Abstract
Introduction:
Early detection and monitoring for malignant arrhythmias is fundamental to prenatal care in long QT syndrome (LQTS). Recently we studied the feasibility of isolating the fetal electrocardiogram (fECG) and measuring electrocardiographic intervals with a non-invasive fECG device using blind source separation with reference signal. Our aim was to evaluate the ability of fECG to diagnose LQTS.
Case Presentations:
We identified three cases of clinically-suspected LQTS based on fetal echocardiogram (2 had sinus bradycardia, 1 had 2nd degree atrioventricular block with negative maternal anti-SSA/SSB antibody titers). With IRB approval, these patients were prospectively enrolled for fECG acquisition. Offline post-processing generated fECG waveforms and calculated QT intervals.
Case 1 and 3 had a maternal history of LQTS. Two of the three fetuses with suspected LQTS had confirmed LQTS by postnatal ECG and genetic testing. FECG was able to identify a prolonged QTc interval in both cases. One of these also had fetal magnetocardiography (fMCG) which yielded similar findings to the fECG. The last case fECG was normal; fMCG and postnatal ECG were also normal.
Conclusions:
In three cases, fECG findings corroborated the diagnosis of LQTS. Non-invasive fECG may offer a novel method for fECG that is portable and more clinically accessible.
Keywords: congenital long QT syndrome, prenatal diagnosis, fetal electrocardiography, non-invasive, arrhythmias
Introduction:
Prenatal diagnosis of congenital LQTS with close fetal monitoring is essential to the management of susceptible fetuses in utero and during postnatal transition. Early detection enables preventive measures such as correcting maternal hypomagnesemia or hypocalcemia, as well as avoiding maternal QT-prolonging medications that could reduce the risk of torsades de pointes (TdP) developing in the LQTS fetus (1). If malignant ventricular arrhythmias such as ventricular tachycardia (VT) or TdP are detected, transplacental treatment is recommended and highly successful (2–5). Additionally, specialized delivery room care can be planned for (6) and primary prevention can be initiated immediately with beta-blockade in the neonate (7).
Prenatal diagnosis of LQTS using conventional antepartum testing is challenging since fetal electrocardiographic tracings are not conventionally available. In addition, the secondary finding of fetal sinus bradycardia or decreased heart rate variability on cardiotocogram is often subtle and may not be recognized as abnormal by routine obstetrical standards (8).
Fetal echocardiography is the conventional tool for evaluating the mechanism of an abnormal fetal heart rhythm, but is limited by its assessment of atrial and ventricular mechanical systoles rather than the cardiac electrical waveform (1,9). It cannot assess the electrical waveform morphology or repolarization characteristics. Fetal magnetocardiography (fMCG) is the current gold standard to precisely characterize fetal heart rhythm and conduction, and has been well described in the diagnosis and management of congenital LQTS (10–12). However, its need for expensive and highly specialized equipment has geographically limited its clinical application (1,13).
The clinical application of fetal electrocardiography (fECG) has been historically limited by technical challenges in extracting the low-amplitude fetal ECG signal from maternal noise (14). A novel signal processing technique of blind source separation has shown improved success in isolating fetal ECG activity and measuring cardiac electrical intervals (15–17).
This case series highlights the use of a non-invasive 12-lead fetal ECG device with blind source separation with reference technique (IRIS™ by Atom Medical Company in Tokyo, Japan) in the diagnosis and management of fetal LQTS. This case series is a part of a IRB-approved research protocol at our institution with this device from which early work has been published (18). Written informed consent was obtained from all expectant women prospectively enrolled in the study.
Table 1 summarizes the results of all three cases. A threshold of QTc interval of ≥ 490ms by fMCG has been shown to correlate with postnatal genetically confirmed LQTS (11).
Table 1:
Summary of results
| Case Number | Maternal LQTS | fECG (GA) | fMCG (GA) | Postnatal ECG | Postnatal genetic testing |
|---|---|---|---|---|---|
| 1 | Yes, KCNE1 and KCNH2 mutations | QTc 526ms (23) Repeat QTc 544ms (27) |
QTc 573ms (25) | Positive, QTc 480–572ms | Variant KCNH2 gene (LQT2) |
| 2 | No | QTc 504ms (24) | Not done | Positive, QTc 484–527ms | Variant KCNQ1 gene (LQT1) |
| 3 | Yes, gene negative | QTc 433ms (27) | QTc 451ms (22) | Normal | Not done |
LQTS long QT syndrome, fECG fetal electrocardiogram, GA gestational age, fMCG fetal magnetocardiography, ms milliseconds
Case Presentations:
Case 1:
A 21-year-old woman with familial LQTS (KCNE1 and probable KCNH2 mutations) with a transvenous ICD, status post left thoracic sympathectomy, and on maintenance beta blockade was referred for a screening fetal echocardiogram at 23 weeks gestation. Fetal echocardiogram revealed a structurally normal heart. Simultaneous pulsed Doppler interrogation of the mitral valve and left ventricular outflow tract revealed normal sinus rhythm with a lower heart rate of 120 bpm. The isovolumetric relaxation time was mildly prolonged at 50 ms, which coupled with the lower heart rate was suggestive of LQTS in the fetus (19,20).
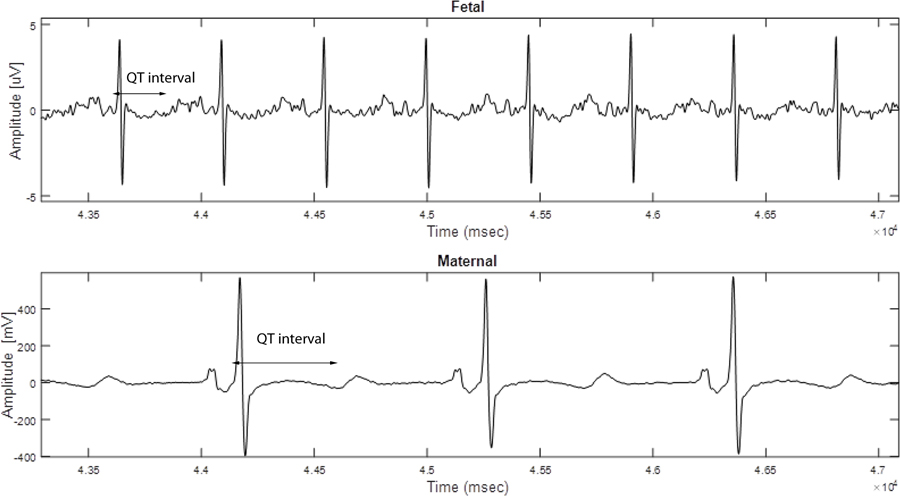
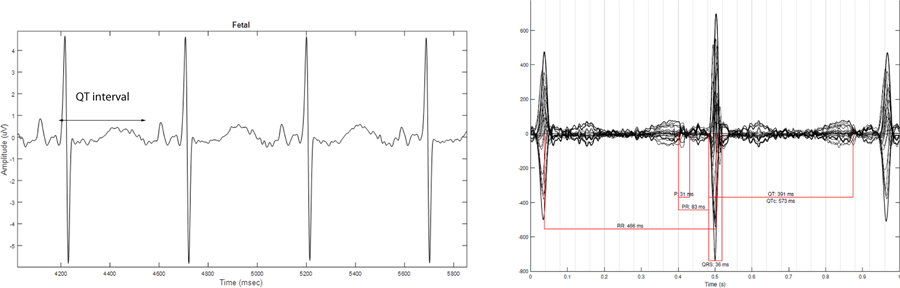
Fetal ECG was performed that appointment and demonstrated normal sinus rhythm with a prolonged QTc interval of 526 ms and T wave flattening, indicative of fetal LQTS. Notably, fECG was able to isolate the fetal signal with a prolonged QT interval from the maternal tracing with LQTS (Figure 1). The mother was referred for fMCG done at 25 weeks gestation which showed an averaged QTc of 573 ms, consistent with LQTS. Fetal ECG was repeated at 27 weeks gestation with similar findings to the prior fECG and the fMCG (Figure 2).
Figure 1.
Case 1 fECG done at 23 weeks showing prolonged QT interval, isolated from the maternal tracing with LQTS.
Figure 2.
Case 1. Panel on the left is fECG tracing done at 27 weeks gestation, demonstrating low fetal heart rate 125 bpm and prolonged QTc 544 ms. Panel on the right shows fMCG done at 25 weeks gestation; also showing a prolonged QT interval with averaged QTc 573 ms. Both consistent with LQTS.
Maternal serum calcium and magnesium levels were monitored regularly. Repeat fetal echocardiogram at 27 weeks gestation was unchanged. She delivered a full-term baby at an outside institution without complications. The baby was admitted to the special care nursery with a QTc of 480–572 ms and biphasic T-waves on postnatal ECG. The baby had no bradycardia or ventricular arrhythmia, and was discharged home with propranolol. Genetic testing returned positive for a variant in the KCNH2 gene, confirming the diagnosis of LQTS type 2.
Case 2:
A 37-year-old woman was referred for fetal echocardiogram at 20 weeks gestation after a routine obstetrical ultrasound found fetal bradycardia. Fetal echocardiogram demonstrated a structurally normal heart. Pulsed Doppler interrogation of rhythm revealed a heart rate of 60 bpm with second degree atrioventricular heart block. Given the possibility of autoimmune-mediated heart block, transplacental treatment with dexamethasone was started and subsequently discontinued once maternal anti-SSA/SSB antibody titers returned negative.
Repeat cardiac evaluations demonstrated persistent fetal sinus bradycardia, raising concern for fetal LQTS. Fetal cardiac function remained normal and there were no signs of fetal hydrops. Fetal ECG at 24 weeks gestation demonstrated sinus bradycardia with an averaged heart rate of 86 bpm and prolonged QTc of 504 ms. An fMCG was unable to be done.
With prenatal electrophysiology consultation, she delivered a full-term baby without complications. In the delivery room, the baby’s heart rate was 140 bpm and no resuscitation was required. Postnatal ECG demonstrated QTc prolongation to 484–527 ms with T-wave abnormalities. After transfer to our institution, the baby was started on propranolol. Genetic testing revealed a pathologic variant in KCNQ1, confirming the diagnosis of LQTS type 1. Notably, parental ECGs were normal and so this likely represented a de novo mutation.
Case 3:
A 26-year-old woman with gene negative LQTS with a transvenous ICD and maintenance beta blockade was referred for a screening fetal echocardiogram at 20 weeks gestation. Fetal echocardiogram demonstrated a structurally normal heart. Pulsed Doppler rhythm interrogation demonstrated normal sinus rhythm. Repeat cardiac evaluations revealed no change in fetal or cardiac status, but the heart rate was lower at 115 bpm in the third trimester.
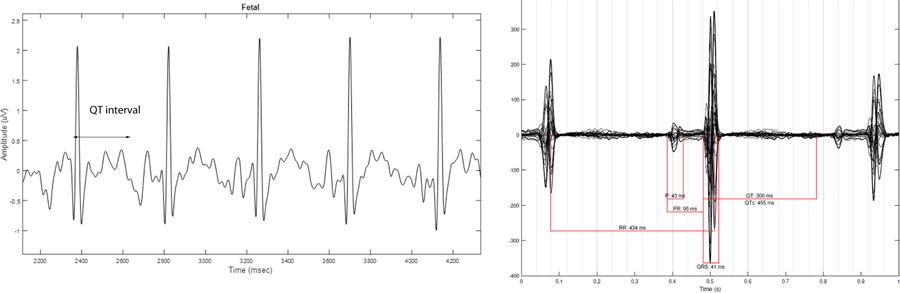
Fetal ECG at 27 weeks found normal sinus rhythm at a rate of 143 bpm with normal QT interval of 433 ms. The mother was referred for fMCG at 22 weeks which demonstrated a normal fetal heart rate and normal averaged QTc interval of 451 ms – i.e., without evidence of LQTS, as seen on fetal ECG (Figure 3).
Figure 3.
Case 3. Panel on left is fECG done at 27 weeks gestation normal QT interval (QTc 433 ms). Panel on right is fMCG at 22 weeks gestation; also normal with an averaged QTc 451 ms.
She delivered a full-term baby without complications. The baby was seen in outpatient cardiology clinic at 1 and 2 months of age with no clinical concerns. ECGs done at those appointments had no concerns for congenital LQTS with normal QTc intervals and normal T-wave morphology.
Discussion:
This case series is the first to feature the application of fetal ECG using blind source separation in the diagnosis of fetal LQTS compared to the current gold standard tests of fMCG or postnatal testing. In the two cases of confirmed LQTS on postnatal ECG and genetic testing, fECG was able to identify a prolonged QT interval in both cases. Significantly, one of these cases had maternal LQTS and fECG was able to isolate the fetal signal with prolonged QT from the maternal tracing with LQTS. That case also had an fMCG done whose findings suggestive of LQTS were similar to that seen on fECG. The third case of clinically suspected LQTS based on fetal echocardiogram had a normal QT interval on both fECG and fMCG with normal postnatal ECG testing, reinforcing the known limitations of echocardiography to predict electrocardiographic abnormalities. Thus in our series of three cases, fECG had high diagnostic accuracy for LQTS compared to conventional gold standard testing.
Blind-source separation with reference technique may offer a novel, non-invasive method for fECG that is portable and able to be acquired in a standard patient examination room and thus, more clinically accessible (18). This is especially valuable in suspected congenital LQTS in which frequent evaluation of the fetal heart rate and rhythm is essential. Furthermore, fetuses at risk for or with clinically suspected LQTS should perhaps be screened more than once since literature on fMCG in the diagnosis of LQTS has shown that a prolonged QT interval may not be evident until later in gestation on repeat testing (11). Additionally, repeated evaluations in LQTS suspected fetuses are needed to monitor for malignant arrhythmias such as long QT induced atrioventricular block and ventricular tachycardia (1). Frequent screening and monitoring are not as feasible by fMCG in which practice locations are limited. In addition, the acquisition time of fECG being only 10 minutes makes it easy to integrate into a busy clinical practice. Therefore, the high clinical accessibility of fECG has the potential to significantly alter the clinical care of LQTS susceptible fetuses.
At this time, there is limited data on the clinical application of fECG and further advancements in the technology are needed for clearer fECG signal acquisition that can more precisely filter out maternal noise and baseline wandering. Also, at this time fECG post-processing and measurements of cardiac time intervals are made as part of clinical research by a specialized team and not widely available for independent analysis. Early results of fECG including this case series are promising however; more data is needed to understand its diagnostic accuracy in fetal arrhythmias and conduction system disease.
Established Facts and Novel Insights:
Established Facts:
Early detection and monitoring for malignant arrhythmias is fundamental to prenatal care in congenital long QT syndrome (LQTS) to prevent perinatal mortality.
Fetal magnetocardiography (fMCG) is the gold standard to precisely characterize fetal heart rhythm and conduction, but has limitations.
Historically, fetal electrocardiography (fECG) has been limited by technical challenges in extracting the low-amplitude fetal ECG signal from maternal noise.
Novel Insights:
A novel signal processing technique of blind source separation has shown improved success in isolating the fetal ECG and measuring cardiac intervals.
This case series highlights the application of a non-invasive fECG device with blind source separation (IRIS™ by Atom Medical Company in Tokyo, Japan).
In three cases of clinically-suspected LQTS, fECG had high diagnostic accuracy compared to current gold-standard tests of fMCG and postnatal testing.
More research on fECG in fetal arrhythmia and conduction disease is needed, but early results are promising.
Acknowledgments:
Funding Sources: Dr. Krishnan received funding for this work from the Seelig Cardiac Research Fund (Children’s National Hospital). The work of Dr. Ronald Wakai with fetal magnetocardiography is supported by NIH Grant RO1HL063174. In this case series, two cases underwent fetal magnetocardiography.
Footnotes
Statement of Ethics: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional review board on human research at Children’s National Hospital and with the 1964 World Medical Association Declaration of Helsinki and its later amendments or comparable ethical standards. Informed consent: Written informed consent was obtained from all individual participants included in the study.
Disclosure Statement: The authors have no conflicts of interest to declare.
References:
- 1.Donofrio MT, Moon-Grady AJ, Hornberger LK, Copel JA, Sklansky MS, Abuhamad A, et al. Diagnosis and treatment of fetal cardiac disease: A scientific statement from the american heart association. Circulation. 2014;129(21):2183–242. [DOI] [PubMed] [Google Scholar]
- 2.Miyake A, Sakaguchi H, Miyazaki A, Miyoshi T, Aiba T, Shiraishi I. Successful prenatal management of ventricular tachycardia and second-degree atrioventricular block in fetal long QT syndrome. Hear Case Reports [Internet]. 2017;3(1):53–7. Available from: 10.1016/j.hrcr.2016.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang I-K, Shyu M-K, Lee C-N, Kau M-L, Ko Y-H, Chow S-N, et al. Prenatal diagnosis and treatment of fetal long QT syndrome: a case report. Prenat Diagn [Internet]. 2002. December;22(13):1209–12. Available from: http://doi.wiley.com/10.1002/pd.475 [DOI] [PubMed] [Google Scholar]
- 4.Simpson JM, Maxwell D, Rosenthal E, Gill H. Fetal ventricular tachycardia secondary to long QT syndrome treated with maternal intravenous magnesium: Case report and review of the literature. Ultrasound Obstet Gynecol. 2009;34(4):475–80. [DOI] [PubMed] [Google Scholar]
- 5.Cuneo BF, Ovadia M, Strasburger JF, Zhao H, Petropulos T, Schneider J, et al. Prenatal diagnosis and In Utero treatment of Torsades de Pointes associated with congenital long QT syndrome. Am J Cardiol. 2003;91(11):1395–8. [DOI] [PubMed] [Google Scholar]
- 6.Greene EA, Berul CI, Donofrio MT. Prenatal diagnosis of long QT syndrome: Implications for delivery room and neonatal management. Cardiol Young. 2013;23(1):141–5. [DOI] [PubMed] [Google Scholar]
- 7.Cuneo BF, Strasburger JF, Wakai RT. The natural history of fetal long QT syndrome. J Electrocardiol [Internet]. 2016;49(6):807–13. Available from: 10.1016/j.jelectrocard.2016.07.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ishikawa S, Yamada T, Kuwata T, Morikawa M, Yamada T, Matsubara S, et al. Fetal presentation of long QT syndrome - Evaluation of prenatal risk factors: A systematic review. Fetal Diagn Ther. 2013;33(1):1–7. [DOI] [PubMed] [Google Scholar]
- 9.Strasburger JF, Cheulkar B, Wakai RT. Magnetocardiography for fetal arrhythmias. Hear Rhythm. 2008;5(7):1073–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Menéndez T, Achenbach S, Beinder E, Hofbeck M, Schmid O, Singer H, et al. Prenatal diagnosis of QT prolongation by magnetocardiography. PACE - Pacing Clin Electrophysiol. 2000;23(8):1305–7. [DOI] [PubMed] [Google Scholar]
- 11.Cuneo BF, Strasburger JF, Yu S, Horigome H, Hosono T, Kandori A, et al. In utero diagnosis of long QT syndrome by magnetocardiography. Circulation. 2013;128(20):2183–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hosono T, Kawamata K, Chiba Y, Kandori A, Tsukada K. Prenatal diagnosis of long QT syndrome using magnetocardiography: a case report and review of the literature. Prenat Diagn [Internet]. 2002. March;22(3):198–200. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11920893 [DOI] [PubMed] [Google Scholar]
- 13.Wacker-gussmann A, Strasburger JF, Cuneo BF, Wakai RT. NIH Public Access. 2015;31(7):617–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kimura Y, Sato N, Sugawara J, Velayo C, Hoshiai T, Ito T, et al. Recent Advances in Fetal Electrocardiography. Open Med Devices J [Internet]. 2012. May;4(1):7–12. Available from: http://benthamopen.com/ABSTRACT/TOMDJ-4-7 [Google Scholar]
- 15.Agostinelli A, Grillo M, Biagini A, Giuliani C, Burattini L, Fioretti S, et al. Noninvasive Fetal Electrocardiography: An Overview of the Signal Electrophysiological Meaning, Recording Procedures, and Processing Techniques. Ann Noninvasive Electrocardiol [Internet]. 2015. July;20(4):303–13. Available from: http://doi.wiley.com/10.1111/anec.12259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sato M, Kimura Y, Chida S, Ito T, Katayama N, Okamura K, et al. A novel extraction method of fetal electrocardiogram from the composite abdominal signal. IEEE Trans Biomed Eng. 2007;54(1):49–58. [DOI] [PubMed] [Google Scholar]
- 17.Sato N, Hoshiai T, Ito T, Owada K, Chisaka H, Aoyagi A, et al. Successful Detection of the Fetal Electrocardiogram Waveform Changes during Various States of Singletons. Tohoku J Exp Med [Internet]. 2011;225(2):89–94. Available from: http://joi.jlc.jst.go.jp/JST.JSTAGE/tjem/225.89?from=CrossRef [DOI] [PubMed] [Google Scholar]
- 18.Doshi AN, Mass P, Cleary KR, Moak JP, Funamoto K, Kimura Y, et al. Feasibility of Non - invasive Fetal Electrocardiographic Interval Measurement in the Outpatient Clinical Setting. Pediatr Cardiol [Internet]. 2019;40(6):1175–82. Available from: 10.1007/s00246-019-02128-w [DOI] [PubMed] [Google Scholar]
- 19.Clur SAB, Vink AS, Etheridge SP, Robles De Medina PG, Rydberg A, Ackerman MJ, et al. Left Ventricular Isovolumetric Relaxation Time Is Prolonged in Fetal Long-QT Syndrome. Circ Arrhythmia Electrophysiol. 2018;11(4). [DOI] [PubMed] [Google Scholar]
- 20.Cuneo B, Robles de Medina P, Blom N, Wilde AA, Donofrio MT, Benson DW, et al. 247: Isovolumic relaxation time is prolonged in fetal long QT syndrome. Am J Obstet Gynecol [Internet]. 2017. January;216(1):S154 Available from: https://linkinghub.elsevier.com/retrieve/pii/S0002937816311310 [Google Scholar]