Case presentation
A 69 year old female patient presented with 5 days of intermittent chest pain in a referring institution. The patient is an active smoker, has a history of chronic obstructive pulmonary disease and a family history of coronary artery disease. The electrocardiogram revealed inferolateral repolarization disorders identical to prior ECGs. High sensitive troponin peaked at 2587 ng/L (162x/ULN) and renal function was normal. With a diagnosis of high risk non-ST segment elevation myocardial infarction the patient underwent urgent coronary angiography at the referring non percutaneous coronary intervention (PCI) institution. Angiography showed non-significant disease in the left coronary system and an unusual filling defect in the middle segment of the right coronary artery (RCA) suspected of being either a recanalized thrombus or a calcified lesion with TIMI III flow.
The patient was referred to our institution for re-evaluation and potential treatment of the RCA 3 days later and coronary angiography confirmed the initially observed intraluminal filling defect (figure 1A). The patient was included in the POLARIS-I registry, designed to assess the added value of polarization sensitive optical frequency domain imaging (PS-OFDI) in patients presenting with acute coronary syndrome. PS-OFDI provides polarimetric measurements that are intrinsically co-registered with the standard intensity data through a conventional OFDI catheter (Fastview, Terumo) (1). The intensity data revealed a honeycomb-like structure with multiple intraluminal microchannels confirming the presence of a recanalized thrombus (figure 2). The lesion site was subsequently treated with a 3.5 × 18 mm Resolute Onyx Zotarolimus eluting stent (Medtronic, Santa Rosa, California, USA).
Figure 1.
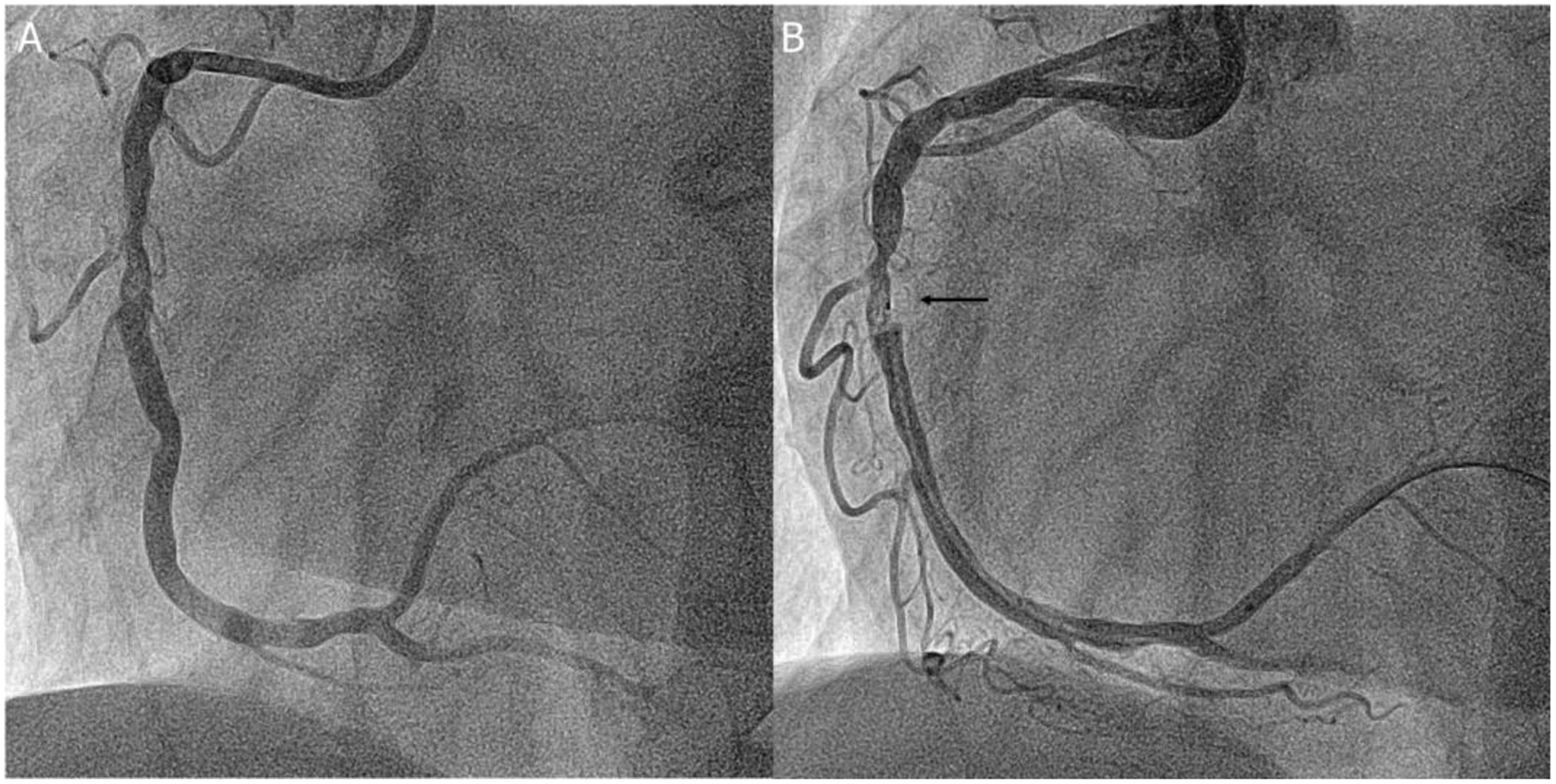
Coronary angiography of the right coronary artery
A: Coronary angiography of the right coronary artery; B: The black arrow denoted the imaging probe of the PS-OFDI during the automated pullback
Figure 2.
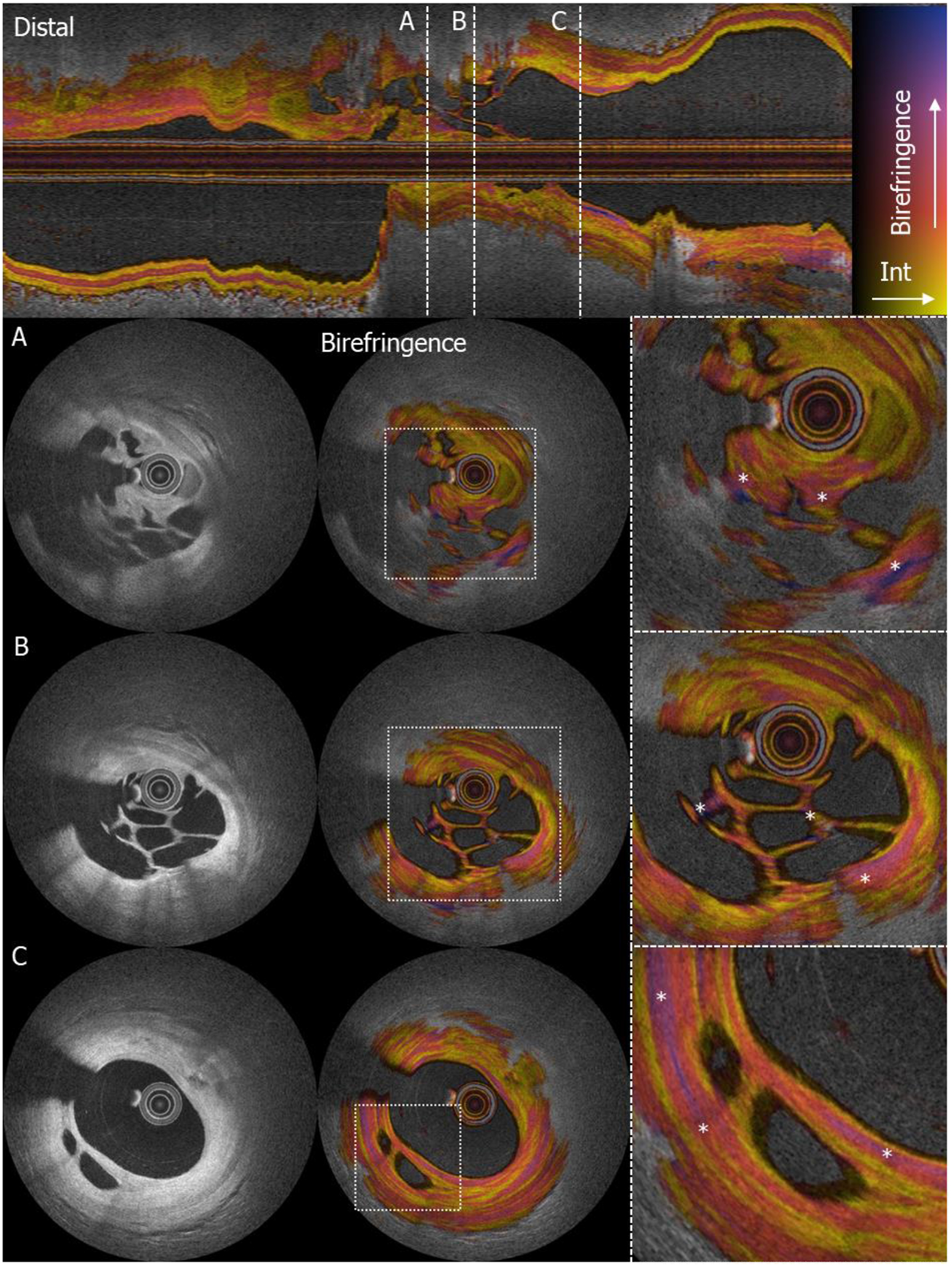
Intensity and polarimetric properties of organized thrombus with microchannels
Upper panel: longitudinal view of the culprit lesion in the right coronary artery. The dashed white lines denote the cross-sectional locations for A, B and C. (A,B,C) Three locations with clear recanalization in organized thrombus; from left to right: intensity image, birefringence image, zoomed in birefringence image (* denotes zones of high birefringence in the microchannels).
Previous histopathological work identified three different stages in the evolution of coronary thrombus (2). First, fresh thrombus (<1 day) which is comprised of platelet aggregates, erythrocytes, intact granulocytes, and fibrin is formed. Second, fresh thrombus evolves into lytic thrombus (1 to 5 days), featuring areas of necrosis and granulocytes which slowly transform into organized thrombus (>5 days), characterized by the presence of smooth muscle cells (SMCs), homogeneous or hyaline fibrin, and depositions of connective tissue and capillary vessel ingrowth (3, 4). Optical Coherene Tomography and OFDI permit the operator to accurately identify thrombus characteristics and to differentiate red from white thrombus (5, 6). Pioneering research with PS-OFDI in cadaveric human hearts by Villiger et al. demonstrated the ability of PS-OFDI to quantitatively measure birefringence and use this endogenous contrast to differentiate between morphometric plaque properties (1). Especially collagen and SMCs display higher birefringence as compared to other tissue due to their fibrillary architecture (1).
In the present case, (figure 2 (* in right panel)), high birefringence signals can be recognized in the connective tissue between the microchannels indicating a high likelihood for presence of collagen and SMCs.
To date, the progression and consistency of thrombus can only be reliably assessed using histopathological examination, which has no place in an acute setting (4). PS-OFDI might be a valuable new imaging modality to help further study age, stability and morphometric characteristics of coronary thrombus.
Funding:
This work was supported by the National Institutes of Health (grants P41EB-015903 and R01HL-119065) and by Terumo Corporation. Dr. Bouma was supported in part by the Professor Andries Querido visiting professorship of the Erasmus University Medical Center in Rotterdam. Dr. Otsuka acknowledges partial support from the Japan Heart Foundation/ Bayer Yakuhin Research Grant Abroad, the Uehara Memorial Foundation Postdoctoral Fellowship, and the Japan Society for the Promotion of Science Overseas Research Fellowship.
Footnotes
Disclosures: Massachusetts General Hospital and the Erasmus University Medical Center have patent licensing arrangements with Terumo Corporation. Drs. Bouma and Villiger have the right to receive royalties as part of the licensing arrangements.
References
- 1.Villiger M, Otsuka K, Karanasos A, et al. Coronary Plaque Microstructure and Composition Modify Optical Polarization: A New Endogenous Contrast Mechanism for Optical Frequency Domain Imaging. J Am Coll Cardiol Img. 2018;11(11):1666–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Silvain J, Collet J-P, Nagaswami C, et al. Composition of coronary thrombus in acute myocardial infarction. J Am Coll Cardiol. 2011;57(12):1359–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carol A, Bernet M, Curos A, et al. Thrombus age, clinical presentation, and reperfusion grade in myocardial infarction. Cardiovasc Pathol 2014;23(3):126–30. [DOI] [PubMed] [Google Scholar]
- 4.Rittersma SZ, van der Wal AC, Koch KT, et al. Plaque instability frequently occurs days or weeks before occlusive coronary thrombosis: a pathological thrombectomy study in primary percutaneous coronary intervention. Circulation 2005;111(9):1160–5. [DOI] [PubMed] [Google Scholar]
- 5.Kume T, Akasaka T, Kawamoto T, et al. Assessment of coronary arterial thrombus by optical coherence tomography. Am J Cardiol 2006;97(12):1713–7. [DOI] [PubMed] [Google Scholar]
- 6.Porto I, Mattesini A, Valente S, Prati F, Crea F, Bolognese L. Optical coherence tomography assessment and quantification of intracoronary thrombus: Status and perspectives. Cardiovasc Revasc Med 2015;16(3):172–8. [DOI] [PubMed] [Google Scholar]


