Abstract
Aims
To investigate associations of lifetime hazardous and binge drinking with biomarkers of cardiometabolic health, liver function, cardiovascular disease (CVD) and mortality.
Design
Prospective cohort study with median follow-up time to CVD incidence of 4.5 years.
Setting
London, UK: Civil servants within the Whitehall II Study.
Participants
4,820 drinkers aged 59–83 years with biological measurements, during the 2011–2012 survey.
Measurements
Hazardous drinking was defined as having an AUDIT-C score ≥5 calculated at each decade of life, forming the following groups: never hazardous drinker, former early (stopping before age 50), former later (stopping after age 50), current hazardous drinker and consistent hazardous drinker (hazardous drinker at each decade of life)
Findings
Over half of the sample had been hazardous drinkers at some point in their lifetime, comprising former early (<age 50) (19%), former later (≥age 50) (11%), current (21%), and consistent hazardous drinker (AUDIT-C ≥5 across life (5%). After adjusting for covariates, waist circumference was larger with more persistent hazardous drinking (e.g. compared with never hazardous drinkers, former early had increased waist circumference by 1.17cm (95% Confidence Interval [CI] 0.25 to 2.08); former later by 1.88cm (CI: 0.77 to 2.98); current by 2.44cm (CI: 1.50 to 3.34) and consistent hazardous drinker by 3.85cm (CI: 2.23 to 5.47). Current hazardous drinkers had higher systolic blood pressure (2.44mmHg, CI: 1.19 to 3.68), and fatty liver index scores (4.05 mmHg, CI: 2.92 to 5.18) than never hazardous drinkers. Current hazardous drinkers (Hazard Ratio [HR] =2.75, CI: 1.44 to 5.22) had an elevated risk of stroke, and former later hazardous drinkers had an elevated risk of non-CVD mortality (HR=1.93, CI: 1.19 to 3.14) than never hazardous drinkers. Lifetime binge drinking was associated with larger waist circumferences and poorer liver function compared with never binge drinkers.
Conclusion
Hazardous drinking may increase cardio-metabolic risk factors; this is made worse by persistent hazardous drinking across life, particularly in relation to weight gain, suggesting benefits of early intervention.
Introduction
Alcohol use disorders, despite common perception, are common among an older population (1), with the number of alcohol-related admissions which list either the primary and secondary diagnoses as linked to alcohol, being highest among 55–74 year olds in the English population (10). There is also concern over how alcohol may be impacting on cardiovascular health at an age where people are at risk of comorbidities and may be more sensitive to the physical effects of alcohol. Research suggests that older drinkers may not be aware of the risks of their level of consumption (2). Establishing the level of hazardous drinking among older adults and its consequences may enable effective public health interventions to be generated to raise awareness of the effects of alcohol among older adults and ultimately reduce associated harms. Screening tools such as the Alcohol Use Disorders Identification Test (AUDIT), developed by the World Health Organisation (WHO) provide a simple method to identify risky drinking providing a ‘framework for intervention to help hazardous and harmful drinkers reduce or cease alcohol consumption’ (13), and are now commonly used in primary care settings.
Alcohol consumption has been found to vary across the life course (3), taking a single snapshot of consumption may mask the cumulative effects of drinking, including past heavy drinking in individuals currently moderate in their consumption. Whilst there have been several large-scale observational studies of the association between alcohol consumption and cardiovascular disease (CVD), (4–8) relatively little is known about how hazardous drinking as defined by AUDIT-C is associated with CVD or various biological markers of cardiovascular health. The drinking habits adopted by individuals earlier in life may have long-term effects, accelerating the age-of-onset of disease (11, 12). Capturing the effects of hazardous drinking across life, may enhance the usefulness of the screening tool, providing further support for reduction at different stages of life.
This study sought to examine associations between lifetime measures of hazardous drinking as classified by responses to the AUDIT-C and a range of cardiometabolic and liver biomarkers, incident CVD and mortality. Given that the focus of the study is on a screening tool which is used to identify harmful patterns of alcohol consumption, and possible health selection effects into non-drinking (15, 16) the study is based on current drinkers only.
Methods
Sample and Design
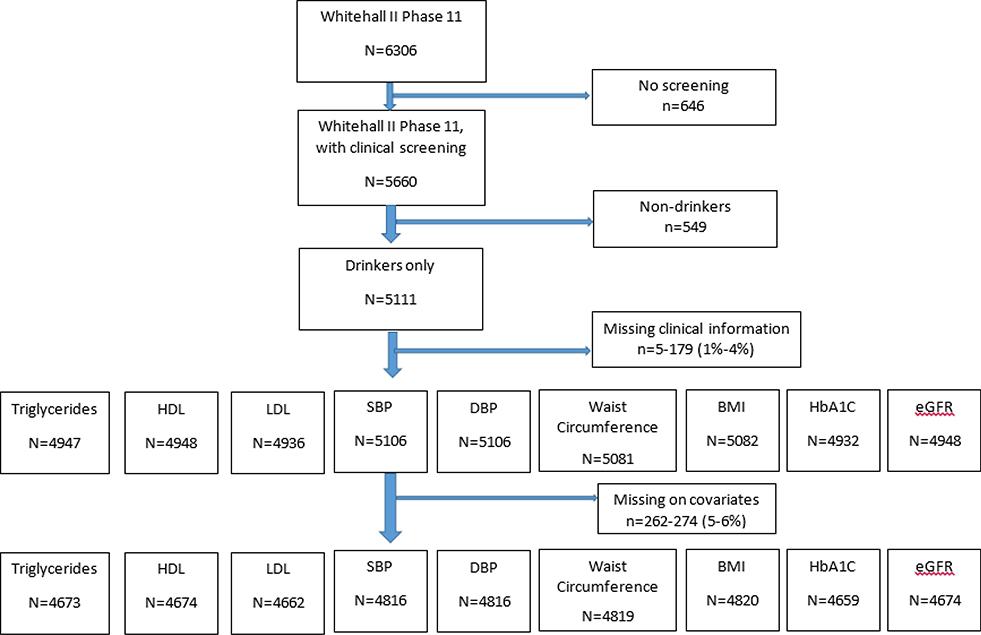
This study uses data from Whitehall II cohort study, which has been prospectively collecting information from 10,308 (33% female) UK civil servants aged 34–56 years at baseline since 1985–1988. (17) Data for this study were drawn from the questionnaire and clinical examination stage of Phase 11 collected in 2012–2013, when participants were aged between 59 to 84 years (N=6306). 646 participants were not clinically screened. Our sample was further limited to drinkers within the previous year, given the focus of the study on a screening tool used among drinkers to identify harmful patterns of consumption, and health selection effects into former and lifetime non-drinking (15, 16) (Figure 1). The final analytical sample was limited to participants with information on all covariates, resulting in around 5–6% missing due to item-non-response (N=4,820 to 4,662). For Cox-proportional hazard models participants with a history of clinically verified CVD were also excluded (n=467).
Figure 1.
Flow diagram of included participants in analytical sample. BMI = body mass index; DBP = diastolic blood pressure; eGFR = estimated glomerular filtration rate; HbA1C = glycated haemoglobin; HDL = high‐density lipoprotein; LDL = low‐density lipoprotein; SBP = systolic blood pressure.
Ethical approval for the Whitehall II study was received from the University College London Medical School Committee on the ethics of human research, and participants gave written informed consent. Whitehall II data are available to bona‐fide researchers for research purposes. The Whitehall II data‐sharing policy is available at http://www.ucl.ac.uk/whitehallII/data-sharing.
Lifetime hazardous drinking
In line with current guidance from Public Health England, a hazardous drinker was defined as someone who scored a total of five or more on the AUDIT-C, this cut-off is recognised by Public Health England as being suggestive of an increasing risk drinker (18) The AUDIT-C involves three questions with five response categories scored from 0 to 5 in order of increasing volume. The questions focus on frequency of drinking (never, to four or more times per week), usual number of drinks consumed in a single drinking session (0 to 2, to 10 or more), and number of days drinking heavily (six drinks or more) on a single occasion (Never, to daily or almost daily). The standard questionnaire can be found here (19)
Participants were assigned an AUDIT-C score during each decade of life, from 16–19 years up to 80+ years, using a retrospective AUDIT-C life grid method. This information was used to form the following lifetime hazardous drinking categories; (1) Never hazardous drinker; (2) Former early hazardous drinker (stopped drinking hazardously before age 50); (3) Former later hazardous drinker (stopped at age 50 or after); (4) Current hazardous drinker; and (5) Consistent hazardous drinker (hazardous drinker during every decade of their life) (Table 1). Age 50 was used as the cut-off for the two types of former drinkers, given the concern of the rise of substance misuse in older people aged 50 and over.(22)
Table 1:
Categorisation of lifetime hazardous drinking groups
| Hazardous drinker | |||
|---|---|---|---|
| Before age 50 | After age 50 | Current | |
| Never hazardous drinker | X | X | X |
| Former early hazardous drinker | √ | X | X |
| Former later hazardous drinker | √ or X | √ | X |
| Current hazardous drinker | √/ or X | √ | √ |
| Consistent hazardous drinker | √ | √ | √ |
X=Not a Hazardous drinker; √=Hazardous drinker
As an additional analyses, to distinguish between frequent moderate drinkers who may have scored positive on AUDIT-C and heavy episodic drinkers, lifetime binge drinking categories were defined using the single item (AUDIT-3) which asks how often the participant has had six or more drinks on one occasion. Binge drinkers were defined as those who indicated doing so at least monthly.
Measurement of Biomarkers
We examined the effect on lifetime hazardous drinking on objective health, utilising the clinical measurements taken during the medical examination in Phase 11. These included cardiometabolic biomarkers such as triglycerides (mmol/L), HDL cholesterol (mmol/L), LDL cholesterol (mmol/L), systolic blood pressure (mmHg), diastolic blood pressure (mmHg), waist circumference (cm), Body Mass Index (BMI, kg/m2), glycated haemoglobin (HbA1C, (mmol/mol), and estimated glomerular filtration rate using serum creatinine and the CKD-Epi 2009 equation (eGFR, ml/min/1.73m2).
We also assessed the relationship of lifetime hazardous drinking and standard liver function tests including gamma-glutamyl transferase (GGT, IU/L), alanine aminotransferase (ALT, IU/L), aspartate aminotransferase (AST, IU/L), bilirubin (IU/L), and derived Fatty Liver Index (FLI). The FLI was calculated using a standard algorithm based on triglycerides, GGT, waist circumference and BMI (23), which resulted in an index ranging from 0 to 100, with higher scores reflecting a greater likelihood of having a fatty liver.
Ascertainment of incident CVD events and mortality
Participants’ data were linked to the national mortality register governed by the National Health Services (NHS) Central register, in which information on mortality was ascertained up to 31st August 2017. Follow-up for clinically verified fatal and non-fatal stroke, myocardial infarction (MI), coronary heart disease (CHD) and aggregate CVD was ascertained up to 31st March 2017. Full ICD codes for classification of these diseases can be found elsewhere (24).
Covariates
Covariates added to each model included sex, age, and occupational grade (low, medium, high) in relation to participants’ last known occupation, and self-reported ethnicity (white, non-white). Health and health behaviours also adjusted for included BMI, self-reported smoking status (never, ex-smoker, current smoker), physical activity, and fruit and vegetable consumption. Physical activity was dichotomised into whether a participant met or exceeded the World Health Organisation (WHO) recommendation of 2.5 hours of moderate-to-vigorous activity per week versus not meeting recommendations. A question relating to frequency of eating fresh fruit and vegetables was categorised into ‘seldom- 3 to 4 times week’, ‘daily’, ‘4+ a day’. We also adjusted for whether participants had a history of any CVD, or previous diagnosis of diabetes based on self-report at phase 11 (no/yes).
Statistical Analyses
A series of linear regression models were fitted with each biomarker as an outcome variable and lifetime hazardous drinking groups as the primary exposure, adjusting for all covariates listed above. Models with waist circumference or BMI as the outcome did not adjust for the alternative. The same models were repeated for lifetime binge drinking groups, which also adjusted for lifetime hazardous drinking (shown as supplementary). Where residuals were not normally distributed the outcome variable was natural log transformed, this applied to triglycerides, HbA1c, ALT, AST and bilirubin. To ease interpretation of models where the outcome variable was log-transformed, regression coefficients were multiplied by 100 and interpreted as a percentage difference. (25)
The associations between lifetime hazardous or binge drinking and incident CVD and mortality were estimated using Cox proportional hazard models. Survival time was calculated from date of interview in Phase 11 to date of CVD event or censoring (CVD death or last date of data linkage up to 31st March 2017, whichever came first), or to death or censoring (indicating no death up to 31st August 2017). For CVD incidence, median and maximum follow up time was 4.5 and 5.3 years. Hazard ratios (HR) and 95% confidence intervals (95%CI) were calculated for each lifetime drinking category, with the never hazardous/binge drinker group treated as reference category. Due to the small number of incident CVD events, current and consistent hazardous drinkers were combined into the one category; ‘current hazardous drinkers’. There was no evidence of deviation from the proportionality assumption in Cox models after assessing Schoenfeld residuals. Survival models were adjusted for the same covariates as the linear regression models, with the exception of having a history of CVD as these participants were excluded from analyses.
Results
Sample characteristics
Of 4,820 participants (mean age 69.4 years, 25% female), over half of drinkers had been hazardous drinkers at some point in their life, with 21% being current hazardous drinkers and 5% being consistent hazardous drinkers (see Table 2). In total, 11% were former early hazardous drinkers, and 19% were former later hazardous drinkers. Current and consistent hazardous drinkers were mostly male (80% and 82% respectively) and predominately white. Those from higher occupational grades were more likely to be current and consistent hazardous drinkers (61%), followed by former later hazardous drinkers (56%), former early hazardous drinkers (53%), and finally, never hazardous drinkers (44%). The highest proportion of those who experienced a previous CVD event or diabetes diagnoses were former later hazardous drinkers (19%), the lowest was current and consistent hazardous drinkers (13%). The 262 participants who were excluded due to having missing information on covariates, were more likely to be female (32%, p=0.022), and non-white (10%, p=0.001), no other results were significant (Supplementary Table 1).
Table 2.
Characteristics of Lifetime hazardous drinking groups, Whitehall II study 2011–2012
| Never hazardous drinker | Former early hazardous drinker | Former later hazardous drinker | Current hazardous drinker | Consistent hazardous drinker | Total | p-value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | N | % | N | % | ||
| All | 2110 | 44 | 932 | 19 | 539 | 11 | 1009 | 21 | 230 | 5 | 4,820 | 100 | |
| Sex | |||||||||||||
| Male | 1,347 | 64 | 753 | 81 | 439 | 81 | 838 | 83 | 221 | 96 | 3,598 | 75 | |
| Female | 763 | 36 | 179 | 19 | 100 | 19 | 171 | 17 | 9 | 4 | 1,222 | 25 | p<0.001 |
| Occupational grade | |||||||||||||
| High | 929 | 44 | 496 | 53 | 303 | 56 | 618 | 61 | 141 | 61 | 2487 | 52 | |
| Medium | 940 | 45 | 388 | 42 | 213 | 40 | 354 | 35 | 84 | 37 | 1979 | 41 | |
| Low | 241 | 11 | 48 | 5 | 23 | 4 | 37 | 4 | 5 | 2 | 354 | 7 | p<0.001 |
| Ethnicity | |||||||||||||
| White | 1,943 | 92 | 914 | 98 | 520 | 96 | 980 | 97 | 228 | 99 | 4,585 | 95 | |
| Non-white | 167 | 8 | 18 | 2 | 19 | 4 | 29 | 3 | 2 | 1 | 235 | 5 | p<0.001 |
| Smoking status | |||||||||||||
| Never smoker | 1189 | 56 | 400 | 43 | 201 | 37 | 336 | 33 | 46 | 20 | 2172 | 45 | |
| Ex-smoker | 873 | 41 | 500 | 54 | 317 | 59 | 630 | 62 | 168 | 73 | 2488 | 52 | |
| Current smoker | 48 | 2 | 32 | 3 | 21 | 4 | 43 | 4 | 16 | 7 | 160 | 3 | p<0.001 |
| Physical activity | |||||||||||||
| < recommendations | 1483 | 70 | 634 | 68 | 363 | 67 | 673 | 67 | 141 | 61 | 3294 | 68 | |
| >= recommendations | 627 | 30 | 298 | 32 | 176 | 33 | 336 | 33 | 89 | 39 | 1526 | 32 | 0.033 |
| Fruit & vegetable consumption | |||||||||||||
| Seldom-3–4 times a week | 430 | 20 | 192 | 21 | 93 | 17 | 227 | 22 | 50 | 22 | 992 | 21 | |
| Daily | 442 | 21 | 170 | 18 | 117 | 22 | 210 | 21 | 45 | 20 | 984 | 20 | |
| 4 or more a day | 1238 | 59 | 570 | 61 | 329 | 61 | 572 | 57 | 135 | 59 | 2844 | 59 | 0.264 |
| Previous CVD or Diabetes | 369 | 17 | 151 | 16 | 100 | 19 | 136 | 13 | 31 | 13 | 787 | 16 | 0.022 |
| Mean (s.e) | N | ||||||||||||
| Age (years) | 70.6 | (5.87) | 67.9 | (5.51) | 70 | (5.50) | 68.8 | (5.47) | 66.2 | (4.06) | 69.4 | (0.46) | 4820 |
| Triglycerides(mmol/L) | 1.2 | (0.55) | 1.2 | (0.67) | 1.2 | (0.64) | 1.2 | (0.74) | 1.3 | (0.74) | 70.4 | (0.97) | 4673 |
| HDL cholesterol (mmol/L) | 1.7 | (0.46) | 1.5 | (0.42) | 1.7 | (0.49) | 1.7 | (0.49) | 1.6 | (0.40) | 1.7 | (0.46) | 4674 |
| LDL cholesterol (mmol/L) | 2.9 | (0.99) | 2.9 | (0.96) | 2.8 | (0.96) | 2.9 | (0.96) | 2.9 | (0.97) | 2.9 | (0.97) | 4662 |
| Systolic bp (mmHg) | 127.3 | (16.55) | 126 | (15.86) | 128 | (16.47) | 130 | (15.97) | 129.6 | (14.82) | 128 | (16.26) | 4816 |
| Diastolic bp (mmHg) | 70.2 | (9.62) | 70.3 | (9.79) | 71 | (10.12) | 72.4 | (10.16) | 72.9 | (9.35) | 70.9 | (9.86) | 4816 |
| Waist (cm) | 94.5 | (12.34) | 96.8 | (12.23) | 98 | (12.03) | 98.2 | (11.97) | 100.5 | (11.34) | 96.4 | (12.29) | 4819 |
| BMI (kg/m2) | 26.4 | (4.53) | 26.7 | (4.49) | 27 | (4.15) | 26.9 | (4.15) | 27.5 | (3.92) | 26.7 | (4.38) | 4820 |
| HbA1c (mmol) | 5.9 | (0.65) | 5.8 | (0.64) | 5.9 | (0.74) | 5.7 | (0.50) | 5.8 | (0.52) | 5.8 | (0.62) | 4659 |
| eGFR (ml/min/1.73m2) | 77.5 | (16.96) | 78.8 | (15.87) | 79 | (16.87) | 81.6 | (16.72) | 82.3 | (16.70) | 79 | (16.76) | 4674 |
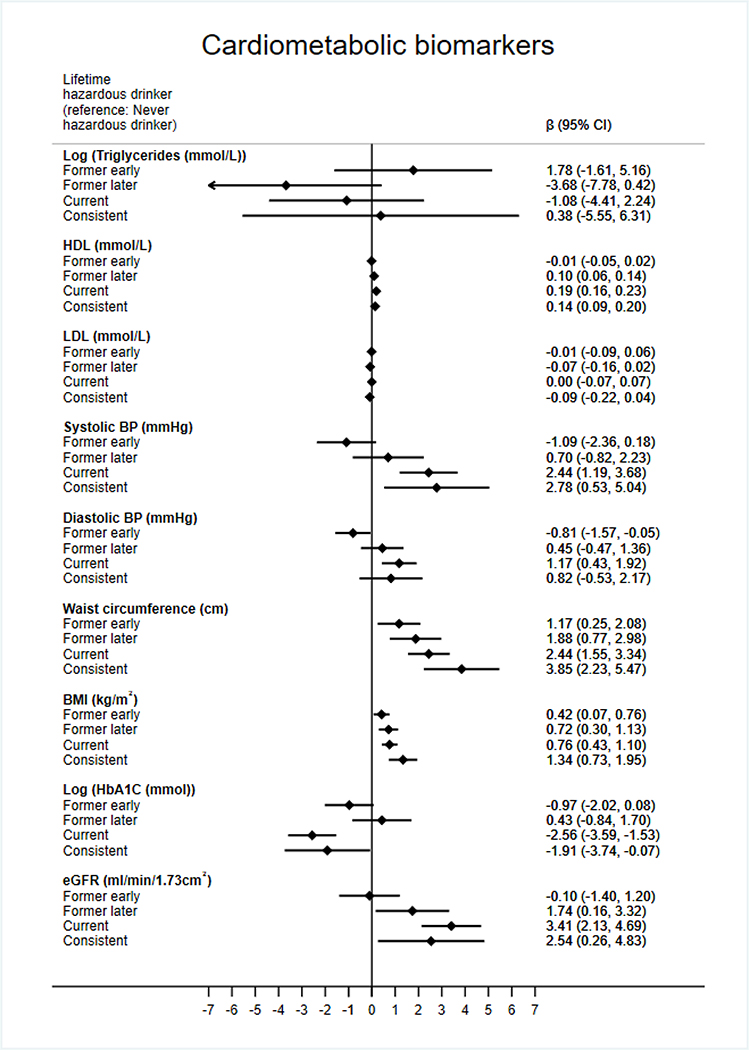
Displayed in Figure 2 are associations between lifetime hazardous drinking and markers of cardiometabolic health. Former later (β=0.10mmol/L, 95% CI 0.06 to 0.14, p<0.001), current (0.19mmol/L, 0.16 to 0.23, p<0.001), and consistent hazardous drinkers (0.14mmol/L, 0.09 to 0.20, p<0.001), had significantly higher HDL cholesterol than never hazardous drinkers. Current (2.44mmHg, 1.19 to 3.68, p<0.001) and consistent hazardous drinkers (2.78mmHg, 0.53 to 5.04, p=0.02) had higher systolic blood pressure than never hazardous drinkers. Current hazardous drinkers also had higher diastolic blood pressure (1.17mmHg, 0.43 to 1.92, p=0.02). Results for triglycerides and LDL cholesterol did not reveal any material differences between drinking categories.
Figure 2.
Linear regression results for lifetime hazardous consumption groups and cardiometabolic biomarkers. Adjusted for sex, age, occupational grade, ethnicity smoking status, BMI, physical activity, fruit & vegetable consumption, and previous CVD or diabetes diagnosis. Models with BMI or Waist circumference as the outcome did not adjust for BMI
Lifetime hazardous drinkers had significantly larger waist circumference and BMI than never hazardous drinkers with the magnitude increasing with more current and consistent hazardous drinking. For example, for waist circumference, former early hazardous drinkers on average had a 1.17cm (0.25 to 2.08, p=0.01) larger waist than never hazardous drinkers, whereas former later hazardous drinkers, current hazardous drinkers and consistent hazardous drinkers had 1.88cm (0.77 to 2.98, p<0.01), 2.44cm (1.55 to 3.34, p<0.01) and 3.85cm (2.23 to 5.47, p<0.01) cm larger waist circumferences, respectively.
Current (−2.56mmol/mol, −3.59 to −1.53, p<0.001) and consistent hazardous drinkers (−1.91mmol/mol, −3.74 to −0.07, p=0.04) had lower HbA1c values than never hazardous drinkers. Former later (1.74ml/min/1.73m2, 0.16 to 3.32, p=0.03), current (3.41ml/min/1.73m2, 2.13 to 4.69, p<0.001) and consistent hazardous drinkers (2.54ml/min/1.73m2, 0.26 to 4.83, p=0.03) had higher eGFR values than never hazardous drinkers. This relationship maintained for current hazardous drinkers, even with the inclusion of systolic blood pressure, HbA1c and HDL in the model (2.62ml/min/1.732, 1.33 to 3.92, p<0.001), however became non-significant for consistent hazardous drinkers (1.96ml/min/1.732, −0.32 to 4.25, p=0.09) (results not shown).
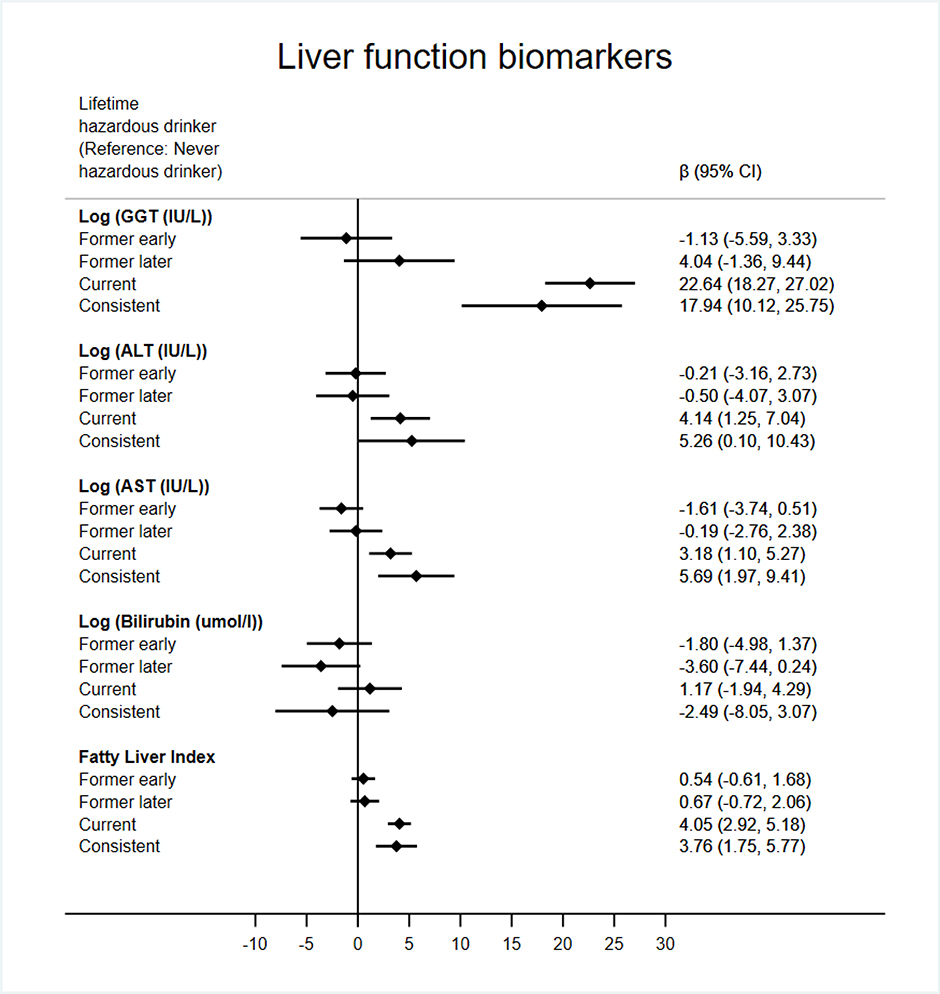
Figure 3 shows similar comparisons between never hazardous drinkers and other lifetime drinking categories across a range of liver function biomarkers. With the exception of bilirubin, current and consistent hazardous drinkers had values indicative of poorer liver function than never hazardous drinkers; (GGT; current (22.64 IU/L, 18.27 to 27.02, p<0.001), consistent (17.94IU/L, 10.10 to 25.75, p<0.001), ALT; current (4.14 IU/L, 1.25 to 7.04, p=0.01), consistent (5.26 IU/L, 0.10 to 10.43, p=0.04), AST; current (3.18 IU/L, 1.10 to 5.27, p<0.01), consistent (5.69 IU/L, 1.97 to 9.41, p<0.01) and Fatty Liver Index; current (4.05, 2.92 to 5.18, p<0.001), consistent (3.76, 1.75 to 5.77, p<0.001). Results for former early and former later hazardous drinkers were non-significant. As a sensitivity analysis, models with FLI as the outcome that did not adjust for BMI found a greater effect of current (8.39, 6.30 to 10.48), and consistent hazardous drinking (11.03, 7.29 to 14.76) as anticipated, but remained in the same direction (results not shown).
Figure 3.
Linear regression results for lifetime hazardous consumption groups and liver function biomarkers. Adjusted for sex, age, occupational grade, ethnicity smoking status, BMI, physical activity, fruit & vegetable consumption, and previous CVD or diabetes diagnosis
Former binge drinkers and current binge drinkers had larger waist circumferences than never binge drinkers. Similarly, all former and current binge drinkers had a greater BMI than never binge drinkers. All other effects failed to reach significance. Current binge drinkers on average had much higher GGT (18.42 IU/L, 11.23 to 25.61, p<0.001), AST (5.80 IU/L, 2.38 to 9.23, p<0.01) and Fatty Liver Index scores than never binge drinkers (3.49, 1.65 to 5.34, p<0.001). Results for consistent binge drinkers failed to reach significance with the exception of FLI (4.73, 1.20 to 8.27). (Table S2)
We observed no statistically significant findings for lifetime hazardous drinking groups and risk of incident MI, any CHD event or aggregate CVD (Table 3). For stroke incidence, current hazardous drinkers had almost a three-fold greater risk than never hazardous drinkers (HR=2.75, 95% CI 1.44 to 5.22), for the other groups results were not significant. No significant findings were found between lifetime hazardous drinking groups and risk of all-cause mortality. Former later hazardous drinkers had approximately two-fold higher risk of non-CVD mortality (1.93, 1.19 to 3.14) than never hazardous drinkers. Risk of mortality for other groups were non-significant.
Table3.
Associations between lifetime hazardous drinking and incident CVD events, and mortality
|
CHD incidence |
Stroke incidence |
|||||||||
| No of cases | Total No | HR | 95% (CI) | p-value | No of cases | Total No | HR | 95% (CI) | p-value | |
| Never hazardous drinker | 88 | 1881 | 1.00 (Ref) | 18 | 1881 | 1.00 | ||||
| Former early hazardous drinker | 41 | 830 | 1.10 | (0.75, 1.62) | 0.615 | 9 | 831 | 1.46 | (0.64, 3.31) | 0.367 |
| Former later hazardous drinker | 24 | 482 | 0.91 | (0.57, 1.44) | 0.686 | 9 | 982 | 1.94 | (0.86, 4.38) | 0.112 |
| Current hazardous drinker | 49 | 1128 | 0.85 | (0.59, 1.23) | 0.399 | 25 | 1127 | 2.75 | (1.44, 5.22) | 0.002 |
|
MI incidence |
CVD incidence |
|||||||||
| No of cases | Total No | HR | 95% (CI) | p-value | No of cases | Total No | HR | 95% (CI) | p-value | |
| Never hazardous drinker | 20 | 1881 | 1.00 | 111 | 1182 | 1.00 | ||||
| Former early hazardous drinker | 14 | 830 | 1.50 | (0.74, 3.04) | 0.266 | 50 | 831 | 1.15 | (0.81, 1.62) | 0.434 |
| Former later hazardous drinker | 6 | 482 | 0.92 | (0.37, 2.33) | 0.864 | 32 | 482 | 1.01 | (0.68, 1.51) | 0.946 |
| Current hazardous drinker | 7 | 1127 | 0.46 | (0.19, 1.13) | 0.091 | 75 | 1128 | 1.13 | (0.83, 1.54) | 0.445 |
|
Total mortality |
Non-CVD mortality |
|||||||||
| No of cases | Total No | HR | 95% (CI) | p-value | No of cases | Total No | HR | 95% (CI) | p-value | |
| Never hazardous drinker | 75 | 1893 | 1.00 | 56 | 1893 | 1.00 | ||||
| Former early hazardous drinker | 28 | 836 | 1.14 | (0.73, 1.78) | 0.573 | 22 | 836 | 1.18 | (0.71, 1.96) | 0.524 |
| Former later hazardous drinker | 26 | 485 | 1.48 | (0.93, 2.33) | 0.095 | 25 | 486 | 1.93 | (1.19, 3.14) | 0.008 |
| Current hazardous drinker | 33 | 1134 | 0.94 | (0.61, 1.44) | 0.771 | 29 | 1134 | 1.10 | (0.69, 1.77) | 0.687 |
Participants with clinically verified CVD were excluded from analyses (n=467). Adjusted for sex, age, occupational grade, ethnicity, smoking status, BMI, physical activity, fruit & vegetable consumption.
Results for lifetime binge drinkers failed to reach significance (Table S3)
Discussion
Hazardous drinking was prevalent among this older aged cohort, with over half of the participants scoring five or more on the AUDIT-C at some point in their life. Current and consistent hazardous drinkers had worse liver function, larger BMI and waist circumference, higher systolic and diastolic blood pressure, and an increased risk of stroke, compared to never hazardous drinkers suggesting benefits in these domains from reducing hazardous consumption. Additionally former hazardous drinkers had larger waist circumferences and BMI, and increased risk of non-CVD mortality than never hazardous drinkers. Reducing hazardous consumption earlier in life may have positive benefits, in particular with relation to weight gain.
It is well established that higher levels of alcohol consumption increase the risk of liver disease.(26) In this respect, our results were as anticipated; current and consistent hazardous had higher values of almost all liver function markers examined in this study. Hazardous drinkers who had quit (either before age 50 or after age 50) did not have worse liver function scores than never hazardous drinkers which could reflect the ability of the liver to regenerate itself.(27) Liver disease is the only major chronic disease to be steadily increasing in the UK(28) yet as this study shows hazardous drinking remains common among an older cohort. Stopping hazardous drinking at any point in the life course is likely to be beneficial for liver health, doing so may help to tackle the rapidly growing burden of liver disease in the population.
Larger BMI and waist circumferences were found with more persistent lifetime hazardous drinking; increasing in magnitude with chronicity of being a hazardous drinker, suggesting the benefits of stopping hazardous drinking in relation to weight gain cannot come too early. Other studies have found that lifetime alcohol use (measured in total volume consumed) is associated with higher waist circumference, particularly in men.(29) Our results suggest that even hazardous drinking or binge drinking before age 50 can contribute to greater BMI and waist circumference in later-life, both of which are major risk factors for heart disease, type 2 diabetes and cancer. (30–32)
Our finding that current hazardous drinkers have increased blood pressure is consistent with other studies,(33) and this might be one pathway implicated in the increased risk of stroke found in this study (34) and elsewhere. (6, 35) We also found an association among former later-life hazardous drinking and increased risk of non-CVD mortality compared with never hazardous drinkers. This may be due to people who have reduced hazardous drinking in later-life due to pre-existing illness,(16), which may be as a result from hazardous consumption.
Relationships with lifetime hazardous drinking in the opposing direction (better for health) were found with HDL, HbA1c, and eGFR. The relationship between alcohol consumption and increased HDL levels has been observed in many other studies.(4) However, we did not find any significant association between lifetime hazardous drinking and CVD incidence which is not necessarily surprising; HDL cholesterol may not have a causal role in reducing the risk of myocardial infarction. (36) The relationship between alcohol and type 2 diabetes is likely to be complex, with many previous studies finding reduced risk for moderate drinkers, particularly among women (37). Hazardous alcohol consumption was associated with better renal function as assessed through eGFR. Other studies have found similar results (38–40) and this counter-intuitive finding requires further investigation. Our investigation into lifetime hazardous consumption and various cardiometabolic biomarkers highlights the complex relationship between alcohol and health; as drinking appears to affect multiple pathways in different ways.
Strengths and Limitations
The strengths of this study include the ability to explore the effects of hazardous drinking across life, where many studies are constrained to assessing alcohol consumption during a short-period only. We also assessed hazardous consumption in relation to a range of objective biomarkers of health, providing a comprehensive overview of the effects of alcohol on an array of biological systems, highlighting the complex association of alcohol with health in the process
Our study is, of course, not without limitations. We have used phase 11 of the study where subjects have been lost to attrition at every follow-up since baseline. This is likely to have resulted in a more affluent sample as those who drop-out tend to be from a lower social grade (41) Furthermore the sample is an occupational cohort and therefore may not be representative of the population.(42) This may have resulted in a lower prevalence of disease in an arguably more affluent sample than the general population and therefore caution should be heeded in generalising the results. Despite this, associations between risk factors and disease observed in the Whitehall II cohort have been shown to be comparable to those in general population (43). The ability to explore the association of lifetime alcohol consumption with a range of biomarkers in a relatively large sample arguably balances out this disadvantage. We used a retrospective measure of lifetime hazardous drinking over a long time period, which is likely to give rise to recall bias. However a previous validation study on this measure in Whitehall II, showed it to be reliable (44). Similar retrospective measures have been used elsewhere,(45, 46) but as with all self-reported data there remains the possibility of bias. Furthermore, due to data constraints there is the possibility of residual confounding, including not accounting for a more comprehensive measure of nutrition. Our short follow-up period made it difficult to assess the longer-term effects of hazardous drinking (e.g. we observed only a small number of events) and this might partly explain the many null findings, and inability to examine the effects of consistent hazardous drinking in survival models in more detail. Unfortunately, we were not able to split stroke by sub-type. Our categorisation of lifetime hazardous consumption may have resulted in a loss of information, however the groups were large enough to facilitate interpretation in particular the effects of being a hazardous drinker before midlife only. Finally, study participants were aged 59 years and over, therefore we may have not captured the full effects of hazardous drinking across life, particularly among younger hazardous drinkers who may have dropped out of the study.
Conclusion
Hazardous drinking, as identified through the AUDIT-C screening tool, is common among older adults and may increase cardio-metabolic risk factors. Population reductions in hazardous drinking are likely to result in improvements in liver function, blood pressure in the elderly and confer a reduced risk of stroke. Longer lasting gains in health and wellbeing may accrue with earlier intervention in the life course, particularly in relation to weight gain
Supplementary Material
Supplementary Table 2 Cardiometabolic and liver function biomarkers and lifetime binge drinking
Acknowledgements
We would like to thank the fieldwork staff, clinical nurses and participants of Whitehall II Study who make the research possible
Funding: The Alcohol Life Course Project was funded by the UK Medical Research Council/Alcohol Research UK (MR/M006638/1) and European Research Council (ERC-StG-2012-309337_AlcoholLifecourse). The Whitehall II study is supported by the UK Medical Research Council (MR/K013351/1; G0902037), British Heart Foundation (RG/13/2/30098), and the US National Institutes of Health (R01HL36310; R01AG013196). The funders had no part to play in the collection, analysis, interpretation, write-up of the study or decision to submit the article for publication. All researchers had full access to the data (including statistical reports and tables in the study) and can take responsibility for the integrity of the data and analyses.
Footnotes
Conflict of Interest Disclosures: None to report
Data sharing: Data, protocols, and other metadata of the Whitehall II study are available to the scientific community. Please refer to the Whitehall II study data sharing policy at www.ucl.ac.uk/whitehallII/data-sharing.
Ethics approval
Ethical approval for the Whitehall II study was received from the University College London Medical School Committee on the ethics of human research, and participants gave written informed consent. Whitehall II data are available to bona‐fide researchers for research purposes. The Whitehall II data‐sharing policy is available at http://www.ucl.ac.uk/whitehallII/data-sharing.
Transparency statement
This manuscript is an honest, accurate, and transparent account of the study being report; no important aspects of the study have been omitted. These analyses were not pre-registered; results should be considered exploratory.
References
- 1.O’Connell H, Chin V, Cunningham C, Lawlor B. Alcohol use disorders in elderly people--redefining an age old problem in old age. BMJ (Clinical research ed). 2003;327(7416):664–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bareham BK, Kaner E, Spencer LP, Hanratty B. Drinking in later life: a systematic review and thematic synthesis of qualitative studies exploring older people’s perceptions and experiences. Age and Ageing. 2018:afy069–afy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Britton A, Ben-Shlomo Y, Benzeval M, Kuh D, Bell S. Life course trajectories of alcohol consumption in the United Kingdom using longitudinal data from nine cohort studies. BMC medicine. 2015;13:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brien SE, Ronksley PE, Turner BJ, Mukamal KJ, A Ghali W. Effect of alcohol consumption on biological markers associated wtih risk of coronary heart disease: systematic review and meta-analysis of interventional studies. BMJ. 2011;312(d636). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bell S, Daskalopoulou M, Rapsomaniki E, George J, Britton A, Bobak M, et al. Association between clinically recorded alcohol consumption and initial presentation of 12 cardiovascular diseases: population based cohort study using linked health records. BMJ. 2017;356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wood AM, Kaptoge S, Butterworth AS, Willeit P, Warnakula S, Bolton T, et al. Risk thresholds for alcohol consumption: combined analysis of individual-participant data for 599,912 current drinkers in 83 prospective studies. The Lancet. 2018;391(10129):1513–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alcohol use and burden for 195 countries and territories, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. The Lancet. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Neill D, Britton A, Hannah MK, Goldberg M, Kuh D, Khaw KT, et al. Association of longitudinal alcohol consumption trajectories with coronary heart disease: a meta-analysis of six cohort studies using individual participant data. BMC medicine. 2018;16(1):124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Green MA, Strong M, Conway L, Maheswaran R. Trends in alcohol-related admissions to hospital by age, sex and socioeconomic deprivation in England, 2002/03 to 2013/14. BMC Public Health. 2017;17:412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Office for National Statistics. Statistics on Alcohol, England 2018 01/05/18 [29/06/2018]. Available from: https://digital.nhs.uk/data-and-information/publications/statistical/statistics-on-alcohol/2018/part-1.
- 11.Bell S Alcohol Consumption, Hypertension, and Cardiovascular Health Across the Life Course: There Is No Such Thing as a One-Size-Fits-All Approach. Journal of the American Heart Association. 2018;7(13). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Charakida M, Georgiopoulos G, Dangardt F, Chiesa ST, Hughes AD, Rapala A, et al. Early vascular damage from smoking and alcohol in teenage years: the ALSPAC study. European Heart Journal. 2018:ehy524–ehy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Babor TFH-B JC. Saunders JB. Monteiro MG. The Alcohol Use Disorders Identification Test (AUDIT) Guidelines for Use in Primary Care. Geneva, Switzerland.; 2001. [Google Scholar]
- 14.World Health Organization. Global status report on alcohol and health. Geneva: World Health Organisation; 2018. [Google Scholar]
- 15.Ng Fat L, Shelton N. Associations between self-reported illness and non-drinking in young adults. Addiction. 2012;107(9):1612–20. [DOI] [PubMed] [Google Scholar]
- 16.Ng Fat L, Cable N, Shelton N. Worsening of Health and a Cessation or Reduction in Alcohol Consumption to Special Occasion Drinking Across Three Decades of the Life Course. Alcoholism: Clinical and Experimental Research. 2015;39(1):166–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marmot M, Brunner E. Cohort Profile: The Whitehall II study. International Journal of Epidemiology. 2005;34(2):251–6. [DOI] [PubMed] [Google Scholar]
- 18.Public Health England. Alcohol use screening tests 2017. [Available from: https://www.gov.uk/government/publications/alcohol-use-screening-tests)..
- 19.Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA, for the Ambulatory Care Quality Improvement P. The AUDIT alcohol consumption questions (AUDIT-C): An effective brief screening test for problem drinking. Archives of internal medicine. 1998;158(16):1789–95. [DOI] [PubMed] [Google Scholar]
- 20.Towers A, Stephens C, Dulin P, Kostick M, Noone J, Alpass F. Estimating older hazardous and binge drinking prevalence using AUDIT-C and AUDIT-3 thresholds specific to older adults. Drug Alcohol Depend. 2011;117(2–3):211–8. [DOI] [PubMed] [Google Scholar]
- 21.Mauri A, Hannu A, HJ T, Kaija S The alcohol use disorders identification test (AUDIT) and its derivatives in screening for heavy drinking among the elderly. International Journal of Geriatric Psychiatry. 2011;26(9):881–5. [DOI] [PubMed] [Google Scholar]
- 22.Rao R, Roche A. Substance misuse in older people. BMJ. 2017;358. [DOI] [PubMed] [Google Scholar]
- 23.Bedogni G, Bellentani S, Miglioli L, Masutti F, Passalacqua M, Castiglione A, et al. The Fatty Liver Index: a simple and accurate predictor of hepatic steatosis in the general population. BMC gastroenterology. 2006;6:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kivimäki M, Batty GD, Singh-Manoux A, Britton A, Brunner EJ, Shipley MJ. Validity of Cardiovascular Disease Event Ascertainment Using Linkage to UK Hospital Records. Epidemiology (Cambridge, Mass). 2017;28(5):735–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cole TJ, Altman DG. Statistics Notes: Percentage differences, symmetry, and natural logarithms. BMJ. 2017;358. [DOI] [PubMed] [Google Scholar]
- 26.Maher JJ. Exploring alcohol’s effects on liver function. Alcohol health and research world. 1997;21(1):5–12. [PMC free article] [PubMed] [Google Scholar]
- 27.Mao SA, Glorioso JM, Nyberg SL. Liver regeneration. Translational Research. 2014;163(4):352–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Williams R, Aspinall R, Bellis M, Camps-Walsh G, Cramp M, Dhawan A, et al. Addressing liver disease in the UK: a blueprint for attaining excellence in health care and reducing premature mortality from lifestyle issues of excess consumption of alcohol, obesity, and viral hepatitis. The Lancet. 2014;384(9958):1953–97. [DOI] [PubMed] [Google Scholar]
- 29.Bergmann MM, Schütze M, Steffen A, Boeing H, Halkjaer J, Tjonneland A, et al. The association of lifetime alcohol use with measures of abdominal and general adiposity in a large-scale European cohort. European Journal Of Clinical Nutrition. 2011;65:1079. [DOI] [PubMed] [Google Scholar]
- 30.Lyall DM, Celis-Morales C, Ward J, et al. Association of body mass index with cardiometabolic disease in the UK Biobank: A Mendelian randomization study. JAMA Cardiology. 2017;2(8):882–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stokes A, Collins JM, Grant BF, Scamuffa RF, Hsiao C-W, Johnston SS, et al. Obesity Progression Between Young Adulthood and Midlife and Incident Diabetes: A Retrospective Cohort Study of U.S. Adults. Diabetes Care. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bhaskaran K, Douglas I, Forbes H, dos-Santos-Silva I, Leon DA, Smeeth L. Body-mass index and risk of 22 specific cancers: a population-based cohort study of 5.24 million UK adults. The Lancet. 2014;384(9945):755–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roerecke M, Kaczorowski J, Tobe SW, Gmel G, Hasan OSM, Rehm J. The effect of a reduction in alcohol consumption on blood pressure: a systematic review and meta-analysis. The Lancet Public Health. 2017;2(2):e108–e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hillbom M, Saloheimo P, Juvela S. Alcohol Consumption, Blood Pressure, and the Risk of Stroke. Current Hypertension Reports. 2011;13(3):208–13. [DOI] [PubMed] [Google Scholar]
- 35.Larsson SC, Wallin A, Wolk A, Markus HS. Differing association of alcohol consumption with different stroke types: a systematic review and meta-analysis. BMC medicine. 2016;14(1):178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Voight BF, Peloso GM, Orho-Melander M, Frikke-Schmidt R, Barbalic M, Jensen MK, et al. Plasma HDL cholesterol and risk of myocardial infarction: a mendelian randomisation study. The Lancet.380(9841):572–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Knott C, Bell S, Britton A. Alcohol Consumption and the Risk of Type 2 Diabetes: A Systematic Review and Dose-Response Meta-analysis of More Than 1.9 Million Individuals From 38 Observational Studies. Diabetes Care. 2015;38(9):1804–12. [DOI] [PubMed] [Google Scholar]
- 38.Koning SH, Gansevoort RT, Mukamal KJ, Rimm EB, Bakker SJL, Joosten MM. Alcohol consumption is inversely associated with the risk of developing chronic kidney disease. Kidney International. 2015;87(5):1009–16. [DOI] [PubMed] [Google Scholar]
- 39.Schaeffner ES, Kurth T, de Jong PE, Glynn RJ, Buring JE, Gaziano JM. Alcohol consumption and the risk of renal dysfunction in apparently healthy men. Archives of internal medicine. 2005;165(9):1048–53. [DOI] [PubMed] [Google Scholar]
- 40.Chung F-M, Yang Y-H, Shieh T-Y, Shin S-J, Tsai JCR, Lee Y-J. Effect of alcohol consumption on estimated glomerular filtration rate and creatinine clearance rate. Nephrology Dialysis Transplantation. 2005;20(8):1610–6. [DOI] [PubMed] [Google Scholar]
- 41.Mein G, Johal S, Grant RL, Seale C, Ashcroft R, Tinker A. Predictors of two forms of attrition in a longitudinal health study involving ageing participants: An analysis based on the Whitehall II study. BMC Medical Research Methodology. 2012;12(1):164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Britton A, Bell S. The protective effects of moderate drinking: lies, damned lies, and… selection biases? Addiction. 2016;112(2):218–9. [DOI] [PubMed] [Google Scholar]
- 43.Batty GD, Shipley M, Tabák A, Singh-Manoux A, Brunner E, Britton A, et al. Generalizability of Occupational Cohort Study Findings. Epidemiology (Cambridge, Mass). 2014;25(6):932–3. [DOI] [PubMed] [Google Scholar]
- 44.Bell S, Britton A. Reliability of a retrospective decade‐based life‐course alcohol consumption questionnaire administered in later life. Addiction. 2015;110(10):1563–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ricci C, Wood A, Muller D, Gunter MJ, Agudo A, Boeing H, et al. Alcohol intake in relation to non-fatal and fatal coronary heart disease and stroke: EPIC-CVD case-cohort study. BMJ. 2018;361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bergmann MM, Rehm J, Klipstein-Grobusch K, Boeing H, Schutze M, Drogan D, et al. The association of pattern of lifetime alcohol use and cause of death in the European prospective investigation into cancer and nutrition (EPIC) study. Int J Epidemiol. 2013;42(6):1772–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 2 Cardiometabolic and liver function biomarkers and lifetime binge drinking