Abstract
Importance:
Antihypertension drugs help prevent cardiovascular events, though less is known about the comparative effectiveness of different drug classes.
Objective:
To compare the contemporary aggregated first in-trial cardiovascular events among hypertensive patients without significant co-morbidities.
Data Sources:
We systematically searched the PubMed, EMBASE, and Cochrane Library databases for articles published between January 1, 1990 and October 24, 2017.
Study selection:
We included randomized clinical trials testing commonly used antihypertension drugs (i.e. angiotensin-converting-enzyme inhibitors, dihydropyridine and non-dihydropyridine calcium-channel blockers, beta blockers, angiotensin receptor blockers, and diuretics) that reported on selected cardiovascular outcomes over at least 6 months of follow-up.
Data Extraction and Synthesis:
The analysis was conducted from October 2017 to December 2019. Two reviewers extracted the number of cardiovascular events at the end of treatment for all study arms. For each outcome, we used frequentist network meta-analysis to compare risk reductions between drug classes (random-effects models weighted by the inverse variance). We estimated the dose-response relationship of 10 mmHg reduction of systolic and 5 mmHg reduction of diastolic blood pressure with risk of first in-trial cardiovascular events.
Main Outcomes and Measures:
First in-trial cardiovascular events, including cardiovascular death, myocardial infarction, stroke, and revascularization.
Results:
We pooled data from 46 eligible trials (n=248,887, mean age 65.6 years, 52.8% male). In the network meta-analysis, compared with placebo, angiotensin-converting-enzyme inhibitors, dihydropyridine calcium-channel blockers and thiazide diuretics were similarly effective in reducing overall cardiovascular events (25%), cardiovascular death (20%), and stroke (35%); angiotensin-converting-enzyme inhibitors were the most effective in lowering risk of myocardial infarction (28%); diuretics were the most effective in lowering revascularization (33%). In meta-regression analyses, each 10mmHg reduction in systolic blood pressure and 5mmHg reduction in diastolic blood pressure was significantly associated with lower risk of cardiovascular death, stroke and overall cardiovascular events.
Conclusion and Relevance:
In this network meta-analysis among hypertensive patients without significant co-morbidities, different classes of blood pressure lowering drugs showed similar benefits in reducing cardiovascular events. Future studies should compare the effectiveness of combinations of antihypertension drugs in reducing cardiovascular events.
INTRODUCTION
Hypertension is the most prevalent risk factor for mortality and disability-adjusted life-years1 worldwide. Cardiovascular disease (CVD) remains the leading cause of death globally, accounting for 17.7 million deaths in 2015, representing 31% of all global deaths.2 Hypertension is a strong driver of CVD3 and, in 2010, it was estimated that a third of the world’s adult population of adults had hypertension.4 The introduction of the ACC/AHA hypertension guidelines in 2017 resulted in higher estimates of those affected by hypertension,5,6 and knowing the optimal first-line blood pressure-lowering drugs for prevention of CVD events and mortality will be important for clinical decision making. Moreover, identifying which treatments are most effective for controlling hypertension, lowering downstream CVD events and mortality, and have the least harms are imperative to guide clinicians and lower CVD burdens worldwide.
Previous meta-analyses have demonstrated the efficacy of antihypertension treatments for reducing cardiovascular events,7,8 which have employed pair-wise comparisons of only two classes of antihypertension drugs, while pair-wise meta-analysis does not enable comparison of multiple classes of drugs. Only one network meta-analysis has compared the effectiveness of different classes of antihypertension drugs in preventing cardiovascular events, but it was published over 15 years ago9 and included drugs such as alpha blockers that are less frequently used in contemporary healthcare.
To provide an updated perspective on comparative efficacy of antihypertension drugs, we conducted a network meta-analysis to compare the effects of different classes of antihypertension drugs in current use in reducing risks of individual cardiovascular event (cardiovascular death, myocardial infarction, stroke, revascularization, as well as the effect in reducing overall risk of any cardiovascular event. Findings from this study will be relevant for contemporary clinical management of hypertension, especially in light of the new ACC/AHA hypertension guidelines.10
METHODS
We conducted a network meta-analysis of randomized clinical trials testing antihypertension drugs. We followed the Preferred Reporting Items of Systematic Reviews incorporating Network Meta-Analyses (PRISMA) guideline for this study.11
Data Sources and Searches
We systematically searched the PubMed, EMBASE, and Cochrane Library databases for articles published between January 1, 1990 and October 24, 2017. Search terms used are listed in the appendix. There were no language restrictions.
We included studies meeting the following criteria: (1) randomized controlled trials; (2) published in or after 1990; (3) included non-pregnant adults aged 18 years or older without chronic kidney disease, diabetic nephropathy, or organ transplants (which are risk factors for secondary hypertension), and without myocardial infarction and/or stroke within the previous 6 months; (3) evaluated antihypertension drugs including angiotensin-converting-enzyme inhibitors (ACEi), dihydropyridine calcium-channel blockers (dCCB), non-dihydropyridine calcium-channel blockers (ndCCB), beta blockers (BB), angiotensin receptor blockers (ARB), and diuretics compared to controls receiving placebo, treatment as usual, or health education; (4) reported incidence of cardiovascular disease events (cardiovascular death, myocardial infarction, stroke, and/or coronary revascularization [either percutaneous coronary intervention or coronary artery bypass grafting]); (5) reported data from at least six months of follow-up. In addition, we excluded studies that reported myocardial infarction and/or stroke within the previous 3 months; studies focused on patients with chronic kidney disease, diabetic nephropathy, or organ transplants (which are risk factors for secondary hypertension); studies not focused on hypertensive patients. In order to minimize concerns about heterogeneity of outcome ascertainment across studies, we only included studies where cardiovascular events were adjudicated by physicians using similar criteria and assessing patients’ medical records.
Study Selection
Study selection followed 3 steps. First, 2 reviewers (JW and AK) independently screened titles of studies. Second, the same 2 reviewers screened and selected abstracts by and disagreements were resolved by a third reviewer (MKA). Then, the team reviewed full-texts of articles for confirmation of inclusion. Disagreements were resolved by consensus or, when necessary, by a third reviewer.
Data Extraction and Quality Assessment
Two reviewers (JW and AK) independently extracted data from studies that met inclusion criteria using a standardized extraction form. The data extracted included sample size, participant characteristics (age, sex), study country, follow-up duration, types of antihypertension drugs and comparator groups, and number of first in-trial cases for each outcome.
Cardiovascular death was defined as death related to cardiovascular disease, or can be calculated using all-cause mortality subtracted by non-cardiovascular related death. We used non-fatal myocardial infarction and stroke if they are indicated or can be calculated, and we used total (including fatal and nonfatal) myocardial infarction and stroke as outcomes if numbers of nonfatal myocardial infarction and stroke could not be derived. Revascularization included percutaneous coronary intervention or coronary artery bypass grafting reported in the trials. The overall cardiovascular events were calculated as the aggregation of cardiovascular death, myocardial infarction, stroke and revascularization.
We assessed the quality of the included studies using the JADAD Scale12 for randomized controlled trials. This scale assesses risk of bias based on 3 domains: randomization (including mention of randomization, appropriate method of randomization), blinding (including mention of blinding, appropriate blinding), and an account of all patients (the ending of all patients in the trial is known, and if there are no data, the reason is stated). A study could be awarded a maximum of 2 points each for domain of randomization and blinding, and 1 point for an account of all patients, for a possible maximum total score of 5. Two reviewers (JW and AK) conducted the quality assessment, assigning quality scores (continuous measure) for each study. Studies scoring ≥3 or more points were deemed as having low risk of bias and studies scoring <3 points were deemed to have high risk of bias.
Data Synthesis and Analysis
We first conducted pairwise meta-analysis of placebo-controlled trials to estimate the direct effect of each agent in reducing cardiovascular events when compared with placebo. Risk difference (as per 1000 persons) and numbers needed to treat (NNT) were calculated for each type of drug.
We conducted frequentist network meta-analysis with random-effects models to estimate the aggregate reductions in CVD events and revascularization for each type of antihypertension drug, compared with placebo and then to each other.13
We used Stata 14.0 (State Corp, College Station, TX) for all analyses using the ‘network’ command.14 This is an extension of method proposed by DerSimonian and Laird, and the performance of this model has been satisfactory.15 This model contains a component of inconsistency variance, which is a source of variation in addition between-study heterogeneity.16 We reported risk ratios (RRs) and corresponding 95% confidence intervals (95% CI), and calculated a pooled RR and 95% CI for each intervention arm separately to placebo.
For each drug class, we assessed heterogeneity across studies using the maximum likelihood method.16 We examined the magnitude of a common heterogeneity variance for the network (tau square [τ2]) as an indicator of the extent of heterogeneity among included studies, in terms of the range of expected treatment estimates (RRs and 95% CIs). τ2 values under 0.25 were considered acceptable, between 0.25 to 1.0 moderately high, and greater than 1.0 represented very high heterogeneity.
We assessed the general within-network inconsistency between direct effects (comparison between specific agents with placebo) and indirect comparisons (comparisons other than direct comparisons within each outcome) for each outcome using chi-square tests. If no inconsistency was detected in general, inconsistency between each two agents was tested by calculating the differences in direct effects and indirect comparisons with their standard errors.17,18 We considered evidence of inconsistency if p-values for the tests were smaller than 0.05.
We assessed for potential publication bias by inspecting symmetry of funnel plots for each outcome.19
We conducted meta-regression analyses to examine the dose-response relationship between each within-treatment 10 mmHg reduction in systolic and 5 mmHg reduction in diastolic blood pressure (regardless of intervention arms) over time and incidence of cardiovascular events (including overall and individual types of cardiovascular events, with lnRR of cardiovascular events as dependent variables). The coefficients of meta-regression is weighted by 1/(σi2 + τ2), where σi2 stands for the standard error of the estimated effect in the particular trial, and τ2 stands for the between-study variance.20 In addition, we reported rates of side effects, such as edema, headache, cough, and hypotension/dizziness that are associated with antihypertension drugs and placebo among studies with available information.
RESULTS
Our systematic search yielded a total of 4,933 articles (Figure 1). We identified an additional 71 articles from bibliographies of relevant reports and reviews. In total, we included 47 published articles from 46 trials with 248,887 participants and 28,658 first in-trial cardiovascular events in the systematic review and network meta-analysis. Due to the small number of studies reporting on different drug combination permutations and ndCCB, we did not report data for fixed dose blood pressure lowering combination medications in the present network meta-analysis.
Figure 1.
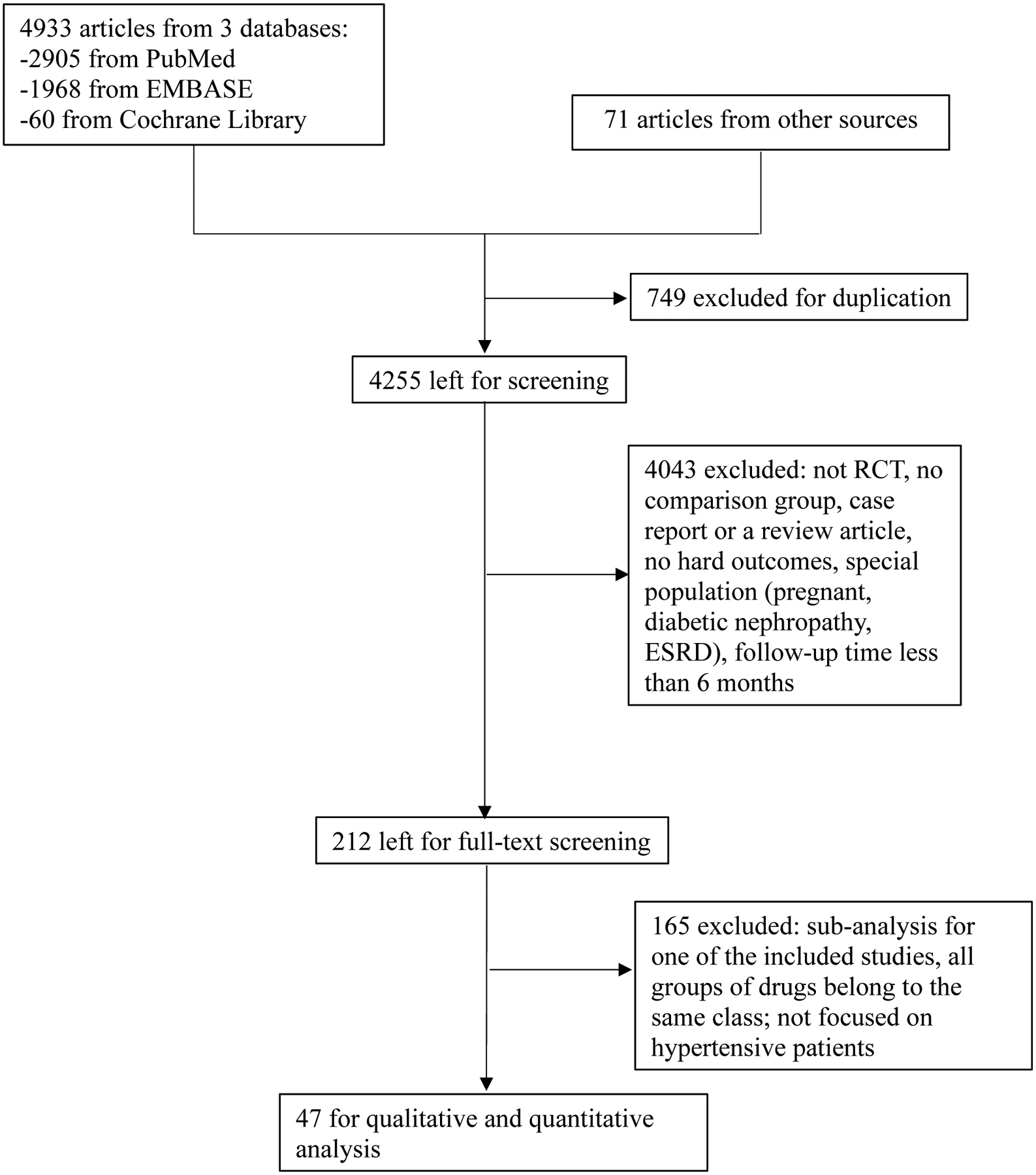
PRISMA diagram of literature search on the network meta-analysis.
Figure 2 shows the network plot depicting the different antihypertension drug classes and comparisons tested. Among included studies, 15 tested angiotensin-converting-enzyme inhibitors, 23 dihydropyridine calcium channel blockers, 4 non-dihydropyridine calcium-channel blockers, 8 beta blockers, 12 angiotensin receptor blockers, and 13 thiazide diuretics. There were 4 studies from North America, 18 from Europe, 16 from Asia, 1 from Oceania, and 7 from multiple regions across continents.
Figure 2.
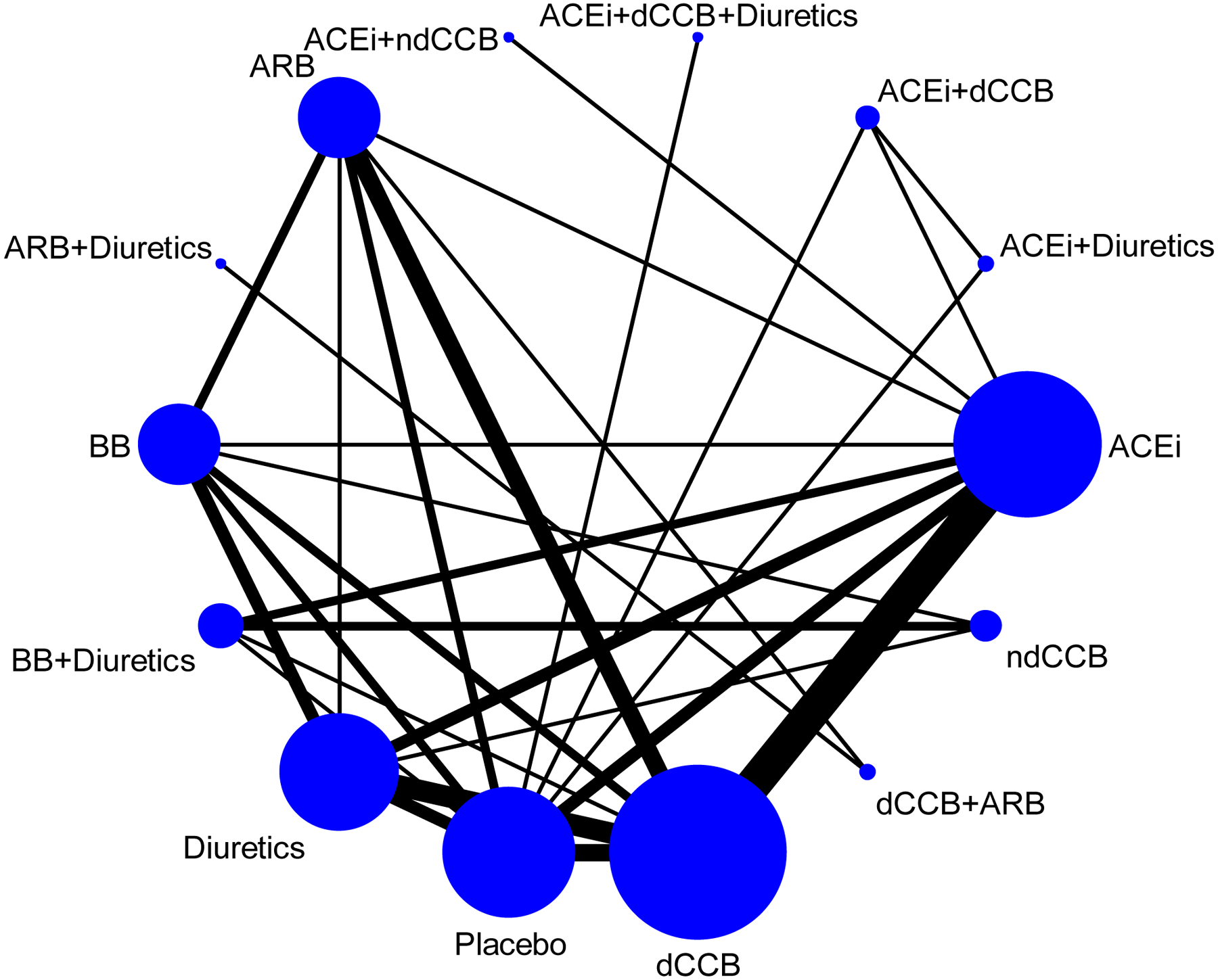
Network plot of antihypertension drugs in included articles.
ACEi: angiotensin-converting-enzyme inhibitors; dCCB: dihydropyridine calcium channel blockers; ndCCB: non-dihydropyridine calcium channel blockers; BB: beta blockers; ARB: angiotensin receptor blockers.
Participant average age was 65.6 years (ranging from 51.8 to 83.8 years), percentage of male participants was 52.8% (ranging from 28.2% to 100%). The baseline systolic blood pressure was 161.3 mmHg (ranging from 129 mmHg to 195 mmHg). There were 14 studies that included placebo/health education/conventional education as control arms, and 35 studies reported on direct (head-to-head) treatment comparisons (including 3 studies includes placebo and 2 treatment agents). The mean follow-up time was 3.7 years (ranging from 1 years to 10 years). Across all included trials with particular classes of antihypertension drugs, the frequency of using each type of antihypertension drug was similar between studies with participants’ mean age over and below 65 years. By regions of studies, ACEi, BB and diuretics were more frequently used in Europe; dCCB was more frequently used in both Asia and Europe; ARB was more frequently used in Asia. The prevalence of baseline cardiovascular disease and diabetes was similar among participants using each class of antihypertension drugs. See characteristics of all 48 included studies in eTable 1.
For all 48 trials included in the analysis, the average overall systolic and diastolic blood pressure changes among those reported was −18.0 mmHg and −10.1 mmHg, respectively. The overall risk of cardiovascular events was 11.5%. Among the side effect profile, edema was prevalent among 0.5% of participants who took ACEi, 17.1% of participants taking dCCB or ndCCB, 8.7% of participants taking BB, 15.1% of participants taking ARB, 9.9% of participants taking diuretics and 3.7% of participants taking placebo; cough was prevalent among 8.3% of participants who took ACEi, 9.6% of participants taking dCCB or ndCCB, 4.1% of participants taking BB, 2.7% of participants taking ARB, 5.4% of participants taking diuretics and 16.1% of participants taking placebo; headache/hypotension was prevalent among 0.7% of participants who took ACEi, 7.9% of participants taking dCCB or ndCCB, 1.4% of participants taking BB, 10.8% of participants taking ARB, 7.4% of participants taking diuretics and 8.8% of participants taking placebo; dizziness was prevalent among 1.7% of participants who took ACEi, 7.5% of participants taking dCCB or ndCCB, 9.1% of participants taking BB, 14.8% of participants taking ARB, 9.0% of participants taking diuretics and 10.2% of participants taking placebo.
Comparative efficacy of antihypertension drugs
Direct comparison among placebo-controlled trials
The absolute risk differences in cardiovascular events comparing blood pressure lowering drugs to placebo in placebo-controlled trials showed that all five types of antihypertension drugs caused larger decreases in cardiovascular events than placebo. Compared to placebo, dCCB lowered the risk of cardiovascular death by 7 cases per 1000 persons; ACEi and diuretics lowered MI risk by 24, 10 cases per 1000 persons, respectively; dCCB and diuretics lowered stroke risk by 16, 21 cases per 1000 persons, respectively (eTable 2). Based on these, 143 people would need to be treated with dCCB to prevent one cardiovascular death. To prevent one MI, 42 and 100 people would need to be treated with ACEi and diuretics, respectively. To prevent one stroke, 63 and 48 people would need to be treated with dCCB and diuretics, respectively.
Network meta-analysis for all included trials
In the network meta-analysis comparing placebo versus drug arms, for cardiovascular death, we noted that ACEi, dCCB, ARB and diuretics were all associated with 15–22% relative risk reductions for cardiovascular death. For myocardial infarction, ACEi, dCCB and BB were effective in lowering risk by 20–28%. For stroke, all classes of drugs were associated with a 19–39% relative risk reduction. For revascularization, diuretics was associated with a 33% relative risk reduction. For overall cardiovascular events, all classes of drugs were each associated with a 17–29% relative risk reduction (Figure 3). According to effect sizes, ACEi, dCCB, diuretics achieved similarly significant reductions in the risk of overall cardiovascular events and cardiovascular death. ACEi achieved the greatest reductions in myocardial infarction risk. dCCB and diuretics achieved similarly significant reductions in the risk of stroke. Diuretics achieved similarly significant reductions in the risk of revascularization. However, their 95% CIs overlapped.
Figure 3.
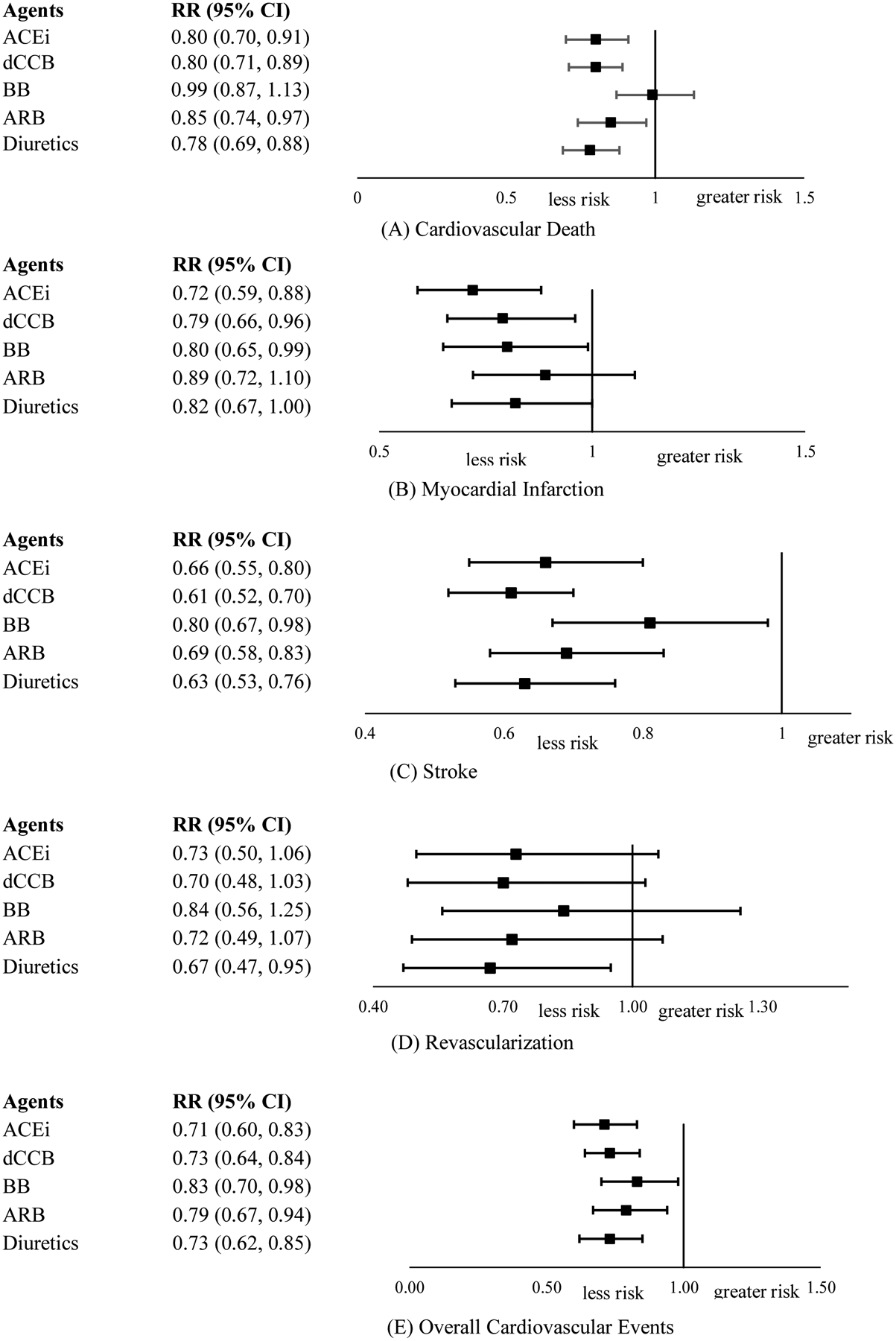
Results of network meta-analysis comparing single class of antihypertension drugs with placebo on cardiovascular events.
ACEi: angiotensin-converting-enzyme inhibitors; dCCB: dihydropyridine calcium channel blockers; ndCCB: non-dihydropyridine calcium channel blockers; BB: beta blockers; ARB: angiotensin receptor blockers; RR: risk ratio; CI: confidence interval.
Consistency, Heterogeneity, Risk of Bias Assessment, and Sensitivity Analysis
In terms of risk of bias assessment, we found that studies were of moderate quality (mean score=4.0 out of 5), where randomization scored 1.8 out of 2, blinding 1.1 out of 2 points, and consideration of all patients 1 out of 1 possible point. No study was considered high risk of bias. Visual examination of funnel plots showed that there was no significant publication bias present for cardiovascular death, myocardial infarction, stroke, revascularization and overall cardiovascular events (eFigure 1).
No general inconsistency of treatment effect on each outcome was found, with all p-values greater than 0.05. Split by individual treatment, there was no significant inconsistency detected among different classes of antihypertension drugs (eTable 3). No significant treatment effect heterogeneity was detected, as none of the τ2s achieving values equal or greater than 0.25 (eFigure 2).
In meta-regressions of blood pressure reduction with cardiovascular events, blood pressure reduction was associated with a trend of reducing cardiovascular events. In particular, each 10mmHg reduction in systolic blood pressure was significantly associated with 13% lower risk of cardiovascular death (RR=0.87, [0.77, 0.99]), 17% lower risk for stroke (RR=0.69, [0.48, 0.97]), and 14% lower risk for overall cardiovascular events (RR=0.86, [0.78, 0.96]); each 5mmHg reduction in diastolic blood pressure was significantly associated with 14% lower risk of cardiovascular death (RR=0.86, [0.74, 1.00]), 20% lower risk for stroke (RR=0.80, [0.67, 0.95]), and 16% lower risk for overall cardiovascular events (RR=0.84, [0.74, 0.96]) (eTable 4).
DISCUSSION
From 46 randomized controlled trials that examined the effects of major antihypertension drugs in preventing cardiovascular events, pooled results showed that ACEi, dCCB and diuretics were similarly effective in preventing cardiovascular death (about 20% reduction, compared with placebo), stroke (about 35%), and overall cardiovascular events (about 30% reduction, compared with placebo). ACEi was the most effective in preventing myocardial infarction (about 30% reduction, compared with placebo). Diuretics was the most effective in reducing revascularization (about 30%, compared with placebo). Our study provides the most up-to-date evidence on comparative efficacy of antihypertension drugs for reducing cardiovascular events in randomized controlled trials. Furthermore, to our knowledge, this is the first meta-analysis pooling results of studies testing antihypertension drugs for reducing risk of revascularization.
Our results are consistent with those reported in previous meta-analyses of randomized controlled trials. Psaty et al.9 concluded that low-dose diuretics were the most effective treatment for preventing the occurrence of CVD mortality. Law et al.8 concluded that all antihypertension drug classes have similar risk reduction effects for stroke for a given reduction in blood pressure. Our findings further supported the evidence that the differences of effects in reducing cardiovascular events between drug classes are small. With newly published articles included in the network meta-analysis, we provided more up-to-date information of efficacy of antihypertension drugs on reducing cardiovascular events. In a recent systematic, multinational analysis of the large-scale evidence generation and evaluation across a network of databases for hypertension (LEGEND-HTN) study, which utilized observational data encompassing millions of patients found that diuretics shows advantages in reducing various cardiovascular events over other classes of drugs,21 while this is different from our study showing that diuretics is similarly effective in reducing cardiovascular events compared to ACEi and dCCB, and ACEi appears to work best on reducing myocardial infarction in our study. The difference may be due to varied study designs (randomized controlled trials vs observational studies), and it is not clear to what extent the study may be subject to selection bias.
Taking advantage of network meta-analysis, we were able to compare drug classes with placebo both directly (i.e. among included placebo-controlled trials) and indirectly (i.e. among all included studies). The comparisons between drugs and placebo showed slight differences between direct and overall comparisons. The network comparisons were more precise (narrower 95% CIs).
It is also worth noting that the associations between lowering blood pressure and reduced cardiovascular events is smaller than those obtained from observational studies.22,23 The reason may be that most participants in trials, including those receiving placebo, are motivated and in a number of cases, were also treated with other drugs. In addition, participant motivation may have led to engaging in healthy lifestyle habits like choosing healthier diet patterns, exercising, and avoiding tobacco and alcohol use. This also calls for future studies that examine the interactions between antihypertension drugs and lifestyle in preventing cardiovascular events among patients with hypertension. However, as small as the effect size is for the association between lowering blood pressure and reduced cardiovascular events, some of the differences were statistically significant.
Our study is limited by a relatively small number of studies included in the network meta-analysis, so there is a lack of statistical power to conduct subgroup analysis to see whether the effects of antihypertension on reducing cardiovascular events could be affected by different factors (e.g., age, sex, baseline blood pressure level). While the results from analysis may serve as a great source of reference, a comprehensive consideration based on demographic factors and comorbidities is needed when deciding which class of antihypertension drug should be used for reducing cardiovascular outcomes. The newly published ACC/AHA guidelines for hypertension identifies a wider range of individuals with early hypertension and pre-hypertension, this study guide first line medication choices.24
Another important limitation of our present study is that we did not include results for combinations of antihypertension drugs, since there were too few studies with data for each permutation of combinations. However, this suggested that the efficacy of several group of combinations, such as BB/diuretics may be studied more frequently in the future. Recent findings have shown that treatment with low doses of 3 antihypertensive drugs led to an increased proportion of patients achieving target blood pressure compared to usual care,25 and quarter-dose therapy with combined medications have been shown more effective than standard monotherapy in reducing blood pressure, and it conferred fewer adverse events.26
In conclusion, the present network meta-analysis indicated that major first-line antihypertension drugs, including ACEi, dCCB, BB, ARB, and diuretics were all effective in reducing cardiovascular events, compared with use of placebo. Furthermore, ACEi, dCCB and diuretics appear to similarly well in reducing cardiovascular deaths, stroke, and overall cardiovascular events, while ACEi appears to be the drug of choice over other antihypertension drugs in prevention of myocardial infarction, and diuretics appear to be an optimal choice in reducing revascularization. The differences between drug classes are generally small in terms of reducing cardiovascular events. Future studies may compare the effectiveness of multiple antihypertension drugs in combination to individual antihypertension drugs in reducing cardiovascular events.
Supplementary Material
Key Points.
Question:
Which classes of blood pressure lowering drugs are most effective in reducing first cardiovascular events?
Findings:
In this systematic review and network meta-analysis of randomized controlled trials comparing individual antihypertension drug classes head-to-head in typical patients with hypertension and no significant co-morbidities, angiotensin-converting-enzyme inhibitors, dihydropyridine calcium-channel blockers and diuretics were similarly effective in reducing cardiovascular death, stroke, and overall cardiovascular events. Angiotensin-converting-enzyme inhibitors and diuretics were the most effective in lowering myocardial infarction and revascularization, respectively.
Meaning:
The effects of different blood pressure lowering drug classes were largely similar with only nuanced differences.
Acknowledgments
JW performed the initial literature search, data collection, data analysis and drafted the manuscript. KIG helped edit the manuscript and assisted with data analysis. AK performed the initial literature search, data collection and edited the manuscript. MJM and JSH contributed to data collection and edited the manuscript. KVN and MKA designed the study, assisted with data analysis and interpretation of findings, and helped edit the manuscript. JW had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding Source
This project was funded by the Disease Control Priorities Network grant to the Institute for Health Metrics and Evaluation from the Bill & Melinda Gates Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Disclosures
None.
REFERENCES
- 1.Forouzanfar MH, Alexander L, Anderson HR, et al. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386(10010):2287–2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO. Cardiovascular diseases (CVDs). 2017; http://www.who.int/mediacentre/factsheets/fs317/en/. Accessed 07/01, 2017.
- 3.Franklin SS, Wong ND. Hypertension and cardiovascular disease: contributions of the framingham heart study. Glob Heart. 2013;8(1):49–57. [DOI] [PubMed] [Google Scholar]
- 4.Bloch MJ. Worldwide prevalence of hypertension exceeds 1.3 billion. Journal of the American Society of Hypertension : JASH. 2016;10(10):753–754. [DOI] [PubMed] [Google Scholar]
- 5.Khera R, Lu Y, Lu J, et al. Impact of 2017 ACC/AHA guidelines on prevalence of hypertension and eligibility for antihypertensive treatment in United States and China: nationally representative cross sectional study. BMJ (Clinical research ed). 2018;362:k2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Venkateshmurthy NS, Geldsetzer P, Jaacks LM, Prabhakaran D. Implications of the New American College of Cardiology Guidelines for Hypertension Prevalence in India. JAMA internal medicine. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ettehad D, Emdin CA, Kiran A, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet (London, England). 2016;387(10022):957–967. [DOI] [PubMed] [Google Scholar]
- 8.Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ (Clinical research ed). 2009;338:b1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Psaty BM, Lumley T, Furberg CD, et al. Health outcomes associated with various antihypertensive therapies used as first-line agents: a network meta-analysis. JAMA : the journal of the American Medical Association. 2003;289(19):2534–2544. [DOI] [PubMed] [Google Scholar]
- 10.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension (Dallas, Tex : 1979). 2018;71(6):1269–1324. [DOI] [PubMed] [Google Scholar]
- 11.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ (Clinical research ed). 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Controlled clinical trials. 1996;17(1):1–12. [DOI] [PubMed] [Google Scholar]
- 13.Rucker G, Schwarzer G. Ranking treatments in frequentist network meta-analysis works without resampling methods. BMC medical research methodology. 2015;15:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chaimani A, Higgins JP, Mavridis D, Spyridonos P, Salanti G. Graphical tools for network meta-analysis in STATA. PloS one. 2013;8(10):e76654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jackson D, Law M, Barrett JK, et al. Extending DerSimonian and Laird’s methodology to perform network meta-analyses with random inconsistency effects. Statistics in medicine. 2016;35(6):819–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Law M, Jackson D, Turner R, Rhodes K, Viechtbauer W. Two new methods to fit models for network meta-analysis with random inconsistency effects. BMC medical research methodology. 2016;16:87–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higgins JP, Jackson D, Barrett JK, Lu G, Ades AE, White IR. Consistency and inconsistency in network meta-analysis: concepts and models for multi-arm studies. Research synthesis methods. 2012;3(2):98–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shim S, Yoon BH, Shin IS, Bae JM. Network meta-analysis: application and practice using Stata. Epidemiology and health. 2017;39:e2017047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sedgwick P. Meta-analyses: how to read a funnel plot. BMJ : British Medical Journal. 2013;346. [Google Scholar]
- 20.Harbord RM, Higgins JPT. Meta-Regression in Stata. The Stata Journal. 2008;8(4):493–519. [Google Scholar]
- 21.Suchard MA, Schuemie MJ, Krumholz HM, et al. Comprehensive comparative effectiveness and safety of first-line antihypertensive drug classes: a systematic, multinational, large-scale analysis. Lancet (London, England). 2019;394(10211):1816–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MacMahon S, Peto R, Cutler J, et al. Blood pressure, stroke, and coronary heart disease. Part 1, Prolonged differences in blood pressure: prospective observational studies corrected for the regression dilution bias. Lancet (London, England). 1990;335(8692):765–774. [DOI] [PubMed] [Google Scholar]
- 23.Lawes CM, Rodgers A, Bennett DA, et al. Blood pressure and cardiovascular disease in the Asia Pacific region. Journal of hypertension. 2003;21(4):707–716. [DOI] [PubMed] [Google Scholar]
- 24.Carey RM, Whelton PK. Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Synopsis of the 2017 American College of Cardiology/American Heart Association Hypertension Guideline. Annals of internal medicine. 2018;168(5):351–358. [DOI] [PubMed] [Google Scholar]
- 25.Webster R, Salam A, de Silva HA, et al. Fixed Low-Dose Triple Combination Antihypertensive Medication vs Usual Care for Blood Pressure Control in Patients With Mild to Moderate Hypertension in Sri Lanka: A Randomized Clinical Trial. Jama. 2018;320(6):566–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bennett A, Chow CK, Chou M, et al. Efficacy and Safety of Quarter-Dose Blood Pressure-Lowering Agents: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Hypertension (Dallas, Tex : 1979). 2017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


