Abstract
Although over 100 studies and reviews have examined the ergogenic effects of dietary nitrate (NO3−) supplementation in young, healthy men and women, it is unclear if participant and environmental factors modulate the well-described ergogenic effects—particularly relevant factors include biological sex, aerobic fitness and fraction of inspired oxygen (FiO2) during exercise. To address this limitation, the literature was systematically reviewed for randomized, cross-over, placebo-controlled studies reporting exercise performance outcome metrics with NO3− supplementation in young, healthy adults. Of the 2,033 articles identified, 80 were eligible for inclusion in the meta-analysis. Random-effects meta-analysis demonstrated that exercise performance improved with NO3− supplementation compared to placebo (d=0.174, 95% CI [0.120–0.229], P<0.001). Subgroup analyses conducted on biological sex, aerobic fitness and FiO2 demonstrated that the ergogenic effect of NO3− supplementation was: 1) not observed in studies with only women (n=6, d=0.116, 95% CI [−0.126–0.358], P=0.347), 2) not observed in well-trained endurance athletes (≥65 mL∙kg−1∙min−1; n=26, d=0.021, 95% CI [−0.103–0.144], P=0.745) and 3) was not modulated by FiO2 (hypoxia vs. normoxia). Together, the meta-analyses demonstrated a clear ergogenic effect of NO3− supplementation in recreationally-active, young, healthy men across different exercise paradigms and NO3− supplementation parameters; however, the effect size of NO3− supplementation was objectively small (d = 0.174). NO3− supplementation has more limited utility as an ergogenic aid in participants with excellent aerobic fitness that have optimized other training parameters. Mechanistic research and studies incorporating a wide variety of subjects (e.g. women) are needed to advance the study of NO3− supplementation; however, additional descriptive studies of young, healthy men may have limited utility.
Keywords: dietary nitrate, exercise performance, sex differences, beetroot juice, nitric-oxide
Introduction
The study of the ergogenic effects of dietary nitrate/beetroot (NO3−) supplementation has been a prominent topic in human performance for the last decade. As previously reviewed (1), early studies demonstrate that NO3− supplementation improves exercise tolerance in healthy humans with blunted effects in trained athletes (2). Although it is generally well-accepted that NO3− supplementation may improve exercise tolerance in healthy, young men (1, 3–18), there is substantial variability within and between studies, and previous systematic reviews have failed to find a (significant) performance-enhancing effect with NO3− supplementation under some conditions (7, 13, 17). Indeed, nearly 70% of studies examining the potential performance-enhancing effects of NO3− supplementation do not observe a difference in performance with NO3− supplementation compared to placebo. The variability of the ergogenic effects of NO3− supplementation is likely due to several descriptive factors including aerobic fitness, dose and timing of NO3− supplementation (19–21), environmental factors (e.g. hypoxia) (22), biological sex (23) and inter-individual variability in pharmacodynamics and dose-response relationships (19–21). Thus, the primary purposes of this systematic review are to determine the magnitude of the potential ergogenic effect of NO3− supplementation and the influence of the aforementioned descriptive factors that contribute to variability of the ergogenic effects of NO3− supplementation.
Nitric oxide has long been recognized for vasculoprotective effects (24), effects on mitochondrial respiration (25), and effects on fatigue development (26). In addition to the biosynthesis of endogenous nitric oxide (24), exogenous dietary sources of NO3− (e.g. green leafy vegetables and beetroot) can markedly increase the bioavailability of nitric oxide (1, 27–29). Following ingestion of a NO3− supplement, plasma nitrate levels peak after 1–2 hours and plasma nitrite levels peak after 2–3 hours, both levels gradually return to baseline after about 24 hours (30). Thus, it is not surprising that NO3− supplementation has been shown to enhance exercise performance in some instances. For example, in 2009, it was shown that beetroot ingestion of 5.5 mmol NO3− per day for 6 days improved time to exhaustion during intense cycling exercise in eight young, healthy men compared to placebo (NO3-: 675 ± 203 vs. placebo: 585 ± 145 s, P < 0.05) (31). Similarly, beetroot ingestion of ~6.2 mmol NO3− in nine competitive male cyclists improved performance by ~3% during laboratory-based simulated cycling races using a 16.1 km fixed-distance time trial compared to a placebo (NO3-: 1614 ± 108 vs. placebo: 1662 ± 126 s, P < 0.01) (32). Despite the growing number of studies demonstrating augmented performance with NO3− supplementation, there are twice as many studies demonstrating no performance-enhancing effect of NO3− supplementation.
Thus, several important questions persist regarding the potential ergogenic effect of NO3− supplementation: 1) what is the magnitude and effect size of the potential ergogenic effect?, 2) are there sex differences? (23), 3) what is the role of aerobic fitness? (6), 4) what is the role of the fraction of inspired oxygen? (22), and 5) what are the optimal dosage, duration, and timing of NO3− supplementation? Thus, the purpose of this systematic review and meta-analysis was to examine these five questions. The information garnered may better inform appropriate dosing for future studies and optimal use of NO3− supplementation as an ergogenic aid for athletes and coaches.
Methods
Methods of the analysis and inclusion criteria were specified a priori and conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist (33).
Literature Search
Studies were initially identified from a search of the online PubMed database through August 2019. Keywords used in the search included: (exercise) AND [(beetroot juice) OR (beetroot) OR (beet) OR (nitrite) OR (nitrate) OR (nitrate supplement) OR (dietary nitrate)], using the following limits: humans, English. The references of all eligible studies were also reviewed to identify other potentially eligible studies that may have been missed using the approaches outline above. Only published material was used.
Identification and Study Selection
In order to be considered eligible for inclusion, studies had to meet the following criteria: 1) all subjects were previously healthy and mean age of study participants was between 18 – 40 years, 2) the study must have used a single or double-blind, within subjects cross-over, placebo controlled study, with a randomized or counter balanced study design, and 3) results had to be reported for both nitrate supplement and placebo as mean ± SD or SE. If the required performance data were provided in figure format, but not numerical text, authors were contacted to obtain the numerical data. If the data could not be provided, the study was excluded to avoid potential bias due to estimation of values.
Selected studies were uploaded into a screening tool (Covidence). Three independent investigators (C.C.W., J.W.S., and R.J.R.) screened the titles and abstracts of all studies identified by the search methodologies to determine potential eligibility. Studies that did not have an abstract, along with studies that were deemed potentially eligible, had their full text reviewed in order to determine if they met the criteria for inclusion in the meta-analysis. Additionally, reference lists of included studies were carefully inspected, and any relevant articles not initially captured in the systematic search but met the inclusion criteria were included. Disagreement was resolved by consensus.
Quality Assessment
Risk of bias was assessed with the Cochrane Risk of Bias (34). This standardized appraisal tool consists of 7 components: 1) sequence generation, 2) allocation concealment, 3) blinding of participants and personnel, 4) blinding of outcome assessors, 5) incomplete outcome data, 6) selective outcome reporting, and 7) other sources of bias. For each included study, components were rated as “high”, “low”, or “unclear” risk of bias based on the detail definitions and standardized criteria provided by the quality assessment tool (34). Because the present systematic review included randomized or counter balanced, single or double-blind, cross-over, placebo controlled, within-group study design, components 1, 2, 3, and 4 were all considered ‘low risk of bias’. Risk of bias was conducted independently by two authors (J.W.S. and R.J.R.) and disagreements were resolved by review of a third author (C.C.W.). No studies were excluded based on the quality assessment. To assess publication bias in the included studies we used visual inspection of the funnel plot and the Egger’s regression test to statistically quantify funnel plot asymmetry (35).
Data Extraction and Analysis
Participants’ characteristics (number, sex, age, and aerobic fitness ()) were identified from the selected studies. Exercise type (cycling, handgrip, kayaking, knee extension, roller-skiing, rowing, running, swimming), task end criteria (time trial, work trial, time to exhaustion, trials to exhaustion, fatigue index, or distance trial), NO3− supplementation (daily amount, total amount, and timing relative to exercise initiation) and performance outcome metric were extracted. Data including means and standard deviations (SD) were extracted independently by two authors (J.W.S. and R.J.R.) and disagreements were resolved by review of a third author (C.C.W.). To account for differences in exercise tasks [e.g. time trial (s) vs. distance trial (m)], all data were transformed so that a positive mean difference denoted ‘better performance with NO3− supplementation’ and a negative mean difference denoted ‘better performance with placebo’. For the exercise tasks in which a smaller value indicates ‘better performance’ (time trial, fatigue index), the mean performance metrics for placebo and NO3− supplementation were replaced with the arithmetic opposite values. Using the example cycling time trial from Lansley and colleagues (32), values of −1614 and −1662 s were input for NO3− and placebo, respectively, such that the standardized mean difference would be a positive value indicating ‘better performance with NO3− supplementation’.
Narrative Synthesis
Initially, a narrative synthesis of studies was conducted. Studies were first grouped based on participant sex (men, mixed or women only), fraction of inspired oxygen (FiO2) during exercise bout (normoxia or hypoxia), then the studies were listed in alphabetical order based on the last name of the first author, and then the studies were listed in chronological order based on publication year from the earliest to most recent. This summary table is provided a supplemental content (see Table, Supplemental Digital Content 1, Included studies characteristics and result).
Meta-analysis and subgroup analyses
To support the narrative synthesis, a meta-analysis of pooled data and subgroup analyses were conducted. Initially, effect sizes were calculated for each study using a general inverse variance and weighted using Cohen’s d (d) for differences in performance outcome between placebo and NO3− supplement. Thresholds for very small, small, moderate and large effect sizes were 0.15, 0.2, 0.5, and 0.8, respectively. Data were pooled with both fixed (inverse-variance method) and random effects (DerSimonian and Laird method (36)) models. Although both models returned similar main effects, we only reported the results of the random-effects analyses. The a priori level of significance for all comparisons was P < 0.05. Pooled data are presented as (Cohen’s d standardized mean difference (SMD) [95% confidence intervals]; z-statistic, p-value) unless otherwise indicated. Comprehensive Meta Analysis Software (Biostat) version 3.3.070 was used for all analyses.
Results
Study Selection
A total of 2,033 articles were identified in the initial search and duplicates were removed (n = 2). After screening titles and abstracts, 1,830 articles were deemed ineligible for inclusion. Of the remaining 201 full-text studies, 77 studies were included. The references of the included 77 studies were then carefully inspected (3,723 references, including 1,729 unique references), 41 articles were added to the full-text screening (242 total) and an additional 8 articles were deemed eligible for inclusion. In total, 162 studies were excluded for various reasons including: no reported performance outcome data (n = 40), no placebo (n = 20), between group study design (n = 15), incorrect subject population (e.g. older adults, patient population) (n = 34), no exhaustive exercise included in the study (n = 36), or an inappropriate control/placebo performance trial (n = 17). Thus, 80 studies were included in the narrative synthesis and meta-analyses. Within these 80 studies, two presented data from men and women separately, six presented data from different NO3− supplementation separately, seven presented data from different exercises separately and eight presented data from different ambient oxygen concentrations separately, resulting in 111 data sets (2, 19, 31, 32, 37–115). A schematic of the search stratagem is presented in Fig 1.
Figure 1.
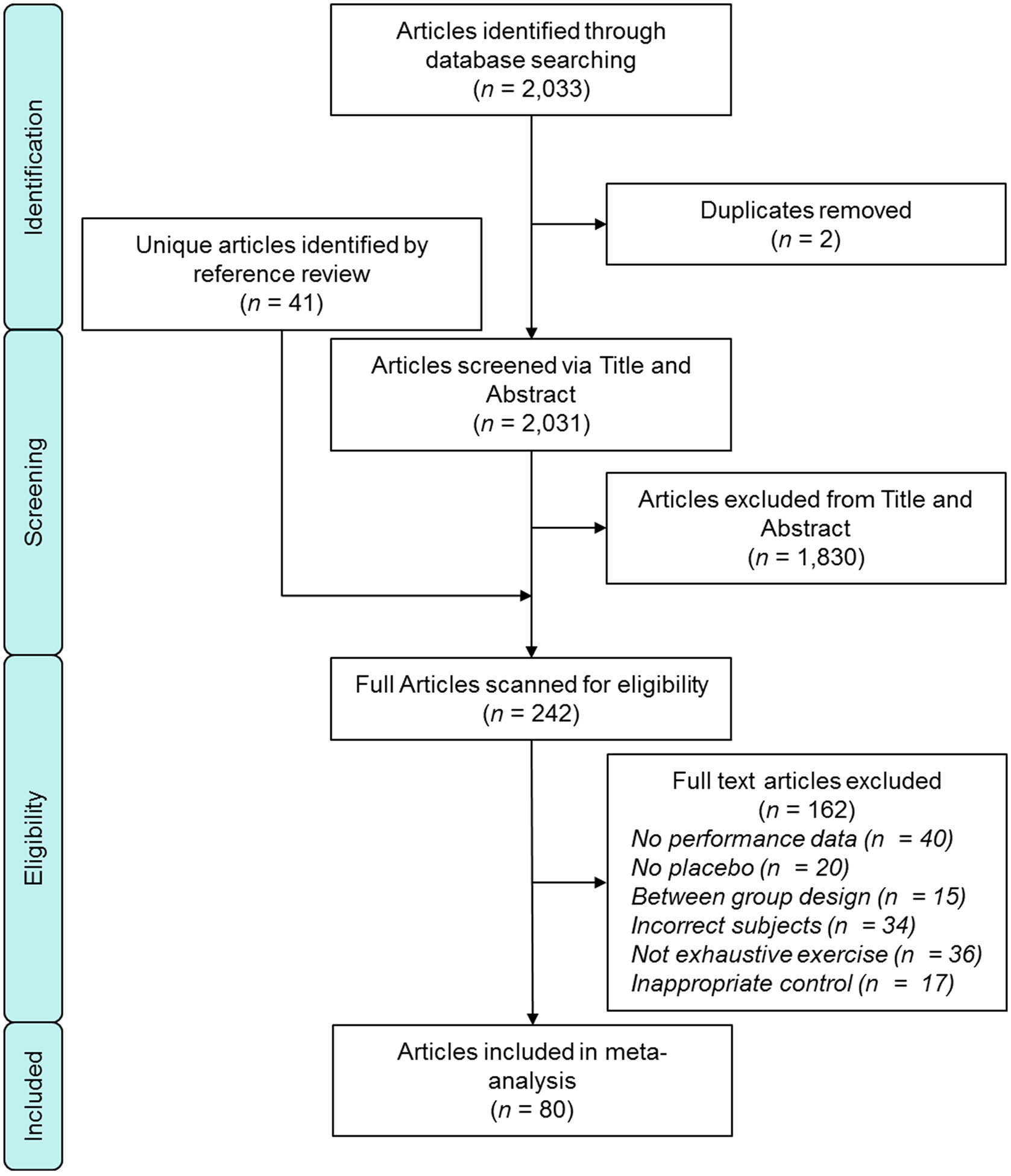
Flow chart of the study selection.
Study Characteristics: Narrative Review
A summary of the 80 included studies and the 113 data sets is provided in the supplemental content (see Table, Supplemental Digital Content 1, Included studies characteristics and result). Studies included were published between 2009 and 2019. A total of 1,179 men and 156 women were included in the selected studies; most data sets (79%, n=90) were composed of men only, 16% (n=17) included both men and women, and 5% (n=6) included exclusively women. The primary performance metrics of studies included time to complete a fixed-distance (time trial, TT; n = 52), time to complete a relative total work (work trial, WT; n = 5), maximal distance covered in a fixed time period (distance trial, DT; n = 6), reduction in maximal strength or power during a task (fatigue index, FI; n = 13), endurance time maintaining a submaximal task (time-to-exhaustion, TTE; n = 32) or number of trials of a submaximal task to exhaustion (trials to exhaustion, TrTE; n = 4). NO3− supplementation was performed between 40 – 210 minutes prior to exercise initiation with a concentration between 1.6 – 28.7 mmol for 1 – 15 days resulting in a cumulative NO3− of 4.2 mmol – 208 mmol. Most studies did not observe a difference in performance (76 of 111 studies; 68%) but no studies reported worsened performance with NO3− supplementation compared to placebo. Generally, most studies examined sustained, endurance-style exercise; however, several studies incorporate single sprint exercises (e.g. 500m kayak time trial; n = 5) or repeated sprint exercises (e.g. three sequential Wingate tests; n = 8). Most studies incorporating ‘sprint’ exercises are encompassed within the ‘300[s] or less’ exercise time.
Pooled Analysis
Considering all studies included in the quantitative synthesis (Fig. 2), exercise performance was improved (faster time, longer distance, lower fatigue index or more trials) with NO3− supplementation compared to placebo with negligible NO3− (0.174 [0.120 – 0.229]; z = 6.299, P < 0.001). Although significant, the effect size was very small (d < 0.2), thus, it is not surprising that only ~32% studies demonstrated significant improved performance with NO3− supplementation compared to placebo. There is substantial variability in the response to NO3− supplementation (21), indicating that other factors may be contributing to the change in performance other than NO3− supplementation. Factors that may contribute to variability of the effects of NO3− supplementation include inter-individual differences, such as biological sex and aerobic fitness and inter-study differences, such as performance parameters (ambient oxygen concentration, exercise time and exercise type), and/or NO3− supplementation parameters (daily dose, dosing period or timing). Thus, sub-analyses were undertaken to examine each identified, potential source of variability. Statistical power analyses indicated that ~6 datasets are required within each category for sub-analyses. To reduce the potential effects of learning, all studies included familiarization procedures for the exhaustive exercise bout and 95% of the studies included a ‘control’ session with completion of the exhaustive exercise without supplementation (placebo or NO3−).
Figure 2.
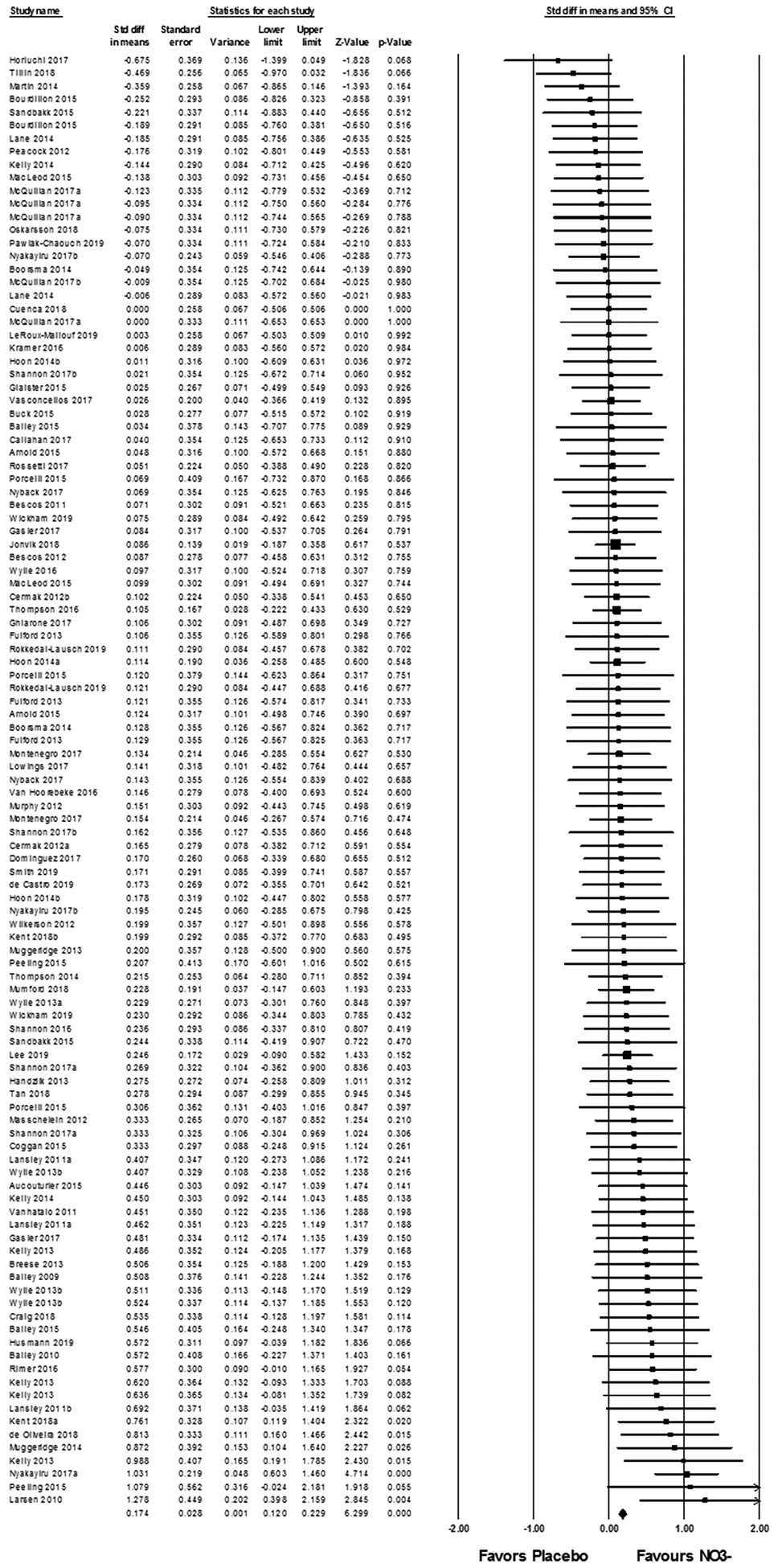
Forest plot displaying random effects meta-analysis of exercise performance after placebo or NO3- supplementation. The vertical line represents the mean overall effect. Symbol size reflects weight of the effect for each individual study. Symbols on the left of the continuous black line at 0 show better exercise performance after placebo supplementation, whereas studies on the right of the black line demonstrate better exercise performance after NO3- supplementation.
Subgroup analysis: biological sex and aerobic fitness
Comparison of men only, mixed sex (men and women) and women only studies revealed a blunted overall effect of NO3− supplementation for women compared to mixed sex and men only studies (Fig. 3A). As has been suggested by Wickham and colleagues in a recent investigation (112) and review (23), women are heavily underrepresented in this field of research as is generally observed in biomedical research (116). Six studies that examined women only or presented data separately for women found no effect of NO3− supplementation on exercise performance (p=0.347). However, a study examining kayak performance in elite, international-level athletes found that NO3− supplementation improved 500-m time-trial performance for five women (p=0.004) but not 4-minute distance trial performance for six men (p=0.110) (95). As reviewed previously (23), although there are physiologically-based sex differences that could potentially reduce the efficacy of NO3− supplementation as an ergogenic aid for women, there is a clear underrepresentation of women that should be addressed in future investigations.
Figure 3.
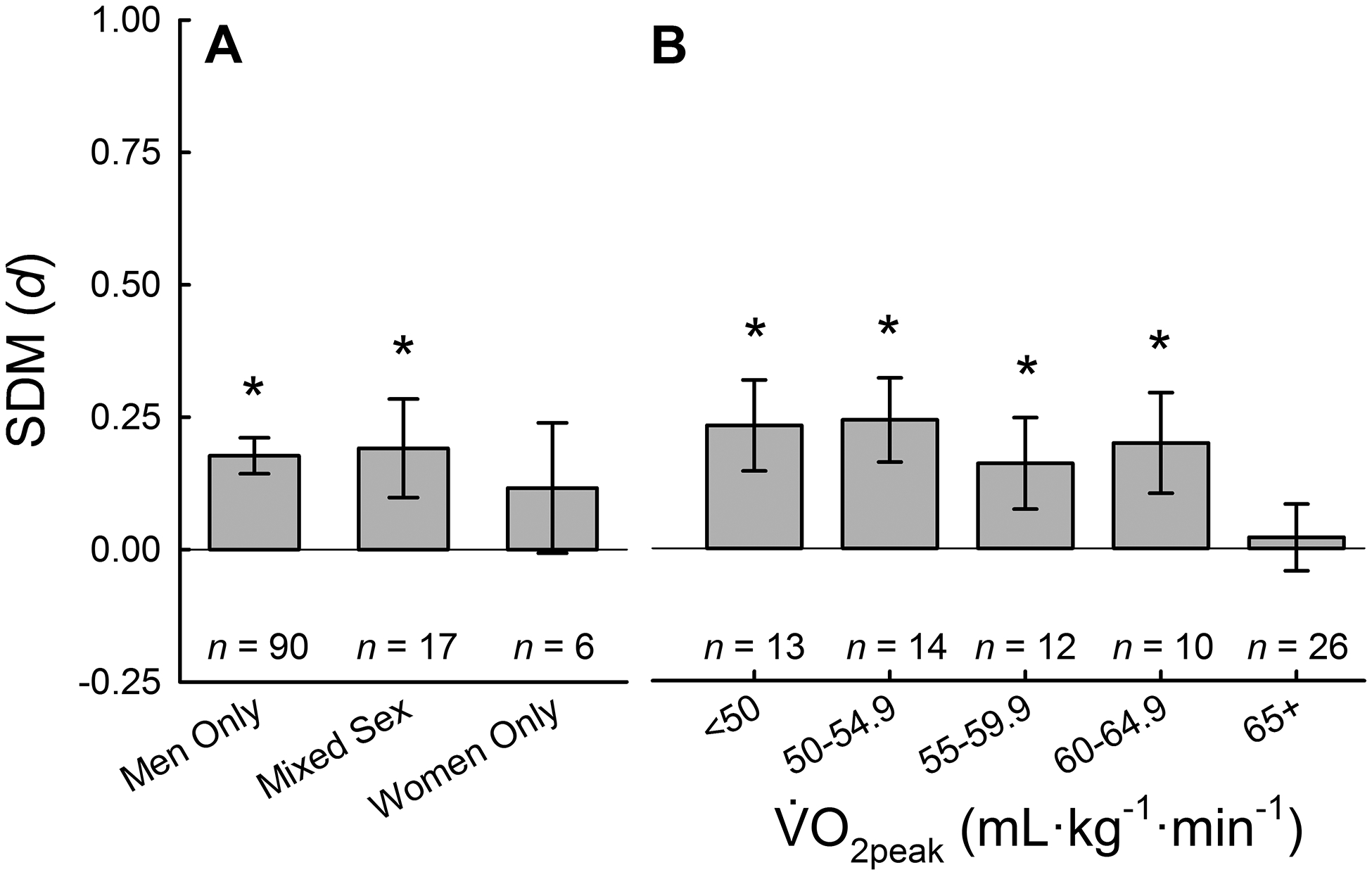
Subgroup analysis of biological sex and aerobic fitness. Standard mean differences (SMD) of NO3− supplementation compared to placebo for biological sex of included participants (A) and (B) calculated used random effects meta-analyses. * denotes better performance after NO3− supplementation compared with placebo, P < 0.05.
A priori, was delineated increments of 5 mL∙kg∙min−1 resulting in six categories (<45, 45–49.9…65+ mL∙kg∙min−1). However, the lowest category was underpowered and highly variable (n=4, d=0.168, 95% CI [−0.134–0.469], P=0.276), thus, was combined with the next lowest category (45 – 49.9 mL∙kg∙min−1). Comparison across demonstrates that the ergogenic effect of NO3− supplementation is observed across a large range of values (~40 – 65 mL∙kg∙min−1), however, the ergogenic effect is not observed in highly fit athletes ( > 64.9 mL∙kg−1∙min−1; Fig 3B). Although a recent systematic review suggested the data were inconclusive (6), with the inclusion of additional data, the role of can be more clearly observed. These data are in agreement with a study from Porcelli and colleagues (96). In a cohort of 21 men with low, moderate and high (~40 vs. ~50 vs. ~70 mL∙kg−1∙min−1), 3-km running time was improved with a six day supplementation of 5.5 mmol∙day−1 of NO3− compared to placebo for men with low (~3% improvement) and moderate (~1.5% improvement) but not high (0.2% improvement) (96). Based on these data, the ergogenic effect of NO3− supplementation is not observed in highly trained athletes with likely optimal training adaptations ( > 64.9 mL∙kg−1∙min−1). However, in moderately trained and untrained men without optimized fitness, a significant ergogenic effect of NO3− supplementation is observed.
Subgroup analysis: exercise parameters
Comparison of hypoxic and normoxic conditions revealed a similar overall effect of NO3− supplementation for both conditions (Fig. 4A). In line with a previous review from Shannon and colleagues (22), these data suggest that NO3− supplementation is a promising ergogenic aid for exercising in low-O2 environments. However, these data do not suggest that the ergogenic effects of NO3− supplementation are greater in hypoxia than normoxia, as previously suggested (22). Albeit, these data are primarily from simulated altitude using normobaric hypoxia with short hypoxic exposure times, and future investigations at terrestrial altitude (hypobaric hypoxia) may be warranted, as formerly suggested (22).
Figure 4.
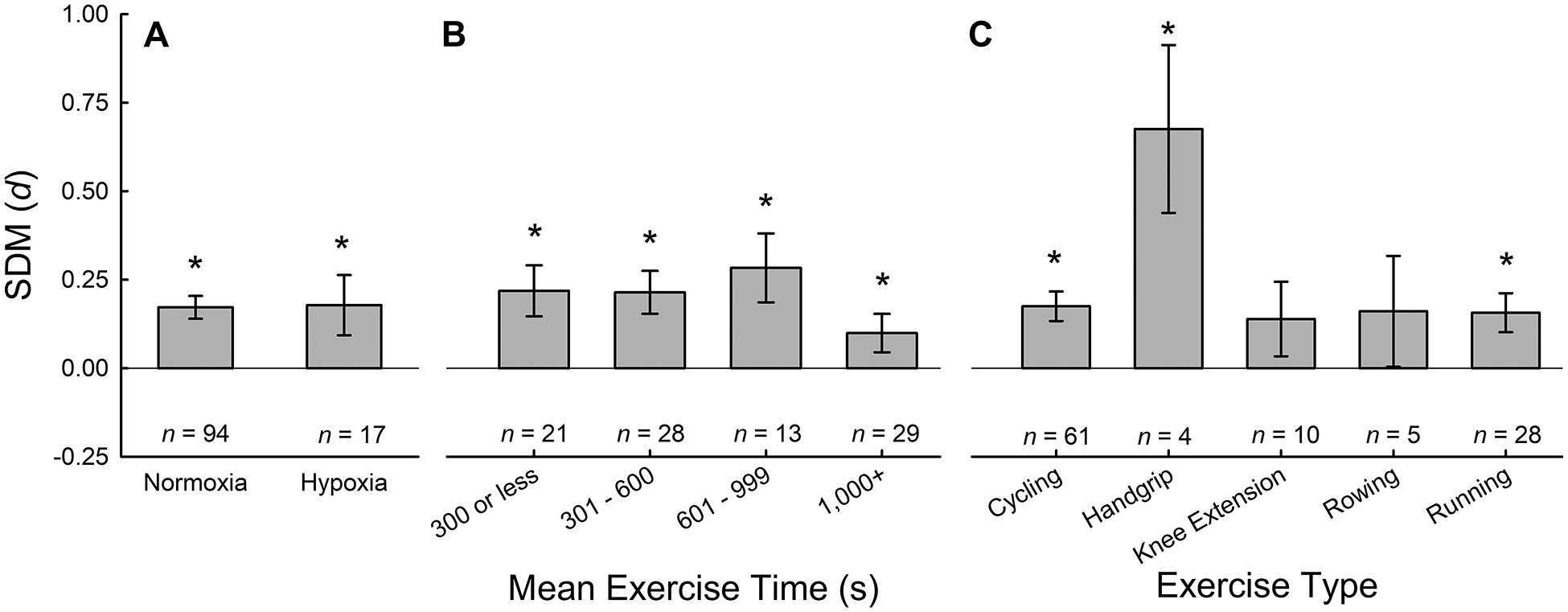
Subgroup analysis of exercise parameters. Standard mean differences (SMD) of NO3− supplementation compared to placebo for fraction of inspired oxygen (normoxia vs. hypoxia; A), mean exercise time (B) and exercise type (C) calculated used random effects meta-analyses. * denotes better performance after NO3− supplementation compared with placebo, P < 0.05.
Comparison across different exercise parameters (time and type) revealed heterogeneous results for exercise type and limited effect of NO3− supplementation in long-duration exercise (1,000s or more). As previously reviewed (8, 9), long-duration exercise that by virtue is lower intensity than short-duration exercise, likely minimizes hypoperfusion of metabolically active tissue during exercise and reduces the requirement for NO production through the reduction of nitrite. This physiological rationale likely underlies the finding that NO3− supplementation is more beneficial for short-duration exercise (<15 minutes) than long-duration exercise (Fig. 4B). Most studies (~80%) used cycling or running as exercise modalities, and the ergogenic effect of NO3− supplementation was not different between running and cycling. However, for the other exercise types (handgrip, kayaking, knee extension, roller-skiing, rowing, and swimming) there was markedly less data and more heterogeneity due to small sample sizes (Fig. 4C). These data may suggest that NO3− supplementation has larger effects in small muscle exercise (handgrip), which is likely limited by peripheral factors (tissue perfusion and metabolic accumulation) rather than cardiac output, compared to whole body or large muscle exercise.
Previous systematic reviews have demonstrated an ergogenic effect of NO3− supplementation that is dependent upon the criteria for exercise termination. As examples, both Hoon et al. (7) and McMahon et al. (13) demonstrated a significant ergogenic effect of NO3− supplementation for TTE exercise but not time trials or graded-exercise performance tests. The current data demonstrated a consistent ergogenic effect of NO3− supplementation across all exercises regardless of criteria for exercise termination, including: time trials (n = 52, 0.086 [0.002 0.0173]; z = 2.000, P = 0.045), distance trials (n = 6, 0.318 [0.136 0.499]; z = 3.430, P = 0.001), fatigue index tasks (n = 13, 0.175 [0.036 0.313]; z = 2.473, P = 0.013) and TTE tasks (n = 32, 0.324 [0.213 0.436]; z = 5.690, P < 0.001). In line with findings from Hoon et al. (7) and McMahon et al. (13), the smallest effect size was observed for studies utilizing time trial performances and the largest effect size was observed for TTE protocols.
Subgroup analysis: NO3− dosage and timing
The influences of NO3− supplementation parameters were also explored. The ergogenic effect of NO3− supplementation was not different between daily concentrations of NO3− supplementation greater than 5 mmol∙day−1 (range: 5.1 – 28.7 mmol∙day−1), however, there was no ergogenic effect with low NO3− supplementation (range: 1.6 – 5.0 mmol∙day−1; p=0.085). The ergogenic effect of NO3− supplementation was not different between the number of days of NO3− supplementation (range: 1 – 15 days). However, the ergogenic effect of NO3− supplementation was different with the timing of NO3− supplementation relative to exercise performance (range: 5 – 210 minutes prior to exercise), see Fig. 5. The optimal timing of NO3− supplementation is 2–3.5 hours prior to the onset of exercise. Dr. Jones’ groups has previously examined the pharmacokinetics and dose response of NO3− supplementation on plasma [NO3−], and determined that the timing of the peak plasma [NO3−] is dependent upon the NO3− dose ingested (19). The potential interaction of NO3− dose and timing was explored in this data; however, no significant interaction was evidenced. Based on these data, the optimal ergogenic effect of NO3− supplementation is with the following parameters of NO3− supplementation: 1) any dose between 5.1 mmol∙day−1 and ~25 mmol∙day−1, 2) at least one day of supplementation, and 3) ingestion of NO3− 2 – 3.5 hours prior to initiation of exercise.
Figure 5.
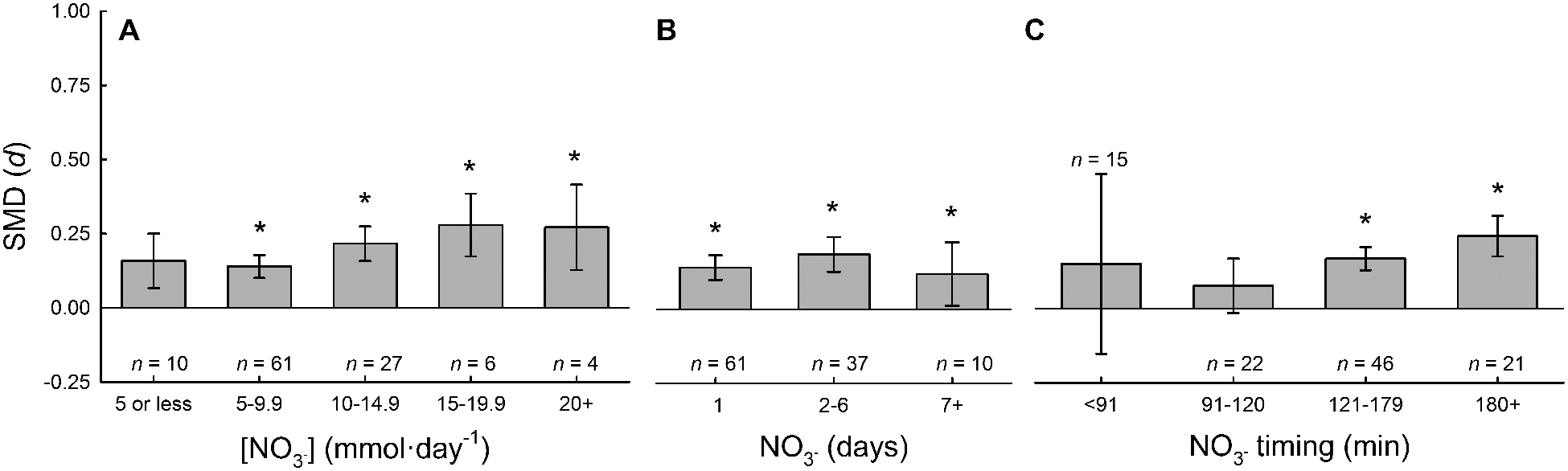
Subgroup analysis of NO3- supplementation parameters. Standard mean differences (SMD) of NO3- supplementation compared to placebo for different daily concentrations of NO3- supplementation (A), different number of days of NO3- supplementation (B) and different timing of NO3- supplementation relative to commencement of exercise (C) calculated used random effects meta-analyses. * denotes better performance after NO3- supplementation compared with placebo, P < 0.05.
Based on these data, a secondary pooled analysis was performed after removing the studies that administered NO3− in an inadequate dose (≤5 mmol∙day−1; n = 10) or with insufficient time to adequately metabolize the NO3− prior to exercise (<91 minutes before exercise; n = 15). The results of this secondary pooled analysis are similar to our primary pooled analysis (0.185 [0.125 – 0.244]; z = 6.102, P < 0.001). Similarly, removal of these 25 data sets with ineffective NO3− supplementation did not change interpretations of any of the subgroup analyses but marginally increased Cohen’s d SDM by ~0.02.
Despite the heterogeneity with NO3− supplementation parameters, a vast proportion of studies used similar type of commercial NO3− supplementation and placebo from Beet it (James White Drinks, Ipswich, UK). The placebo is created by passage of the beetroot juice, before pasteurization, through a column containing Purolite A520E ion-exchange resign, which selectively removes NO3− ions (39). Thus, the placebo is an identical version of the beetroot juice in appearance and taste, with negligible levels of NO3− ions.
Risk of Bias
Publication bias was assessed using a funnel plot (Fig. 6). Visual inspection of the funnel plot shows three studies fall below 95% CI and three studies are above 95% CI. Egger’s regression test suggests there is no significant asymmetry of the plot (intercept = 1.18, p = 0.07).
Figure 6.
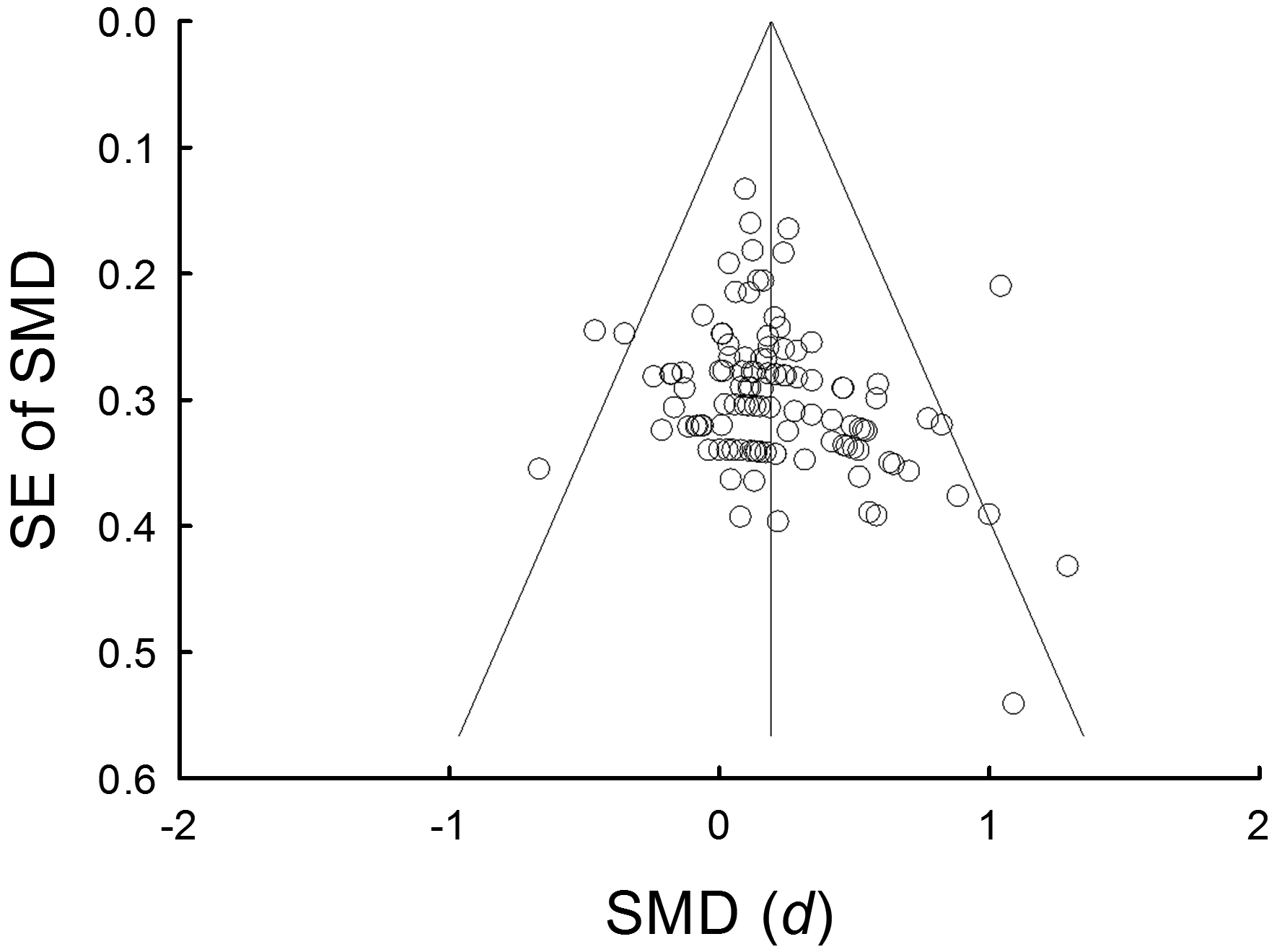
Funnel plot of the standard error (SE) and standardized effect for each study. The angled lines define the area including the 95% confidence interval (CI) of the standardized mean differences (SMD) and the vertical line defines the middle of the funnel at the mean SMD. Visual inspection of the funnel plot shows three studies fall below 95% CI and three studies are above 95% CI.
Discussion
This systematic review incorporated 80 studies investigating exercise performance after ingestion of NO3− supplementation or placebo supplement with negligible NO3− content in ~1,300 subjects. Contrary to much of the literature (~2/3 studies), there was a clear ergogenic effect of NO3− supplementation across many different exercises modalities in normoxic and hypoxic conditions in young men, but not women or elite athletes ( > 64.9 mL∙kg−1∙min−1). In agreement with previous recommendations, the ergogenic effect of NO3− supplementation is limited in highly trained athletes and lower-intensity/long-duration exercise (>15 minutes) (8, 9). The optimal NO3− supplementation was between 5 and ~25 mmol∙day−1 ingested 2 – 3.5 hours before exercise. These data provide robust support of previous contentions from individual studies (19, 53) and narrative reviews (1, 9, 117) considering optimal NO3− supplementation parameters. Thus, the general parameters for “successful” NO3− supplementation gleaned from our analysis are not novel; however, the current meta-analyses substantiate previous claims from leading experts regarding “best practices” for NO3− supplementation. These data may also be useful in designing studies that consider the effects of NO3− supplementation on other modes of exercise in other demographic groups.
The effect size of NO3− supplementation was objectively small (d = 0.174) even with the removal of studies administering sub-optimal NO3− supplementation (d = 0.185), and this small effect size likely explains the large number of studies (~68%) that do not observe an ergogenic effect of NO3− supplementation on exercise performance. Although the effect size is small, these data demonstrate a quantitative and repeatable enhancement of exercise performance by ~3% (e.g. 48s in 16.1 km cycling time trial (32)) across many different exercise modalities and performances. In the context of athletic competition, the ~3% ergogenic effect of NO3− supplementation may be highly meaningful and is not dissimilar to the potential ergogenic effect of new running shoes with embedded carbon-fiber plates (e.g. Nike Next%) (118). Thus, although the effect size of NO3− supplementation is small, these data suggest NO3− as a viable ergogenic aid.
Inorganic vs. Organic Nitrates.
Although often underreported, it is nearly ubiquitous for studies to use inorganic nitrate for supplementation for enhanced exercise performance. The differences between organic and inorganic nitrate are related to their underlying chemical structure—with organic nitrates primarily used in medicine (e.g. glyceryl trinitrate (GTN)) and inorganic nitrates are primarily found in plants, particularly green leafy vegetables and beetroot plants (119). As reviewed previously, the pharmacokinetic properties of organic vs. inorganic nitrates are markedly different, and within the current data, all studies collated utilized inorganic nitrate supplementation (119). Thus, the effect of organic nitrate supplementation as an ergogenic aid is unknown but may be limited by developed tolerance and potential endothelial dysfunction with prolonged use (119, 120).
Oral Microbiome.
The ergogenic potential of NO3− supplementation is largely dependent on the reduction of concentrated NO3− to nitrite (NO2−) which is regulated by anaerobic bacteria in the oral cavity (121–123). Although the oral microbiome may be disturbed by many oral substances (e.g. antibiotics, antibacterial mouthwash, gum chewing, etc.) (124), only about 50% of studies reported controlled environments for the oral microbiome, for example, ‘subjects were asked to abstain from using antibacterial mouthwash and chewing gum…’ (115). Thus, variability in the oral microbiome may contribute to the observed variability in the efficacy of NO3− supplementation. Further, recent studies have demonstrated that under controlled conditions, the reduction of NO3− to NO2− in biological fluids varies substantially within individuals across repeated visits (121). The large variability in the performance-enhancing effects of NO3− supplementation may be due to the profound biological variability of the oral microbiome (121), and this postulation warrants future investigation. One approach to reduce the potential impact of the variability of oral microbiome is to provide NO3− supplementation for several days before an exercise test, which has been shown to increase abundance of some bacteria capable of NO3− reduction (123) which may optimize the effectiveness of NO3− supplementation.
Sex Differences.
As previously reviewed (23), there is a clear underrepresentation of women in the study of NO3− supplementation, and more generally, in science (116). In the current data, women account for ~10% of the total sample size and there was no ergogenic effect of NO3− supplementation. The absence of an ergogenic effect of NO3− supplementation in women is likely spurious due to a dearth of studies including women, and the sex bias in studies of NO3− supplementation has created a field that is ripe with opportunities for future study. A recent review from Wickham and Spriet (23) explicitly provides a strong rationale for potential sex differences in response to NO3− supplementation and highlights areas for future scientific inquiry.
Conclusion
This systematic review and meta-analysis clearly demonstrate a ~3% performance enhancing effect after optimal NO3− supplementation for healthy, young men that is often not observed in individual studies likely due to the small effect size. Importantly, these data support previous assertions from narrative reviews regarding optimal NO3− supplementation parameters and highlight a dearth of studies including women. The performance enhancing effect of NO3− supplementation was not observed with administration of low doses of NO3− (≤ 5 mmol∙day−1) or NO3− dose within 90 minutes prior to exercise, nor in participants with excellent values (>64.9 mL∙kg∙min−1). Thus, NO3− supplementation was demonstrated to be an effective ergogenic aid for young, healthy men; however, additional mechanistic research and studies incorporating a wide variety of subjects (e.g. women) are warranted to advance the study of NO3− supplementation as an ergogenic aid.
Supplementary Material
Supplementary Table 1. docx—Included studies characteristics and results.
Acknowledgements
Authors have no professional relationships with companies or manufacturers who will benefit from the results of the present study. Results of the present study do not constitute endorsement by ACSM. Results of the study are presented clearly, honestly and without fabrication, falsification, or inappropriate data manipulation.
Funding Sources
This work was supported by a National Heart, Lung, and Blood Institute (NHLBI) grant R-35-HL-139854 to MJJ. JWS and CCW were supported by National Institute of Diabetes and Digestive and Kidney Disease grant T32-DK0007352, PBD was supported by a post-doctoral fellowship from the Natural Sciences and Engineering Research Council of Canada, and SEB was supported by NHLBI grant F32-HL-131151.
References
- 1.Jones AM, Thompson C, Wylie LJ, Vanhatalo A. Dietary Nitrate and Physical Performance. Annu Rev Nutr. 2018;38:303–28. [DOI] [PubMed] [Google Scholar]
- 2.Bescos R, Ferrer-Roca V, Galilea PA et al. Sodium nitrate supplementation does not enhance performance of endurance athletes. Med Sci Sports Exerc. 2012;44(12):2400–9. [DOI] [PubMed] [Google Scholar]
- 3.Bescos R, Sureda A, Tur JA, Pons A. The effect of nitric-oxide-related supplements on human performance. Sports Med. 2012;42(2):99–117. [DOI] [PubMed] [Google Scholar]
- 4.Burke LM. To beet or not to beet? Journal of Applied Physiology. 2013;115(3):311–2. [DOI] [PubMed] [Google Scholar]
- 5.Clements WT, Lee SR, Bloomer RJ. Nitrate ingestion: a review of the health and physical performance effects. Nutrients. 2014;6(11):5224–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dominguez R, Cuenca E, Mate-Munoz JL et al. Effects of Beetroot Juice Supplementation on Cardiorespiratory Endurance in Athletes. A Systematic Review. Nutrients. 2017;9(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoon MW, Johnson NA, Chapman PG, Burke LM. The effect of nitrate supplementation on exercise performance in healthy individuals: a systematic review and meta-analysis. Int J Sport Nutr Exerc Metab. 2013;23(5):522–32. [DOI] [PubMed] [Google Scholar]
- 8.Jones AM. Influence of dietary nitrate on the physiological determinants of exercise performance: a critical review. Appl Physiol Nutr Metab. 2014;39(9):1019–28. [DOI] [PubMed] [Google Scholar]
- 9.Jones AM. Dietary nitrate supplementation and exercise performance. Sports Med. 2014;44 Suppl 1:S35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones AM, Bailey SJ, Vanhatalo A. Dietary nitrate and O(2) consumption during exercise. Med Sport Sci. 2012;59:29–35. [DOI] [PubMed] [Google Scholar]
- 11.Jones AM, Vanhatalo A, Bailey SJ. Influence of dietary nitrate supplementation on exercise tolerance and performance. Nestle Nutr Inst Workshop Ser. 2013;75:27–40. [DOI] [PubMed] [Google Scholar]
- 12.Jonvik KL, Nyakayiru J, van Loon LJ, Verdijk LB. Can elite athletes benefit from dietary nitrate supplementation? J Appl Physiol (1985). 2015;119(6):759–61. [DOI] [PubMed] [Google Scholar]
- 13.McMahon NF, Leveritt MD, Pavey TG. The Effect of Dietary Nitrate Supplementation on Endurance Exercise Performance in Healthy Adults: A Systematic Review and Meta-Analysis. Sports Med. 2017;47(4):735–56. [DOI] [PubMed] [Google Scholar]
- 14.Naderi A, de Oliveira EP, Ziegenfuss TN, Willems MT. Timing, Optimal Dose and Intake Duration of Dietary Supplements with Evidence-Based Use in Sports Nutrition. J Exerc Nutrition Biochem. 2016;20(4):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pawlak-Chaouch M, Boissiere J, Gamelin FX, Cuvelier G, Berthoin S, Aucouturier J. Effect of dietary nitrate supplementation on metabolic rate during rest and exercise in human: A systematic review and a meta-analysis. Nitric Oxide. 2016;53:65–76. [DOI] [PubMed] [Google Scholar]
- 16.Poortmans JR, Gualano B, Carpentier A. Nitrate supplementation and human exercise performance: too much of a good thing? Curr Opin Clin Nutr Metab Care. 2015;18(6):599–604. [DOI] [PubMed] [Google Scholar]
- 17.Van De Walle GP, Vukovich MD. The Effect of Nitrate Supplementation on Exercise Tolerance and Performance: A Systematic Review and Meta-Analysis. J Strength Cond Res. 2018;32(6):1796–808. [DOI] [PubMed] [Google Scholar]
- 18.Vitale K, Getzin A. Nutrition and Supplement Update for the Endurance Athlete: Review and Recommendations. Nutrients. 2019;11(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wylie LJ, Kelly J, Bailey SJ et al. Beetroot juice and exercise: pharmacodynamic and dose-response relationships. J Appl Physiol (1985). 2013;115(3):325–36. [DOI] [PubMed] [Google Scholar]
- 20.Wylie LJ, Ortiz de Zevallos J, Isidore T et al. Dose-dependent effects of dietary nitrate on the oxygen cost of moderate-intensity exercise: Acute vs. chronic supplementation. Nitric Oxide. 2016;57:30–9. [DOI] [PubMed] [Google Scholar]
- 21.Wylie LJ, Park JW, Vanhatalo A et al. Human skeletal muscle nitrate store: influence of dietary nitrate supplementation and exercise. J Physiol. 2019;597(23):5565–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shannon OM, McGawley K, Nyback L et al. “Beet-ing” the Mountain: A Review of the Physiological and Performance Effects of Dietary Nitrate Supplementation at Simulated and Terrestrial Altitude. Sports Med. 2017;47(11):2155–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wickham KA, Spriet LL. No longer beeting around the bush: a review of potential sex differences with dietary nitrate supplementation (1). Appl Physiol Nutr Metab. 2019;44(9):915–24. [DOI] [PubMed] [Google Scholar]
- 24.Moncada S, Higgs A. The L-arginine-nitric oxide pathway. N Engl J Med. 1993;329(27):2002–12. [DOI] [PubMed] [Google Scholar]
- 25.Brown GC, Cooper CE. Nanomolar concentrations of nitric oxide reversibly inhibit synaptosomal respiration by competing with oxygen at cytochrome oxidase. FEBS Lett. 1994;356(2–3):295–8. [DOI] [PubMed] [Google Scholar]
- 26.Percival JM, Anderson KN, Huang P, Adams ME, Froehner SC. Golgi and sarcolemmal neuronal NOS differentially regulate contraction-induced fatigue and vasoconstriction in exercising mouse skeletal muscle. J Clin Invest. 2010;120(3):816–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lundberg JO, Gladwin MT, Ahluwalia A et al. Nitrate and nitrite in biology, nutrition and therapeutics. Nat Chem Biol. 2009;5(12):865–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hord NG, Tang Y, Bryan NS. Food sources of nitrates and nitrites: the physiologic context for potential health benefits. Am J Clin Nutr. 2009;90(1):1–10. [DOI] [PubMed] [Google Scholar]
- 29.Bryan NS. Nitrite in nitric oxide biology: cause or consequence? A systems-based review. Free Radic Biol Med. 2006;41(5):691–701. [DOI] [PubMed] [Google Scholar]
- 30.Webb AJ, Patel N, Loukogeorgakis S et al. Acute blood pressure lowering, vasoprotective, and antiplatelet properties of dietary nitrate via bioconversion to nitrite. Hypertension. 2008;51(3):784–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bailey SJ, Winyard P, Vanhatalo A et al. Dietary nitrate supplementation reduces the O2 cost of low-intensity exercise and enhances tolerance to high-intensity exercise in humans. J Appl Physiol (1985). 2009;107(4):1144–55. [DOI] [PubMed] [Google Scholar]
- 32.Lansley KE, Winyard PG, Bailey SJ et al. Acute dietary nitrate supplementation improves cycling time trial performance. Med Sci Sports Exerc. 2011;43(6):1125–31. [DOI] [PubMed] [Google Scholar]
- 33.Liberati A, Altman DG, Tetzlaff J et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Higgins JP, Altman DG, Gotzsche PC et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88. [DOI] [PubMed] [Google Scholar]
- 37.Arnold JT, Oliver SJ, Lewis-Jones TM, Wylie LJ, Macdonald JH. Beetroot juice does not enhance altitude running performance in well-trained athletes. Appl Physiol Nutr Metab. 2015;40(6):590–5. [DOI] [PubMed] [Google Scholar]
- 38.Aucouturier J, Boissiere J, Pawlak-Chaouch M, Cuvelier G, Gamelin FX. Effect of dietary nitrate supplementation on tolerance to supramaximal intensity intermittent exercise. Nitric Oxide. 2015;49:16–25. [DOI] [PubMed] [Google Scholar]
- 39.Lansley KE, Winyard PG, Fulford J et al. Dietary nitrate supplementation reduces the O2 cost of walking and running: a placebo-controlled study. J Appl Physiol (1985). 2011;110(3):591–600. [DOI] [PubMed] [Google Scholar]
- 40.Larsen FJ, Schiffer TA, Borniquel S et al. Dietary inorganic nitrate improves mitochondrial efficiency in humans. Cell Metab. 2011;13(2):149–59. [DOI] [PubMed] [Google Scholar]
- 41.Lane SC, Hawley JA, Desbrow B et al. Single and combined effects of beetroot juice and caffeine supplementation on cycling time trial performance. Appl Physiol Nutr Metab. 2014;39(9):1050–7. [DOI] [PubMed] [Google Scholar]
- 42.Kent GL, Dawson B, McNaughton LR, Cox GR, Burke LM, Peeling P. The effect of beetroot juice supplementation on repeat-sprint performance in hypoxia. J Sports Sci. 2019;37(3):339–46. [DOI] [PubMed] [Google Scholar]
- 43.Bailey SJ, Fulford J, Vanhatalo A et al. Dietary nitrate supplementation enhances muscle contractile efficiency during knee-extensor exercise in humans. J Appl Physiol (1985). 2010;109(1):135–48. [DOI] [PubMed] [Google Scholar]
- 44.Bailey SJ, Varnham RL, DiMenna FJ, Breese BC, Wylie LJ, Jones AM. Inorganic nitrate supplementation improves muscle oxygenation, O2 uptake kinetics and exercise tolerance at high but not low pedal rates. J Appl Physiol (1985). 2015:jap 01141 2014. [DOI] [PubMed] [Google Scholar]
- 45.Boorsma RK, Whitfield J, Spriet LL. Beetroot Juice Supplementation Does Not Improve Performance of Elite 1500-m Runners. Medicine & Science in Sports & Exercise. 2014;46(12):2326–34. [DOI] [PubMed] [Google Scholar]
- 46.Bescos R, Rodriguez FA, Iglesias X, Ferrer MD, Iborra E, Pons A. Acute administration of inorganic nitrate reduces VO(2peak) in endurance athletes. Med Sci Sports Exerc. 2011;43(10):1979–86. [DOI] [PubMed] [Google Scholar]
- 47.Buck CL, Henry T, Guelfi K, Dawson B, McNaughton LR, Wallman K. Effects of sodium phosphate and beetroot juice supplementation on repeated-sprint ability in females. Eur J Appl Physiol. 2015;115(10):2205–13. [DOI] [PubMed] [Google Scholar]
- 48.Cermak NM, Res P, Stinkens R, Lundberg JO, Gibala MJ, van Loon LJ. No improvement in endurance performance after a single dose of beetroot juice. Int J Sport Nutr Exerc Metab. 2012;22(6):470–8. [DOI] [PubMed] [Google Scholar]
- 49.Craig JC, Broxterman RM, Smith JR, Allen JD, Barstow TJ. Effect of dietary nitrate supplementation on conduit artery blood flow, muscle oxygenation, and metabolic rate during handgrip exercise. J Appl Physiol (1985). 2018;125(2):254–62. [DOI] [PubMed] [Google Scholar]
- 50.de Oliveira GV, Nascimento L, Volino-Souza M, Mesquita JS, Alvares TS. Beetroot-based gel supplementation improves handgrip strength and forearm muscle O2 saturation but not exercise tolerance and blood volume in jiu-jitsu athletes. Appl Physiol Nutr Metab. 2018;43(9):920–7. [DOI] [PubMed] [Google Scholar]
- 51.Garnacho-Castano MV, Palau-Salva G, Cuenca E et al. Effects of a single dose of beetroot juice on cycling time trial performance at ventilatory thresholds intensity in male triathletes. J Int Soc Sports Nutr. 2018;15(1):49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Glaister M, Pattison JR, Muniz-Pumares D, Patterson SD, Foley P. Effects of dietary nitrate, caffeine, and their combination on 20-km cycling time trial performance. J Strength Cond Res. 2015;29(1):165–74. [DOI] [PubMed] [Google Scholar]
- 53.Hoon MW, Jones AM, Johnson NA et al. The effect of variable doses of inorganic nitrate-rich beetroot juice on simulated 2,000-m rowing performance in trained athletes. Int J Sports Physiol Perform. 2014;9(4):615–20. [DOI] [PubMed] [Google Scholar]
- 54.Jonvik KL, Nyakayiru J, Van Dijk JW et al. Repeated-sprint performance and plasma responses following beetroot juice supplementation do not differ between recreational, competitive and elite sprint athletes. Eur J Sport Sci. 2018;18(4):524–33. [DOI] [PubMed] [Google Scholar]
- 55.Bourdillon N, Fan JL, Uva B, Muller H, Meyer P, Kayser B. Effect of oral nitrate supplementation on pulmonary hemodynamics during exercise and time trial performance in normoxia and hypoxia: a randomized controlled trial. Front Physiol. 2015;6:288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Callahan MJ, Parr EB, Hawley JA, Burke LM. Single and Combined Effects of Beetroot Crystals and Sodium Bicarbonate on 4-km Cycling Time Trial Performance. Int J Sport Nutr Exerc Metab. 2017;27(3):271–8. [DOI] [PubMed] [Google Scholar]
- 57.Christensen PM, Nyberg M, Bangsbo J. Influence of nitrate supplementation on VO(2) kinetics and endurance of elite cyclists. Scand J Med Sci Sports. 2013;23(1):e21–31. [DOI] [PubMed] [Google Scholar]
- 58.Cuenca E, Jodra P, Perez-Lopez A et al. Effects of Beetroot Juice Supplementation on Performance and Fatigue in a 30-s All-Out Sprint Exercise: A Randomized, Double-Blind Cross-Over Study. Nutrients. 2018;10(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dominguez R, Garnacho-Castano MV, Cuenca E et al. Effects of Beetroot Juice Supplementation on a 30-s High-Intensity Inertial Cycle Ergometer Test. Nutrients. 2017;9(12):1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gasier HG, Reinhold AR, Loiselle AR, Soutiere SE, Fothergill DM. Effects of oral sodium nitrate on forearm blood flow, oxygenation and exercise performance during acute exposure to hypobaric hypoxia (4300 m). Nitric Oxide. 2017;69:1–9. [DOI] [PubMed] [Google Scholar]
- 61.Handzlik MK, Gleeson M. Likely additive ergogenic effects of combined preexercise dietary nitrate and caffeine ingestion in trained cyclists. ISRN Nutr. 2013;2013:396581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Breese BC, McNarry MA, Marwood S, Blackwell JR, Bailey SJ, Jones AM. Beetroot juice supplementation speeds O2 uptake kinetics and improves exercise tolerance during severe-intensity exercise initiated from an elevated metabolic rate. Am J Physiol Regul Integr Comp Physiol. 2013;305(12):R1441–50. [DOI] [PubMed] [Google Scholar]
- 63.Cermak NM, Gibala MJ, van Loon LJ. Nitrate supplementation’s improvement of 10-km time-trial performance in trained cyclists. Int J Sport Nutr Exerc Metab. 2012;22(1):64–71. [DOI] [PubMed] [Google Scholar]
- 64.Coggan AR, Leibowitz JL, Kadkhodayan A et al. Effect of acute dietary nitrate intake on maximal knee extensor speed and power in healthy men and women. Nitric Oxide. 2015;48:16–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Horiuchi M, Endo J, Dobashi S, Handa Y, Kiuchi M, Koyama K. Muscle oxygenation profiles between active and inactive muscles with nitrate supplementation under hypoxic exercise. Physiol Rep. 2017;5(20). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kelly J, Vanhatalo A, Wilkerson DP, Wylie LJ, Jones AM. Effects of nitrate on the power-duration relationship for severe-intensity exercise. Med Sci Sports Exerc. 2013;45(9):1798–806. [DOI] [PubMed] [Google Scholar]
- 67.Kent GL, Dawson B, Cox GR et al. Dietary nitrate supplementation does not improve cycling time-trial performance in the heat. J Sports Sci. 2018;36(11):1204–11. [DOI] [PubMed] [Google Scholar]
- 68.Kramer SJ, Baur DA, Spicer MT, Vukovich MD, Ormsbee MJ. The effect of six days of dietary nitrate supplementation on performance in trained CrossFit athletes. J Int Soc Sports Nutr. 2016;13:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kelly J, Vanhatalo A, Bailey SJ et al. Dietary nitrate supplementation: effects on plasma nitrite and pulmonary O2 uptake dynamics during exercise in hypoxia and normoxia. Am J Physiol Regul Integr Comp Physiol. 2014;307(7):R920–30. [DOI] [PubMed] [Google Scholar]
- 70.Husmann F, Bruhn S, Mittlmeier T, Zschorlich V, Behrens M. Dietary Nitrate Supplementation Improves Exercise Tolerance by Reducing Muscle Fatigue and Perceptual Responses. Front Physiol. 2019;10:404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hoon MW, Hopkins WG, Jones AM et al. Nitrate supplementation and high-intensity performance in competitive cyclists. Appl Physiol Nutr Metab. 2014;39(9):1043–9. [DOI] [PubMed] [Google Scholar]
- 72.Ghiarone T, Ataide-Silva T, Bertuzzi R, McConell GK, Lima-Silva AE. Effect of acute nitrate ingestion on VO2 response at different exercise intensity domains. Appl Physiol Nutr Metab. 2017;42(11):1127–34. [DOI] [PubMed] [Google Scholar]
- 73.Fulford J, Winyard PG, Vanhatalo A, Bailey SJ, Blackwell JR, Jones AM. Influence of dietary nitrate supplementation on human skeletal muscle metabolism and force production during maximum voluntary contractions. Pflugers Arch. 2013;465(4):517–28. [DOI] [PubMed] [Google Scholar]
- 74.de Castro TF, Manoel FA, Figueiredo DH, Figueiredo DH, Machado FA. Effect of beetroot juice supplementation on 10-km performance in recreational runners. Appl Physiol Nutr Metab. 2019;44(1):90–4. [DOI] [PubMed] [Google Scholar]
- 75.Le Roux-Mallouf T, Laurent J, Besset D et al. Effects of acute nitric oxide precursor intake on peripheral and central fatigue during knee extensions in healthy men. Exp Physiol. 2019;104(7):1100–14. [DOI] [PubMed] [Google Scholar]
- 76.Lee S, Abel MG, Thomas T, Symons TB, Yates JW. Acute beetroot juice supplementation does not attenuate knee extensor exercise muscle fatigue in a healthy young population. J Exerc Nutrition Biochem. 2019;23(1):55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lowings S, Shannon OM, Deighton K, Matu J, Barlow MJ. Effect of Dietary Nitrate Supplementation on Swimming Performance in Trained Swimmers. Int J Sport Nutr Exerc Metab. 2017;27(4):377–84. [DOI] [PubMed] [Google Scholar]
- 78.MacLeod KE, Nugent SF, Barr SI, Koehle MS, Sporer BC, MacInnis MJ. Acute Beetroot Juice Supplementation Does Not Improve Cycling Performance in Normoxia or Moderate Hypoxia. Int J Sport Nutr Exerc Metab. 2015;25(4):359–66. [DOI] [PubMed] [Google Scholar]
- 79.Martin K, Smee D, Thompson KG, Rattray B. No improvement of repeated-sprint performance with dietary nitrate. Int J Sports Physiol Perform. 2014;9(5):845–50. [DOI] [PubMed] [Google Scholar]
- 80.Masschelein E, Van Thienen R, Wang X, Van Schepdael A, Thomis M, Hespel P. Dietary nitrate improves muscle but not cerebral oxygenation status during exercise in hypoxia. J Appl Physiol (1985). 2012;113(5):736–45. [DOI] [PubMed] [Google Scholar]
- 81.McQuillan JA, Casadio JR, Dulson DK, Laursen PB, Kilding AE. The Effect of Nitrate Supplementation on Cycling Performance in the Heat in Well-Trained Cyclists. Int J Sports Physiol Perform. 2018;13(1):50–6. [DOI] [PubMed] [Google Scholar]
- 82.McQuillan JA, Dulson DK, Laursen PB, Kilding AE. Dietary Nitrate Fails to Improve 1 and 4 km Cycling Performance in Highly Trained Cyclists. Int J Sport Nutr Exerc Metab. 2017;27(3):255–63. [DOI] [PubMed] [Google Scholar]
- 83.Montenegro CF, Kwong DA, Minow ZA, Davis BA, Lozada CF, Casazza GA. Betalain-rich concentrate supplementation improves exercise performance and recovery in competitive triathletes. Appl Physiol Nutr Metab. 2017;42(2):166–72. [DOI] [PubMed] [Google Scholar]
- 84.Mosher SL, Sparks SA, Williams EL, Bentley DJ, Mc Naughton LR. Ingestion of a Nitric Oxide Enhancing Supplement Improves Resistance Exercise Performance. J Strength Cond Res. 2016;30(12):3520–4. [DOI] [PubMed] [Google Scholar]
- 85.Muggeridge DJ, Howe CC, Spendiff O, Pedlar C, James PE, Easton C. The effects of a single dose of concentrated beetroot juice on performance in trained flatwater kayakers. Int J Sport Nutr Exerc Metab. 2013;23(5):498–506. [DOI] [PubMed] [Google Scholar]
- 86.Muggeridge DJ, Howe CC, Spendiff O, Pedlar C, James PE, Easton C. A single dose of beetroot juice enhances cycling performance in simulated altitude. Med Sci Sports Exerc. 2014;46(1):143–50. [DOI] [PubMed] [Google Scholar]
- 87.Mumford PW, Kephart WC, Romero MA et al. Effect of 1-week betalain-rich beetroot concentrate supplementation on cycling performance and select physiological parameters. Eur J Appl Physiol. 2018;118(11):2465–76. [DOI] [PubMed] [Google Scholar]
- 88.Murphy M, Eliot K, Heuertz RM, Weiss E. Whole beetroot consumption acutely improves running performance. J Acad Nutr Diet. 2012;112(4):548–52. [DOI] [PubMed] [Google Scholar]
- 89.Nyakayiru J, Jonvik KL, Trommelen J et al. Beetroot Juice Supplementation Improves High-Intensity Intermittent Type Exercise Performance in Trained Soccer Players. Nutrients. 2017;9(3):314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nyakayiru JM, Jonvik KL, Pinckaers PJ, Senden J, van Loon LJ, Verdijk LB. No Effect of Acute and 6-Day Nitrate Supplementation on VO2 and Time-Trial Performance in Highly Trained Cyclists. Int J Sport Nutr Exerc Metab. 2017;27(1):11–7. [DOI] [PubMed] [Google Scholar]
- 91.Nyback L, Glannerud C, Larsson G, Weitzberg E, Shannon OM, McGawley K. Physiological and performance effects of nitrate supplementation during roller-skiing in normoxia and normobaric hypoxia. Nitric Oxide. 2017;70:1–8. [DOI] [PubMed] [Google Scholar]
- 92.Oskarsson J, McGawley K. No individual or combined effects of caffeine and beetroot-juice supplementation during submaximal or maximal running. Appl Physiol Nutr Metab. 2018;43(7):697–703. [DOI] [PubMed] [Google Scholar]
- 93.Pawlak-Chaouch M, Boissiere J, Munyaneza D et al. Beetroot Juice Does Not Enhance Supramaximal Intermittent Exercise Performance in Elite Endurance Athletes. J Am Coll Nutr. 2019;38(8):729–38. [DOI] [PubMed] [Google Scholar]
- 94.Peacock O, Tjonna AE, James P et al. Dietary nitrate does not enhance running performance in elite cross-country skiers. Med Sci Sports Exerc. 2012;44(11):2213–9. [DOI] [PubMed] [Google Scholar]
- 95.Peeling P, Cox GR, Bullock N, Burke LM. Beetroot Juice Improves On-Water 500 M Time-Trial Performance, and Laboratory-Based Paddling Economy in National and International-Level Kayak Athletes. Int J Sport Nutr Exerc Metab. 2015;25(3):278–84. [DOI] [PubMed] [Google Scholar]
- 96.Porcelli S, Ramaglia M, Bellistri G et al. Aerobic Fitness Affects the Exercise Performance Responses to Nitrate Supplementation. Med Sci Sports Exerc. 2015;47(8):1643–51. [DOI] [PubMed] [Google Scholar]
- 97.Rimer EG, Peterson LR, Coggan AR, Martin JC. Increase in Maximal Cycling Power With Acute Dietary Nitrate Supplementation. Int J Sports Physiol Perform. 2016;11(6):715–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rokkedal-Lausch T, Franch J, Poulsen MK et al. Chronic high-dose beetroot juice supplementation improves time trial performance of well-trained cyclists in normoxia and hypoxia. Nitric Oxide. 2019;85:44–52. [DOI] [PubMed] [Google Scholar]
- 99.Rossetti GMK, Macdonald JH, Wylie LJ et al. Dietary nitrate supplementation increases acute mountain sickness severity and sense of effort during hypoxic exercise. J Appl Physiol (1985). 2017;123(4):983–92. [DOI] [PubMed] [Google Scholar]
- 100.Sandbakk SB, Sandbakk O, Peacock O et al. Effects of acute supplementation of L-arginine and nitrate on endurance and sprint performance in elite athletes. Nitric Oxide. 2015;48:10–5. [DOI] [PubMed] [Google Scholar]
- 101.Shannon OM, Barlow MJ, Duckworth L et al. Dietary nitrate supplementation enhances short but not longer duration running time-trial performance. Eur J Appl Physiol. 2017;117(4):775–85. [DOI] [PubMed] [Google Scholar]
- 102.Shannon OM, Duckworth L, Barlow MJ et al. Effects of Dietary Nitrate Supplementation on Physiological Responses, Cognitive Function, and Exercise Performance at Moderate and Very-High Simulated Altitude. Front Physiol. 2017;8(401):401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shannon OM, Duckworth L, Barlow MJ et al. Dietary nitrate supplementation enhances high-intensity running performance in moderate normobaric hypoxia, independent of aerobic fitness. Nitric Oxide. 2016;59:63–70. [DOI] [PubMed] [Google Scholar]
- 104.Smith K, Muggeridge DJ, Easton C, Ross MD. An acute dose of inorganic dietary nitrate does not improve high-intensity, intermittent exercise performance in temperate or hot and humid conditions. Eur J Appl Physiol. 2019;119(3):723–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tan R, Wylie LJ, Thompson C et al. Beetroot juice ingestion during prolonged moderate-intensity exercise attenuates progressive rise in O2 uptake. J Appl Physiol (1985). 2018;124(5):1254–63. [DOI] [PubMed] [Google Scholar]
- 106.Thompson C, Vanhatalo A, Jell H et al. Dietary nitrate supplementation improves sprint and high-intensity intermittent running performance. Nitric Oxide. 2016;61:55–61. [DOI] [PubMed] [Google Scholar]
- 107.Thompson KG, Turner L, Prichard J et al. Influence of dietary nitrate supplementation on physiological and cognitive responses to incremental cycle exercise. Respir Physiol Neurobiol. 2014;193:11–20. [DOI] [PubMed] [Google Scholar]
- 108.Tillin NA, Moudy S, Nourse KM, Tyler CJ. Nitrate Supplement Benefits Contractile Forces in Fatigued but Not Unfatigued Muscle. Med Sci Sports Exerc. 2018;50(10):2122–31. [DOI] [PubMed] [Google Scholar]
- 109.Van Hoorebeke JS, Trias CO, Davis BA, Lozada CF, Casazza GA. Betalain-Rich Concentrate Supplementation Improves Exercise Performance in Competitive Runners. Sports (Basel). 2016;4(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Vanhatalo A, Fulford J, Bailey SJ, Blackwell JR, Winyard PG, Jones AM. Dietary nitrate reduces muscle metabolic perturbation and improves exercise tolerance in hypoxia. J Physiol. 2011;589(Pt 22):5517–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Vasconcellos J, Henrique Silvestre D, Dos Santos Baiao D, Werneck-de-Castro JP, Silveira Alvares T, Paschoalin VM. A Single Dose of Beetroot Gel Rich in Nitrate Does Not Improve Performance but Lowers Blood Glucose in Physically Active Individuals. J Nutr Metab. 2017;2017:7853034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wickham KA, McCarthy DG, Pereira JM et al. No effect of beetroot juice supplementation on exercise economy and performance in recreationally active females despite increased torque production. Physiol Rep. 2019;7(2):e13982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wilkerson DP, Hayward GM, Bailey SJ, Vanhatalo A, Blackwell JR, Jones AM. Influence of acute dietary nitrate supplementation on 50 mile time trial performance in well-trained cyclists. Eur J Appl Physiol. 2012;112(12):4127–34. [DOI] [PubMed] [Google Scholar]
- 114.Wylie LJ, Bailey SJ, Kelly J, Blackwell JR, Vanhatalo A, Jones AM. Influence of beetroot juice supplementation on intermittent exercise performance. Eur J Appl Physiol. 2016;116(2):415–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wylie LJ, Mohr M, Krustrup P et al. Dietary nitrate supplementation improves team sport-specific intense intermittent exercise performance. Eur J Appl Physiol. 2013;113(7):1673–84. [DOI] [PubMed] [Google Scholar]
- 116.Clayton JA, Collins FS. Policy: NIH to balance sex in cell and animal studies. Nature. 2014;509(7500):282–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bailey SJ, Vanhatalo A, Jones AM. Nitrate in exercise performance In: Bryan NS, Loscalzo J editors. Nitrite and Nitrate in Human Health and Disease Humana Press; 2017, pp. 293–310. [Google Scholar]
- 118.Hoogkamer W, Kipp S, Frank JH, Farina EM, Luo G, Kram R. A Comparison of the Energetic Cost of Running in Marathon Racing Shoes. Sports Med. 2018;48(4):1009–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Omar SA, Artime E, Webb AJ. A comparison of organic and inorganic nitrates/nitrites. Nitric Oxide. 2012;26(4):229–40. [DOI] [PubMed] [Google Scholar]
- 120.McNally B, Griffin JL, Roberts LD. Dietary inorganic nitrate: From villain to hero in metabolic disease? Mol Nutr Food Res. 2016;60(1):67–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Liddle L, Burleigh MC, Monaghan C et al. Variability in nitrate-reducing oral bacteria and nitric oxide metabolites in biological fluids following dietary nitrate administration: An assessment of the critical difference. Nitric Oxide. 2019;83:1–10. [DOI] [PubMed] [Google Scholar]
- 122.Duncan C, Dougall H, Johnston P et al. Chemical generation of nitric oxide in the mouth from the enterosalivary circulation of dietary nitrate. Nat Med. 1995;1(6):546–51. [DOI] [PubMed] [Google Scholar]
- 123.Vanhatalo A, Blackwell JR, L’Heureux JE et al. Nitrate-responsive oral microbiome modulates nitric oxide homeostasis and blood pressure in humans. Free Radic Biol Med. 2018;124:21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Govoni M, Jansson EA, Weitzberg E, Lundberg JO. The increase in plasma nitrite after a dietary nitrate load is markedly attenuated by an antibacterial mouthwash. Nitric Oxide. 2008;19(4):333–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. docx—Included studies characteristics and results.


