Abstract
The capacity to stabilize the content of attention over time varies among individuals and its impairment is a hallmark of several mental illnesses. Impairments in sustained attention in patients with attention disorders have been associated with increased trial-to-trial variability in reaction time and event-related potential (ERP) deficits during attention tasks. At present, it is unclear whether the ability to sustain attention and its underlying brain circuitry are transformable through training. Here, we show, with dichotic listening task performance and electroencephalography (EEG), that training attention, as cultivated by meditation, can improve the ability to sustain attention. Three months of intensive meditation training reduced variability in attentional processing of target tones, as indicated by both enhanced theta-band phase consistency of oscillatory neural responses over anterior brain areas and reduced reaction time variability. Furthermore, those individuals who showed the greatest increase in neural response consistency showed the largest decrease in behavioral response variability. Notably, we also observed reduced variability in neural processing, in particular in low-frequency bands, regardless of whether the deviant tone was attended or unattended. Focused attention meditation may thus affect both distracter and target processing, perhaps by enhancing entrainment of neuronal oscillations to sensory input rhythms; a mechanism important for controlling the content of attention. These novel findings highlight the mechanisms underlying focused attention meditation, and support the notion that mental training can significantly affect attention and brain function.
Keywords: Noise, Prefrontal, Dopamine, Training, Attention, event-related
Introduction
The capacity to sustain attention is critical for maintaining performance over a period of time. Although this capacity shows some heritability (Kuntsi and Stevenson, 2001), it is also influenced by factors such as fatigue, and task familiarity (Aston-Jones, Rajkowski and Cohen, 1999). Deficits in sustained attention are a core feature of several mental illnesses, including attention-deficit hyperactivity disorder (ADHD) and schizophrenia (Castellanos et al., 2006, Winterer et al., 2000). Recent work has linked sustained attention impairment in these patient groups to increased trial-to-trial variability in reaction time and in neural responses (Castellanos et al., 2006, Winterer et al., 2000). In normal individuals, attention also frequently lapses, requiring a refocusing of attention. Such ongoing fluctuations in attention stability are thought to reflect competitive interactions between task-related and task-unrelated processes (e.g. mind-wandering) (Kelly et al., 2008, Weissman et al., 2006).
Contemplative traditions suggest that specific mental training regimes, such as a meditation practice that involves the focusing of attention (Focused Attention (FA) meditation), enhance the stability of attention and reduce the need to invoke executive skills that regulate the focus of attention from moment-to-moment (Gunaratana, 2002, Gyatso, Tenzin (the XIV Dalai Lama) and Jinpa, 1995)). Here, we investigated longitudinally whether 3-months of intensive meditation training, which included FA meditation as a major component, can increase attentional stability as one may predict based on Buddhist descriptions of FA. We probed attentional stability using a dichotic listening task that has previously been used to characterize attentional function in normal adults (Hillyard et al., 1973, Polich and Criado, 2006) and in patients with ADHD (Manassis, Tannock and Masellis, 1996) and schizophrenia (Winterer et al., 2000). Participants were presented with frequent standard, and rare deviant tones in both ears, and had to detect an intermittent deviant tone in the attended ear channel. We investigated the effects of FA meditation on moment-to-moment stability of attention by quantifying the cross-trial variability of: a) reaction time in response to attended deviant tones (standard deviation (SD)) of reaction time (RT), (Castellanos et al., 2006)) and b) of the phase of oscillatory neural activity in response to attended and unattended deviant tones, as indexed by the Phase-Locking Factor (PLF, (Palva, Palva and Kaila, 2005)). PLF is a frequency domain measure adapted specifically to measure trial-to-trial stability of stimulus-evoked neural responses. Several studies have shown that PLF is reduced in schizophrenic patients when compared to controls in similar auditory attention tasks, highlighting its potential relevance as a marker of attentional stability (e.g. (Winterer et al., 2000)).
Our main hypothesis was that intensive meditation training would significantly improve attentional stability. We predicted that intensive meditation training would reduce variability in attentional responses to deviant attended tones, as indicated by decreased SD of RT measurements and by increased PLF to target tones. In addition, we explored the possibility that FA meditation increases the monitoring of both target stimuli and distractions and thus affects stimulus processing in general. This unspecific meditation-related effect would be indicated by changes in PLF to attended and unattended deviant tones.
Materials and Methods
Participants
Seventeen practitioners (7 male; median age 41 years, range 22–64 years, median education = 18 years) were recruited prior to the start of a three-month meditation retreat at the Insight Meditation Society in Barre, MA (the practitioner group). Twenty-three matched controls (9 male; median age 41 years, range 20–62 years, median education = 17 years) with no prior meditation experience were recruited via advertisements in local newspapers directed at individuals interested in learning about meditation (the novice group). The mean age and the standard deviation of age did not differ between groups (respectively, t-test, t(1,29)=−0.5, p=0.60 and F(16,13)=1.25, p=0.35). The participants had no history of mental or neurological illness, and gave informed consent to participate. The practitioners self-selected for the meditation group and all had prior experience with meditation. Their average life experience with meditation was 2967 hours (standard deviation of 3162 hours). This average includes daily practice and days in retreat (we counted 8 hours of meditation per day of retreat). This research was approved by UW-Madison institutional review board. Informed consent was obtained from all participants.
Meditation training
The practitioners participated in a 3-month meditation retreat during which they meditated for 10–12 hours per day. Retreat participants were trained in Vipassana meditation (Focused Attention (FA) and Open Monitoring (OM) meditations; see (Lutz et al., 2008a)) and also received some training in ‘Metta’, a loving kindness and compassion meditation. The novice group attended a one-hour group instruction class on Vipassana meditation prior to each visit to the laboratory and meditated daily for 20 minutes. Their daily practice prior to data collection was assessed at each visit.
Vipassana meditation can be understood as a combination of two styles of practices that we conceptualized as Focus Attention meditation (FA) and Open Monitoring meditation (OM) (Lutz et al., 2008a). The first style, FA meditation, entails voluntary focusing attention on a chosen object in a sustained fashion. The second style, OM meditation, involves nonreactively monitoring the content of experience from moment to moment, primarily as a means to recognize the nature of emotional and cognitive patterns. The current study focused on the long-term impact of FA meditation. Our main assumption was that the mental processes trained during FA meditation are required for performance of sustained attention tasks, such as the dichotic listening task used in our study (see below for details). FA meditation is a widespread style of Buddhist practice and involves sustaining selective attention moment by moment on a chosen object, such as a subset of localized sensations caused by respiration (for details see (Gunaratana, 2002)). To sustain this focus, the practitioner must also constantly monitor the quality of attention. At the beginning of the practice, the attention may wander away from the chosen object, and the typical instruction is to recognize the wandering and then restore attention to the chosen object. For example, while intending to focus on localized sensations around the nostrils caused by breathing, one might notice that one’s attention has been lost and that one has become unwillingly busy with thoughts unrelated to the task. One then simply releases this distraction, and returns to the intended object. Thus, while cultivating the acuity and stability of sustained attention on a chosen object, this practice also develops three skills regulative of attention: the first is the monitoring faculty that remains vigilant to distractions without destabilizing the intended focus. The next skill is the ability to disengage from a distracting object without further involvement. The last involves the ability to redirect focus promptly to the chosen object. Progress in this form of meditation is measured, in part, by the degree of effort required to sustain the intended focus. The novice contends with more distractions, and the three regulative skills are frequently exercised. As one advances, the three regulative skills can be developed to the point that, for example, advanced practitioners have an especially acute ability to notice when the mind has wandered. Eventually, FA induces a trait change, whereby the attention rests more readily and stably on the chosen focus. At the most advanced levels, the regulative skills are invoked less and less frequently, and the ability to sustain focus thus becomes progressively effortless.
Stimuli and Task
Participants first performed an attention blink task to examine effects of intensive meditation on temporal attention (see (Slagter et al., 2007)). This task was followed, after a 5-minute break, by two versions of a standard selective attention auditory task, the dichotic listening task (Tiitinen et al., 1993). In one version (‘Focused Attention version’), participants were instructed to attend to tone pips presented in one ear (e.g., the left ear), and to press a button each time they detected an intermittent deviant tone among the frequent (‘standard’) tones in that ear. They were asked to ignore concurrent tone pips in the opposite ear. Of importance, participants were explicitly instructed to do the Focused Attention task version as a FA meditation practice. In the second task version, participants were asked to press a button each time they detected a deviant tone in either ear (i.e., the left or the right ear; ‘Open attention version’). During this version of the task they were asked to cultivate OM meditation (data not presented here). The order of the two task versions was randomized across participants. Participants were instructed to keep fixation on a central cross and to minimize eye movements and blinks during the experiment. They were sensitized to the issue of muscle artefacts by watching their EEG signal on the screen while moving their eyes and blinking, and while squeezing their jaw muscles. Participants were asked to take this instruction as a recommendation rather than an additional task.
Each task version began with a 45-second eye open baseline, which was followed by a 3-minute meditation (either FA meditation with the breath sensations as object (FA version) or OM meditation (Open Attention version)). This meditation period was followed by four 5-minutes blocks of 350 auditory stimuli (80dB, 60-ms duration, rise and fall time 10 ms each). The inter-stimulus interval was randomized between 700 and 1100 ms. Each block contained 300 standard (one ear 1000Hz, the other ear, 500Hz) and 50 deviant (one ear 1,050 Hz and the other 475Hz) stimuli. The low and high tones were randomly presented in each ear across blocks in an order randomized across participants. Practitioners performed both versions of the dichotic listening task before and after the 3-month retreat. Novices also performed the two task versions twice with a 3-month period in between sessions. The first session occurred one week after the first instruction class. The second session occurred about 3 months later, one week after the second instruction class.
Electrophysiological (EEG) data
EEG was recorded at 512 Hz from 64 Ag AgCl electrodes using the Active-Two system (BioSemi, Amsterdam, Netherlands). With BioSemi systems, every electrode or combination of electrodes can be the “reference”. We re-referenced the data offline to the average of both mastoids. Additional electrodes recorded the potential above the right and left mastoids and the electrooculogram. The elecooculogram was place above and below the right eye.
Data Analysis
Behavioral Data Analysis
We examined effects of intensive meditation training on several behavioral measures: the mean reaction time (RT), the standard deviation (ST) of RT and the sensitivity index (d′), which indexes the ability to discern target stimuli (Swets et al., 1978). Fourteen practitioners and seventeen novices were included in the behavioral and EEG analysis. Criteria of exclusions included misunderstanding of the instructions, data recording difficulties (battery or software failures, headphone malfunction) and participant drop out at time 2. Repeated measures ANOVAs were run on RT, SD and d′ with Group (practitioners, novices) as a between-subject factor and Time (time 1, time 2) as a within-subject factor. As the standard deviation might change depending on the mean RT, we also ran a control analysis on the SD normalized compared to the mean RT. Importantly, as when using the non-normalized SD values (see main text), we found a mental training-related reduction in reaction time variability as indexed by a significant Group by Time interaction when using the normalized SD values (F(1,29)=13.5, p<0.005). Trials in which a response was recorded to the standard tone immediately following a deviant tone which was faster relative to the onset of the standard tone than the fastest response measured to any deviant tone in the session were defined as ‘ambiguous’ and not included in our analyses. Our behavioral results did not change when we included these ambiguous trials (less than 0.08% on average across participants), as was shown in an additional control analysis (not reported here).
EEG data: Analyses
EEGLAB (Delorme and Makeig, 2004) and Matlab (Mathworks, Inc., Natick, MA) were used for off-line EEG data processing. Data were band-pass filtered (1–50Hz), re-referenced to the average of both mastoids, and cleared of large movement-related artifacts. ICA as implemented in EEGLAB (Delorme and Makeig, 2004) was then used to remove ocular and muscle artefacts (for a description of the method see (Delorme, Sejnowski and Makeig, 2007)). Down-sampled (256 Hz) data were epoched (−1000ms to 1500ms after each tone) and baseline corrected (50ms preceding tones). Trials with remaining artifacts (exceeding ± 100 microvolts) were removed. For each condition (‘attended’ and ‘unattended’ deviant tones), group and time point separately, the EEG signals were averaged in synchrony with tone onset to create ERPs. Only trials used in the behavioral analysis were included in any EEG/ERP analysis.
Phase-locking factor (PLF)
To investigate effects of FA meditation on the variability of brain responses, we examined stimulus locking of ongoing cortical activity using the phase-locking factor (PLF), which quantifies the non-uniformity of a phase distribution (Palva, Palva and Kaila, 2005). PLF is an amplitude-independent measure, and is as such less sensitive than the classical ERP measure to inter-trial amplitude variability related to noise or small remaining artefacts, particularly for small number of trials. The reason being that each trial in the PLF approach is a unitary complex vector (i.e. amplitude equal to 1), and so contributes equally to mean PLF value. On the contrary, the amplitude of each trial used in the ERP analysis can fluctuate more, due to a greater sensitivity to noise, small remaining artefacts, and small changes in impedance. PLF therefore provides a particularly sensitive measure of trial-to-trial stability of evoked responses. PLF ranges from 0 to 1, with the value 1 indicating a δ-function distribution and the values approaching 0 indicating a uniform distribution. For N complex values zi, i=1… N, on the unit circle, the PLF is given by N−1 |Σzi| 50 (Sinkkonen, Tiitinen and Naatanen, 1995). The complex value zi is computed as the complex phase of the Hilbert transform of a signal bandpass filtered in a frequency range of interest (Palva, Palva and Kaila, 2005). If values zi are uncorrelated and randomly drawn from a uniform distribution, the PLFs of different sets of zi obey the Rayleigh distribution p(λ) = λσ−2exp[−0.5λ2σ−2], where λ denotes the PLF for a given realization of zi, s=λm (0.5π)−0.5, with λm being the mean of the distribution. The null hypothesis that the samples were drawn from a uniform distribution was tested using the Rayleigh statistic in which λm is simply defined by the number of trials. Because the Rayleigh distribution is a function of its mean (λm) only, PLFs were normalized by λμ to express the strength of phase locking directly in a statistically meaningful manner (Palva, Palva and Kaila, 2005). PLF/λm > 1.95 corresponds to p < 0.05 and PLF/λm > 2.42 to p < 0.01.Normalized PLF, or nPLF, were computed for the standard narrow-band frequency bands: delta (1–3Hz), theta (3–7Hz), alpha (8–12Hz), beta (13–30Hz) and gamma (30–45Hz). Our first analysis focused on the average nPLF across frequency bands between 1–30Hz, as this frequency range contains most of the spectral power of the event-related potentials. In a second analysis, we examined mental training-related changes in phase locking for each of the different standard frequency bands separately. Gamma was not included in the broadband PLF analysis as we did not find any meditation training-related effects on the phase locking of the gamma frequency band.
Despite the normalization procedure, the PLF measure is sensitive to the number of trials used to compute phase locking. We therefore equated the number of trials used to compute PLF values between Time (time 1, time 2) and Condition (attention, unattended deviant tones) separately for each participant. To this end, we first determined the minimum number of artifact-free trials separately for each session and condition. The surplus number of trials was then randomly removed from the relevant conditions (e.g., attended deviant tones at time 1). In-house simulations showed that a minimum of 40 trials is necessary to obtain reliable phase locking estimates (Slagter et al., 2008) and all participants had at least 40 trials of each condition at each session. The mean number of artifact-free trials used in the analysis was 79 for the practitioners and 78 for the novices. This number did not differ between the two groups (t-test, t(1,29)=, p>0.80). To describe the meditation related effect on PLF at each sample and channel, we submitted nPLF values to a repeated-measures ANOVA with Time (time 1 or time 2) and Condition (attended or unattended deviant tones) as within-subject factors, and Group (practitioners or novices) as a between-subjects factor (e.g. Figure 2A–B). The inference statistical analysis for the broad-band analysis consisted of 9 t-tests performed on the interaction term (group by time by condition) computed on the average PLF in three spatial regions (anterior, central and posterior electrodes) and in three temporal intervals (50–150ms, 150–300ms, 300–500ms). This selection was based on visual inspection of our data and on the latency and scalp topography of the classical ERP components N100, P200, P300. We corrected for multiple corrections at p < 0.05 using a nonparametric single threshold test (Nichols and Holmes, 2002). To this end, participants were randomly assigned 2000 times to two groups whose sizes were identical to the current group sizes. We computed two-tailed t-tests comparing the mean changes in PLF in attended versus unattended deviant tones between time 2 and time 1 for each of these regions and intervals (3 by 3 tests). We then computed the permutation distribution of the maximal voxel statistic over these 9 tests. We reject the omnibus hypothesis at level alpha = 0.975 if the maximal statistic for the actual labeling of the experiment is in the top 100*alpha% of the permutation distribution for the maximal statistic. The thresholds used for correcting at p<0.05 were 3.1 for the Group by Time effect and 3.2 for the Group by Time by Condition effect. The statistical analysis on the narrow-band analysis was similar but used 36 t-tests (3 spatial regions, 3 time interval and 4 frequency bands). The thresholds used for correcting at p<0.05 were 3.7 for the Group by Time effect and 3.8 for the Group by Time by Condition effect.
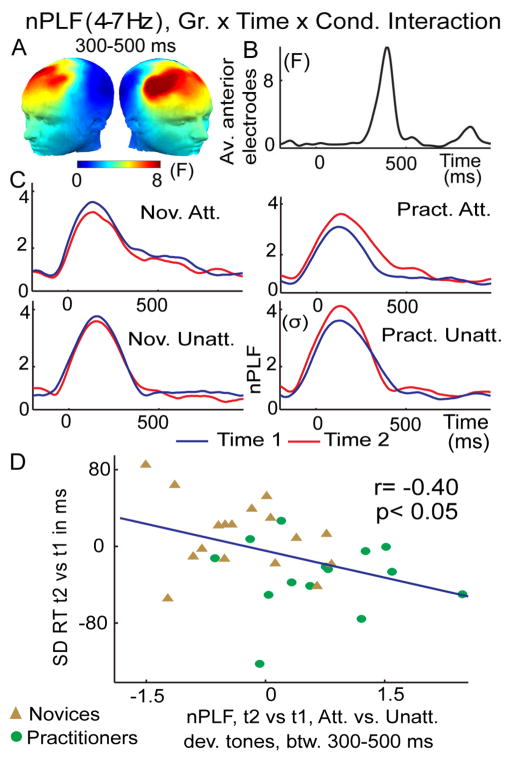
Figure 2. Intensive mental training increases trial-to-trial consistency of brain responses to attended deviant tones.
(A) Spatial distribution of mental training-related increase in theta-band (4–7 Hz) phase consistency (indexed by normalized PLF) to target tones, as indexed by a 3-way interaction between Group (Gr.; practitioners, novices), Time (time 1, time 2), and Condition (Cond; attended, unattended deviant tones). F values are averaged between 300–500 ms. (B) Time course of this 3-way interaction across these significant electrodes. (C) Normalized PLF values averaged across the electrodes sites showing a significant 3-way interaction in (A–B), separately for the practitioners (Pract) and novices (Nov) and attention (Att) and unattended (Unatt) deviant tones. Note that the observed increase in phase consistency to attended deviant tones over time was only observed for the practitioner group. (D) The correlation plot shows that the observed change in the trial-to-trial variability of brain responses, as indexed by nPLF, predicted the observed behavioral change in the trial-to-trial variability of the reaction time (RT) (standard deviation (SD)).
Event-related desynchronization (ERD)
The nonstimulus-locked amplitude fluctuations in oscillatory activity were obtained by time-frequency decompositions of EEG signals during the brain responses to deviant tones. Previous studies have shown most pronounced ERD in the alpha (8–12Hz) and beta (13–30Hz) frequency bands (Pfurtscheller and Lopes da Silva, 1999). We therefore focused our ERD analysis on these bands. The amplitude envelop was computed by taking the amplitude of the Hilbert transform of the signal bandpass filtered in these frequency ranges. For each electrode and each frequency separately, we averaged the power of the amplitude envelopes across trials for each Condition (Attention vs. Unattended deviant tones) and each Time (Time 1 vs. Time 2). The average envelope was then convoluted to a unitary vector corresponding to a time window of 100ms so as to smooth the envelope. Event-related synchronization (ERD) was then calculated by converting the power of the mean amplitude envelop at each frequency into percentage signal change relative to the average power in a reference pre-stimulus interval from −600ms to −200ms. We chose the baseline interval distant from the onset of the stimulation (at −200ms) to prevent the baseline to be affected by the induced response. To describe the meditation related effect on ERD, we applied in alpha and beta frequency bands repeated-measures ANOVA with Time (time 1 or time 2) and Condition (attended or unattended deviant tones) as within-subject factors and Group (practitioners or novices) as a between-subjects factor. The inference statistical analysis consisted of 6 t-tests performed on the interaction term (group by time by condition) computed from the average ERD in three spatial regions (anterior, central and posterior electrodes) and in two temporal intervals (250–500ms) and (500–750ms). As above, we corrected these tests for multiple comparisons corrected at p < 0.05. The threshold used for correcting at p<0.05 was 3.0 for the Group by Time by Condition effect. These temporal intervals were chosen around the peak of the ERD which occurred at 500ms (Figure 5).
Figure 5. Mental training selectively reduced cognitive effort as indexed by ERD.
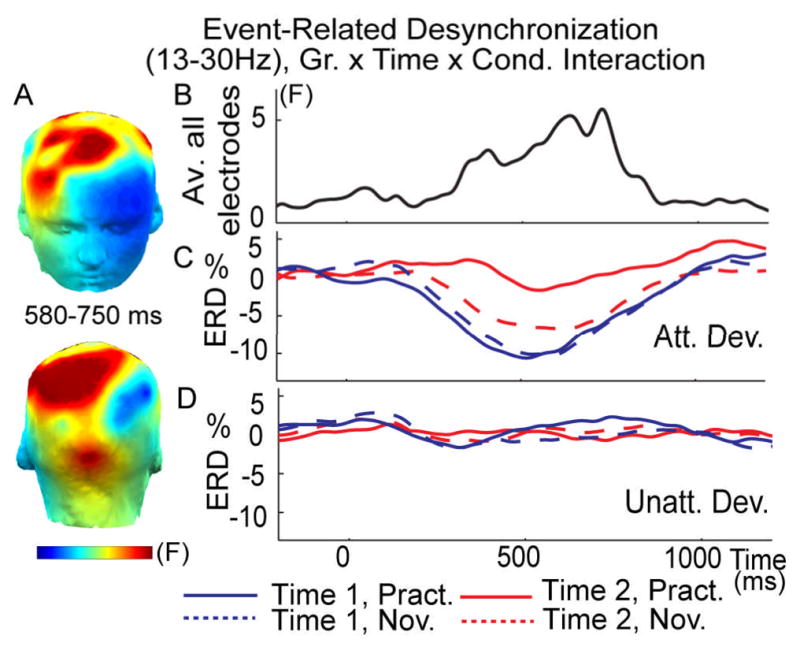
(A) Spatial distribution of the mental training-related decrease in beta-band (13–30 Hz) ERD to attended versus unattended deviant tones (3-way interaction between Group (Gr.; practitioners, novices), Time (time 1, time 2), and Condition (Cond; attended, unattended deviant tones). F values are averaged between 500–750 ms. Note that this interaction is significant (p<0.05, corrected), above the three a priori groups of anterior, central and posterior electrodes. Time course of this 3-way interaction (F value) (B) and of the ERD for attended (C) and unattended (D) deviant tones tones (Dev) on average across the 3 a priori groups of electrodes (anterior, central and posterior) for the practitioners (Pract) and novices (Nov).
Results
We collected data from 17 participants at the beginning and end of a 3-month meditation retreat, during which participants meditated for 10–12 hours per day (practitioner group, see methods for details on the retreat). We also collected control data from 23 participants interested in learning to meditate (novice group), who attended a 1-hour meditation class and were asked to meditate for 20 min daily for 1 week prior to each session. Participants performed the dichotic listening task (see Methods) as a variation of FA meditation on breath sensations. Specifically, they were asked to sustain attention from moment-to-moment on the attended side in order to detect target tones, while also constantly monitoring the quality of this attention. We examined our predictions using separate repeated measure ANOVAs with behavioral performance (RT, SD, and target detection rates (d′)), PLF and ERD as the dependent measure and the between-subject variable “Group” (experts vs. novices) and within-subject variables “Time” (time 1 and time 2) and/or “Condition” (attended, unattended).
Behavior
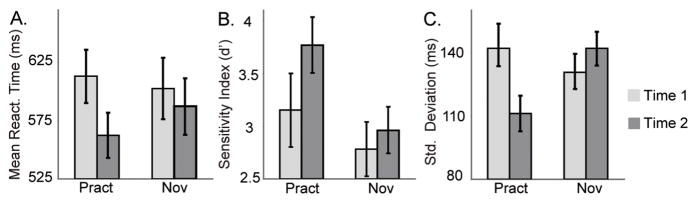
We found that both groups performed better at the task at time 2 versus 1. These generic practice effects were reflected in faster mean reaction times (main effect of Time, repeated measures analysis of variance (ANOVA), F(1,29)=5.9, p<0.05, Figure 1A) and increased target detection rates, as indexed by d′ (main effect of Time, F(1,29)= 7.3, p<0.01, Figure 1B). The overall increase in d′ was produced by an increase in hit rates at time 2 (main effect of Time, F(1,29)= 8.6, p<0.01). For experts, the mean hit rates were 80% (SD 20%) at time 1 and 91% (SD 10%) at time 2. For novices, the mean hit rates were 81% (SD 18%) at time 1 and 84% (SD 17%) at time 2. There was no significant change in false alarm rates over time in either group (paired t-test, t(1,13)=−1.7, p=0.1, for practitioner group, t(1,16)=0.1, p=0.9, for novice group). In addition, next to these generic practice effects, and in line with our prediction, we found that intensive mental training reduced behavioural response time variability. Thus, the practitioners showed a significantly greater reduction in RT variability (SDs) than the novices at time 2 versus time 1 (Group by Time interaction, repeated measures ANOVA, F(1,29)=10.2, p<0.005, Figure 1C). This effect was driven by the practitioner group only (paired t-test on the difference in SD at time 2 versus time 1, t(1,13)=3.1, p<0.01; novices: t(1,16)=−1.3, p=0.2). Mental training did not affect mean RT (Group by Time interaction effect, F(1,29)=1.8, p=0.18) or target tone detection rates (Group by Time interaction effect, F(1,29)=1.97, p=0.17). Of importance, there were no differences between groups in performance at time 1 (RT, SD, or d′ measures: no main effect of Group, all p’s>0.28).
Figure 1. Intensive mental training reduces intra-individual variability of behavioral performance.
(A) Average mean reaction time, (B) sensitivity index d′ and (C) standard deviation of reaction time (plus standard error) in response to target (attended deviant) tones, separately for each session (time 1, time 2) and group (practitioners, novices). Note that the practitioners showed significantly lower variability of reaction times (SDs) than the novices at time 2 versus time 1.
Phase-Locking Factor and Event-Related Potentials
We next predicted that the observed FA meditation-related reduction in reaction time variability would be associated with reduced variability of target-evoked brain responses, as indexed by the Phase-Locking Factor (PLF;(Palva, Palva and Kaila, 2005)). PLF is an amplitude-independent measure (i.e. PLF depends on the phase but not on the amplitude of the spectral representation of the signal; see Methods) adapted specifically to measure trial-to-trial stability of stimulus-evoked brain responses. To describe mental training-related effects on neural response variability, we first ran a repeated measures ANOVA for selected electrodes and time windows separately, using the average normalized PLF (nPLF) across frequency bands from 1 to 30Hz as the dependent measure (see Methods). The analysis of stimulus locking of broadband phase allows the evaluation of the precise time course of the phase-locked events, but does not disclose the spectral characteristics of the underlying activities. Therefore, we examined, in addition, stimulus locking within standard narrow-band frequency bands (δ(1–3Hz), θ (4–7Hz), α (8–13Hz) and β (13–30Hz)). To correct for multiple comparisons, we used a nonparametric single threshold test (Nichols and Holmes, 2002). To reduce the number of tests, statistical examination of effects of FA meditation focused on three spatial regions (anterior, central and posterior electrodes), three temporal intervals (50–150ms, 150–300ms, and 300–500ms) and four frequency bands. This selection was based on visual inspection of the data and the topography and chronomometry of standard evoked brain responses (N100, P200, and P300, respectively) (see Methods).
To test our hypothesis that intensive FA meditation would enhance attentional stability at the neural level, we examined whether FA meditation affected the phase relationship between the recorded signals at a given latency and the presented stimuli across the trials, using the PLF. Mental-training related increases in phase locking of broadband (1–30Hz) neural activity to attended deviant tones did not survive correction for multiple comparisons. Yet, narrow-band frequency analyses showed that, in line with our prediction, mental training was associated with decreased cross-trial variability in the phase of oscillatory activity in the theta band between 300–500ms after attended deviant tones over frontal scalp regions, as indicated by a significant 3-way interaction (Group by Time by Condition (attended, unattended deviant); F(1,29)=5.7, corrected p<0.05; see Methods). This effect peaked around 400ms over frontal scalp regions (Figure 2A–B), and was only found for the practitioner group (Figure 2C; paired t-test for practitioners on the difference t2 versus t1 attended versus unattended, between 300–500ms over anterior electrodes t(1,13)=3.1, p<0.01; for the novices t(1,16)=−1.7, p=0.1). Importantly, and further in line with our hypothesis that mental training would reduce sustained attention task performance variability by enhancing the stability of cortical signal processing, these training-related changes in theta PLF predicted the observed training-related reduction in trial-to-trial reaction time variability (Figure 2D). Specifically, between 300–500ms (see Figure 2B), over anterior scalp regions, a negative cross-subject correlation was observed between the increase across sessions in the consistency with which the brain responded to attended versus unattended deviant tones and the decrease in RT variability to attended deviant tones across sessions (Figure 2D, r=−0.40, p<0.05 for practitioners and novices). Such relationship was not seen with mean reaction time (r=−0.14, p=0.46) or d′ (r=0.13, p=0.45). To summarize, mental training reduced variability in attentional responses to task-relevant stimuli, as indicated by increased PLF to target tones and reduced reaction time variability.
In the dichotic listening paradigm, discrimination between target and non-target stimuli usually produces an event-related potential in response to target stimuli (called the P3a), that is sensitive to demands of attention ((Polich, 2007)). The P3a is thought to reflect stimulus-driven disruption of frontal attention engagement (Polich, 2007). Notably, this component has a similar latency and scalp topography as the observed mental training-related increase in phase consistency to target tones, and we therefore explored effects of FA meditation on the amplitude of the P3a. In our data, replicating prior studies (for review (Polich, 2007)).), we also observed a greater P3a to attended vs. unattended deviant tones over frontal scalp regions between 300–500 ms (main effect of Condition at electrode Fz, t(1,29)=4.3, p<0.0002, for all participants across sessions, Figure 3A). Yet, mental training did not affect P3a amplitude (Group by Time by Condition interaction, F(1,29)=0.41, p=0.53 at Fz, and F(1,29)=2.1, p=0.16 at Cz. For practitioners paired t-tests on P3a amplitude comparing time 2 versus time 1 at Cz and Fz, p>0.5). This is likely due to the fact that the ERP is more susceptible to trial-to-trial amplitude variability than PLF for relatively small numbers of trials and/or that this finding is specific to theta-band frequencies. In summary, mental training increased theta-band phase locking to target tones only and this effect was not reflected in an event-related potential measures of selective attention (P3a).
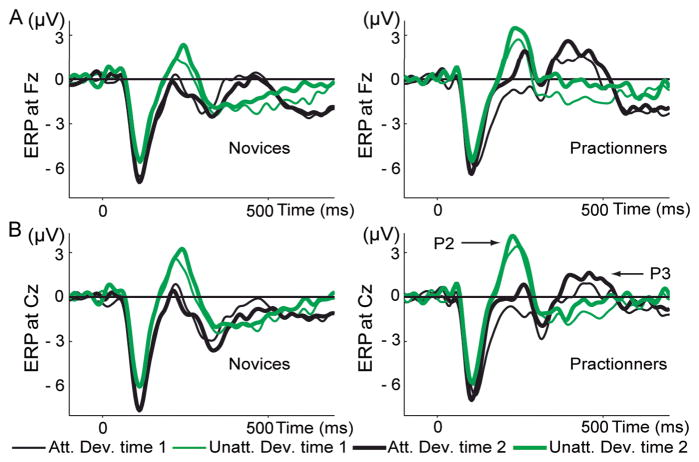
Figure 3. Event-related potentials (ERPs).
This panel shows ERPs (1–30Hz) to attended (black line) and unattended (green line) deviant tones at Fz (A) and Cz (B) at time 1 (thin line) and time 2 (thick line), separately for the novices (on the left) and the practitioners (on the right). In line with previous studies a smaller P200 (averaged between 150–300ms) was observed to attended vs. unattended deviant tones (main effect of condition, t(1,30)>6.4, p<1.10^−6 above Fz and Cz). The amplitude of the P200 increased across conditions only for the practitioners at time 2 (t(1,13)>2.7, p<0.05 over Fz and Cz). Note that in line with previous studies, attended target tones elicited a bigger P3a than unattended tones (main effect of Condition over electrode Fz, t(1,29)=4.3, p<0.0002, for all participants across sessions).
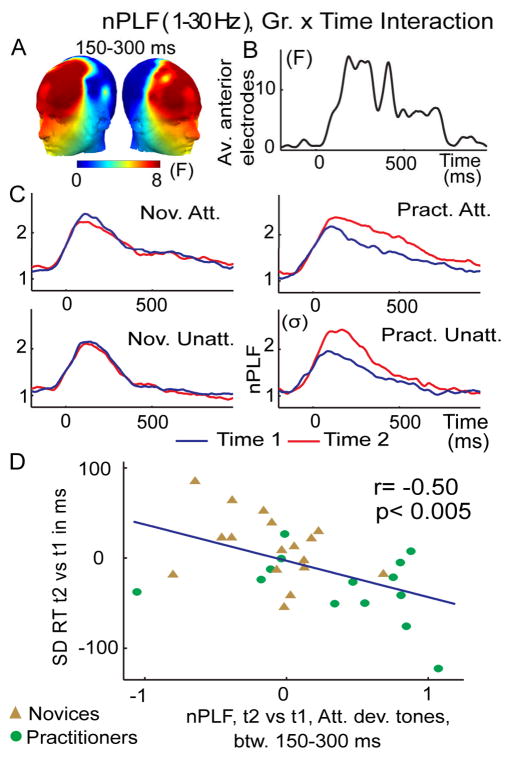
In addition, we explored the possibility that FA meditation - given that it is also assumed to affect the processing of distractions and task-unrelated thought - may affect stimulus processing in general (see Methods and (Lutz et al., 2008b)). Specifically, we examined whether FA meditation may modulate both distracter and target processing, as indicated by changes in PLF to deviant tones regardless of whether they were presented in the attended or unattended ear channel. Indeed, we found a robust meditation-related increase in broadband phase locking of neural activity at frontal electrodes between 150–300ms to any deviant tone (Group by Time interaction; on average F(1,29)=8.4, p<0.01, corrected for multiple comparisons at p<0.05, see Figure 4A–C). The effect was driven by stronger phase locking to deviant tones at time 2 in the practitioner group (paired t-test on the different t2 versus t1 for unattended deviant tones t(1,13)=2.4, p<0.05, and, attended deviant tones t(1,13)=5.8, p<0.0001), an effect that was not observed for the novice group (t(1,16)=−1.1, p=0.27 and t(1,16)=−0.42, p=0.68, respectively). The observed meditation-related increase in broadband phase locking PLF effects to attended deviant tones also predicted corresponding changes in intra-individual variability in reaction time (r=−0.50, p< 0.005, Figure 4D), d′ (r=0.48, p<0.01) and reaction time (r=−0.35, p=0.06). This early mental training-related PLF effect remained significant over anterior electrodes between 300–500ms, although the effect was weaker (corrected p<0.05, F(1,29)=5.9, p<0.05).
Figure 4. Intensive mental training increases trial-to-trial consistency of brain responses to any deviant tones.
(A–D) The graphs show the Group by Time interaction on broadband (1–30 Hz) nPLF following the display conventions and analysis from Figure 2. A mental training-related increase in phase locking to deviant tones in general (attended and unattended) was significant (p<0.05, corrected) between 150–300ms across the a priori group of frontal electrodes (A–C) and correlated negatively, for the attended deviant tones, with the observed reduction in RT SD (D). A similar negative correlation (r=−0.30) was found for the unattended deviant tones, even if this effect did not reach significant p=0.11.
Notably, the scalp topography and latency of the early mental training-related, attention-independent PLF effect corresponds to the scalp topography and latency of the P200 (Figure 3B), as confirmed by an additional ERP analysis: the topography of the P200 (averaged between 150–300ms) positively correlated with the topography of the broadband PLF between 150–300ms (r= 0.48, p<0.0001 for practitioners and r= 0.26, p<0.05 for controls). For the practitioners only, the topography of the P200 variations between time 1 and time 2 correlated with the topography of the PLF variations between time 1 and time 2 (r=0.72, p<0.0001). The amplitude of the P200 increased for the practitioners at time 2 (paired t-test on time 2 versus time 1 at Fz, t(1,13)=2.8, p<0.05 and at Cz, t(1,13)=2.7, p<0.05) but not for novices (paired t-test, time 2 versus time 1 for novices, at Fz, t(1,16)=0.7, p=0.49 and at Cz, t(1,13)=0.5, p=0.66). These results suggest that mental training may have also affected processes reflected by the P200. The P200 is typically associated with the beginning of an executive process responsible for stimulus identification and the initiation of decision making, and is also modulated by attention (Lindholm and Koriath, 1985). In line with previous studies, a smaller P200 (averaged between 150–300ms) was observed to attended vs. unattended deviant tones (paired t-test for all participants attended vs. unattended deviant tones averaged across time 1 and time2, p<0.000001, t(1,30)>6.4 above Fz and Cz). As the PLF frontal effect remained significant during the interval 300–500ms, we also explored possible mental training-related changes in ERPs in this later interval at electrodes Fz and Cz. Indeed, we found an increase in P3 amplitude for the practitioners only at time 2 (repeated ANOVAs, Group by Time interaction on average ERP between 300–500ms, F(1,29)=4.5, p<0.05 at Fz and F(1,29)=5.4, p<0.05 at Cz. Paired t-test for time 2 versus time 1 for all deviants tones for practitioners t(1,13)=2.3, p<0.05 at Fz and t(1,13)=2.1, p=0.06 at Cz, and for novices t(1,16)=−1.0, p=0.33 at Fz and t(1,16)=−0.15, p=0.88 at Cz. Figure 3).
Finally, we also examined mental training-related changes in stimulus locking to any deviant tone within standard narrow-band frequency bands for the same electrode sites and time windows for which broadband effects were observed to shed light on the spectral characteristics of this effect. Mental training only affected phase locking to any deviant tone in the delta (1–3Hz) frequency band (frontal electrodes, interval 300–500ms, F(1,29)=8.2, p<0.05 corrected). Notably, recent work suggests that when task-relevant input is regular or “rhythmic”, attention can enforce oscillatory entrainment to the input stream, thereby optimizing performance ((Jones and Boltz, 1989, Schroeder and Lakatos, 2009)). As in our task, the stimulus presentation rate fell within the frequency of the delta band (with a mean stimulus onset asynchrony (SOA) of 950ms), an intriguing possibility is thus that the observed mental training-related modulation of general stimulus processing in the delta band reflects a increase in oscillatory entrainment to the task-input rhythmic of the auditory tones. To explore this possibility, we analyzed mental training-related effects of phase locking to any deviant tone separately for a frequency band centred on the task-input rhythmic (estimated to be [0.85–1.3Hz] based on the range of SOAs) and the higher part of the delta band (1.5–3Hz) (for this analysis the EEG signals were band-pass filtered between 0.5–30Hz). Both analyses replicated the above findings for the 1–3Hz band, with mental training enhancing phase locking of oscillatory activity above frontal electrodes in both delta sub-bands (0.85–1.3Hz band: t-test comparing practitioners versus novices at time 2 versus time 1, t(1,29)=3, p<0.01), albeit more strongly so in the higher delta band (1.5Hz–3Hz) than in the task-input rhythmic band (t-test, t(1,29)= 2.4, p<0.05). This effect was driven by the practitioners showing higher PLF amplitude in high delta band than [0.85–1.3Hz] at time 2 compared to time 1 (paired t-test, t(1,13)=5, p<0.005). Nevertheless, the fact that mental training enhanced phase locking of oscillatory activity in a band corresponding to the auditory input frequency suggests that FA meditation may have lead attention to operate more steadily in a “rhythmic” mode of attention.
One could also argue that observed increase in phase locking to unattended deviant tones simply reflects an increase in general arousal level for meditators at time 2 rather than an increase in monitoring of any deviant tone or a reduction in task-unrelated processes. Higher arousal is generally thought to increase the system’s sensitivity to any sensory stimulus, and has typically been associated with a reduction in EEG power in the alpha band (8–12Hz)(Niedermeyer, 1999). To exclude the possibility that our effects merely reflect differences between groups in changes in arousal over time, we computed the global EEG power in the alpha band. Importantly, we did not find a group by time effect (repeated ANOVA, F(1,28)= 0.02, p=0.88). Rather, for both groups, we observed an increase in global alpha power (main effect of time, F(1,28)=4.6, p<0.05, mean power in a 2 second window for practitioners, 2.9×10^5 μV^2 at time 1 and 4.1×10^5 μV^2 at time 2, and for SC, 2.4×10^5 μV^2 at time 1 and 3.3×10^5 μV^2 at time 2). This finding argues against the possibility that a selective increase in arousal at time 2 in the practitioner group can explain our findings.
To summarize the above findings, mental training enhanced trial-to-trial phase consistency of oscillatory brain responses to attended deviant tones, as well as to deviant tones in general (i.e., independent of selective attention). The mental training-related increase in phase consistency to any deviant tone correlated with a mental training-related increase in the amplitude of the P200 ERP. These meditation-related effects could not simply be explained by a mere change in arousal level at time 2 for the meditators, as global alpha power increased similarly for both groups.
Event-Related Desynchronization
In addition, as we previously found that meditation training affects the distribution of limited brain resources ((Slagter et al., 2007)), we estimated how FA meditation affects cortical processing during the task. We indexed the extent of cortical engagement required to meet tasks demands by quantifying event-related desynchronization (ERD) to target tones. ERD reflects the blocking of alpha (8–12Hz) and beta (13–30Hz) EEG oscillatory rhythms by stimulus processing, in particular during motor preparation and execution, and is thought to reflect increased cellular excitability in thalamocortical systems during cortical information processing (Pfurtscheller and Lopes da Silva, 1999). Effort exerted during task performance, task difficulty, advanced age and low IQ are all factors that enhance ERD (for review (Pfurtscheller and Lopes da Silva, 1999)). By contrast, cognitive task practice has been found to reduce beta ERD (Romero et al., 2008). In line with the hypothesis that FA practice results in a decreased need for active engagement (Lutz et al., 2008b), we found that intensive mental training was associated with a reduction in ERD of beta oscillatory activity to attended deviant tones over the three spatial regions of interest as reflected by a Group by Time by Condition interaction between 500–750ms (F(1,29)=4.8, p< 0. 05, F(1,29)=5.2, p<0.05, F(1,29)=4.7, p<0.05, across anterior, middle, and posterior electrodes, respectively, p< 0.05 corrected, Figure 5). This interaction was driven by the practitioners showing less ERD at time 2 in the attended condition only (t-test comparing ERD to target minus ERD to unattended deviant tones at time 2 vs time 1, t(1,13)=3.4, p<0.005), while no such effect was observed for the novices (t(1,16)=−1.1, p=0.29) (Figure 5). The peak of this effect was located over frontal and left centro-posterior electrodes (Figure 5A). The lateralization of this ERD pattern above somato-sensory electrodes contralateral to the hand executing the motor response (paired t-test between the average across electrodes CP1, CP3, CP5, P1, P3, P5 and the average across CP2, CP4, CP6, P2, P4, P6, t(1,30)=−2.84, p<0.01) is in line with the proposal that beta-band ERD in part reflects motor selection processes ((Doyle, Yarrow and Brown, 2005)). Different from the observed mental training-related increase in phase consistency, these mental training-related changes in ERD did not correlate with the observed mental training-related behavioral changes in attentional stability (i.e., variability of reaction time). This latter finding may indicate that the observed effects of mental training on the stability of attention and task engagement were independent. This may not be surprising given that ERD is thought to index the amount of cortical engagement allocated by the system to perform the task, rather than reflect the actual performance of the system during the task (Pfurtscheller and Lopes da Silva, 1999). Alpha ERD was not affected by mental training, although this frequency band showed the greatest reduction in ERD to attended versus unattended deviant tones in general (data not shown). To summarize, the observed mental training-induced changes in ERD of beta band activity suggests a reduction in the amount of resources or engagement necessary to perform the task as a result of training.
Discussion
Our study examined whether 3-months of intensive mental training, as cultivated by FA meditation, can enhance i) attentional stability as reflected by decreased trial-to-trial variability in brain and behavioral responses to deviant attended tones and ii) reduce resource allocation or cortical engagement (i.e., promote more efficient processing). There were three main findings. First, we found a mental training-related increase in phase consistency of theta-band oscillatory neural responses over anterior scalp regions to target stimuli only (Figure 2). Importantly, this change in cortical signal stability predicted the observed reduction in reaction time variability (Figure 1). Second, mental training reduced cortical engagement, as reflected by a reduction in event-related desynchronization (ERD) to target tones in the beta (13–30Hz) frequency band (Figure 5). Third and lastly, mental training was associated with enhanced phase-consistency of the brain responses to any deviant tone (Figure 4). Together, these novel findings confirm first-person reports that FA meditation can affect the stability of attentional processes, and reveal the neural mechanisms underlying an improved ability to sustain attention. They extend prior work showing effects of a variety of meditation styles on various mental skills (Lutz et al., 2008a, Slagter et al., 2007, Slagter et al., 2008, Lutz et al., 2008b, Jha, Krompinger and Baime, 2007, Cahn and Polich, 2008, Tang et al., 2007, Brefczynski-Lewis et al., 2007), and corroborate the idea that purely mental training, as cultivated by meditation, can significantly affect brain and mental function (Lutz, 2008). Below we will discuss these three principal findings and their implications in more detail.
Effect of mental training on attentional stability
In support of our first hypothesis, we found a mental training-related increase in phase consistency of theta-band oscillatory neural responses over anterior scalp regions to target stimuli only, which predicted the observed reduction in reaction time variability. The observation that increases in theta phase locking over anterior regions were accompanied by less variable performance is consistent with a role of theta in cognitive control (e.g. (Hanslmayr et al., 2008)}, or in the formation of mnemonic neuronal representations (e.g. (Jensen and Lisman, 1996, Kahana, Seelig and Madsen, 2001)), important for sustaining attention on the task from moment-to-moment. Thus, the observed mental training-related increase in the consistency with which the brain can respond to a target stimulus from moment to moment may reflect increased cognitive control capacity, for example an increased ability to suppress task-unrelated thoughts or counter distractions, in line with phenomenological descriptions of this technique ((Lutz et al., 2008a)). This would be reflected in increased cortical signal-to-noise (e.g., PLF) in networks important for sustaining a performance over time. Similar proposals have been made to characterize attention disorders in several major psychiatric disorders (Rolls et al., 2008). For instance, simulations using biologically realistic neural networks have related the EEG-measured reduction of cortical phase consistency in schizophrenia (Winterer et al., 2000) to a diminished stability of these networks and associated reduced network stability to alterations of dopamine levels in the prefrontal cortex (Loh, Rolls and Deco, 2007). Next to dopamine, noradrenergic projections originating from the locus coeruleus are also important for sensory signal transmission in cortical areas (Aston-Jones, Rajkowski and Cohen, 1999). Thus, the here observed increase in phase consistency after intensive FA meditation training might be linked to cortical signal-to-noise of attentional networks in the brain and prefrontal dopaminergic and/or noradrenergic function. Future studies will need to incorporate brain measures of neurotransmitter functions (e.g. PET) and first-person reports of mind-wandering ((Christoff et al., 2009)) to explicitly test this assumption. To summarize, FA meditation may have improved signal-to-noise ratios in neural networks involved in attention.
Effect of mental training on cortical engagement
In support of our second hypothesis, we found that intense mental training affected cortical engagement, as reflected by a concomitant reduction in event-related desynchronization (ERD) to target tones in the beta (13–30Hz) frequency band (Figure 5). ERD is usually viewed as a correlate of increased cellular excitability in thalamocortical systems during cortical information processing (Pfurtscheller and Lopes da Silva, 1999). Within this framework, previous studies have interpreted reductions in beta ERD after practice of external tasks as decreased cognitive effort (Pfurtscheller and Lopes da Silva, 1999)(Romero et al., 2008). A mental training-related reduction in task effort would be consistent with traditional accounts of progress in this practice (Gunaratana, 2002, Gyatso, Tenzin (the XIV Dalai Lama) and Jinpa, 1995). At a more advanced level of training, the regulative attentional skills allegedly are invoked less and less frequently, and the ability to sustain focus thus becomes progressively ‘effortless’ (see (Lutz et al., 2008b) for a recent discussion). Previous fMRI findings from our group, which showed less BOLD activity in attention-related networks in expert meditators with the most hours of practice during FA meditation, also indicate that one long-term effect of FA meditation may be reduction in task effort (Brefczynski-Lewis et al., 2007). In the present study, this interpretation is however limited by the lack of first-person report on perceived effort, or of psychophysiological measures of cognitive effort (e.g. pupillometry). A more parsimonious reading of the finding is that mental training affected the oscillatory mode of the brain such that less cortical processing was needed to meet task demands. As discussed below, such change in neuronal oscillations of the system might be have been achieved by reducing task-irrelevant processes and/or by simply facilitating a sustained oscillatory mode throughout the task.
Enhanced processing of task-related auditory inputs during FA meditation
As mentioned above, next to increases in attention-related cortical signal stability, we also observed increases in stimulus-related neural processing regardless of whether the deviant tone was attended or unattended. Focused attention meditation may thus affect both distracter and target processing. This possibility is consistent with phenomenological descriptions of this style of meditation. While cultivating the acuity and stability of sustained attention on a chosen object, this practice is also said to develop three skills regulative of attention ((Gunaratana, 2002, Gyatso, Tenzin (the XIV Dalai Lama) and Jinpa, 1995, Lutz et al., 2008a), see Methods): the first is the monitoring faculty that remains vigilant to distractions without destabilizing the intended focus. The next skill is the ability to disengage from a distracting object without further involvement. The last involves the ability to redirect focus promptly to the chosen object (Lutz et al., 2008a). FA meditation training is thus thought to result not only in a high frequency of moments of attention on the attended object (or increased attentional stability), but also to improve one’s ability to remain vigilant and monitor distractors without losing focus. Consistently with this description of FA meditation effects, we observed a reduction in phase variability to any deviant tone during a time interval (150–300m; i.e., prior to the selective mental training effect on attentional processing (300–500ms)). This general increase in phase consistency was focused over anterior scalp regions and accounted for 22% of the variance in behavior (30% for the attended deviant tone, Figure 4). This effect was also highly similar both in time course and scalp topography to the P200 (Figure 3). This ERP component is typically associated with the beginning of executive processes responsible for stimulus identification and the initiation of decision-making and is also modulated by attention (Lindholm and Koriath, 1985). This point is important if one assumes that monitoring of distractors is one important mechanism for regulating task-unrelated thoughts processes during task performance (Lutz et al., 2008a). The persistence of this effect in the later time interval (300–500ms) for both PLF and ERP (P3, Figure 3), is consistent with this interpretation (for an opposite effect of meditation on the P3 to distractors, see (Cahn and Polich, 2008)).
As discussed above, it is possible that FA meditation reduced cortical signal-to-noise (e.g., PLF) in attention-related networks, and that this stabilized responding to target and distracter stimuli alike. Another, albeit not mutually exclusive possibility is that FA training led attention to operate more steadily into a ‘rhythmic’ mode ((Schroeder and Lakatos, 2009, Lakatos et al., 2008)), in tune with the temporal structure of the stimuli. Temporal expectations of rhythmic sensory stimuli stream spontaneously from and can optimize task demands ((Jones and Boltz, 1989)). Typically, perceptual judgments are enhanced for stimuli occurring in trials with more regular rhythm (i.e. (Schroeder and Lakatos, 2009, Jones, Johnston and Puente, 2006)). In our task, the auditory tones were presented with some temporal regularity, with the average time interval between the onset of a first stimulus and the onset of a second stimulus being around 900ms (+/− 150ms). Thus, training to sustain attention could have led practitioners to better anticipate the temporal aspects of the auditory stream. Importantly, during such dynamic attending ((Jones and Boltz, 1989)). ), or ‘rhythmic’ mode of attention ((Schroeder and Lakatos, 2009)), neural oscillations, in particularly low-frequency ones (e.g. delta (1–3Hz), see (Lakatos et al., 2008)), become entrained to the sensory inputs. In this situation, the high excitability phases of these oscillations tend to coincide with the temporal structures of the stimuli, consequently amplifying neural input responses ((Schroeder and Lakatos, 2009, Lakatos et al., 2008)), as such modulating signal-to-noise ratios. It is notable in this respect that we also observed a mental training-related increase in phase locking of low-frequency oscillatory activity (1–3Hz) to both attended and unattended stimuli (Figure 4). Although this effect was not maximal at the intrinsic oscillatory mode of the stimuli stream ([.85–1.3Hz]), it may include an amplified entrainment of neuronal oscillations to sensory input rhythms in the delta frequency band. The assumption about the ‘rhythmic’ mode attention during FA should be further investigated, in particular by looking at the influence of sensory input rhythms on the phase of the delta frequency band oscillations and by contrasting FA meditation with Open Monitoring meditation. OM meditation cultivates a monitoring state that does not focus or hold attention from moment-to-moment on an explicit object (Lutz et al., 2008a, Slagter et al., 2008). We would therefore predict that attention will operate less in a rhythmic mode during this state than during FA meditation.
The observed increase in phase consistency to deviant tones in general cannot simply be explained as a general mental training-related increase in arousal level for two reasons. First, as noted above, we observed a reduction in cortical engagement, as reflected by a mental training-related decrease in ERD, and effort to do a task is typically positively linked to some increase in arousal (Critchley et al., 2003)). Secondly, both groups showed an increase in global alpha power over time, and increases in alpha are generally associated with decreased arousal (Niedermeyer, 1999). To summarize, FA meditation may have reduced cortical signal-to-noise (e.g., PLF) in attention-related networks or may have led attention to operate more steadily into a ‘rhythmic’ mode, thereby increasing signals to task-relevant stimuli. Future studies are necessary to distinguish between these two accounts.
Limitations of the study and future directions
One important limitation of our study is that subjects self-selected to participate in the 3-month retreat. Although the controls were also interested in learning to meditate, it is possible that the two groups differed in some variable prior to the study. Yet, no differences in performance on the sustained attention task were observed between the two groups at time 1. Future studies should randomly assign participants to the retreat group or the control group, or use a randomized wait-list control group design.
Summary/Conclusions
To summarize, the current findings suggest that 3-months of intensive training in FA meditation 1) enhances attentional stability, 2) reduces task effort and 3) amplifies the phase-consistency of the brain responses to task-related sensory inputs. We propose that these mental training-related effects might be produced by a reduction in cortical noise, and/or by an enhancement of the ‘rhythmic’ mode of attention. Future studies will have to test these ideas and examine to what extent purely mental training of attentional skills can also impact other mental domains, which depend on attention regulation, such as emotion regulation and self regulation
Acknowledgments
We thank the Barre Insight Meditation Society, and P. Babe, S. Harkness, and Z. Moran for assistance in data collection and Matthieu Ricard, James Austin, and three anonymous reviewers for their comments on an earlier version of the manuscript. We thank J. Davis for teaching the novices meditation. We also thank the Mind and Life Institute for helping to facilitate this work. This work was supported by grants from the National Institute of Mental Health (to R.J.D.), the National Center for Complementary and Alternative Medicine (to R.J.D. and A.L.), and gifts from Bryant Wangard and Ralph Robinson, Keith and Arlene Bronstein and the John W. Kluge Foundation (to R.J.D.).
Footnotes
Author contributions AL, HAS, and RJD designed the study. AL, HAS, NBR and ADF were involved in data collection. AL, NBR, ADF and LLG analyzed the data. AL wrote the paper. All authors discussed the results and commented on the manuscript.
References
- Aston-Jones G, Rajkowski J, Cohen J. Role of locus coeruleus in attention and behavioral flexibility. Biol Psychiatry. 1999;46:1309–1320. doi: 10.1016/s0006-3223(99)00140-7. [DOI] [PubMed] [Google Scholar]
- Brefczynski-Lewis JA, Lutz A, Schaefer HS, Levinson DB, Davidson RJ. Neural correlates of attentional expertise in long-term meditation practitioners. Proc Natl Acad Sci U S A. 2007;104:11483–11488. doi: 10.1073/pnas.0606552104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahn BR, Polich J. Meditation (vipassana) and the P3a event-related brain potential. Int J Psychophysiol. 2008 doi: 10.1016/j.ijpsycho.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos FX, Sonuga-Barke EJ, Milham MP, Tannock R. Characterizing cognition in ADHD: Beyond executive dysfunction. Trends Cogn Sci. 2006;10:117–123. doi: 10.1016/j.tics.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Christoff K, Gordon AM, Smallwood J, Smith R, Schooler JW. Experience sampling during fMRI reveals default network and executive system contributions to mind wandering. Proc Natl Acad Sci U S A. 2009;106:8719–8724. doi: 10.1073/pnas.0900234106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD, Mathias CJ, Josephs O, O’Doherty J, Zanini S, Dewar BK, Cipolotti L, Shallice T, Dolan RJ. Human cingulate cortex and autonomic control: Converging neuroimaging and clinical evidence. Brain. 2003;126:2139–2152. doi: 10.1093/brain/awg216. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Delorme A, Sejnowski T, Makeig S. Enhanced detection of artifacts in EEG data using higher-order statistics and independent component analysis. Neuroimage. 2007;34:1443–1449. doi: 10.1016/j.neuroimage.2006.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle LM, Yarrow K, Brown P. Lateralization of event-related beta desynchronization in the EEG during pre-cued reaction time tasks. Clin Neurophysiol. 2005;116:1879–1888. doi: 10.1016/j.clinph.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Gunaratana H. Mindfulness in plain english. Wisdom Publications; 2002. [Google Scholar]
- Gyatso, Tenzin (the XIV Dalai Lama), Jinpa GT (1995) The world of tibetan buddhism: An overview of its philosophy and practice. Wisdom Publications.
- Hanslmayr S, Pastotter B, Bauml KH, Gruber S, Wimber M, Klimesch W. The electrophysiological dynamics of interference during the stroop task. J Cogn Neurosci. 2008;20:215–225. doi: 10.1162/jocn.2008.20020. [DOI] [PubMed] [Google Scholar]
- Hillyard SA, Hink RF, Schwent VL, Picton TW. Electrical signs of selective attention in the human brain. Science. 1973;182:177–180. doi: 10.1126/science.182.4108.177. [DOI] [PubMed] [Google Scholar]
- Jensen O, Lisman JE. Theta/gamma networks with slow NMDA channels learn sequences and encode episodic memory: Role of NMDA channels in recall. Learn Mem. 1996;3:264–278. doi: 10.1101/lm.3.2-3.264. [DOI] [PubMed] [Google Scholar]
- Jha AP, Krompinger J, Baime MJ. Mindfulness training modifies subsystems of attention. Cogn Affect Behav Neurosci. 2007;7:109–119. doi: 10.3758/cabn.7.2.109. [DOI] [PubMed] [Google Scholar]
- Jones MR, Boltz M. Dynamic attending and responses to time. Psychol Rev. 1989;96:459–491. doi: 10.1037/0033-295x.96.3.459. [DOI] [PubMed] [Google Scholar]
- Jones MR, Johnston HM, Puente J. Effects of auditory pattern structure on anticipatory and reactive attending. Cogn Psychol. 2006;53:59–96. doi: 10.1016/j.cogpsych.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Kahana MJ, Seelig D, Madsen JR. Theta returns. Curr Opin Neurobiol. 2001;11:739–744. doi: 10.1016/s0959-4388(01)00278-1. [DOI] [PubMed] [Google Scholar]
- Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Competition between functional brain networks mediates behavioral variability. Neuroimage. 2008;39:527–537. doi: 10.1016/j.neuroimage.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Kuntsi J, Stevenson J. Psychological mechanisms in hyperactivity: II. the role of genetic factors. J Child Psychol Psychiatry. 2001;42:211–219. [PubMed] [Google Scholar]
- Lakatos P, Karmos G, Mehta AD, Ulbert I, Schroeder CE. Entrainment of neuronal oscillations as a mechanism of attentional selection. Science. 2008;320:110–113. doi: 10.1126/science.1154735. [DOI] [PubMed] [Google Scholar]
- Lindholm E, Koriath JJ. Analysis of multiple event related potential components in a tone discrimination task. Int J Psychophysiol. 1985;3:121–129. doi: 10.1016/0167-8760(85)90032-7. [DOI] [PubMed] [Google Scholar]
- Loh M, Rolls ET, Deco G. A dynamical systems hypothesis of schizophrenia. PLoS Comput Biol. 2007;3:e228. doi: 10.1371/journal.pcbi.0030228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz A, Brefczynski-Lewis J, Johnstone T, Davidson RJ. Regulation of the neural circuitry of emotion by compassion meditation: Effects of meditative expertise. PLoS ONE. 2008a;3:e1897. doi: 10.1371/journal.pone.0001897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz A, Slagter HA, Dunne JD, Davidson RJ. Attention regulation and monitoring in meditation. Trends Cogn Sci. 2008b;12:163–169. doi: 10.1016/j.tics.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manassis K, Tannock R, Masellis M. Cognitive differences between anxious, normal, and ADHD children on a dichotic listening task. Anxiety. 1996;2:279–285. [PubMed] [Google Scholar]
- Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: A primer with examples. Hum Brain Mapp. 2002;15:1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedermeyer E. The normal EEG of the waking adult. In: Niedermeyer E, Lopes Da Silva F, editors. Electroencephalography: Basic principles, clinical application, and related fields. Maryland, USA: Lippincott Williams & Wilkins; 1999. [Google Scholar]
- Palva JM, Palva S, Kaila K. Phase synchrony among neuronal oscillations in the human cortex. J Neurosci. 2005;25:3962–3972. doi: 10.1523/JNEUROSCI.4250-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfurtscheller G, Lopes da Silva FH. Event-related EEG/MEG synchronization and desynchronization: Basic principles. Clin Neurophysiol. 1999;110:1842–1857. doi: 10.1016/s1388-2457(99)00141-8. [DOI] [PubMed] [Google Scholar]
- Polich J. Updating P300: An integrative theory of P3a and P3b. Clin Neurophysiol. 2007;118:2128–2148. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polich J, Criado JR. Neuropsychology and neuropharmacology of P3a and P3b. Int J Psychophysiol. 2006;60:172–185. doi: 10.1016/j.ijpsycho.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Loh M, Deco G, Winterer G. Computational models of schizophrenia and dopamine modulation in the prefrontal cortex. Nat Rev Neurosci. 2008;9:696–709. doi: 10.1038/nrn2462. [DOI] [PubMed] [Google Scholar]
- Romero SG, McFarland DJ, Faust R, Farrell L, Cacace AT. Electrophysiological markers of skill-related neuroplasticity. Biol Psychol. 2008;78:221–230. doi: 10.1016/j.biopsycho.2008.03.014. [DOI] [PubMed] [Google Scholar]
- Schroeder CE, Lakatos P. Low-frequency neuronal oscillations as instruments of sensory selection. Trends Neurosci. 2009;32:9–18. doi: 10.1016/j.tins.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinkkonen J, Tiitinen H, Naatanen R. Gabor filters: An informative way for analysing event-related brain activity. J Neurosci Methods. 1995;56:99–104. doi: 10.1016/0165-0270(94)00111-s. [DOI] [PubMed] [Google Scholar]
- Slagter HA, Lutz A, Greischar LL, Nieuwenhuis S, Davidson RJ. Theta phase synchrony and conscious target perception: Impact of intensive mental training. J Cogn Neurosci. 2008 doi: 10.1162/jocn.2009.21125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slagter HA, Lutz A, Greischar LL, Francis AD, Nieuwenhuis S, Davis JM, Davidson RJ. Mental training affects distribution of limited brain resources. PLoS Biol. 2007;5:e138. doi: 10.1371/journal.pbio.0050138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swets JA, Green DM, Getty DJ, Swets JB. Signal detection and identification at successive stages of observation. Percept Psychophys. 1978;23:275–289. doi: 10.3758/bf03199711. [DOI] [PubMed] [Google Scholar]
- Tang YY, Ma Y, Wang J, Fan Y, Feng S, Lu Q, Yu Q, Sui D, Rothbart MK, Fan M, Posner MI. Short-term meditation training improves attention and self-regulation. Proc Natl Acad Sci U S A. 2007;104:17152–17156. doi: 10.1073/pnas.0707678104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiitinen H, Sinkkonen J, Reinikainen K, Alho K, Lavikainen J, Naatanen R. Selective attention enhances the auditory 40-hz transient response in humans. Nature. 1993;364:59–60. doi: 10.1038/364059a0. [DOI] [PubMed] [Google Scholar]
- Weissman DH, Roberts KC, Visscher KM, Woldorff MG. The neural bases of momentary lapses in attention. Nat Neurosci. 2006;9:971–978. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]
- Winterer G, Ziller M, Dorn H, Frick K, Mulert C, Wuebben Y, Herrmann WM, Coppola R. Schizophrenia: Reduced signal-to-noise ratio and impaired phase-locking during information processing. Clin Neurophysiol. 2000;111:837–849. doi: 10.1016/s1388-2457(99)00322-3. [DOI] [PubMed] [Google Scholar]