Abstract
Although neuronal communication is thought to be summated within local dendritic segments, no technique is currently available to monitor activity in vivo at this level of resolution. To overcome this challenge, we developed an optical reporter of neuronal activity utilizing the coding sequence of Venus, flanked by short stretches of the 5' and 3' untranslated regions from CAMKIIα. This reporter takes advantage of the fact that CAMKIIα mRNA is transported to the dendrite and locally translated in an activity dependent manner (Aakalu et al., 2001). Using adeno associated virus (AAV), we used this reporter to study neuronal activity in adult mice. Exposure of the mice to an enriched environment led to enhancement of Venus expression in dendritic segments of somatosensory cortex, demonstrating in vivo that dendritic mRNA translocation and local transcription occur in response to physiologically relevant stimuli. We then utilized this system to examine the impact of Alzheimer related local amyloid-β deposits on neural system function, to test the hypothesis that plaques are toxic. In APPswe/PS1d9 (APP/PS1) mice, neurons close to plaques, and dendritic segments close to plaques, both showed diminished fluorescent intensity and therefore neuronal activity. In contrast to wildtype mice, fluorescent intensity in neurons near plaques in transgenic mice did not increase after environmental enrichment. These data indicate that neuronal activity in dendritic segments and neurons in the vicinity of a plaque is decreased compared to wildtype mice, supporting the idea that plaques are a focal lesion leading to impaired neural system function.
Keywords: Alzheimer’s disease, senile plaque, neuritic alterations, APP/PS1 transgenic mice, Adeno associated virus, calcium/calmodulin-dependent kinase II
Introduction
In vivo functional studies of neurons to date have either relied on population characteristics (e.g. LTP phenomenon) or reflect signal integration at the level of the cell body (e.g. sharp electrode studies; calcium imaging). However, much signal integration occurs at the level of the dendritic segment, yet this cell compartment is difficult to interrogate using physical methods. We report herein an optical method designed to detect activity levels in individual dendritic segments, as a first step towards higher resolution mapping of structure-function relationships in intact neural systems, and as a means of exploring the functional consequences of local pathological changes. The probe design is based on the observation that some messenger RNA’s are translocated to the dendrite and locally translated in an activity dependent fashion. One of the best characterized of these is calcium/calmodulin-dependent kinase II (CAMKIIα), whose protein is highly enriched at synapses, is regulated by neural activity, and is important in synaptic plasticity, learning and memory (Mayford et al., 1996). The 3’ and 5’ untranslated region (UTR) of CAMKIIα encode sequences that direct translocation to the dendrite and prevent translation unless the neuron is active (Mori et al., 2000). Compelling evidence from cell culture experiments support the hypothesis that new protein synthesis can appear in dendrites after a given stimulus (Aakalu et al., 2001; Sutton et al., 2004; Smith et al., 2005), but this has not been examined in the intact brain.
In order to establish methodology whereby the function of an individual dendrite can be evaluated we used a neuronal activity reporter in which the coding sequence of Venus is flanked by 5' and truncated 3' UTRs of CAMKIIα. A CAMKIIα UTR Venus adeno associated virus (AAV) was used to examine in vivo whether its synthesis in neurons and especially dendrites change in response to environmental enrichment. We found increased Venus expression after environmental stimulation, supporting the idea that the probe reflects in vivo neural system activation.
In Alzheimer Disease (AD) as well as in transgenic animal models of AD pathology, amyloid β (Aβ)-containing plaques are fibrillar deposits scattered throughout the neuropil in the cortex. Morphological studies have suggested that amyloid plaques disrupt neural systems by leading to abnormal axonal sprouting (Cotman et al., 1990; Phinney et al., 1999) and by distortion of the dendritic tree (Knowles et al., 1999; Le et al., 2001), yet the functional significance of these changes is unknown. By contrast, recent studies have challenged the idea that plaques are pathophysiological (Shankar et al 2008), and have even led to the suggestion that they are protective as a depot of otherwise more toxic soluble species of Aβ. We used the CAMKIIα Venus reporter to test the hypothesis that plaques focally disrupt neural system function in the cortex. In the setting of microscopic amyloid plaque lesions in APP/PS1 transgenic mice, the reporter revealed diminished baseline activity and diminished response to environmental enrichment in dendritic segments closest to the amyloid deposits. Our results indicate that neuronal activity is decreased in neurons of these transgenic mice and that their functional neuronal response to environmental stimulation is diminished in the vicinity of plaques.
Material and Methods
Cell culture
Primary neuronal cell cultures were prepared from mixed cortical-hippocampal neurons generated from CD1 mice at embryonic day 15–16. Cells were plated on poly-d-lysine-coated coverslipped 35-mm dishes (MatTek Cultureware, Ashland, MA) in Neurobasal media (Invitrogen) containing 10% fetal bovine serum. The neurons were maintained and allowed to mature in Neurobasal media containing 2% B27 supplement (Invitrogen) for 6–12 days before use. 1 week after plating the neurons they were transfected either with the pcDNA3.1-CAMKIIα-5’UTR-myr.eGFP-3’UTR, pcDNA3.1-CAMKIIα-5’UTR-myr.Venus-3’UTR construct or with pcDNA3.1-CAMKIIα-5’UTR-myr. Venus construct using lipofectamin2000 (Invitrogen) according to manufacturer’s protocol. 48 hours post- transfection, dishes were exposed either to BDNF (50 ng/ml) or left in their original media and 2–4 neurons were imaged per experiment with a Zeiss LSM510 Meta confocal microscope every 15 minutes up to 75 minutes. Cells were observed at 63x for quantification of pixel intensity. In all cell culture experiments, identical acquisition parameters and settings were used for both control and BDNF treated neurons on a given experimental day. During the experiments, cultured neurons were maintained at 37°C and 5% CO2 level over the entire 75 minutes duration of the experiment. Fluorescence intensity of manually outlined cell bodies and neurites was measured as a function of time. The intensity was further calculated by dividing the intensity at a given time point by the intensity at time point 0 (baseline level of time point 0 was set as 1). Statistical differences in reporter expression between groups were assessed by analysis of variance (ANOVA) in the StatView program (SAS Institute, Inc, Cary, NC) followed by Fisher’s LSD post hoc tests. The level of significance was set at p≤ 0.05.
Constructs
The CAMKIIα-5’UTR-myr.EGFP-3’UTR construct was a kind gift from Dr. Erin Schuman (Aakalu et al., 2001) and the sequence was verified using standard sequencing procedures. We first cloned Venus into the position of EGFP. The Venus fragment was created using the following PCR primers: 5’-ctagtctagagtgagcaagggcgaggagc-3’ (forward primer) and 5’-ttttccttttgcggccgcgaattcctacttgtacagctcgtc-3’ (reverse primer) and Venus was then digested with XbaI and EcoRI and ligated into the CAMKIIα-5’UTR-myr. EGFP-3’UTR construct with XbaI and NotI. To generate CAMKIIα-5’UTR-myr. Venus the above construct was digested with NotI and ApaI to excise the 3’UTR and the vector was religated using Klenow fragment and blunt end ligation.
Virus production
AAV (serotype 8) was generated using plasmid triple-transfection of the AAV vector plasmid, packaging (encoding rep and cap genes), and adenovirus miniplasmid into HEK293A cells. Viral particles were purified by iodixanol gradient. Concentrated virus was isolated and titered by dot blot hybridization yielding 6.1×1012 particles/ml (virus was kindly provided by the Harvard Gene Therapy Initiative, Harvard Medical School).
Animals and surgical procedures
APPswe/PS1d9 transgenic mice at 9–11 months of age (APP/PS1; obtained from Jackson lab, Bar Harbor, Maine) and nontransgenic control mice were used in this study (8 transgenic, 8 controls) (Jankowsky et al., 2004). APP/PS1 mice start to develop plaques in the neocortex between 5 and 7 months of age. All animal work conformed to NIH and institutional guidelines. For intracortical injections of adeno-associated virus (AAV) containing the gene for Venus, mice were anesthetized with avertin (1.3% 2,2,2-tribromethanol, 0.8%tert-pentylalcohol; 250 mg/kg) and placed in a stereotaxic apparatus. The surgical site was sterilized with betadyne and isopropyl alcohol, and an incision was made in the scalp along the midline. Burr holes were drilled in the scull 1mm lateral to bregma bilaterally. Using a Hamilton syringe, 3 µl of virus was injected 1 mm deep at a rate of 0.2 µl/min. After the injection, the scalp was sutured, and the mouse recovered from anesthesia on a heating pad.
After a 3 weeks incubation period to allow expression of Venus in neurons, half of the mice (n=4 per group) were assigned to novel environment exploration without showing any signs of motor deficits and behavioral differences between transgenic and nontransgenic mice. These mice were transferred to a new cage containing 3 objects and allowed to explore the new environment for 30 minutes before they went back to their home cages for 20 minutes before they were euthanized with an overdose of avertin (400 mg/kg), transcardially perfused with ice cold PBS and 4% PFA and the brains fixed in 4% paraformaldehyde in phosphate buffer with 15% glycerol cryoprotectant.
Immunohistochemistry
The brains were frozen and 30 µm-thick serial coronal sections were cut on a freezing sliding microtome. Sections were immunostained with the polyclonal antibody against GFP (ab 6556, 1:5000; Abcam) followed by secondary anti-rabbit conjugated to Alexa 488 (Molecular Probes). Images were acquired using a Zeiss LSM510 Meta laser scanning confocal microscope equipped with a 488nm argon laser and 543 helium/neon laser and a 720–930nm chameleon mode locked ti/saph laser. All images were acquired in 1 µm sections, and z-series were set to span the entire neuronal volume. Image analysis was conducted on z-compressed image stacks. Contrast and intensity parameters were kept constant across all image acquisitions. By necessity, some soma and proximal dendrites had saturated image intensity to allow visualization of the entire dendritic tree. This saturation effect will underestimate any observed effects of genotype or enrichment. For cortical postmortem intensity measurements 20 images of fields of Venus infected neurons were taken per animal and fluorescence intensity was measured using Adobe Photoshop 7 software (Adobe, San Jose, CA). Adobe Photoshop was used to convert the average GFP/Venus fluorescence of each neuron to average pixel intensity. Data were expressed as mean ± standard deviation from the mean. Group means were compared using Student T-test or analysis of variance (ANOVA) in the StatView program (SAS Institute, Inc, Cary, NC). The level of significance was set at p≤ 0.05.
Results
Neuronal activity can be measured via dendritic protein synthesis in cultured neurons
In order to measure neuronal activity in living neurons, we used a green fluorescent protein (GFP) reporter in which the coding sequence is flanked by the 5’ and 3’ untranslated regions of CAMKIIα (Aakalu et al., 2001). Previous work has shown that the 3’and 5’ UTR of the CAMKIIα mRNA contains information sufficient for its dendritic localization (Mayford et al., 1996; Mori et al., 2000) and that BDNF stimulates protein synthesis especially in dendrites (Aakalu et al., 2001). In an initial experiment we transfected primary neurons with the GFP reporter and measured fluorescent intensity over time after BDNF application to the media. In most untreated neurons expression of the reporter was relatively weak in the cell body and neurites (Fig. 1a). However, neurons that were exposed to BDNF exhibited an increase in GFP synthesis that was evident in both the cell body and the neurites within tens of minutes, confirming previously published work (Aakalu et al., 2001)(Fig. 1b,c). The presence or absence of a myristoylation site did not alter these effects (data not shown). In an effort to enhance the signal for in vivo experiments in the mouse brain we replaced GFP by Venus and sequentially decreased the size of the 3’UTR from 3100 bp to 420 bp. Venus is a variant of YFP and the brightest yellow variant to date (Nagai et al., 2002). A small (420 bp) 3’ UTR containing construct still responded to BDNF treatment (Fig. 1g,h) and was small enough to be efficiently packaged into AAV. As expected, untreated control neurons remained stable (Fig. 1d,e) but BDNF treated neurons had significantly higher intensity in cell bodies and neurites already evident after 30 minutes of BDNF exposure (Fig. 1f,i).
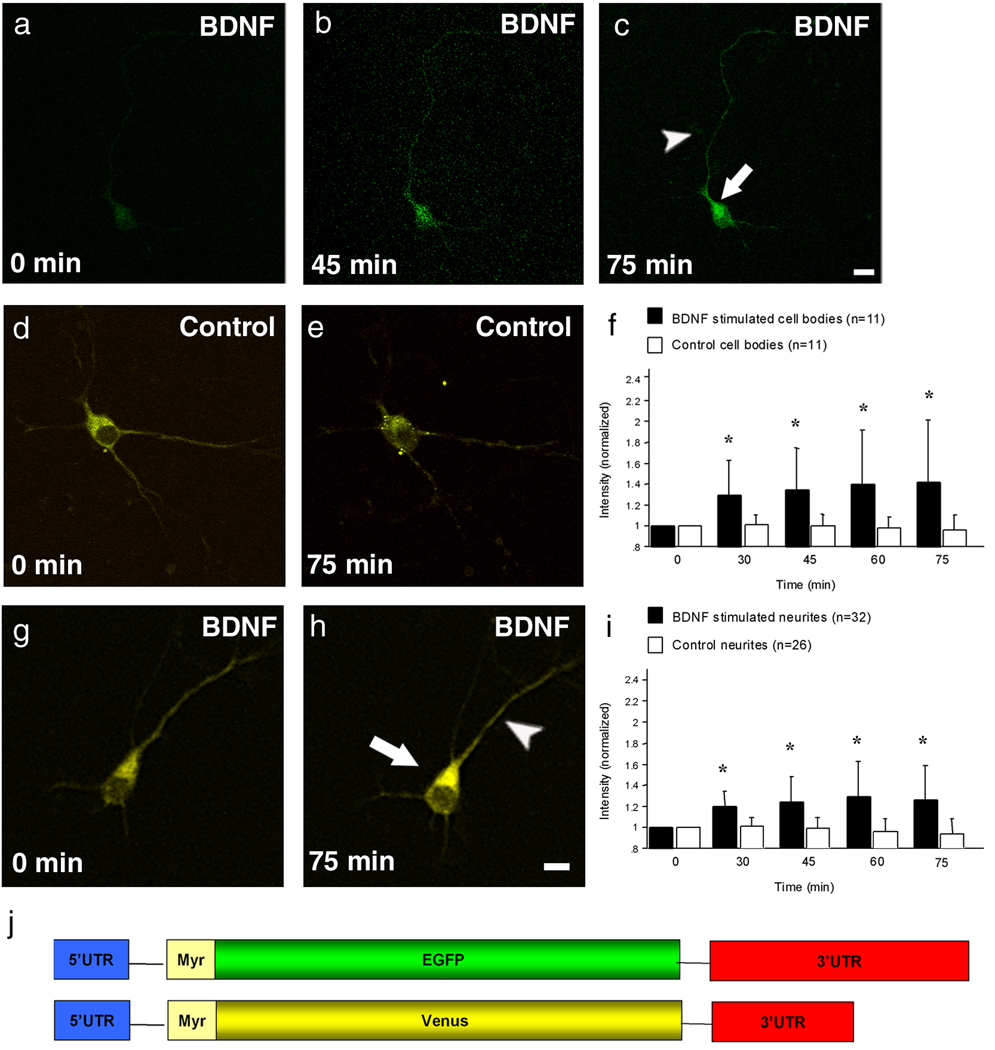
Figure 1. BDNF stimulates protein synthesis of the GFP and Venus reporter in primary neurons.
(a–c) Shown is a neuron transfected with 3’ UTR CAMKIIαEGFP 5’ UTR reporter and treated for 75 minutes with BDNF. Before BDNF application the GFP signal is very modest (a). After 45 and 75 minutes the fluorescent signal increases dramatically in the cell body (arrow) as well as in the neurites (arrowhead) (b,c).
(d,g) Shown is an untreated and BDNF treated cultured neuron expressing the Venus reporter. The BDNF treated cell shows enhanced fluorescence after 75 minutes in the cell body (arrow) and neurites (arrowhead) (h) in contrast to the untreated control cell (e). (f,i) Summary data for all untreated and BDNF treated cell bodies and neurites. The intensity remains unchanged in the control neurons and only changes in the stimulated neurons. Note that the intensity is significantly higher after only 30 minutes of BDNF stimulation in both the cell body (f) and neurites (i) and increases over time (*p<0.0001). (j) A schematic representation of the constructs used for this experiment.
Scale bars, 15 µm (a–c) and 15 µm (d,e,g,h).
To further characterize and determine the necessity of the 3’UTR in directing protein synthesis to the dendrites in an activity dependent manner we generated another construct completely missing the 3’UTR. In contrast to the above-mentioned effect of BDNF on transfected neurons those neurons were unresponsive and did not differ from untreated control neurons (supplemental Fig. 1) showing that the 3’UTR of CAMKIIα is a critical element in activity dependent neuronal protein synthesis.
Neurons from APP/PS1 mice have decreased neuronal activity and are unaffected by environmental stimulation
We next generated a 3’ UTR CAMKIIαVenus 5’ UTR AAV and performed intracortical injections into the somatosensory cortex of adult APP/PS1 and wildtype mice. APP/PS1 mice, a transgenic model of Alzheimer’s disease, overexpress mutant human APP and PS1, leading to the robust deposition of amyloid-β plaques. After an incubation time of 3 weeks that allowed viral transduction of neurons, half of the mice explored a new enriched environment for 30 minutes before the brains were analyzed postmortem by immunostaining to label Venus expressing neurons. Similar injection of 3’ UTR CAMKIIα Venus 5’ UTR AAV under identical conditions surprisingly led to decreased neuronal Venus fluorescence intensity in the vicinity of plaques when compared to wildtype neurons (Fig. 2 a,e). Moreover, neuronal cell bodies distant (>100 µm) from plaques were clearly brighter (p<0.0001, Fig. 2 c,g,h ). This effect was also observed, perhaps even more dramatically, in individual dendritic segments near a plaque. Dendritic segments closest to a plaque were substantially dimmer than adjacent segments on either side (p<0.0001, Fig. 3 a–c). Based on this finding we also tested whether the reporter would show a response if the transgenic mice were allowed to explore an enriched environment. In contrast to wildtype mice, neurons from APP/PS1 transgenic mice near plaques remained unaltered after environmental enrichment (Fig. 2b,g,h), whereas cell bodies distant from a plaque (>100 µm away) have a detectable response to environmental stimulation (Fig. 2d,g). Thus our data indicate that neurons in the vicinity of plaques have diminished neuronal function and are unresponsive to environmental stimulation, in contrast to neurons from the same mouse that are distant from a plaque, or, most obviously, from nontransgenic mice.
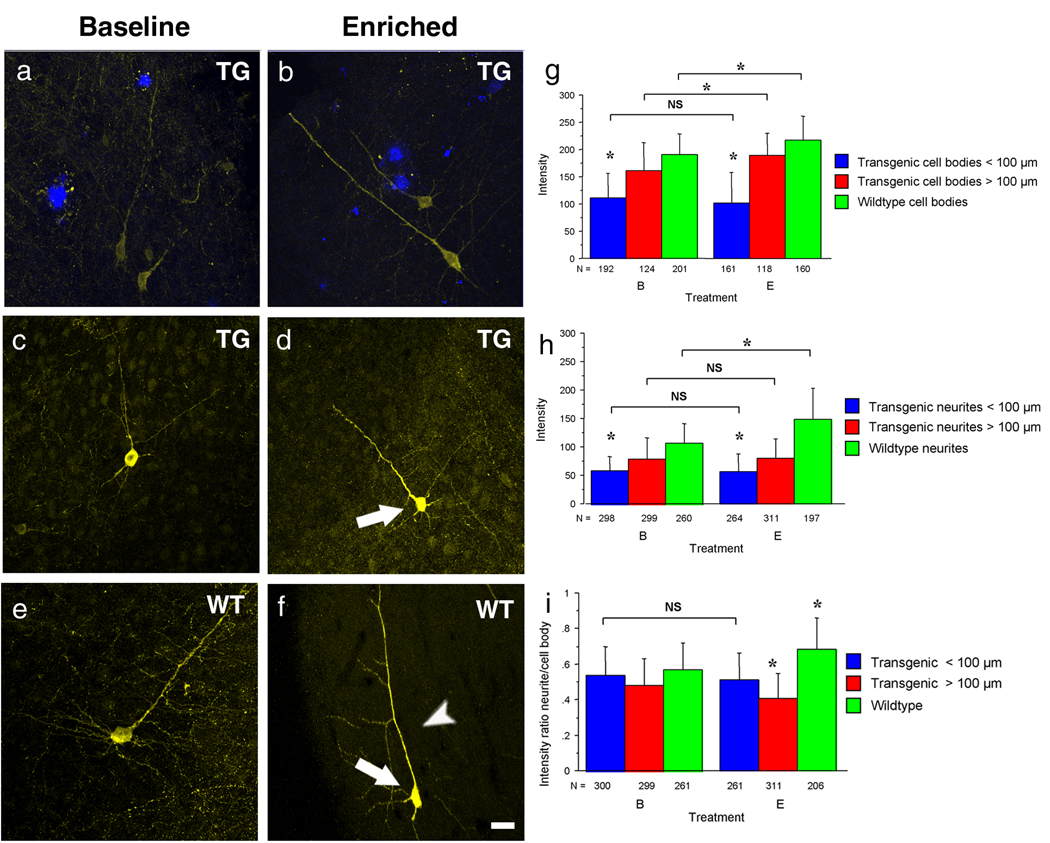
Figure 2. Fluorescence intensity measured postmortem is decreased in neurons surrounding plaques and remains unaltered after environmental stimulation.
(a–f) Representative neurons from wildtype and transgenic mice that differ in their fluorescence intensity. Fluorescence intensity from transgenic neurons in the vicinity of plaques (a, shown in blue) was greatly overall reduced compared to wildtype neurons and remained unchanged after environmental stimulation (b,e). Interestingly fluorescence intensity from neurons greater than 100 µm away from plaques (c) was significantly higher than those from close to plaques and increased in the cell body (arrow) after environmental stimulation (d). Note that the intensity in the wildtype neurons started at an even higher level (e) and increased further after enriched environment (f) in the cell body (arrow) as well as in neurites (arrowhead). (g,h,i) Intensity measurements confirmed that transgenic neurons had significantly decreased intensity (*p<0.0001) and only wildtype neurons increased intensity in both cell bodies (*p<0.0001) (G) as well as in neurites (*p<0.0001) (h) after environmental enrichment.
Scale bar, 20 µm (a–f).
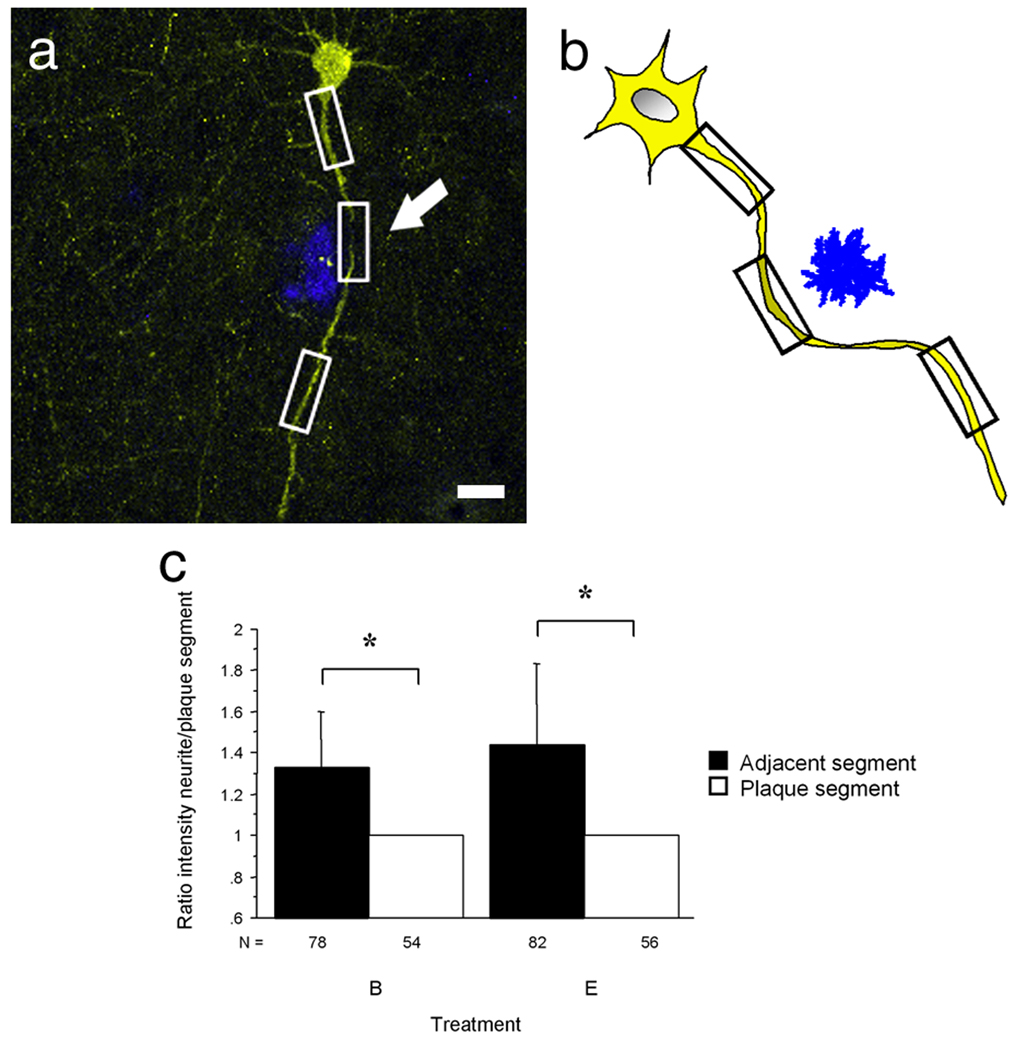
Figure 3. Individual dendritic segments in close proximity to a plaque have decreased neuronal activity.
(a,b) A neuron and a schematic illustration of a neuron filled with Venus in the vicinity of a plaque (shown in blue). White and black boxes mark the segments where intensity was measured. Note the decreased intensity in the segment close to the plaque indicated by the arrow. (c) Dendritic segments farther from the plaque had a statistically significant higher intensity when compared to the dendritic segments nearest to the plaque in unstimulated as well as in stimulated neurons (*p<0.0001).
Scale bar, 10 µm (a).
Discussion
In the current study, we re-engineered a reporter molecule previously shown to respond to BDNF application in primary neuronal culture to initially test the hypothesis that dendritic mRNA transport and local translation occur after behaviorally relevant stimuli in vivo. The 3’ and 5’ UTR of CAMKIIα encodes sequences that direct translocation to the dendrite and direct local translation (Aakalu et al., 2001; Sutton et al., 2004; Smith et al., 2005). In initial experiments we found that the proximal 420 bp of the 3’ UTR contains sequences that are critical for activity dependent protein synthesis.
Alzheimer’s disease is a neurodegenerative disorder that is characterized by severe memory impairment due, in part, to synaptic dysfunction. Amyloid plaques are deposited throughout the cortex, but the role of plaques in neurodegeneration and in neural system function is uncertain, and has recently been challenged by the idea that soluble forms of amyloid-β, rather than plaques, are the toxic species since soluble oligomers are sufficient to induce deficits in LTP and behavior in rodent models (Shankar et al., 2008). By contrast, plaques are associated with inflammatory responses, neuronal morphological alterations, and disturbances in local calcium homeostasis (D'Amore et al., 2003; Spires et al., 2005; Busche et al., 2008; Kuchibhotla et al., 2008; Meyer-Luehmann et al., 2008). We utilized the CAMKIIα UTR Venus AAV to test the hypothesis that plaques would be associated with dysfunction of neural system integration after behaviorally relevant stimuli at the level of the dendritic segment and cell body.
Surprisingly, we found only very limited neuronal Venus expression in plaque bearing APP/PS1 transgenic mice in contrast to the robust fluorescence in wildtype neurons. Neurons and dendrites distant from a plaque in the APP/PS1 cortex showed higher levels of the Venus reporter, arguing for a plaque specific effect on the microenvironment near an amyloid deposit. Our data argue that reduced reporter synthesis occurs in transgenic neurons as a result of diminished activation near plaques, rather than due to technical issues of reporter expression in transgenic animals, since (1) analysis showed that the least fluorescence intensity in transgenic dendrites occurs in the vicinity of plaques, so that the portion of the dendrite closest to the plaque has less Venus than the average of the segments proximal and distal to the plaque of the same dendrite; (2) neurons whose cell bodies are close to plaques have diminished reporter expression compared to neurons in the same mice only a 100 µm away, but distant from a plaque and (3) a differential response to environmental enrichment was observed in neurons close to or far from plaques.
APP transgenic mice show reduced expression of Arc and other immediate early genes known to be activity dependent (Dickey et al., 2004; Palop et al., 2005). We observed that environmental enrichment induced a stimulation of local dendritic reporter synthesis in the somatosensory cortex in wildtype mice. Although transgenic mice likewise explored the enriched environment, neurons and isolated dendritic segments near plaques failed to respond upon behavioral stimulation. The deficit in activation specifically in dendritic segments likely leads to impaired integration of somatosensory signaling, impaired reporter activity in the cell bodies and failure of broader neural system function as the impairment in dendritic integration plays out throughout the arborizations associated with these dendritic elements.
In summary, our data show that 1) mRNA translocates into dendrites and can be translated there in response to physiologic stimuli in vivo; 2) that this property can be utilized to monitor neural activity within individual dendritic segments, opening a new level of structure-function relationships to understand signal integration properties on a subcellular basis; 3) this tool also allowed us to explore the consequences of amyloid-β deposits on neural system function in a mouse model of Alzheimer disease. Amyloid plaques appear to be a focal lesion, around which there is impaired response, suggesting that plaques (and potentially elevated Aβ soluble species in equilibrium near plaques (Koffie et al., 2009)) directly impact neurons’ response to physiological stimuli. Taken together with recent observations showing alteration of neuronal calcium homeostasis near plaques, the presence of morphological axonal and dendritic alterations and dendritic spine loss specifically near plaques (D'Amore et al., 2003; Spires et al., 2005; Kuchibhotla et al., 2008; Meyer-Luehmann et al., 2008), and electrophysiological data implicating plaque-dependent disruption of neural system integration (Stern et al., 2004), we conclude that individual plaques serve as focal pathophysiological lesions with a wide ranging impact on nearby and distant neural systems. We postulate that numerous plaques thus impair neural system function by altering dendritic integration of information in a distributed fashion throughout the cortex, preventing the activation of otherwise intact neuronal structures. This construct may be the underlying basis of changes in neural system activation observed by fMRI studies of AD patients, and help explain the concordance between the pattern of altered activation in resting fMRI studies of AD patients and amyloid plaque deposition (Buckner et al., 2005; Buckner et al., 2009).
Supplementary Material
Acknowledgements
This work was supported by NIH Grant AG08487 (B.T.H.) and NIH Grant EB000768 (B.J.B.). We also would like to thank Erin Schuman (Cal Tech, Pasadena) for the gift of the CAMKIIαGFP construct.
References
- Aakalu G, Smith WB, Nguyen N, Jiang C, Schuman EM. Dynamic visualization of local protein synthesis in hippocampal neurons. Neuron. 2001;30:489–502. doi: 10.1016/s0896-6273(01)00295-1. [DOI] [PubMed] [Google Scholar]
- Bacskai BJ, Kajdasz ST, McLellan ME, Games D, Seubert P, Schenk D, Hyman BT. Non-Fc-mediated mechanisms are involved in clearance of amyloid-beta in vivo by immunotherapy. J Neurosci. 2002;22:7873–7878. doi: 10.1523/JNEUROSCI.22-18-07873.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Sepulcre J, Talukdar T, Krienen FM, Liu H, Hedden T, Andrews-Hanna JR, Sperling RA, Johnson KA. Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer's disease. J Neurosci. 2009;29:1860–1873. doi: 10.1523/JNEUROSCI.5062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Snyder AZ, Shannon BJ, LaRossa G, Sachs R, Fotenos AF, Sheline YI, Klunk WE, Mathis CA, Morris JC, Mintun MA. Molecular, structural, and functional characterization of Alzheimer's disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci. 2005;25:7709–7717. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busche MA, Eichhoff G, Adelsberger H, Abramowski D, Wiederhold KH, Haass C, Staufenbiel M, Konnerth A, Garaschuk O. Clusters of hyperactive neurons near amyloid plaques in a mouse model of Alzheimer's disease. Science. 2008;321:1686–1689. doi: 10.1126/science.1162844. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Geddes JW, Kahle JS. Axon sprouting in the rodent and Alzheimer's disease brain: a reactivation of developmental mechanisms? Prog Brain Res. 1990;83:427–434. doi: 10.1016/s0079-6123(08)61266-2. [DOI] [PubMed] [Google Scholar]
- D'Amore JD, Kajdasz ST, McLellan ME, Bacskai BJ, Stern EA, Hyman BT. In vivo multiphoton imaging of a transgenic mouse model of Alzheimer disease reveals marked thioflavine-S-associated alterations in neurite trajectories. J Neuropathol Exp Neurol. 2003;62:137–145. doi: 10.1093/jnen/62.2.137. [DOI] [PubMed] [Google Scholar]
- Dickey CA, Gordon MN, Mason JE, Wilson NJ, Diamond DM, Guzowski JF, Morgan D. Amyloid suppresses induction of genes critical for memory consolidation in APP + PS1 transgenic mice. J Neurochem. 2004;88:434–442. doi: 10.1111/j.1471-4159.2004.02185.x. [DOI] [PubMed] [Google Scholar]
- Jankowsky JL, Slunt HH, Gonzales V, Jenkins NA, Copeland NG, Borchelt DR. APP processing and amyloid deposition in mice haplo-insufficient for presenilin 1. Neurobiol Aging. 2004;25:885–892. doi: 10.1016/j.neurobiolaging.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Klunk WE, Bacskai BJ, Mathis CA, Kajdasz ST, McLellan ME, Frosch MP, Debnath ML, Holt DP, Wang Y, Hyman BT. Imaging Abeta plaques in living transgenic mice with multiphoton microscopy and methoxy-X04, a systemically administered Congo red derivative. J Neuropathol Exp Neurol. 2002;61:797–805. doi: 10.1093/jnen/61.9.797. [DOI] [PubMed] [Google Scholar]
- Knowles RB, Wyart C, Buldyrev SV, Cruz L, Urbanc B, Hasselmo ME, Stanley HE, Hyman BT. Plaque-induced neurite abnormalities: implications for disruption of neural networks in Alzheimer's disease. Proc Natl Acad Sci U S A. 1999;96:5274–5279. doi: 10.1073/pnas.96.9.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffie RM, Meyer-Luehmann M, Hashimoto T, Adams KW, Mielke ML, Garcia-Alloza M, Micheva KD, Smith SJ, Kim ML, Lee VM, Hyman BT, Spires-Jones TL. Oligomeric amyloid beta associates with postsynaptic densities and correlates with excitatory synapse loss near senile plaques. Proc Natl Acad Sci U S A. 2009;106:4012–4017. doi: 10.1073/pnas.0811698106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchibhotla KV, Goldman ST, Lattarulo CR, Wu HY, Hyman BT, Bacskai BJ. Abeta plaques lead to aberrant regulation of calcium homeostasis in vivo resulting in structural and functional disruption of neuronal networks. Neuron. 2008;59:214–225. doi: 10.1016/j.neuron.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le R, Cruz L, Urbanc B, Knowles RB, Hsiao-Ashe K, Duff K, Irizarry MC, Stanley HE, Hyman BT. Plaque-induced abnormalities in neurite geometry in transgenic models of Alzheimer disease: implications for neural system disruption. J Neuropathol Exp Neurol. 2001;60:753–758. doi: 10.1093/jnen/60.8.753. [DOI] [PubMed] [Google Scholar]
- Mayford M, Baranes D, Podsypanina K, Kandel ER. The 3'-untranslated region of CaMKII alpha is a cis-acting signal for the localization and translation of mRNA in dendrites. Proc Natl Acad Sci U S A. 1996;93:13250–13255. doi: 10.1073/pnas.93.23.13250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Luehmann M, Spires-Jones TL, Prada C, Garcia-Alloza M, de Calignon A, Rozkalne A, Koenigsknecht-Talboo J, Holtzman DM, Bacskai BJ, Hyman BT. Rapid appearance and local toxicity of amyloid-beta plaques in a mouse model of Alzheimer's disease. Nature. 2008;451:720–724. doi: 10.1038/nature06616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori Y, Imaizumi K, Katayama T, Yoneda T, Tohyama M. Two cis-acting elements in the 3' untranslated region of alpha-CaMKII regulate its dendritic targeting. Nat Neurosci. 2000;3:1079–1084. doi: 10.1038/80591. [DOI] [PubMed] [Google Scholar]
- Nagai T, Ibata K, Park ES, Kubota M, Mikoshiba K, Miyawaki A. A variant of yellow fluorescent protein with fast and efficient maturation for cell-biological applications. Nat Biotechnol. 2002;20:87–90. doi: 10.1038/nbt0102-87. [DOI] [PubMed] [Google Scholar]
- Palop JJ, Chin J, Bien-Ly N, Massaro C, Yeung BZ, Yu GQ, Mucke L. Vulnerability of dentate granule cells to disruption of arc expression in human amyloid precursor protein transgenic mice. J Neurosci. 2005;25:9686–9693. doi: 10.1523/JNEUROSCI.2829-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phinney AL, Deller T, Stalder M, Calhoun ME, Frotscher M, Sommer B, Staufenbiel M, Jucker M. Cerebral amyloid induces aberrant axonal sprouting and ectopic terminal formation in amyloid precursor protein transgenic mice. J Neurosci. 1999;19:8552–8559. doi: 10.1523/JNEUROSCI.19-19-08552.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, Brett FM, Farrell MA, Rowan MJ, Lemere CA, Regan CM, Walsh DM, Sabatini BL, Selkoe DJ. Amyloid-beta protein dimers isolated directly from Alzheimer's brains impair synaptic plasticity and memory. Nat Med. 2008;14:837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith WB, Starck SR, Roberts RW, Schuman EM. Dopaminergic stimulation of local protein synthesis enhances surface expression of GluR1 and synaptic transmission in hippocampal neurons. Neuron. 2005;45:765–779. doi: 10.1016/j.neuron.2005.01.015. [DOI] [PubMed] [Google Scholar]
- Spires TL, Meyer-Luehmann M, Stern EA, McLean PJ, Skoch J, Nguyen PT, Bacskai BJ, Hyman BT. Dendritic spine abnormalities in amyloid precursor protein transgenic mice demonstrated by gene transfer and intravital multiphoton microscopy. J Neurosci. 2005;25:7278–7287. doi: 10.1523/JNEUROSCI.1879-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern EA, Bacskai BJ, Hickey GA, Attenello FJ, Lombardo JA, Hyman BT. Cortical synaptic integration in vivo is disrupted by amyloid-beta plaques. J Neurosci. 2004;24:4535–4540. doi: 10.1523/JNEUROSCI.0462-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton MA, Wall NR, Aakalu GN, Schuman EM. Regulation of dendritic protein synthesis by miniature synaptic events. Science. 2004;304:1979–1983. doi: 10.1126/science.1096202. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.