Abstract
Virus-like particles (VLPs) present viral antigens in a native conformation and are effectively recognized by the immune system and therefore are considered as suitable and safe vaccine candidates against many viral diseases.
Here we demonstrate that chimeric VLPs containing Rift Valley fever virus (RVFV) glycoproteins GN and GC, nucleoprotein N and the gag protein of Moloney murine leukemia virus represent an effective vaccine candidate against Rift Valley fever, a deadly disease in humans and livestock. Long-lasting humoral and cellular immune responses are demonstrated in a mouse model by the analysis of neutralizing antibody titers and cytokine secretion profiles. Vaccine efficacy studies were performed in mouse and rat lethal challenge models resulting in high protection rates.
Taken together, these results demonstrate that replication-incompetent chimeric RVF VLPs are an efficient RVFV vaccine candidate.
Introduction
Rift Valley fever virus (RVFV) is a devastating mosquito-borne viral zoonotic disease that causes serious morbidity and mortality in both humans and livestock. In ruminants, RVF is characterized by substantial mortality of young animals (especially of lambs), fetal deformities and abortion (Flick and Bouloy, 2005; Gerdes, 2004; Swanepoel and Coetzer, 2003). In humans the disease is often associated with benign fever but can lead to more complicated cases such as retinal vasculitis, encephalitis, neurologic deficits, hepatic necrosis, or fatal hemorrhagic fever (Flick and Bouloy, 2005; Geisbert and Jahrling, 2004; Meegan, 1979). Interestingly, human case fatality rates increased significantly during the last several years. While historically less than 2% of infected individuals developed a fatal hemorrhagic fever, analysis from recent outbreaks (2007/2008) reveal a 20 to 30% fatality rate in humans (LaBeaud et al., 2008). However, differences in case definition, accuracy in disease surveillance methods and data gathering methodology likely impact these numbers.
RVFV is a member of the Bunyaviridae family, which includes more than 300 viruses grouped into five genera (Orthobunyavirus, Hantavirus, Nairovirus, Phlebovirus, and Tospovirus). Bunyaviruses are enveloped viruses with a tripartite, single-stranded RNA genome of negative and sometimes ambisense polarity (Elliott, 1996; Elliott, Schmaljohn, and Collett, 1991; Schmaljohn and Hooper, 2001). The large (L) genomic RNA segment encodes the RNA-dependent RNA polymerase (L), the medium (M) segment the glycoprotein precursor, which is post-translationally processed into the two mature spike proteins (G1 and G2, or by the new convention: GN and GC), and in some viruses a non-structural protein (NSM), while the small (S) segment encodes the nucleoprotein (N), and in some viruses a non-structural protein NSS (Elliott, 1996; Schmaljohn and Hooper, 2001).
RVFV has traditionally caused recurrent outbreaks affecting humans and ruminants predominantly in Sub-Saharan Africa, but spread to Egypt in 1977 and to the Arabian Peninsula in 2000 (Al-Hazmi et al., 2003; Anonymous, 2000; Balkhy and Memish, 2003; Madani et al., 2003; Shoemaker et al., 2002). More recently, RVFV circulated in East Africa causing serious epidemics in Kenya, Tanzania, Somalia, Sudan and was reported in the Comoros Islands (LaBeaud et al., 2008; WHO, 2007) in 2007, and subsequently expanded to Madagascar and South Africa in 2008 [http://www.fao.org/docs/eims/upload//242253/EW_rvf_apr08.pdf].
RVFV is a prototype of emerging/re-emerging pathogens and is classified as a Category A High Priority Pathogen by the National Institute for Allergy and Infectious Diseases (NIAID) (http://www3.niaid.nih.gov/topics/BiodefenseRelated/Biodefense/research/CatA.htm), is on the Center for Disease Control (CDC) Bioterror Agent list (http://www.bt.cdc.gov/agent/agentlist-category.asp#a) and is also classified as a Department of Health and Human Services (HHS), United States Department of Agriculture (USDA) overlap Select Agent (USDA, 2005).
The lack of prophylactic and therapeutic measures, the potential for human-to-human transmission, and the significant threat to livestock associated with RVFV make infection with this pathogen a serious public health concern not only in endemic, developing countries, but also in many non-endemic developed countries due to recent bioterror threats, and clearly illustrates the need for more RVFV vaccine research and development.
MP12, a highly attenuated (by 5-fluorouracil treatment in cell culture) human virus isolate of RVFV (Caplen, Peters, and Bishop, 1985; Vialat et al., 1997), has recently been tested in a phase II safety/efficacy clinical trial (ClinicalTrials.gov identifier: NCT00415051) to determine if it is safe to give to humans (results not yet published). MP-12 also has potential veterinary applications (Hunter, Erasmus, and Vorster, 2002).
A formalin-inactivated RVFV vaccine, TSI-GSD-200, has been developed, however it is not licensed and not commercially available (Pittman et al., 1999). TSI-GSD-200 is only provided to veterinarians working in endemic areas, high containment laboratory workers and others at high risk for contracting RVFV (Pittman et al., 1999). Unfortunately, this vaccine is (i) expensive, (ii) difficult to produce, (iii) in short supply, (iv) requires larger dose relative to an attenuated vaccine and three initial inoculations followed by a 6-month booster (v) and requires continued annual boosters to maintain protective immunity (Frank-Peterside, 2000; Kark, Aynor, and Peters, 1982; Kark, Aynor, and Peters, 1985; Niklasson et al., 1985).
The use of virus like particles (VLPs) is a promising approach for the development of a safe and efficient RVFV vaccine. Expression of structural proteins of many non-enveloped and enveloped viruses leads to the formation of VLPs (Garcea and Gissmann, 2004; Grgacic and Anderson, 2006; Noad and Roy, 2003). Such VLPs frequently exhibit a morphology very similar to that of wild-type (wt) viruses (Johnson and Chiu, 2000). Since VLPs have a tropism similar to that of the wt virus, and show comparable cellular uptake and intracellular trafficking, the formation of VLPs can be used to study virus assembly and morphogenesis, budding processes, genome packaging, receptor binding, and virus entry (Bos, Luytjes, and Spaan, 1997; Johnson and Chiu, 2000; Li et al., 2003; Licata et al., 2004; Overby et al., 2006; Schmitt et al., 2002; Ye et al., 2006). Especially for viruses classified as high containment agents, e.g., RVFV and Ebolavirus, the development of VLP systems are of practical use because subsequent work can be performed under lower biosafety conditions (Habjan et al., 2009; Naslund et al., 2009; Warfield et al., 2004; Watanabe et al., 2004). VLPs present viral antigens in a native conformation and are effectively recognized by the immune system (Grgacic, 2006; Grgacic and Anderson, 2006; Noad and Roy, 2003).
Many promising vaccine candidates based on VLPs are at various stages of development, including vaccine candidates for hepatitis B virus (HBV), human papillomavirus (HPV), Norwalk virus, human polyomavirus, Bluetongue virus, rotavirus, retroviruses, bunyaviruses and filoviruses (Garcea and Gissmann, 2004; Grgacic, 2006; Habjan et al., 2009; Naslund et al., 2009; Noad and Roy, 2003).
These promising attempts to generate VLP-based vaccines against many different animal and human pathogens encouraged us to evaluate RVF VLPs as vaccine candidates against RVFV. Here we describe the generation of chimeric RVF VLPs, a novel concept for bunyaviruses, the optimization of VLP production and their successful use as vaccine candidates. Vaccine efficacy was analyzed through immunological studies of vaccinated mice and in lethal challenge studies in two different rodent models. High protection rates and robust and long-lasting immune response of vaccinated animals demonstrate that chimeric RVF VLPs are a promising approach to generate safe and efficient RVFV vaccines.
Materials and Methods
RVFV expression vectors
RVFV glycoprotein (G) expression plasmids
RNA was purified from MP12-infected Vero cells using a QIAamp Viral RNA Mini Kit (QIAGEN). MP12 sequences were chosen as the source of the glycoprotein gene because it allowed us to perform initial challenge studies with MP12 under lower containment conditions (data not shown). cDNA was prepared using RT PCR (Thermoscript RT-PCR Kit) with oligo dT primers. A 3.3kb 5’-truncated fragment of the RVFV M segment was amplified from cDNA using Phusion HF polymerase (Phusion High Fidelity PCR Mastermix, Finnzymes) and RVFV M segment-specific primers: 5’GCAATCGATGCAGGGATTGCAATGACAGTC and 5’GCACTCGAGCTATGAGGCCTTCTTAGTGGCAG. This results in tranlsation of the RVFV glycoprotein precursor from the 4th available start codon to facilitate optimal glycoprotein expression (Collett et al., 1985; Gerrard and Nichol, 2007; Suzich, Kakach, and Collett, 1990). Next, a Kozak sequence (Kozak, 1984a; Kozak, 1984b; Kozak, 1987; Kozak, 2002) was added immediately 5’ to the ATG start codon by PCR amplification using Phusion HF polymerase with the following RVFV M segment specific oligos: 5’TTAATGAATTCGCCACCATGGCAGGGATTGCAATGACAGTCCTTCC and 5’GCACTCGAGCTATGAGGCCTTCTTAGTGGCAG. This PCR product was cloned downstream of the chicken β-actin promoter into the pCAGGS expression vector (Kobasa et al., 1997; Niwa, Yamamura, and Miyazaki, 1991) (kindly provided by Yoshihiro Kawaoka, University of Wisconsin-Madison, USA) via EcoRI/XhoI restriction endonuclease sites. Plasmid DNA used for transfections was prepared (0.323 mg/ml, cesium chloride (CsCl)-purified) by the University of Texas Medical Branch (UTMB) Recombinant DNA Laboratory (Galveston, TX, USA). A codon-optimized (codon usage as described in (Babcock et al., 2004)) version of the truncated RVFV glycoprotein precursor gene was synthesized by GenScript (Piscataway, NJ, USA; GenBank accession number GQ148915) that contains a Kozak sequence immediately 5’ to the ATG start codon and 5’ and 3’ EcoRI and XhoI restriction sites for subsequent cloning steps. This gene was cloned into pCAGGS expression vector via the EcoRI/XhoI restriction endonuclease sites. The plasmid preparations used for transfections was prepared (0.5mg/ml, NucleoBond column-purified (Machery-Nagel) by the Nature Technology Corporation (Lincoln, NE, USA).
RVFV N expression plasmid
RVFV N cDNA was amplified from MP12 RNA by RT-PCR (Thermoscript RT-PCR Kit) with primers specific for the S segment: 5’ATTATGGTACCGCCACCATGGACAACTATCAAGAGCTTGCGATC and 5’ATTATCTCGAGTTAGGCTGCTGTCTTGTAAGCCTGAGC. This generates a product with a Kozak sequence (Kozak, 1984a; Kozak, 1984b; Kozak, 1987; Kozak, 2002) immediately 5’ to the start codon and 5’ and 3’ KpnI and XhoI restriction sites, respectively. This product was cloned into pCAGGS expression vector via KpnI/XhoI restriction endonuclease sites. Plasmid DNA used for transfections was prepared (3.024mg/ml, CsCl-purified) by the Recombinant DNA Laboratory, Sealy Center for Molecular Medicine, UTMB (Galveston, TX, USA).
RVF VLP preparation
293-gag cells (HEK-293 cells (CRL-1573, ATCC) that constitutively express MoMLV gag and pol) were cultured in poly-d-lysine coated 150mm tissue culture dishes (Falcon) at 1.2 × 107 cells per plate in DMEM (Gibco) with 10% FBS (Hyclone) with 1% penicillin/streptomycin (pen/strep, 1000U/ml and 1000µg/ml, Gibco), and 2mM L-glutamine (Gibco) at 37°C, 5% CO2 overnight. Media was removed and transfection of RVFV G and N expression plasmids was performed using Lipofectamine 2000 reagent (Invitrogen) with Opti-MEM I media (Gibco). Transfection media was removed at 4 h post transfection and replaced with 30ml DMEM media plus 10% FBS, 1% pen/strep and 1% L-glutamine. Supernatants were harvested at 24, 36, 48, 72, 96 and 120 h post transfection, and each time the cells were cultured with 30ml of fresh medium. Supernatants were pooled and clarified by centrifugation at 2,700 × g at 4°C for 10 min. Samples were concentrated to 150ml via tangential flow filtration through Pellicon® 2 “Mini” Filter (0.1m2 Biomax® 300K polyethersulfone, screen type C, Millipore). Purification of RVF chimVLPs was performed by centrifugation of concentrated VLP preparations through a 20% sucrose cushion in PBS using Beckman Ultraclear ultracentrifuge tubes in a SW28 rotor at 26,000 rpm at 4°C for 2 h with a Beckman L-80 ultracentrifuge. Samples were then resuspended in 5ml of sterile 0.9% NaCl (Baxter). RVF VLPs from 293 cells were prepared using the same methodology.
Western blot analysis
RVFV chimVLPs were combined with 4x LDS buffer (Invitrogen) and 50mM Dithiothreitol (DTT, Sigma), heated to 95°C for 10 min, then fractionated by NuPAGE 4–12% Bis-Tris Gels (Invitrogen); for protein size comparison a pre-stained protein molecular weight marker (SeeBlue Plus 2, Invitrogen) was used. Proteins were then transferred to methanol-activated PVDF membrane (Invitrogen) which was subsequently incubated 16 h in 1% nonfat dry milk in PBS. Membranes were washed 3X for 10 min in 0.05% Tween20 in PBS and probed with primary antibodies for 1 h at room temperature (RT): monoclonal RVFV GN antibodies at 1:8,000 (ProSci Inc., Poway, CA, USA, 4F8C8, developed against the GN-specific peptide AEDPHLRNRPGKGH), monoclonal RVFV GC antibodies 1:5,000 (ProSci Inc., 14G1B11, developed against the GC-specific peptide QTRNDKTFAASKGN), RVFV N ascites 1:2,000 (kindly provided by Dr. Robert B. Tesh, University of Texas Medical Branch, USA) and rabbit polyclonal MoMLV gag antibodies 1:5,000 (kindly provided by Dr. Chinglai Wang, Emory University, USA) for 1 h at RT. Membranes were washed 3X as above, then incubated with either AP-conjugated goat anti-mouse antibodies at 1:5,000 (for GN, GC, and N; Jackson ImmunoResearch, West Grove, PA, USA ) or AP-conjugated rabbit anti-goat (for MoMLV gag; Southern Biotech, Birmingham, Alabama, USA). Membranes were then washed 3X as described above. Protein bands were visualized using 1-Step NBT/BCIP solution (Pierce). For quantitative analysis, Western blots were analyzed using ImageJ software (Burger and Burge, 2008). Image colors were inverted and background subtracted. The average of three integrated density readings per band was determined. Maximum value was set to equal 100% and the remaining values were converted to percentages relative to the highest reading.
Transmission electron microscopy (TEM)
TEM was performed at the University of Iowa Central Microscopy Research Facility (University of Iowa, USA). 293 cells were fixed 12 h with glutaraldehyde (Acros Organics, Geel, Belgium; final concentration of 2.5%) 60 h post transfection of RVFV G and N expression plasmids. Cells were washed with PBS pH 7.2 and then 3X with 0.1M sodium cacodylate buffer. Cells were then fixed 1 h with 1% osmium tetroxide and washed 3X with 0.1M sodium cacodylate buffer. Subsequently, cells were rinsed in distilled water for 1 min and then treated with 2.5% uranyl acetate in distilled water for 20 min. Cells were then equilibrated into ethanol in three 15 min steps (50, 75 and 95%) and then equilibrated in 2:1 ethanol:epon resin (Epon 12, Ted Pella) and then 1:2 ethanol:epon for 1 h and finally 100% epon for 2 h. Samples were then placed in fresh epon at 65°C for 12 h. Blocks were then subjected to microtomy to generate 70nm thin sections. Sections were counterstained with 5% uranyl acetate for 12 min and lead citrate (Reynold’s) for 7 min. Sections were imaged with a JEOL 1230 transmission electron microscope and Gatan UltraScan 1000 CCD camera.
Sucrose density gradient fractionation
RVF VLPs purified from 293 and 293-gag cells were fractionated by centrifugation at 30,000rpm for 1 h through a discontinuous sucrose gradient (10 – 60%) using a Beckman SW 40 Ti rotor. 2ml fractions were collected and diluted with 12ml of cold PBS. Fractions were then collected by centrifugation at 30,000rpm for 1 h in a Beckman SW 40 Ti rotor, and pellets resuspended in 0.1ml cold PBS. 16µl of each sample was combined with 2.5µl 50mM DTT (Sigma) and 6.25µl LDS Sample Buffer (Invitrogen) and fractionated by SDS-PAGE and analyzed by Western blot. MoMLV gag proteins were detected with the MoMLV gag antibodies used at 1:500 dilution and visualized with a alkaline phosphatase-conjugated rabbit anti-goat IgG (Southern Biotech) at 1:5,000 dilution.
Immunoprecipitation
100µl of RVF chimVLPs and MoMLV gag VLPs were combined with 3µl of RVFV GN antibodies and 5µl of Protein A/G PLUS Agarose beads (Santa Cruz Biotechnology, Santa Cruz, CA) and incubated at 4°C for 14 h on a Labquake shaker (Barnstead, Thermo Scientific). Supernatant and beads were then separated by centrifugation at 1000 × g for 5 min at 4°C. The beads were washed 3X by resuspension in 1ml of 1x PBS and centrifugation at 1000 × g for 5 min at 4°C. After final wash beads were resuspended in 100µl PBS. 37µl of SDS lysis buffer (Invitrogen) and 15µl of DTT were added to 100µl of beads or the 100µl of supernatant and heated to 90°C for 10 min. Samples were analyzed by Western blot using RVFV GN antibodies and MoMLV gag antibodies. Immunoprecipitation results were analyzed by measuring the integrated densities of the specific bands visualized by Western analysis using ImageJ software.
Plaque reduction neutralization titer assays (PRNT80)
Mouse serum samples were separated from whole blood by centrifugation in a microcentrifuge for 5 min and were stored at −80°C until use. Sera were diluted 1:10 in maintenance medium (DMEM, 2% FBS, 1% pen/strep), and titrated in two-fold serial dilution steps. Equal volumes (60µl) of RVFV ZH501 dilution containing approximately 60 plaque-forming units (pfu)/100µl, and serum dilutions were mixed and incubated for 1 h at 37°C and 5% CO2. Confluent monolayers of VeroE6 cells (seeded in 12-well plates) were infected with 100µl of the virus–serum mixtures. After 1 h incubation at 37°C and 5% CO2, the inocula were removed and wells overlaid with a mixture of one part 1.6% Gum Tragacanth (Sigma) and one part 2xMEM (Gibco, Invitrogen) supplemented with 4% FBS (Sigma) and 2% penicillin/streptomycin (Sigma). The plates were incubated at 37°C and 5% CO2 for 3 days and then stained with 0.25% crystal violet in 10% buffered formalin. Plates were then washed and the plaques enumerated. Dilutions of RVFV ZH501 with maintenance medium were used as a positive control. The neutralizing antibody titer of a serum was considered “positive” at the lowest initial serum dilution that resulted in >80% (PRNT80) reduction of the number of plaques as compared to the virus control.
Cytokine secretion from cultured splenocytes
BALB/c, H2d haplotype, α1,3 galactosyltransferase-KO transgenic mice (Thall, Maly, and Lowe, 1995), kindly provided by NewLink Genetics Corporation, were immunized on days 0, 9, and 20 with RVFV chimVLPs. Spleens were harvested from three mice and one PBS-vaccinated control mouse (31 days post final vaccination) and each placed in separate petri dishes with sterile Gey’s Balanced Salt Solution (Sigma). Each spleen was slightly minced and pressed through a Cellector™ Tissue Sieve (Bellco). Cells were filtered through a 40µm filter (BD Falcon 40µm strainer) and centrifuged at 800 × g for 10 min. Cells were harvested by resuspension in Gey’s solution and centrifugation through underlayed Lympholyte-M (Cedarlane Labs, Burlington, NC, USA) at 1,500 × g for 20 min (with no brake). Splenocytes were harvested from the interface and washed 3X in Gey’s solution. Viable cells were plated to 24-well dishes (Corning, Corning, NY, USA) at 8 × 106 cells/ml. 1 × 107 TCID50/ml heat-inactivated flu virus (mouse-adapted A/HK/1/68 strain (Abdel-Motal et al., 2007)) or 5.6 × 105 pfu/ml heat-inactivated RVFV MP-12 (Caplen, Peters, and Bishop, 1985) was added to test wells. Cells were maintained at 37°C, 5% CO2, and 90% humidity. Supernatants were harvested for analysis at 24, 96, and 168 h post-stimulation. Cytokines in cell culture supernatant were analyzed by a bead-based multiplex system (Bioplex, BioRad) according to manufacturer’s instructions. Secretion of the following was assessed: IL-2, IL-4, IL-5, and IFN-γ.
Mouse challenge experiments
BALB/c mice (as described above) were immunized subcutaneously (s.c.) 3X with ~6µg (total protein) of RVF VLP-based vaccine candidates combined with 100µl of Sigma Adjuvant System (Sigma; prepared according to manufacturers recommendations) at 9-day intervals. For challenge, mice were transferred to the Robert E. Shope Biosafety Level 4 (BSL-4) facility at the University of Texas Medical Branch at Galveston for intraperitoneal (i.p.) challenge with 1000 pfu of RVFV ZH501. Mice were monitored daily for signs of disease for a 2 to 3 week period. All animal experiments were approved by Iowa State University and the University of Texas Medical Branch Internal Animal Care and Use Committees (IACUC).
Rat challenge experiments
Eight week old female Wistar-Furth rats (Harlan Laboratories, Indianapolis, IN) were immunized 3X at 2 week intervals i.p. with 1ml of RVF chimVLP vaccine candidate or 1ml sterile saline (Baxter, Deerfield, IL) combined with 250µl of Sigma Adjuvant System (prepared according to manufacturers recommendations). Rats were transferred to the Robert E. Shope BSL-4 facility at the University of Texas Medical Branch at Galveston for subcutaneous (s.c.) challenge with 105 pfu of RVFV ZH501 60 days post final booster. Rats were monitored daily for weight change and signs of disease. All animal experiments were approved by Iowa State University and the University of Texas Medical Branch IACUC committees.
Results
Generation and characterization of RVF VLPs
RVF VLPs were generated by transient transfection of HEK-293 or 293-gag cells (HEK-293 cells that constitutively express Moloney murine leukemia virus (MoMLV) gag protein) with expression plasmids encoding the RVFV glycoproteins and the nucleoprotein (N). We initially focused upon the generation of chimeric (MoMLV gag-containing VLPs, designated RVF chimVLPs) because it has been previously shown that the inclusion of retroviral gag can increase the uniformity and quantity ((Gheysen et al., 1989; Haffar et al., 1990; Haynes et al., 1991; Rovinski et al., 1992; Szecsi et al., 2006)) and stability (Hammonds et al., 2003) of VLPs. In addition, MoMLV gag protein could have an adjuvant-like effect. RVF VLPs were harvested from tissue culture supernatants from 48 to 96h post transfection, concentrated by tangential flow filtration and purified by ultracentrifugation through a 20% sucrose cushion. To analyze VLP components, samples were fractionated by SDS-PAGE and analyzed by Western blots using RVFV and MoMLV-specific antibodies.
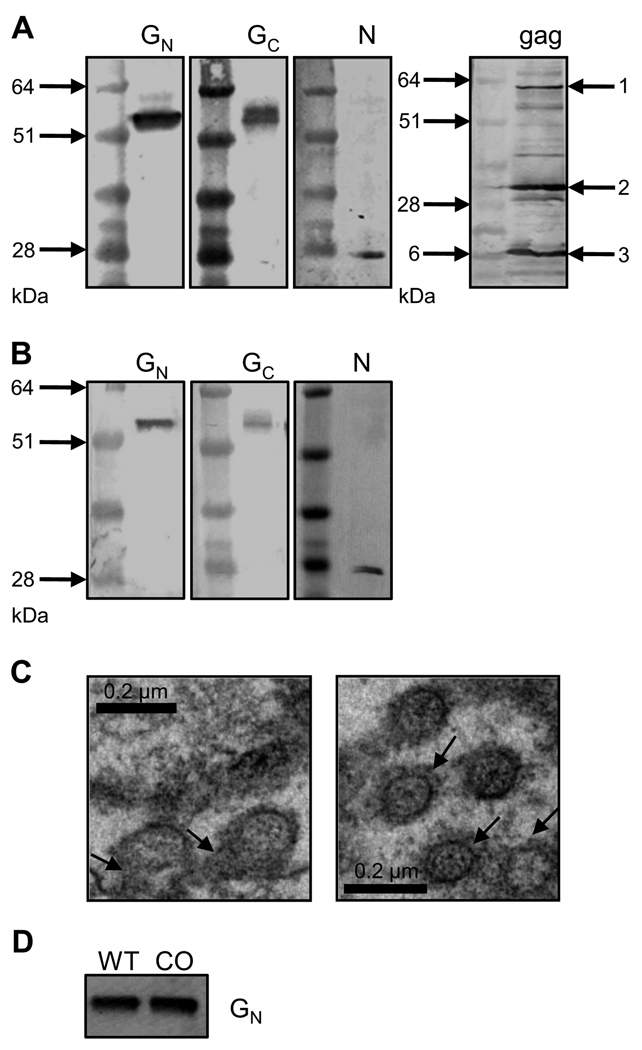
Specific signals were detected confirming the presence of RVFV GN, GC and N in 293 and 293-gag cell-derived VLP preparations (Fig. 1A, first three panels and Fig. 1B), as well as MoMLV gag in the chimeric VLP (chimVLP) preparation derived from 293-gag cells (Fig. 1A, right panel). Note that the gag species are detected as both precursor (arrow 1) and mature, proteolytically processed species (arrows 2 and 3) (Suomalainen, Hultenby, and Garoff, 1996; Yoshinaka et al., 1985).
Figure 1. Characterization of RVF VLPs.
A) Western blot analysis of chimeric RVF VLPs (chimVLP): Concentrated supernatants from 293-gag cells transfected with RVFV G and N expression plasmids analyzed by Western blotting using antibodies specific for RVFV GN, GC, N and Moloney murine leukemia virus (MoMLV) gag. B) Western blot analysis of RVF VLPs: Concentrated supernatants from 293 cells transfected with RVFV G and N expression plasmids analyzed by Western blotting using antibodies specific for RVFV GN, GC and N. C) Negative staining of RVFV G and N transfected 293 cells fixed with glutaraldehyde and stained with uranyl acetate and examined by transmission electron microscopy. Scale bar represents 200nm. Left panel: arrows point to budding VLPs; right panel: arrows indicate RVFV G spikes protruding from the VLP membrane. D) Western blot analysis of RVF chimVLPs: Concentrated supernatants from 293-gag cells transfected with RVFV N and wild type (WT) or codon-optimized (CO) RVFV G sequences.
Transmission electron microscopy was used to analyze the structure of the generated VLP preparations, demonstrating their uniform nature and size. Newly synthesized VLPs are formed at intracellular membranes, presumably the Golgi apparatus (based on studies of bunyavirus glycoproteins, (Andersson et al., 1997; Gerrard and Nichol, 2002; Haferkamp et al., 2005; Shi and Elliott, 2007)), and bud into vesicles analogous to RVF virions (Fig. 1C, left panel). RVFV glycoprotein spikes embedded in the VLP membranes are visible (Fig. 1C, right panel, arrows).
We next attempted to optimize RVF VLP production by using expression plasmids containing a codon-optimized RVFV G gene. Suboptimal codon usage often leads to the inefficient expression of viral protein genes in mammalian cells (Barrett et al., 2006; Haas, Park, and Seed, 1996; Zhou et al., 1999). We therefore compared the amount of RVF chimVLPs generated by 293-gag cells transfected with expression plasmids encoding either native or codon-optimized (Babcock et al., 2004) RVFV G genes. No significant difference in GN content of concentrated and purified VLP preparations was observed when separated by SDS-PAGE and analyzed by Western blot (Fig. 1D). However, the use of the codon-optimized RVFV G appears to have a minimally beneficial effect on VLP yield. Similar results were obtained when RVF VLPs were generated in 293 cells (data not shown). Therefore, for all subsequent experiments the codon-optimized sequence was utilized.
To further characterize the individual components of the RVF VLPs, sucrose density gradient fractionation was employed. This technique has been successfully used in other studies to elucidate the specific components in VLP preparations (Haynes et al., 2009; Ye et al., 2006; Young, Smith, and Ross, 2004). The basis for this approach is that chimVLPs are of a different density than VLPs based on the different protein composition. Results suggest that RVFV GN and MoMLV gag are located within the same particle in the generated chimVLPs (data not shown). Subsequent immunoprecipitation experiments with RVFV GN antibodies and protein A/G-coated agarose beads confirm these results (data not shown).
Optimization of RVF VLP generation
Generation of VLP-based vaccine candidates for RVFV from mammalian cells is labor-intensive and costly in regard to both time and reagents. Therefore it is critical to perform the necessary process development to establish efficient manufacturing procedures. Therefore, additional optimization experiments were performed to maximize RVF VLP production in adherent mammalian cells.
Different amounts of expression plasmids were transfected in the first step to optimize VLP production. Three different amounts (3, 9 and 15µg) of the RVFV G expression plasmid were used and analyzed with increasing amounts of RVFV N expression plasmid (from 0 to 15µg). RVF chimVLPs were harvested at 12 h intervals post-transfection, purified and concentrated as described above and fractionated by SDS-PAGE. RVF chimVLP generation was measured by Western blot analysis using antibodies specific for RVFV GN.
As shown in Fig. 2A, generation of RVF chimVLPs is strongly affected by the amount of the RVFV G- and N-encoding expression plasmids used for transfection of 293-gag cells. A high amount (≥9µg) of RVFV N expression plasmid combined with a lower amount of RVFV G expression plasmid (3µg) (molar ratio RVFV N:G = 4.3) resulted in the best VLP yields (as indicated by GN signal). These conditions also resulted in the most detectable RVFV N in the VLP preparations (data not shown). Interestingly, this analysis also shows that RVF VLPs can be generated without inclusion of the N expression plasmid by transfection of RVFV glycoprotein expression plasmids into 293 cells (Fig. 2A, lanes 4, 8 and 12).
Figure 2. Optimization of RVF VLP production.
A) Different plasmid ratios to determine optimal chimeric RVF VLP production: 293-gag cells were transfected with 15, 9 or 3µg of the RVFV G expression plasmid, and co-transfected with 0, 3, 9 or 15µg RVF N expression plasmid as indicated. Concentrated supernatants of a 60 h post transfection harvest were analyzed by Western blot using antibodies specific for RVFV GN. B) Time course experiment to optimize RVF VLP yields: Western blot analysis was performed using antibodies specific for RVFV GN and GC, as indicated at each blot of RVF chimVLPs and VLPs harvested at select times post-transfection. Densitometric analysis of band intensity, displayed as % maximum band intensity for a particular blot, is represented by histograms above each Western blot. Harvest times are indicated.
Previous studies demonstrate that the inclusion of a minigenome encoding a reporter gene can be a useful tool for the determination of VLP titer and to potentially increase the N content in VLPs (Habjan et al., 2009; Overby et al., 2006). Therefore, an M segment-based minigenome under the control of a RNA polymerase I promoter (Billecocq et al., 2008) was co-transfected in concert with the RVFV G and N expression plasmids to determine if encapsidation of this minigenome with RVFV N and subsequent packaging into the budding VLPs could increase overall N content. However, no significant change in either VLP yields or N content was observed (data not shown).
Next, the optimized transfection scheme (3µg RVFV G and 9µg RVFV N expression plasmids) was used for the determination of the optimal times to harvest RVF VLPs post-transfection to obtain the best yields. Focusing the time of harvest to a shorter interval will clearly save substantial time and reagents. Therefore, time course experiments were performed in which RVF chimVLPs and VLPs, generated in 293-gag and 293 cells, respectively, were harvested at 12 h intervals post-transfection, purified, concentrated and analyzed by Western blots with antibodies specific for RVFV GN and GC. Maximum RVFV GN and GC signals are observed at the 36 and 60 h time points, indicating that generation of VLPs is optimal between 24 and 60 h post-transfection (Fig. 2B). In addition, these results demonstrate that six harvests, at 24, 36, 48, 60, 72 and 84 h post transfection will recover most of the RVF chimVLPs or RVFV VLPs produced. After optimizing expression strategies, transfection conditions (data not shown), plasmid amounts, plasmid ratios and harvest times, we are able to generate sufficient VLP material for subsequent studies.
Immunogenicity of RVF VLPs
We next addressed the question of whether the generated RVF VLPs can be used as a replication-incompetent vaccine candidate. We first analyzed the immune response in mice (neutralizing antibody titers, durability and cytokine expression levels) induced by RVF VLPs and subsequently performed protection studies in two different rodent models.
Viral vaccine efficacy often correlates with seroconversion – specifically the generation of virus-neutralizing antibodies (Khanam, Khanna, and Swaminathan, 2006; Ye et al., 2006). Therefore, we first performed durability studies to determine whether RVF VLPs induce long-lasting RVFV neutralizing antibodies. Mice were immunized subcutaneously (s.c.) three times with RVFV chimVLPs at 9-day intervals, and blood was collected 161 days post 3rd vaccination (179 days post first vaccination). Plaque reduction neutralization PRNT80 assays (Mangiafico et al., 1988) using RVFV ZH501-infected VeroE6 cells were performed by combining serum with 60pfu ZH501 virus. The neutralizing antibody titer is considered positive at the highest serum dilution that inhibits 80% of the plaques compared with the virus control titration. As shown in Fig. 3, neutralizing titers of ≥1:640 were obtained for all RVF chimVLP and RVF VLP - N (no nucleoprotein) vaccinated mice (n=4), while 3 of 4 RVF VLP + N-vaccinated mice developed titers of ≥640 and one of 320. No seroconversion was detected in a control group vaccinated with an unrelated MoMLV-based vaccine (Ebolavirus GP-pseudotyped MoMLV; Control 1) or non-vaccinated mice (Control 2). Such high neutralizing titers observed more than 6 months post vaccination indicates a durable RVFV vaccine candidate and warrants further evaluation of these RVF VLP-based vaccine candidates.
Figure 3. Neutralizing antibody titer in RVF VLP-vaccinated mice determined by plaque reduction neutralization tests.
Mouse sera were collected after three immunizations with RVF chimVLPs, RVF VLPs with or without RVFV N, Ebolavirus GP-pseudotyped MoMLV (Control 1) and from unimmunized mice (Control 2). The neutralizing antibody titer was determined as the reciprocal of the dilution of five two-fold serial dilutions of sera, respectively. Neutralizing antibody titer is considered positive at the lowest initial serum dilution that results in >80% (PRNT80) reduction of the number of plaques as compared to the virus control.
Next, we studied antigen-specific secretion of select cytokines by splenocytes isolated from vaccinated mice to determine whether RVF VLP-based vaccines elicit both humoral and cellular immunity. These responses have been correlated with vaccine efficacy in live challenge models (Wack et al., 2008). Importantly, anti-viral immunity often correlates with the development of cellular immune response (Warfield et al., 2005a). Splenocytes were harvested from mice vaccinated three times with RVF chimVLPs (days 0, 9 and 18) 31 days post final vaccination and cultured in 24-well dishes. Cells were stimulated by the addition of heat-inactivated live attenuated RVFV strain MP12 (Caplen, Peters, and Bishop, 1985) or a control antigen, heat-inactivated influenza A virus strain A/HK/1/68. Supernatant samples were taken at 24, 96, and 168 h and subjected to cytokine analysis using a bead-based multiplex system (Bioplex, BioRad). Splenocytes from vaccinated mice secrete antigen-specific cytokines in response to MP12 stimulation, but not to the unspecific influenza antigen (Fig. 4, blue bars). Splenocytes from the control (unvaccinated) mouse do not respond to either stimulus (Fig. 4, red bars). IL-2, IL-4, IL-5 and IFN-γ production is elicited by the RVFV-specific antigen, consistent with both humoral (TH2) and cellular (TH1) responses (Chung et al., 2008; Fromantin et al., 2001). Interestingly, cytokine levels peak at different times post-stimulation, which suggests that antigen-dependent expansion of T cells is occurring in vitro.
Figure 4. Antigen-specific cytokine secretion by splenocytes of chimeric RVF VLP-vaccinated mice measured by multiplex analysis.
Tissue culture supernatants from splenocytes harvested at 24, 96 or 168 h post-antigen stimulation from mice immunized with RVF chimVLPs, influenza VLPs or an unvaccinated mouse were subjected to multiplex bead analysis to measure select cytokines. Time of harvest and the cytokine measured are described at the top and left of the panels, respectively. Data from splenocytes harvested from the control mouse are indicated by red bars, and data from RVF chimVLP-vaccinated mice are indicated by blue bars. Stimulatory antigens are indicated below the graphs. Insets show the same results as the main graphs except with reduced scales (Y-axis, pg/ml) to show that secretion is detected at many points but is masked by the scale required to illustrate maximal cytokine signal.
RVF VLP vaccine efficacy studies
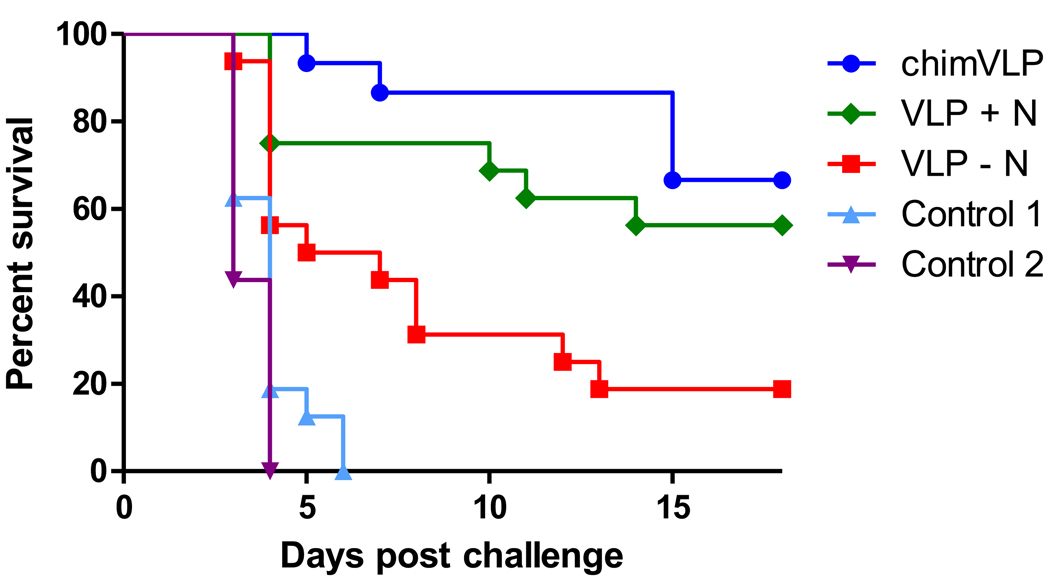
While the immunological data (neutralizing antibody titers and cytokine secretion levels) suggests the tested RVF VLP-based vaccine candidates are immunogenic, these immune correlates alone are not always predictive of efficacy in a live challenge model. Therefore, groups of 20 mice were immunized on days 0, 9, and 18 with select vaccine candidates: chimeric VLPs produced in 293-gag cells (chimVLP), VLPs produced in 293 cells with RVFV G and N (VLP + N) and VLPs produced in 293 cells without RVFV N (VLP - N). Forty-six days after the third vaccination (64 days post first vaccination), 16 of 20 animals were challenged with 103 pfu RVFV ZH501 under biosafety level 4 (BSL-4) conditions, and observed for signs of disease for 18 days (Fig. 5). The remaining four mice per group were used for the PRNT80 experiments (Fig. 3). As expected, all animals vaccinated with PBS only or a non-specific MoMLV-based vaccine (control 1 and 2, respectively) succumbed to RVFV challenge within the first 4 to 6 days. This demonstrates that MoMLV-specific components of the vaccines do not protect mice from RVFV challenge. Nine of 16 (56%) of the VLP + N vaccinated mice survived lethal challenge, while only 3 of 16 (~19%) survived if immunized with VLP - N vaccine. These results show that protection from RVFV challenge is dependent upon the presence of RVFV G proteins and is enhanced by the presence of RVFV N. chimVLP-vaccinated mice showed the best survival rates, as 11 of 16 (68%) mice survived the lethal challenge dose. Significance was determined using the Mantel-Cox test, and significant differences exist between groups including chimVLP and VLP - N (P = 0.001), VLP + N and VLP - N (P = 0.0196), all three vaccinated groups and controls 1 and 2 (P = 0.0001 (chimVLP), P = 0.0001 (VLP + N) and P = 0.0007 (VLP - N). There is no significant difference between the chimVLP and VLP +N groups (P = 0.393).
Figure 5. RVF VLP efficacy studies in mice.
RVF VLP-vaccinated mice (n=16) were challenged with 1×103 pfu of RVFV strain ZH501 under BSL-4 conditions. Mice immunized with an Ebolavirus GP-pseudotyped MoMLV vaccine and unimmunized mice served as controls 1 and 2, respectively. Data are shown in a Kaplan-Meier format.
Rats present another important alternative animal model for RVFV disease and are often considered more relevant than mice (Anderson and Peters, 1988; Anderson et al., 1991b; Bird, Albarino, and Nichol, 2007). Therefore, after promising data were obtained in the mouse model, RVF VLP-based vaccine efficacy (survival) was next analyzed in a lethal rat challenge model. Our objective was to test only the best vaccine candidate (based on the immunological and mouse protection studies) with a minimum number of animals. Groups of six rats were immunized three times at two week intervals with the chimeric RVF VLP vaccine candidate or a sterile saline control. Sixty-seven days post third vaccination (95 days post first vaccination), rats were challenged with a high dose of 105 pfu RVFV ZH501 and examined for signs of disease for 16 days. Body weight was monitored daily as an indication of the overall health of the rats. As shown in Fig. 6A, while all control rats succumbed to disease by day 4 post-challenge, the RVF chimVLP vaccine candidate protected 100% of the vaccinated rats. Unvaccinated control rats showed rapid and substantial weight loss before succumbing to challenge, whereas RVFV chimVLP-vaccinated animals generally maintained their weight throughout the course of the experiment (Fig. 6B).
Figure 6. RVF VLP efficacy studies in rats.
Results are shown as Kaplan-Meier survival curves and weights of VLP-immunized rats after RVFV challenge. A) Rats (n=6) were inoculated with the RVF chimVLP vaccine candidate and then challenged with 1×105 pfu of RVFV strain ZH501. Control rats were immunized with sterile saline. B) The mean and standard deviation of the weight change for RVF chimVLP vaccinated (blue) and control (red) rats are shown for each time point.
Overall, immunological as well as lethal challenge studies in two different rodent models clearly demonstrate that RVF VLP-based vaccines are a promising concept for the development of a vaccine for use in humans and livestock. Additional experiments to determine minimum vaccine dose and vaccination schedule as well as safety and efficacy studies in non-human primates are required to further evaluate this promising vaccine platform.
Discussion
The structural proteins of many viruses can assemble into VLPs (Grgacic and Anderson, 2006). VLPs are often described as being more efficacious in the activation of immune responses than conventional protein immunogens/subunit vaccines because their immunogenic protein components are displayed in a high density, more authentic conformation, often with intact biochemical functionality that is effectively immunogenic (Garcea and Gissmann, 2004; Grgacic, 2006; Noad and Roy, 2003). This is further enhanced by the particulate nature of VLPs that appears to be optimal for uptake by dendritic cells (Fifis et al., 2004). In addition, they are safer than inactivated and attenuated virus vaccines because they are usually free of viral genetic material and therefore are not encumbered by the possible safety-related drawbacks including reversion, recombination and re-assortment. At this stage, the ability to develop VLPs does not appear to be limited to any one type of virus or virus family, or by the complexity of the virus particle.
Mimicking the structure of virus particles allows the use of lower doses of antigen to elicit a similar protective response when compared to subunit vaccines (Noad and Roy, 2003). In addition to their ability to stimulate B-cell-mediated immune responses, VLPs have been shown to be highly effective at stimulating CD4 proliferative and cytotoxic T lymphocyte responses (Murata et al., 2003; Paliard et al., 2000; Schirmbeck, Bohm, and Reimann, 1996). This feature of VLP-based vaccines likely plays a major role in their effectiveness as vaccines against viral diseases. The well-documented immunogenicity of VLPs is likely facilitated by their interaction with dendritic cells (Warfield et al., 2003).
Several VLP-based vaccines are in human clinical trials or are FDA-approved, including those for hepatitis B virus (Andre and Safary, 1987; McAleer et al., 1984) (Sitrin, Wampler, and Ellis, 1993), trivalent influenza H1N1, H3N2 and B vaccine (“FluBlok”) (Cox, Patriarca, and Treanor, 2008; Treanor et al., 2007), H5N1 “bird flu” (Perrone et al., 2009), human papillomavirus (2007; Giannini et al., 2006; Harper et al., 2004; Harro et al., 2001; Joura et al., 2007), human immunodeficiency virus (Young et al., 2006) and Norwalk virus (Tacket et al., 2003). Other VLP-based vaccines with very promising pre-clinical results include VLPs for the severe acute respiratory syndrome (SARS) coronavirus (Lokugamage et al., 2008), human polyomavirus (Goldmann et al., 1999), rotavirus (Ciarlet et al., 1998; El-Attar et al., 2009; Jiang et al., 1999), and Ebola and Marburg viruses (Swenson et al., 2008; Swenson et al., 2005; Warfield et al., 2003; Warfield et al., 2005b; Warfield et al., 2007). Additionally, VLP-vaccines also have important agricultural applications, including promising vaccines for livestock diseases including bluetongue (Noad and Roy, 2003; Roy, 2000; Roy, Boyce, and Noad, 2009; Roy and Noad, 2008) and foot-and-mouth (Li et al., 2008; Remond et al., 2009).
Few studies have been undertaken to develop VLPs for bunyaviruses. Bunyamwera VLPs were generated by co-expression of GN, GC and NSm, in addition to a minigenome system (bunyamwera L, N and minigenome) in mammalian cells and were used to identify viral protein components required for virus assembly (Shi et al., 2009; Shi et al., 2006; Shi et al., 2007). Similarly, it has been shown that the expression of recombinant GN and GC glycoproteins of Uukuniemi (UUK) virus, a phlebovirus closely related to RVFV, leads to the assembly and budding of VLPs from transfected mammalian cells (Overby, Pettersson, and Neve, 2007; Overby et al., 2006; Overby et al., 2007). These VLPs are similar in structure to wt virus and are neutralized by UUK-specific antibodies. However, no immunological in vivo studies were performed to determine immunogenicity.
Generation of RVFV VLPs in insect cells has been demonstrated by Liu et al. (2008) using a single recombinant baculovirus that expresses the RVFV glycoproteins (GN/GC) and the N protein (Liu, Celma, and Roy, 2008). Efficient generation of RVF VLPs in mammalian cells has been recently demonstrated by Habjan et al. (2009) using transfected DNA encoding the complete RVFV M segment and both the RNA polymerase L and a GFP-expressing minigenome (Habjan et al., 2009; Naslund et al., 2009). Näslund et al. (2009) showed that these RVF VLPs can be used for vaccine studies. Three intraperitoneal injections of 1×106 RVF VLPs in mice induces antibody titers from 1:300 to 1:900 against GN and GC proteins but does not result in the development of detectable N-specific antibodies. Importantly, these VLPs protect 11 of 12 vaccinated mice from lethal virus challenge (2.4×104 pfu), whereas only 1 of 12 survived in the unvaccinated control group (Naslund et al., 2009).
The generation of chimeric RVF VLPs and its successful use as a vaccine candidate is unique to the field of bunyaviruses. We have established an efficient system to generate RVFV chimVLPs and VLPs from 293-gag and 293 cells (Figure 1 and Figure 2). These VLPs are immunogenic as indicated by the generation of neutralizing antibodies in vivo by immunized mice (Fig. 3) and antigen-specific secretion of immune-related cytokines by splenocytes from vaccinated mice (Fig 4). Furthermore, these VLP-based vaccine candidates are partially protective in mice and 100% protective in rats against lethal challenge (Figure 5 and Figure 6). Interestingly, RVF VLP production requires only the expression of the two glycoproteins GN and GC, and therefore RVFV N is not required. While consistent with findings by Overby et al. (Overby et al., 2006) who was able to generate UUK and bunyamwera VLPs without N (Overby et al., 2006), this contradicts recent findings that suggest RVF VLPs could only be generated through expression of RVFV glycoproteins together with RVFV N (as part of the minireplicon system) (Habjan et al., 2009). A possible explanation for this discrepancy is that the use of different expression systems (e.g., chicken β-actin vs. immediate-early cytomegalovirus promoter) leads to substantially different amount of RVFV G being produced, and RVF VLPs derived only from the expression of RVFV G requires high levels of expression.
The generation of RVF VLPs lacking the N protein facilitates the rational vaccine design for the generation of a safe and highly efficient RVFV vaccine following the DIVA (Differentiating Infected from Vaccinated Animals) concept to differentiate vaccinated from infected individuals (see (Bird et al., 2008; Capua, Cattoli, and Marangon, 2004)). Because RVFV-specific antibodies against the N proteins are easily detected in infected individuals, a vaccine candidate lacking the N antigens facilitates DIVA. However, further studies have to be performed (e.g., increased vaccine dose, different adjuvants) to increase N-lacking VLP vaccine efficiency (see Fig. 5).
chimVLPs containing a retroviral gag protein (either MoMLV or simian immunodeficiency virus (SIV) gag) and the antigen of interest (e.g., influenza hemagglutinin and neuraminidase) have been recently described (Guo et al., 2003; Haynes et al., 2009). Unfortunately, the generation of RVF chimVLPs is more complicated because RVFV G and MoMLV gag localize to the Golgi (Gerrard and Nichol, 2002; Schmaljohn and Hooper, 2001; Wasmoen, Kakach, and Collett, 1988) and plasma membranes (Soneoka, Kingsman, and Kingsman, 1997), respectively, in mammalian cells. However, over-expression of RVFV G leads to some GN/GC localization at the cell surface (Filone et al., 2006; Gerrard and Nichol, 2002; Gerrard and Nichol, 2007; Liu, Celma, and Roy, 2008), which allows the generation of RVF chimVLPs. Further attempts to increase RVFV G surface localization by generating chimeric RVFV G proteins containing the ectodomain of RVFV G and the transmembrane domain and cytoplasmic tail of the MoMLV Env (C-terminal 56aa of the envelope polyprotein, accession number GI:331936), which removes a putative Golgi retention signal of RVFV GN, did not significantly increase RVFV G content on cell surfaces as demonstrated by immunofluorescence studies (data not shown) and did not result in increased chimVLP yields.
Optimization of VLP production is important for the ability to scale-up for the generation of material required for non-human primate studies and ultimately clinical grade vaccine production. First, as seen in Fig. 2A, the ratio of transfected expression plasmid for the RVFV G and N proteins influences VLP yields. Second, optimal generation of VLPs is clearly observed when the N plasmid is included, and increasing amounts of N expression plasmid enhances the generation of RVFV VLPs. Similar findings were also reported for the generation of UUK VLPs (Overby et al., 2006). The addition of a RVFV-specific minigenome did not significantly increase the N content of generated VLPs despite the fact that VLPs were able to package minigenome and transfer reporter gene activity into VLP-infected cells (M. Bouloy and R. Flick, unpublished data). This contradicts previous findings by Overby et al. (Overby et al., 2006) who showed that omission of a minigenome leads to almost no nucleoprotein incorporation into UUK VLPs. Generation of VLPs is optimal between 24 and 60 h post-transfection (Fig. 2B) as determined via time course experiments. Furthermore, multiple harvests during a 120 h time period post transfection results in higher VLP yields compared to less frequent harvests. This is consistent with our previous studies which show that multiple harvests of Ebolavirus and Lassa virus chimVLPs yields substantially more VLPs compared to a single harvest or collections at intervals longer than 12h (data not shown). This might reflect VLP stability or binding to the producing cells.
While inactivated viral vaccines are often ineffective at eliciting neutralizing antibodies (Green et al., 2001), robust vaccines can elicit the development of neutralizing antibodies that are maintained for prolonged times (Kan et al., 2007). A PRNT80 of 1:40 is generally accepted as protective against RVF disease in mice (Peters et al., 1986), rats (Anderson et al., 1991a; Anderson, Slone, and Peters, 1987), hamsters (Niklasson, Meadors, and Peters, 1984) and Rhesus macaques (Peters et al., 1988). Pittman et al. (1999) (Pittman et al., 1999) examined the neutralizing antibody responses in 598 human subjects vaccinated with the TSI-GSD-200 inactivated RVFV vaccine. 540 (90.3%) had serum neutralizing antibody titers >1:40 after their primary series of three injections while 58 individuals (9.7%) had titers of <1:40. PRNT80 >1:40 was maintained for 183 days in 85% of recipients but decreased to only 35% at 1yr indicating that durability might be an issue with this particular vaccine.
Correlation between the development of neutralizing antibodies and protective efficacy for RVFV vaccines has also been shown more recently in several systems. Naslund et al. (Naslund et al., 2009) showed that 5 of 6 mice vaccinated with 1×106 RVF VLPs developed neutralizing antibody titers (PRNT80) from 250 to 1250. Eleven of 12 vaccinated mice were protected from lethal challenge. Bird et al. (2008) (Bird et al., 2008) showed in a rat model that neutralizing antibody titers (PRNT50) of 1:640 – 1:7040 were obtained with a highly attenuated RVFV strain lacking the NSs and NSm genes that is 100% protective from lethal challenge.
Here we demonstrate that RVF VLPs induce neutralizing antibody titers of ≥ 1:640 detectable 6 months post-immunization (Fig. 3), indicating the robustness and durability of the VLP-based RVFV vaccine candidates. Interestingly, as seen in Fig. 3, while similar neutralizing antibody titers are generated by RVF VLPs with or without N, these results do not correlate with vaccine efficacy in the mouse model, as seen in Fig. 5, where N-containing RVF VLPs are substantially more efficacious compared to VLPs lacking RVFV N. As demonstrated with DNA vaccines expressing only the RVFV M ORF, while it is likely that immunity to RVFV is determined by the response to the RVFV G, (Lagerqvist et al., 2009; Spik et al., 2006; Wallace et al., 2006), the results described above suggest that N might represent an important component of an efficacious RVFV vaccine. It has been demonstrated that both RVFV N (Lagerqvist et al., 2009; Wallace et al., 2006) and Toscana virus (a related bunyavirus) N (Gori Savellini et al., 2008) are partly protective against lethal challenge in mice. Interestingly, while previous passive transfer studies would suggest that the generation of neutralizing antibodies to RVFV GN and GC is predictive of vaccine efficacy (Besselaar and Blackburn, 1991; Schmaljohn et al., 1989), our RVF VLP without N, while sufficient for the generation of neutralizing antibodies (see Fig. 3), is not efficacious in the mouse challenge model (see Fig. 5).
Furthermore, we were able to demonstrate that RVF VLPs clearly induce antigen-specific cytokine secretion by isolated splenocytes from vaccinated animals (Fig. 4). Consistent with these results, Ebolavirus VLPs produced in mammalian and insect cells have been shown to stimulate secretion of cytokines such as IL-6, IL-10, IL-12, and TNF-α from dendritic cells (Bosio et al., 2004; Ye et al., 2006). Taken together, measurement of immune correlates clearly demonstrate that VLPs are immunogenic, however, only vaccine efficacy studies can clearly demonstrates the potency of vaccine candidates.
We therefore employed two different rodent models to determine RVF VLP-based vaccine efficacy. The results shown in Figure 5 and Figure 6 clearly show that our RVF VLP vaccine candidates are partially protective in mice and fully protective in rats. Importantly, RVFV ZH501 challenge in rats was performed with a 2 log10 higher dose than is often reported in the literature (see (Bird et al., 2008)) to ensure 100% lethality in the model and to demonstrate vaccine potency.
Overall, this novel approach of using chimeric RVF VLPs as vaccine candidates yielded promising immunological and efficacy data in two different rodent models, and sets a strong precedent for the generation of an efficacious vaccine against RVFV that is urgently needed for the high containment laboratory worker, indigenous people in endemic areas, and for the Strategic National Stockpile and National Veterinary Stockpile as protection against the emergence of this disease and for a potential bioterror event.
Acknowledgements
This work was supported by the National Institute of Health (7U01AI066327) and the United States Medical Research & Material Command (C081-105-0080, CBD08-105). We are thankful to Dawn Bertrand, Larissa Boeck and Tina Mortenson of NewLink Genetics Corporation for their excellent care and vaccinations of mice and rats. We thank Missy Worthy and Terry Juelich for their BSL-4 animal work in the University of Texas Medical Branch Robert E. Shope BSL-4 laboratory. We thank Dr. Chinglai Yang of Emory University for MoMLV gag antibodies, Dr. Robert Tesh of the University of Texas Medical Branch for RVFV N antibodies, and Dr. Wendy Maury of the University of Iowa for helpful advice and discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007;356(19):1915–1927. doi: 10.1056/NEJMoa061741. [DOI] [PubMed] [Google Scholar]
- Abdel-Motal UM, Guay HM, Wigglesworth K, Welsh RM, Galili U. Immunogenicity of influenza virus vaccine is increased by anti-gal-mediated targeting to antigen-presenting cells. J Virol. 2007;81(17):9131–9141. doi: 10.1128/JVI.00647-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Hazmi M, Ayoola EA, Abdurahman M, Banzal S, Ashraf J, El-Bushra A, Hazmi A, Abdullah M, Abbo H, Elamin A, Al-Sammani el T, Gadour M, Menon C, Hamza M, Rahim I, Hafez M, Jambavalikar M, Arishi H, Aqeel A. Epidemic Rift Valley fever in Saudi Arabia: a clinical study of severe illness in humans. Clin Infect Dis. 2003;36(3):245–252. doi: 10.1086/345671. [DOI] [PubMed] [Google Scholar]
- Anderson GW, Jr, Lee JO, Anderson AO, Powell N, Mangiafico JA, Meadors G. Efficacy of a Rift Valley fever virus vaccine against an aerosol infection in rats. Vaccine. 1991a;9(10):710–714. doi: 10.1016/0264-410x(91)90285-e. [DOI] [PubMed] [Google Scholar]
- Anderson GW, Jr, Peters CJ. Viral determinants of virulence for Rift Valley fever (RVF) in rats. Microb Pathog. 1988;5(4):241–250. doi: 10.1016/0882-4010(88)90096-4. [DOI] [PubMed] [Google Scholar]
- Anderson GW, Jr, Rosebrock JA, Johnson AJ, Jennings GB, Peters CJ. Infection of inbred rat strains with Rift Valley fever virus: development of a congenic resistant strain and observations on age-dependence of resistance. Am J Trop Med Hyg. 1991b;44(5):475–480. doi: 10.4269/ajtmh.1991.44.475. [DOI] [PubMed] [Google Scholar]
- Anderson GW, Jr, Slone TW, Jr, Peters CJ. Pathogenesis of Rift Valley fever virus (RVFV) in inbred rats. Microb Pathog. 1987;2(4):283–293. doi: 10.1016/0882-4010(87)90126-4. [DOI] [PubMed] [Google Scholar]
- Andersson AM, Melin L, Bean A, Pettersson RF. A retention signal necessary and sufficient for Golgi localization maps to the cytoplasmic tail of a Bunyaviridae (Uukuniemi virus) membrane glycoprotein. J Virol. 1997;71(6):4717–4727. doi: 10.1128/jvi.71.6.4717-4727.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andre FE, Safary A. Summary of clinical findings on Engerix-B, a genetically engineered yeast derived hepatitis B vaccine. Postgrad Med J. 1987;63 Suppl 2:169–177. [PubMed] [Google Scholar]
- Anonymous. Outbreak of Rift Valley fever, Yemen, August-October 2000. Wkly Epidemiol Rec. 2000;75(48):392–395. [PubMed] [Google Scholar]
- Babcock GJ, Esshaki DJ, Thomas WD, Jr, Ambrosino DM. Amino acids 270 to 510 of the severe acute respiratory syndrome coronavirus spike protein are required for interaction with receptor. J Virol. 2004;78(9):4552–4560. doi: 10.1128/JVI.78.9.4552-4560.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkhy HH, Memish ZA. Rift Valley fever: an uninvited zoonosis in the Arabian peninsula. Int J Antimicrob Agents. 2003;21(2):153–157. doi: 10.1016/s0924-8579(02)00295-9. [DOI] [PubMed] [Google Scholar]
- Barrett JW, Sun Y, Nazarian SH, Belsito TA, Brunetti CR, McFadden G. Optimization of codon usage of poxvirus genes allows for improved transient expression in mammalian cells. Virus Genes. 2006;33(1):15–26. doi: 10.1007/s11262-005-0035-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besselaar TG, Blackburn NK. Topological mapping of antigenic sites on the Rift Valley fever virus envelope glycoproteins using monoclonal antibodies. Arch Virol. 1991;121(1–4):111–124. doi: 10.1007/BF01316748. [DOI] [PubMed] [Google Scholar]
- Billecocq A, Gauliard N, Le May N, Elliott RM, Flick R, Bouloy M. RNA polymerase I-mediated expression of viral RNA for the rescue of infectious virulent and avirulent Rift Valley fever viruses. Virology. 2008;378(2):377–384. doi: 10.1016/j.virol.2008.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird BH, Albarino CG, Hartman AL, Erickson BR, Ksiazek TG, Nichol ST. Rift valley fever virus lacking the NSs and NSm genes is highly attenuated, confers protective immunity from virulent virus challenge, and allows for differential identification of infected and vaccinated animals. J Virol. 2008;82(6):2681–2691. doi: 10.1128/JVI.02501-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird BH, Albarino CG, Nichol ST. Rift Valley fever virus lacking NSm proteins retains high virulence in vivo and may provide a model of human delayed onset neurologic disease. Virology. 2007;362(1):10–15. doi: 10.1016/j.virol.2007.01.046. [DOI] [PubMed] [Google Scholar]
- Bos EC, Luytjes W, Spaan WJ. The function of the spike protein of mouse hepatitis virus strain A59 can be studied on virus-like particles: cleavage is not required for infectivity. J Virol. 1997;71(12):9427–9433. doi: 10.1128/jvi.71.12.9427-9433.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosio CM, Moore BD, Warfield KL, Ruthel G, Mohamadzadeh M, Aman MJ, Bavari S. Ebola and Marburg virus-like particles activate human myeloid dendritic cells. Virology. 2004;326(2):280–287. doi: 10.1016/j.virol.2004.05.025. [DOI] [PubMed] [Google Scholar]
- Burger W, Burge MJ, editors. Digital Image Processing: An algorithmic introduction using Java. New York: Springer-Verlag; 2008. [Google Scholar]
- Caplen H, Peters CJ, Bishop DH. Mutagen-directed attenuation of Rift Valley fever virus as a method for vaccine development. J Gen Virol. 1985;66(Pt 10):2271–2277. doi: 10.1099/0022-1317-66-10-2271. [DOI] [PubMed] [Google Scholar]
- Capua I, Cattoli G, Marangon S. DIVA--a vaccination strategy enabling the detection of field exposure to avian influenza. Dev Biol (Basel) 2004;119:229–233. [PubMed] [Google Scholar]
- Chung YC, Ho MS, Wu JC, Chen WJ, Huang JH, Chou ST, Hu YC. Immunization with virus-like particles of enterovirus 71 elicits potent immune responses and protects mice against lethal challenge. Vaccine. 2008;26(15):1855–1862. doi: 10.1016/j.vaccine.2008.01.058. [DOI] [PubMed] [Google Scholar]
- Ciarlet M, Crawford SE, Barone C, Bertolotti-Ciarlet A, Ramig RF, Estes MK, Conner ME. Subunit rotavirus vaccine administered parenterally to rabbits induces active protective immunity. J Virol. 1998;72(11):9233–9246. doi: 10.1128/jvi.72.11.9233-9246.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett MS, Purchio AF, Keegan K, Frazier S, Hays W, Anderson DK, Parker MD, Schmaljohn C, Schmidt J, Dalrymple JM. Complete nucleotide sequence of the M RNA segment of Rift Valley fever virus. Virology. 1985;144(1):228–245. doi: 10.1016/0042-6822(85)90320-4. [DOI] [PubMed] [Google Scholar]
- Cox MM, Patriarca PA, Treanor J. FluBlok, a recombinant hemagglutinin influenza vaccine. Influenza Other Respi Viruses. 2008;2(6):211–219. doi: 10.1111/j.1750-2659.2008.00053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Attar L, Olivera SL, Mackie A, Charpilienne A, Poncet D, Cohen J, Bridger JC. Comparison of the efficacy of rotavirus VLP vaccines to a live homologous rotavirus vaccine in a pig model of rotavirus disease. Vaccine. 2009;27:3201–3208. doi: 10.1016/j.vaccine.2009.03.043. [DOI] [PubMed] [Google Scholar]
- Elliott RM. In: The Bunyaviridae. E RM, editor. New York and London: Plenum Press; 1996. [Google Scholar]
- Elliott RM, Schmaljohn CS, Collett MS. Bunyaviridae genome structure and gene expression. Curr Top Microbiol Immunol. 1991;169:91–141. doi: 10.1007/978-3-642-76018-1_4. [DOI] [PubMed] [Google Scholar]
- Fifis T, Gamvrellis A, Crimeen-Irwin B, Pietersz GA, Li J, Mottram PL, McKenzie IF, Plebanski M. Size-dependent immunogenicity: therapeutic and protective properties of nano-vaccines against tumors. J Immunol. 2004;173(5):3148–3154. doi: 10.4049/jimmunol.173.5.3148. [DOI] [PubMed] [Google Scholar]
- Filone CM, Heise M, Doms RW, Bertolotti-Ciarlet A. Development and characterization of a Rift Valley fever virus cell-cell fusion assay using alphavirus replicon vectors. Virology. 2006;356(1–2):155–164. doi: 10.1016/j.virol.2006.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flick R, Bouloy M. Rift Valley fever virus. Curr Mol Med. 2005;5(8):827–834. doi: 10.2174/156652405774962263. [DOI] [PubMed] [Google Scholar]
- Frank-Peterside N. Response of laboratory staff to vaccination with an inactivated Rift Valley fever vaccine--TSI-GSD 200. Afr J Med Med Sci. 2000;29(2):89–92. [PubMed] [Google Scholar]
- Fromantin C, Jamot B, Cohen J, Piroth L, Pothier P, Kohli E. Rotavirus 2/6 virus-like particles administered intranasally in mice, with or without the mucosal adjuvants cholera toxin and Escherichia coli heat-labile toxin, induce a Th1/Th2-like immune response. J Virol. 2001;75(22):11010–11016. doi: 10.1128/JVI.75.22.11010-11016.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcea RL, Gissmann L. Virus-like particles as vaccines and vessels for the delivery of small molecules. Curr Opin Biotechnol. 2004;15(6):513–517. doi: 10.1016/j.copbio.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Geisbert TW, Jahrling PB. Exotic emerging viral diseases: progress and challenges. Nat Med. 2004;10(12 Suppl):S110–S121. doi: 10.1038/nm1142. [DOI] [PubMed] [Google Scholar]
- Gerdes GH. Rift Valley fever. Rev Sci Tech. 2004;23(2):613–623. doi: 10.20506/rst.23.2.1500. [DOI] [PubMed] [Google Scholar]
- Gerrard SR, Nichol ST. Characterization of the Golgi retention motif of Rift Valley fever virus G(N) glycoprotein. J Virol. 2002;76(23):12200–12210. doi: 10.1128/JVI.76.23.12200-12210.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerrard SR, Nichol ST. Synthesis, proteolytic processing and complex formation of N-terminally nested precursor proteins of the Rift Valley fever virus glycoproteins. Virology. 2007;357(2):124–133. doi: 10.1016/j.virol.2006.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gheysen D, Jacobs E, de Foresta F, Thiriart C, Francotte M, Thines D, De Wilde M. Assembly and release of HIV-1 precursor Pr55gag virus-like particles from recombinant baculovirus-infected insect cells. Cell. 1989;59(1):103–112. doi: 10.1016/0092-8674(89)90873-8. [DOI] [PubMed] [Google Scholar]
- Giannini SL, Hanon E, Moris P, Van Mechelen M, Morel S, Dessy F, Fourneau MA, Colau B, Suzich J, Losonksy G, Martin MT, Dubin G, Wettendorff MA. Enhanced humoral and memory B cellular immunity using HPV16/18 L1 VLP vaccine formulated with the MPL/aluminium salt combination (AS04) compared to aluminium salt only. Vaccine. 2006;24(33–34):5937–5949. doi: 10.1016/j.vaccine.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Goldmann C, Petry H, Frye S, Ast O, Ebitsch S, Jentsch KD, Kaup FJ, Weber F, Trebst C, Nisslein T, Hunsmann G, Weber T, Luke W. Molecular cloning and expression of major structural protein VP1 of the human polyomavirus JC virus: formation of virus-like particles useful for immunological and therapeutic studies. J Virol. 1999;73(5):4465–4469. doi: 10.1128/jvi.73.5.4465-4469.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gori Savellini G, Di Genova G, Terrosi C, Di Bonito P, Giorgi C, Valentini M, Docquier JD, Cusi MG. Immunization with Toscana virus N-Gc proteins protects mice against virus challenge. Virology. 2008;375(2):521–528. doi: 10.1016/j.virol.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Green TD, Newton BR, Rota PA, Xu Y, Robinson HL, Ross TM. C3d enhancement of neutralizing antibodies to measles hemagglutinin. Vaccine. 2001;20(1–2):242–248. doi: 10.1016/s0264-410x(01)00266-3. [DOI] [PubMed] [Google Scholar]
- Grgacic E, Anderson D. Virus-like particles; passport to immune recognition. Methods. 2006;40(60–65) doi: 10.1016/j.ymeth.2006.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grgacic EV, Anderson DA. Virus-like particles: passport to immune recognition. Methods. 2006;40(1):60–65. doi: 10.1016/j.ymeth.2006.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, Lu X, Kang SM, Chen C, Compans RW, Yao Q. Enhancement of mucosal immune responses by chimeric influenza HA/SHIV virus-like particles. Virology. 2003;313(2):502–513. doi: 10.1016/s0042-6822(03)00372-6. [DOI] [PubMed] [Google Scholar]
- Haas J, Park EC, Seed B. Codon usage limitation in the expression of HIV-1 envelope glycoprotein. Curr Biol. 1996;6(3):315–324. doi: 10.1016/s0960-9822(02)00482-7. [DOI] [PubMed] [Google Scholar]
- Habjan M, Penski N, Wagner V, Spiegel M, Overby AK, Kochs G, Huiskonen JT, Weber F. Efficient production of Rift Valley fever virus-like particles: The antiviral protein MxA can inhibit primary transcription of bunyaviruses. Virology. 2009;385(2):400–408. doi: 10.1016/j.virol.2008.12.011. [DOI] [PubMed] [Google Scholar]
- Haferkamp S, Fernando L, Schwarz TF, Feldmann H, Flick R. Intracellular localization of Crimean-Congo Hemorrhagic Fever (CCHF) virus glycoproteins. Virol J. 2005;2:42. doi: 10.1186/1743-422X-2-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haffar O, Garrigues J, Travis B, Moran P, Zarling J, Hu SL. Human immunodeficiency virus-like, nonreplicating, gag-env particles assemble in a recombinant vaccinia virus expression system. J Virol. 1990;64(6):2653–2659. doi: 10.1128/jvi.64.6.2653-2659.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammonds J, Chen X, Ding L, Fouts T, De Vico A, zur Megede J, Barnett S, Spearman P. Gp120 stability on HIV-1 virions and Gag-Env pseudovirions is enhanced by an uncleaved Gag core. Virology. 2003;314(2):636–649. doi: 10.1016/s0042-6822(03)00467-7. [DOI] [PubMed] [Google Scholar]
- Harper DM, Franco EL, Wheeler C, Ferris DG, Jenkins D, Schuind A, Zahaf T, Innis B, Naud P, De Carvalho NS, Roteli-Martins CM, Teixeira J, Blatter MM, Korn AP, Quint W, Dubin G. Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomised controlled trial. Lancet. 2004;364(9447):1757–1765. doi: 10.1016/S0140-6736(04)17398-4. [DOI] [PubMed] [Google Scholar]
- Harro CD, Pang YY, Roden RB, Hildesheim A, Wang Z, Reynolds MJ, Mast TC, Robinson R, Murphy BR, Karron RA, Dillner J, Schiller JT, Lowy DR. Safety and immunogenicity trial in adult volunteers of a human papillomavirus 16 L1 virus-like particle vaccine. J Natl Cancer Inst. 2001;93(4):284–292. doi: 10.1093/jnci/93.4.284. [DOI] [PubMed] [Google Scholar]
- Haynes JR, Cao SX, Rovinski B, Sia C, James O, Dekaban GA, Klein MH. Production of immunogenic HIV-1 viruslike particles in stably engineered monkey cell lines. AIDS Res Hum Retroviruses. 1991;7(1):17–27. doi: 10.1089/aid.1991.7.17. [DOI] [PubMed] [Google Scholar]
- Haynes JR, Dokken L, Wiley JA, Cawthon AG, Bigger J, Harmsen AG, Richardson C. Influenza-pseudotyped Gag virus-like particle vaccines provide broad protection against highly pathogenic avian influenza challenge. Vaccine. 2009;27(4):530–541. doi: 10.1016/j.vaccine.2008.11.011. [DOI] [PubMed] [Google Scholar]
- Hunter P, Erasmus BJ, Vorster JH. Teratogenicity of a mutagenised Rift Valley fever virus (MVP 12) in sheep. Onderstepoort J Vet Res. 2002;69(1):95–98. [PubMed] [Google Scholar]
- Jiang B, Estes MK, Barone C, Barniak V, O'Neal CM, Ottaiano A, Madore HP, Conner ME. Heterotypic protection from rotavirus infection in mice vaccinated with virus-like particles. Vaccine. 1999;17(7–8):1005–1013. doi: 10.1016/s0264-410x(98)00317-x. [DOI] [PubMed] [Google Scholar]
- Johnson JE, Chiu W. Structures of virus and virus-like particles. Curr Opin Struct Biol. 2000;10(2):229–235. doi: 10.1016/s0959-440x(00)00073-7. [DOI] [PubMed] [Google Scholar]
- Joura EA, Leodolter S, Hernandez-Avila M, Wheeler CM, Perez G, Koutsky LA, Garland SM, Harper DM, Tang GW, Ferris DG, Steben M, Jones RW, Bryan J, Taddeo FJ, Bautista OM, Esser MT, Sings HL, Nelson M, Boslego JW, Sattler C, Barr E, Paavonen J. Efficacy of a quadrivalent prophylactic human papillomavirus (types 6, 11, 16, and 18) L1 virus-like-particle vaccine against high-grade vulval and vaginal lesions: a combined analysis of three randomised clinical trials. Lancet. 2007;369(9574):1693–1702. doi: 10.1016/S0140-6736(07)60777-6. [DOI] [PubMed] [Google Scholar]
- Kan VL, Manischewitz J, King LR, Golding H. Durable neutralizing antibodies after remote smallpox vaccination among adults with and without HIV infection. AIDS. 2007;21(4):521–524. doi: 10.1097/QAD.0b013e32802f7d7c. [DOI] [PubMed] [Google Scholar]
- Kark JD, Aynor Y, Peters CJ. A rift Valley fever vaccine trial. I. Side effects and serologic response over a six-month follow-up. Am J Epidemiol. 1982;116(5):808–820. doi: 10.1093/oxfordjournals.aje.a113471. [DOI] [PubMed] [Google Scholar]
- Kark JD, Aynor Y, Peters CJ. A Rift Valley fever vaccine trial: 2. Serological response to booster doses with a comparison of intradermal versus subcutaneous injection. Vaccine. 1985;3(2):117–122. doi: 10.1016/0264-410x(85)90060-x. [DOI] [PubMed] [Google Scholar]
- Khanam S, Khanna N, Swaminathan S. Induction of neutralizing antibodies and T cell responses by dengue virus type 2 envelope domain III encoded by plasmid and adenoviral vectors. Vaccine. 2006;24(42–43):6513–6525. doi: 10.1016/j.vaccine.2006.06.031. [DOI] [PubMed] [Google Scholar]
- Kobasa D, Rodgers ME, Wells K, Kawaoka Y. Neuraminidase hemadsorption activity, conserved in avian influenza A viruses, does not influence viral replication in ducks. J Virol. 1997;71(9):6706–6713. doi: 10.1128/jvi.71.9.6706-6713.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984a;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Point mutations close to the AUG initiator codon affect the efficiency of translation of rat preproinsulin in vivo. Nature. 1984b;308(5956):241–246. doi: 10.1038/308241a0. [DOI] [PubMed] [Google Scholar]
- Kozak M. An analysis of 5'-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987;15(20):8125–8148. doi: 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Pushing the limits of the scanning mechanism for initiation of translation. Gene. 2002;299(1–2):1–34. doi: 10.1016/S0378-1119(02)01056-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBeaud AD, Muchiri EM, Ndzovu M, Mwanje MT, Muiruri S, Peters CJ, King CH. Interepidemic Rift Valley fever virus seropositivity, northeastern Kenya. Emerg Infect Dis. 2008;14(8):1240–1246. doi: 10.3201/eid1408.080082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagerqvist N, Naslund J, Lundkvist A, Bouloy M, Ahlm C, Bucht G. Characterisation of immune responses and protective efficacy in mice after immunisation with Rift Valley Fever virus cDNA constructs. Virol J. 2009;6:6. doi: 10.1186/1743-422X-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li PP, Naknanishi A, Tran MA, Ishizu K, Kawano M, Phillips M, Handa H, Liddington RC, Kasamatsu H. Importance of Vp1 calcium-binding residues in assembly, cell entry, and nuclear entry of simian virus 40. J Virol. 2003;77(13):7527–7538. doi: 10.1128/JVI.77.13.7527-7538.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Yi Y, Yin X, Zhang Z, Liu J. Expression of foot-and-mouth disease virus capsid proteins in silkworm-baculovirus expression system and its utilization as a subunit vaccine. PLoS ONE. 2008;3(5):e2273. doi: 10.1371/journal.pone.0002273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licata JM, Johnson RF, Han Z, Harty RN. Contribution of ebola virus glycoprotein, nucleoprotein, and VP24 to budding of VP40 virus-like particles. J Virol. 2004;78(14):7344–7351. doi: 10.1128/JVI.78.14.7344-7351.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Celma CC, Roy P. Rift Valley fever virus structural proteins: expression, characterization and assembly of recombinant proteins. Virol J. 2008;5:82. doi: 10.1186/1743-422X-5-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lokugamage KG, Yoshikawa-Iwata N, Ito N, Watts DM, Wyde PR, Wang N, Newman P, Kent Tseng CT, Peters CJ, Makino S. Chimeric coronavirus-like particles carrying severe acute respiratory syndrome coronavirus (SCoV) S protein protect mice against challenge with SCoV. Vaccine. 2008;26(6):797–808. doi: 10.1016/j.vaccine.2007.11.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madani TA, Al-Mazrou YY, Al-Jeffri MH, Mishkhas AA, Al-Rabeah AM, Turkistani AM, Al-Sayed MO, Abodahish AA, Khan AS, Ksiazek TG, Shobokshi O. Rift Valley fever epidemic in Saudi Arabia: epidemiological, clinical, and laboratory characteristics. Clin Infect Dis. 2003;37(8):1084–1092. doi: 10.1086/378747. [DOI] [PubMed] [Google Scholar]
- Mangiafico JA, Sanchez JL, Figueiredo LT, LeDuc JW, Peters CJ. Isolation of a newly recognized Bunyamwera serogroup virus from a febrile human in Panama. Am J Trop Med Hyg. 1988;39(6):593–596. doi: 10.4269/ajtmh.1988.39.593. [DOI] [PubMed] [Google Scholar]
- McAleer WJ, Buynak EB, Maigetter RZ, Wampler DE, Miller WJ, Hilleman MR. Human hepatitis B vaccine from recombinant yeast. Nature. 1984;307(5947):178–180. doi: 10.1038/307178a0. [DOI] [PubMed] [Google Scholar]
- Meegan JM. The Rift Valley fever epizootic in Egypt 1977–78. 1. Description of the epizzotic and virological studies. Trans R Soc Trop Med Hyg. 1979;73(6):618–623. doi: 10.1016/0035-9203(79)90004-x. [DOI] [PubMed] [Google Scholar]
- Murata K, Lechmann M, Qiao M, Gunji T, Alter HJ, Liang TJ. Immunization with hepatitis C virus-like particles protects mice from recombinant hepatitis C virus-vaccinia infection. Proc Natl Acad Sci U S A. 2003;100(11):6753–6758. doi: 10.1073/pnas.1131929100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naslund J, Lagerqvist N, Habjan M, Lundkvist A, Evander M, Ahlm C, Weber F, Bucht G. Vaccination with virus-like particles protects mice from lethal infection of Rift Valley Fever Virus. Virology. 2009;385(2):409–415. doi: 10.1016/j.virol.2008.12.012. [DOI] [PubMed] [Google Scholar]
- Niklasson B, Peters CJ, Bengtsson E, Norrby E. Rift Valley fever virus vaccine trial: study of neutralizing antibody response in humans. Vaccine. 1985;3(2):123–127. doi: 10.1016/0264-410x(85)90061-1. [DOI] [PubMed] [Google Scholar]
- Niklasson BS, Meadors GF, Peters CJ. Active and passive immunization against Rift Valley fever virus infection in Syrian hamsters. Acta Pathol Microbiol Immunol Scand [C] 1984;92(4):197–200. doi: 10.1111/j.1699-0463.1984.tb00074.x. [DOI] [PubMed] [Google Scholar]
- Niwa H, Yamamura K, Miyazaki J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene. 1991;108(2):193–199. doi: 10.1016/0378-1119(91)90434-d. [DOI] [PubMed] [Google Scholar]
- Noad R, Roy P. Virus-like particles as immunogens. Trends Microbiol. 2003;11(9):438–444. doi: 10.1016/s0966-842x(03)00208-7. [DOI] [PubMed] [Google Scholar]
- Overby AK, Pettersson RF, Neve EP. The glycoprotein cytoplasmic tail of Uukuniemi virus (Bunyaviridae) interacts with ribonucleoproteins and is critical for genome packaging. J Virol. 2007;81(7):3198–3205. doi: 10.1128/JVI.02655-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overby AK, Popov V, Neve EP, Pettersson RF. Generation and analysis of infectious virus-like particles of uukuniemi virus (bunyaviridae): a useful system for studying bunyaviral packaging and budding. J Virol. 2006;80(21):10428–10435. doi: 10.1128/JVI.01362-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overby AK, Popov VL, Pettersson RF, Neve EP. The cytoplasmic tails of Uukuniemi Virus (Bunyaviridae) G(N) and G(C) glycoproteins are important for intracellular targeting and the budding of virus-like particles. J Virol. 2007;81(20):11381–11391. doi: 10.1128/JVI.00767-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paliard X, Liu Y, Wagner R, Wolf H, Baenziger J, Walker CM. Priming of strong, broad, and long-lived HIV type 1 p55gag-specific CD8+ cytotoxic T cells after administration of a virus-like particle vaccine in rhesus macaques. AIDS Res Hum Retroviruses. 2000;16(3):273–282. doi: 10.1089/088922200309368. [DOI] [PubMed] [Google Scholar]
- Perrone LA, Ahmad A, Veguilla V, Lu X, Smith G, Katz JM, Pushko P, Tumpey TM. Intranasal vaccination with 1918 influenza virus-like particles protects mice and ferrets from lethal 1918 and H5N1 influenza virus challenge. J Virol. 2009;83(11):5726–5734. doi: 10.1128/JVI.00207-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters CJ, Jones D, Trotter R, Donaldson J, White J, Stephen E, Slone TW., Jr Experimental Rift Valley fever in rhesus macaques. Arch Virol. 1988;99(1–2):31–44. doi: 10.1007/BF01311021. [DOI] [PubMed] [Google Scholar]
- Peters CJ, Reynolds JA, Slone TW, Jones DE, Stephen EL. Prophylaxis of Rift Valley fever with antiviral drugs, immune serum, an interferon inducer, and a macrophage activator. Antiviral Res. 1986;6(5):285–297. doi: 10.1016/0166-3542(86)90024-0. [DOI] [PubMed] [Google Scholar]
- Pittman PR, Liu CT, Cannon TL, Makuch RS, Mangiafico JA, Gibbs PH, Peters CJ. Immunogenicity of an inactivated Rift Valley fever vaccine in humans: a 12-year experience. Vaccine. 1999;18(1–2):181–189. doi: 10.1016/s0264-410x(99)00218-2. [DOI] [PubMed] [Google Scholar]
- Remond M, Da Costa B, Riffault S, Parida S, Breard E, Lebreton F, Zientara S, Delmas B. Infectious bursal disease subviral particles displaying the foot-and-mouth disease virus major antigenic site. Vaccine. 2009;27(1):93–98. doi: 10.1016/j.vaccine.2008.10.036. [DOI] [PubMed] [Google Scholar]
- Rovinski B, Haynes JR, Cao SX, James O, Sia C, Zolla-Pazner S, Matthews TJ, Klein MH. Expression and characterization of genetically engineered human immunodeficiency virus-like particles containing modified envelope glycoproteins: implications for development of a cross-protective AIDS vaccine. J Virol. 1992;66(7):4003–4012. doi: 10.1128/jvi.66.7.4003-4012.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy P. Orbiviruses and their replication. In: Fields BN, editor. Virology. Lippincott-Raven; 2000. [Google Scholar]
- Roy P, Boyce M, Noad R. Prospects for improved bluetongue vaccines. Nat Rev Microbiol. 2009;7(2):120–128. doi: 10.1038/nrmicro2052. [DOI] [PubMed] [Google Scholar]
- Roy P, Noad R. Virus-like particles as a vaccine delivery system: myths and facts. Hum Vaccin. 2008;4(1):5–12. doi: 10.4161/hv.4.1.5559. [DOI] [PubMed] [Google Scholar]
- Schirmbeck R, Bohm W, Reimann J. Virus-like particles induce MHC class I-restricted T-cell responses. Lessons learned from the hepatitis B small surface antigen. Intervirology. 1996;39(1–2):111–119. doi: 10.1159/000150482. [DOI] [PubMed] [Google Scholar]
- Schmaljohn C, Hooper J. Bunyaviridae: The viruses and their replication. In: Howley PM, Howley PM, editors. Fields Virology. 4th ed. Vol. 2. Philadelphia: Lippincott Williams & Wilkins; 2001. pp. 1581–1602. 2 vols. [Google Scholar]
- Schmaljohn CS, Parker MD, Ennis WH, Dalrymple JM, Collett MS, Suzich JA, Schmaljohn AL. Baculovirus expression of the M genome segment of Rift Valley fever virus and examination of antigenic and immunogenic properties of the expressed proteins. Virology. 1989;170(1):184–192. doi: 10.1016/0042-6822(89)90365-6. [DOI] [PubMed] [Google Scholar]
- Schmitt AP, Leser GP, Waning DL, Lamb RA. Requirements for budding of paramyxovirus simian virus 5 virus-like particles. J Virol. 2002;76(8):3952–3964. doi: 10.1128/JVI.76.8.3952-3964.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X, Elliott RM. Analysis of glycoproteins of viruses in the family Bunyaviridae. Methods Mol Biol. 2007;379:137–148. doi: 10.1007/978-1-59745-393-6_10. [DOI] [PubMed] [Google Scholar]
- Shi X, Goli J, Clark G, Brauburger K, Elliott RM. Functional Analysis of the Bunyamwera Orthobunyavirus Gc Glycoprotein. J Gen Virol. 2009 doi: 10.1099/vir.0.013540-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X, Kohl A, Leonard VH, Li P, McLees A, Elliott RM. Requirement of the N-terminal region of orthobunyavirus nonstructural protein NSm for virus assembly and morphogenesis. J Virol. 2006;80(16):8089–8099. doi: 10.1128/JVI.00579-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X, Kohl A, Li P, Elliott RM. Role of the cytoplasmic tail domains of Bunyamwera orthobunyavirus glycoproteins Gn and Gc in virus assembly and morphogenesis. J Virol. 2007;81(18):10151–10160. doi: 10.1128/JVI.00573-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker T, Boulianne C, Vincent MJ, Pezzanite L, Al-Qahtani MM, Al-Mazrou Y, Khan AS, Rollin PE, Swanepoel R, Ksiazek TG, Nichol ST. Genetic analysis of viruses associated with emergence of Rift Valley fever in Saudi Arabia and Yemen, 2000–01. Emerg Infect Dis. 2002;8(12):1415–1420. doi: 10.3201/eid0812.020195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitrin RD, Wampler DE, Ellis RW. Survey of licensed hepatitis B vaccines and their production processes. In: Ellis RW, editor. Hepatitis B vaccines in clinical practice. New York: Marcel Dekker Inc.; 1993. pp. 83–102. [Google Scholar]
- Soneoka Y, Kingsman SM, Kingsman AJ. Mutagenesis analysis of the murine leukemia virus matrix protein: identification of regions important for membrane localization and intracellular transport. J Virol. 1997;71(7):5549–5559. doi: 10.1128/jvi.71.7.5549-5559.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spik K, Shurtleff A, McElroy AK, Guttieri MC, Hooper JW, SchmalJohn C. Immunogenicity of combination DNA vaccines for Rift Valley fever virus, tick-borne encephalitis virus, Hantaan virus, and Crimean Congo hemorrhagic fever virus. Vaccine. 2006;24(21):4657–4666. doi: 10.1016/j.vaccine.2005.08.034. [DOI] [PubMed] [Google Scholar]
- Suomalainen M, Hultenby K, Garoff H. Targeting of Moloney murine leukemia virus gag precursor to the site of virus budding. J Cell Biol. 1996;135(6 Pt 2):1841–1852. doi: 10.1083/jcb.135.6.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzich JA, Kakach LT, Collett MS. Expression strategy of a phlebovirus: biogenesis of proteins from the Rift Valley fever virus M segment. J Virol. 1990;64(4):1549–1555. doi: 10.1128/jvi.64.4.1549-1555.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanepoel R, Coetzer JAW. Rift Valley fever. 2nd edition ed. In: Coetzer JAW, Tustin RC, editors. Infectious diseases of livestock with special reference to South Africa. Cape Town: Oxford University Press Southern Africa; 2003. [Google Scholar]
- Swenson DL, Warfield KL, Larsen T, Alves DA, Coberley SS, Bavari S. Monovalent virus-like particle vaccine protects guinea pigs and nonhuman primates against infection with multiple Marburg viruses. Expert Rev Vaccines. 2008;7(4):417–429. doi: 10.1586/14760584.7.4.417. [DOI] [PubMed] [Google Scholar]
- Swenson DL, Warfield KL, Negley DL, Schmaljohn A, Aman MJ, Bavari S. Virus-like particles exhibit potential as a pan-filovirus vaccine for both Ebola and Marburg viral infections. Vaccine. 2005;23(23):3033–3042. doi: 10.1016/j.vaccine.2004.11.070. [DOI] [PubMed] [Google Scholar]
- Szecsi J, Boson B, Johnsson P, Dupeyrot-Lacas P, Matrosovich M, Klenk HD, Klatzmann D, Volchkov V, Cosset FL. Induction of neutralising antibodies by virus-like particles harbouring surface proteins from highly pathogenic H5N1 and H7N1 influenza viruses. Virol J. 2006;3:70. doi: 10.1186/1743-422X-3-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tacket CO, Sztein MB, Losonsky GA, Wasserman SS, Estes MK. Humoral, mucosal, and cellular immune responses to oral Norwalk virus-like particles in volunteers. Clin Immunol. 2003;108(3):241–247. doi: 10.1016/s1521-6616(03)00120-7. [DOI] [PubMed] [Google Scholar]
- Thall AD, Maly P, Lowe JB. Oocyte Gal alpha 1,3Gal epitopes implicated in sperm adhesion to the zona pellucida glycoprotein ZP3 are not required for fertilization in the mouse. J Biol Chem. 1995;270(37):21437–21440. doi: 10.1074/jbc.270.37.21437. [DOI] [PubMed] [Google Scholar]
- Treanor JJ, Schiff GM, Hayden FG, Brady RC, Hay CM, Meyer AL, Holden-Wiltse J, Liang H, Gilbert A, Cox M. Safety and immunogenicity of a baculovirus-expressed hemagglutinin influenza vaccine: a randomized controlled trial. JAMA. 2007;297(14):1577–1582. doi: 10.1001/jama.297.14.1577. [DOI] [PubMed] [Google Scholar]
- USDA. Agricultural Bioterrorism Protection Act of 2002; possession, use, and transfer of biological agents and toxins. In: A. USDA, editor. Fed Register. Vol. Part II. 2005. pp. 13241–13292. 7 CFR Part 331 and 9 CFR Part 121. [Google Scholar]
- Vialat P, Muller R, Vu TH, Prehaud C, Bouloy M. Mapping of the mutations present in the genome of the Rift Valley fever virus attenuated MP12 strain and their putative role in attenuation. Virus Res. 1997;52(1):43–50. doi: 10.1016/s0168-1702(97)00097-x. [DOI] [PubMed] [Google Scholar]
- Wack A, Baudner BC, Hilbert AK, Manini I, Nuti S, Tavarini S, Scheffczik H, Ugozzoli M, Singh M, Kazzaz J, Montomoli E, Del Giudice G, Rappuoli R, O'Hagan DT. Combination adjuvants for the induction of potent, long-lasting antibody and T-cell responses to influenza vaccine in mice. Vaccine. 2008;26(4):552–561. doi: 10.1016/j.vaccine.2007.11.054. [DOI] [PubMed] [Google Scholar]
- Wallace DB, Ellis CE, Espach A, Smith SJ, Greyling RR, Viljoen GJ. Protective immune responses induced by different recombinant vaccine regimes to Rift Valley fever. Vaccine. 2006;24(49–50):7181–7189. doi: 10.1016/j.vaccine.2006.06.041. [DOI] [PubMed] [Google Scholar]
- Warfield KL, Bosio CM, Welcher BC, Deal EM, Mohamadzadeh M, Schmaljohn A, Aman MJ, Bavari S. Ebola virus-like particles protect from lethal Ebola virus infection. Proc Natl Acad Sci U S A. 2003;100(26):15889–15894. doi: 10.1073/pnas.2237038100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warfield KL, Olinger G, Deal EM, Swenson DL, Bailey M, Negley DL, Hart MK, Bavari S. Induction of humoral and CD8+ T cell responses are required for protection against lethal Ebola virus infection. J Immunol. 2005a;175(2):1184–1191. doi: 10.4049/jimmunol.175.2.1184. [DOI] [PubMed] [Google Scholar]
- Warfield KL, Swenson DL, Demmin G, Bavari S. Filovirus-like particles as vaccines and discovery tools. Expert Rev Vaccines. 2005b;4(3):429–440. doi: 10.1586/14760584.4.3.429. [DOI] [PubMed] [Google Scholar]
- Warfield KL, Swenson DL, Negley DL, Schmaljohn AL, Aman MJ, Bavari S. Marburg virus-like particles protect guinea pigs from lethal Marburg virus infection. Vaccine. 2004;22(25–26):3495–3502. doi: 10.1016/j.vaccine.2004.01.063. [DOI] [PubMed] [Google Scholar]
- Warfield KL, Swenson DL, Olinger GG, Kalina WV, Aman MJ, Bavari S. Ebola virus-like particle-based vaccine protects nonhuman primates against lethal Ebola virus challenge. J Infect Dis. 2007;196 Suppl 2:S430–S437. doi: 10.1086/520583. [DOI] [PubMed] [Google Scholar]
- Wasmoen TL, Kakach LT, Collett MS. Rift Valley fever virus M segment: cellular localization of M segment-encoded proteins. Virology. 1988;166(1):275–280. doi: 10.1016/0042-6822(88)90174-2. [DOI] [PubMed] [Google Scholar]
- Watanabe S, Watanabe T, Noda T, Takada A, Feldmann H, Jasenosky LD, Kawaoka Y. Production of novel ebola virus-like particles from cDNAs: an alternative to ebola virus generation by reverse genetics. J Virol. 2004;78(2):999–1005. doi: 10.1128/JVI.78.2.999-1005.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. Outbreaks of Rift Valley fever in Kenya, Somalia and United Republic of Tanzania, December 2006–April 2007. Wkly Epidemiol Rec. 2007;82(20):169–178. [PubMed] [Google Scholar]
- Ye L, Lin J, Sun Y, Bennouna S, Lo M, Wu Q, Bu Z, Pulendran B, Compans RW, Yang C. Ebola virus-like particles produced in insect cells exhibit dendritic cell stimulating activity and induce neutralizing antibodies. Virology. 2006;351(2):260–270. doi: 10.1016/j.virol.2006.03.021. [DOI] [PubMed] [Google Scholar]
- Yoshinaka Y, Katoh I, Copeland TD, Oroszlan S. Murine leukemia virus protease is encoded by the gag-pol gene and is synthesized through suppression of an amber termination codon. Proc Natl Acad Sci U S A. 1985;82(6):1618–1622. doi: 10.1073/pnas.82.6.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young KR, McBurney SP, Karkhanis LU, Ross TM. Virus-like particles: designing an effective AIDS vaccine. Methods. 2006;40(1):98–117. doi: 10.1016/j.ymeth.2006.05.024. [DOI] [PubMed] [Google Scholar]
- Young KR, Smith JM, Ross TM. Characterization of a DNA vaccine expressing a human immunodeficiency virus-like particle. Virology. 2004;327(2):262–272. doi: 10.1016/j.virol.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Zhou J, Liu WJ, Peng SW, Sun XY, Frazer I. Papillomavirus capsid protein expression level depends on the match between codon usage and tRNA availability. J Virol. 1999;73(6):4972–4982. doi: 10.1128/jvi.73.6.4972-4982.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]