Abstract
Background:
Pre-exposure prophylaxis (PrEP) can reduce HIV acquisition among female sex workers (FSW). However, changes in condomless sex frequency after PrEP initiation could reduce PrEP effectiveness when PrEP adherence is suboptimal as well as increase the risk of acquiring other sexually transmitted infections (STIs). Objective measures of condomless sex may be more accurate for determining changes in sexual behavior than self-reported measures.
Methods:
We longitudinally measured self-reported condom use, number of clients, and presence of Y-chromosomal DNA (Yc-DNA) in vaginal swabs among 267 FSW accessing PrEP at four clinics in Senegal between 2015 and 2016. We assessed trends in sexual behavior over time since PrEP initiation using generalized estimating equations and evaluated predictors of Yc-DNA detection.
Results:
We found no increase in self-reported condomless sex with clients (odds ratio (OR)=0.94; 95% CI: [0.89, 1.00]), main partners (OR=0.99; 95% CI: [0.96, 1.02]) or Yc-DNA detection (OR=0.99; 95% CI: [0.90, 1.08]) over time since initiation. Yc-DNA was detected in 34/154 (22%) of swabs tested and in 15/58 (26%) of swabs from FSW reporting consistent condom use among both clients and main partners. Self-reported condom use with clients or main partners did not predict Yc-DNA detection.
Conclusions:
In a FSW PrEP demonstration project in Senegal, we found no evidence of risk compensation among FSW on PrEP as measured by self-reported behavior or through Yc-DNA detection. Yc-DNA detection was frequently detected among FSW reporting consistent condom use, highlighting limitations of self-reported sexual behavioral measures.
Keywords: Condoms; Biomarker; Y chromosomal DNA; Africa; HIV, HIV-2
Short summary:
A study among female sex workers in Senegal found no evidence of increased sexual risk behavior after PrEP initiation as measured by self-report or Yc-DNA detection.
INTRODUCTION:
Despite increased access to HIV treatment and prevention options, an estimated 1.8 million people globally became newly infected with HIV in 2017.1 Key populations are disproportionately affected by HIV and account for nearly half of new infections.1 World Health Organization guidelines recommend offering pre-exposure prophylaxis (PrEP) to individuals at substantial risk of HIV infection, including female sex workers (FSW).2 Despite the potential benefits of PrEP use among FSW, condom use is still recommended while taking PrEP due to concerns about adherence to daily medication, sexually transmitted infection (STI) acquisition, and pregnancy.3 FSW who benefit from PrEP may be incentivized to reduce condom use secondary to condom availability or costs, client preference, ability to charge more for condomless sex, and concerns about client violence.4,5 If clients are aware of PrEP availability, FSW may face increasing challenges in negotiating condom use within a context of imbalanced power dynamics.5 Decreases in condom use, increases in number of partners, or other forms of sexual risk compensation may increase the risk of sexually transmitted infection (STI) acquisition or attenuate PrEP effectiveness when PrEP adherence is suboptimal.6 Therefore, monitoring changes in sexual behavior and condomless sex among FSW initiating PrEP is needed to assess PrEP delivery programs.
Studies investigating changes in sexual behavior after PrEP initiation have primarily evaluated self-reported measures such as number of partnerships and condom use.7–11 However, self-reported sexual behavior measures have significant limitations, such as recall inaccuracy and social desirability bias.12,13 Biomarkers of condomless sex such as prostate-specific antigen (PSA) and Y-chromosomal DNA (Yc-DNA) have been used to assess self-report accuracy as well as to evaluate changes in sexual behavior.14,15 The presence of male Yc-DNA in vaginal swabs is a validated biomarker for recent condomless sex that can be detected up to 14 days after semen exposure.16–20 A previous study among FSW in West Africa found that 21% of participants who reported no condomless sex in the last 14 days had detectable Yc-DNA in vaginal swab samples.21 However, few studies among FSW have used objective measures to investigate changes in condomless sex frequency after PrEP initiation.22
Sex work has been legal in Senegal since 1969 and is regulated by requiring sex workers to register at a health facility and attend routine check-ups.23 As part of a PrEP demonstration project in Senegal, we investigated condom use and Yc-DNA detection in the genital tract of sex workers initiating oral PrEP at four Ministry of Health FSW Clinics in Senegal, West Africa. Our objectives were to assess changes in self-reported sexual behavior and Yc-DNA detection from time since initiation, to evaluate whether self-reported sexual behavioral measures such as recent partners and condom use were associated with vaginal Yc-DNA detection, and to evaluate predictors of Yc-DNA detection.
MATERIALS AND METHODS:
Study Population
The design, methods, and primary outcomes of the Senegal PrEP Demonstration Project have been described previously.24 Briefly, 267 HIV-negative active female sex workers (reported paid sex within the past six months) medically eligible for PrEP were offered daily oral tenofovir disoproxil fumarate/emtricitabine (300mg/200mg once daily; Truvada®, Gilead Sciences) at four clinics in Senegal and followed at one month, three months, and every three months thereafter for one year. Study visits occurred from 2015 to 2016 and included testing for HIV-1 and HIV-2 and other STIs, medication adherence counseling, condom distribution, risk reduction counseling, clinical examination, and creatinine testing. At each visit, trained staff conducted standardized interviewer-administered questionnaires to assess demographic, clinical, and sexual behavior characteristics among participants continuing to receive PrEP services. Retention in PrEP care was 67% at 12 months after PrEP initiation (Supplemental Digital Content, Table S1). No HIV seroconversions were detected over the course of the study among participants retained in care. The study was registered at ClinicalTrials.gov (NCT02474303). All participants in this study provided written informed consent and the study was approved by the Human Subjects Division Institutional Review Board at the University of Washington, the Institutional Review Board at Westat, and the Senegalese National Ethics Committee for Health Research (CNERS).
Self-Reported Sexual Behavior Measures
At initiation and each follow-up visit, participants were asked to report the following measures: number of clients in the last seven days, frequency of condom use during vaginal or anal sex with clients in the last month (always, almost always, sometimes, almost never, or never), whether the participant has a “main” partner (defined as having regular sexual relations with an individual who did not pay or trade goods or services for sex), number of main partners in the last month, and frequency of condom use during vaginal or anal sex with main partners in the last month (always, almost always, sometimes, almost never, or never). Participants were also asked if they used a condom the last time they had sex with a client and the last time they had sex with a main partner.
Y-Chromosomal DNA Detection
Vaginal swabs were self-collected by women at each visit. We used non-proportional quota sampling (stratified on clinic, sex worker registration status, visit number, and retention status) to choose 156 swabs from 121 FSW for Yc-DNA testing throughout the study period (see Supplemental Digital Content for further details). Vaginal swabs were frozen at −80°C and shipped on dry ice in bulk to the University of Washington in Seattle. DNA extraction was conducted using the QIAamp96 DNA blood kit (Qiagen, Germany) as described previously.25 The Quantifiler® Duo DNA Quantification Kit (Applied Biosystems®, Waltham, Massachussetts) was used for Yc-DNA detection per the manufacturer’s instructions as described previously.20,26 All Yc-DNA polymerase chain reaction (PCR) analyses were conducted by female laboratory staff (to prevent contamination with male DNA) who were blinded to study data. We excluded one sample which produced an invalid result (no detection of human autosomal DNA). Two swabs from the same participant at the same visit (one for Yc-DNA detection and one initially designated for sexually transmitted infection (STI) testing) were inadvertently analyzed and returned discordant results. We excluded the negative result because the likelihood of cross-contamination was low (adjacent samples were negative), the detection level in the positive swab was near the limit of detection, and the assay is highly specific due to low levels of cross-reactivity. Therefore, our sample for our final analysis included 154 swabs from 121 women.
STI testing
Limited STI testing was conducted due to logistical constraints such as equipment failure and reagent stockouts and funding (Supplemental Digital Content, Table S2). Neisseria gonorrhoeae and Chlamydia trachomatis (NG/CT) NAAT testing was performed in Senegal at the Institut de Recherche en Santé de Surveillance Epidemiologique et de Formations (IRESSEF) using the Roche cobas® 4800 System (Roche Molecular Systems, Pleasanton, CA, USA) per the manufacturer’s instructions. Syphilis testing was performed by clinic staff using rapid plasma reagin (RPR) and Treponema pallidum hemagglutination assays (TPHA).
Statistical Analysis
We summarized demographic and behavioral characteristics of participants using descriptive methods. We dichotomized self-reported condom use into always (consistent) or not always (inconsistent). To investigate changes in sexual risk behaviors over time since PrEP initiation, we calculated the average number of clients reported in the last seven days and the proportion of participants reporting inconsistent condom use in the last month with main partners and clients by study visit among all women enrolled in the demonstration project. We also calculated the proportion of swabs with detectable Yc-DNA by study visit. We estimated 95% confidence intervals for proportions using the Wilson score interval to constrain limits between 0 and 1.27 We estimated trends in sexual behavior over time using generalized estimating equations assuming an exchangeable working correlation structure to account for repeated measures on participants.28 We used a binomial model with a logit link to model dichotomous outcomes (condom use and Yc-DNA detection) and a Gaussian model with identity link to model the number of clients reported in the last week. All models included month since initiation as a linear predictor and were adjusted for age, education, ethnic group, and clinic site. To evaluate changes in sexual behavior over time since initiation, we tested whether the estimated coefficient on the month since initiation variable was statistically significantly different from zero (linear models) or one (logistic models).
To compare Yc-DNA detection by reported partnerships, we stratified the proportion of swabs with detectable Yc-DNA by reported sex with a client (in the seven days prior to swab collection), main partner (in the month prior to swab collection), or both. To compare Yc-DNA detection by reported condom use, we calculated the proportion of swabs with detectable Yc-DNA by condom use in the last month with clients, main partners, or both, as well as condom use at most recent sex with clients, main partners, or both. We assessed condom use with main partners in the last month only among those reporting at least one main partner in the last month; since the number of clients was ascertained in the prior week, we did not perform a similar restriction for condom use with clients in the last month. We also compared Yc-DNA detection across baseline demographic and socioeconomic measures hypothesized a priori as potential predictors of condomless sex frequency. We estimated odds ratios (OR) and 95% confidence intervals (CI) using generalized estimating equations assuming an exchangeable working correlation structure. All analyses were conducted using R version 3.6.1.
RESULTS:
Study Population
The demographic and baseline characteristics of the 267 participants enrolled in the PrEP Demonstration Project are shown in Table 1. Sixty-four percent of participants were legally registered sex workers, 41% were of Wolof ethnicity, and 58% reported two or fewer clients in the prior week. Participants reported high levels of condom use at initiation (93% and 66% reported always using condoms in the last month with clients and main partners, respectively). The majority of participants reported feeling “very confident” or “confident” in their ability to use condoms the next time they had sex with clients (90%) and main partners (89%), respectively. Of the 40 percent of participants who received gonorrhea and chlamydia testing at least once during the study period, 7.5% (8/106) tested positive for gonorrhea and 7.5% (8/106) tested positive for chlamydia. Thirty-four of 221 women (15.4%) tested had a positive TPHA test at either baseline or at any point throughout the study period. The distribution of demographic and baseline characteristics among the subsample of participants for Yc-DNA testing was similar to that of all the participants in the demonstration project (Supplemental Digital Content, Table S3).
Table 1:
Demographic and baseline characteristics of all female sex workers participants enrolled in PrEP Demonstration Project (N = 267)
| Age (Median (IQR)) | 38 | (13) |
| Born in Senegal (%) | 263 | (99%)* |
| Registered sex worker (%)1 | 170 | (64%) |
| Site (N (%)) | ||
| Diamniadio | 76 | (28%) |
| Mbao | 52 | (19%) |
| Pikine | 73 | (27%) |
| Rufisque | 66 | (25%) |
| Ethnic Group (N (%)) | ||
| Wolof | 110 | (41%) |
| Fula (Pulaar) | 68 | (25%) |
| Serer | 42 | (16%) |
| Mandinka/Bamabara | 21 | (8%) |
| Jola (Diola) | 5 | (2%) |
| Manjak | 5 | (2%) |
| Soninke | 7 | (3%) |
| Other | 9 | (3%) |
| Number of clients during prior week (N (%))2 | ||
| 0 | 63 | (26%) |
| 1–2 | 76 | (32%) |
| 3+ | 101 | (42%) |
| Reports at least one main partner in prior six months (N (%))3 | 180 | (71%) |
| Condom use with clients in the last month for vaginal or anal sex (N (%))4 | ||
| Always | 228 | (93%) |
| Almost always | 10 | (4%) |
| Sometimes | 5 | (2%) |
| Almost never | 2 | (1%) |
| Never | 0 | (0%) |
| Condom use with main partner in the last month for vaginal or anal sex among participants reporting a main partner (N (%))5 | ||
| Always | 111 | (66%) |
| Almost always | 17 | (10%) |
| Sometimes | 9 | (5%) |
| Almost never | 6 | (4%) |
| Never | 25 | (15%) |
| Confidence in ability to use condom during the next time having sex with clients (N (%))6 | ||
| Very confident | 197 | (79%) |
| Confident | 28 | (11%) |
| Less confident | 20 | (8%) |
| Not at all confident | 3 | (1%) |
| Confidence in ability to use condom during the next time having sex with main partner, among participants reporting a main partner (N (%))7 | ||
| Very confident | 113 | (71%) |
| Confident | 28 | (18%) |
| Less confident | 13 | (8%) |
| Not at all confident | 5 | (3%) |
4 FSW born outside Senegal (Cape Verde, Mali, Burkina Faso, Guinea)
Number missing:
n=1
n=27
-n=14
n=22
n=12
n=19
n=21
Trends in sexual behavior
Figure 1 displays trends in self-reported sexual behavior from initiation to study exit at month 12 among all FSW participating in the demonstration project. The proportion of participants reporting inconsistent condom use with clients over the last month decreased from initiation (6.9%; 95% CI: [4.4%, 10.8%]) to month 12 (1.8%; 95% CI: [0.6%, 5.3%]). Similarly, inconsistent condom use with main partners also decreased from initiation (33.7%; 95% CI: [27.1%, 41.1%]) to month 12 (25.7%; 95% CI: [18.4%, 34.6%]). The average number of clients reported in the last week was 4.7 at initiation (95% CI: [3.8, 5.6]) and 4.3 at month 12 (95% CI: [3.6, 5.1]). Figure 2 shows the proportion of swabs with Yc-DNA detected by month since initiation. The prevalence of detectable Yc-DNA among tested swabs was similar at initiation (18.2%; 95% CI: [8.6, 34.4%]) and at month 12 (18.9%; 95% CI: [9.5%, 34.2%]).
Figure 1:
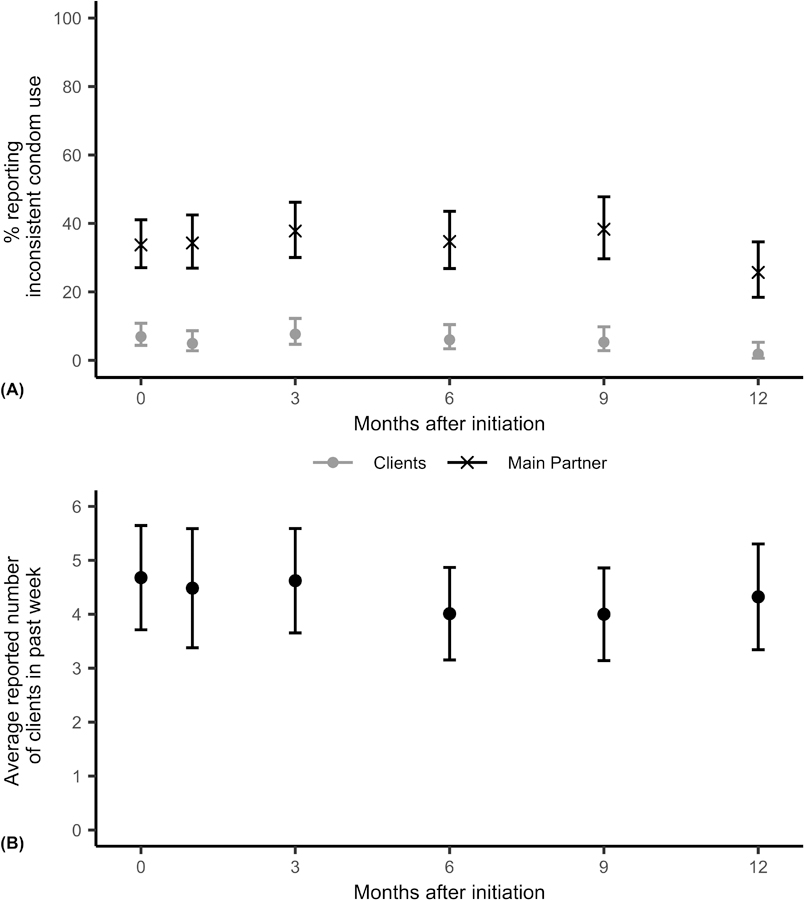
Trends in self-reported sexual behavior among female sex workers participating in the Senegal PrEP demonstration project. Error bars represent 95% confidence intervals. Relationship between outcome and month since initiation estimated from adjusted regression models fit using generalized estimating equations assuming an exchangeable working correlation structure. (A) Percent of participants reporting inconsistent condom usage in the last month with clients (grey circles) and main partners (black Xs). Inconsistent condom use is defined as not reporting always using condoms. Clients: odds ratio = 0.94 (95% confidence interval: [0.89, 1.00], p = 0.053); Main Partner: odds ratio = 0.99 (95% confidence interval: [0.96, 1.02], p = 0.53). (B) Average number of reported clients in the last seven days among participants. Beta = −0.04 (95% confidence interval: [−0.12, 0.044], p = 0.35).
Figure 2:
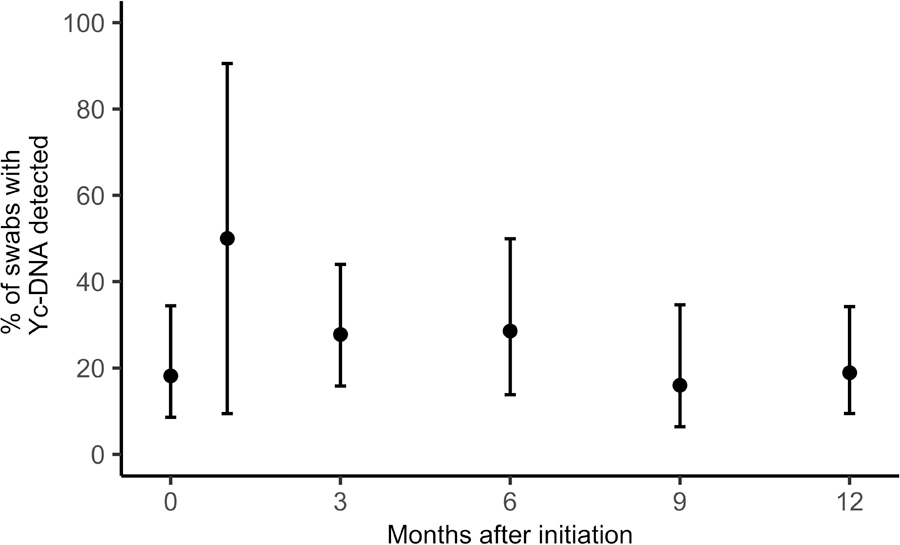
Trends in Y-chromosomal DNA (Yc-DNA) detection among female sex workers participating in the Senegal PrEP demonstration project. Error bars represent 95% confidence intervals. Number of swabs tested: Initiation: n=33; Month 1: n=2; Month 3: n=36; Month 6: n=21; Month 9: n=25; Month 12: n=37. Relationship between Yc-DNA detection and month since initiation estimated from adjusted regression models fit using generalized estimating equations assuming an exchangeable working correlation structure. Odds ratio = 0.99 (95% confidence interval: [0.90, 1.08], p = 0.82).
In adjusted logistic regression models, we estimated the odds ratios of inconsistent condom use for a one month increase in time since PrEP initiation. The odds of inconsistent condom use with clients decreased over time (OR = 0.94, 95% CI: [0.89, 1.00], p = 0.053), while the odds of inconsistent condom use with main partners was stable over time (OR = 0.99, 95% CI: [0.96, 1.02]. p = 0.55). The estimated change in number of reported clients for a one month increase in time since PrEP initiation was negative (β = −0.040, 95% CI: [−0.12, 0.044], p = 0.35), though this difference was not statistically significant. The median number of clients reported in the past seven days did not change over time (Supplemental Digital Content, Figure S3). We found no evidence of changes in the odds of Yc-DNA detection over time (OR = 0.99, 95% CI: [0.90, 1.08], p = 0.82).
Y-chromosomal DNA detection
Thirty-one women of 121 women tested had at least one swab with detectable Yc-DNA (25.6%; 95%CI: [18.7, 34.1%]), and Yc-DNA was detected in 34 of 154 swabs overall (22.1%; 95% CI: [16.3%, 29.3%]). Table 2 displays the frequency of Yc-DNA detection stratified by self-reported partnerships and condom use. Yc-DNA detection was more common among participants who reported having had sex with a main partner in the last month compared to those who did not (28% vs. 14%), but the estimated odds ratio was not statistically significantly different from one (OR = 2.10; 95% CI: [0.84, 5.30], p = 0.11). Yc-DNA detection did not differ by whether participants reported having had sex with a client in the last week (OR = 1.00). Nearly all (96%) of participants reported always using condoms with clients in the month prior to swab collection, but 22% of samples from these participants had detectable Yc-DNA. Of 58 participants who reported always using condoms with both their main partner and clients in the last month, 15 (26%) had swabs with detectable Yc-DNA. The odds of Yc-DNA detection did not differ significantly by whether or not participants reported inconsistent condom use in the last month with clients (OR = 0.75; 95% CI: [0.10, 5.90], p = 0.78), main partners (OR = 0.78; 95% CI: [0.25, 2.43], p = 0.67), or both (OR = 0.80; 95% CI: [0.25, 2.55], p = 0.71). Results were similar when condom use was assessed at most recent sex rather than over the last month. In addition, none of the other variables we evaluated (age, education, ethnic group, FSW registration status, and site) were statistically significantly associated with Yc-DNA detection in unadjusted analyses (see Supplemental Digital Content, Table S5).
Table 2:
Y-chromosomal DNA (Yc-DNA) detection stratified by self-reported sexual partners and condom use (N = 154*)
| Reported sexual
partners | |||
|---|---|---|---|
| Reports sex with client in last 7 days1 | n detected/N analyzed (%) | Odds ratio‡ [95% Conf. int.] | p-value |
| Yes | 25/108 (23%) | 1.00 [0.42, 2.40] | 0.99 |
| No | 8/37 (22%) | - | |
| Reports sex with main partner in last month2 | |||
| Yes | 25/91 (28%) | 2.10 [0.84, 5.30] | 0.11 |
| No | 8/56 (14%) | - | |
| Reports sex with either main partner in last month or client in last 7 days3 | |||
| Yes | 30/131 (23%) | 0.81 [0.24, 2.76] | 0.73 |
| No | 3/14 (21%) | - | |
| Condom use in prior
month | |||
| Condom use with clients4 | n detected/N analyzed (%) | Odds ratio‡ [95% Conf. int.] | p-value |
| Not Always | 1/6 (17%) | 0.75 [0.095, 5.90] | 0.78 |
| Always | 31/140 (22%) | - | |
| Condom use with main partner†,5 | |||
| Not Always | 8/31 (26%) | 0.78 [0.25, 2.43] | 0.67 |
| Always | 16/60 (27%) | - | |
| Condom use with both main partner and clients†,6 | |||
| Not Always | 8/31 (26%) | 0.80 [0.25, 2.55] | 0.71 |
| Always | 15/58 (26%) | - | |
| Condom use at most recent
sex | |||
| Used a condom with client7 | n detected/N analyzed (%) | Odds ratio‡ [95% Conf. int.] | p-value |
| No | 1/3 (33%) | 1.75 [0.15, 20.04] | 0.65 |
| Yes | 31/139 (22%) | - | |
| Used a condom with main partner†,8 | |||
| No | 7/26 (27%) | 1.06 [0.42, 2.65] | 0.91 |
| Yes | 17/64 (27%) | - | |
| Used a condom with both client and main partner†,9 | |||
| No | 7/26 (27%) | 1.01 [0.40, 2.55] | 0.98 |
| Yes | 17/62 (27%) | - | |
154 swabs collected from 121 unique participants
Only among participants reporting at least one main partner in last month
Odds ratios of Yc-DNA detection estimated from unadjusted logistic regression model fit using generalized estimating equations assuming an exchangeable correlation structure
Number missing:
n=9
n=7
n=9
n=8
n=2
n=4
n=12
n=3
n=5.
DISCUSSION:
Our objectives were to assess changes in sexual behavior after PrEP initiation, to compare self-reported measures with Yc-DNA detection among FSW in Senegal, and to assess predictors of Yc-DNA detection. We found no evidence of risk compensation after PrEP initiation as measured by changes in number of clients, condom use, or Yc-DNA detection. While prior studies in Africa have shown that FSW may charge more for condomless sex, and a discrete choice experiment among FSW in South Africa predicted that PrEP use would more than double the frequency of condomless sex, our results are consistent with previous studies in PrEP demonstration projects among FSW in South Africa and Benin that have shown no evidence of risk compensation.5,8,11,22,29,30 A recent analysis of a cohort of FSW initiating PrEP in Benin found no changes in the frequency of PSA or Yc-DNA detection over 24 months since initiation.22 Several reasons could explain the lack of risk compensation observed in our study. It is possible that changes in risk behavior may not manifest within the first year of follow-up during PrEP demonstration projects; alternatively, risk reduction counseling and condom provision during PrEP delivery may counteract any perceived economic incentives for engaging in condomless sex. Furthermore, since condoms protect against other STIs and unwanted pregnancy, PrEP use may not have impacted participants’ desire to use condoms. Prior research has established a relationship between sexual violence and condomless sex that is partially mediated by difficulties in condom negotiation.31 If FSW have little control over condom use, then PrEP use may have little impact on the frequency of condomless sex. While difficulty negotiating condom use is an important consideration, study participants reported high confidence in their ability to use condoms with clients and main partners, suggesting that participants in our study had considerable agency in determining their frequency of condomless sex. As PrEP is rolled out to FSW in resource-limited settings, ongoing assessments of HIV acquisition, STIs and risk behavior will be needed.
Despite high levels of reported condom use, Yc-DNA was detected in 22% of swabs tested, and self-reported condom use, partnerships, and demographic characteristics did not predict Yc-DNA detection. Prior studies among sex workers in sub-Saharan Africa have not identified consistent predictors of misreporting as compared with prostate-specific antigen detection.32–34 In our study, Yc-DNA was detectable in 26% of swabs from participants reporting consistent condom use in the past month with both clients and main partners. This discrepancy may be explained by several factors. Participants may have overreported condom use due to fear of judgment or stigma by health care workers and study staff.35 Incorrect condom usage or condom failure could have resulted in unnoticed semen exposure detected by the Yc-DNA assay.19 Our measures of reported condom use may not adequately capture non-consensual condom removal or forced breakage, which have been reported in other settings.36 In addition, participants may have misinterpreted the questions or misremembered their condom use. The accuracy of self-reported sexual behavior tends to decrease with longer recall periods or when behavior frequency is high.13 These results warrant caution in the use of self-reported sexual behavior for assessing individual-level HIV risk.
Our study builds on prior research of risk compensation by assessing changes in objective biomarker-based measures of sexual behavior over time since PrEP initiation. However, our analysis has several limitations. Our sample size for Yc-DNA analysis was limited by funding and reduced our power to detect predictors of Yc-DNA detection or trends in Yc-detection over time. As condom use was assessed in the month prior to swab collection, and the sensitivity of Yc-DNA assays is limited beyond two weeks after semen exposure, the level of underreporting may be underestimated in our study. Oral sex and/or digital vaginal penetration by clients or main partners may have left traces of Yc-DNA detectable by vaginal swabs, potentially explaining some of the positive Yc-DNA results among women reporting consistent condom use.17 We did not assess menstruation or female genital hygiene practices, such as vaginal washing or douching, that potentially could have affected assay performance. While we ascertained sexual behavior among clients and main partners, we did not ascertain whether participants had other types of partnerships. Our estimate of underreporting may be too high if these partnerships are common and if participants who reported consistent condom use with both clients and main partners would have reported not using a condom with other types of partners. Last, our results may not be generalizable to FSW in other settings or in routine PrEP delivery outside of demonstration projects, where factors influencing condom use, partnerships, and sexual behavior reporting could differ. Nevertheless, these results highlight the limitations of using self-reported measures to assess sexual risk behaviors in this population.
In our study of PrEP initiation among FSW in Senegal, we found no evidence of risk compensation using self-reported condom use, self-reported number of clients, or by Yc-DNA detection. However, condomless sex as measured by detectable Yc-DNA in vaginal swabs was common among FSW who self–reported consistent condom use. Studies that rely on accurate measurement of sexual behavior should consider using biomarker measures when feasible. Additional efforts are needed to improve the accuracy of self-reported sexual behavior data.
Supplementary Material
ACKNOWLEDGEMENTS:
We would like to thank the study participants for their dedication to this project. We thank the clinicians and staff at the participating FSW clinics in Diamniadio, Mbao, Pikine & Rufisque. We thank the Conseil National de Lutte Contre Le SIDA Du Sénégal (CNLS) and Division de Lutte contre le SIDA et les IST (DLSI) for their support. We thank Keith Jerome, Meei-Li Huang and Stacy Selke for Yc-DNA testing (UW Laboratory Medicine). The Senegal PrEP Demonstration Project team includes (alphabetical order): Bill & Melinda Gates Foundation: Mary Aikenhead, Josie Presley, and Papa Salif Sow; Institut de Recherche en Santé de Surveillance Epidémiologique et de Formations: Mame D. Bousso Bao, Saly Amos Diatta, Ousmane Diouf, Daouda Gueye, Coumba Toure Kane, Moustapha Mane, Aminata Mboup, Souleymane Mboup, Anna Julienne Ndiaye, Birahim Pierre Ndiaye, and Ibrahima Traoré; Senegal’s Ministry of Health and government: Aichatou Barry, Diambogne Ndour, Cheikh Tidiane Ndour, Bouna Sall, Cheikh Saadibou Senghor; Mbaye Thiam, and Safiatou Thiam; University of Washington, Seattle: Geoffrey S. Gottlieb, Stephen E. Hawes; Westat: Victoria Kioko, Fatima D. Jones, Moussa Sarr, and Carlos Suarez.
Conflicts of Interests and Sources of Funding:
GSG has received research grants and support from the US National Institutes of Health, University of Washington, Bill & Melinda Gates Foundation, Gilead Sciences, Alere Technologies, Merck & Co., Inc., Janssen Pharmaceutica, Cerus Corporation, ViiV Healthcare, Bristol-Myers Squibb, TheraTechnologies/TaiMed Biologics and Abbott Molecular Diagnostics. SEH has received research grants and support from the US National Institutes of Health, the University of Washington, and the Bill & Melinda Gates Foundation. None of the remaining authors had potential conflicts of interests to disclose. This research was supported by the Bill & Melinda Gates Foundation (OPP1084414). Gilead Sciences provided tenofovir/emtricitabine.
Footnotes
Parts of data presented at CROI 2019 (Seattle, WA, March 2019)
REFERENCES:
- 1.UNAIDS. Global HIV & AIDS Statistics: 2018 Fact Sheet [Internet]. 2018. [cited 2019 Apr 11]. Available from: http://www.unaids.org/en/resources/fact-sheet
- 2.World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. 2016. [PubMed]
- 3.World Health Organization. Consolidated guidelines on HIV prevention, diagnosis, treatment and care for key populations. 2014. 159 p. [PubMed]
- 4.Wang C, Hawes SE, Gaye A, Sow PS, Ndoye I, Manhart LE, et al. HIV prevalence, previous HIV testing, and condom use with clients and regular partners among Senegalese commercial sex workers. Sex Transm Infect 2007. December 1;83(7):534–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Quaife M, Vickerman P, Manian S, Eakle R, Cabrera-Escobar MA, Delany-Moretlwe S, et al. The effect of HIV prevention products on incentives to supply condomless commercial sex among female sex workers in South Africa. Health Econ 2018. October 1;27(10):1550–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grant H, Mukandavire Z, Eakle R, Prudden H, Gomez BG, Rees H, et al. When are declines in condom use while using PrEP a concern? Modelling insights from a Hillbrow, South Africa case study. J Int AIDS Soc 2017;20(1):21744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mugwanya KK, Donnell D, Celum C, Thomas KK, Ndase P, Mugo N, et al. Sexual behaviour of heterosexual men and women receiving antiretroviral pre-exposure prophylaxis for HIV prevention: a longitudinal analysis. Lancet Infect Dis 2013. December;13(12):1021–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mboup A, Béhanzin L, Guédou FA, Geraldo N, Goma-Matsétsé E, Giguère K, et al. Early antiretroviral therapy and daily pre-exposure prophylaxis for HIV prevention among female sex workers in Cotonou, Benin: a prospective observational demonstration study. J Int AIDS Soc 2018. November 1;21(11):e25208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gust DA, Soud F, Hardnett FP, Malotte CK, Rose C, Kebaabetswe P, et al. Evaluation of Sexual Risk Behavior Among Study Participants in the TDF2 PrEP Study Among Heterosexual Adults in Botswana. JAIDS J Acquir Immune Defic Syndr 2016. December 15;73(5):556–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maljaars LP, Gill K, Smith PJ, Gray GE, Dietrich JJ, Gomez GB, et al. Condom migration after introduction of pre-exposure prophylaxis among HIV-uninfected adolescents in South Africa: A cohort analysis. South Afr J HIV Med 2017. September 21;18(1):712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eakle R, Gomez GB, Naicker N, Bothma R, Mbogua J, Cabrera Escobar MA, et al. HIV pre-exposure prophylaxis and early antiretroviral treatment among female sex workers in South Africa: Results from a prospective observational demonstration project. Bekker L-G, editor. PLOS Med 2017. November 21;14(11):e1002444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Treibich C, Lépine A. Estimating misreporting in condom use and its determinants among sex workers: Evidence from the list randomisation method. Health Econ 2019. January 1;28(1):144–60. [DOI] [PubMed] [Google Scholar]
- 13.Fenton KA, Johnson AM, McManus S, Erens B. Measuring sexual behaviour: methodological challenges in survey research. Sex Transm Infect 2001. April 1;77(2):84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Snead MC hristin., Black CM, Kourtis AP. The use of biomarkers of semen exposure in sexual and reproductive health studies. J Womens Health (Larchmt) 2014. October 1;23(10):787–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rattray C, Wiener J, Legardy-Williams J, Costenbader E, Pazol K, Medley-Singh N, et al. Effects of initiating a contraceptive implant on subsequent condom use: A randomized controlled trial. Contraception. 2015. December 1;92(6):560–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zenilman JM, Yuenger J, Galai N, Turner CF, Rogers SM. Polymerase chain reaction detection of Y chromosome sequences in vaginal fluid: preliminary studies of a potential biomarker for sexual behavior. Sex Transm Dis 2005. February;32(2):90–4. [DOI] [PubMed] [Google Scholar]
- 17.Ghanem KG, Melendez JH, McNeil-Solis C, Giles JA, Yuenger J, Smith TD, et al. Condom Use and Vaginal Y-Chromosome Detection: The Specificity of a Potential Biomarker. Sex Transm Dis 2007. February;PAP. [DOI] [PubMed]
- 18.Penrose KJ, Richardson BA, Besson G, Dezzutti CS, Herold BC, Abdool Karim SS, et al. Y Chromosome and HIV DNA Detection in Vaginal Swabs as Biomarkers of Semen and HIV Exposure in Women. Sex Transm Dis 2014. November;41(11):674–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jamshidi R, Penman-Aguilar A, Wiener J, Gallo MF, Zenilman JM, Melendez JH, et al. Detection of two biological markers of intercourse: prostate-specific antigen and Y-chromosomal DNA. Contraception. 2013. December;88(6):749–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heffron R, Parikh UM, Penrose KJ, Mugo N, Donnell D, Celum C, et al. Objective Measurement of Inaccurate Condom Use Reporting Among Women Using Depot Medroxyprogesterone Acetate for Contraception. AIDS Behav 2017. July;21(7):2173–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giguère K, Béhanzin L, Guédou FA, Leblond FA, Goma-Matsétsé E, Zannou DM, et al. Biological Validation of Self-Reported Unprotected Sex and Comparison of Underreporting Over Two Different Recall Periods Among Female Sex Workers in Benin. Open Forum Infect Dis 2019. February 1;6(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giguère K, Béhanzin L, Guédou FA, Talbot D, Leblond FA, Goma-Matsétsé E, et al. PrEP Use Among Female Sex Workers. JAIDS J Acquir Immune Defic Syndr 2019. July;1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ngugi EN, Roth E, Mastin T, Nderitu MG, Yasmin S. Female sex workers in Africa: Epidemiology overview, data gaps, ways forward. SAHARA J 2012;9(3):148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Diouf O, Sarr M, Gueye D, Mane M, Mboup A, Toure Kane C, et al. Retention in care for HIV pre-exposure prophylaxis (PrEP) among sex workers of four public health centers in Senegal. In: 22nd International AIDS Conference Amsterdam; 2018. [Google Scholar]
- 25.Casper C, Krantz E, Selke S, Kuntz SR, Wang J, Huang M, et al. Frequent and Asymptomatic Oropharyngeal Shedding of Human Herpesvirus 8 among Immunocompetent Men. J Infect Dis 2007. January 1;195(1):30–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.ThermoFisher Scientific. Quantifiler™ Duo DNA Quantification Kit User Guide 2018.
- 27.Wilson EB. Probable Inference, the Law of Succession, and Statistical Inference. J Am Stat Assoc 1927. June;22(158):209. [Google Scholar]
- 28.Fitzmaurice GM, Laird NM, Ware JH. Applied longitudinal analysis. Wiley; 2011. 701 p. [Google Scholar]
- 29.Robinson J, Yeh E. Transactional Sex as a Response to Risk in Western Kenya. Am Econ J Appl Econ 2011. January;3(1):35–64. [Google Scholar]
- 30.George G, Nene S, Beckett S, Durevall D, Lindskog A, Govender K. Greater risk for more money: the economics of negotiating condom use amongst sex workers in South Africa. AIDS Care. 2019. January 7;1–4. [DOI] [PubMed]
- 31.Wirtz AL, Schwartz S, Ketende S, Anato S, Nadedjo FD, Ouedraogo HG, et al. Sexual violence, condom negotiation, and condom use in the context of sex work: Results from two West African countries. J Acquir Immune Defic Syndr 2015. March 1;68:S171–9. [DOI] [PubMed] [Google Scholar]
- 32.Gallo MF, Behets FM, Steiner MJ, Thomsen SC, Ombidi W, Luchters S, et al. Validity of self-reported “safe sex” among female sex workers in Mombasa, Kenya—PSA analysis. Int J STD AIDS 2007. January 25;18(1):33–8. [DOI] [PubMed] [Google Scholar]
- 33.Gallo MF, Steiner MJ, Hobbs MM, Weaver MA, Hoke TH, Van Damme K, et al. Predictors of unprotected sex among female sex workers in madagascar: Comparing semen biomarkers and self-reported data. AIDS Behav 2010. December;14(6):1279–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aho J, Koushik A, Diakité SL, Loua KM, Nguyen V-K, Rashed S. Biological Validation of Self-Reported Condom Use Among Sex Workers in Guinea. AIDS Behav 2010. December 13;14(6):1287–93. [DOI] [PubMed] [Google Scholar]
- 35.Guest G, Bunce A, Johnson L, Akumatey B, Adeokun L. Fear, hope and social desirability bias among women at high risk for HIV in West Africa. J Fam Plan Reprod Heal care. 2005. October 1;31(4):285–7. [DOI] [PubMed] [Google Scholar]
- 36.Eakle R, Bothma R, Bourne A, Gumede S, Motsosi K, Rees H. “I am still negative”: Female sex workers’ perspectives on uptake and use of daily pre-exposure prophylaxis for HIV prevention in South Africa. Rendina HJ, editor. PLoS One. 2019. April 9;14(4):e0212271. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


