Abstract
Self–other discrimination is fundamental to social interaction, however, little is known about the neural systems underlying this ability. In a previous functional magnetic resonance imaging study, we demonstrated that a right fronto-parietal network is activated during viewing of self-faces as compared with the faces of familiar others. Here we used image-guided repetitive transcranial magnetic stimulation (rTMS) to create a ’virtual lesion’ over the parietal component of this network to test whether this region is necessary for discriminating self-faces from other familiar faces. The current results indeed show that 1 Hz rTMS to the right inferior parietal lobule (IPL) selectively disrupts performance on a self–other discrimination task. Applying 1 Hz rTMS to the left IPL had no effect. It appears that activity in the right IPL is essential to the task, thus providing for the first time evidence for a causal relation between a human brain area and this high-level cognitive capacity.
Keywords: self-awareness, self-recognition, social cognition, inferior parietal lobule, mirror neurons
INTRODUCTION
Distinguishing the self from others is a key aspect of social behaviour. During development, the ability to discriminate self from others appears to emerge earlier than the ability to explicitly self-recognize. Infants as young as 4 months of age show signs of discriminating between self and others (Rochat and Striano, 2002), whereas the ability to recognize oneself in front of a mirror typically emerges around 2 years of age (Amsterdam, 1972), coinciding with the onset of the use of self-referential pronouns (‘I’ and ‘me’) (Preyer, 1889). This evidence suggests a developmental progression from a differentiation of the self from others to explicit identification of the self-image as self-awareness emerges over the first few years of life. It appears that understanding of one’s body as differentiated from the bodies of others develops into the conceptual understanding that images of the self are also representations of one’s body (Rochat and Striano, 2002).
Only recently cognitive and social neuroscientists have begun to explore the topic of the self using functional imaging methods. The emerging picture from the current literature seems to suggest a special role of the right hemisphere (RH) in self-related cognition (Decety and Chaminade, 2003; Platek et al., 2004), own-body perception (Blanke et al., 2002, 2005), self-awareness (Stuss, 1991; Andelman et al., 2004; Barnacz et al., 2004) and autobiographical memory (Fink et al., 1996; Levine et al., 1998; Greenberg et al., 2005). Additional support for a role of the RH in various aspects of self-representation comes from studies of neuropsychological patients (Devinsky, 2000; Breen et al., 2001), behavioural studies (Keenan et al., 2000; Platek and Gallup, 2002) and transcranial magnetic stimulation (TMS) studies (Theoret et al., 2004; Molnar-Szakacs et al., 2005a).
In particular, a right fronto-parietal network in the human brain seems critical for distinguishing the self from others (Decety and Sommerville, 2003). Within this network, the right inferior parietal lobule (IPL) has long been linked with own-body perception; indeed, lesions in this area often lead to disruption of body schema and corporeal awareness, such as that seen in anosognosia (Berlucchi and Aglioti, 1997). Likewise, direct cortical stimulation of the right IPL has been associated with the phenomenon of out-of-body experience (Blanke et al., 2002). Recent functional neuroimaging studies have also implicated the right IPL in self-face recognition (Uddin et al., 2005b; Sugiura et al., 2005; Platek et al., 2006).
Although these recent studies have attempted to reveal the neural bases of self-processing, there is still much debate in the literature regarding whether, and to what extent, these processes are lateralized, and their precise anatomical localizations (Gillihan and Farah, 2005). While there is considerable evidence that self-face recognition involves largely RH processes and neural networks (Keenan et al., 2001; Uddin et al., 2005b; Sugiura et al., 2005), there have also been some reports of left hemisphere (LH) bias related to viewing of self-faces in commissurotomy patients (Turk et al., 2002) (but see Keenan et al., 2003; Uddin et al., 2005a). Likewise, there exists some imaging evidence for involvement of bilateral networks during similar tasks (Sugiura et al., 2000; Kircher et al., 2001).
A limitation of some of the previous neuroimaging work is that functional magnetic resonance imaging (fMRI) provides only correlational information about the relationship between a given brain area and a particular cognitive task. Causal relationships between brain and behaviour can be tested with TMS, a technique involving transient disruption of normal brain activity using focal magnetic pulses that target specific brain areas. Low-frequency (1 Hz or less) repetitive TMS (rTMS) over a particular cortical region may produce a ‘virtual lesion’ that results in reduced performance, if this region is essential to the performance of the task (Pascual-Leone et al., 2000). In this study, we used the fMRI data acquired from our previous study to guide the TMS coil over the parietal site of the network activated by a self-recognition task. We performed low-frequency rTMS to determine if the functional mechanisms implemented by the inferior parietal area previously reported (Uddin et al., 2005b) are indeed necessary for successful self–other discrimination.
MATERIALS AND METHODS
Subjects
Eight healthy volunteers (2 males, 6 females, mean age: 26.6) who were also subjects in our previous fMRI experiment (Uddin et al., 2005b) were recruited for this TMS study. All were right-handed, as assessed by a modified version of the Oldfield Handedness Questionnaire (Oldfield, 1971). Participants were screened for neurological, psychiatric and medical problems, drug use and contraindications to TMS. Written informed consent was obtained prior to the experiment, which was approved by the UCLA Institutional Review Board, and conformed to The Code of Ethics of the World Medical Association (Declaration of Helsinki).
Stimuli and Task
Each subject participated in two TMS sessions, occurring on separate days to avoid residual effects of TMS between sessions. During each session, subjects performed a behavioural task consisting of watching static morphed images of themselves and a highly familiar other and indicating by a button press whether the image they saw looked more like ‘self’ or ‘other’. Stimuli were individually tailored to each subject, and consisted of a series of static colour images constructed from pictures of the subjects’ own face and the face of a gender-matched highly familiar ‘other’, to control for familiarity of the self-face. Images were constructed from digital pictures of subjects’ and control faces acquired with a Kodak 3400C digital camera. Subjects chose a personal friend or colleague they encountered on a daily, or almost daily, basis to be used as a familiar control. Morph Editor (Soft Key Corporation, Cambridge, MA) was used to create digital morphs between the subjects and the familiar face, resulting in six unique faces, each morphed to a varying extent (0, 20, 40, 60, 80 and 100%). Images were edited using Adobe Photoshop 7.0 to remove external features (hair, ears) and to create a uniform gray background. The software package Presentation (Neurobehavioral Systems Inc., http://www.neuro-bs.com/) was used to present stimuli and record responses.
During four 2 min runs, each of six morphed faces was presented ten times in a random sequence. Each of the four runs consisted of a different random sequence. Each stimulus was presented for 1 s, with a 1 s inter-stimulus interval. Subjects were asked to press a button with their index finger if the image presented looked like ‘self’, and another button with their middle finger if it looked like an ‘other’ face. This task was followed by 20 min of rTMS at 1 Hz either over the LH or RH. Subsequently, subjects completed the behavioural task again, such that performance before and after TMS could be compared. Each subject switched response hand midway through behavioural testing, and starting hand order and stimulation site (LH, RH) was counterbalanced between subjects. Counterbalancing procedures are outlined in Table 1.
Table 1.
Subject counterbalancing
| Subject | Session 1 (Starting hand, stimulation location) | Session 2 (Starting hand, stimulation location) |
|---|---|---|
| S1 | Right hand, right IPL | Right hand, left IPL |
| S2 | Right hand, reft IPL | Right hand, right IPL |
| S3 | Left hand, right IPL | Left hand, left IPL |
| S4 | Left hand, left IPL | Left hand, right IPL |
| S5 | Right hand, right IPL | Right hand, left IPL |
| S6 | Right hand, left IPL | Right hand, right IPL |
| S7 | Left hand, right IPL | Left hand, left IPL |
| S8 | Left hand, left IPL | Left hand, right IPL |
Figure 1 shows examples of stimuli presented during the task (1A), and depicts the cortical region (IPL) individually targeted for each subject (1B).
Fig. 1.
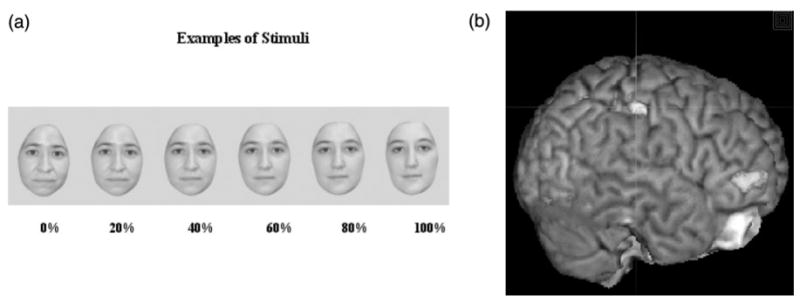
(a) Subjects viewed morphed self-images presented at random for 1 s each and used a button-box to indicate whether the image presented was ‘self’ or ‘other’. Zero% indicates no morphing (i.e. all ‘self’). (b) The right inferior parietal lobule was targeted for TMS in each individual subject by superimposing previously acquired functional imaging data at the individual subject level onto high-resolution structural images.
TMS Protocol
For each subject, resting motor threshold (MT) of the LH was determined according to conventional criteria [minimal stimulator output that induced motor-evoked potentials (MEPs) from the contralateral first dorsal interosseous (FDI) hand muscle of at least 50 μV in five out of ten trials (Rossini et al., 1994)]. The average MT for the subjects was 61.6% maximal stimulator output. Recordings from hand muscles were made with surface Ag/AgCl electrodes, and a Magstim Rapid stimulator (Magstim Company Ltd, Whitland, UK) powered by two booster modules connected to a vacuum-cooled figure-eight coil was used to deliver pulses. Subsequently, all subjects were stimulated at 100% MT (except one, stimulated at 90% MT due to facial muscle twitch). As we had previously used fMRI to reveal cortical activations associated with self-face recognition, we were able to superimpose each subjects’ functional activation during the self–other discrimination task on their own high-resolution structural brain image (magnetization-prepared rapid acquisition gradient echo, MP-RAGE). Stimulation sites for each subject were targeted using Brain Sight, a system for frameless stereotaxy (Rogue Research Inc., Montreal, Canada).
Landmarks on the subject’s head (nose tip, nose bridge, left ear, right ear) were co-registered with landmarks on each subject’s structural MRI to allow tracking of the TMS coil position with respect to the underlying cortex. The coordinates of activity in the regions of interest (right IPL) obtained from functional MRI sessions were marked on the structural MRI of each subject. We chose the left IPL as a control site, as it was not activated during self–other discrimination in our previous study (Uddin et al., 2005b). As a result, we used anatomical landmarks (approximated by the location of the intraparietal sulcus) to localize this region.
RESULTS
As expected, subjects had little difficulty completing the task, and the number of ‘self’ responses decreased as the images morphed increasingly into ‘other’ (Figure 2A). We used signal detection methods to compute d′ as a measure of sensitivity to detect ‘self’ stimuli. Images morphed <50% were designated as ‘self’ trials, and those morphed >50% towards ‘other’ were designated as ‘other’. Hits were defined as ‘self’ responses to trials where the image contained mostly ‘self’ (0, 20 and 40% morphs). False alarms were defined as ‘self’ responses to trials where the image contained mostly ‘other’ (60, 80 and 100% morphs). Thus, a response was considered a hit if the subject identified a <50% morph as ‘self’. The proportion of hits was defined as the proportion of <50% morph stimuli responded to as ‘self’. We found no significant difference in d′ between response hands, therefore we collapsed data across response hand in the final analysis. The values for hits and false alarms for each condition are presented in Table 2.
Fig. 2.
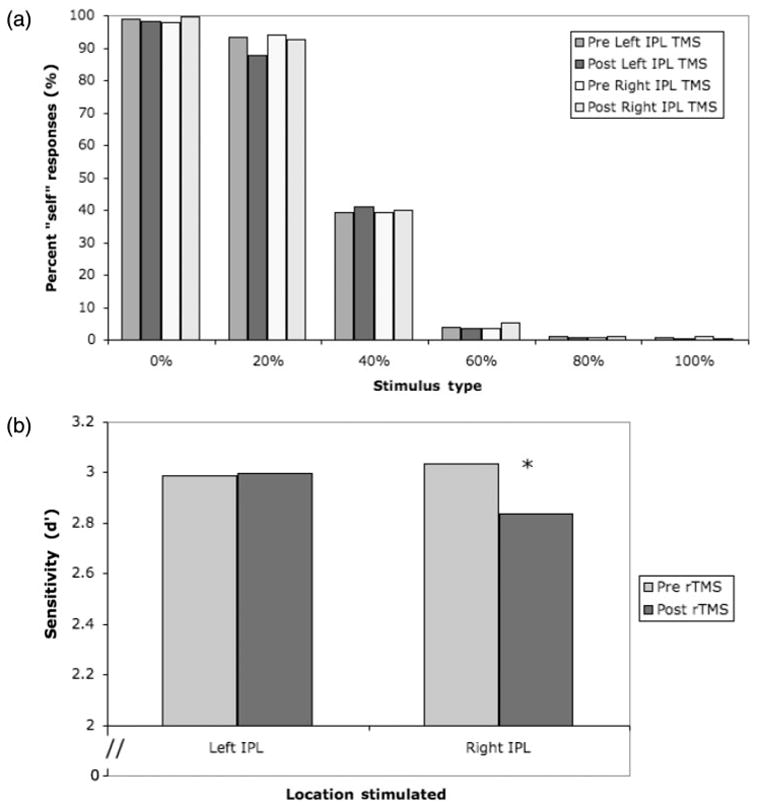
(a) Subjects responded ‘self’ more when the stimulus contained mostly the self-face (b) Stimulation over the right IPL significantly impaired performance on the self–other discrimination task (P = 0.02). Subjects’ performance was not affected by TMS over the left IPL (P = 0.45).
Table 2.
Hits and false alarms used to compute d′
| Pre-rTMS, Left IPL | Post-rTMS, Left IPL | Pre-rTMS, Right IPL | Post-rTMS, Right IPL | |
|---|---|---|---|---|
| Hits | 0.772 | 0.757 | 0.774 | 0.773 |
| False alarms | 0.019 | 0.018 | 0.020 | 0.029 |
Based on our a priori hypothesis regarding laterality from our imaging data (Uddin et al., 2005b), we conducted t-tests on the d′ values. Paired one-tailed t-tests (comparing pre-and post-TMS scores) revealed that rTMS over the right IPL produced a significant decrease in sensitivity to detect self-faces (P = 0.02). Stimulation of the left IPL produced no effect (P = 0.45) (Figure 2B). Similar one-tailed t-tests between β values revealed a marginally significant bias due to rTMS over the right IPL (P = 0.08) and no bias due to rTMS over the left IPL (P = 0.43).
DISCUSSION
In the present study, we found that rTMS over the right IPL significantly impaired the subjects’ performance on discriminating self-faces from other-faces. Subjects’ responses included more false alarms (identifying an image containing mostly ‘other’ as ‘self’) after right IPL stimulation. Stimulation of a control site, the anatomically homologous region in the LH, produced no significant difference in performance. Thus, the right IPL appears to be an essential component of the neural network for visual self-other discrimination. The exact nature of the impairment, as seen in a heightened false alarm rate, can be interpreted either as subjects’ overinclusion of stimuli containing any elements of ‘self’ (i.e. less conservative ‘self’ criteria) or a more conservative strategy when responding to ‘other’. This finding is also in line with neuropsychological evidence for right parietal involvement in own-body awareness (Berlucchi and Aglioti, 1997; Blanke et al., 2002) and agency (Farrer and Frith, 2002; Chaminade and Decety, 2002).
We propose that self-recognition is one component of a system representing the self, and specialized in the RH. This system has two complementary roles. First, it may use a mirror neuron mechanism to establish shared representations between self and others to enable social communication and intersubjectivity (Gallese, 2003). Second, it implements a mechanism to distinguish representation of the self from that of others, which is critical for maintaining an individual sense of unity and agency. The mirror neuron system occupies the IPL and inferior frontal cortex (Rizzolatti et al., 1996; Gallese et al., 1996; Fogassi et al., 2005) and similar fronto-parietal networks are implicated in self–other discrimination (Decety and Sommerville, 2003). Indeed, in our previous fMRI study, we reported that activity in a fronto-parietal network in the RH increases the more a face resembles the self (Uddin et al., 2005b). This RH network overlapped with mirror neuron areas previously identified by separate experiments in our lab (Iacoboni et al., 2005). In line with this suggestion, a recent fMRI study shows that a dysfunction of the mirror neuron system may account for some of the social deficits in children with autism spectrum disorder (ASD) (Dapretto et al., 2006). Indeed, the failure to develop a ‘theory of mind’ is prominent in autism (Frith and Happe, 1999), i.e. a separate representation of self and others.
In humans, the mirror neuron system has been shown to be involved in action observation and imitation (Iacoboni et al., 1999; Rizzolatti and Craighero, 2004; Molnar-Szakacs et al., 2005b). It has also been found that children who have developed the ability to self-recognize are more likely to engage in synchronic imitation with other children (Asendorpf and Baudonniere, 1993). Thus, it appears that there is a developmental link between self-recognition and imitation, which may in part be supported by the mirror neuron system. Furthermore, this link between self-recognition and social engagement through imitation may indicate that indeed the neural mechanisms of self–other discrimination and social interaction are at least in part overlapping. Although the present study cannot provide definitive proof that the behavioural effect reported here is due to a disruption of mirror neurons in the right IPL, we believe that this is a plausible explanation of our findings, as the previous imaging data that guided our stimulation site (Uddin et al., 2005b) overlap quite well with the inferior parietal mirror neuron area identified in previous experiments in our lab (Iacoboni et al., 2005).
Mirror neurons are typically associated with action observation and dynamic visual stimuli, whereas subjects in our experiment watched static images of neutral faces. However, the activation of mirror neuron areas in response to static biological stimuli has been previously observed (Johnson-Frey et al., 2003; Carr et al., 2003; Dapretto et al., 2006). Thus, a mirror neuron response to our stimuli is probable.
It is unlikely that a disruption in the neuronal pool of inferior parietal mirror neurons can account for the whole effect reported here. In fact, mirror neuron areas identified by previous fMRI studies tend to be quite bilateral (Iacoboni et al., 2005; Molnar-Szakacs et al., 2005b), whereas the effect reported here is clearly lateralized to the RH. As discussed, the right IPL has often been associated with the implementation of self-awareness (Spence et al., 1997) and body schema (Berlucchi and Aglioti, 1997). It is likely that the essential role of the right IPL in self–other discrimination revealed by rTMS results from a combination of bilateral functional mechanisms implemented by mirror neurons and lateralized functional mechanisms associated with more general aspects of self-representation and body schema.
A possible concern is that the effect we report may not be specific to self-recognition, and may reflect disruption of processing in more general face discrimination tasks. Due to practical constraints in stimulation procedures that make it difficult to run multiple tasks in multiple cortical areas, we did not run a control task condition in which subjects had to discriminate other non-self familiar faces. However, we believe it highly unlikely that our result would be seen for a general face discrimination task, as most neuroimaging studies of general face discrimination show activity in superior temporal gyrus and fusiform gyrus (Andrews and Ewbank, 2004; Grill-Spector et al., 2004). Conversely, our stimulation site was guided by previous imaging data that demonstrated right IPL activation during self-face recognition (Uddin et al., 2005b).
It is known that rTMS stimulation to a given brain region has both local and potentially interhemispheric effects. For this reason, we included as a control stimulation condition the homologous cortical region of the LH (left IPL). Though it is possible that some of the effects on behaviour we see after right IPL stimulation may result from alteration of activity in regions highly interconnected to this area, the effect of stimulation should be strongest on the targeted site and relatively much weaker at transcallosal sites. One target of particular interest to this study is the right inferior frontal gyrus, the other component of the fronto-parietal system responsive to self-faces. Assessing the effects on behaviour of targeting this region with rTMS is a direction for future research.
Another possible control condition is that of sham TMS. Though sham stimulation may be used as a possible control, there is still much unresolved debate about the validity of sham stimulation. In a systematic study designed to assess the validity of sham stimulation, Loo and colleagues (2000) found that ‘none of the coil positions studies met the criteria for an ideal sham’. Furthermore, Lisanby and colleagues (2001) found that some sham TMS conditions produce substantial cortical stimulation, casting further doubt on the validity of this control. Additionally, it is often obvious to the subject that he/she is receiving no actual stimulation. Therefore, we chose an active stimulation condition as a control by using the anatomically homologous site in the LH.
In conclusion, we show for the first time that disruption of processing in the right IPL is sufficient to degrade self-face recognition performance, suggesting a causal role for this region in self–other discrimination. This is the first evidence demonstrating that a human brain area is necessary for the discrimination of the self-face from other familiar faces, a correlate of maintaining a distinct representation of self while engaging with others.
Abbreviation
- FDI
first dorsal interosseous
- fMRI
functional magnetic resonance imaging
- IPL
inferior parietal lobule
- LH
left hemisphere
- MEP
motor-evoked potential
- MT
motor threshold
- RH
right hemisphere
- rTMS
repetitive transcranial magnetic stimulation
Footnotes
We would like to thank Lisa Koski, Allan Wu, Lisa Aziz-Zadeh, Eric Mooshagian, Jan Rayman and Jonas Kaplan for their technical assistance. This study was supported by a National Science Foundation graduate research fellowship to the first author, Brain Mapping Medical Research Organization, Brain Mapping Support Foundation, Pierson-Lovelace Foundation, The Ahmanson Foundation, Tamkin Foundation, Jennifer Jones-Simon Foundation, Capital Group Companies Charitable Foundation, Robson Family, William M. and Linda R. Dietel Philanthropic Fund at the Northern Piedmont Community Foundation, Northstar Fund, the National Center for Research Resources grants RR12169, RR13642 and RR08655.
References
- Amsterdam B. Mirror self-image reactions before age two. Developmental Psychobiology. 1972;5:297–305. doi: 10.1002/dev.420050403. [DOI] [PubMed] [Google Scholar]
- Andelman F, Zuckerman-Feldhay E, Hoffien D, Fried I, Neufeld MY. Lateralization of deficit in self-awareness of memory in patients with intractable epilepsy. Epilepsia. 2004;45:826–33. doi: 10.1111/j.0013-9580.2004.51703.x. [DOI] [PubMed] [Google Scholar]
- Andrews TJ, Ewbank MP. Distinct representations for facial identity and changeable aspects of faces in the human temporal lobe. Neuroimage. 2004;23:905–13. doi: 10.1016/j.neuroimage.2004.07.060. [DOI] [PubMed] [Google Scholar]
- Asendorpf JB, Baudonniere PM. Self-awareness and other-awareness: mirror self-recognition and synchronic imitation among unfamiliar peers. Developmental Psychology. 1993;29:88–95. [Google Scholar]
- Barnacz AL, Johnson A, Constantino P, Keenan JP. Schizotypal personality traits and deception: the role of self-awareness. Schizophrenia Research. 2004;70:115–6. doi: 10.1016/j.schres.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Berlucchi G, Aglioti S. The body in the brain: neural bases of corporeal awareness. Trends in Neurosciences. 1997;20:560–4. doi: 10.1016/s0166-2236(97)01136-3. [DOI] [PubMed] [Google Scholar]
- Blanke O, Ortigue S, Landis T, Seeck M. Stimulating illusory own-body perceptions. Nature. 2002;419:269–70. doi: 10.1038/419269a. [DOI] [PubMed] [Google Scholar]
- Blanke O, Mohr C, Michel CM, et al. Linking out-of-body experience and self processing to mental own-body imagery at the temporoparietal junction. Journal of Neuroscience. 2005;25:550–7. doi: 10.1523/JNEUROSCI.2612-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breen N, Caine D, Coltheart M. Mirrored-self misidentification: two cases of focal onset dementia. Neurocase. 2001;7:239–54. doi: 10.1093/neucas/7.3.239. [DOI] [PubMed] [Google Scholar]
- Carr L, Iacoboni M, Dubeau MC, Mazziotta JC, Lenzi GL. Neural mechanisms of empathy in humans: a relay from neural systems for imitation to limbic areas. Proceedings of National Academy of Sciences USA. 2003;100:5497–502. doi: 10.1073/pnas.0935845100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaminade T, Decety J. Leader or follower? Involvement of the inferior parietal lobule in agency. Neuroreport. 2002;13:1975–8. doi: 10.1097/00001756-200210280-00029. [DOI] [PubMed] [Google Scholar]
- Dapretto M, Davies MS, Pfeifer JH, et al. Understanding emotions in others: mirror neuron dysfunction in children with autism spectrum disorders. Nature Neuroscience. 2006;9:28–30. doi: 10.1038/nn1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety J, Chaminade T. When the self represents the other: a new cognitive neuroscience view on psychological identification. Consciousness and Cognition. 2003;12:577–96. doi: 10.1016/s1053-8100(03)00076-x. [DOI] [PubMed] [Google Scholar]
- Decety J, Sommerville JA. Shared representations between self and other: a social cognitive neuroscience view. Trends in Cognitive Sciences. 2003;7:527–33. doi: 10.1016/j.tics.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Devinsky O. Right cerebral hemisphere dominance for a sense of corporeal and emotional self. Epilepsy & Behavior. 2000;1:60–73. [Google Scholar]
- Farrer C, Frith CD. Experiencing oneself vs another person as being the cause of an action: the neural correlates of the experience of agency. Neuroimage. 2002;15:596–603. doi: 10.1006/nimg.2001.1009. [DOI] [PubMed] [Google Scholar]
- Fink GR, Markowitsch HJ, Reinkemeier M, Bruckbauer T, Kessler J, Heiss WD. Cerebral representation of one’s own past: neural networks involved in autobiographical memory. Journal of Neuroscience. 1996;16:4275–82. doi: 10.1523/JNEUROSCI.16-13-04275.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogassi L, Ferrari PF, Gesierich B, Rozzi S, Chersi F, Rizzolatti G. Parietal lobe: from action organization to intention understanding. Science. 2005;308:662–7. doi: 10.1126/science.1106138. [DOI] [PubMed] [Google Scholar]
- Frith U, Happe F. Theory of mind and self-consciousness: what is it like to be autistic? Mind & Language. 1999;14:82–9. [Google Scholar]
- Gallese V. The roots of empathy: the shared manifold hypothesis and the neural basis of intersubjectivity. Psychopathology. 2003;36:171–80. doi: 10.1159/000072786. [DOI] [PubMed] [Google Scholar]
- Gallese V, Fadiga L, Fogassi L, Rizzolatti G. Action recognition in the premotor cortex. Brain. 1996;119:593–609. doi: 10.1093/brain/119.2.593. Pt 2. [DOI] [PubMed] [Google Scholar]
- Gillihan SJ, Farah MJ. Is self special? A critical review of evidence from experimental psychology and cognitive neuroscience. Psychological Bulletin. 2005;131:76–97. doi: 10.1037/0033-2909.131.1.76. [DOI] [PubMed] [Google Scholar]
- Greenberg DL, Rice HJ, Cooper JJ, Cabeza R, Rubin DC, Labar KS. Co-activation of the amygdala, hippocampus and inferior frontal gyrus during autobiographical memory retrieval. Neuropsychologia. 2005;43:659–74. doi: 10.1016/j.neuropsychologia.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Knouf N, Kanwisher N. The fusiform face area subserves face perception, not generic within-category identification. Nature Neuroscience. 2004;7:555–62. doi: 10.1038/nn1224. [DOI] [PubMed] [Google Scholar]
- Iacoboni M, Woods RP, Brass M, Bekkering H, Mazziotta JC, Rizzolatti G. Cortical mechanisms of human imitation. Science. 1999;286:2526–8. doi: 10.1126/science.286.5449.2526. [DOI] [PubMed] [Google Scholar]
- Iacoboni M, Molnar-Szakacs I, Gallese V, Buccino G, Mazziotta JC, Rizzolatti G. Grasping the intentions of others with one’s own mirror neuron system. PLoS Biology. 2005;3:e79. doi: 10.1371/journal.pbio.0030079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson-Frey SH, Maloof FR, Newman-Norlund R, Farrer C, Inati S, Grafton ST. Actions or hand-object interactions? Human inferior frontal cortex and action observation. Neuron. 2003;39:1053–8. doi: 10.1016/s0896-6273(03)00524-5. [DOI] [PubMed] [Google Scholar]
- Keenan JP, Nelson A, O’Connor M, Pascual-Leone A. Self-recognition and the right hemisphere. Nature. 2001;409:305. doi: 10.1038/35053167. [DOI] [PubMed] [Google Scholar]
- Keenan JP, Freund S, Hamilton RH, Ganis G, Pascual-Leone A. Hand response differences in a self-face identification task. Neuropsychologia. 2000;38:1047–53. doi: 10.1016/s0028-3932(99)00145-1. [DOI] [PubMed] [Google Scholar]
- Keenan JP, Wheeler M, Platek SM, Lardi G, Lassonde M. Self-face processing in a callosotomy patient. European Journal of Neuroscience. 2003;18:2391–5. doi: 10.1046/j.1460-9568.2003.02958.x. [DOI] [PubMed] [Google Scholar]
- Kircher TT, Senior C, Phillips ML, et al. Recognizing one’s own face. Cognition. 2001;78:1–15. doi: 10.1016/s0010-0277(00)00104-9. [DOI] [PubMed] [Google Scholar]
- Levine B, Black SE, Cabeza R, et al. Episodic memory and the self in a case of isolated retrograde amnesia. Brain. 1998;121:1951–73. doi: 10.1093/brain/121.10.1951. Pt 10. [DOI] [PubMed] [Google Scholar]
- Lisanby SH, Gutman D, Luber B, Schroeder C, Sackeim HA. Sham TMS: intracerebral measurement of the induced electrical field and the induction of motor-evoked potentials. Biological Psychiatry. 2001;49:460–3. doi: 10.1016/s0006-3223(00)01110-0. [DOI] [PubMed] [Google Scholar]
- Loo CK, Taylor JL, Gandevia SC, McDarmont BN, Mitchell PB, Sachdev PS. Transcranial magnetic stimulation (TMS) in controlled treatment studies: are some ‘‘sham’’ forms active? Biological Psychiatry. 2000;47:325–31. doi: 10.1016/s0006-3223(99)00285-1. [DOI] [PubMed] [Google Scholar]
- Molnar-Szakacs I, Uddin LQ, Iacoboni M. Right-hemisphere motor facilitation by self-descriptive personality-trait words. European Journal of Neuroscience. 2005a;21:2000–6. doi: 10.1111/j.1460-9568.2005.04019.x. [DOI] [PubMed] [Google Scholar]
- Molnar-Szakacs I, Iacoboni M, Koski L, Mazziotta JC. Functional segregation within pars opercularis of the inferior frontal gyrus: evidence from fMRI studies of imitation and action observation. Cerebral Cortex. 2005b;15:986–94. doi: 10.1093/cercor/bhh199. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh Inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Walsh V, Rothwell J. Transcranial magnetic stimulation in cognitive neuroscience–virtual lesion, chronometry, and functional connectivity. Current Opinion in Neurobiology. 2000;10:232–7. doi: 10.1016/s0959-4388(00)00081-7. [DOI] [PubMed] [Google Scholar]
- Platek SM, Gallup GG. Self-face recognition is affected by schizotypal personality traits. Schizophrenia Research. 2002;57:81–5. doi: 10.1016/s0920-9964(01)00310-3. [DOI] [PubMed] [Google Scholar]
- Platek SM, Keenan JP, Gallup GG, Jr, Mohamed F. Where am I? The neurological correlates of self and other. Brain Research Cognitive Brain Research. 2004;19:114–22. doi: 10.1016/j.cogbrainres.2003.11.014. [DOI] [PubMed] [Google Scholar]
- Platek SM, Loughead JW, Gur RC, et al. Neural substrates for functionally discriminating self-face from personally familiar faces. Human Brain Mapping. 2006;27:91–8. doi: 10.1002/hbm.20168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preyer W. The Mind of the Child Part II: The Development of the Intellect. New York: Appleton; 1889. [Google Scholar]
- Rizzolatti G, Craighero L. The mirror-neuron system. Annual Review of Neuroscience. 2004;27:169–92. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fadiga L, Gallese V, Fogassi L. Premotor cortex and the recognition of motor actions. Brain Research Cognitive Brain Research. 1996;3:131–41. doi: 10.1016/0926-6410(95)00038-0. [DOI] [PubMed] [Google Scholar]
- Rochat P, Striano T. Who’s in the mirror? Self-other discrimination in specular images by four- and nine-month-old infants. Child Development. 2002;73:35–46. doi: 10.1111/1467-8624.00390. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Barker AT, Berardelli A, et al. Non-invasive electrial and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalography and Clinical Neurophysiology. 1994;91:79–92. doi: 10.1016/0013-4694(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Spence SA, Brooks DJ, Hirsch SR, Liddle PF, Meehan J, Grasby PM. A PET study of voluntary movement in schizophrenic patients experiencing passivity phenomena (delusions of alien control) Brain. 1997;120:1997–2011. doi: 10.1093/brain/120.11.1997. Pt 11. [DOI] [PubMed] [Google Scholar]
- Stuss DT. Self, awareness, and the frontal lobes: A neuropsychological perspective. In: Strauss J, Goethals GR, editors. The self: Interdisciplinary Approaches. New York: Springer-Verlag; 1991. pp. 255–78. [Google Scholar]
- Sugiura M, Watanabe J, Maeda Y, Matsue Y, Fukuda H, Kawashima R. Cortical mechanisms of visual self-recognition. Neuroimage. 2005;24:143–49. doi: 10.1016/j.neuroimage.2004.07.063. [DOI] [PubMed] [Google Scholar]
- Sugiura M, Kawashima R, Nakamura K, et al. Passive and active recognition of one’s own face. Neuroimage. 2000;11:36–48. doi: 10.1006/nimg.1999.0519. [DOI] [PubMed] [Google Scholar]
- Theoret H, Kobayashi M, Merabet L, Wagner T, Tormos JM, Pascual-Leone A. Modulation of right motor cortex excitability without awareness following presentation of masked self-images. Brain Research Cognitive Brian Research. 2004;20:54–7. doi: 10.1016/j.cogbrainres.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Turk DJ, Heatherton TF, Kelley WM, Funnell MG, Gazzaniga MS, Macrae CN. Mike or me? Self-recognition in a split-brain patient. Nature Neuroscience. 2002;5:841–2. doi: 10.1038/nn907. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Rayman J, Zaidel E. Split-brain reveals separate but equal self-recognition in the two cerebral hemispheres. Consciousness and Cognition. 2005a;14:633–40. doi: 10.1016/j.concog.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Kaplan JT, Molnar-Szakacs I, Zaidel E, Iacoboni M. Self-face recognition activates a frontoparietal ‘‘mirror’’ network in the right hemisphere: an event-related fMRI study. Neuroimage. 2005b;25:926–35. doi: 10.1016/j.neuroimage.2004.12.018. [DOI] [PubMed] [Google Scholar]


