Abstract
Importance:
National guidelines discourage the use of continuous pulse oximetry monitoring in hospitalized children with bronchiolitis not requiring supplemental oxygen.
Objective:
Measure continuous pulse oximetry use in bronchiolitis.
Design, Setting, and Participants:
A multicenter cross-sectional study was performed on pediatric wards in 56 US and Canadian hospitals of the Pediatric Research in Inpatient Settings Network from December 1, 2018 through March 31, 2019. Participants included a convenience sample of patients 8 weeks through 23 months old with bronchiolitis but without active supplemental oxygen administration. Patients with extreme prematurity, cyanotic congenital heart disease, pulmonary hypertension, home respiratory support, neuromuscular disease, immunodeficiency, and cancer were excluded.
Exposure:
Hospitalization with bronchiolitis but without active supplemental oxygen administration.
Main Outcomes and Measures:
Primary outcome, receipt of continuous pulse oximetry, was measured using direct observation. Continuous pulse oximetry use proportions were risk-standardized using the following variables: nighttime (11PM – 7AM), age combined with preterm birth, time since weaning from supplemental oxygen or flow, apnea or cyanosis during the present illness, neurologic impairment, and presence of an enteral feeding tube.
Results:
The sample included 3612 patient observations in 33 freestanding children’s hospitals, 14 children’s hospitals within hospitals, and 9 community hospitals. Patients were 59% male, 56% white, and 15% black; their ages were 48% 8 weeks – 5 months, 28% 6 months – 11 months, 16% 12 months – 17 months, and 9% 18 months – 23 months. The overall continuous pulse oximetry monitoring use proportion in these patients, none of whom were receiving any supplemental oxygen or nasal cannula flow, was 46% (95% confidence interval [CI] 40–53%). Hospital-level unadjusted continuous pulse oximetry use ranged from 2% to 92%. After risk standardization, use ranged from 6% to 82%. Intraclass correlation coefficient suggested that 27% (95% CI 19–36%) of observed variation was attributable to unmeasured hospital level factors.
Conclusions and Relevance:
In a convenience sample of children hospitalized with bronchiolitis but without active supplemental oxygen administration, monitoring with continuous pulse oximetry was frequent and varied widely among hospitals. Because of the apparent absence of a guideline- or evidence-based indication for continuous monitoring in this population, this practice may represent overuse.
INTRODUCTION
Continuous pulse oximetry (SpO2) monitoring has enabled timely detection of oxygen desaturation and improved outcomes in operating rooms1 and other high-risk settings2 over the past 50 years. Continuous monitoring use has since expanded to hospital wards without supporting evidence of benefit, likely due to perceptions that it improves safety with little downside.3
Acute viral bronchiolitis is the leading cause of infant hospitalization.4 Bronchiolitis hospital care is primarily supportive, including nasopharyngeal suctioning, nasogastric or intravenous fluids, and supplemental oxygen. Continuous SpO2 monitoring in children with bronchiolitis who are not also requiring supplemental oxygen has been recognized as a form of medical overuse.5–7
Risks associated with continuous SpO2 monitoring in bronchiolitis include prolonged length of stay,8–11 increased costs attributable to delayed discharge, supplemental oxygen, and oximeter probes,12 and potential for iatrogenic harm.13 Monitor alarms also contribute to alarm fatigue among nurses, which is associated with delays in alarm response time.14,15
Appropriate use of continuous SpO2 monitoring in bronchiolitis is guided by an American Academy of Pediatrics (AAP) Clinical Practice Guideline5 and Society of Hospital Medicine (SHM) Choosing Wisely recommendations.6 The AAP Guideline states “Clinicians may choose not to use continuous pulse oximetry for children with a diagnosis of bronchiolitis.” Choosing Wisely recommendations state “Do not use continuous pulse oximetry routinely in children with acute respiratory illness unless they are on supplemental oxygen.”6
The primary objective of this study was to determine the extent of continuous SpO2 monitoring in a population in whom continuous monitoring is not indicated: hospitalized children with bronchiolitis not requiring supplemental oxygen. The primary hypothesis was that continuous SpO2 monitoring use would exceed 30% in the population specified above across sites. The 30% cut point was selected as a guide to inform the decision to subsequently perform a deimplementation trial.
METHODS
Design
We performed a multicenter cross-sectional study using in-person observation to sample the practice of continuous SpO2 monitoring during bronchiolitis season, December 1, 2018 through March 31, 2019. An overview of this study’s protocol and the projects that will follow was previously published.16 For US sites, the Institutional Review Board (IRB) at Children’s Hospital of Philadelphia approved the study and the remaining US sites established reliance agreements with the reviewing IRB. Research Ethics Boards at University of Calgary and The Hospital for Sick Children also reviewed and approved the study. All sites granted waivers of consent, assent, parental permission, and HIPAA authorization.
Setting
We performed this study in 56 US and Canadian hospitals participating in the Pediatric Research in Inpatient Settings Network (PRIS). PRIS is an independent, hospital-based research network that aims to improve the health of and healthcare delivery to hospitalized children and their families. Member hospitals were categorized as (a) freestanding children’s hospitals (hospitals devoted entirely to the care of children including a full range of pediatric subspecialty services), (b) children’s hospitals within hospitals (general medical hospitals that care mainly for adult patients and include a pediatric department offering a full range of pediatric subspecialty services), and (c) community hospitals (general medical centers that care mainly for adult patients and include a pediatric department offering limited or no pediatric subspecialty services). We performed observations only on acute care pediatric inpatient units not classified as intensive care.
Patients
We included patients 8 weeks through 23 months old. Eligible patients had an active primary diagnosis of bronchiolitis in the hospital chart and were not receiving any supplemental oxygen or nasal cannula flow (even with room air [21% fraction of inspired oxygen]) at the time of data collection. While the majority of children with bronchiolitis receive supplemental oxygen at some point during their hospital admission, some require only supportive care for respiratory distress (e.g. frequent nasal suctioning) or feeding difficulties (e.g. intravenous fluids or nasogastric feedings).17 Included patients were cared for by generalist services. We excluded patients documented as having experienced “premature” or “preterm” birth without a numeric gestational age listed and those with documented extreme prematurity (< 28 weeks gestation), cyanotic congenital heart disease, pulmonary hypertension, home oxygen or positive pressure ventilation requirement, tracheostomy, neuromuscular disease, immunodeficiency, or cancer.
Data collection
Observational rounds for primary outcome
Staff at each hospital performed observational rounds during the study period by walking to the bedside of each patient who met the criteria outlined above. Investigators determined the continuous monitoring status of the patients based on visual confirmation of waveforms and data displayed on the bedside monitor. Each Site Principal Investigator used convenience sampling based on the availability of their data collection team to determine which dates to perform observational rounds. We restricted observational rounds to occur only during certain hours, designated as “daytime” (10AM - 5PM) or “nighttime” (11PM - 7AM). We asked sites to aim to collect at least 60 observations during the bronchiolitis season, targeting approximately 50% of observations during nighttime hours. Weekends were not specifically targeted for data collection. The end time of daytime was extended from 4PM (as in the original protocol16) to 5PM at the request of Site Principal Investigators prior to the start of data collection to increase feasibility.
While we did not collect patient identifiers, we required that each observational rounds data collection session be separated by at least 36 hours to limit within-patient repeated measures given that the median length of stay for bronchiolitis is 2 days.18
Chart review for demographic and clinical variables (covariates)
Following the in-person data collection, investigators reviewed patients’ charts for demographic and clinical information including age, gestational age, previous respiratory support during the same hospitalization, presence of feeding tube, apnea or cyanosis during the present illness, prior intensive care unit stay during the present hospitalization, and the presence of conditions associated with neurologic impairment. Patient family-reported race and ethnicity were abstracted from charts in categories defined by the Standards for the Classification of Federal Data on Race and Ethnicity, in compliance with NIH inclusion reporting policies.19 In addition to reporting, we planned to analyze race and ethnicity as variables possibly associated with continuous SpO2 monitoring, which could suggest important disparities in care based on race or ethnicity.
Analysis
We estimated the frequency of within-patient repeated measures by first generating a patient “phenotype variable” for each unique combination of hospital, unit, age category, gestational age category, race, ethnicity, sex, presence of gastrostomy, and neurologic impairment. Based on bronchiolitis length of stay data from a randomized trial,12 we considered observations of the same patient phenotype that were separated by less than 4 days (approximately the 75th percentile of length of stay in the trial’s usual care group) to possibly represent the same patient.
Because of the straightforward approach to data collection, with basic elements collected from the chart combined with in-person direct observation of monitoring, we expected only trivial amounts of missing data. However, we anticipated missing numeric gestational age documentation in some patients, and designed the data collection form to accommodate this issue. If a numeric gestational age was not listed in the chart, the data collector reviewed the chart for qualitative descriptions of the patient as “full term,” “premature,” or “preterm.” Patients described as premature or preterm in the absence of a documented gestational age were assumed to be born prior to 28 weeks and were excluded. Those described as full term or without a qualitative description of gestational age were included. In the analysis, we dichotomized included patients as preterm (28 0/7 to 33 6/7 weeks documented in chart) or not preterm. We did not perform imputation or use any other methods to replace missing data with values.
Unadjusted
We calculated the unadjusted observed continuous SpO2 monitoring use proportion for each hospital as a simple proportion of the total number of observations during which patients were continuously monitored divided by the total number of observations performed at that hospital, comprised exclusively of patients not receiving any supplemental oxygen or nasal cannula flow. We estimated the 95% confidence interval (CI) of the unadjusted monitoring proportion accounting for clustering at the hospital level using linear regression with a sandwich estimator for the standard errors allowing for intra-hospital correlation (Stata “regress” command with “vce cluster” option). We performed a one-sample test of this proportion against the hypothesized proportion of 30%, specifying a conservative intraclass correlation of 40% to account for the hospital-level clustering (Stata “prtest” command with “cluster” and “rho” options).
We then examined the bivariable associations of the chart-abstracted demographic and clinical covariates listed above with continuous monitoring use using fixed-effects logistic regression. Given that in clinical practice, gestational age and chronological age are often considered in combination when thinking about risk, we used dichotomous preterm status and categorical chronological age jointly as an interaction term in all models (categories shown in Table 1).
Table 1.
Characteristics of sampled patients with bronchiolitis not receiving any supplemental oxygen or nasal cannula flow.
| Variable | Observations, n (%) | |
|---|---|---|
| Patient Demographics | ||
| Age | ||
| 8 weeks through 5 months | 1742 | (48%) |
| 6 months through 11 months | 1001 | (28%) |
| 12 months through 17 months | 560 | (16%) |
| 18 months through 23 months | 309 | (9%) |
| Gestational age | ||
| Preterm (28 0/7 to 33 6/7 weeks documented in chart) | 361 | (10%) |
| Not preterma | 3251 | (90%) |
| Sex | ||
| Male | 2125 | (59%) |
| Female | 1485 | (41%) |
| Not specified | 2 | (<1%) |
| Raceb | ||
| White | 2,034 | (56%) |
| Black or African American | 553 | (15%) |
| Specified as “Other” | 500 | (14%) |
| Specified as “Unknown” | 279 | (8%) |
| Asian | 144 | (4%) |
| More than one race | 56 | (2%) |
| Native Hawaiian or Pacific Islander | 30 | (1%) |
| American Indian or Alaska Native | 16 | (<1%) |
| Ethnicityb | ||
| Not Hispanic or Latino | 2454 | (68%) |
| Hispanic or Latino | 766 | (21%) |
| Unknown | 259 | (7%) |
| Other | 133 | (4%) |
| Illness characteristics at time of observation | ||
| Time since weaning from supplemental oxygen or flow | ||
| Never received | 1190 | (33%) |
| Off < 1 hr | 80 | (2%) |
| Off 1 - < 2 hrs | 148 | (4%) |
| Off 2 - < 4 hrs | 244 | (7%) |
| Off 4 - < 6 hrs | 234 | (6%) |
| Off 6 - < 12 hrs | 505 | (14%) |
| Off 12 - < 24 hrs | 687 | (19%) |
| Off 24 hrs + | 499 | (14%) |
| Unknown | 25 | (<1%) |
| Prior intensive care unit stay during present hospitalization | 884 | (24%) |
| Apnea or cyanosisc | 235 | (7%) |
| Comorbid condition associated with neurologic impairmentd | 93 | (3%) |
| Enteral feeding tube in place (nasogastric or gastrostomy) | 305 | (8%) |
| Hospital typee | ||
| Freestanding (n=33) | 2667 | (74%) |
| Children’s hospital within hospital (n=14) | 591 | (16%) |
| Community (n=9) | 354 | (10%) |
| Time of day observation performed | ||
| Day (10AM - 5PM) | 2073 | (57%) |
| Night (11PM - 7AM) | 1539 | (43%) |
Note: For some variables, the sum of percentages does not equal 100% due to rounding.
Not preterm included the following: documented gestational age 34 0/7 weeks and above, or absence of gestational age but documented as full term, or absence of gestational age but not labeled in chart as preterm or premature.
Patient family-reported race and ethnicity were abstracted from charts in categories defined by the Standards for the Classification of Federal Data on Race and Ethnicity, in compliance with NIH inclusion reporting policies.
Includes documentation of apnea or cyanosis occurring at home or in hospital during the present illness.
Static encephalopathy, cerebral palsy, hydrocephalus, spina bifida, epilepsy/seizure disorder, or hypotonia.
Number of observations by hospital type (median, IQR): Freestanding (70, 61–95); Hospital within hospital (38, 24–62); Community (35, 29–57).
Adjusted
We then performed multivariable analysis to compare hospitals’ monitoring proportions in a standardized way, accounting for differences in the patient-level variables potentially associated with monitoring. The purpose of this risk-standardization was to approximate what we would have found if we had hospitalized a similar cohort of infants in each of the hospitals, and to permit identification of statistical outlier hospitals. We chose this approach because we anticipated that patient-level factors associated with use would differ between sites due in part to site-level differences in patient populations with different degrees of risk20–23 and in part due to differences in sampling. To do this, we used methods developed for the Centers for Medicare & Medicaid Services (CMS) for public reporting of hospital quality based on administrative data.24,25 These methods adjust for case mix differences among hospitals using patient-level factors, thus permitting comparison of hospital performance.25 This approach also assumes that there are underlying differences between hospitals, allowing us to distinguish within-hospital variation from between-hospital variation in continuous SpO2 monitoring use.26
For each hospital, we first calculated the “expected” continuous SpO2 monitoring use proportion given the hospital-specific differences in case mix using patient-level variables. We used a fixed effects multivariable logistic regression model that included the covariates meeting pre-specified criteria of having composite P values <.2 for being continuously SpO2 monitored in the model described in the “Unadjusted” section above. We retained variables in this model with P values that remained <.2 when included in the multivariable model. This expected use proportion estimates the monitoring proportion if the set of patients observed at this hospital were treated at the average hospital.26
We then calculated the “predicted” use proportion for each hospital by incorporating the hospital-specific random effect into the multivariable fixed effects model (resulting in the final mixed effects regression model that accounts for hospital-level clustering). We computed a risk-standardized monitoring proportion for each hospital as the ratio of the predicted to expected use proportions multiplied by the unadjusted overall proportion across all hospitals. We constructed percentile-based 95% CIs for the risk-standardized proportions of each hospital based on 1000 samples.25,26 We considered hospitals to be “statistical high use outliers” if the lower bound of the 95% CI was higher than the overall observed monitoring proportion, and “statistical low use outliers” if the upper bound of the 95% CI was lower than the overall proportion.25 We excluded hospitals that submitted fewer than 20 observations from the hospital comparisons.
We used data collection forms designed in REDCap and hosted centrally at Children’s Hospital of Philadelphia.27 We used SAS software (SAS Institute Inc.) version 9.4 and Stata (StataCorp LLC) version 15.1 for analysis. We used publicly available statistical code in the 2018 CMS Mortality Measures “SAS Pack” to calculate the risk-standardized monitoring proportion for each hospital and to construct percentile-based 95% CIs. Statistical significance was indicated by P <.05 in 2-sided tests.
RESULTS
We collected 3612 observations in 33 freestanding children’s hospitals, 14 children’s hospitals within hospitals, and 9 community hospitals during the 4-month study period (Figure 1). Seven hospitals collected fewer than 20 observations and were excluded from hospital comparisons. Of the 49 hospitals with at least 20 observations, the median number of observations per hospital was 63 (IQR 50–89). Two hospitals were in Canada; the remainder were in the US.
Figure 1.
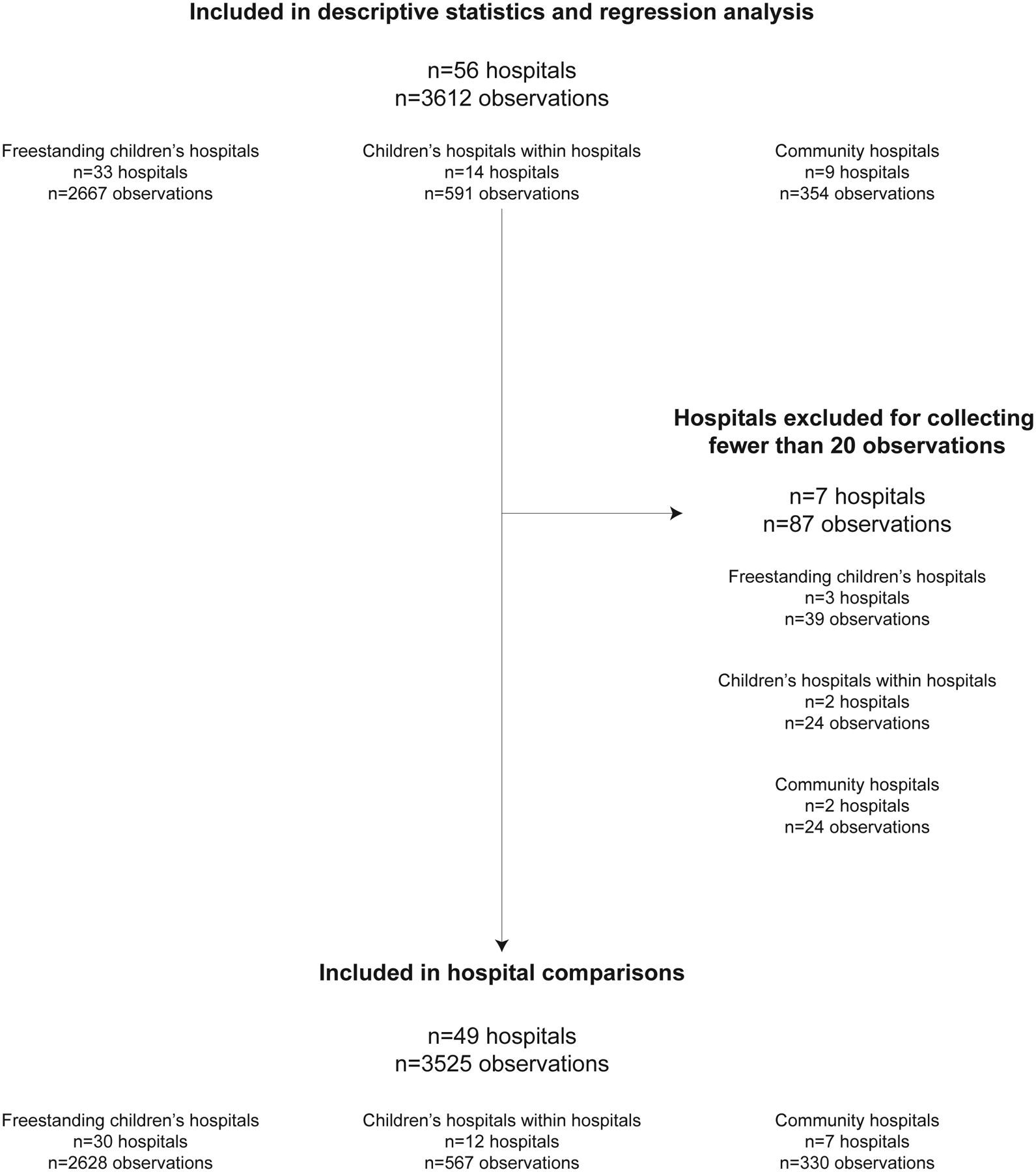
Flow diagram of hospitals and observations used in the study.
The study population of children with bronchiolitis was 59% male, 56% white, 15% black, and 21% Hispanic or Latino. Their ages were 48% 8 weeks through 5 months, 28% 6 months through 11 months, 16% 12 months through 17 months, and 9% 18 months through 23 months. Overall, 66% of patients had received supplemental oxygen or flow earlier during their current admission. Investigators performed 43% of observations during nighttime hours (11PM - 7AM). We found that 10% of observations had another observation of the same patient phenotype in the preceding 4 days. Other characteristics are in Table 1.
In the patients with bronchiolitis we included, none of whom were receiving any supplemental oxygen or nasal cannula flow at the time of data collection, the overall proportion with continuous SpO2 monitoring use was 46% (95% CI 40–53% accounting for clustering at the hospital level, 2-sided P <.001 rejecting the null hypothesis that the proportion was 30%). Of the 49 hospitals that collected at least 20 observations, the hospital-level unadjusted continuous SpO2 monitoring use proportions ranged from 2% to 79% for the 30 freestanding children’s hospitals (hospital-level median 40%), from 7% to 92% for the 12 children’s hospitals within hospitals (hospital-level median 58%), and from 22% to 77% for the 7 community hospitals (hospital-level median 48%).
In unadjusted fixed effects analysis, the following variables met the pre-specified criteria to be included in the multivariable model: nighttime, age combined with preterm birth, time since weaning from supplemental oxygen or flow, documented history of apnea or cyanosis during the present illness, neurologic impairment, and presence of an enteral feeding tube (Table 2). Ethnicity met initial criteria to enter the multivariable model based on having a bivariable association P value <.2 but was eliminated from the multivariable model for a composite P value of .34.
Table 2.
Continuous pulse oximetry use in patients with bronchiolitis not receiving any supplemental oxygen or nasal cannula flow.
| Unadjusted | Adjustedb | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Total, n | Continuously monitored with pulse oximetry, n | Continuously monitored with pulse oximetry, % | OR for use of continuous pulse oximetry | 95% CI | Category P value | Composite P valuea | OR for use of continuous pulse oximetry | 95% CI | Category P value | Composite P valuea |
| Overall(n=56 hospitals) | 3612 | 1679 | 46 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Age and gestational age categoryc | |||||||||||
| 8 weeks through 5 months, preterm | 183 | 103 | 56 | 1.78 | 1.12–2.83 | .02 | .11 | 2.58 | 1.65–4.02 | <.001 | <.001 |
| 8 weeks through 5 months, not preterm | 1559 | 758 | 49 | 1.31 | 1.03–1.66 | .03 | 1.51 | 1.12–2.03 | .007 | ||
| 6 months through 11 months, preterm | 107 | 47 | 44 | 1.08 | 0.74–1.59 | .68 | 1.21 | 0.72–2.05 | .48 | ||
| 6 months through 11 months, not preterm | 894 | 402 | 45 | 1.13 | 0.89–1.43 | .31 | 1.26 | 0.93–1.73 | .14 | ||
| 12 months through 17 months, preterm | 48 | 20 | 42 | 0.99 | 0.52–1.86 | .97 | 0.77 | 0.38–1.58 | .48 | ||
| 12 months through 17 months, not preterm | 512 | 219 | 43 | 1.03 | 0.74–1.45 | .85 | 1.01 | 0.72–1.42 | .95 | ||
| 18 months through 23 months, preterm | 23 | 10 | 43 | 1.06 | 0.46–2.47 | .89 | 0.50 | 0.18–1.37 | .18 | ||
| 18 months through 23 months, not preterm | 286 | 120 | 42 | [reference] | [reference] | ||||||
| Sex | |||||||||||
| Male | 2125 | 977 | 46 | [reference] | .52 | Not includedb | |||||
| Female | 1485 | 702 | 47 | 1.05 | 0.90–1.23 | .52 | |||||
| Raced | |||||||||||
| White | 2034 | 938 | 46 | [reference] | .61 | Not includedb | |||||
| Other | 1025 | 502 | 49 | 1.12 | 0.82–1.53 | .47 | |||||
| Black or African American | 553 | 239 | 43 | 0.90 | 0.62–1.28 | .53 | |||||
| Ethnicityd | |||||||||||
| Not Hispanic or Latino | 2454 | 1088 | 44 | [reference] | .11 | Not includedb | |||||
| Hispanic or Latino | 766 | 410 | 54 | 1.45 | 1.05–1.98 | .02 | |||||
| Unknown | 259 | 123 | 48 | 1.14 | 0.77–1.68 | .53 | |||||
| Other | 133 | 58 | 44 | 0.97 | 0.62–1.51 | .90 | |||||
| Time since weaning from supplemental oxygen or flowe | |||||||||||
| Never received | 1190 | 442 | 37 | [reference] | <.001 | [reference] | <.001 | ||||
| Off < 1 hr | 80 | 59 | 74 | 4.75 | 1.90–11.93 | .001 | 5.01 | 2.76–9.07 | <.001 | ||
| Off 1 - < 2 hrs | 148 | 108 | 73 | 4.57 | 2.50–8.35 | <.001 | 5.97 | 3.84–9.30 | <.001 | ||
| Off 2 - < 4 hrs | 244 | 166 | 68 | 3.60 | 2.31–5.61 | <.001 | 5.55 | 3.91–7.89 | <.001 | ||
| Off 4 - < 6 hrs | 234 | 135 | 58 | 2.31 | 1.49–3.58 | <.001 | 2.96 | 2.13–4.13 | <.001 | ||
| Off 6 - < 12 hrs | 505 | 276 | 55 | 2.04 | 1.42–2.93 | <.001 | 2.12 | 1.65–2.72 | <.001 | ||
| Off 12 - < 24 hrs | 687 | 302 | 44 | 1.33 | 0.98–1.80 | .07 | 1.16 | 0.93–1.45 | .20 | ||
| Off 24 hrs + | 499 | 179 | 36 | 0.95 | 0.68-1-31 | .74 | 0.75 | 0.58–0.97 | .03 | ||
| Intensive care unit stay during present hospitalization | |||||||||||
| Yes | 884 | 424 | 48 | 1.08 | 0.84–1.39 | .54 | .54 | Not includedb | |||
| No | 2728 | 1255 | 46 | [reference] | |||||||
| Apnea or cyanosisf | |||||||||||
| Yes | 235 | 128 | 54 | 1.41 | 1.07–1.86 | .02 | .02 | 1.40 | 1.01–1.93 | .04 | .04 |
| No | 3377 | 1551 | 46 | [reference] | [reference] | ||||||
| Comorbid condition associated with neurologic impairmentg | |||||||||||
| Yes | 93 | 51 | 55 | 1.41 | 0.96–2.06 | .08 | .08 | 1.50 | 0.93–2.43 | .10 | .10 |
| No | 3519 | 1628 | 46 | [reference] | [reference] | ||||||
| Enteral feeding tube in placeh | |||||||||||
| Yes | 305 | 176 | 58 | 1.64 | 1.23–2.17 | <.001 | <.001 | 1.98 | 1.46–2.67 | <.001 | <.001 |
| No | 3307 | 1503 | 45 | [reference] | [reference] | ||||||
| Hospital type | |||||||||||
| Freestanding (n=33) | 2667 | 1198 | 45 | [reference] | .58 | Not includedb | |||||
| Children’s hospital within hospital (n=14) | 591 | 317 | 54 | 1.42 | 0.74–2.73 | .29 | |||||
| Community (n=9) | 354 | 164 | 46 | 1.06 | 0.55–2.02 | .86 | |||||
| Time of day observation performed | |||||||||||
| Day (10AM - 5PM) | 2073 | 870 | 42 | [reference] | <.001 | [reference] | <.001 | ||||
| Night (11PM - 7AM) | 1539 | 809 | 53 | 1.53 | 1.27–1.85 | <.001 | 2.07 | 1.76–2.43 | <.001 | ||
Abbreviations: CI, confidence interval; OR, odds ratio.
In variables with multiple categories, composite P-value obtained using Wald test.
The following variables met the pre-specified criteria (see Methods) to be included in the multivariable model: Age and gestational age category, time since weaning from supplemental oxygen or flow, apnea or cyanosis, comorbid condition associated with neurologic impairment, enteral feeding tube in place, and time of day observation performed. Ethnicity met initial criteria to enter the model based on having a bivariable association composite P value <.2 but was eliminated from the multivariable model for a composite P value of .34.
Not preterm included the following: documented gestational age 34 0/7 weeks and above, or absence of gestational age but documented as full term, or absence of gestational age but not labeled in chart as preterm or premature.
Patient family-reported race and ethnicity were abstracted from charts in categories defined by the Standards for the Classification of Federal Data on Race and Ethnicity, in compliance with NIH inclusion reporting policies. In this Table, “Other” race combines all of the following categories: American Indian or Alaska Native, Asian, Native Hawaiian or Pacific Islander, specified as more than one race, specified as other, specified as unknown.
Unknown time since weaning (n=25) excluded from Table.
Includes documentation of apnea or cyanosis occurring at home or in hospital during the present illness.
Static encephalopathy, cerebral palsy, hydrocephalus, spina bifida, epilepsy/seizure disorder, or hypotonia.
Nasogastric or gastrostomy.
In the final adjusted mixed effects regression analysis (Table 2), the following variables were significantly associated with being continuously SpO2-monitored: age combined with preterm birth (e.g. odds ratio [OR] of age 8 weeks through 5 months and born preterm = 2.58, 95% CI 1.65–4.02, P <.001 relative to reference group of age 18 months through 23 months and not born preterm), time since weaning from supplemental oxygen or flow (e.g. OR of patients off supplemental oxygen for 2 - < 4 hrs = 5.55, 95% CI 3.91–7.89, P<.001 relative to reference group of never having received supplemental oxygen or flow), documented history of apnea or cyanosis during the present illness (OR 1.40, 95% CI 1.01–1.93, P=.041), presence of an enteral feeding tube (OR 1.98, 95% CI 1.46–2.67, P<.001), and nighttime (OR 2.07, 95% CI 1.76–2.43, P<.001).
Risk-standardized proportions of continuous SpO2 monitoring use ranged from 6% to 82% (Figure 2). Seventeen hospitals were statistical high use outliers (9 freestanding, 6 children’s hospitals within hospitals, and 2 community hospitals), and 10 hospitals were statistical low use outliers (6 freestanding, 2 children’s hospitals within hospitals, and 2 community hospitals). The adjusted model’s intraclass correlation coefficient suggested that 27% (95%CI 19–36%) of the observed variation was attributable to unmeasured hospital level factors.
Figure 2.
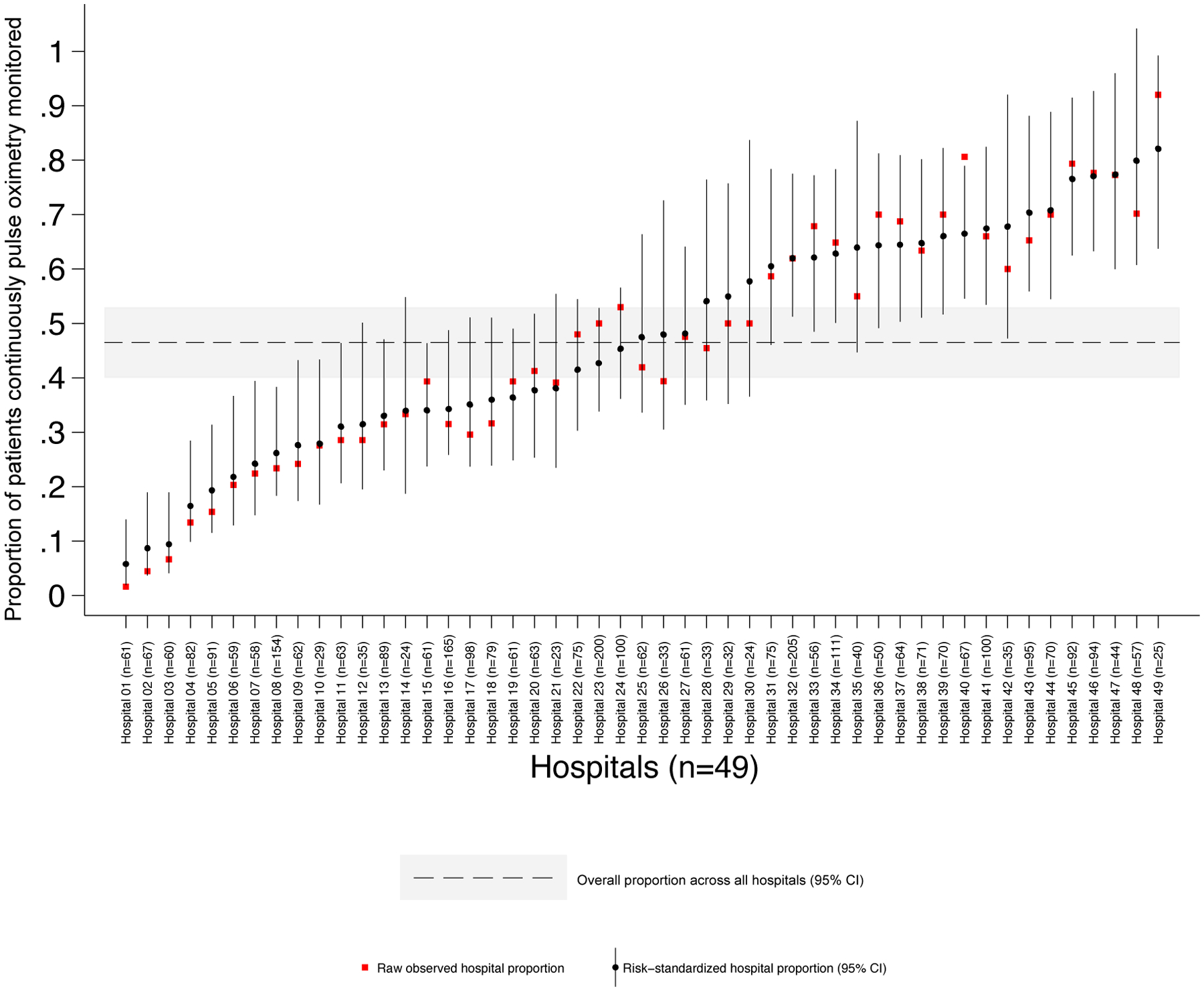
Continuous pulse oximetry use in patients with bronchiolitis not receiving any supplemental oxygen or nasal cannula flow at 49 hospitals. Patients were 8 weeks through 23 months old. Points represent the proportion of patients with bronchiolitis actively monitored with continuous pulse oximetry, measured using direct observation. The risk-standardized proportion for each hospital is the ratio of the predicted to expected use proportions multiplied by the overall proportion across all hospitals. Hospitals are ordered by risk-standardized proportion of patients monitored.
DISCUSSION
In this multicenter cross-sectional study involving a convenience sample of children hospitalized with bronchiolitis, but not actively receiving supplemental oxygen, continuous SpO2 monitoring occurred frequently and this practice varied widely among hospitals.
To our knowledge, this is the first study to measure continuous SpO2 monitoring use in bronchiolitis using direct observation. In a multicenter pediatric quality improvement collaborative, use of continuous SpO2 monitoring in patients with bronchiolitis off supplemental oxygen was assumed if an active monitoring order existed at the time the patient was discharged, but the investigators did not measure the use of continuous SpO2 monitoring at other points in the hospitalization.28 A single center quality improvement project targeting length of stay reduction in bronchiolitis also used orders as a measure of continuous versus intermittent SpO2 monitoring practice.11 Neither project validated the presence of orders against actual monitoring at the bedside. A second single center quality improvement project identified continuous SpO2 monitoring status in children with wheezing by examining monitor data that was directly integrated into the electronic health record in order to quantify time on continuous SpO2 monitoring after patients were weaned to every 2-hour albuterol treatments or off supplemental oxygen to room air.29
This work provides evidence suggesting continuous SpO2 monitoring overuse in bronchiolitis despite national guidelines discouraging its use in this population, and also has broader implications. Recent estimates suggested that the total cost of waste from overtreatment or low-value care in United States ranges from $75.7 billion to $101.2 billion.30 Since the publication of a landmark 2010 article challenging medical specialty societies to create “Top 5 lists” of frequently ordered tests or treatments that provide little benefit,31 attention to minimizing the use of low-value, ineffective, or unproven health care practices increased.32–34 There is an emerging science of deimplementation, the systematic, structured reduction or elimination of a low value care practices, that may inform efforts to reduce monitoring overuse.35,36 This project represents essential first steps in deimplementing an overused low value care practice: measuring “baseline” or “usual care” practices, measuring contextual contributors to overuse, and identifying outlier sites to begin the process of assessing barriers and facilitators to deimplementation.37
Limitations
This study has several limitations. First, it is possible that the convenience sampling approach resulted in a sample not representative of the entire population of stable patients with bronchiolitis. This pragmatic approach was necessary to include a diverse set of hospitals, many of which had limited resources for data collection. However, since at some hospitals, data collectors were physicians and nurses, it is possible that during very high census days in the hospital those individuals were required to provide direct patient care and thus were unavailable to collect data. If, during high census days, monitor use was more prevalent, this convenience sampling approach would have biased our findings toward the null. As physiologic monitoring data become more easily accessible, it is likely that future studies will determine continuous monitoring status using electronic health record data only, eliminating the need for in-person data collection. Second, freestanding children’s hospitals were overrepresented in the sample. There is a need to include more community hospitals in research since less than 30% of pediatric hospitalizations in the United States take place in freestanding children’s hospitals.38 Third, the relationships of other hospital-level factors (e.g. presence of clinical pathways, which have been shown to improve quality of care and reduce overuse in pediatric asthma,39 or characteristics of the nurse work environment associated with patient safety40) and other patient-level factors (e.g. work of breathing, respiratory rate, other comorbidities) were not analyzed in this study but might contribute to continuous SpO2 monitoring use. Fourth, since observers only visited each bedside once during data collection rounds, it is possible that some patients were classified as being continuously monitored at time points when they were actually having intermittent vital sign measurements. Fifth, no data were available to determine if actions were taken to change monitoring practice during the study period in response to occurrence of the observational data collection rounds. Actively changing individual practice was discouraged by requiring that the data collectors not be simultaneously involved in the care of the patients whose data were being collected. Actively changing group practice (e.g. at the unit or department level) in response to feedback of continuous SpO2 use results was prevented by hosting and managing the REDCap database centrally. Individual sites had data entry access only and could not generate reports or download their raw data. Continuous SpO2 use data were shared with hospitals after the data collection period ended. Sixth, the statistical analysis accounted for clustering at the hospital level but could not account for patient, nurse, or physician clustering due to limitations of the data collected.
Conclusions
In a convenience sample of children hospitalized with bronchiolitis but without active supplemental oxygen administration, continuous SpO2 monitoring was frequent and varied widely among hospitals. Because of the apparent absence of a guideline- or evidence-based indication for continuous monitoring in this population, this practice may represent overuse.
KEY POINTS.
Question:
What proportion of children hospitalized with viral bronchiolitis who are not receiving any supplemental oxygen are continuously monitored with pulse oximetry?
Findings:
In this cross-sectional study that included 56 hospitals and 3612 observations of children hospitalized with bronchiolitis, but without receipt of supplemental oxygen, pulse oximetry use ranged from 2% to 92%, with a mean of 46%.
Meaning:
Continuous pulse oximetry monitoring among a sample of hospitalized children with bronchiolitis but without an apparent indication for its use had high prevalence.
ACKNOWLEDGEMENTS
We thank the Center for Outcomes Research & Evaluation at Yale School of Medicine for sharing the CMS SAS Pack for risk standardization, which is freely available to the public upon request from cmsmortalitymeasures@yale.edu.
We acknowledge the NHLBI scientists who contributed their expertise to this project as part of the U01 Cooperative Agreement funding mechanism as federal employees conducting their official job duties: Lora Reineck, MD, MS, Karen Bienstock, MS, and Cheryl Boyce, PhD.
We thank Justin Lakkis, BA, a PhD student at the University of Pennsylvania, for his contributions to statistical analysis. He did not receive compensation for his role in the study.
We thank the Executive Council of the Pediatric Research in Inpatient Settings Network for their contributions to the early scientific development of this project. The Network assessed a Collaborative Support Fee for access to the hospitals and support of this project.
We thank the PRIS Network collaborators for their major contributions to data collection:
Akron Children’s Hospital: Jaclyn Urquiola Sorzano, DO; Karuna Ramcharran, MPH; Prabi Rajbhandari, MD, FAAP; Thomas Mike, MD. Alberta Children’s Hospital: Christopher Andrews, BSc. (H), MD, FRCP(C); Lindsay Long, BSc.,MD, FRCP(C); Michelle Bailey, BSc. (H), MSc., MD, FRCP(C). American Family Children’s Hospital: Kristin Shadman, MD; Rhonda Yngsdal-Krenz, MBA; Sarah MacKay, MD. Ann & Robert H. Lurie Children’s Hospital of Chicago: Kate Lucey, MD, MS; Kristin Van Genderen, MD; M. Katherine Stone, MD, MPH; Michael Spewak, MD; Victoria A. Rodriguez, MD; Waheeda Samady, MD, MSCI. Antelope Valley Hospital: Della Archambo, MSN, RN-BC; Lynne Ellison, DO, MT. Boston Children’s Hospital: Deanna Chieco, MD; Elizabeth Pingree, MD; Patricia Stoeck, MD. C.S. Mott Children’s Hospital: Hiral Mehta, MD; Katrina Foo, MD; Kimberly Monroe, MD, MS; Luzum Matthew, MD, MPH; Mayya Malakh, MD; Nora Biary, MD; Rebekah Shaw, MD. Children’s Hospital & Medical Center Omaha: Chelsea Bloom Anderson, MD; Gregory Johnson, MD; Jacquie Hanks, DNP; Jodi Cantrel, MD; Katherine MacKrell, MD; Melissa England, MD; Russell McCulloh, MD; Sharon Stoolman, MD; Sheilah Snyder, MD. Children’s Hospital at Montefiore: Alyssa Silver, MD; Priya Jain, MD. Children’s Hospital Colorado: Amy Tyler, MD; Michael Tchou, MD. Children’s Hospital Los Angeles: Christopher Russell, MD, MS; Maria Santos, MD; Phillip Abarca, BA; Susan Wu, MD; Vivian Lee, MD. Children’s Hospital New Orleans: Amanda Messer, MD; George Hescock, MD. Children’s Hospital of Oklahoma: Monique Naifeh, MD, MPH; Rachna May, MD; Stephanie Deleon, MD. Children’s Hospital of Philadelphia: Laura El-Hage, MD; Padmavathy Parthasarathy, MD; Stan Oliveira, RN, BSN. Children’s Hospital of Richmond at Virginia Commonwealth University: Amy Spinella, NP; Christine Sirota, NP; Hadi Anwar, MD; Jennifer Schrecengost, NP. Children’s Hospital of The King’s Daughters: Bradley Sieckman, MD; Hope Breckenridge, MSN; Judith Roberts, MSN, RN; Kyrie Shomaker, MD; Megan Brinkley, MSN; Mishi Bhushan, MD, MPH. Children’s Medical Center Dallas: Caitlin Layton, BSN, RN; Courtney Solomon, MD; Danielle Dukellis, MD; Hailee Scoggins, BSN; Mayra Garcia, DNP, RN, PCNS-BC. Children’s Mercy Kansas City: Amita Amonker, MD; Ashley Daly, MD; Kathleen Berg, MD; Matthew Johnson, MD. Children’s National Medical Center: Lynsey Watry, MD; Margaret Rush, MD, MSHS; Tamara Gayle, MD, MEd; Tina Halley, MD. CHOP Care Network at Virtua: Rashida Pittalwala, MD; Shraddha Mittal, MD. Cincinnati Children’s Hospital Medical Center: Sarah Ferris, BA. Cohen Children’s Medical Center: Alexandra Kilinsky, DO; Alyssa Churchill, MD; Ann Le, DO; Erin P. Allmer, MD; Hayley Wolfgruber, MD; Kimberly Lau, MD; Kriti Gupta, MD; Nicole Irgens-Moller, MD. Connecticut Children’s Medical Center: Amy Blodgett, MD; Aseel Dabbagh, DO; Chelsea Lepus, DO; Danielle Klima, DO; Ilana Waynik, MD; Owen Kahn, MD. Cook Children’s Medical Center: Amy Turner, NP; Karen Schultz, MD; Stacey VanVliet, MD. Cox Medical Center South: Jessica Sears, MD; Kayce Morton, DO. Dayton Children’s Hospital: Beth Sullivan, MD; Merrilee Cox, MD. Diamond Children’s Medical Center: Adam Walpert, MD; Chan Lowe, MD; Geetha Gopalakrishnan, MD; Janet Lau, MD; Jasna Seserinac, MD; Melissa Cox, DO; Rachel Cramton, MD. Grand View Hospital: Andrew Chu, MD; Kathleen Shafer, RN; Krista Zehr, RN; Mary Nicolai, RN; Sheila Knerr, MD; Valarie Polk, RN. Hassenfeld Children’s Hospital at NYU Langone: Jasmine Gadhavi, MD. Inova Children’s Hospital: Alexandru Firan, MD; Carolina Saldarriaga Perez, MD; Meredith Carter, MD, MEd. Lucile Packard Children’s Hospital Stanford: Alan Schroeder, MD; Kevin Chi, MD. Mary Washington Hospital: Allison Markowsky, MD, MSHS; Katherine Donowitz, MD; Nailah Coleman, MD; Summer Peters, DO. MUSC Children’s Hospital: Ronald Teufel II, MD, MSCR; Sasha Wee, MD. Nationwide Children’s Hospital: Allison Heacock, MD; Kimberly Tartaglia, MD; Matthew Emery, MD; Michael Perry, MD; Nancy Liao, MD; Ryan Bode, MD; Stephanie Kwon, MD. Nemours / A.I. duPont Hospital for Children: Samuel Stubblefield, MD. Poudre Valley Hospital & Medical Center of the Rockies: Elizabeth Ballard, MD. Primary Children’s Hospital: Ashley Dennis, MD; Glen Huff, MD; John Mulcaire-Jones, MD; Karee Nicholson, RN, MSN; Katie Mailey, MD; Robert Willer, DO. Princeton Medical Center: Alicia Brennan, MD; Anupa Dalal, MD; Geetha Lingasubramanian, MD; Joel Krauss, MD; Julianne Prasto, MD; Koel Guha, MD; Marissa Castellano, MD. Riverton Hospital: Glen Huff, MD. Seattle Children’s Hospital: Kaitlyn McQuistion, MD; Sarah Zaman, MD. St Louis Children’s Hospital: Christine Hrach, MD; Erik Hoefgen, MD; Laura Hulteen, MD; Shakila Mathew, MD; Tosin Adeyanju, MD. St. Mary’s Hospital: Ann Allen, MD. Texas Children’s Hospital: Imgard Carolina Molleda Castro, MD; Mohammad Ovais Aziz, MD; Mohammed Nassif, MD; Ricardo Quinonez, MD. The Hospital for Sick Children: Amna Hilal, MD; Brie Yama, MD, MSc, MED (c), FRCPC, FAAP; Brigitte Parisien, MD; Catherine Diskin, MB, BCH, BAO, MRCPI (Paid) MSc; Dana Arafeh, BSc, CAPM; Sanjay Mahant, MD MSc FRCPC. Tufts Medical Center Floating Hospital for Children: Elena Aragona, MD, MS; Jana Leary, MD, MS. UCSF Benioff Children’s Hospital Mission Bay: Glenn Rosenbluth, MD; Manisha Israni-Jiang, MD; Matt Pantell, MD, MS. University Hospitals Rainbow Babies and Children’s Hospital: Allayne Stephans, MD; Amanda Lansell, MD. University of Iowa Stead Family Children’s Hospital: Guru Bhoojhawon, MBBS, MD; Katherine Patrick, MD; Kelly Wood, MD; Kristen Sandgren, MD. University of Vermont Children’s Hospital: Leigh-Anne Cioffredi, MD/MPH. UPMC Children’s Hospital of Pittsburgh: Kishore Vellody, MD; Sylvia Choi, MD. Upstate Golisano Children’s Hospital: John Andrake, MD; Melissa Schafer, MD. Valley Children’s Hospital: Angela Veesenmeyer, MD, MPH; Katie Chan-Boeckh, RN; Laura Grant, RN; Nicole Webb, MD. Vanderbilt University Medical Center: Derek Williams, MD, MPH; Emily Datyner, MD; Gregory Plemmons, MD; Jakobi Johnson, BS. Wake Forest Baptist Medical Center: Jeanna Auriemma, MD; John Darby, MD; Nicholas Potisek, MD; Sean Ervin, MD, PhD. WVU Children’s Hospital: Chickajajur Vijay, MD, MBBS; Christy Glass, MSN; Kamakshya Patra, MD; Kudora Maize, MSN, APRN; Meghan Williams, MSN, FNP-C; Travis Kennedy, MSN, APRN, FNP-BC. Yale-New Haven Children’s Hospital: Adam Berkwitt, MD.
Funding/Support:
Research reported in this publication was supported by a Cooperative Agreement from the National Heart, Lung, and Blood Institute (NHLBI) of the National Institutes of Health under award number U01HL143475 (Bonafide, PI).
Role of the Funder/Sponsor:
As a Cooperative Agreement, NIH scientists participated in study conference calls and provided ongoing feedback on the conduct and findings of the study. The funding organization had no role in the design of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of Interest Disclosures:
Dr. Beidas reports receiving royalties from Oxford University Press and has provided paid consultation to Merck and Camden Coalition of Healthcare Providers.
Dr. McLeod participated in this project when she was a faculty member at Children’s Hospital Colorado. She is now employed by Array BioPharma. Her work at Array BioPharma is unrelated to this project.
Previous presentation of the information reported in the manuscript:
Presented at the Pediatric Hospital Medicine Annual Meeting in Seattle, WA on July 26, 2019.
REFERENCES
- 1.Cullen DJ, Nemeskal AR, Cooper JB, Zaslavsky A, Dwyer MJ. Effect of pulse oximetry, age, and ASA physical status on the frequency of patients admitted unexpectedly to a postoperative intensive care unit and the severity of their anesthesia-related complications. Anesth Analg. 1992;74(2):181–188. [DOI] [PubMed] [Google Scholar]
- 2.Ochroch EA, Russell MW, Hanson WC, et al. The impact of continuous pulse oximetry monitoring on intensive care unit admissions from a postsurgical care floor. Anesth Analg. 2006;102(3):868–875. [DOI] [PubMed] [Google Scholar]
- 3.Watkins T, Whisman L, Booker P. Nursing assessment of continuous vital sign surveillance to improve patient safety on the medical/surgical unit. J Clin Nurs. 2016;25(1–2):278–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hasegawa K, Tsugawa Y, Brown DFM, Mansbach JM, Camargo CA. Trends in bronchiolitis hospitalizations in the United States, 2000–2009. Pediatrics. 2013;132(1):28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ralston SL, Lieberthal AS, Meissner HC, et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134(5):e1474–e1502. [DOI] [PubMed] [Google Scholar]
- 6.Quinonez RA, Garber MD, Schroeder AR, et al. Choosing wisely in pediatric hospital medicine: Five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):479–485. [DOI] [PubMed] [Google Scholar]
- 7.Quinonez RA, Coon ER, Schroeder AR, Moyer VA. When technology creates uncertainty: pulse oximetry and overdiagnosis of hypoxaemia in bronchiolitis. BMJ. 2017;358:j3850. [DOI] [PubMed] [Google Scholar]
- 8.Cunningham S, Rodriguez A, Adams T, et al. Oxygen saturation targets in infants with bronchiolitis (BIDS): a double-blind, randomised, equivalence trial. Lancet. 2015;386(9998):1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schroeder AR, Marmor AK, Pantell RH, Newman TB. Impact of pulse oximetry and oxygen therapy on length of stay in bronchiolitis hospitalizations. Arch Pediatr Adolesc Med. 2004;158(6):527. [DOI] [PubMed] [Google Scholar]
- 10.Cunningham S, McMurray A. Observational study of two oxygen saturation targets for discharge in bronchiolitis. Arch Dis Child. 2012;97(4):361–363. [DOI] [PubMed] [Google Scholar]
- 11.Mittal S, Marlowe L, Blakeslee S, et al. Successful use of quality improvement methodology to reduce inpatient length of stay in bronchiolitis through judicious use of intermittent pulse oximetry. Hosp Pediatr. 2019;9(2):73–78. [DOI] [PubMed] [Google Scholar]
- 12.Cunningham S, Rodriguez A, Boyd KA, McIntosh E, Lewis SC, on behalf of the BIDS Collaborators Group. Bronchiolitis of Infancy Discharge Study (BIDS): a multicentre, parallel-group, double-blind, randomised controlled, equivalence trial with economic evaluation. Health Technol Assess. 2015;19(71):1–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McBride SC, Chiang VW, Goldmann DA, Landrigan CP. Preventable adverse events in infants hospitalized with bronchiolitis. Pediatrics. 2005;116(3):603–608. [DOI] [PubMed] [Google Scholar]
- 14.Bonafide CP, Lin R, Zander M, et al. Association between exposure to nonactionable physiologic monitor alarms and response time in a children’s hospital. J Hosp Med. 2015;10(6):345–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bonafide CP, Localio AR, Holmes JH, et al. Video analysis of factors associated with response time to physiologic monitor alarms in a children’s hospital. JAMA Pediatr. 2017;171(6):524–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rasooly IR, Beidas RS, Wolk CB, et al. Measuring overuse of continuous pulse oximetry in bronchiolitis and developing strategies for large-scale deimplementation: study protocol for a feasibility trial. Pilot Feasibility Stud. 2019;5(1):68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Unger S, Cunningham S. Effect of oxygen supplementation on length of stay for infants hospitalized with acute viral bronchiolitis. Pediatrics. 2008;121(3):470–475. [DOI] [PubMed] [Google Scholar]
- 18.Cleve WCV, Christakis DA. Unnecessary care for bronchiolitis decreases with increasing inpatient prevalence of bronchiolitis. Pediatrics. 2011;128(5):e1106–e1112. [DOI] [PubMed] [Google Scholar]
- 19.NIH Policy and Guidelines on The Inclusion of Women and Minorities as Subjects in Clinical Research | grants.nih.gov. https://grants.nih.gov/policy/inclusion/women-and-minorities/guidelines.htm. Accessed January 27, 2020. [Google Scholar]
- 20.Schroeder AR, Mansbach JM, Stevenson M, et al. Apnea in children hospitalized with bronchiolitis. Pediatrics. 2013;132(5):e1194–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parker MJ, Allen U, Stephens D, Lalani A, Schuh S. Predictors of major intervention in infants with bronchiolitis. Pediatr Pulmonol. 2009;44(4):358–363. [DOI] [PubMed] [Google Scholar]
- 22.Freire G, Kuppermann N, Zemek R, et al. Predicting escalated care in infants with bronchiolitis. Pediatrics. 2018;142(3):e20174253. [DOI] [PubMed] [Google Scholar]
- 23.Schuh S, Kwong JC, Holder L, Graves E, Macdonald EM, Finkelstein Y. Predictors of critical care and mortality in bronchiolitis after emergency department discharge. J Pediatr. 2018;199:217–222.e1. [DOI] [PubMed] [Google Scholar]
- 24.Krumholz HM, Wang Y, Mattera JA, et al. An administrative claims model suitable for profiling hospital performance based on 30-day mortality rates among patients with an acute myocardial infarction. Circulation. 2006;113(13):1683–1692. [DOI] [PubMed] [Google Scholar]
- 25.2019 Condition-Specific Mortality Measures Updates and Specifications Report. Submitted by Yale New Haven Health Services Corporation – Center for Outcomes Research & Evaluation. Prepared for Centers for Medicare & Medicaid Services. http://www.qualitynet.org/dcs/ContentServer?c=Page&pagename=QnetPublic%2FPage%2FQnetTier4&cid=1163010421830. Published March 2019. Accessed February 1, 2020. [Google Scholar]
- 26.Lagu T, Pekow PS, Stefan MS, et al. Derivation and validation of an in‐hospital mortality prediction model suitable for profiling hospital performance in heart failure. J Am Heart Assoc. 2018;7(4):e005256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inf. 2009;42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ralston SL, Garber MD, Rice-Conboy E, et al. A multicenter collaborative to reduce unnecessary care in inpatient bronchiolitis. Pediatrics. 2016;137(1):e20150851. [DOI] [PubMed] [Google Scholar]
- 29.Schondelmeyer AC, Simmons JM, Statile AM, et al. Using quality improvement to reduce continuous pulse oximetry use in children with wheezing. Pediatrics. 2015;135(4):e1044–e1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shrank WH, Rogstad TL, Parekh N. Waste in the US health care system: estimated costs and potential for savings. JAMA. 2019;322(15):1501–1509. [DOI] [PubMed] [Google Scholar]
- 31.Brody H Medicine’s ethical responsibility for health care reform — the top five list. N Engl J Med. 2010;362(4):283–285. [DOI] [PubMed] [Google Scholar]
- 32.Cassel CK, Guest JA. Choosing wisely: helping physicians and patients make smart decisions about their care. JAMA. 2012;307(17):1801–1802. [DOI] [PubMed] [Google Scholar]
- 33.Morgan DJ, Brownlee S, Leppin AL, et al. Setting a research agenda for medical overuse. BMJ. 2015;351:h4534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coon ER, Quinonez RA, Morgan DJ, et al. 2018 update on pediatric medical overuse: a review. JAMA Pediatr. 2019;173(4):379–384. [DOI] [PubMed] [Google Scholar]
- 35.Bodegom-Vos L van, Davidoff F, Marang-van de Mheen PJ. Implementation and de-implementation: two sides of the same coin? BMJ Qual Saf. 2017;26(6):495–501. [DOI] [PubMed] [Google Scholar]
- 36.Norton WE, Chambers DA, Kramer BS. Conceptualizing de-implementation in cancer care delivery. J Clin Oncol. November 2018:JCO.18.00589. [DOI] [PubMed] [Google Scholar]
- 37.Niven DJ, Mrklas KJ, Holodinsky JK, et al. Towards understanding the de-adoption of low-value clinical practices: a scoping review. BMC Med. 2015;13:255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leyenaar JK, Ralston SL, Shieh M-S, Pekow PS, Mangione-Smith R, Lindenauer PK. Epidemiology of pediatric hospitalizations at general hospitals and freestanding children’s hospitals in the United States. J Hosp Med. 2016;11(11):743–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaiser SV, Rodean J, Bekmezian A, et al. Effectiveness of pediatric asthma pathways for hospitalized children: a multicenter, national analysis. J Pediatr. 2018;197:165–171.e2. [DOI] [PubMed] [Google Scholar]
- 40.Lake ET, Roberts KE, Agosto PD, et al. The association of the nurse work environment and patient safety in pediatric acute care. J Patient Saf. Published online December 28, 2018. doi: 10.1097/PTS.0000000000000559 [DOI] [PMC free article] [PubMed] [Google Scholar]


