Summary
Understanding how flowers form is an important problem in plant biology, as the human food supply depends on flower and seed production. Flower development also provides an excellent model for understanding how cell division, expansion and differentiation are coordinated during organogenesis. In the model plant Arabidopsis thaliana, floral organogenesis requires AINTEGUMENTA (ANT) and AINTEGUMENTA-LIKE6 (AIL6)/PLETHORA3 (PLT3), two members of the Arabidopsis AINTEGUMENTA-LIKE/PLETHORA (AIL/PLT) transcription factor family. Together, ANT and AIL6/PLT3 regulate aspects of floral organogenesis including floral organ initiation, growth, identity specification, and patterning. Previously, we used RNA-Seq to identify thousands of genes with disrupted expression in ant ail6 mutant flowers, indicating that ANT and AIL6/PLT3 influence a vast transcriptional network. However, the immediate downstream targets of ANT and AIL6/PLT3 in flowers are unknown. To identify direct targets of ANT regulation, we performed a RNA-Seq time course experiment in which we induced ANT activity in transgenic plants bearing an ANT-glucocorticoid receptor fusion construct. In addition, we performed a ChIP-Seq experiment that identified ANT binding sites in developing flowers. These experiments identified 200 potential ANT target genes based on their proximity to ANT binding sites and differential expression in response to ANT. These 200 candidate target genes were involved in functions such as polarity specification, floral organ development, meristem development and auxin signaling. In addition, we identified several genes associated with lateral organ growth that may mediate ANT’s role in organ size control. These results reveal new features of the ANT transcriptional network by linking ANT to previously unknown regulatory targets.
Keywords: Arabidopsis thaliana, flower development, AINTEGUMENTA (ANT), AINTEGUMENTA-LIKE (AIL)/PLETHORA (PLT), RNA-Seq, ChIP-Seq, organ polarity, organ growth, auxin signaling
Introduction
Flowers supply fruits, seeds and grains to the human diet and are a subject of fascination for their beauty and morphological diversity. Molecular genetic studies in Arabidopsis have identified many regulatory factors that control the initiation and subsequent development of flowers. Flower primordia arise in the periphery of the inflorescence meristem at sites of auxin maxima, during the reproductive phase of the plant life cycle (Benkova et al. 2003, Heisler et al. 2005, Reinhardt et al. 2003). At these sites, AUXIN RESPONSE FACTOR5/MONOPTEROS (ARF5/MP) upregulates the expression of LEAFY (LFY) and two AINTEGUMENTA-LIKE/PLETHORA (AIL/PLT) genes: AINTEGUMENTA (ANT) and AINTEGUMENTA-LIKE6 (AIL6)/PLETHORA3 (PLT3) to specify these primordia as flowers and promote their outgrowth (Yamaguchi et al. 2013). Within flower primordia, floral organ primordia are initiated at defined positions within whorls and subsequently adopt one of four fates according to the ABCE model [reviewed in (Krizek and Fletcher 2005)]. Class A and E gene activities in whorl one specify sepal identity; class A, B and E gene activities in whorl two specify petal identity; class B, C and E gene activities in whorl three specify stamen identity, and class C and E gene activities in whorl four specify carpel identity.
Most class A, B, C and E floral organ identity genes encode MADS domain transcription factors that act in higher order protein complexes to regulate gene expression (Smaczniak et al. 2012). Genomic studies on these transcription factors have begun to reveal the gene regulatory networks that control the development of each floral organ type (Kaufmann et al. 2009, Kaufmann et al. 2010, O’Maoiléidigh et al. 2013, Wuest et al. 2012). These studies indicate that floral organ identity proteins regulate a large number of genes throughout floral organ development with distinct genes being regulated at different stages of organ development [reviewed in (Stewart et al. 2016, Yan et al. 2016)]. Direct regulatory targets include other transcription factors as well as genes involved in plant hormone signaling pathways and developmental processes such as pattern formation and morphogenesis. Furthermore, the floral organ identity proteins appear to repress the expression of genes that specify a leaf developmental program (O’Maoiléidigh et al. 2013).
Genetic studies have uncovered multiple roles for ANT and AIL6/PLT3 during the initiation and subsequent development of floral organ primordia. ANT and AIL6/PLT3 promote the initiation of floral organ primordia at defined positions within the floral meristem and also prevent premature differentiation of both primordial and floral meristem cells (Krizek 2009, Krizek and Eaddy 2012). ANT and AIL6/PLT3 function is required for proper expression of the class B and C floral organ identity genes APETALA3 (AP3) and AGAMOUS (AG), respectively and consequently the elaboration of petal and stamen fates (Krizek 2009). ANT and AIL6/PLT3 also regulate the growth of developing floral organs, thus contributing to morphogenesis and the attainment of correct organ size (Elliott et al. 1996, Klucher et al. 1996, Krizek 1999, Mizukami and Fischer 2000). Despite their well-established importance in many facets of flower development, we currently know very little about the downstream target genes that are activated or repressed by ANT and AIL6/PLT3. Without this knowledge, we lack understanding of the biological processes regulated by these transcription factors that contribute to the sculpting of each floral organ type.
A genomic study comparing the transcriptomes of wild type and ant ail6 double mutants identified thousands of differentially expressed (DE) genes, consistent with the numerous roles of ANT and AIL6/PLT3 in floral organogenesis and the severe phenotypic consequences of losing ANT and AIL6/PLT3 functions (Krizek et al. 2016). This experiment suggested that ANT and AIL6/PLT3 functions are intimately coupled within a vast transcriptional network regulating floral organogenesis. To distinguish between short-term and longer-term consequences of loss of ANT and AIL6/PLT3 activities, we have utilized more focused genomic studies. We performed two complementary experiments: RNA-Seq analysis of floral buds at two, four, and eight hours after induction of ANT activity, and ChIP-Seq analysis of stage 6/7 flowers. Together, these studies identified 200 genes that are both differentially expressed after ANT induction and are bound by ANT and are thus likely to be direct targets of ANT regulation. Our experiments suggest that ANT controls floral organogenesis through direct regulation of growth and patterning genes as well as auxin responses.
Results
RNA-Seq identifies genes differentially expressed after activation of ANT-GR
To identify direct targets of ANT regulation, we created a line of transgenic plants containing an inducible form of ANT in which the ligand binding domain of the glucocorticoid receptor (GR) was fused to the coding region of ANT. In this system, the GR domain blocks migration of the ANT-GR fusion partner into the nucleus, rendering it incapable of activating or repressing the expression of target genes. Applying the steroid dexamethasone (dex) causes a conformational change in GR that permits entry of ANT-GR into the nucleus, where it can bind to target promoters and regulate gene expression. Applying dex to 35S:ANT-GR inflorescences led to the production of larger flowers and caused male sterility, similar to the phenotypes observed in 35S:ANT inflorescences (Figure S1) (Krizek 1999, Yamaguchi et al. 2013). This established that the ANT-GR line contained inducible ANT activity that could be triggered by application of dex.
We performed a time course experiment using the 35S:ANT-GR line in which floral buds were collected two, four, and eight hours following dex treatment or treatment with a solvent only (mock) negative control. Whole inflorescences corresponding to floral buds stage 1–12 were collected as four matched pairs of treatment and control samples and processed for RNA sequencing. Following sequencing and alignment to the Arabidopsis reference genome, each sample yielded between 13 and 29 million aligned fragments, which were then analyzed for differential expression. Using a false discovery rate (FDR) of 0.05, we identified 1,195 genes that were DE at one or more time points (Data S1). More DE genes were present at four (746) and eight hours (609) post treatment as compared with two hours (324) (Figure 1A). The log2 fold change (logFC) values of the DE genes ranged from 3.15 to −1.80. There were 106 genes that were DE at all three time points. Approximately equal numbers of upregulated (836) and downregulated (843) genes were identified.
Figure 1. Genes differentially expressed (DE) in mock and dex treated 35S:ANT-GR inflorescences.

A. Venn diagram showing overlap between DE genes identified at two, four and eight hours after treatment. The Venn diagram was created with BioVenn (Hulsen et al. 2008). B. Biological process GO terms enriched in DE genes.
To visualize gene expression changes over time, we developed an interactive R Shiny app (called “Show gene expression”) that plots individual gene expression data over the time course of the experiment (available at https://bitbucket.org/lorainelab/inducible-ant-rna-seq/) (Figure S2A–D). After launching the Shiny App in the RStudio application, users can generate plots showing expression of a gene of interest after entering its gene identifier. Gene expression can be visualized as “Sample RPKM” with individual treatment and time points selected, “Group RPKM” which averages the four replicates for each sample, or “Expression over time”, in which the data can be visualized with lines, points or arrows that indicate up or down regulation between the control and treated samples. The “gene info” tab provides a link to TAIR (The Arabidopsis Information Resource) for further information about the gene. We used this app to investigate expression of candidate target genes and explore their functions.
DE genes were enriched in terms associated with development and hormone physiology
We used enrichment analysis to identify Gene Ontology (GO) and KEGG functional annotation categories overrepresented among the 1,195 DE genes. We further investigated individual gene functions using TAIR for members of these categories.
Many DE genes had functions related to lateral organ development, consistent with ANT’s role as a regulator of organogenesis. These included: leaf formation (GO:0010338), polarity specification of adaxial/abaxial axis specification (GO:0009944), and stamen development (GO:0048443) (Figure 1B). Seven genes that regulate lateral organ polarity were differentially expressed (Table S1). These genes included three YABBY genes [CRABS CLAW (CRC), YAB3, YAB5], one class III HD-ZIP gene [PHABULOSA (PHB)], one KANADI gene (KAN2) as well as ASYMMMETRIC LEAVES 1 (AS1) and BLADE ON PETIOLE 1 (BOP1). Thus both adaxial (PHB, AS1, BOP1) and abaxial (CRC, YAB3, YAB5, KAN2) genes were identified as potential targets of ANT regulation. The identification of genes regulating adaxial/abaxial axis specification is consistent with previous genetic work suggesting that ANT contributes to organ polarity (Nole-Wilson and Krizek 2006). Leaf development GO category genes include several genes regulating organ growth including KLUH (KLU/CYP78A5), GROWTH REGULATING FACTORS (GRF3, GRF6, GRF8) and ANGUSTIFOLIA3/GRF1-INTERACTING FACTOR1 (AN3/GIF1).
Several genes with known roles in floral organ development were DE. These included floral organ identity genes APETALA1 (AP1), APETALA2 (AP2), and SEPALLATA3 (SEP3), as well as genes that function in later aspects of stamen development [SQUAMOSA PROMOTER-BINDING PROTEIN-LIKE 8 (SPL8), EXCESS MICROSPOROCYTES1 (EMS1)] and carpel development [SPATULA (SPT), CRC, STRUBBELIG (SUB)] (Table S1).
In addition to genes involved in lateral organ development, seven DE genes involved in meristem maintenance (GO:0010073), were identified: CORYNE (CRN), BARELY ANY MERISTEM 3 (BAM3), CLAVATA3 INSENSITIVE RECEPTOR KINASE 4 (CIK4), MINI ZINC FINGER 2 (MIF2), and FANTASTIC FOUR 1, 2, 3 (FAF1, FAF2, FAF3) (Table S1). This suggests that ANT has a role in meristems maintenance in addition to its role in lateral organ development, consistent with genetic work showing the importance of ANT and AIL6/PLT3 in preventing premature differentiation of floral meristem cells (Krizek and Eaddy 2012).
Many hormone-related functions were enriched in the DE genes (Data S2) suggesting that ANT function is closely linked with multiple aspects of hormone physiology. These GO terms included: negative regulation of cytokinin-activated signaling pathway (GO: 0080037), regulation of jasmonic acid mediated signaling pathway (GO:2000022), regulation of auxin mediated signaling pathway (GO:0010928), jasmonic acid mediated signaling pathway (GO:0009867), gibberellin mediated signaling pathway (GO:0010476), auxin-activated signaling pathway (GO:0009734)], hormone biosynthesis [(indoleacetic acid biosynthetic process (GO:000009684), jasmonic acid biosynthetic process (GO:0009695)], hormone transport [auxin efflux (GO:0010315), and auxin transport (GO:0060918)] (Figure 1B). Additional analysis using the KEGG pathway framework also found links with cytokinin, auxin, and gibberellin hormone signaling pathways (Figure S3). DE genes associated with the synthesis/metabolism, transport, signaling and response for cytokinin, auxin, gibberellin, abscisic acid and jasmonic acid are shown in Table S2. These results suggest that ANT plays roles in the metabolism of auxin and jasmonic acid (and perhaps cytokinin and gibberellin) while also influencing signaling pathways downstream of cytokinin, auxin, gibberellin, abscisic acid and jasmonic acid. Our earlier RNA-Seq investigation of ant ail6 double mutants inflorescences previously linked ANT function with auxin biosynthesis and JA signaling but not cytokinin or gibberellin signaling (Krizek et al. 2016).
ChIP-Seq identifies ANT genomic binding sites in stage 6/7 flowers
To identify genome-wide ANT binding sites, we performed chromatin immunoprecipitation in combination with next generation sequencing (ChIP-Seq). For these studies, we used a transgenic line in which an ANT-VENUS gene fusion was expressed under the control of the ANT promoter in the ant mutant background. Fusion of ANT with VENUS, a rapidly folding variant of YELLOW FLUORESCENT PROTEIN (YFP), allows immunoprecipitation of DNA bound to ANT by a commercially available antibody against GREEN FLUORESCENT PROTEIN (GFP). The transgene fully rescued the ant-4 mutant phenotype as assayed by petal measurements and floral organ counts, indicating that the ANT-VENUS fusion protein has full ANT activity (Tables S3, S4). The ANT:ANT-VENUS ant-4 line was crossed into a genetic background in which flower development can be synchronized (i.e. AP1:AP1-GR ap1 cal) (O’Maoiléidigh et al. 2015), allowing us to collect large numbers of flowers of the same developmental stage. Inflorescences from two biological replicates of AP1:AP1-GR ap1 cal and AP1:AP1-GR ap1 cal ANT:ANT-VENUS ant-4 were harvested five days after dex treatment when they are composed of stage 6/7 flowers.
In our ChIP-Seq experiment, we identified 1,113 ANT binding peaks in stage 6/7 flowers using visual analytics and the Integrated Genome Browser (IGB). These peaks were associated with 1,081 unique genes (Data S3). Almost half of the peaks (48%) are present upstream of the gene near the transcriptional start site (TSS) with the remaining peaks either overlapping the start of transcription (18%), within the gene (15%), overlapping the end of transcription (5%), downstream of the gene (14%) or encompassing the gene (1%) (Figure 2A, B).
Figure 2. Position of ANT ChIP-Seq peaks relative to closest gene.

A. Pie chart showing the position of ANT ChIP-Seq binding peaks relative to the closest gene. Almost half of the peaks are upstream of the closest gene (48%). The remaining peaks either overlap the start of the gene (18.0%), are within the gene (15%), overlap the end of the gene (5%), are downstream of the gene (14%) or overlap the entire gene (1%). B. Position of ANT binding peak relative to the transcriptional start site (TSS) of the closest gene.
Genes associated with ANT binding sites include regulators of polarity specification, floral organ development and meristem development
To gain insight into the set of genes associated with ANT ChIP-Seq peaks, we performed a GO enrichment analysis (Data S4; Figure 3). Several of the identified GO terms were the same or similar to those identified in the ANT-GR RNA-Seq experiment. Within the biological process GO category, a number of developmental terms were identified that relate to adaxial/abaxial polarity, floral organ development and meristem development including the following: polarity specification of adaxial/abaxial axis (GO:0009944), floral organ formation (GO:0048449), meristem determinacy (GO:0010022), meristem initiation (GO:0010014), plant ovule development (GO:0048481), stamen development (GO:0048443), and regulation of flower development (GO:0009909) (Figure 3A). Other overrepresented developmental GO biological process terms include: stomatal complex morphogenesis (GO:0010103), cell fate specification (GO:0001708), and leaf development (GO:0048366) (Figure 3A). Two hormone-related overrepresented GO biological process terms are the following: response to gibberellin (GO:0009739) and auxin-activated signaling pathway (GO:0009734), which were also identified in the RNA-Seq experiment (Figure 3A). However, several other hormone-related GO terms such as those related to cytokinin and JA signaling identified in the RNA-Seq experiment were not enriched in the ChIP-Seq experiment.
Figure 3. GO enrichment analyses on genes associated with ANT ChIP-Seq binding peaks.
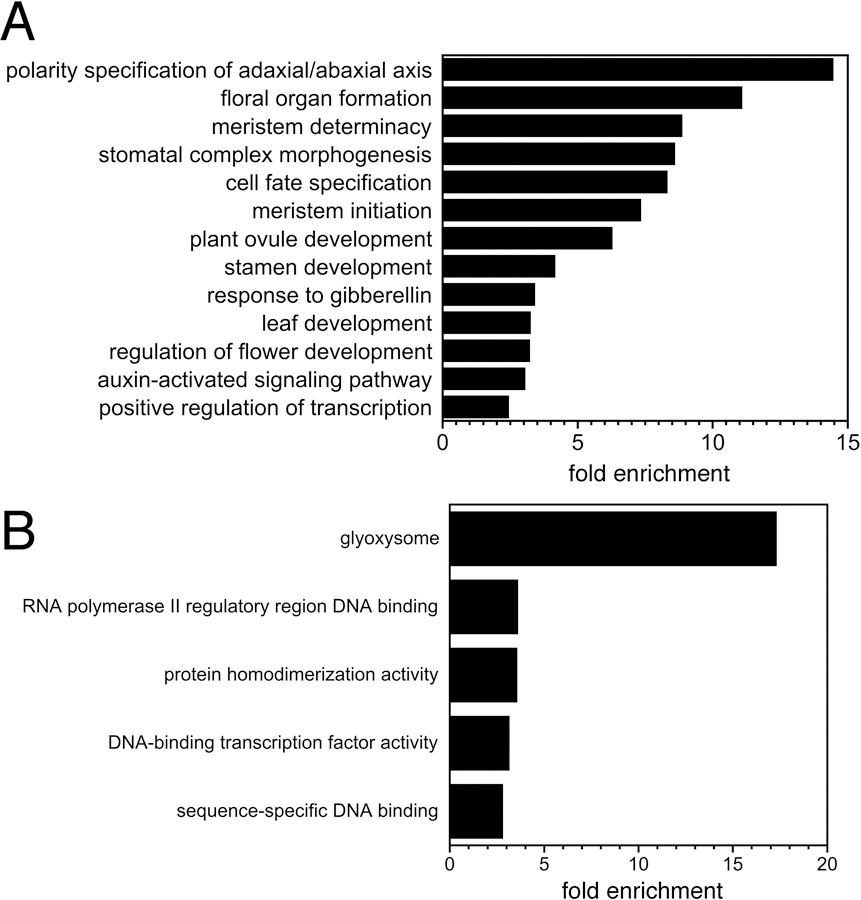
A. Biological process GO terms enriched in genes associated with ANT binding peaks. B. Molecular function and cellular component GO terms enriched in genes associated with ANT binding peaks.
The biological process GO term “positive regulation of transcription, DNA-templated” (GO:0045893) was also overrepresented, which suggests that ANT regulates the expression of other transcription factors (Figure 3A). This is also supported by the identification of the following enriched molecular function GO terms: RNA polymerase II regulatory region DNA binding (GO:0001012), DNA-binding transcription factor activity (GO:0003700), and sequence-specific DNA binding (GO:0043565) (Figure 3B). Another enriched molecular function GO term was protein homodimerization activity (GO:0042803) (Figure 3B).
The enriched cellular component GO term glyoxysome (GO:0009514) was also identified (Figure 3B). This category includes two enzymes, isocitrate lyase (ICL) and malate synthase (MS), specific to the glyoxylate cycle which occurs in glyoxysomes.
200 DE genes are bound by ANT and likely direct targets of ANT regulation
The most likely direct targets of ANT regulation are genes that are both bound by ANT and are differentially expressed in response to changes in ANT activity. There were 200 genes common between the set of RNA-Seq DE genes and the set of ChIP-Seq bound genes (Figure 4A; Data S5). While the total set of DE genes (1,195) consists of slightly less upregulated genes (523) than downregulated genes (672), a much larger number of upregulated genes (154) are associated with ANT binding peaks as compared with downregulated genes (46).
Figure 4. DE genes that are bound by ANT.
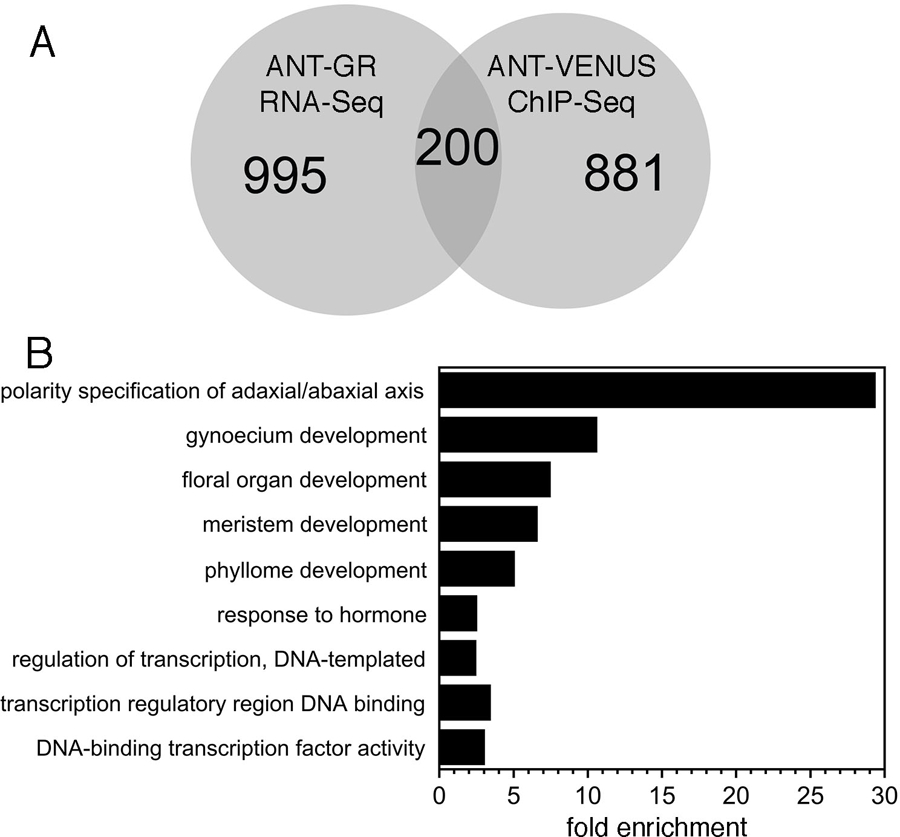
A. Venn diagram showing overlap between DE genes identified and those bound by ANT. The Venn diagram was created with BioVenn (Hulsen et al. 2008). B. Biological process and molecular function GO terms enriched in genes that are both DE and bound by ANT.
GO term enrichment analysis on this set of 200 genes identifies several GO terms similar to or the same as those identified for the entire set of ANT ChIP-Seq associated genes (Data S6; Figure 4). In particular, three terms were enriched in both sets: polarity specification of adaxial/abaxial axis (GO:0009944), regulation of transcription, DNA-templated (GO:0006355), and DNA-binding transcription factor activity (GO:0003700) (Figure 4). Five polarity genes were identified: PHB, BOP1, AS1, KAN2 and YAB3 (Table 1). Other enriched GO terms were the following: gynoecium development (GO:0048467), floral organ development (GO:0048437), meristem development (GO:0048507), phyllome development (GO:0048827), and response to hormone (GO:0009725). Floral organ development genes include those involved in specifying floral organ identity (AP1, AP2, SEP3), regulating cellular differentiation (EMS1, SPL8) and controlling morphogenesis (SUB, SPT) (Table 1). Nine auxin genes were part of the hormone responses category including those involved in biosynthesis (TAA1), signaling (AFB2, ARF6, ARF11, ARF18, IAA3/SHY2, IAA27, PAP2) and responses (SAUR50, SAUR14) (Table 2). To confirm our RNA-Seq and ChIP-Seq results, we selected eight genes from this set of 200. The selected genes were not previously known to be regulated by ANT and were associated with several different biological processes. The eight genes included two polarity genes KAN2 and PHB; two hormone signaling genes BES1/BZR1 HOMOLOG 4 (BEH4), REPRESSOR OF GA (RGA); the reproductive organ development gene SPL8; and three genes that regulate lateral organ growth: AN3/GIF1, XYLOGLUCAN ENDOTRANSGLUCOSYLASE/HYDROLASE 9 (XTH9) and SMALL RUBBER PARTICLE PROTEIN2 (SRP2) (Table 1).
Table 1.
Developmental genes DE after ANT-GR activation and bound by ANT
| Polarity specification | Name | log2 fold change (hr) | ChIP-Seq |
|---|---|---|---|
| AT2G34710 | PHB | 0.256 (4); 0.346 (8) | upstream; overlap start |
| AT3G57130 | BOP1 | −0.449 (4) | upstream |
| AT2G37630 | AS1 | −0.235 (4); −0.175 (8) | upstream |
| AT1G32240 | KAN2 | 0.227 (4); 0.255 (8) | inside |
| AT4G00180 | YAB3 | 0.269 (2); 0.262 (4); 0.374 (8) | upstream |
| Floral organ development | |||
| AT1G69120 | AP1 | −0.144 (4) | upstream; overlap start |
| AT4G36920 | AP2 | 0.22 (2) | upstream |
| AT1G24260 | SEP3 | 0.175 (8) | inside |
| AT1G02065 | SPL8 | −0.341 (2); −0.589 (4); −0.534 (8) | overlap start |
| AT5G07280 | EMS1 | −0.14 (4) | upstream |
| AT1G11130 | SUB | 0.154 (4); 0.165 (8) | overlap start |
| AT4G36930 | SPT | 0.269 (2); 0.297 (4); 0.418 (8) | upstream |
| Growth genes | |||
| AT1G13710 | KLU/CYP78A5 | −0.281 (2); −0.272 (4) | upstream |
| AT4G24150 | GRF8 | 0.317 (8) | inside |
| AT5G28640 | AN3/GIF1 | 0.244 (2); 0.25 (4); 0.403 (8) | overlap start, inside |
| AT4G03210 | XTH9 | 0.315 (2); 0.351 (4); 0.405 (8) | upstream |
| AT2G47780 | SRP2 | 0.482 (4); 0.718 (8) | overlap start |
| AT2G32710 | KRP4 | 0.293 (2); 0.299 (4); 0.331 (8) | overlap end |
Table 2.
Hormone genes DE after ANT-GR activation and bound by ANT
| AGI locus code | Gene | log2 fold change (hr) | ChIP-Seq |
|---|---|---|---|
| Auxin | |||
| AT1G70560 | TAA1/WEI8 | 0.297 (4); 0.444 (8) | inside |
| AT3G26810 | AFB2 | 0.185 (8) | upstream |
| AT1G30330 | ARF6 | 0.132 (4) | upstream |
| AT2G46530 | ARF11 | 0.376 (8) | upstream |
| AT3G61830 | ARF18 | 0.198 (8) | overlap end |
| AT1G04240 | SHY2/IAA3 | 0.285 (8) | overlap start |
| AT4G29080 | PAP2/IAA27 | 0.14 (4) | upstream |
| AT4G34760 | SAUR50 | 0.346 (8) | upstream |
| AT4G38840 | SAUR14 | −0.478 (4) | overlap start |
| Gibberellin | |||
| AT1G14920 | GAI | 0.133 (4); 0.181 (8) | upstream |
| AT2G01570 | RGA | 0.246 (2); 0.259 (4); 0.334 (8) | upstream |
| Brassinosteroid | |||
| AT1G78700 | BEH4 | 0.664 (2); 0.734 (4); 0.918 (8) | inside |
| Abscisic Acid | |||
| AT2G40330 | PYL6 | 0.552 (2) | overlap start |
| Ethylene | |||
| AT1G15360 | WIN1/SHN1 | −0.212 (8) | overlap start |
We examined the expression of these eight genes in an independent batch of mock and dex treated 35S:ANT-GR inflorescences. To determine if activation of these target genes required de novo protein synthesis, we included inflorescences treated with the protein synthesis inhibitor cycloheximide (chx). Inflorescences were collected four hours after the respective treatment. Similar changes in gene expression in inflorescences treated with dex+chx versus chx alone as compared with those treated with dex versus mock support direct regulation of the gene by ANT. Seven of the eight DE genes showed expression changes independent of protein synthesis in 35S:ANT-GR inflorescences (Figure 5). The lone exception was SRP2 which showed similar expression levels in chx and dex+chx samples (Figure 5). Thus, SRP2 may not be a direct target of ANT regulation, although it is possible that the use of whole inflorescences in the RT-qPCR experiment obscured developmental stage-specific regulation of this gene by ANT in stage 6/7 flowers.
Figure 5. Several tested DE genes show protein synthesis independent gene expression changes after induction of ANT activity in 35S:ANT-GR.
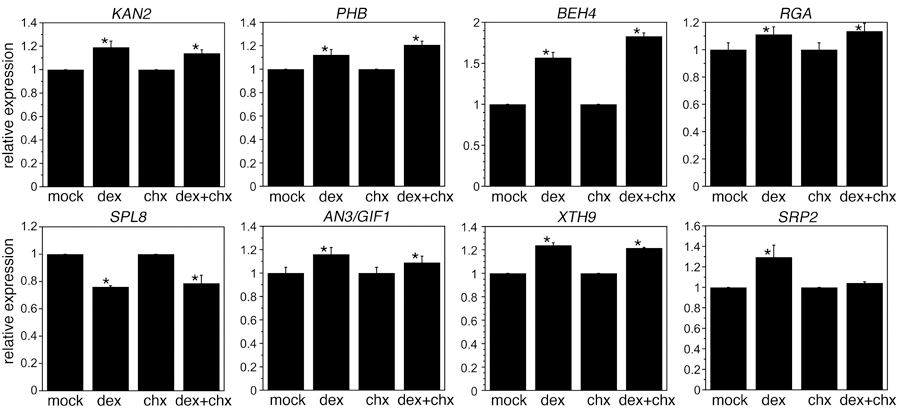
Graphs show relative expression of KAN2, PHB, BEH4, RGA, SPL8, AN3/GIF1, XTH9, and SRP2 after mock, dexamethasone (dex), cycloheximide (chx), dex+chx treatments. Relative expression refers to mRNA levels in dex samples compared to mock samples and mRNA levels in dex+chx samples compared to chx samples. * indicates dex samples statistically different than mock samples and dex+chx samples statistically different than chx samples as determined by Students t-test (P value less than 0.05). mRNA levels were measured 4 hours after treatment. Graphs show mean ± SD of two biological replicates.
ChIP-qPCR experiments performed on these eight genes with an independent batch of ChIP DNA gave results that are similar to those seen by ChIP-Seq (Figure 6, Figure S4). ANT binds to regions upstream or overlapping the 5’ UTR (PHB, RGA, SPL8, AN3/GIF1, XTH9, SRP2) or within the gene body (KAN2, BEH4) in stage 6/7 flowers. No enrichment was observed in AP1:AP1-GR ap1 cal inflorescences lacking ANT:ANT-VENUS. ANT binds to genomic regions of both upregulated (KAN2, PHB, BEH4, RGA, AN3/GIF1, XTH9, SRP2) and a downregulated (SPL8) gene.
Figure 6. ChIP-qPCR confirms that ANT binds to genomic regions upstream or within genes associated with stamen development (SPL8) and growth (AN3/GIF1, XTH9, SRP2).
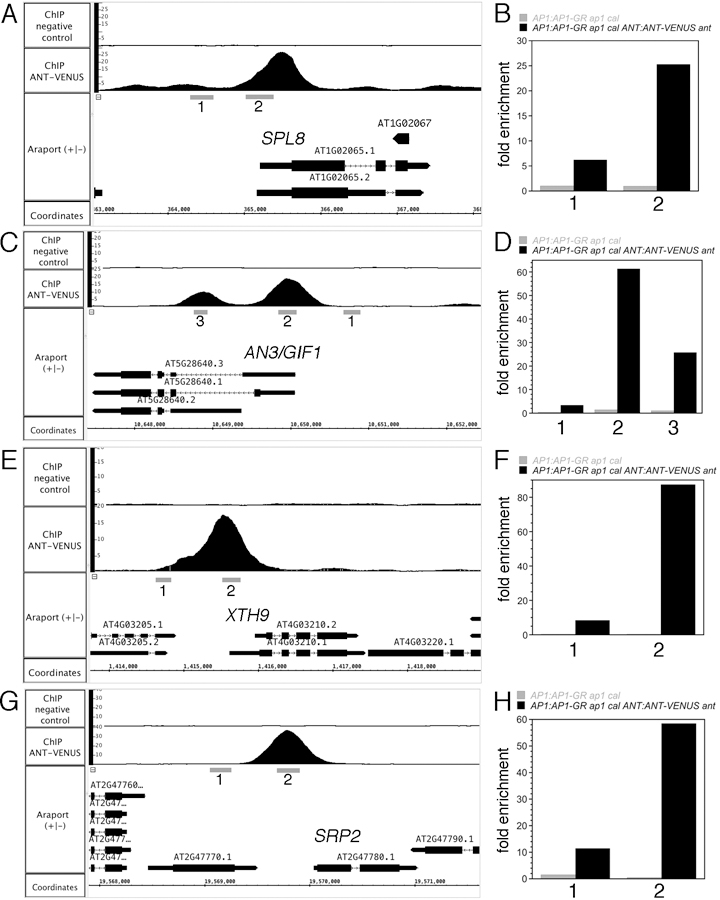
ChIP-Seq coverage graphs for SPL8 (A), AN3/GIF1 (B), XTH9 (E), and SRP2 (G). Numbers below the gene indicate the regions tested for ANT binding by ChIP-qPCR. ChIP-qPCR data for SPL8 (B), AN3/GIF1 (D), XTH9 (F) and SRP2 (H). Grey bars show results from AP1:AP1-GR ap1 cal and black bars show results from AP1:AP1-GR ap1 cal ANT:ANT-VENUS ant. Numbers on the x axis correspond to the genomic regions indicated in the ChIP-Seq coverage graphs.
DNA sequence motif analyses of ChIP-Seq peaks
In previous work using SELEX, we identified the in vitro DNA binding motif of ANT (Nole-Wilson and Krizek 2000). To determine whether ANT binding peaks contain DNA sequences with similarity to this motif, we mapped putative ANT binding sites on a genome-wide level using the FIMO program from the MEME software suite (Grant et al. 2011). We then compared the position of the FIMO predicted sites to both the ChIP-Seq peaks and to a set of randomized peaks of the same size. 66.7% of the ChIP-Seq peaks and 43.8% of the randomized peaks overlapped a FIMO site. Thus, ChIP-Seq peaks were more likely than randomized peaks to contain a FIMO predicted ANT binding site. However, nearly a third of the ChIP-Seq peaks did not contain a FIMO predicted ANT binding site.
We used MEME-ChIP from the MEME Suite to perform de novo motif discovery (Machanick and Bailey 2011). Our analysis used the DAP-Seq database for motif discovery which includes several AIL/PLT binding sites but not that of ANT (O’Malley et al. 2016). MEME-ChIP identified seven motifs with an e-value of 1.00E-10 or lower (Table 3). Two of these motifs: MEME-1 (HNNNHGGCACRNWTH) and MEME-3 (RCACRRWWHYCRAKG) were similar to the PLT1 and AIL6/PLT3 DAP-Seq binding motifs, respectively (Table 3; Figure 7A). The PLT1 and AIL6/PLT3 binding sites consisted of a fairly long sequence with several conserved residues near each end of the site and less conserved nucleotides in the center. The ANT SELEX determined in vitro binding motif is similar to both of these sites (Figure 7A) (Nole-Wilson and Krizek 2000). The MEME-1 motif had similarity to the first conserved part of AIL/PLT sites, while the MEME-3 motif had similarity to both conserved parts of AIL/PLT sites (Figure 7A). Thus, the identification of MEME-1 and MEME-3 motifs in ANT binding peaks suggests that the in vivo binding specificity of ANT resembles that determined in vitro but with less conservation at several positions within the motif. Interestingly, in some MEME-1 sites, a second motif was identified at a conserved distance from the MEME-1 motif and resembled the second half of AIL/PLT binding sites (Figure S5).
Table 3.
MEME-ChIP analysis of ANT ChIP-Seq peaks
| Motif | Motif ID | Width | Sites | e-value | Most similar motif |
|---|---|---|---|---|---|
| HNNNHGGCACRNWTH | MEME-1 | 15 | 267 | 5.80E-251 | PLT1 (AP2/ERF) |
| YTYTBTCTYTYTYTY | MEME-2 | 15 | 400 | 2.00E-169 | BPC5 (BBR/BPC) |
| RCACRRWWHYCRAKG | MEME-3 | 15 | 263 | 7.30E-99 | AIL6/PLT3 (AP2/ERF) |
| CGWGSC | DREME-1 | 6 | 221 | 8.70E-19 | BPE/bHLH31 (bHLH) |
| ARAGARAR | DREME-2 | 8 | 348 | 5.30E-16 | BPC1 (BBR/BPC) |
| AAARGHRGARARARAAADARAAVAAMAAA | MEME-4 | 29 | 64 | 2.60E-15 | VRN1 (ABI3/VP1) |
| GYRRRTSCCACGTG | MEME-5 | 14 | 39 | 8.30E-11 | PIF7 (bHLH) |
Figure 7. MEME-ChIP identifies sequences with similarity to AIL/PLT sites as well as other transcription factors.

A. Sequence logos representing the DNA binding specificities of ANT, AIL6/PLT3, PLT1 and two motifs (MEME-1 and MEME-3) identified within ANT ChIP-Seq binding peaks. B. Sequence logos representing the DNA binding specificity of BPC1 and two related motifs (MEME-2, DREME-2) identified within ANT ChIP-Seq binding peaks. C. Two bHLH binding sites from bHLH31/BPE and PIF7 and two related motifs (DREME-1 and MEME-5) identified within ANT ChIP-Seq binding peaks.
MEME-ChIP identified several other enriched motifs in ANT binding peaks. These had similarity to the binding sites of other transcription factors. Two such motifs, MEME-2 (YTYTBTCTYTYTYTY) and DREME-2 (ARAGARAR), resembled the binding sites of BASIC PENTACYSTEINE (BPC) transcription factors (Table 3, Figure 7B). These transcription factors bind GA repeat sequences present in many plant promoters. Recently BPC proteins were shown to bind to Polycomb response elements (PREs) and interact with components of Polycomb repressive complex 2 (PRC2) to mediate the silencing of gene expression by PRC2 (Xiao et al. 2017). Two additional motifs, MEME-5 (GYRRRTSCCACGTG), and DREME-1 (CGWGSC), resembled the binding sites of basic HELIX LOOP HELIX (bHLH) transcription factors (Table 3, Figure 7C). In particular the identified motifs most closely match the DAP-Seq binding sites of BIG PETAL (BPE)/bHLH31 and PHYTOCHROME INTERACTING FACTOR7 (PIF7).
The ANT ChIP-Seq gene set exhibited limited overlap with those from AP1, JAG and PLT2
To investigate where ANT acts within the hierarchy of known flower development regulators, we compared the set of genes bound by ANT to genes bound by other transcription factors involved in flower development as determined by ChIP-chip or ChIP-Seq experiments. We compared ANT with the floral meristem identity protein LFY, the floral organ identity proteins AP1, AP3, PI, AG and SEP3, the growth regulator JAGGED (JAG), and AUXIN RESPONSE FACTOR 3/ETTIN (ARF3/ETT) which regulates gynoecium development (O’Maoiléidigh et al. 2013, Pajoro et al. 2014, Schiessl et al. 2014, Simonini et al. 2017, Winter et al. 2011, Wuest et al. 2012). Fisher’s exact tests were performed using the R package GeneOverlap (Shen and Sinai 2019). GeneOverlap also calculates the Jaccard index to assess the overlap of two gene lists. A Jaccard index of 0 indicates no similarity between the gene lists while a value of 1 indicates the lists are identical. Comparisons between the gene set of ANT with gene sets from LFY, AP3, PI, AG, SEP3, and ETT each gave a Jaccard index of 0.1 while the comparison between ANT and JAG and that between ANT and AP1 gave a Jaccard index of 0.2 (Figure S6). This degree of overlap is much less than that observed among the floral organ identity proteins AP3, PI and AG which exhibit Jaccard indices of 0.4 or 0.5 (Figure S6). JAG also showed a Jaccard Index of 0.2 with the floral organ identity proteins SEP3, AP1, and PI.
We also compared the set of ANT ChIP-Seq bound genes with genes bound by two other AIL/PLT transcription factors: PLT2, which specifies stem cell identity in the root and regulates shoot phyllotaxy and BABYBOOM (BBM), which promotes somatic embryogenesis (Aida et al. 2004, Boutilier et al. 2002, Prasad et al. 2011). The PLT2 ChIP-Seq experiment utilized roots and the BBM ChIP-Seq experiment used somatic embryos (BBM) (Horstman et al. 2015, Santuari et al. 2016). The comparison with PLT2 gave a Jaccard index of 0.2 while that with BBM gave a Jaccard index of 0.1. It is interesting that ANT showed more overlap with the related PLT2 transcription factor, which primarily functions in roots, versus most non-related transcription factors that regulate floral organ identity.
ANT may repress SPL8 to promote petal growth
One of the 200 likely direct targets of ANT regulation is SPL8, a gene that acts in micro- and megasporogenesis (Unte et al. 2003). spl8 mutants produce slightly thinner flowers than wild type and have stamens with smaller anthers and shorter filaments (Unte et al. 2003). SPL8 mRNA levels are reduced in dex-treated 35S:ANT-GR inflorescences, and ANT binds to its 5’ UTR (Figures 5, 6). This suggests that ANT can directly repress SPL8 expression. To investigate a possible genetic interaction between ANT and SPL8, we generated ant-4 spl8–1 double mutants. ant-4 spl8–1 flowers exhibit a partial suppression of the petal growth defects of ant-4 (Figure 8A). Petal width and petal area are larger in ant-4 spl8–1 flowers as compared with ant-4 flowers (Figure 8B). This finding suggests that SPL8 acts as a repressor of petal growth in the ant-4 background and that one means by which ANT promotes petal growth is through downregulation of SPL8. A role in petal development has not previously been noted for SPL8 although the gene is expressed in the margins of petals in stage 8 flowers (Unte et al. 2003). The molecular mechanism by which SPL8 may repress petal growth in ant mutants is not clear as spl8–1 mutants have smaller petals than those in wild type flowers (Figure S7).
Figure 8. Mutations in SPL8 partially rescue the petal size defects of ant flowers.

A. ant-4 flower (left) and ant-4 spl8–1 flower (right). B. Petal width, length and area in ant-4 and ant-4 spl8–1 flowers. * indicates values with a P value less than 0.05 (Student’s t-test). Size bar is 1mm.
Discussion
ANT is a key regulator of floral organ growth that acts in a redundant manner with AIL6/PLT3 to regulate the initiation, identity and patterning of floral organs (Elliott et al. 1996, Klucher et al. 1996, Krizek 1999, Krizek 2009, Mizukami and Fischer 2000). Using transcriptional profiling and genome-wide mapping of ANT binding sites, we identified 200 genes that both responded to changes in ANT activity and were bound by ANT. This set of genes included auxin signaling genes as well as genes that specify floral organ identity, establish polarity, regulate growth, and promote cell differentiation. Thus our work begins to reveal the molecular means by which ANT regulates growth and patterning during floral organogenesis and identifies candidate direct target genes in the larger transcriptional network surrounding ANT.
Our studies reveal that ANT can act directly as both a transcriptional activator and transcriptional repressor. The set of 200 likely direct targets of ANT regulation include both upregulated and downregulated genes. More upregulated genes (154) are associated with ANT binding peaks compared with downregulated genes (46), suggesting that ANT primarily acts as a transcriptional activator. While our work suggests that ANT binds in vivo to DNA sequences with similarity to the in vitro determined ANT binding motif, not all ANT binding peaks contain an AIL/PLT-like site, as defined by the MEME discovered MEME-1 and MEME-3 motifs. Thus, ANT may bind directly to other DNA sequences and/or be recruited to different DNA sites via interaction with other transcription factors. The identification of DNA binding motifs of BPC and bHLH transcription factors within ANT peaks suggests that ANT may be recruited to some DNA sites via interaction with other classes of transcription factors.
A comparison of the set of genes bound by ANT with those bound by other floral regulators did not reveal strong overlap with any other gene set as measured by the Jaccard index. This is consistent with ANT having functions that are distinct from transcription factors specifying floral meristem or floral organ identity. One limitation of these comparisons is the different tissues used in these studies. The LFY, JAG and ETT experiments were performed with whole inflorescences while the SEP3, AP1, AP3, PI, and AG used stage 5 flowers. Among floral regulators tested, the highest overlap of ANT was seen with AP1 and JAG. JAG is a regulator of floral organ shape, which suggests that ANT and JAG may regulate some common target genes mediating floral organ growth. We also observed higher overlap with the root stem cell regulator PLT2 despite the different tissues used in the ChIP-Seq experiments (stage 6/7 flowers versus seedlings). The similar DNA binding specificities of ANT and PLT2 may result in the regulation of some common targets despite the distinct morphologies and functions of roots and flowers.
ANT directly regulates genes involved in auxin signaling
Genomic studies in the root have shown that AIL/PLT transcription factors directly regulate genes involved in auxin biosynthesis and transport. Our studies here imply that ANT directly regulates auxin signaling in developing flowers (Santuari et al. 2016, Zúñiga-Mayo et al. 2019). While our RNA-Seq experiment suggested that ANT could regulate other hormone signaling pathways including cytokinin, gibberellin, and JA, the DE genes in these categories were not bound by ANT and are thus likely to be indirect targets of ANT regulation. However, it is also possible that these genes may be bound by ANT at a different stage of flower development, since the RNA-Seq experiment utilized floral buds of stage 1–12 while the ChIP-Seq experiment utilized stage 6/7 buds.
Auxin is linked to many aspects of flower development including floral organ initiation, primordium growth, stamen filament elongation, pollen maturation and gynoecium patterning, several of which overlap with ANT function (Cheng et al. 2006, Marsch-Martînez and de Folter 2016). Patterning of distinct tissues and cell types within the gynoecium appears to involve complex transcriptional networks and the precise distributions of hormones, particularly auxin and cytokinin (Zúñiga-Mayo et al. 2019). Within the gynoecium, ANT acts redundantly with several other genes including REV to promote the development of the carpel marginal meristem, a meristematic region within the medial domain of the ovary that gives rise to multiple tissues including the placentae, ovules and transmitting tract (Azhakanandam et al. 2008, Krizek 2009, Liu et al. 2000, Nole-Wilson and Krizek 2006). In ant rev double mutants, medial domain development is disrupted and there is partial loss of the carpel marginal meristem (Nole-Wilson et al. 2010). These defects are associated with reduced expression of the auxin biosynthetic enzyme TAA1 in stage 7 gynoecium (Nole-Wilson et al. 2010). We found that ANT induction activated TAA1 expression and that ANT bound to TAA1. These results suggest that ANT may be a direct regulator of TAA1 in this tissue. Mutation of the ANT binding site within the TAA1 gene would help to reveal whether ANT is required for expression of this gene within the gynoecium.
ANT directly regulates genes that specify floral organ identity
Three of the 200 likely direct targets of ANT regulation were floral organ identity genes: the class A genes AP1 and AP2 and the class E gene SEP3. AP1 was downregulated after ANT induction while AP2 and SEP3 were upregulated (Table 1). Expression of AP1, AP2 and SEP3 is initiated early in flower development (stage 1 for AP1 and AP2 and stage 2 for SEP3) and maintained in developing flowers (Jofuku et al. 1994, Mandel et al. 1992, Mandel and Yanofsky 1998). Thus, the binding of ANT to the regulatory regions of these genes in stage 6/7 flowers may reflect a role in maintaining (AP2, SEP3) or limiting (AP1) expression in later stages of flower development.
The combined activities of class A and E genes specify sepal identity which is not disrupted in ant single mutants or ant ail6 double mutants. However, in combination with class B genes, class A and E genes also contribute to the specification of petal identity in the second whorl. Petals are not present in ant ail6 flowers and expression of the class B gene AP3 is reduced (Krizek 2009, Krizek et al. 2016). While we detected binding of ANT to the regulatory regions of AP3 in stage 6/7 flowers (Dataset S3), we failed to observe statistically significant differential expression of AP3 following induction of ANT activity. Failure to detect a significant difference between treatment and control samples in any time point was not due to low AP3 expression, as AP3 was highly expressed in these samples. Instead, this negative result may have been due to the timing of the floral stage examined, the bulk nature of the tissue collected, or because ANT requires some other unknown factor to alter AP3 expression.
ANT directly regulates genes acting later in floral organogenesis that control growth and differentiation
We found that ANT directly regulates genes that act in the elaboration of organ size and shape and the differentiation of distinct cell types. We showed that ANT binds to two genes involved in stamen development: EXCESS MICROSPOROCYTES1 (EMS1) and SPL8. EMS1 encodes a leucine-rich repeat receptor kinase that is required for tapetal cell differentiation (Huang et al. 2016, Zhao et al. 2002) and SPL8 encodes an SPB-box transcription factor that is required for the normal development of anther sporogenic tissue (Unte et al. 2003, Xing et al. 2010). Both EMS1 and SPL8 were downregulated after induction of ANT activity, consistent with a role for ANT in inhibiting differentiation in early stages of floral organogenesis (Krizek and Eaddy 2012).
Several known regulators of lateral organ growth were identified in the set of 200 DE genes bound by ANT. These were KLU, GRF8, AN3/GIF1, XTH9, and SRP2 which act as growth-promoting genes and KIP-RELATED PROTEIN 4 (KRP4) which acts as a growth repressor (Table 1) (Anastasiou et al. 2007, Bemis and Torii 2007, Hyodo et al. 2003, Kim et al. 2016, Kim et al. 2003, Kim and Kende 2004). GRF8, AN3/GIF1, XTH9 and SRP2 were upregulated after ANT induction suggesting they mediate ANT’s role in promoting organ growth. Furthermore, AN3/GIF1 and XTH9, have largely overlapping expression patterns with ANT which is consistent with ANT acting as an activator of these genes (Figure S8) (Hyodo et al. 2003).
The identification of members of the GRF/GIF pathway (GRF8, AN3/GIF) as potential targets of ANT regulation is interesting, as GRFs and their miRNA regulator miR396 are key regulators of growth in many plant tissues [reviewed in (Liebsch and Palatnik 2020)]. In the root, GRF/GIF complexes repress AIL/PLT expression in transit-amplifying cells to promote proliferation of these cells, while PLT1 and PLT2 activate miR396 within the root stem cell niche to repress GRF expression and maintain stem cell identity (Rodriguez et al. 2015). The miR396-GRF/GIF module also controls floral organ growth and meristematic competence within reproductive organs, but a connection with AIL/PLT function has not been described (Lee et al. 2014, Lee et al. 2018). ant an3 double mutants exhibit more severe defects in leaf growth than either single mutant but there was no enhancement of the carpel marginal meristem defects within the gynoecium (Lee et al. 2014). Extensive genetic redundancies in the AIL/PLT, GRF and GIF gene families may complicate interpretation of these results (Lee et al. 2014). Future studies will need to address whether AIL/PLT might directly regulate GRF/GIF expression or act indirectly through miR396 in the carpel marginal meristem and whether AIL/PLT genes are targets of GRF/GIF regulation.
ANT may promote organ growth through direct regulation of polarity genes
Seven genes associated with lateral organ polarity were identified in our RNA-Seq experiment, of which five genes (PHB, BOP1, AS1, KAN2, and YAB3) were also next to an ANT binding site (Table 1). The differentially expressed genes included both upregulated and downregulated genes. We found ANT binding sites near or within four other genes that were not detected as differentially expressed. These included adaxial genes REVOLUTA (REV) and BOP2 and the abaxial genes KAN2 and FILAMENTOUS FLOWER (FIL). The ANT binding peaks upstream of FIL and YAB3 overlapped an in vitro defined ANT binding site (Nole-Wilson and Krizek 2006).
Previous genetic work has suggested a role for ANT in lateral organ polarity. While ant single mutants do no show defects in organ polarity, mutations in ANT combined with mutations in FIL produce plants with smaller leaves that exhibit polarity defects on both the adaxial and abaxial surfaces of leaves (Nole-Wilson and Krizek 2006). ant fil yab3 triple mutants exhibit even more severe defects in leaf polarity and growth, and YAB3 and FIL expression is reduced in ant ail6 double mutants (Krizek et al. 2016, Nole-Wilson and Krizek 2006). Polarity defects were also observed in ant fil floral organs and these defects were associated with reduced expression of PHB (Nole-Wilson and Krizek 2006). Together, these data suggest that ANT regulates organ polarity through regulation of both adaxial and abaxial-specifying genes. Since the juxtaposition of adaxial and abaxial cell types is required for outgrowth of the leaf lamina, our work suggests that one mechanism by which ANT controls lateral organ growth is through direct regulation of polarity genes to establish distinct adaxial and abaxial domains within developing lateral organ primordia (Yamaguchi et al. 2012).
Overall, the work described here reveals that ANT can directly regulate the expression of target genes involved in various aspects of flower development including floral organ identity, polarity, growth and cellular differentiation. Furthermore, our findings connect ANT function with several hormone pathways that may provide positional information for growth and patterning events during flower development.
Experimental Procedures
Plant materials, growth conditions, genotyping and treatments
35S:ANT-GR plants were grown on a soil mixture of Fafard 4P:perlite:vermiculite (8:1:1) in 16 hour days at a light intensity of approximately 160 micromol/m2/s at 20°C. ANT:ANT-VENUS ant-4 AP1:AP1-GR ap1 cal inflorescences were grown on a soil mixture of Fafard 4P:perlite:vermiculite (8:1:1) in 24 hour days at a light intensity of approximately 160 micromol/m2/s at 20°C. ant-4 and spl8–1 were grown on a soil mixture of Fafard 4P:perlite:vermiculite (8:1:1) in 16 hour days at a light intensity of approximately 160 micromol/m2/s at 22°C. ant-4 spl8–1 double mutants were identified by genotyping for ant-4 and spl8–1 as described previously (Krizek 2009, Unte et al. 2003). 35S:ANT-GR plants for RNA-Seq and RT-qPCR were treated by pipetting a mock (0.1% ethanol + 0.015% Silwet), dex (10μM dexamethasone +0.015% Silwet), chx (10μM cycloheximide + 0.015% Silwet + 0.1% ethanol), or dex+chx (10μM dexamethasone + 10μM cycloheximide + 0.015% Silwet) solution onto the inflorescences. AP1:AP1-GR ap1 cal and AP1:AP1-GR ap1 cal ANT:ANT-VENUS ant-4 plants for ChIP-Seq and ChIP-qPCR were treated by pipetting a dex (10μM dexamethasone +0.015% Silwet) solution onto the inflorescences.
RNA-Seq
35S:ANT-GR inflorescences containing unopened floral buds (flowers stages 1–12) were collected in four batches at each time point (two, four and eight hours after treatment) consisting of two flats per batch, where dexamethasone was applied to one flat and a mock treatment was applied to the other flat. RNA was extracted from inflorescences using Trizol following the manufacturer’s instructions with cleanup and DNase treatment on a RNeasy column (Qiagen). Sequencing libraries were prepared from four biological replicates using TruSeq Stranded mRNA Sample preparation kit (Illumina) and sequenced on the Illumina HiSeq 2500 producing 100 base single-end reads. Sequence reads were aligned to the reference Arabidopsis thaliana genome (version TAIR9, released June 2009) using tophat and bowtie2. Reads per gene were counted using featureCounts. Read counts were analyzed using the edgeR software. Differentially expressed genes were identified using an additive linear model with adjustment for batch (flat) effects. Source code for differential expression analysis is available in the project “git” repository https://bitbucket.org/lorainelab/inducible-ant-rna-seq/. Gene Ontology analyses were performed with AmiGO 2 (http://amigo.geneontology.org/amigo).
RT-qPCR
RNA was isolated as described above for RNA-Seq. First strand complementary DNA (cDNA) synthesis was performed using Quanta qScript cDNA SuperMix (Quanta BioSciences) following the manufacturer’s instructions. qPCR was performed on a BioRad CFX96 using PerfeCTa SYBR Green FastMix for iQ (Quanta BioSciences) and primers listed in Table S5. Data analyses were carried out as described previously (Krizek and Eaddy 2012). Two biological replicates were analyzed for each experiment.
ChIP-Seq and ChIP-qPCR
Chromatin immunoprecipitation was performed similarly to that described in (Yamaguchi et al. 2014) with the same buffers and solutions. Approximately 600mg of inflorescence tissue consisting of stage 6/7 flowers from AP1:AP1-GR ap1 cal and ANT:ANT-VENUS ant-4 AP1:AP1-GR ap1 cal plants was collected five days after dex treatment into a 2 ml tube filled with 1.5ml cold 1x PBS on ice. The PBS was then removed and replaced with 10mls of room temperature 1% methanol-free formaldehyde (Thermo Scientific) in 1xPBS and 0.015% Silwet L-77 for 15 mins at room temperature. During this time, the tissue was vacuum infiltrated three times for two minutes each time. The fixative was removed and crosslinking stopped with the addition of 10mls of 0.125M glycine and incubated for 5 mins. During this time, the tissue was vacuum infiltrated once for two mins. The tissue was rinsed three times with 10mls cold 1x PBS while on ice, dried briefly on paper towels, frozen in liquid nitrogen, and stored at −80°C. The tissue was ground in liquid nitrogen and 2.5ml nuclei extraction buffer with protease inhibitors and beta-mercaptoethanol was added. The samples were filtered twice through Miracloth and centrifuged at 10,000g for 5 mins at 4°C. The pellet was resuspended in 107 μl of nuclei lysis buffer and left on ice for 30 mins with occasional stirring with a pipet tip. 893 μl of ChIP dilution buffer without Triton X-100 was added to bring the volume to 1 ml. The sample was loaded into a milliTUBE 1ml AFA Fiber tube (Covaris) and chromatin shearing was performed with a Covaris M220 Focused ultra-sonicator (14 cycles of 75% peak power, 5 duty factor, 200 cycles/burst at 7°C). After sonication, 200 μl of ChIP dilution buffer with Triton X-100 and 53 μl of 22% Triton X-100 was added to each sample. The samples were centrifuged twice at 12,000g for 10 mins at 4°C. The sample was pre-cleared by adding 50 μl of Dynabeads-Protein A and incubating for 2 hours at 4°C on a tube rotator. The sample was removed using a magnetic stand and transferred into a 1.5 ml low adhesion tube. A 12.5 μl sample was removed as the Input sample. 50 μl of GFP (Invitrogen A6455) coated Dynabeads was added to each sample and incubated for 4 hours at 4°C.The samples were washed twice (5 mins each at 4°C) with the following four cold wash buffers: low salt wash buffer, high salt wash buffer, 250mM LiCl buffer, 0.5x TE. Immunoprecipitated DNA was eluted from the Dynabeads by the addition of 50 μl of Nuclei Lysis Buffer and a 30 minute incubation at 65°C on a Thermomixer. The elution was repeated a second time and the samples combined. Crosslinks were reversed by the addition of 6 μl of 5M NaCl to the ChIP samples and an overnight incubation at 65°C. 87.5 μl of Nuclei Lysis buffer and 6 μl of 5M NaCl was added to the input samples followed by overnight incubation at 65°C. The input and ChIP DNA was purified using Qiagen PCR purification kit. Primers for ChIP-qPCR are listed in Table S5. Fold enrichment was determined relative to a negative control, the transposon TA3.
Sequencing libraries were prepared from two biological replicates of input and ChIP DNA for stage 6/7 flowers for both AP1:AP1-GR ap1 cal and AP1:AP1-GR ap1 cal ANT:ANT-VENUS ant-4 using Accel-NGS 2S DNA library kit (Swift Biosciences). The libraries were quantitated using the NEBNext Library Quant Kit for Illumina (New England Biolabs) and sequenced on an Illumina HiSeq 2500 producing 150 base paired-end reads. Sequence reads were aligned to the reference Arabidopsis thaliana genome (version TAIR9, released June 2009) using bowtie2. Examination of the coverage graphs revealed high reproducibility between the two ChIP-Seq replicates. In addition, the input samples closely resembled the control untagged AP1:AP1-GR ap1 cal samples. ANT binding peaks were identified using a visual analytics approach within the Integrated Genome Brower (IGB) (Freese et al. 2016). Specifically, coverage graphs were generated for the combined data from the two replicates. A difference coverage graph was generated by subtracting coverage graphs of the untagged sample (AP1:AP1-GR ap1 cal) from the coverage graphs for the tagged sample (AP1:AP1-GR ap1 cal ANT:ANT-VENUS ant-4). Peaks were defined using the thresholding feature. A thresholding value of 5000 identified 90 peaks while a thresholding value of 1000 identified 11,133 peaks. Further analyses were performed using the 1,113 peaks identified with a threshold value of 1,000. For each identified peak, ChIPpeakAnno was used to identify the gene with the closest transcription start site (TSS) (Zhu et al. 2010). GeneOverlap was used to compare gene lists for different ChIP-Seq data sets. Gene Ontology analyses were performed with AmiGO 2 (http://amigo.geneontology.org/amigo). Source code for bioinformatic analyses are available in the project “git” repository https://bitbucket.org/lorainelab/ant-chip/.
Motif analysis with the MEME Suite
Genomic locations of putative ANT binding sites were determined using the FIMO tool of the MEME Suite (Grant et al. 2011). The in vitro defined ANT binding motif was used as a position specific prior (Nole-Wilson and Krizek 2000). Putative ANT binding sites were identified using a p value of 0.001 or smaller. De novo motif discovery was performed with MEME-ChIP.
Petal measurements
Petal width, length and area were measured as described previously (Krizek 2015).
Accession Numbers
RNA-Seq sequences are available from Sequence Read Archive (https://www.ncbi.nlm.nih.gov/sra) accession number PRJNA539947. ChIP-Seq sequences are available from Sequence Read Archive (https://www.ncbi.nlm.nih.gov/sra) accession number PRJNA593434. Version-controlled source code used to process and analyze data are available from https://bitbucket.org/krizeklab. Sequence alignments and coverage graphs are available for interactive visualization within the Integrated Genome Browser (Nicol et al. 2009). To view the data in IGB, readers may download and install the software from https://bioviz.org. Once installed, data sets from the study can be opened within IGB by selecting the latest Arabidopsis thaliana genome and then choosing RNA-Seq and ChIP-Seq folders within the Available Data Sets section of the Data Access Panel.
Supplementary Material
Supporting Materials
Figure S1. Dex treatment of 35S:ANT-GR inflorescences results in larger flowers and male sterility. Mock (left) and dex (right) treated 35S:ANT-GR flowers. Size bar is 1mm.
Figure S2. Interactive R Shiny app tool to display gene expression data in control (C) and treated (T) 35S:ANT-GR samples. Control samples correspond to mock-treated 35S:ANT-GR inflorescences while treated samples correspond to dex-treated 35S:ANT-GR inflorescences. A. Sample RPKM for AT1G48660 that shows gene expression values for each of four biological replicates. B. Group RPKM for AT1G48660 which show average RPKM for the four replicates. C. Expression over time for AT1G48660. D. Gene info links for AT1G48660.
Figure S3. Hormone signaling pathways associated with changes in ANT activity. Blue indicates genes that are downregulated after induction of ANT activity. Orange indicates genes that are upregulated after induction of ANT activity. Mixed colored rectangles indicate classes in which some genes were downregulated while others were upregulated. The circles above and below the rectangle represent the number of downregulated (blue) and upregulated (orange) genes for such classes. Abbreviations: TFs, transcription factors.
Figure S4. ChIP-qPCR confirms that ANT binds to genomic regions upstream or within genes associated with polarity specification (KAN2, PHB) and hormone signaling (BEH4, RGA). ChIP-Seq coverage graphs for KAN2 (A), PHB (B), BEH4 (E), and RGA (G). Numbers below the gene indicate the regions tested for ANT binding by ChIP-qPCR. ChIP-qPCR data for KAN2 (B), PHB (D), BEH4 (F) and RGA (H). Grey bars show results from AP1:AP1-GR ap1 cal and black bars show results from AP1:AP1-GR ap1 cal ANT:ANT-VENUS ant. Numbers on the x axis correspond to the genomic regions indicated in the ChIP-Seq coverage graphs.
Figure S5. Secondary motif identified in some MEME-1 sites. Sequence logos for the DAP-Seq site of PLT1 (top), the MEME-1 motif (middle), and the secondary motif identified in some MEME-1 sites (bottom).
Figure S6. Pairwise comparison heat map displaying the Jaccard index degree of overlap among whole genome ChIP datasets of floral regulators and AIL/PLT transcription factors. The ANT data set shows the highest amount of overlap with those of AP1, JAG and PLT2. This degree of overlap is less than that observed among the floral organ identity proteins AP3, PI, and AG. The heat map was created with Heatmapper (heatmapper.ca) (Sabicki et al. 2016).
Figure S7. spl8–1 flowers are smaller than wild-type flowers. A. Col flower (left) and spl8–1 flower (right). B. Graph showing petal width, length and area for Col and spl8–1 flowers. * indicates values with a P value less than 0.05 (Student’s t-test). Size bar is 1mm.
Figure S8. AN3/GIF1 and XTH9 expression within developing flowers overlaps with ANT expression. AN3/GIF1 mRNA expression in the inflorescence meristem (A), stage 2 flower (A), stage 4 flower (B), stage 6 flower (C), stage 8 flower (D), and in the developing carpel (E). XTH9 mRNA expression in the inflorescence meristem (F), stage 2 flower (F), stage 4 flower (G), stage 7 flower (H), stage 8 flower (I), and in the developing carpel (J). All pictures taken at the same magnification. Abbreviations: IM (inflorescence meristem); st 2 (stage 2 flowers); st 4 (stage 4 flowers); st 6 (stage 6 flowers); st 7 (stage 7 flower); and st 8 (stage 8 flowers). Size bar is 50μm. All pictures were taken at the same magnification.
Table S1. Developmental genes differentially expressed after ANT-GR activation
Table S2. Hormone genes differentially expressed after ANT-GR activation
Table S3. Petal area, length and width in Ler, ant-4 and ANT:ANT-VENUS ant-4 flowers
Table S4. Floral organ counts in Ler, ant-4 and ANT:ANT-VENUS ant-4 flowers at positions 1–30 on the inflorescence
Table S5. Primers used in this study
Data S1. Genes differentially expressed in 35S:ANT-GR inflorescences after dex treatment
Data S2. Overrepresented Gene Ontology (GO) terms for 35S:ANT-GR DE genes
Data S4. Overrepresented Gene Ontology (GO) terms for genes associated with ANT ChIP-Seq peaks
Data S3. Genes associated with ANT ChIP-Seq peaks
Data S5. Genes DE in 35S:ANT-GR and bound by ANT
Data S6. Overrepresented Gene Ontology (GO) terms for genes DE in 35S:ANT-GR and bound by ANT
Significance Statement.
“Flower development has been extensively studied, but our knowledge of gene regulatory networks generating floral organs with characteristic forms remains incomplete. This study used a highly targeted genomic approach to identify 200 genes likely to be direct targets of regulation by AINTEGUMENTA (ANT), a transcription factor regulating flower patterning and growth. Our set of identified target genes include those with known roles in polarity specification, floral organ development, lateral organ growth, and auxin signaling.”
Acknowledgements
This work was supported by National Science Foundation (NSF) grant IOS 1354452. The Integrated Genome Browser software was supported by National Institutes of Health grants R01-GM103463 and R01-GM121927. Data hosting is provided by the SciDas project, funded by NSF award 1659300 to PI Frank (Alex) Feltus. We thank Roger Deal for helpful suggestions for ChIP experiments and ChIP-Seq library preparation.
Footnotes
Conflict of Interest
The authors declare that they have no conflicts of interest.
References
- Aida M, Beis D, Heidstra R, Willemsen V, Blilou I, Galinha C, Nussaume L, Noh Y-S, Amasino R and Scheres B (2004) The PLETHORA genes mediate patterning of the Arabidopsis root stem cell niche. Cell, 119, 109–120. [DOI] [PubMed] [Google Scholar]
- Anastasiou E, Kenz S, Gerstung M, MacLean D, Timmer J, Fleck C and Lenhard M (2007) Control of plant organ size by KLUH/CYP78A5-dependent intercellular signaling. Dev Cell, 13, 843–856. [DOI] [PubMed] [Google Scholar]
- Azhakanandam S, Nole-Wilson S, Bao F and Franks RG (2008) SEUSS and AINTEGUMENTA mediate patterning and ovule initiation during gynoecium medial domain development. Plant Physiol, 146, 1165–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bemis SM and Torii KU (2007) Autonomy of cell proliferation and developmental programs during Arabidopsis aboveground organ morphogenesis. Dev Biol, 304, 367–381. [DOI] [PubMed] [Google Scholar]
- Benkova E, Michniewicz M, Sauer M, Teichmann T, Seifertova D, Jurgens G and Friml J (2003) Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell, 115, 591–602. [DOI] [PubMed] [Google Scholar]
- Boutilier K, Offringa R, Sharma VK, Kieft H, Ouellet T, Zhang L, Hattori J, Liu C-M, van Lammeren AAM, Miki BLA, Custers JBM and van Lookeren Campagne MM (2002) Ectopic expression of BABY BOOM triggers a conversion from vegetative to embryonic growth. Plant Cell, 14, 1737–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Dai X and Zhao Y (2006) Auxin biosynthesis by the YUCCA flavin monooxygenase controls the formation of floral organs and vascular tissues in Arabidopsis. Genes Dev, 20, 1790–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott RC, Betzner AS, Huttner E, Oakes MP, Tucker WQJ, Gerentes D, Perez P and Smyth DR (1996) AINTEGUMENTA, an APETALA2-like gene of Arabidopsis with pleiotropic roles in ovule development and floral organ growth. Plant Cell, 8, 155–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freese NH, Norris DC and Loraine AE (2016) Integrated genome browser: visual analytics platform for genomics. Bioinformatics, 32, 2089–2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant CE, Bailey TL and Noble WS (2011) FIMO: Scanning for occurrences of a given motif. Bioinformatics, 27, 1017–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisler MG, Ohno C, Das P, Sieber P, Reddy GV, Long JA and Meyerowitz EM (2005) Patterns of auxin transport and gene expression during primodium development revealed by live imaging of the Arabidopsis inflorescence meristem. Curr Biol, 15, 1899–1911. [DOI] [PubMed] [Google Scholar]
- Horstman A, Fukuoka H, Muiño JM, Nitsch L, Guo C, Passarinho P, Sanchez-Perez GF, Immink R, Angenent GC and Boutilier K (2015) AIL and HDG proteins act antagonistically to control cell proliferation. Development, 142, 454–464. [DOI] [PubMed] [Google Scholar]
- Huang J, Zhang T, Linstroth L, Tillman Z, Otegui MS, Owen HA and Zhao D (2016) Control of anther cell differentiation by the small protein ligand TPD1 and its receptor EMS1 in Arabidopsis. PLoS Genet, 12, e1006147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulsen T, de Vlieg J and Aikema W (2008) BioVenn-a web application for the comparison and visualization of biological lists using area-proportional Venn diagrams. BMC Genomics, 9, 488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyodo H, Yamakawa S, Takeda Y, Tsuduki M, Yokota A, Nishitani K and Kohchi T (2003) Active gene expression of a xyloglucan endotransglucosylate/hydrolase gene, XTH9, in inflorescence apices is related to cell elongation in Arabidopsis thaliana. Plant Mol Biol, 52, 473–482. [DOI] [PubMed] [Google Scholar]
- Jofuku KD, den Boer BGW, Van Montagu M and Okamuro JK (1994) Control of Arabidopsis flower and seed development by the homeotic gene APETALA2. Plant Cell, 6, 1211–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann K, Muino JM, Jauregui R, Airoldi CA, Smaczniak C, Krajewski P and Angenent GC (2009) Target genes of the MADS transcription factor SEPALLATA3: Integration of developmental and hormonal pathways in the Arabidopsis flower. PLOS Biol, 2009, e1000090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann K, Wellmer F, Muino JM, Ferrier T, Wuest SE, Kumar V, Serrano-Mislata A, Madueno F, Krajewski P, Meyerowitz EM, Angenent GC and Riechmann JL (2010) Orchestration of floral initiation by APETALA1. Science, 328, 85–89. [DOI] [PubMed] [Google Scholar]
- Kim EY, Park KY, Seo YS and Kim WT (2016) Arabidopsis small rubber particle protein homolog SRPs play dual roles as positive factors for tissue growth and development and in drought stress responses. Plant Physiol, 170, 2494–2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Choi D and Kende H (2003) The AtGRF family of putative transcription factos is involved in leaf and cotyledon growth in Arabidopsis. Plant J, 36, 94–104. [DOI] [PubMed] [Google Scholar]
- Kim JH and Kende H (2004) A transcriptional coactivator, AtGIF1, is involved in regulating leaf growth and morphology in Arabidopsis. Proc Natl Acad Sci U S A, 101, 13374–13379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klucher KM, Chow H, Reiser L and Fischer RL (1996) The AINTEGUMENTA gene of Arabidopsis required for ovule and female gametophyte development is related to the floral homeotic gene APETALA2. Plant Cell, 8, 137–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krizek BA (1999) Ectopic expression of AINTEGUMENTA in Arabidopsis plants results in increased growth of floral organs. Dev Genet, 25, 224–236. [DOI] [PubMed] [Google Scholar]
- Krizek BA (2009) AINTEGUMENTA and AINTEGUMENTA-LIKE6 act redundantly to regulate Arabidopsis floral growth and patterning. Plant Physiol, 150, 1916–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krizek BA (2015) AINTEGUMENTA-LIKE genes have partly overlapping functions with AINTEGUMENTA but make distinct contributions to Arabidopsis thaliana flower development. J Exp Bot, 66, 4537–4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krizek BA, Bequette CJ, Xu K, Blakley I, Fu ZQ, Stratmann JW and Loraine AE (2016) RNA-Seq links the transcription factors AINTEGUMENTA and AINTEGUMENTA-LIKE6 to cell wall remodeling and plant defense pathways. Plant Physiol, 171, 2069–2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krizek BA and Eaddy M (2012) AINTEGUMENTA-LIKE6 regulates cellular differentiation in flowers. Plant Mol Biol, 78, 199–209. [DOI] [PubMed] [Google Scholar]
- Krizek BA and Fletcher JC (2005) Molecular mechanisms of flower development: an armchair guide. Nat Rev Genet, 6, 688–698. [DOI] [PubMed] [Google Scholar]
- Lee BH, Wynn AN, Franks RG, Hwang Y. s., Lim J and Kim JH (2014) The Arabidopsis thaliana GRF-INTERACTING FACTOR gene family plays an essential role in control of male and female reproductive development. Dev Biol, 386, 12–24. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Lee BH, Jung JH, Park SK, Song JT and Kim JH (2018) GROWTH-REGULATING FACTOR and GRF-INTERACTING FACTOR specify meristematic cells of gynoecia and anthers. Plant Physiol, 176, 717–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebsch D and Palatnik JF (2020) MicroRNA miR396, GRF transcription factors and GIF co-regulators: a conserved plant organ regulatory module with potential for breeding and biotechnology. Curr Opin Plant Biol, 53, 31–42. [DOI] [PubMed] [Google Scholar]
- Liu Z, Franks RG and Klink VP (2000) Regulation of gynoecium marginal tissue formation by LEUNIG and AINTEGUMENTA. Plant Cell, 12, 1879–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machanick P and Bailey TL (2011) MEME-ChIP: motif analysis of large DNA datasets. Bioinformatics, 27, 1696–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel MA, Gustafson-Brown C, Savidge B and Yanofsky MF (1992) Molecular characterization of the Arabidopsis floral homeotic gene APETALA1. Nature, 360, 273–277. [DOI] [PubMed] [Google Scholar]
- Mandel MA and Yanofsky MF (1998) The Arabidopsis AGL9 MADS box gene is expressed in young flower primordia. Sex Plant Reprod, 11, 22–28. [Google Scholar]
- Marsch-Martînez N and de Folter S (2016) Hormonal control of the development of the gynoecium. Curr Opin Plant Biol, 29, 104–114. [DOI] [PubMed] [Google Scholar]
- Mizukami Y and Fischer RL (2000) Plant organ size control: AINTEGUMENTA regulates growth and cell numbers during organogenesis. Proc Natl Acad Sci USA, 97, 942–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicol JW, Helt GA, Blanchard SGJ, Raja A and Loraine AE (2009) The Integrated Genome Browser: free software for distribution and exploration of genome-scale datasets. Bioinformatics, 25, 2730–2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nole-Wilson S, Azhakanandam S and Franks RG (2010) Polar auxin transport together with AINTEGUMENTA and REVOLUTA coordinate early Arabidopsis gynoecium development. Dev Biol, 346, 181–195. [DOI] [PubMed] [Google Scholar]
- Nole-Wilson S and Krizek BA (2000) DNA binding properties of the Arabidopsis floral development protein AINTEGUMENTA. Nucleic Acids Res, 28, 4076–4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nole-Wilson S and Krizek BA (2006) AINTEGUMENTA contributes to organ polarity and regulates growth of lateral organs in combination with YABBY genes. Plant Physiol, 141, 977–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Malley RC, Huang SC, Song L, Lewsey MG, Bartlett A, Nery JR, Galli M, Gallavotti A and Ecker JR (2016) Cistrome and epicistrome features shape the regulatory DNA landscape. Cell, 165, 1280–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Maoiléidigh DS, Thomson B, Raganelli A, Wuest SE, Ryan PT, Kwasniewska K, Carles CC, Graciet E and Wellmer F (2015) Gene network analysis of Arabidopsis thaliana flower development through dynamic gene perturbations. Plant J, 83, 344–358. [DOI] [PubMed] [Google Scholar]
- O’Maoiléidigh DS, Wuest SE, Rae L, Raganelli A, Ryan PT, Kwasniewska K, Das P, Lohan AJ, Loftus B, Graciet E and Wellmer F (2013) Control of reproductive floral organ identity specification in Arabidopsis by the C function regulator AGAMOUS. Plant Cell, 25, 2482–2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pajoro A, Madrigal P, Muiño JM, Matus JT, Jin J, Mecchia MA, Debernardi JM, Palatnik JF, Balazadeh S, Arif M, O’Maoilédigh DS, Wellmer F, Krajewski P, Riechmann JL, Angenent GC and Kaufmann K (2014) Dynamics of chromatin accessibility and gene regulation by MADS-domain transcription factors in flower development. Genome Biol, 15, R41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad K, Grigg SP, Barkoulas M, Yadav RK, Sanchez-Perez GF, Pinon V, Blilou I, Hofhuis H, Dhonukshe P, Galinha C, Mahonen AP, Muller WH, Raman S, Verkleij AJ, Snel B, Reddy GV, Tsiantis M and Scheres B (2011) Arabidopsis PLETHORA transcription factors control phyllotaxis. Curr Biol, 21, 1123–1128. [DOI] [PubMed] [Google Scholar]
- Reinhardt D, Eva-Rachele P, Stieger P, Mandel T, Baltensperger K, Bennett M, Traas J, Friml J and Kuhlemeier C (2003) Regulation of phyllotaxis by polar auxin transport. Nature, 426, 255–260. [DOI] [PubMed] [Google Scholar]
- Rodriguez RE, Ercoli MF, Debernardi JM, Breakfield NW, Mecchia MA, Sabatini M, Cools T, De Veylder L, Benfey PN and Palatnik JF (2015) MicroRNA miR396 regulates the switch between stem cells and transit-amplifying cells in Arabidopsis roots. Plant Cell, 27, 3354–3366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabicki S, Arndt D, Marcu A, Lian Y, Grant JR, Maciejewski A and Wishart DS (2016) Heatmapper: web-enabled heat mapping for all. Nucleic Acids Res, 44, W147–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santuari L, Sanchez-Perez GF, Luijten M, Rutjens B, Terpstra I, Berke L, Gorte M, Prasad K, Bao D, Timmermans-Hereijgers JLPM, Maeo K, Nakamura K, Shimotohno A, Pencik A, Novak O, Ljung K, van Heesch S, de Bruijn E, Cuppen E, Willemsen V, Mähönen AP, Lukowitz W, Snel B, de Ridder D, Scheres B and Heidstra R (2016) The PLETHORA gene regulatory network guides growth and cell differentiation in Arabidopsis roots. Plant Cell, 28, 2937–2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiessl K, Muiño JM and Sablowski R (2014) Arabidopsis JAGGED links floral organ patterning to tissue growth by repressing Kip-related cell cycle inhibitors. Proc Natl Acad Sci U S A, 111, 2830–2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L and Sinai M (2019) GeneOverlap: Test and visualize gene overlaps. R package version 1220. [Google Scholar]
- Simonini S, Bencivenga S, Trick M and Ostergaard L (2017) Auxin-induced modulation of ETTIN activity orchestrates gene expression in Arabidopsis. Plant Cell, 29, 1864–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smaczniak C, Immink RGH, Muino JM, Blanvillain R, Busscher M, Busscher-Lange J, Dinh QD, Liu S, Westphal AH, Boeren S, Parcy F, Xu L, Carles CC, Angenent GC and Kaufmann K (2012) Characterization of MADS-domain transcription factor complexes in Arabidopsis flower development. Proc Natl Acad Sci USA, 109, 1560–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart D, Graciet E and Wellmer F (2016) Molecular and regulatory mechanisms controlling floral organ development. FEBS J, 283, 1823–1830. [DOI] [PubMed] [Google Scholar]
- Unte US, Sorensen A-M, Pesaresi P, Gandikota M, Leister D, Saedler H and Huijser P (2003) SPL8, an SBP-Box gene that affects pollen sac development in Arabidopsis. Plant Cell, 15, 1009–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter CM, Austin RS, Blanvillain-Baufumé S, Reback MA, Monniaux M, Wu M-F, Sang Y, Yamaguchi A, Yamaguchi N, Parker JE, Parcy F, Jensen ST, Li H and Wagner D (2011) LEAFY target genes reveal floral regulatory logic, cis motifs, and a link to biotic stimulus response. Dev Cell, 20, 430–443. [DOI] [PubMed] [Google Scholar]
- Wuest SE, O’Maoiléidigh DS, Rae L, Kwasniewska K, Raganelli A, Hanczaryk K, Lohan AJ, Loftus B, Graciet E and Wellmer F (2012) Molecular basis for the specification of floral organs by APETALA3 and PISTILLATA. Proc Natl Acad Sci USA, 109, 13452–13457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao J, Jin R, Yu X, Shen M, Wagner JD, Pai A, Song C, Zhuang M, Klasfeld S, He C, Santos AM, Helliwell C, Pruneda-Paz JL, Kay SA, Lin X, Cui S, Garcia MF, Clarenz O, Goodrich J, Zhang X, Austin RS, Bonasio R and Wagner D (2017) Cis and trans determinants of epigenetic silencing by Polycomb repressive comples 2 in Arabidopsis. Nat Genet, 49, 1546–1552. [DOI] [PubMed] [Google Scholar]
- Xing S, Salinas M, Höhmann S, Berndtgen R and Huijser P (2010) miR156-targeted and nontargeted SBP-box transcription factors act in concert to secure male fertility in Arabidopsis. Plant Cell, 22, 3935–3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi N, Winter CM, Wu M-F, Kwon CS, William DA and Wagner D (2014) Chromatin Immunoprecipitation from Arabidopsis tissue. The Arabidopsis Book, e0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi N, Wu M-F, Winter CM, Berns MC, Nole-Wilson S, Yamaguchi A, Coupland G, Krizek BA and Wagner D (2013) A molecular framework for auxin-mediated initiation of flower primordia. Dev Cell, 24, 271–282. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Nukazuka A and Tsukaya H (2012) Leaf adaxial-abaxial polarity specification and lamina outgrowth: evolution and development. Plant Cell Physiol, 53, 1180–1194. [DOI] [PubMed] [Google Scholar]
- Yan W, Chen D and Kaufmann K (2016) Molecular mechanisms of floral organ specification by MADS domain proteins. Curr Opin Plant Biol, 29, 154–162. [DOI] [PubMed] [Google Scholar]
- Zhao DZ, Wang GF, Speal B and Ma H (2002) The EXCESS MICROSPOROCYTES1 gene encodes a putative leucine-rich repeat receptor protein kinase that controls somatic and reproductive cell fares in the Arabidopsis anther. Genes Dev, 16, 2021–2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu LJ, Gazin C, Lawson ND, Pagés H, LIn SM, Lapointe DS and Green MR (2010) ChIPpeakAnno: a Bioconductor package to annotate ChIP-seq and ChIP-chip data. BMC Bioinformatics, 11, 237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zúñiga-Mayo VM, Gómez-Felipe A, Herrera-Ubaldo H and de Folter S (2019) Gynoecium development: networks in Arabidopsis and beyond. J Exp Bot, 70, 1447–1460. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Materials
Figure S1. Dex treatment of 35S:ANT-GR inflorescences results in larger flowers and male sterility. Mock (left) and dex (right) treated 35S:ANT-GR flowers. Size bar is 1mm.
Figure S2. Interactive R Shiny app tool to display gene expression data in control (C) and treated (T) 35S:ANT-GR samples. Control samples correspond to mock-treated 35S:ANT-GR inflorescences while treated samples correspond to dex-treated 35S:ANT-GR inflorescences. A. Sample RPKM for AT1G48660 that shows gene expression values for each of four biological replicates. B. Group RPKM for AT1G48660 which show average RPKM for the four replicates. C. Expression over time for AT1G48660. D. Gene info links for AT1G48660.
Figure S3. Hormone signaling pathways associated with changes in ANT activity. Blue indicates genes that are downregulated after induction of ANT activity. Orange indicates genes that are upregulated after induction of ANT activity. Mixed colored rectangles indicate classes in which some genes were downregulated while others were upregulated. The circles above and below the rectangle represent the number of downregulated (blue) and upregulated (orange) genes for such classes. Abbreviations: TFs, transcription factors.
Figure S4. ChIP-qPCR confirms that ANT binds to genomic regions upstream or within genes associated with polarity specification (KAN2, PHB) and hormone signaling (BEH4, RGA). ChIP-Seq coverage graphs for KAN2 (A), PHB (B), BEH4 (E), and RGA (G). Numbers below the gene indicate the regions tested for ANT binding by ChIP-qPCR. ChIP-qPCR data for KAN2 (B), PHB (D), BEH4 (F) and RGA (H). Grey bars show results from AP1:AP1-GR ap1 cal and black bars show results from AP1:AP1-GR ap1 cal ANT:ANT-VENUS ant. Numbers on the x axis correspond to the genomic regions indicated in the ChIP-Seq coverage graphs.
Figure S5. Secondary motif identified in some MEME-1 sites. Sequence logos for the DAP-Seq site of PLT1 (top), the MEME-1 motif (middle), and the secondary motif identified in some MEME-1 sites (bottom).
Figure S6. Pairwise comparison heat map displaying the Jaccard index degree of overlap among whole genome ChIP datasets of floral regulators and AIL/PLT transcription factors. The ANT data set shows the highest amount of overlap with those of AP1, JAG and PLT2. This degree of overlap is less than that observed among the floral organ identity proteins AP3, PI, and AG. The heat map was created with Heatmapper (heatmapper.ca) (Sabicki et al. 2016).
Figure S7. spl8–1 flowers are smaller than wild-type flowers. A. Col flower (left) and spl8–1 flower (right). B. Graph showing petal width, length and area for Col and spl8–1 flowers. * indicates values with a P value less than 0.05 (Student’s t-test). Size bar is 1mm.
Figure S8. AN3/GIF1 and XTH9 expression within developing flowers overlaps with ANT expression. AN3/GIF1 mRNA expression in the inflorescence meristem (A), stage 2 flower (A), stage 4 flower (B), stage 6 flower (C), stage 8 flower (D), and in the developing carpel (E). XTH9 mRNA expression in the inflorescence meristem (F), stage 2 flower (F), stage 4 flower (G), stage 7 flower (H), stage 8 flower (I), and in the developing carpel (J). All pictures taken at the same magnification. Abbreviations: IM (inflorescence meristem); st 2 (stage 2 flowers); st 4 (stage 4 flowers); st 6 (stage 6 flowers); st 7 (stage 7 flower); and st 8 (stage 8 flowers). Size bar is 50μm. All pictures were taken at the same magnification.
Table S1. Developmental genes differentially expressed after ANT-GR activation
Table S2. Hormone genes differentially expressed after ANT-GR activation
Table S3. Petal area, length and width in Ler, ant-4 and ANT:ANT-VENUS ant-4 flowers
Table S4. Floral organ counts in Ler, ant-4 and ANT:ANT-VENUS ant-4 flowers at positions 1–30 on the inflorescence
Table S5. Primers used in this study
Data S1. Genes differentially expressed in 35S:ANT-GR inflorescences after dex treatment
Data S2. Overrepresented Gene Ontology (GO) terms for 35S:ANT-GR DE genes
Data S4. Overrepresented Gene Ontology (GO) terms for genes associated with ANT ChIP-Seq peaks
Data S3. Genes associated with ANT ChIP-Seq peaks
Data S5. Genes DE in 35S:ANT-GR and bound by ANT
Data S6. Overrepresented Gene Ontology (GO) terms for genes DE in 35S:ANT-GR and bound by ANT


