Abstract
Free radical-induced oxidative damage reactions, and membrane lipid peroxidation (LP) in particular, are one of the best validated secondary injury mechanisms in preclinical traumatic brain injury models. In addition to the disruption of the membrane phospholipid architecture, LP results in the formation of cytotoxic aldehyde-containing products that bind to cellular proteins and impair their normal functions. This article reviews the progress over the past three decades in regards to the preclinical discovery and attempted clinical development of antioxidant drugs designed to inhibit free radical-induced LP and its neurotoxic consequences via different mechanisms including the O2•- scavenger superoxide dismutase (SOD) and the lipid peroxidation inhibitor tirilazad. In addition, various other antioxidant agents that have been shown to have efficacy in preclinical TBI models are briefly presented such as the LP inhibitors U83836E, resveratrol, curcumin, OPC-14177 and lipoic acid; the iron chelator deferoxamine and the nitroxide-containing antioxidants such as α-phenyl-tert-butyl nitrone and tempol. A relatively new antioxidant mechanistic strategy for acute TBI is aimed at the scavenging of aldehydic LP by-products that are highly neurotoxic with “carbonyl scavenging” compounds. Finally, it is proposed that the most effective approach to interrupt posttraumatic oxidative brain damage after TBI might involve the combined treatment with mechanistically-complementary antioxidants that simultaneously scavenge LP-initiating free radicals, inhibit LP propagation and lastly remove neurotoxic LP byproducts.
Keywords: traumatic brain injury, lipid peroxidation, oxidative damage, antioxidants
INTRODUCTION
At present, there are no FDA-approved pharmacological therapies for acute treatment of traumatic brain injury (TBI) patients that are conclusively proven to mitigate the often devastating neurological effects of their injuries. Nevertheless, the possibility of an effective treatment is based upon the fact that even though some of the neural injury is due to the primary mechanical events (i.e. shearing of nerve cells and blood vessels), the majority of post-traumatic neurodegeneration is due to a pathochemical and pathophysiological cascade of secondary events occurring during the first minutes, hours and days following the injury which exacerbate the damaging effects of the primary injury. Arguably, one of the most validated “secondary injury” mechanisms revealed in experimental TBI studies involves oxygen radical-induced oxidative damage to lipids, proteins and nucleic acids. This review briefly outlines the key sources of reactive oxygen species (ROS) including their derived highly reactive free radicals, the mechanisms associated with their neural damage and the past, present and future of pharmacological antioxidants that should be able to produce a clinically demonstrable neuroprotective effect, if properly applied.
OXIDATIVE DAMAGE IN TRAUMATIC BRAIN INJURY
Superoxide Radical
The first body of work showing a role of oxygen radicals in acute TBI pathophysiology was conducted by Kontos and colleagues who demonstrated an almost immediate post-injury increase in brain microvascular superoxide radical (O2•-) production associated with compromise of autoregulatory function in fluid percussion TBI models 1, 2. These early investigators also demonstrated that scavengers of O2•- decrease the post-traumatic superoxide levels and protect against the loss of microvascular autoregulatory competency. Within the injured nervous system, a number of possible sources of O2•- may be operative during the first minutes and hours after injury including: the arachidonic acid cascade (i.e. prostaglandin synthase and 5-lipoxygenase activity), enzymatic or autoxidation of biogenic amine neurotransmitters (e.g. dopamine, norepinephrine, 5-hydroxytryptamine), “mitochondrial leak”, xanthine oxidase activity and the oxidation of extravasated hemoglobin. Activated microglia and infiltrating neutrophils and macrophages provide additional sources of O2•- at later timepoints.
Superoxide, which is formed by the single electron reduction of oxygen, may act as either an oxidant or reductant. While, O2•- itself is reactive, its direct reactivity toward biological substrates in aqueous environments is relatively weak. Moreover, once formed, O2•- undergoes spontaneous dismutation to form hydrogen peroxide (H2O2) in a reaction that is markedly accelerated by the enzyme superoxide dismutase (SOD): O2−• - + O2− + 2H+ ➔ H2O2 + O2 3. In solution, O2•- actually exists in equilibrium with the hydroperoxyl radical (HO2·): O2• - + H+ ➔ HO2•, which is considerably more lipid soluble and a far more powerful oxidizing or reducing agent (9). Since the pKa of the O2•-/HO2• is 4.8, as the pH of a solution falls (i.e. tissue acidosis), the equilibrium between O2• - and HO2· shifts in favor of HO2• which is much more reactive than O2•-, particularly toward lipids
Iron and Hydroxyl Radical
The CNS is an extremely rich source of iron and its regional distribution varies in parallel with the sensitivity of various regions to oxidative damage 4. Under normal circumstances, low molecular weight forms of redox-active iron are maintained at extremely low levels. In plasma, the iron transport protein transferrin tightly binds iron in the Fe+++ form. Intracellularly, Fe+++ is sequestered by the iron storage protein ferritin. While both ferritin and transferrin have very high affinity for iron at neutral pH and effectively maintain iron in a non-catalytic state 3, both proteins readily give up their iron at pH values of 6.0 or less which is a level of acidosis that has been shown to be reached in the injured brain. Once iron is released from ferritin or transferring, it can actively catalyze oxygen radical reactions. Therefore, within the traumatized brain, where pH in injured areas is typically lowered, conditions are favorable for the potential release of iron from storage proteins 3. In the case of ferritin, its iron can also be released by reductive mobilization by O2• -.
A second source of catalytically active iron is hemoglobin. Hemorrhage resulting from mechanical trauma provides an obvious source of hemoglobin. While hemoglobin itself has been reported to stimulate oxygen radical reactions, it is more likely that iron released from hemoglobin is responsible for hemoglobin-mediated oxidative damage 5, 6. Iron is released from hemoglobin by either H2O2 or by lipid hydroperoxides (LOOH; see below) and this release is further enhanced as the pH falls to 6.5 or below. Therefore, hemoglobin may catalyze oxygen radical formation and LP either directly or through the release of iron by H2O2, LOOH and/or acidic pH.
Free iron or iron chelates participate in free radical reactions at two levels. The autoxidation of Fe++ results in the formation of O2• - 3: Fe++ + O2 ➔ Fe+++ + O2• -. Secondly, Fe++ is also oxidized in the presence of H2O2 to form hydroxyl radical (•OH) (Fenton reaction): Fe++ + H2O2 ➔ Fe+++ + •OH + OH−. Using the salicylate trapping method, a rise in brain ·OH levels has also been documented in a mouse diffuse and rat focal TBI models by the senior author 7, 8 and others 8–10. As with the work of Kontos and colleagues discussed above, the cerebral microvasculature appears to be the initial source of post-traumatic ·OH production.
Chemistry of Lipid Peroxidation and Target Mechanisms for it’s Pharmacological Inhibition
The most studied mechanism of oxidative damage in models of TBI concerns free radical-induced lipid peroxidation (LP). The process of LP is presented in Figure 1 in the context of the ●OH-induced peroxidation of the LP-susceptible arachidonic acid (AA) which is highly enriched in brain cell membranes. “Initiation” of LP occurs when a radical species such as ●OH reacts with and removes an allylic carbon (carbon surrounded by adjacent double bonds) and extracts a hydrogen and it’ single electron from AA (AA + R• ➔ AA• + RH). In the process, the initiating radical is quenched by receipt of an electron (hydrogen) from the polyunsaturated AA. This, however, converts the AA into a lipid or “alkyl” radical (AA●). This sets the stage for a series of “propagation” reactions which begins when the alkyl radical takes on a mole of oxygen creating a lipid peroxyl radical (AA-OO•; AA• + O2 ➔ AA-OO•). The peroxyl radical then reacts with a neighboring AA within the membrane and steals its electron forming a lipid hydroperoxide (AA-OOH) and a second alkyl radical (AA•; AA-OO• + AA ➔ AA-OOH + AA•).
1.
Chemistry involved in the initiation, propagation and termination reactions of arachidonic acid during lipid peroxidation with the resulting formation of the aldehydic end-product 4-hydroxynonenal (4-HNE).
Once LP begins the propagation phase, iron may participate in driving the process as lipid •hydroperoxides are decomposed by reactions with either ferrous iron (Fe++), ferric iron (Fe+++). In the case of Fe++, the reaction results in formation of a lipid alkoxyl radical (AA-O•; AA-OOH + Fe++ ➔ AA-O• + OH− + Fe+++). If, however, the reaction involves Fe+++, the AA-OOH is converted back into a lipid peroxyl radical (AA-OO•; AA-OOH + Fe+++ ➔ AA-OO• + Fe++). Both of the reactions of AA-OOH with iron have acidic pH optima causing them to be augmented by tissue acidosis. Either alkoxyl (AA-O·) or peroxyl (AA-OO·) radicals arising from AA-OOH decomposition by iron can initiate so called lipid hydroperoxide-dependent LP resulting in “chain branching” reactions: (AA-OO• + AA ➔ AA-OOH + AA• or AA-O• + AA ➔ AA-OH +AA•).
Ultimately, the LP process leads to “fragmentation” or “scission” reactions in which the peroxidized AA breaks down to give rise to the neurotoxic aldehydes 4-HNE or 2-propenal (acrolein). The 4-HNE (as well as acrolein) produces neurotoxicity by binding to basic amino acids such as lysine or histidine as well as sulfhydryl-containing cysteine residues in cellular proteins as illustrated in Figure 2. The resulting chemical modifications have been shown to inhibit the function of a variety of structural and enzymatic cellular proteins.
2.
Chemical reactions of 4-HNE with amino acids that lead to impairment of protein structure and function.
Other Forms of Oxidative Damage
The central nervous system is exquisitely sensitive to LP because of its high content of peroxidation-susceptible lipids such as AA, linoleic acid, linolenic acid and docosahexaenoic acid and the high levels of iron. While LP disrupts the normal phospholipid architecture of cellular and subcellular organellar membranes, end-products of LP, most notably 4-HNE and acrolein, can bind to proteins, modifying their structure and compromising function. However, primary radical-mediated oxidative damage can also occur in proteins. For instance, iron-catalyzed, •OH mechanisms can target certain basic amino acids (e.g. lysine, arginine, histidine) leading to the formation of “protein carbonyl” moieties. Another form of protein oxidative damage involves the oxidation of cysteine sulfhydryl groups which can lead to the formation of abnormal disulfide bridges.
Nucleic acids, both DNA and RNA, are also susceptible to oxidative medication by inorganic and organic (i.e. lipid) radicals. In addition to potentially compromising DNA replication, transcription and mRNA translation, DNA oxidative damage also triggers DNA repair mechanisms that can greatly stress cellular function and survival. One such mechanism concerns the activation of poly ADP ribose polymerase (PARP) whose action can lead to severe depletion of cellular stores of ATP. In addition, DNA-protein cross-linking can occur (e.g. thymine-tyrosine) 3. However, compared to the numerous studies that have documented post-traumatic LP and protein oxidative damage in TBI models, very little examination of nucleic acid oxidation has occurred.
Peroxynitrite
Nearly 20 years ago, Beckman and coworkers introduced the theory that the principal ROS involved in producing tissue injury in a variety of neurological disorders is the “reactive nitrogen species” peroxynitrite (PN; ONOO−), which is formed by the combination of NOS-generated ·NO radical and O2•-: O2•- + •NO ➔ ONOO− 11. Since that time, the biochemistry of PN has been in large part defined. PN-mediated oxidative damage is actually caused by PN decomposition products that possess potent free radical characteristics. These are formed in one of two ways. The first involves the protonation of PN to form peroxynitrous acid (ONOOH) which can undergo homolytic decomposition to form the highly reactive nitrogen dioxide radical (·NO2) and ·OH; (ONOOH ➔ ·NO2 + ·OH). Perhaps more important physiologically, PN will react with carbon dioxide (CO2) to form nitrosoperoxocarbonate (ONOOCO2) which can decompose into ·NO2 and carbonate radical (·CO3); (ONOOCO2 ➔ ·NO2 + ·CO3).
Each of the PN-derived radicals (·OH, ·NO2 and ·CO3) can initiate LP cellular damage by abstraction of an electron from a hydrogen atom bound to an allylic carbon in polyunsaturated fatty acids or cause protein carbonylation by reaction with susceptible amino acids (e.g. lysine, cysteine, arginine). Additionally, ·NO2 can nitrate the 3 position of tyrosine residues in proteins; 3-NT is a specific footprint of PN-induced cellular damage. Peroxynitrite-mediated protein nitration can involve the initial oxidation of a tyrosine moiety by a lipid peroxyl or alkoxyl radical followed by nitration by •NO2.
The implication of PN in post-TBI pathophysiology is derived from four lines of evidence. First of all, all three NOS isoforms (endothelial, neuronal and inducible) are known to be up regulated during the first 24 hrs after TBI in rodents 12–14. Secondly, several laboratories have shown that the acute treatment of injured mice or rats with NOS inhibitors can exert a neuroprotective effect and/or improve neurological recovery 15–22. Thirdly, biochemical footprints of PN-mediated damage have been documented in rodent TBI paradigms including an increase in 3-NT levels 15, 21, and ADP ribosylation (evidence of PARP activation). Fourthly, the notion that these markers of PN-mediated damage are pathophysiologically important is supported by the finding that the NOS inhibitor L-NAME can lessen the accumulation of 3-NT in injured brains 15, 21 at the same doses which improve neurological recovery 23.
MECHANISMS FOR PHARMACOLOGICAL INHIBITION OF OXIDATIVE DAMAGE IN TRAUMATIC BRAIN INJURY
Based upon this outline of the steps involved in oxygen radical-induced oxidative damage, and LP in particular, a number of potential mechanisms for its inhibition are apparent which fall into three categories. The first category includes compounds that inhibit the initiation of LP and other forms of oxidative damage by preventing the formation of ROS or RNS species. For instance, NOS inhibitors, discussed above exert an indirect antioxidant effect by limiting •NO production and thus PN formation. However, they also have the potential to interfere with the physiological roles that •NO is responsible for including antioxidant effects which are due it’s important role as a scavenger of lipid peroxyl radicals (e.g. AAOO• + •NO ➔ AAOONO) 24. Another approach to blocking posttraumatic radical formation is the inhibition of the enzymatic (e.g. cyclooxygenase, 5-lipoxygenases) AA cascade during which the formation of O2•- is produced as a by-product of prostanoid and leukotriene synthesis. Kontos and colleagues 2, 25 and Hall and coworkers 26, 27 have shown that cyclooxygenase inhibiting non-steroidal anti-inflammatory agents (e.g. indomethacin, ibuprofen) are vaso-and neuro-protective in TBI models.
A second indirect LP inhibitory approach involves chemically scavenging the radical species (e.g. O2•-, •OH, •NO2, •CO3) before they have a chance to steal an electron from a polyunsaturated fatty acid and thus initiate LP. The use of pharmacologically-administered SOD represents an example of this strategy. Another example concerns the use of the nitroxide antioxidant tempol which has been shown to catalytically scavenge the PN-derived free radicals •NO2 and •CO3 28. In either case, a general limitation to these first two approaches is that they would be expected to have a short therapeutic window and would have to be administered rapidly in order to have a chance to interfere with the initial posttraumatic “burst” of free radical production that has been documented in TBI models 2, 29. While it is believed that ROS, including PN production persists several hrs after injury, the major portion is an early event that peaks in the first 60 minutes after injury making it clinically impractical to pharmacologically inhibit, unless the antioxidant compound is already “on board” when the injury occurs or available for administration immediately thereafter.
In contrast to the above indirect-acting antioxidant mechanisms, the third category involves stopping the “chain reaction” propagation of LP once it has begun. The most demonstrated way to accomplish this is by scavenging of lipid peroxyl (LOO•) or alkoxyl (LO•) radicals. The endogenous scavenger of these lipid radicals is alpha tocopherol or vitamin E (Vit E) which can donate an electron from its phenolic hydroxyl moiety to quench LOO●. However, the scavenging process is stoichiometric (1 Vit E can only quench 1 LOO•) and in the process vitamin E loses its antioxidant efficacy and becomes Vitamin E radical (LOO• + Vit E ➔ LOOH + Vit E•). Although Vit E• is relatively unreactive (i.e. harmless), it also cannot scavenge another LOO• until it is reduced back to its active form by receiving an electron from other endogenous antioxidant reducing agents such as ascorbic acid (Vitamin C) or glutathione (GSH). While this tripartite LOO• antioxidant defense system (Vit E, Vit C, GSH) works fairly effectively in the absence of a major oxidative stress, numerous studies have shown that each of these antioxidants is rapidly consumed during the early min. and hrs. after TBI. Thus, it has long been recognized that more effective pharmacological LOO• and LO• scavengers are needed. Furthermore, it is expected that compounds that could interrupt the LP process after it has begun would be able to exert a more practical neuroprotective effect (i.e. possess longer antioxidant therapeutic window).
A second approach to inhibiting the propagation of LP reactions is to chelate free iron, either ferrous (Fe++) or ferric (Fe+++), which potently catalyzes the breakdown of lipid hydroperoxides (LOOH), an essential event in the continuation of LP chain reactions in cellular membranes. The prototypical iron-chelating drug which chelates Fe+++, is the bacterially (streptomyces pilosus)-derived tri-hydroxamic acid compound deferoxamine.
NEUROPROTECTIVE EFFECTS OF PHARMACOLOGICAL ANTIOXIDANTS
TBI Clinical Trial Results with PEG-SOD, Tirilazad and Dexanabinol
During the past 25 years, there has been an intense effort to discover and develop pharmacological agents for acute treatment of TBI. This has included multiple compounds that possess free radical scavenging/antioxidant properties including polyethylene glycol-conjugated superoxide dismutase (PEG-SOD), the LP inhibitor tirilazad 30–32 and more recently the mixed glutamate antagonist/antioxidant compound dexanabinol 33. However, each of these trials was a therapeutic failure in that no overall benefit has been documented in moderate and severe TBI patient populations which was the primary goal in each case. These failures can be attributed to several factors. Perhaps most importantly, the preclinical assessment of compounds destined for acute TBI trials has often been woefully inadequate in regards to the definition of neuroprotective dose-response relationships, pharmacokinetic-pharmacodynamic correlations, therapeutic window and optimum dosing regimen and treatment duration. However, a number of other issues related to design of the clinical trials are also believed to be involved 32. The following sections briefly review the TBI histories of PEG-SOD and tirilazad. Dexanabinol (HU211) is not discussed further since it is a mixed glutamate antagonist/antioxidant compound that was studied very little in preclinical TBI paradigms prior to being the subject of clinical development for that indication.
PEG-SOD
As mentioned earlier, the earliest studies of free radical scavenging compounds in TBI models were carried out with Cu/Zn SOD based upon the work of Kontos and colleagues who showed that post-traumatic microvascular dysfunction was initiated by O2• 2212 generated as a by-product of the arachidonic acid cascade which is massively activated during the first minutes and hours after TBI 1, 2, 25. Their work showed that administration of SOD prevented the post-traumatic microvascular dysfunction. This lead to clinical trials in which the more metabolically stable polyethylene glycol (PEG)-conjugated SOD was examined in moderate and severe TBI patients when administered within the first 8 hrs after injury. Although an initial small phase II study showed a positive trend, subsequent multi-center phase III studies failed to show a significant benefit in terms of increased survival or improved neurological outcomes 34. Although many explanations for these negative results may be postulated, one reason may be that a large protein like SOD is unlikely to have much brain penetrability and therefore its radical scavenging effects may be limited to the microvasculature. A second reason may be that attempting to scavenge the short-lived inorganic radical O2• - may be associated with a very short therapeutic window, as suggested above. Indeed, the time course of measurable post-traumatic •OH formation in the injured rodent brain has been shown to largely run its course by the end of the first hour after TBI 9, 29. A more rational strategy would be to inhibit the LP that is triggered by the initial burst of inorganic radicals. A comparison of the time course of LP with that of post-traumatic •OH shows that LP reactions continue to build beyond the first post-traumatic hr 9 and may continue for 3–4 days 35. Despite the failure of PEG-SOD in human TBI, experimental studies have shown that transgenic mice that over-express Cu/Zn SOD are significantly protected against post-TBI pathophysiology and neurodegeneration 36–40. This fully supports the importance of post-traumatic O2• - in post-traumatic secondary injury, despite the fact that targeting this primordial radical is probably not the best antioxidant strategy for acute CNS injury compared to trying to stop the downstream LP process that is initiated by the early increases in O2• -, •OH, •NO2 and •CO3.
Tirilazad
Consistent with that rationale, the 21-aminosteroid LP inhibitor tirilazad (aka. U74006F) was discovered which inhibits free radical-induced LP by a combination of LOO• scavenging and a membrane-stabilizing action that limits the propagation of LP reactions between an LOO• and an adjacent polyunsaturated fatty acid. The protective efficacy of tirilazad has been demonstrated in multiple animal models of acute TBI in mice 41, rats 42 and cats 43. While the compound is largely localized in the microvascular endothelium, the post-traumatic disruption of the BBB is known to allow the successful penetration of tirilazad into the brain parenchyma as noted earlier 44. Other mechanistic data derived from the rat controlled cortical impact and the mouse diffuse concussive head injury models have definitively shown that a major effect of tirilazad is to lessen post-traumatic BBB opening 9, 44.
Tirilazad was taken into clinical development in the early 1990s and following a small phase II dose-escalation study that demonstrated the drug’s safety in TBI patients was evaluated in two phase III multi-center clinical trials for it’s ability to improve neurological recovery in moderately and severely injured closed TBI patients. One trial was conducted in North America and the other in Europe. In both trials, TBI patients were treated within 4 hrs after injury with either vehicle or tirilazad (2.5 mg/kg i.v. q6h for 5 days). The North American trial was never published due to a major confounding imbalance in the randomization of the patients to placebo or tirilazad in regards to injury severity and pre-treatment neurological status. In contrast, the European trial had much better randomization balance and has been published 30. The results failed to show a significant beneficial effect of tirilazad in either moderate (GCS = 9–12) or severe (GCS = 4–8) patient categories. However, a post hoc analysis showed that moderately-injured male TBI patients with traumatic SAH has significantly less mortality after treatment with tirilazad (6%) compared to placebo (24%, p<0.026). In severely injured males with tSAH (tSAH) tirilazad also lessened mortality from 43% in placebo-treated to 34% (p<0.071) 30, 45. This result is consistent with the fact that this compound is also highly effective in animal models of SAH 7. Nevertheless, additional trials would have been required in order to establish the neuroprotective utility of tirilazad in certain human TBI subgroups and to gain FDA approval in the U.S. However, the sponsoring company Pharmacia & Upjohn opted not to continue the compound’s development for TBI although it was successfully approved and marketed for use in aneurysmal SAH in several western European and Australasian countries based upon its demonstrated efficacy in phase III SAH trials 46, 47.
Effects of Direct and Indirect-Acting Lipid Peroxidation Inhibitors
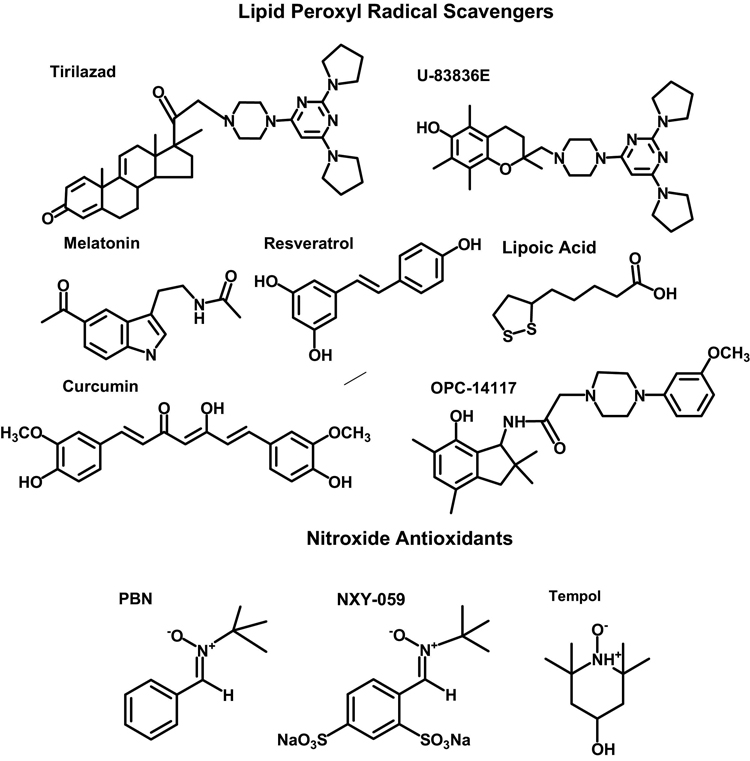
In addition to tirilazad, several other LP inhibitors have been reported to be effective neuroprotectants in TBI models. These include the lipid peroxyl radical (LOO•) scavenging 2-methylaminochromans U-78517F and U-83836E 48, the pyrrolopyrimidine U-101033E 48, 49, OPC-14117 50 and the naturally-occurring LOO• scavengers curcumin 51, 52 and resveratrol 53, 54, the indoleamine melatonin 21, 55–58 and lastly, the endogenous antioxidant lipoic acid 59 (see Figure 3). In the case of curcumin and resveratrol, these are potent LOO• scavengers due to their possession of multiple phenolic hydroxyl groups that can donate electrons to LOO• radicals. Melatonin also has LOO• scavenging capability 60, but in addition appears to react with PN 61, 62. Lipoic acid may also have LOO• scavenging effects, but these are more likely to be indirect via the regeneration (i.e. re-reduction) of other endogenous electron-donating antioxidants including vitamin E, glutathione and vitamin C.
3.
Chemical structures of lipid peroxyl radical scavenging and nitroxide-containing antioxidants shown to be neuroprotective in TBI models.
Among these LP inhibitors, the most potent and effective LOO• scavenging LP inhibitors yet discovered is the 2-methylaminochroman compound U-83836E which combines the LOO• scavenging antioxidant chroman ring structure of vitamin E with the bis-pyrrolopyrimidine moiety of tirilazad. The phenolic chroman antioxidant moiety can be re-reduced by endogenous ascorbic acid (vitamin C) or glutathione (GSH) after it has donated its phenolic electron to an initial LOO• making it able to quench a second and then a third LOO•, etc. The bis-pyrrolopyrimidine moiety, on the other hand, can also scavenge multiple moles of LOO•, but by a true catalytic mechanism 49, 63. Thus, U-83836E, is a dual functionality LOO• scavenger that is understandably more effective than either vitamin E, tirilazad 63 and possibly the other naturally-occurring LOO• scavengers such as curcumin, resveratrol, melatonin and lipoic acid. Furthermore, U-83836E possesses a high degree of lipophilicity endowing it with a high affinity for membrane phospholipids where LP takes place. Recent studies from the authors’ laboratory in the mouse CCI-TBI model have shown that U-83836E is able to reduce post-traumatic LP and protein nitration and preserve mitochondrial respiratory function in injured cortical tissue and mitochondria 64.
Nitroxide Antioxidants and Peroxynitrite Scavengers
In addition to the lipid peroxyl (LOO•) radical scavengers, the neuroprotective effects of a family of nitroxide-containing antioxidants have also been examined in experimental TBI models. These are sometimes referred to as “spin-trapping agents” and include α-phenyl-tert-butyl nitrone (PBN) and its thiol analog NXY-059 and tempol (see bottom of Figure 3). Both PBN and tempol have been shown to be protective in rodent TBI paradigms 65, 66. As mentioned earlier, tempol has been shown by the author and colleagues to catalytically scavenge PN-derived ·NO2 and ·CO3 28, 67, and to reduce post-traumatic oxidative damage (both LP and protein nitration), preserve mitochondrial function, decrease calcium-activated, calpain-mediated cytoskeletal damage and reduce neurodegeneration in mice subjected to a severe controlled cortical impact-induced focal TBI 68. Another laboratory has reported that tempol can reduce post-traumatic brain edema and improve neurological recovery in rat contusion injury model 69, 70. However, the neuroprotective effect of tempol, administered alone, is associated with a therapeutic window of an hr or less in the mouse controlled cortical impact TBI (CCI-TBI) model. Moreover, tempol is not effective at directly inhibiting LP in the latter model 68.
Effects of the Iron Chelator Deferoxamine
The prototype iron chelator deferoxamine which binds ferric (Fe+++) iron and thereby would lessen the catalytic effects of iron on LP, has also been reported to have beneficial actions in preclinical TBI or TBI-related models 71, 72. However, deferoxamine is hindered by its lack of brain penetration and rapid plasma elimination rate. To partially counter the latter limitation, a dextran-coupled deferoxamine has been synthesized that has been reported to significantly improve early neurological recovery in a mouse diffuse TBI model 73. Much of this activity, however, is probably due to microvascular antioxidant protection because of limited brain penetrability. Another caveat to the iron-chelation antioxidant neuroprotective approach that is at least relevant to the ferric iron chelators such as deferoxamine is that they can cause a pro-oxidant effect in that their binding of Fe+++ can actually drive the oxidation of ferrous to ferric iron which can increase superoxide radical formation in the process (Fe++ + O2 ➔ Fe+++ + O2•-).
Carbonyl Scavenging as an Approach to Inhibit 4-HNE and Acrolein Binding to Proteins
As pointed out earlier (Fig. 2), the LP-derived aldehydic (carbonyl-containing) breakdown products 4-HNE and acrolein have high affinity for binding to selected protein amino acid residues including histidine, lysine, arginine and cysteine. These modifications have been shown to inhibit the activities of a variety of enzymatic proteins 3. Several compounds have been recently identified that are able to antagonize this “carbonyl stress” by covalently binding to reactive LP-derived aldehydes. For instance, D-penicillamine has been demonstrated to form an irreversible bond to primary aldehydes. We have previously demonstrated that penicillamine is able to scavenge PN 74 and to protect brain mitochondria from PN-induced respiratory dysfunction in isolated rat brain mitochondria 75. This latter action was observed along with an attenuation of 4-HNE-modified mitochondrial proteins 75. The PN scavenging action of penicillamine along with its carbonyl scavenging capability may jointly explain our previous findings that acutely administered penicllamine can improve early neurological recovery of mice subjected to moderately severe concussive TBI 76.
More recently, it has been demonstrated that a variety of hydrazine (-NH-NH2)-containing compounds such as the anti-hypertensive agent hydralazine, the anti-depressant phenelzine and the anti-tubercular agent iproniazid can react with the carbonyl (CHO) moieties of 4-HNE or acrolein which prevents the latter from binding to susceptible amino acids in proteins 77. Consistent with this effect being neuroprotective, others have shown that hydralazine inhibits either compression of acrolein-mediated injuries to ex vivo spinal cord 78. Hydralazine, which is a potent vasodilator would be difficult to administer in vivo after either spinal cord injury or TBI in which hypotension is already a common pathophysiological problem. However, other hydrazine-containing compounds such as phenelzine and iproniazid do not compromise blood pressure as readily as hydralazine and have a long history of clinical use although never having been examined in acute neurotrauma models. Most impressive is the fact that the application of hydrazines can rescue cultured cells from 4-HNE toxicity even when administered after the 4-HNE has already covalently bound to cellular proteins 77. Such an effect could be associated with a favorable neuroprotective therapeutic window.
RATIONALE FOR COMBINATION ANTIOXIDANT TREATMENT OF TBI
Antioxidant neuroprotective therapeutic discovery directed at acute TBI has consistently been focused upon attempting to inhibit the secondary injury cascade by pharmacological targeting of a single oxidative damage mechanism. As presented above, these efforts have included either enzymatic scavenging of superoxide radicals with SOD 34 or inhibition of LP with tirilazad 30. While each of these strategies has shown protective efficacy in animal models of TBI, phase III clinical trials with either compound failed to demonstrate a statistically significant positive effect although post hoc subgroup analysis suggests that the microvascularly localized tirilazad may have efficacy in moderate and severe TBI patients with tSAH 30. While many reasons have been identified as possible contributors to the failure, one logical explanation has to with the possible need to interfere at multiple points in the oxidative damage portion of the secondary injury cascade either simultaneously or in a phased manner in order to achieve a clinically demonstrable level of neuroprotection.
In addition to the antioxidant strategy of scavenging the initiating radicals and stopping the propagation of LP reactions in the injured brain tissue, recent work has shown that carbonyl (CHO) scavenging compounds can also act to protect cellular proteins from the binding of neurotoxic LP-derived aldehydes. Thus, we are presently exploring the neuroprotective efficacy of three of the prototypes of this new class of compound alone and in combination with the PN-radical scavenging tempol and/or the LP-inhibiting U-83836E. Figure 4 summarizes the overall rationale for a multi-mechanistic antioxidant therapy for TBI. It is anticipated that the combination of two or three antioxidant mechanistic strategies may improve the extent of neuroprotective efficacy, lessen the variability of the effect and possibly provide a longer therapeutic window of opportunity compared to the window for the individual strategies. If these theoretical combinatorial benefits are confirmed in preclinical TBI models this should greatly enhance the chance of neuroprotective success in future clinical trials in contrast to previous failures with single antioxidant agents.
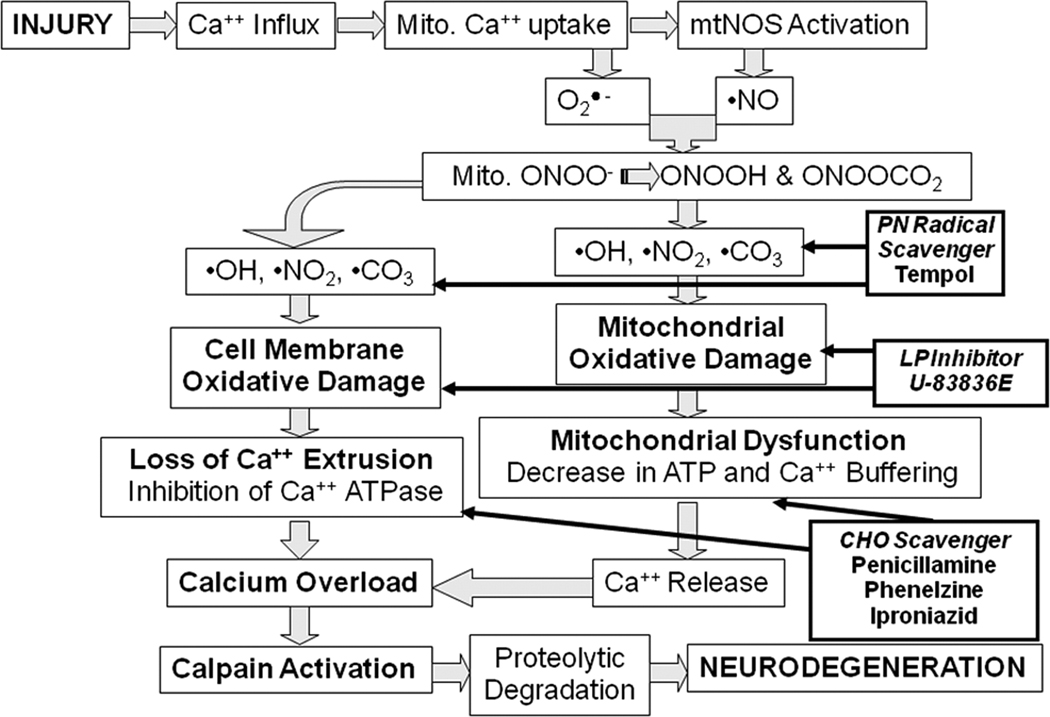
4.
Rationale for combination antioxidant therapy for TBI. Injury triggers an increase in cytoplasmic Ca++ via voltage dependent and glutamate receptor-operated channels. The increase in intracellular Ca++ initiates activation of cytoplasmic calpain. Mitochondrial Ca++ uptake (buffering) stresses the mitochondria and contributes to mitochondrial dysfunction Specifically, Ca++ uptake by the mitochondria leads O2•- leakage from the electron transport chain and activation of Ca++-activated mitochondrial nitric oxide synthase (NOS). The O2•- and NO● combine to form the potent reactive nitrogen species PN which is able to which in turn gives rise to the highly reactive nitrogen dioxide (•NO2), hydroxyl (•OH) and carbonate (•CO3) radicals which cause oxidative damage to the mitochondria as well as other cellular structures due to PN’s large diffusion radius. When this becomes severe, there is a decrease in the mitochondrial ATP production and membrane potential (ΔΨ). This leads to catastrophic mitochondrial failure (mitochondrial permeability transition, MPT) and the dumping of mitochondrial Ca++ into the cytoplasm where it exacerbates cytoplasmic calpain activation and proteolysis of a cytoskeletal proteins and other substrates. The combination of the antioxidant tempol which catalytically reacts with PN-derived radicals with a chain-breaking LP inhibitor such as U-83836E or a carbonyl (CHO) scavenging compound should produce a better neuroprotective effect than any of these compounds alone.
ACKNOWLEDGEMENTS
Portions of the work reviewed in this article were supported by funding from 5R01 NS046566, 5P30 NS051220 and 5P01 NS58484 and from the Kentucky Spinal Cord & Head Injury Research Trust.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Kontos HA, Povlishock JT. Oxygen radicals in brain injury. Cent Nerv Syst Trauma. 1986;3:257–263. doi: 10.1089/cns.1986.3.257. [DOI] [PubMed] [Google Scholar]
- 2.Kontos HA, Wei EP. Superoxide production in experimental brain injury. J Neurosurg. 1986;64:803–807. doi: 10.3171/jns.1986.64.5.0803. [DOI] [PubMed] [Google Scholar]
- 3.Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine. 3rd ed. Oxford University Press; 2008. [Google Scholar]
- 4.Zaleska MM, Floyd RA. Regional lipid peroxidation in rat brain in vitro: possible role of endogenous iron. Neurochem Res. 1985;10:397–410. doi: 10.1007/BF00964608. [DOI] [PubMed] [Google Scholar]
- 5.Sadrzadeh SM, Graf E, Panter SS, Hallaway PE, Eaton JW. Hemoglobin. A biologic fenton reagent. J Biol Chem. 1984;259:14354–14356. [PubMed] [Google Scholar]
- 6.Sadrzadeh SM, Eaton JW. Hemoglobin-mediated oxidant damage to the central nervous system requires endogenous ascorbate. J Clin Invest. 1988;82:1510–1515. doi: 10.1172/JCI113759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hall ED, McCall JM, Means ED. Therapeutic potential of the lazaroids (21-aminosteroids) in acute central nervous system trauma, ischemia and subarachnoid hemorrhage. Adv Pharmacol. 1994;28:221–268. doi: 10.1016/s1054-3589(08)60497-4. [DOI] [PubMed] [Google Scholar]
- 8.Hall ED, Braughler JM. Free radicals in CNS injury. Res Publ Assoc Res Nerv Ment Dis. 1993;71:81–105. [PubMed] [Google Scholar]
- 9.Smith SL, Andrus PK, Zhang JR, Hall ED. Direct measurement of hydroxyl radicals, lipid peroxidation, and blood-brain barrier disruption following unilateral cortical impact head injury in the rat. J Neurotrauma. 1994;11:393–404. doi: 10.1089/neu.1994.11.393. [DOI] [PubMed] [Google Scholar]
- 10.Globus MY, Alonso O, Dietrich WD, Busto R, Ginsberg MD. Glutamate release and free radical production following brain injury: effects of posttraumatic hypothermia.PG - 1704-11. J Neurochem. 1995;65 doi: 10.1046/j.1471-4159.1995.65041704.x. [DOI] [PubMed] [Google Scholar]
- 11.Beckman JS. The double-edged role of nitric oxide in brain function and superoxide-mediated injury. J Dev Physiol. 1991;15:53–59. [PubMed] [Google Scholar]
- 12.Cobbs CS, Fenoy A, Bredt DS, Noble LJ. Expression of nitric oxide synthase in the cerebral microvasculature after traumatic brain injury in the rat. Brain Res. 1997;751:336–338. doi: 10.1016/s0006-8993(96)01429-1. [DOI] [PubMed] [Google Scholar]
- 13.Rao VL, Dogan A, Bowen KK, Dempsey RJ. Traumatic injury to rat brain upregulates neuronal nitric oxide synthase expression and L-[3H]nitroarginine binding. J Neurotrauma. 1999;16:865–877. doi: 10.1089/neu.1999.16.865. [DOI] [PubMed] [Google Scholar]
- 14.Gahm C, Holmin S, Mathiesen T. Temporal profiles and cellular sources of three nitric oxide synthase isoforms in the brain after experimental contusion. Neurosurgery. 2000;46:169–177. [PubMed] [Google Scholar]
- 15.Mesenge C, Charriaut-Marlangue C, Verrecchia C, Allix M, Boulu RR, Plotkine M. Reduction of tyrosine nitration after N(omega)-nitro-L-arginine-methylester treatment of mice with traumatic brain injury. Eur J Pharmacol. 1998;353:53–57. doi: 10.1016/s0014-2999(98)00432-4. [DOI] [PubMed] [Google Scholar]
- 16.Mesenge C, Verrecchia C, Allix M, Boulu RR, Plotkine M. Reduction of the neurological deficit in mice with traumatic brain injury by nitric oxide synthase inhibitors.PG - 209-14. J Neurotrauma. 1996;13 [PubMed] [Google Scholar]
- 17.Wada K, Alonso OF, Busto R, et al. Early treatment with a novel inhibitor of lipid peroxidation ( LY341122) improves histopathological outcome after moderate fluid percussion brain injury in rats. Neurosurgery. 1999;45:601–608. doi: 10.1097/00006123-199909000-00031. [DOI] [PubMed] [Google Scholar]
- 18.Wada K, Chatzipanteli K, Busto R, Dietrich WD. Role of nitric oxide in traumatic brain injury in the rat. J Neurosurg. 1998;89:807–818. doi: 10.3171/jns.1998.89.5.0807. [DOI] [PubMed] [Google Scholar]
- 19.Wada K, Chatzipanteli K, Busto R, Dietrich WD. Effects of L-NAME and 7-NI on NOS catalytic activity and behavioral outcome after traumatic brain injury in the rat. J Neurotrauma. 1999;16:203–212. doi: 10.1089/neu.1999.16.203. [DOI] [PubMed] [Google Scholar]
- 20.Wallis RA, Panizzon KL, Girard JM. Traumatic neuroprotection with inhibitors of nitric oxide and ADP-ribosylation. Brain Res. 1996;710:169–177. doi: 10.1016/0006-8993(95)01278-8. [DOI] [PubMed] [Google Scholar]
- 21.Mesenge C, Margaill I, Verrecchia C, Allix M, Boulu RG, Plotkine M. Protective effect of melatonin in a model of traumatic brain injury in mice. J Pineal Res. 1998;25:41–46. doi: 10.1111/j.1600-079x.1998.tb00384.x. [DOI] [PubMed] [Google Scholar]
- 22.Wada K, Chatzipanteli K, Kraydieh S, Busto R, Dietrich WD. Inducible nitric oxide synthase expression after traumatic brain injury and neuroprotection with aminoguanidine treatment in rats. Neurosurgery. 1998;43:1427–1436. doi: 10.1097/00006123-199812000-00096. [DOI] [PubMed] [Google Scholar]
- 23.Mesenge C, Verrecchia C, Allix M, Boulu RR, Plotkine M. Reduction of the neurological deficit in mice with traumatic brain injury by nitric oxide synthase inhibitors. J Neurotrauma. 1996;13:11–16. doi: 10.1089/neu.1996.13.11. [DOI] [PubMed] [Google Scholar]
- 24.Hummel SG, Fischer AJ, Martin SM, Schafer FQ, Buettner GR. Nitric oxide as a cellular antioxidant: a little goes a long way. Free Radic Biol Med. 2006;40:501–506. doi: 10.1016/j.freeradbiomed.2005.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kontos HA. Oxygen radicals in CNS damage. Chem Biol Interact. 1989;72:229–255. doi: 10.1016/0009-2797(89)90001-x. [DOI] [PubMed] [Google Scholar]
- 26.Hall E. Beneficial effects of acute intravenous ibuprofen with inhibition of thromboxane A2 synthetase or 5-lipoxygenase. CNS Trauma. 1986;2:75–83. doi: 10.1089/cns.1985.2.75. [DOI] [PubMed] [Google Scholar]
- 27.Hall E. Beneficial effects of acute intravenous ibuprofen on neurological recovery of head injured mice: Comparison of cyclooxygenase inhibition of thromboxane A2 synthetase or 5-lipoxygenase. CNS Trauma. 1986;2:75–83. doi: 10.1089/cns.1985.2.75. [DOI] [PubMed] [Google Scholar]
- 28.Carroll RT, Galatsis P, Borosky S, et al. 4-Hydroxy-2,2,6,6-tetramethylpiperidine-1-oxyl (Tempol) inhibits peroxynitrite-mediated phenol nitration. Chem Res Toxicol. 2000;13:294–300. doi: 10.1021/tx990159t. [DOI] [PubMed] [Google Scholar]
- 29.Hall ED, Andrus PK, Yonkers PA. Brain hydroxyl radical generation in acute experimental head injury. J Neurochem. 1993;60:588–594. doi: 10.1111/j.1471-4159.1993.tb03189.x. [DOI] [PubMed] [Google Scholar]
- 30.Marshall LF, Maas AI, Marshall SB, et al. A multicenter trial on the efficacy of using tirilazad mesylate in cases of head injury.PG - 519-25. J Neurosurg. 1998;89 doi: 10.3171/jns.1998.89.4.0519. [DOI] [PubMed] [Google Scholar]
- 31.Langham J, Goldfrad C, Teasdale G, Shaw D, Rowan K. Calcium channel blockers for acute traumatic brain injury. Cochrane Database Syst Rev. 2000 doi: 10.1002/14651858.CD000565. CD000565. [DOI] [PubMed] [Google Scholar]
- 32.Narayan RK, Michel ME, Ansell B, et al. Clinical trials in head injury. J Neurotrauma. 2002;19:503–557. doi: 10.1089/089771502753754037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maas AI, Murray G, Henney H, 3rd, et al. Efficacy and safety of dexanabinol in severe traumatic brain injury: results of a phase III randomised, placebo-controlled, clinical trial. Lancet Neurol. 2006;5:38–45. doi: 10.1016/S1474-4422(05)70253-2. [DOI] [PubMed] [Google Scholar]
- 34.Muizelaar JP, Kupiec JW, Rapp LA. PEG-SOD after head injury. J Neurosurg. 1995;83:942. doi: 10.3171/jns.1995.83.5.0942. [DOI] [PubMed] [Google Scholar]
- 35.Du L, Bayir H, Lai Y, et al. Innate gender-based proclivity in response to cytotoxicity and programmed cell death pathway. J Biol Chem. 2004;279:38563–38570. doi: 10.1074/jbc.M405461200. [DOI] [PubMed] [Google Scholar]
- 36.Chan PH, Epstein CJ, Li Y, et al. Transgenic mice and knockout mutants in the study of oxidative stress in brain injury. J Neurotrauma. 1995;12:815–824. doi: 10.1089/neu.1995.12.815. [DOI] [PubMed] [Google Scholar]
- 37.Mikawa S, Kinouchi H, Kamii H, et al. Attenuation of acute and chronic damage following traumatic brain injury in copper, zinc-superoxide dismutase transgenic mice. J Neurosurg. 1996;85:885–891. doi: 10.3171/jns.1996.85.5.0885. [DOI] [PubMed] [Google Scholar]
- 38.Lewen A, Matz P, Chan PH. Free radical pathways in CNS injury. J Neurotrauma. 2000;17:871–890. doi: 10.1089/neu.2000.17.871. [DOI] [PubMed] [Google Scholar]
- 39.Xiong Y, Shie FS, Zhang J, Lee CP, Ho YS. Prevention of mitochondrial dysfunction in post-traumatic mouse brain by superoxide dismutase. J Neurochem. 2005;95:732–744. doi: 10.1111/j.1471-4159.2005.03412.x. [DOI] [PubMed] [Google Scholar]
- 40.Gladstone DJ, Black SE, Hakim AM. Toward wisdom from failure: lessons from neuroprotective stroke trials and new therapeutic directions. Stroke. 2002;33:2123–2136. doi: 10.1161/01.str.0000025518.34157.51. [DOI] [PubMed] [Google Scholar]
- 41.Hall ED, Yonkers PA, McCall JM, Braughler JM. Effects of the 21-aminosteroid U74006F on experimental head injury in mice. J Neurosurg. 1988;68:456–461. doi: 10.3171/jns.1988.68.3.0456. [DOI] [PubMed] [Google Scholar]
- 42.McIntosh TK, Thomas M, Smith D, Banbury M. The novel 21-aminosteroid U74006F attenuates cerebral edema and improves survival after brain injury in the rat. J Neurotrauma. 1992;9:33–46. doi: 10.1089/neu.1992.9.33. [DOI] [PubMed] [Google Scholar]
- 43.Dimlich RV, Tornheim PA, Kindel RM, Hall ED, Braughler JM, McCall JM. Effects of a 21-aminosteroid (U-74006F) on cerebral metabolites and edema after severe experimental head trauma. Adv Neurol. 1990;52:365–375. [PubMed] [Google Scholar]
- 44.Hall ED, Yonkers PA, Andrus PK, Cox JW. Anderson DK. Biochemistry and pharmacology of lipid antioxidants in acute brain and spinal cord injury. J Neurotrauma. 1992;9 Suppl 2:S425–S442. [PubMed] [Google Scholar]
- 45.Lyons WE, George EB, Dawson TM, Steiner JP, Snyder SH. Immunosuppressant FK506 promotes neurite outgrowth in cultures of PC12 cells and sensory ganglia. Proc Natl Acad Sci U S A. 1994;91:3191–3195. doi: 10.1073/pnas.91.8.3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kassell NF, Haley EC, Jr, Apperson-Hansen C, Alves WM. Randomized, double-blind, vehicle-controlled trial of tirilazad mesylate in patients with aneurysmal subarachnoid hemorrhage: a cooperative study in Europe, Australia, and New Zealand. J Neurosurg. 1996;84:221–228. doi: 10.3171/jns.1996.84.2.0221. [DOI] [PubMed] [Google Scholar]
- 47.Lanzino G, Kassell NF. Double-blind, randomized, vehicle-controlled study of high-dose tirilazad mesylate in women with aneurysmal subarachnoid hemorrhage. Part II. A cooperative study in North America. J Neurosurg. 1999;90:1018–1024. doi: 10.3171/jns.1999.90.6.1018. [DOI] [PubMed] [Google Scholar]
- 48.Hall ED. The mouse head injury model: utility in the discovery of acute cerebroprotective agents. In: STOO T, editor. In Central Nervous System Trauma Research Techniques. CRC Press: Boca Raton, FL; 1995. pp. 213–233. [Google Scholar]
- 49.Hall ED, Andrus PK, Smith SL, Oostveen JA, scherch HM, Lutzke BS, Raub TJ, Sawada GA, Palmer JR, Banitt LS, Tustin JM, Belonga KL, Ayer DE, Bundy GL. Neuroprotective efficacy of microvascularly-localized versus brain-penetraiting antioxidants. acta neurochir(Suppl) 1995;66 doi: 10.1007/978-3-7091-9465-2_19. [DOI] [PubMed] [Google Scholar]
- 50.Mori T, Kawamata T, Katayama Y, et al. Antioxidant, OPC-14117, attenuates edema formation, and subsequent tissue damage following cortical contusion in rats. Acta Neurochir Suppl (Wien) 1998;71:120–122. doi: 10.1007/978-3-7091-6475-4_36. [DOI] [PubMed] [Google Scholar]
- 51.Sharma S, Zhuang Y, Ying Z, Wu A, Gomez-Pinilla F. Dietary curcumin supplementation counteracts reduction in levels of molecules involved in energy homeostasis after brain trauma. Neuroscience. 2009;161:1037–1044. doi: 10.1016/j.neuroscience.2009.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu A, Ying Z, Gomez-Pinilla F. Dietary curcumin counteracts the outcome of traumatic brain injury on oxidative stress, synaptic plasticity, and cognition. Exp Neurol. 2006;197:309–317. doi: 10.1016/j.expneurol.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 53.Ates O, Cayli S, Altinoz E, et al. Neuroprotection by resveratrol against traumatic brain injury in rats. Mol Cell Biochem. 2007;294:137–144. doi: 10.1007/s11010-006-9253-0. [DOI] [PubMed] [Google Scholar]
- 54.Sonmez U, Sonmez A, Erbil G, Tekmen I, Baykara B. Neuroprotective effects of resveratrol against traumatic brain injury in immature rats. Neurosci Lett. 2007;420:133–137. doi: 10.1016/j.neulet.2007.04.070. [DOI] [PubMed] [Google Scholar]
- 55.Beni SM, Kohen R, Reiter RJ, Tan DX, Shohami E. Melatonin-induced neuroprotection after closed head injury is associated with increased brain antioxidants and attenuated late-phase activation of NF-kappaB and AP-1. FASEB J. 2004;18:149–151. doi: 10.1096/fj.03-0323fje. [DOI] [PubMed] [Google Scholar]
- 56.Cirak B, Rousan N, Kocak A, Palaoglu O, Palaoglu S, Kilic K. Melatonin as a free radical scavenger in experimental head trauma. Pediatr Neurosurg. 1999;31:298–301. doi: 10.1159/000028879. [DOI] [PubMed] [Google Scholar]
- 57.Ozdemir D, Tugyan K, Uysal N, et al. Protective effect of melatonin against head trauma-induced hippocampal damage and spatial memory deficits in immature rats. Neurosci Lett. 2005;385:234–239. doi: 10.1016/j.neulet.2005.05.055. [DOI] [PubMed] [Google Scholar]
- 58.Ozdemir D, Uysal N, Gonenc S, et al. Effect of melatonin on brain oxidative damage induced by traumatic brain injury in immature rats. Physiol Res. 2005;54:631–637. [PubMed] [Google Scholar]
- 59.Toklu HZ, Hakan T, Biber N, Solakoglu S, Ogunc AV, Sener G. The protective effect of alpha lipoic acid against traumatic brain injury in rats. Free Radic Res. 2009;43:658–667. doi: 10.1080/10715760902988843. [DOI] [PubMed] [Google Scholar]
- 60.Longoni B, Salgo MG, Pryor WA, Marchiafava PL. Effects of melatonin on lipid peroxidation induced by oxygen radicals. Life Sci. 1998;62:853–859. doi: 10.1016/s0024-3205(98)00002-2. [DOI] [PubMed] [Google Scholar]
- 61.Zhang H, Squadrito GL, Pryor WA. The reaction of melatonin with peroxynitrite: formation of melatonin radical cation and absence of stable nitrated products. Biochem Biophys Res Commun. 1998;251:83–87. doi: 10.1006/bbrc.1998.9426. [DOI] [PubMed] [Google Scholar]
- 62.Zhang H, Squadrito GL, Uppu R, Pryor WA. Reaction of peroxynitrite with melatonin: A mechanistic study. Chem Res Toxicol. 1999;12:526–534. doi: 10.1021/tx980243t. [DOI] [PubMed] [Google Scholar]
- 63.Hall ED, Braughler JM, Yonkers PA, et al. U-78517F: a potent inhibitor of lipid peroxidation with activity in experimental brain injury and ischemia. J Pharmacol Exp Ther. 1991;258:688–694. [PubMed] [Google Scholar]
- 64.Mustafa AG, Singh IN, Carrico KM, Hall ED. Mitochondrial protection after traumatic brain injury by scavenging lipid peroxyl radicals. J Neurotrauma. 2009;26:A 93. doi: 10.1111/j.1471-4159.2010.06749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Awasthi D, Church DF, Torbati D, Carey ME, Pryor WA. Oxidative stress following traumatic brain injury in rats. Surg Neurol. 1997;47:575–581. doi: 10.1016/s0090-3019(96)00461-2. discussion 581-572. [DOI] [PubMed] [Google Scholar]
- 66.Marklund N, Clausen F, Lewen A, Hovda DA, Olsson Y, Hillered L. alpha-Phenyl-tert-N-butyl nitrone (PBN) improves functional and morphological outcome after cortical contusion injury in the rat. Acta Neurochir (Wien) 2001;143:73–81. doi: 10.1007/s007010170141. [DOI] [PubMed] [Google Scholar]
- 67.Bonini MG, Mason RP, Augusto O. The Mechanism by which 4-hydroxy-2,2,6,6-tetramethylpiperidene-1-oxyl (tempol) diverts peroxynitrite decomposition from nitrating to nitrosating species.PG - 506-11. Chem Res Toxicol. 2002;15 doi: 10.1021/tx015571z. [DOI] [PubMed] [Google Scholar]
- 68.Deng-Bryant Y, Singh IN, Carrico KM, Hall ED. Neuroprotective effects of tempol, a catalytic scavenger of peroxynitrite-derived free radicals, in a mouse traumatic brain injury model. J Cereb Blood Flow Metab. 2008;28:1114–1126. doi: 10.1038/jcbfm.2008.10. [DOI] [PubMed] [Google Scholar]
- 69.Zhang R, Shohami E, Beit-Yannai E, Bass R, Trembovler V, Samuni A. Mechanism of brain protection by nitroxide radicals in experimental model of closed-head injury.PG - 332-40. Free Radic Biol Med. 1998;24 doi: 10.1016/s0891-5849(97)00267-0. [DOI] [PubMed] [Google Scholar]
- 70.Beit-Yannai E, Zhang R, Trembovler V, Samuni A, Shohami E. Cerebroprotective effect of stable nitroxide radicals in closed head injury in the rat.PG - 22-8. Brain Res. 1996;717 doi: 10.1016/0006-8993(95)01492-6. [DOI] [PubMed] [Google Scholar]
- 71.Long DA, Ghosh K, Moore AN, Dixon CE, Dash PK. Deferoxamine improves spatial memory performance following experimental brain injury in rats. Brain Res. 1996;717:109–117. doi: 10.1016/0006-8993(95)01500-0. [DOI] [PubMed] [Google Scholar]
- 72.Gu Y, Hua Y, Keep RF, Morgenstern LB, Xi G. Deferoxamine reduces intracerebral hematoma-induced iron accumulation and neuronal death in piglets. Stroke. 2009;40:2241–2243. doi: 10.1161/STROKEAHA.108.539536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Panter SS, Braughler JM, Hall ED. Dextran-coupled deferoxamine improves outcome in a murine model of head injury. J. Neurotrauma. 1992;9:47–53. doi: 10.1089/neu.1992.9.47. [DOI] [PubMed] [Google Scholar]
- 74.Althaus JS, Oien TT, Fici GJ, Scherch HM, Sethy VH, VonVoigtlander PF. Structure activity relationships of peroxynitrite scavengers an approach to nitric oxide neurotoxicity. Res Commun Chem Pathol Pharmacol. 1994;83:243–254. [PubMed] [Google Scholar]
- 75.Singh IN, Sullivan PG, Hall ED. Peroxynitrite-mediated oxidative damage to brain mitochondria: Protective effects of peroxynitrite scavengers. J Neurosci Res. 2007;85:2216–2223. doi: 10.1002/jnr.21360. [DOI] [PubMed] [Google Scholar]
- 76.Hall ED, Kupina NC, Althaus JS. Peroxynitrite scavengers for the acute treatment of traumatic brain injury. Ann N Y Acad Sci. 1999;890:462–468. doi: 10.1111/j.1749-6632.1999.tb08025.x. [DOI] [PubMed] [Google Scholar]
- 77.Galvani S, Coatrieux C, Elbaz M, et al. Carbonyl scavenger and antiatherogenic effects of hydrazine derivatives. Free Radic Biol Med. 2008;45:1457–1467. doi: 10.1016/j.freeradbiomed.2008.08.026. [DOI] [PubMed] [Google Scholar]
- 78.Hamann K, Nehrt G, Ouyang H, Duerstock B, Shi R. Hydralazine inhibits compression and acrolein-mediated injuries in ex vivo spinal cord. J Neurochem. 2008;104:708–718. doi: 10.1111/j.1471-4159.2007.05002.x. [DOI] [PubMed] [Google Scholar]