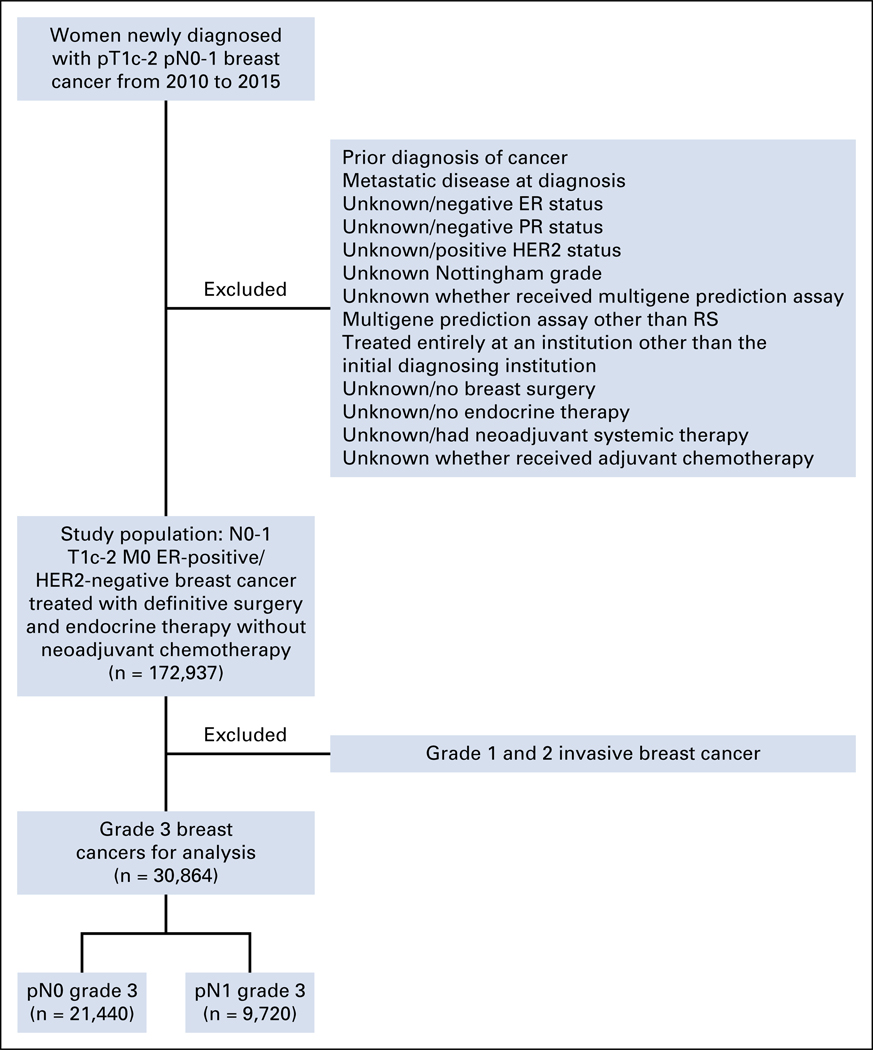
The registry-derived, hospital-based National Cancer Database (NCDB) curated by the American College of Surgeons and American Cancer Society incorporates more than 70% of newly diagnosed cancers in the United States.14 The NCDB routinely collects information on patient, tumor, demographic, and hospital characteristics in addition to information on any treatments administered within the first year of diagnosis. Patients newly diagnosed with invasive breast cancer (ie, third edition of the WHO International Classification of Diseases for Oncology morphologic codes 8500 and 8520 to 8524, with invasive behavior code 3, and breast topographic codes C50.0 to 50.9) from 2010 to 2015 were identified.15 The NCDB began incorporating HER2 (using the 2007 ASCO/College of American Pathologists grading guidelines) and multigene prediction assay data as of 2010.16–18 Women with invasive ductal, lobular, or mixed carcinoma histologies; Nottingham grade data; and hormone receptor–positive, HER2-negative, pT1c to 2, and pN0 to 1 resected cancers without neoadjuvant systemic therapy, as determined from collaborative staging, breast cancer–specific site factors, were included (Fig 1).19 Patients were excluded if they were younger than 20 years of age, had a prior diagnosis of cancer, were diagnosed at an index institution but treated entirely elsewhere, had evidence of distant metastasis, or did not receive surgery or hormonal therapy. Patients also were excluded if they lacked data about whether they received adjuvant chemotherapy or had RS testing. RS risk groups were categorized as low (less than 18), intermediate (18 to 30), and high (31 or higher) as originally defined by Paik et al.1 Variables were coded according to the Facility Oncology Registry Data Standards Manual revised for 2013.20
FIG 1.
Flow diagram of inclusion and exclusion criteria. ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; PR, progesterone receptor; RS, recurrence score.
Variables of Interest
Our primary outcomes of interest were receipt of RS testing (yes/no; with no defined as multigene signature testing neither ordered nor performed) and overall survival (OS) using National Death Index data provided by the NCDB and defined as the time from date of diagnosis to death, with patients censored at the date of last follow-up available in the NCDB (December 31, 2015). Because of limited followup, the NCDB does not include survival information for patients diagnosed in the most recent year, which for this release was 2015. Our independent variables of interest for survival analyses were adjuvant chemotherapy receipt (yes/no) and RS score (low, intermediate, high). Adjuvant chemotherapy and radiotherapy receipt was defined as therapy documented postoperatively within 1 year of diagnosis. Control clinicopathologic variables were age, year of diagnosis, race/ethnicity, insurance status, Charlson-Deyo comorbidity index,21 histology, AJCC pT classification, PR status, geographic location, Commission on Cancer–designated hospital type, and additional treatments received beyond chemotherapy (ie, surgery type [breast conserving surgery/mastectomy], adjuvant radiotherapy [yes/no]).
Statistical Analyses
RS testing by clinicopathologic characteristics for all patients was compared using χ2 tests and t tests, as appropriate. Multivariable logistic regression for receipt of RS testing was then performed, with stratification by pN status. To examine unadjusted differences in OS by chemotherapy receipt and RS, Kaplan-Meier method and log-rank test were used. The OS associated with adjuvant chemotherapy also was evaluated with multivariable Cox proportional hazards regression, after adjustment for all clinicopathologic variables of interest. Potential interaction effects of RS testing with adjuvant chemotherapy were explored using a previously described methodology.22 To evaluate the accuracy of RS testing and Nottingham/Bloom-Richardson grade encoding, patients treated for breast cancer from 2010 to 2015 were queried from the cancer registry–submitted data from Brigham and Women’s Hospital and Dana-Farber Cancer Institute. The concordance between registry-submitted encoded data and the corresponding Oncotype DX–reported RS and breast pathologist–assigned grade were evaluated for each patient. Statistical analyses were performed using Stata 14.2 software (StataCorp, College Station, TX), with a two-sided α-level of 0.05 selected as significant. This study was approved by the Partners Healthcare institutional review board (2019P000950).
RESULTS
A total of 172,937 women with pT1c to 2 invasive breast carcinoma were included in our analysis. Of 126,827 patients with pN0 disease, 16.7% had grade 3 tumors (n = 21,144), and of the 46,110 patients with pN1 disease, 21.1% had grade 3 tumors (n = 9,720). Clinicopathologic characteristics are listed in Table 1.
TABLE 1.
Characteristics Associated With RS Testing for Grade 3 Disease, Stratified by pN Status
| Characteristic | pN0 | pN1 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total (No.) | Had RS Testing (%) | Multivariable Logistic Regression | Total (No.) | Had RS Testing (%) | Multivariable Logistic Regression | |||||
| OR | 95% CI | P | OR | 95% CI | P | |||||
| No. of patients | 21,144 | 64.1 | NA | 9,720 | 29.2 | NA | ||||
| Age, years | ||||||||||
| < 50 | 4,940 | 63.6 | 0.81 | 0.74 to 0.89 | < .001 | 2,865 | 22.8 | 0.80 | 0.70 to 0.92 | .002 |
| 50–59 | 5,552 | 70.6 | Ref | 2,699 | 29.4 | Ref | ||||
| 60–69 | 6,201 | 71.2 | 1.06 | 0.97 to 1.16 | .21 | 2,340 | 36.5 | 1.46 | 1.28 to 1.67 | < .001 |
| ≥ 70 | 4,451 | 46.7 | 0.41 | 0.37 to 0.46 | < .001 | 1,816 | 29.6 | 1.14 | 0.96 to 1.36 | .15 |
| Year of diagnosis | ||||||||||
| 2010 | 2,671 | 53.2 | Ref | 1,339 | 16.9 | Ref | ||||
| 2011 | 3,207 | 58.8 | 1.28 | 1.14 to 1.44 | < .001 | 1,495 | 24.5 | 1.65 | 1.35 to 2.00 | < .001 |
| 2012 | 3,567 | 63.1 | 1.54 | 1.38 to 1.73 | < .001 | 1,670 | 28.9 | 1.99 | 1.64 to 2.40 | < .001 |
| 2013 | 3,937 | 64.7 | 1.65 | 1.48 to 1.85 | < .001 | 1,835 | 31.5 | 2.33 | 1.93 to 2.80 | < .001 |
| 2014 | 3,858 | 68.3 | 1.94 | 1.73 to 2.17 | < .001 | 1,706 | 34.2 | 2.67 | 2.22 to 3.21 | < .001 |
| 2015 | 3,904 | 72.3 | 2.46 | 2.19 to 2.75 | < .001 | 1,675 | 36.0 | 2.90 | 2.41 to 3.49 | < .001 |
| Comorbidity index | ||||||||||
| 0 | 17,494 | 65.2 | Ref | 8,115 | 29.3 | Ref | ||||
| 1 | 3,021 | 60.1 | 0.90 | 0.83 to 0.99 | .03 | 1,280 | 29.1 | 0.95 | 0.83 to 1.09 | .48 |
| 2 | 478 | 55.0 | 0.75 | 0.61 to 0.91 | .004 | 249 | 28.9 | 0.88 | 0.66 to 1.18 | .40 |
| 3 | 151 | 55.6 | 0.87 | 0.61 to 1.24 | .44 | 76 | 25.0 | 0.71 | 0.41 to 1.22 | .22 |
| Race/ethnicity | ||||||||||
| White | 16,551 | 65.1 | Ref | 7,380 | 30.2 | Ref | ||||
| Black | 2,377 | 60.3 | 0.80 | 0.72 to 0.89 | < .001 | 1,198 | 26.4 | 0.87 | 0.75 to 1.02 | .08 |
| Asian/Pacific Islander | 873 | 65.2 | 0.95 | 0.80 to 1.11 | .50 | 412 | 27.7 | 0.99 | 0.77 to 1.26 | .93 |
| Hispanic | 1,046 | 58.0 | 0.76 | 0.66 to 0.88 | < .001 | 572 | 23.1 | 0.75 | 0.60 to 0.95 | .02 |
| Other/unknown | 297 | 61.3 | 0.76 | 0.59 to 0.99 | .04 | 158 | 32.9 | 1.14 | 0.79 to 1.66 | .48 |
| Primary payer | ||||||||||
| Not insured | 335 | 56.1 | Ref | 224 | 22.8 | Ref | ||||
| Private insurance | 12,072 | 68.9 | 1.50 | 1.17 to 1.90 | .001 | 5,729 | 29.0 | 1.25 | 0.88 to 1.79 | .21 |
| Medicaid | 1,392 | 60.2 | 1.27 | 0.97 to 1.65 | .08 | 789 | 24.0 | 1.02 | 0.69 to 1.51 | .91 |
| Medicare | 6,948 | 56.9 | 1.35 | 1.05 to 1.74 | .02 | 2,784 | 31.4 | 1.14 | 0.79 to 1.66 | .48 |
| Other government | 185 | 62.2 | 1.30 | 0.86 to 1.97 | .22 | 112 | 35.7 | 1.70 | 0.98 to 2.96 | .06 |
| Unknown | 212 | 66.0 | 1.51 | 1.02 to 2.23 | .04 | 82 | 28.1 | 1.03 | 0.55 to 1.91 | .93 |
| Hospital type | ||||||||||
| Community cancer program | 1,923 | 57.9 | Ref | 869 | 27.9 | Ref | ||||
| Comprehensive cancer center | 9,565 | 63.3 | 1.22 | 1.09 to 1.35 | < .001 | 4,282 | 28.8 | 1.03 | 0.87 to 1.22 | .72 |
| Academic/research | 6,264 | 69.0 | 1.42 | 1.27 to 1.60 | < .001 | 2,875 | 32.9 | 1.27 | 1.07 to 1.52 | .01 |
| Integrated cancer network program | 2,180 | 64.7 | 1.24 | 1.08 to 1.42 | .003 | 923 | 29.9 | 1.09 | 0.88 to 1.35 | .43 |
| Hospital location | ||||||||||
| New England | 1,266 | 66.8 | Ref | 540 | 27.0 | Ref | ||||
| Middle Atlantic | 3,107 | 70.7 | 1.31 | 1.13 to 1.52 | < .001 | 1,556 | 33.2 | 1.33 | 1.06 to 1.66 | .01 |
| South Atlantic | 4,247 | 65.8 | 1.12 | 0.97 to 1.29 | .14 | 1,846 | 30.1 | 1.25 | 0.99 to 1.56 | .06 |
| East North Central | 3,736 | 66.3 | 1.08 | 0.94 to 1.25 | .29 | 1,634 | 32.1 | 1.31 | 1.04 to 1.64 | .02 |
| East South Central | 1,127 | 64.2 | 0.96 | 0.80 to 1.15 | .67 | 438 | 32.0 | 1.32 | 0.99 to 1.77 | .06 |
| West North Central | 1,796 | 64.3 | 0.97 | 0.82 to 1.14 | .70 | 833 | 29.5 | 1.13 | 0.88 to 1.45 | .35 |
| West South Central | 1,232 | 56.0 | 0.73 | 0.61 to 0.87 | < .001 | 535 | 23.6 | 0.90 | 0.67 to 1.20 | .46 |
| Mountain | 932 | 64.8 | 1.07 | 0.88 to 1.30 | .48 | 435 | 32.6 | 1.45 | 1.08 to 1.94 | .01 |
| Pacific | 2,489 | 56.9 | 0.74 | 0.64 to 0.87 | < .001 | 1,132 | 26.5 | 1.08 | 0.85 to 1.38 | .53 |
| AJCC pT | ||||||||||
| 1c | 11,687 | 69.0 | Ref | 3,507 | 36.1 | Ref | ||||
| 2 | 9,457 | 58.1 | 0.66 | 0.62 to 0.70 | < .001 | 6,213 | 25.3 | 0.65 | 0.59 to 0.72 | <.001 |
| Histology | ||||||||||
| IDC | 18,854 | 64.2 | Ref | 8,625 | 29.1 | Ref | ||||
| ILC | 826 | 61.6 | 0.99 | 0.85 to 1.16 | .89 | 328 | 33.5 | 1.26 | 0.98 to 1.61 | .07 |
| IMC | 1,464 | 64.8 | 1.01 | 0.90 to 1.15 | .82 | 767 | 29.2 | 0.97 | 0.82 to 1.15 | .74 |
| PR status | ||||||||||
| Negative | 3,941 | 55.4 | Ref | 1,439 | 22.1 | Ref | ||||
| Positive | 17,182 | 66.1 | 1.51 | 1.39 to 1.63 | < .001 | 8,265 | 30.5 | 1.47 | 1.28 to 1.69 | < .001 |
| Standard surgery/RT | ||||||||||
| Yes (lumpectomy with RT or mastectomy only) | 18,898 | 65.5 | Ref | 7,085 | 32.6 | Ref | ||||
| No (lumpectomy only) | 832 | 38.2 | 0.44 | 0.38 to 0.51 | < .001 | 238 | 21.9 | 0.53 | 0.38 to 0.75 | < .001 |
| No (mastectomy with RT) | 515 | 52.8 | 0.69 | 0.57 to 0.84 | < .001 | 2,317 | 19.3 | 0.55 | 0.48 to 0.62 | < .001 |
NOTE. Boldface indicates significance at P < .05.
Abbreviations: AJCC, American Joint Committee on Cancer; IDC, invasive ductal carcinoma; ILC, invasive lobular carcinoma; IMC, invasive mixed carcinoma; NA, not applicable; OR, odds ratio; PR, progesterone receptor; Ref, reference; RS, recurrence score; RT, radiotherapy.
Characteristics Associated With RS Testing for pN0 Disease
In patients with pN0 grade 3 tumors, 64.1% (n = 13,558) had RS testing compared with 59.2% (n = 22,267) and 65.7% (n = 44,708) with grade 1 and 2 tumors, respectively (P < .001). There was increasing use of RS testing between 2010 to 2015 in grade 3 disease (from 53.2% to 72.3%; P < .001). In multivariable logistic analyses, receipt of RS testing for grade 3 disease was significantly associated with ages 50 to 59 years; a more recent (v earlier) diagnosis; lower comorbidity score; white race; private or Medicare insurance (v uninsured); and tumors that were pT1c, PR positive, or treated with standard local therapy (v nonstandard; Table 1).
Characteristics Associated With RS Testing for pN1 Disease
In patients with pN1 grade 3 tumors, 29.2% (n = 2,840) had RS testing compared with 47.4% (n = 4,937) and 42.7% (n = 11,083) with grade 1 and 2 tumors, respectively (P < .001). We observed an increasing use of RS testing between 2010 and 2015 in pN1 grade 3 disease (from 16.9% to 36.0%; P < .001). In multivariable logistic analyses, receipt of RS testing was significantly associated with ages 50 to 69 years; a more recent diagnosis; lower comorbidity score; non-Hispanic race/ethnicity; and tumors that were pT1c, PR positive, or treated with standard local therapy (Table 1).
Associations of Chemotherapy and OS for pN0 Grade 3 Disease by RS
In multivariable logistic regression analyses, intermediate RS (odds ratio [OR], 15.23; 95% CI, 13.20 to 17.58; P < .001) and high RS (OR, 141.55; 95% CI, 118.58 to 168.97; P < .001) were associated with receipt of adjuvant chemotherapy compared with low RS (Table 2). Adjuvant chemotherapy receipt also was associated with younger patients; patients who were diagnosed earlier in the study period (v more recently); patients without comorbidity, pT2 tumors, invasive ductal carcinoma, and PR-negative status; and patients who received standard local therapy (v nonstandard; Table 2).
TABLE 2.
Characteristics Associated With Adjuvant Chemotherapy for Grade 3 Disease, Stratified by pN Status
| Characteristic | pN0 | pN1 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total (No.) | Had CT (%) | Multivariable Logistic Regression | Total (No.) | Had CT (%) | Multivariable Logistic Regression | |||||
| OR | 95% CI | P | OR | 95% CI | P | |||||
| No. of patients | 21,144 | 53.1 | NA | 9,720 | 76.0 | NA | ||||
| RS | ||||||||||
| No RS | 7,586 | 52.2 | 16.66 | 14.42 to 19.25 | < .001 | 6,880 | 82.8 | 21.87 | 17.87 to 26.77 | < .001 |
| Low | 3,591 | 9.1 | Ref | 821 | 25.9 | Ref | ||||
| Intermediate | 5,304 | 54.9 | 15.23 | 13.20 to 17.58 | < .001 | 1,188 | 64.4 | 6.62 | 5.28 to 8.30 | < .001 |
| High | 4,332 | 89.4 | 141.55 | 118.58 to 168.97 | < .001 | 726 | 88.3 | 36.92 | 26.73 to 50.99 | < .001 |
| Age, years | ||||||||||
| < 50 | 4,940 | 74.8 | 1.83 | 1.63 to 2.05 | < .001 | 2,865 | 91.8 | 1.39 | 1.13 to 1.71 | .002 |
| 50–59 | 5,552 | 63.1 | Ref | 2,699 | 85.7 | Ref | ||||
| 60–69 | 6,201 | 49.9 | 0.57 | 0.51 to 0.63 | < .001 | 2,340 | 75.9 | 0.63 | 0.53 to 0.76 | < .001 |
| ≥ 70 | 4,451 | 21.1 | 0.12 | 0.11 to 0.14 | < .001 | 1,816 | 37.0 | 0.09 | 0.08 to 0.12 | < .001 |
| Year of diagnosis | ||||||||||
| 2010 | 2,671 | 56.8 | Ref | 1,339 | 78.4 | Ref | ||||
| 2011 | 3,207 | 55.0 | 0.95 | 0.83 to 1.10 | .53 | 1,495 | 77.1 | 1.11 | 0.88 to 1.40 | .37 |
| 2012 | 3,567 | 52.3 | 0.93 | 0.81 to 1.07 | .33 | 1,670 | 76.9 | 1.12 | 0.89 to 1.39 | .34 |
| 2013 | 3,937 | 53.1 | 0.93 | 0.81 to 1.07 | .33 | 1,835 | 76.6 | 1.12 | 0.90 to 1.40 | .30 |
| 2014 | 3,858 | 53.1 | 0.85 | 0.74 to 0.98 | .02 | 1,706 | 73.3 | 0.84 | 0.67 to 1.05 | .12 |
| 2015 | 3,904 | 49.9 | 0.74 | 0.64 to 0.85 | < .001 | 1,675 | 74.5 | 1.09 | 0.87 to 1.36 | .45 |
| Comorbidity index | ||||||||||
| 0 | 17,494 | 54.8 | Ref | 8,115 | 78.3 | Ref | ||||
| 1 | 3,021 | 46.4 | 0.89 | 0.80 to 1.00 | .04 | 1,280 | 67.9 | 0.83 | 0.70 to 0.99 | .03 |
| 2 | 478 | 38.9 | 0.79 | 0.62 to 1.01 | .06 | 249 | 57.0 | 0.57 | 0.41 to 0.79 | .001 |
| 3 | 151 | 34.4 | 0.65 | 0.42 to 1.02 | .06 | 76 | 36.8 | 0.31 | 0.18 to 0.55 | < .001 |
| Race/ethnicity | ||||||||||
| White | 16,551 | 51.5 | Ref | 7,380 | 74.9 | Ref | ||||
| Black | 2,377 | 58.4 | 0.99 | 0.87 to 1.12 | .82 | 1,198 | 78.6 | 0.89 | 0.73 to 1.07 | .22 |
| Asian/Pacific Islander | 873 | 61.5 | 1.17 | 0.97 to 1.42 | .11 | 412 | 80.3 | 1.04 | 0.75 to 1.45 | .81 |
| Hispanic | 1,046 | 58.4 | 1.08 | 0.90 to 1.29 | .42 | 572 | 82.5 | 1.27 | 0.95 to 1.71 | .11 |
| Other/unknown | 297 | 57.9 | 1.04 | 0.76 to 1.43 | .81 | 158 | 76.0 | 1.29 | 0.78 to 2.14 | .32 |
| Primary payer | ||||||||||
| Not insured | 335 | 61.2 | Ref | 224 | 84.8 | Ref | ||||
| Private insurance | 12,072 | 63.2 | 1.20 | 0.89 to 1.62 | .23 | 5,729 | 85.8 | 1.70 | 1.10 to 2.61 | .02 |
| Medicaid | 1,392 | 63.2 | 0.94 | 0.68 to 1.31 | .73 | 789 | 84.9 | 1.31 | 0.81 to 2.14 | .27 |
| Medicare | 6,948 | 33.0 | 0.80 | 0.59 to 1.09 | .16 | 2,784 | 52.5 | 1.04 | 0.66 to 1.62 | .87 |
| Other government | 185 | 61.6 | 1.41 | 0.85 to 2.32 | .18 | 112 | 82.1 | 1.38 | 0.66 to 2.88 | .39 |
| Unknown | 212 | 50.9 | 0.78 | 0.49 to 1.26 | .32 | 82 | 76.8 | 1.52 | 0.72 to 3.21 | .27 |
| Hospital type | ||||||||||
| Community cancer program | 1,923 | 48.5 | Ref | 869 | 73.5 | Ref | ||||
| Comprehensive cancer center | 9,565 | 50.2 | 1.09 | 0.96 to 1.25 | .20 | 4,282 | 73.4 | 0.91 | 0.73 to 1.13 | .39 |
| Academic/research | 6,264 | 53.2 | 1.08 | 0.94 to 1.24 | .26 | 2,875 | 75.3 | 0.88 | 0.70 to 1.10 | .27 |
| Integrated cancer network program | 2,180 | 52.3 | 1.13 | 0.95 to 1.34 | .16 | 923 | 76.6 | 1.02 | 0.77 to 1.34 | .90 |
| Hospital location | ||||||||||
| New England | 1,266 | 51.1 | Ref | 540 | 79.1 | Ref | ||||
| Middle Atlantic | 3,107 | 49.6 | 1.03 | 0.86 to 1.24 | .72 | 1,556 | 72.6 | 0.85 | 0.63 to 1.14 | .28 |
| South Atlantic | 4,247 | 51.1 | 0.92 | 0.77 to 1.10 | .35 | 1,846 | 74.2 | 0.83 | 0.62 to 1.11 | .21 |
| East North Central | 3,736 | 52.9 | 1.10 | 0.92 to 1.31 | .30 | 1,634 | 74.9 | 0.94 | 0.69 to 1.26 | .66 |
| East South Central | 1,127 | 53.1 | 1.12 | 0.90 to 1.40 | .30 | 438 | 75.3 | 0.92 | 0.63 to 1.35 | .67 |
| West North Central | 1,796 | 52.2 | 1.08 | 0.89 to 1.32 | .42 | 833 | 76.1 | 1.05 | 0.75 to 1.46 | .79 |
| West South Central | 1,232 | 50.7 | 0.92 | 0.74 to 1.14 | .44 | 535 | 74.8 | 0.88 | 0.61 to 1.26 | .48 |
| Mountain | 932 | 49.1 | 0.88 | 0.70 to 1.12 | .30 | 435 | 70.3 | 0.68 | 0.46 to 0.99 | .04 |
| Pacific | 2,489 | 50.1 | 0.86 | 0.72 to 1.04 | .12 | 1,132 | 73.6 | 0.74 | 0.54 to 1.02 | .07 |
| AJCC pT | ||||||||||
| 1c | 11,687 | 48.3 | Ref | 3,507 | 74.1 | Ref | ||||
| 2 | 9,457 | 59.1 | 1.66 | 1.54 to 1.80 | < .001 | 6,213 | 77.1 | 1.08 | 0.95 to 1.22 | .27 |
| Histology | ||||||||||
| IDC | 18,854 | 54.8 | Ref | 8,625 | 76.5 | Ref | ||||
| ILC | 826 | 30.8 | 0.61 | 0.50 to 0.74 | < .001 | 328 | 66.8 | 0.93 | 0.69 to 1.26 | .64 |
| IMC | 1,464 | 44.4 | 0.84 | 0.72 to 0.97 | .02 | 767 | 74.8 | 0.93 | 0.74 to 1.16 | .53 |
| PR status | ||||||||||
| Negative | 3,941 | 75.0 | Ref | 1,439 | 83.7 | Ref | ||||
| Positive | 17,182 | 48.1 | 0.44 | 0.40 to 0.49 | < .001 | 8,265 | 74.7 | 0.62 | 0.51 to 0.75 | < .001 |
| Standard surgery/RT | ||||||||||
| Yes (lumpectomy with RT or mastectomy only) | 18,898 | 54.5 | Ref | 7,085 | 73.1 | Ref | ||||
| No (lumpectomy only) | 832 | 21.9 | 0.36 | 0.28 to 0.44 | < .001 | 238 | 47.1 | 0.51 | 0.37 to 0.71 | < .001 |
| No (mastectomy with RT) | 515 | 71.7 | 2.06 | 1.60 to 2.66 | < .001 | 2,317 | 88.2 | 2.30 | 1.94 to 2.74 | < .001 |
NOTE. Boldface indicates significance at P < .05.
Abbreviations: AJCC, American Joint Committee on Cancer; CT, chemotherapy; IDC, invasive ductal carcinoma; ILC, invasive lobular carcinoma; IMC, invasive mixed carcinoma; NA, not applicable; OR, odds ratio; PR, progesterone receptor; Ref, reference; RS, recurrence score; RT, radiotherapy.
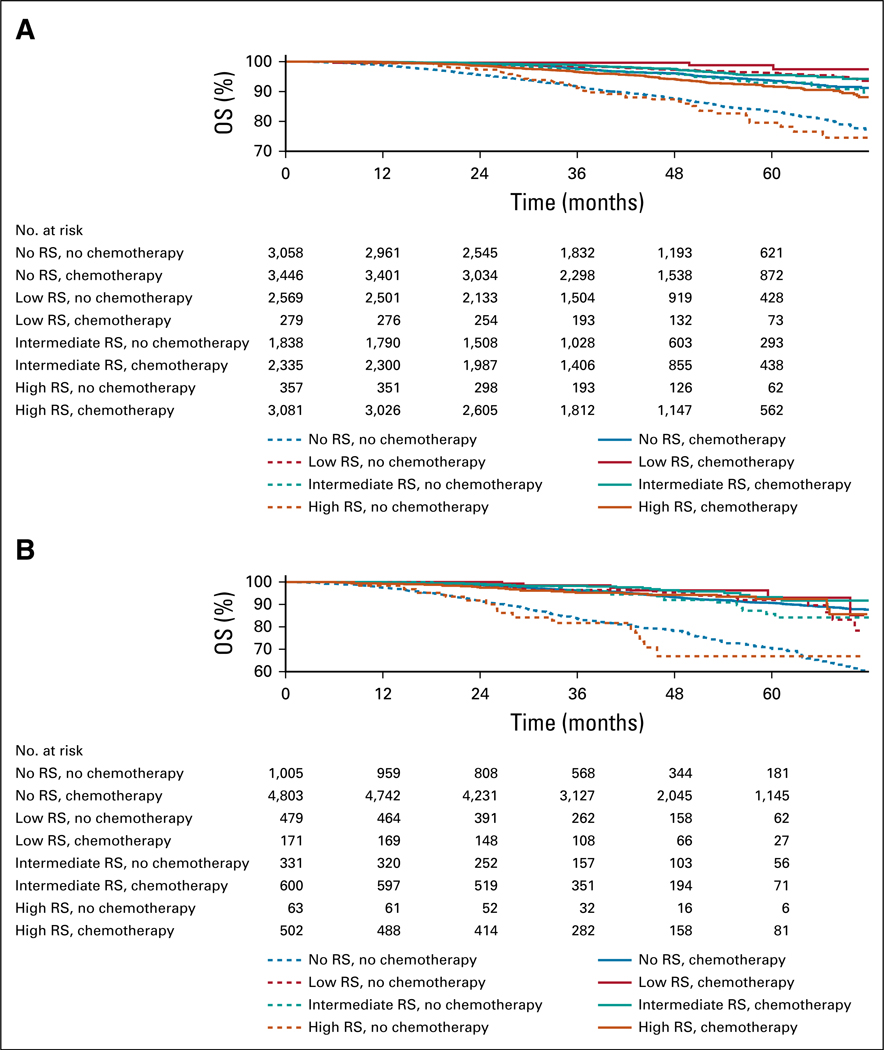
The median follow-up for patients with pN0 grade 3 disease was 41.1 months (interquartile range, 28.8–56.3 months), and 5.3% of patients died during the study period. In the group of patients without RS testing, 52.2% received chemotherapy in addition to endocrine therapy, which demonstrated an unadjusted absolute improvement in 5-year OS rate of 10.3% (P < .001; Table 3; Fig 2A). For patients with low RS, 9.1% received chemotherapy, which was not associated with a significantly increased 5-year unadjusted OS rate (P = .07; Table 3; Fig 2A). In contrast, 54.9% of patients with intermediate RS and 89.4% of patients with high RS received chemotherapy, with both groups demonstrating associations with an improved unadjusted 5-year OS rate of 2.5% (P = .002) and 12.2% (P <.001), respectively (Table 3; Fig 2A).
TABLE 3.
Kaplan-Meier Estimates of Overall Survival Associated With Adjuvant Chemotherapy in Patients With Grade 3 Invasive Breast Cancer, Stratified by pN Status and RS
| RS* | Total (No.) | Had Chemotherapy (%) | 5-Year Overall Survival Rate, % (95% CI) | P | |
|---|---|---|---|---|---|
| No Chemotherapy | Chemotherapy | ||||
| pN0 | |||||
| Low | 3,591 | 9.1 | 96.3 (95.0 to 97.2) | 98.8 (94.9 to 99.7) | .07 |
| Intermediate | 5,304 | 54.9 | 93.0 (90.8 to 94.7) | 95.5 (94.0 to 96.6) | .002 |
| High | 4,332 | 89.4 | 79.6 (72.4 to 85.1) | 91.8 (90.2 to 93.1) | < .001 |
| No RS | 7,586 | 52.2 | 83.3 (81.3 to 85.0) | 93.6 (92.4 to 94.7) | < .001 |
| pN1 | |||||
| Low | 821 | 25.9 | 92.0 (86.9 to 95.2) | 93.0 (80.7 to 97.6) | .27 |
| Intermediate | 1,188 | 64.4 | 85.7 (77.5 to 91.1) | 93.2 (88.4 to 96.1) | .02 |
| High | 726 | 88.3 | 66.9 (48.6 to 79.9) | 92.4 (88.1 to 95.2) | < .001 |
| No RS | 6,880 | 82.8 | 70.6 (66.5 to 74.3) | 90.7 (89.5 to 91.8) | < .001 |
NOTE. Boldface indicates significance at P < 05.
Abbreviation: RS, recurrence score.
In 2.4% of patients (n = 331) with pN0 and 3.7% (n = 105) with pN1 grade 3 tumors who underwent RS testing, the RS score was not available.
FIG 2.
Overall survival (OS) in patients with grade 3 invasive breast cancer, stratified by recurrence score (RS). Adjuvant chemotherapy OS estimated by Kaplan-Meier method for patients with (A) pN0 and (B) pN1 grade 3 invasive breast cancer, stratified by RS and adjuvant chemotherapy, with an underlying number at risk table. Adjuvant chemotherapy (solid lines) was associated with significantly better median OS than no adjuvant chemotherapy (dashed lines) for intermediate, high, and no RS.
The OS improvements associated with adjuvant chemotherapy in patients with pN0 grade 3 disease also were assessed in multivariable Cox proportional hazards regression analyses (Table 4). In patients with grade 3 disease with high RS or who had no RS testing, adjuvant chemotherapy demonstrated significantly improved OS in multivariable analyses compared with those who did not receive chemotherapy (hazard ratio [HR], 0.63 [95% CI, 0.43 to 0.90; P = .01] and 0.51 [95% CI, 0.40 to 0.64; P < .001], respectively), whereas low and intermediate RS were not associated with an OS benefit from adjuvant chemotherapy (reference no chemotherapy; HR, 0.50 [95% CI, 0.16 to 1.61; P = .25] and 0.71 [95% CI, 0.49 to 0.90; P =.07], respectively). Increasing age at diagnosis, comorbidity index, pT classification, and nonstandard lumpectomy-only management all demonstrated worse OS, whereas Asian/ Pacific Islander and Hispanic race (reference white), management at an academic/research institution (reference community cancer program), invasive lobular carcinoma histology, and PR-positive status were associated with improved OS (Table 4).
TABLE 4.
Multivariable Cox Proportional Hazards Regression for the Overall Survival Associated With Adjuvant Chemotherapy in Patients With Grade 3 Invasive Breast Cancer, Stratified by pN Status
| Variable | pN0 | pN1 | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| RS by chemotherapy | ||||||
| No RS | ||||||
| Chemotherapy (Ref no chemotherapy) | 0.51 | 0.40 to 0.64 | < .001 | 0.42 | 0.33 to 0.53 | < .001 |
| Low | ||||||
| Chemotherapy (Ref no chemotherapy) | 0.50 | 0.16 to 1.61 | .25 | 0.81 | 0.33 to 1.98 | .64 |
| Intermediate | ||||||
| Chemotherapy (Ref no chemotherapy) | 0.71 | 0.49 to 1.03 | .07 | 0.67 | 0.35 to 1.27 | .22 |
| High | ||||||
| Chemotherapy (Ref no chemotherapy) | 0.63 | 0.43 to 0.90 | .01 | 0.24 | 0.13 to 0.47 | < .001 |
| Age, years | ||||||
| < 50 | 0.90 | 0.67 to 1.20 | .46 | 0.71 | 0.53 to 0.96 | .03 |
| 50–59 | Ref | Ref | ||||
| 60–69 | 1.29 | 1.02 to 1.62 | .03 | 1.00 | 0.77 to 1.30 | 1.00 |
| ≥ 70 | 2.07 | 1.59 to 2.70 | < .001 | 1.64 | 1.20 to 2.23 | .002 |
| Year of diagnosis | ||||||
| 2010 | Ref | Ref | ||||
| 2011 | 1.06 | 0.88 to 1.28 | .55 | 1.37 | 1.08 to 1.72 | .008 |
| 2012 | 0.96 | 0.77 to 1.19 | .70 | 1.28 | 0.99 to 1.66 | .06 |
| 2013 | 1.05 | 0.82 to 1.34 | .69 | 1.33 | 0.99 to 1.79 | .06 |
| 2014 | 1.29 | 0.95 to 1.75 | .11 | 1.79 | 1.25 to 2.58 | .002 |
| Comorbidity index | ||||||
| 0 | Ref | Ref | ||||
| 1 | 1.43 | 1.20 to 1.69 | < .001 | 1.35 | 1.10 to 1.67 | .004 |
| 2 | 2.83 | 2.15 to 3.73 | < .001 | 1.76 | 1.20 to 2.58 | .004 |
| 3 | 2.95 | 1.89 to 4.60 | < .001 | 3.68 | 2.26 to 6.01 | < .001 |
| Race/ethnicity | ||||||
| White | Ref | Ref | ||||
| Black | 1.02 | 0.82 to 1.26 | .88 | 1.06 | 0.83 to 1.35 | .65 |
| Asian/Pacific Islander | 0.50 | 0.29 to 0.87 | .02 | 0.66 | 0.38 to 1.17 | .15 |
| Hispanic | 0.63 | 0.40 to 0.99 | .045 | 0.67 | 0.43 to 1.04 | .08 |
| Other/unknown | 0.63 | 0.28 to 1.42 | .26 | 0.59 | 0.24 to 1.43 | .24 |
| Primary payer | ||||||
| Not insured | Ref | Ref | ||||
| Private insurance | 0.74 | 0.40 to 1.36 | .34 | 0.74 | 0.43 to 1.26 | .27 |
| Medicaid | 0.92 | 0.47 to 1.80 | .81 | 0.85 | 0.47 to 1.57 | .61 |
| Medicare | 0.99 | 0.53 to 1.83 | .97 | 0.91 | 0.52 to 1.57 | .72 |
| Other government | 1.64 | 0.69 to 3.88 | .26 | 0.91 | 0.33 to 2.53 | .86 |
| Unknown | 0.77 | 0.29 to 1.99 | .58 | 1.29 | 0.49 to 3.34 | .61 |
| Hospital type | ||||||
| Community cancer program | Ref | Ref | ||||
| Comprehensive cancer center | 0.86 | 0.69 to 1.07 | .17 | 0.74 | 0.57 to 0.97 | .03 |
| Academic/research | 0.74 | 0.59 to 0.94 | .01 | 0.84 | 0.63 to 1.11 | .21 |
| Integrated cancer network program | 0.83 | 0.62 to 1.11 | .21 | 0.92 | 0.66 to 1.30 | .65 |
| Hospital location | ||||||
| New England | Ref | Ref | ||||
| Middle Atlantic | 1.08 | 0.78 to 1.51 | .64 | 1.82 | 1.14 to 2.92 | .01 |
| South Atlantic | 0.92 | 0.66 to 1.27 | .61 | 1.84 | 1.15 to 2.94 | .01 |
| East North Central | 1.07 | 0.78 to 1.48 | .66 | 1.57 | 0.98 to 2.52 | .06 |
| East South Central | 1.06 | 0.72 to 1.57 | .75 | 1.95 | 1.10 to 3.44 | .02 |
| West North Central | 0.93 | 0.65 to 1.34 | .71 | 1.79 | 1.08 to 2.96 | .02 |
| West South Central | 0.75 | 0.49 to 1.14 | .18 | 1.94 | 1.14 to 3.31 | .02 |
| Mountain | 0.97 | 0.64 to 1.48 | .90 | 1.55 | 0.86 to 2.79 | .14 |
| Pacific | 0.82 | 0.57 to 1.16 | .26 | 1.70 | 1.03 to 2.79 | .04 |
| AJCC pT | ||||||
| 2 (Ref 1c) | 1.99 | 1.73 to 2.30 | < .001 | 1.45 | 1.20 to 1.75 | < .001 |
| Histology | ||||||
| IDC | Ref | Ref | ||||
| ILC | 0.60 | 0.41 to 0.88 | .01 | 1.26 | 0.86 to 1.86 | .23 |
| IMC | 0.81 | 0.61 to 1.09 | .17 | 0.94 | 0.69 to 1.29 | .71 |
| PR status | ||||||
| Positive (Ref negative) | 0.70 | 0.59 to 0.82 | < .001 | 0.54 | 0.45 to 0.66 | < .001 |
| Standard surgery/RT | ||||||
| Yes (lumpectomy with RT or mastectomy only) | Ref | Ref | ||||
| No (lumpectomy only) | 1.94 | 1.55 to 2.44 | < .001 | 1.99 | 1.46 to 2.72 | < .001 |
| No (mastectomy with RT) | 1.03 | 0.66 to 1.59 | .91 | 0.97 | 0.78 to 1.21 | 80 |
NOTE. Boldface indicates significance at P < .05.
Abbreviations: AJCC, American Joint Committee on Cancer; HR, hazard ratio; IDC, invasive ductal carcinoma; ILC, invasive lobular carcinoma; IMC, invasive mixed carcinoma; PR, progesterone receptor; Ref, reference; RS, recurrence score; RT, radiotherapy.
Associations of Chemotherapy and OS for pN1 Grade 3 Disease by RS Testing
In multivariable logistic analyses, intermediate (OR, 6.62; 95% CI, 5.28 to 8.30; P < .001) and high (OR, 36.92; 95% CI, 26.73 to 50.99; P < .001) RS were associated with receiving adjuvant chemotherapy compared with low RS (Table 2). Treatment with adjuvant chemotherapy also was associated with patients who were younger, privately insured, without comorbidity, and PR negative and who had received standard local therapy (Table 2).
The median follow-up of patients with pN1 grade 3 disease was 41.9 months (interquartile range, 29.0–56.7 months), and 8.0% of patients died during the study period. In the group of patients without RS testing, 82.8% received chemotherapy in addition to endocrine therapy, which was associated with an absolute improvement in 5-year OS rate of 20.1% (P < .001; Table 3; Fig 2B). For patients with low RS, 25.9% received chemotherapy, and this was not associated with a significantly increased unadjusted 5-year OS (P = .27; Table 3; Fig 2B). In contrast, 64.4% of patients with intermediate RS and 88.3% with high RS received chemotherapy, with both groups demonstrating absolute improvements in 5-year OS rate of 7.5% (P = .02) and 25.5% (P < 0.001), respectively (Table 3; Fig 2B).
The OS benefits of adjuvant chemotherapy in patients with pN1 grade 3 disease also were assessed in multivariable Cox proportional hazards regression analyses (Table 4). As in pN0 disease, patients with pN1 grade 3 disease with high RS and no RS testing showed significantly improved OS with adjuvant chemotherapy (HR, 0.24 [95% CI, 0.13 to 0.47; P < .001] and 0.42 [95% CI, 0.33 to 0.53; P < .001], respectively), whereas low and intermediate RS did not (HR, 0.81 [95% CI, 0.33 to 1.98; P = .64] and 0.67 [95% CI, 0.35 to 1.27; P = 0.22], respectively). In addition to increasing age at diagnosis, comorbidity index, AJCC pT classification, PR-negative status, and nonstandard lumpectomy-only management all demonstrated worse OS. OS also significantly varied by geographical location (Table 4).
Evaluation of Grade 3 and RS Coding Accuracy
We also examined our multi-institutional cohort to validate registry-submitted grade and RS encoding. Of 351 adults with invasive breast carcinoma who had RS testing between 2010 and 2015, 74.7% (n = 259) were encoded and submitted for inclusion into cancer registries. Nottingham/ Bloom-Richardson grade was missing or incorrectly encoded in 6.6% of patients (n = 17), including 8.1% (n = 3) with grade 3 disease. The numerical RS was missing in 15.4% of registry-submitted patients (n = 40) and incorrectly encoded in 3.9% (n = 10). In particular, of the 37 grade 3 registry-submitted institutional patients with RS testing, 16.2% (n = 6) had missing scores in registry data, and only 3.2% (n = 1) were incorrectly encoded (as no RS instead of an actual RS of 22).
DISCUSSION
In this large national cohort, we evaluated the use of RS, use of adjuvant chemotherapy, and OS for women with breast cancer, with a focus on high tumor grade. We found that 30.0% of pN0 and 27.1% of pN1 grade 3 invasive breast cancers had a low RS, which was not associated with an OS benefit from chemotherapy. Of note, chemotherapy was associated with significant OS improvements in grade 3 invasive breast cancers with high RS, and although associated with improved OS in univariable analyses, intermediate RS was not predictive of significant chemotherapy OS benefit when risk adjusted for clinicopathologic characteristics. The incorporation of RS testing into the clinical decision making for grade 3 invasive breast cancers may help to tailor treatment recommendations for these patients. To our knowledge, these findings represent the largest analysis to date of the potential impact of RS on the outcomes and management of grade 3 tumors and suggest that the assumption that all pT1c/2 pN0/1, ER-positive histopathologic grade 3 tumors are high risk and will consequently benefit from adjuvant chemotherapy may be unmerited.
Although we await the RxPONDER trial final results, the data presented here suggest that patients with a pT1c/2 N0/1 grade 3 tumor with high RS derive benefit from chemotherapy (92% 5-year OS with chemotherapy v 67% to 79% without), whereas grade 3 tumors with low and intermediate RS do not. Our results further reinforce the utility of broad RS testing in grade 3 tumors and suggest that RS can help to distinguish the anticipated chemotherapy benefit among this heterogeneous group of tumors.
The rate of RS testing is less in pN1 disease than in pN0 disease (36% in 2015 v 72% in pN0) but is expanding in both groups because the importance of tumor biology is increasingly recognized. In our study, the increased use of RS testing in pN0 grade 3 disease (64.1%) and lower rate of chemotherapy (9.1%) in patients with low RS suggest that clinical practice is increasingly incorporating RS results into decision trees for pN0 grade 3 disease. However, our findings also suggest that a significant proportion of patients with pN1 grade 3 disease who do not undergo RS testing may be overtreated and receive chemotherapy with no added OS benefit. We also find that nationally, significant variability exists in grade 3 RS testing patterns by hospital type and geographic location and even in patient insurance status and race/ethnicity, which suggests opportunities for more-detailed guidance from national guidelines for RS testing, particularly for grade 3 tumors.
We found that a high proportion of patients designated as having a high histologic grade had a low RS (less 18)— 27.1% with pN0 and 30.0% with pN1 disease—results that are notably higher than those reported in the PlanB trial (18% of grade 3 tumors).13 However, our findings are in keeping with those reported in the Microarray in Node-Negative (or 1–3 Positive Lymph Node) Disease May Avoid Chemotherapy (MINDACT) trial, where patients were stratified by both anatomic and genomic risk using a 70-gene signature. In MINDACT, 28.6% of tumors deemed to be of high clinical risk had low genomic risk and were unlikely to benefit from chemotherapy.23 In the published TAILORx trial data, intermediate RS (11 to 25) tumors in postmenopausal women did not demonstrate clear benefit from chemotherapy; however, this trial was in patients with N0 disease with mostly small tumors, excluded those with an intermediate RS of 26 to 30, and only included a small proportion (17.8%) of patients with grade 3 disease, all of which are limiting comparisons within our study that included a substantial number of T2 tumors in N0 disease.12
Our analyses are constrained by several limitations of the NCDB. Of note, despite representing a majority of patients with cancer in the United States and forming the basis of AJCC staging guidelines, the lack of centralized clinical or pathologic review may limit the accuracy of encoded data.
However, in our multi-institutional cohort of registry-submitted patients, 92% with grade 3 disease demonstrated accurate grade coding of whom only 3% had an incorrectly encoded RS, which suggests that key breast cancer–specific factors are encoded into national registries with encouraging accuracy. In addition, the NCDB only includes OS data, which precludes assessment of breast cancer–specific, progression-free, or recurrence-free survival, end points that may be of greater relevance in the clinical setting. Because of limited follow-up, the NCDB does not incorporate survival data for patients diagnosed in the most recent year. As such, our median follow-up is only 41.3 months and the event rate only 6.2%; nevertheless, we incorporated a large sample size that powered the detection of clinically relevant differences in RS predictive value. Finally, the NCDB lacks detailed information about type, dose, and duration of chemotherapeutic and hormonal treatments and, in particular, has limited granular data about the factors that influence clinical decision making about when to administer adjuvant chemotherapy.
For example, across all patients with grade 3 disease who were not administered chemotherapy, the NCDB only encoded a reason in 27% (21% because chemotherapy was contraindicated and 7% because chemotherapy was recommended but refused by the patient). To help to address the lack of data about patient contraindications, we included Charlson-Deyo comorbidity index data in our risk-adjusted analyses, and higher indices were associated with reduced chemotherapy use. The NCDB also lacks patient menopausal status and only began encoding HER2 status as of 2010. Furthermore, the NCDB does not have information on why RS was or was not sent, which introduces the potential for bias in whom is anticipated to benefit or not benefit from chemotherapy on the basis of additional patient and physician factors that were not captured in our data set.
In conclusion, our data show significant clinical value for RS testing in patients with grade 3 breast cancer to predict which patients are likely to show early benefit or not from the addition of chemotherapy. Furthermore, our findings show significant variability in national patterns of RS testing and chemotherapy use for grade 3 disease, which suggests opportunities for more comprehensive national guidelines for RS testing in high-grade tumors. These results fill a gap as we await the final RxPONDER trial results and provide additional data to the reported results of the TAILORx trial to help clinicians to identify patients who may safely omit chemotherapy without compromising their outcomes.
CONTEXT.
Key Objective
Although grade 3 is considered a poor prognostic factor in estrogen receptor–positive, human epidermal growth factor receptor 2–negative breast cancer, the predictive benefit of multigene recurrence score (RS) testing in this subgroup remains unclear.
Knowledge Generated
Our analysis of the National Cancer Database that comprises more than 70% of all newly diagnosed cancers in the United States reveals that RS results in grade 3 T1c/T2 N0 and N1 breast cancer provide important discriminatory information with regard to chemotherapy benefit. In addition, our findings reveal significant variability in national patterns of RS testing and chemotherapy use for grade 3 tumors.
Relevance
Expanding national clinical guidelines with regard to the value of RS testing and increasing use of RS testing in grade 3 tumors may facilitate de-escalation of therapy in those with a low RS
ACKNOWLEDGMENT
We thank Tari King, MD, for helpful input and the cancer registrars at Brigham and Women’s Hospital and Dana-Farber Cancer Institute for invaluable assistance in data acquisition.
SUPPORT
Supported by the National Institutes of Health (5T32-HL007627; J.B.I.), the American Cancer Society (125912-MRSG-14–240-01-CPPB; R.A.F.), and Susan G. Komen (CCRCR18552788; R.A.F.).
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst=My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwcorascopubs.org/po/author-center.
Rachel A. Freedman
Research Funding: Puma Biotechnology (Inst), Eisai (Inst)
Susan C. Lester
Employment: Novartis Institutes for BioMedical Research (I), resTORbio (I)
Leadership: Blade Therapeutics (I)
Stock and Other Ownership Interests: Novartis
Patents, Royalties, Other Intellectual Property: More than 20 patents issued and pending related to health care therapies (I)
Travel, Accommodations, Expenses: Novartis (I), Blade Therapeutics (I)
Elizabeth A. Mittendorf
Honoraria: Physician Education Resource
Consulting or Advisory Role: Peregrine Pharmaceuticals, TapImmune, Sellas Life Sciences, Merck, Genomic Health
Research Funding: Galena Biopharma (Inst), Genentech (Inst), Roche (Inst), AstraZeneca (Inst), EMD Serono (Inst)
Footnotes
PRIOR PRESENTATION
Presented at the USCAP 2018 Annual Meeting, Vancouver, British Columbia, Canada, March 17–23, 2018.
No other potential conflicts of interest were reported.
REFERENCES
- 1.Paik S, Shak S, Tang G, et al. : A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med 351:2817–2826, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Paik S, Tang G, Shak S, et al. : Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol 24:3726–3734, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Albain KS, Barlow WE, Shak S, et al. : Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: A retrospective analysis of a randomised trial. Lancet Oncol 11:55–65, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dowsett M, Cuzick J, Wale C, et al. : Prediction of risk of distant recurrence using the 21-gene recurrence score in node-negative and node-positive postmenopausal patients with breast cancer treated with anastrozole or tamoxifen: A TransATAC study. J Clin Oncol 28:1829–1834, 2010 [DOI] [PubMed] [Google Scholar]
- 5.National Comprehensive Cancer Network: Breast Cancer, version 1.2018. https://www.nccn.org/professionals/physician_gls/pdf/breast_blocks.pdf
- 6.Harris LN, Ismaila N, McShane LM, et al. : Use of biomarkers to guide decisions on adjuvant systemic therapy for women with early-stage invasive breast cancer: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 34:1134–1150, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.American Joint Committee on Cancer: AJCC Cancer Staging Manual (ed 8). New York, NY, Springer, 2017 [Google Scholar]
- 8.Sanders MA, Wong SM, Iorgulescu JB, et al. : Changes and clarifications in the eighth edition of the AJCC cancer staging system for breast cancer. AJSP Rev Rep 23:113–117, 2018 [Google Scholar]
- 9.Giuliano AE, Connolly JL, Edge SB, et al. : Breast cancer-major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 67:290–303, 2017 [DOI] [PubMed] [Google Scholar]
- 10.Sparano JA, Gray RJ, Makower DF, et al. : Prospective validation of a 21-gene expression assay in breast cancer. N Engl J Med 373:2005–2014, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wong WB, Ramsey SD, Barlow WE, et al. : The value of comparative effectiveness research: Projected return on investment of the RxPONDER trial (SWOG S1007). Contemp Clin Trials 33:1117–1123, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sparano JA, Gray RJ, Makower DF, et al. : Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. N Engl J Med 379:111–121, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gluz O, Nitz UA, Christgen M, et al. : West German Study Group Phase III PlanB Trial: First prospective outcome data for the 21-gene recurrence score assay and concordance of prognostic markers by central and local pathology assessment. J Clin Oncol 34:2341–2349, 2016 [DOI] [PubMed] [Google Scholar]
- 14.Boffa DJ, Rosen JE, Mallin K, et al. : Using the National Cancer Database for outcomes research: A review. JAMA Oncol 3:1722–1728, 2017 [DOI] [PubMed] [Google Scholar]
- 15.Tavassoli FA, Devilee P (eds): World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of the Breast and Female Genital Organs. Lyon, France, IARC Press, 2003 [Google Scholar]
- 16.Orucevic A, Bell JL, McNabb AP, et al. : Oncotype DX breast cancer recurrence score can be predicted with a novel nomogram using clinicopathologic data. Breast Cancer Res Treat 163:51–61, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roberts MC, Miller DP, Shak S, et al. : Breast cancer-specific survival in patients with lymph node-positive hormone receptor-positive invasive breast cancer and Oncotype DX recurrence score results in the SEER database. Breast Cancer Res Treat 163:303–310, 2017 [DOI] [PubMed] [Google Scholar]
- 18.Bhutiani N, Egger ME, Ajkay N, et al. : Multigene signature panels and breast cancer therapy: Patterns of use and impact on clinical decision making. J Am Coll Surg 226:406–412.e1, 2018 [DOI] [PubMed] [Google Scholar]
- 19.American Joint Committee on Cancer: AJCC Cancer Staging Manual (ed 7). New York, NY, Springer, 2010 [Google Scholar]
- 20.Commission on Cancer. Facility Oncology Registry Data Standards Manual: 2013 Revision. Chicago, IL, American College of Surgeons, 2013 [Google Scholar]
- 21.Deyo RA, Cherkin DC, Ciol MA: Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 45:613–619, 1992 [DOI] [PubMed] [Google Scholar]
- 22.Iorgulescu JB, Harary M, Zogg CK, et al. : Improved risk-adjusted survival for melanoma brain metastases in the era of checkpoint blockade immunotherapies: Results from a national cohort. Cancer Immunol Res 6:1039–1045, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cardoso F, van’t Veer LJ, Bogaerts J, et al. : 70-Gene signature as an aid to treatment decisions in early-stage breast cancer. N Engl J Med 375:717–729, 2016 [DOI] [PubMed] [Google Scholar]