Abstract
The medical, public health and scientific communities are grappling with monumental imperatives to contain COVID-19, develop effective vaccines, identify efficacious treatments for the infection and its complications, and find biomarkers that detect patients at risk of severe disease. The focus of this communication is on a potential biomarker, short telomere length (TL), that might serve to identify patients more likely to die from the SARS-CoV-2 infection, regardless of age. The common thread linking these patients is lymphopenia, which largely reflects a decline in the numbers of CD4/CD8 T cells but not B cells. These findings are consistent with data that lymphocyte TL dynamics impose a limit on T cell proliferation. They suggest that T cell lymphopoiesis might stall in individuals with short TL who are infected with SARS-CoV-2.
Keywords: Telomeres, COVID-19, T cells, lymphopenia
Introduction
The following topics are briefly covered in this communication: (a) major features suggesting that telomere length (TL) is short in leukocytes of persons at higher risk of dying from COVID-19; (b) principles of hematopoietic cell TL dynamics relevant to the immune response in the face of SARS-CoV-2 infection; (c) the advantage of having a longer leukocyte TL (LTL) to mount an immune response against SARS-CoV-2 infection; (d) ramifications stemming from the potential role of TL in COVID-19 outcome; and (e) contextualizing the pandemic from the standpoint of evolutionary forces shaping TL in humans.
Major features of COVID-19
The majority of individuals who died from COVID-19 are elderly, adults with cardiovascular disease (CVD) and diabetes, and men (1–3). In contrast, infants and children typically had a milder clinical course (4–6). At the population level, comparatively short telomeres in the elderly, persons with cardio-metabolic diseases and men (7) may be the common thread linking the worse COVID-19 outcomes
This conclusion is supported by another finding: the association of severe lymphopenia with fatal outcomes of COVID-19 (8–10). Lymphopenia also characterizes severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS), two other diseases caused by betacoronaviruses (11–15). As described below, short LTL might partially explain the COVID-19- associated lymphopenia, which primarily reflects a decline in the number and exhaustion of T lymphocytes (16).
Hematopoietic cell telomere length dynamics and its importance in the face COVID-19-associated lymphopenia
LTL is a highly heritable human trait (17, 18) that displays wide inter-individual variation (range 3–4 kilo base (kb) after adjustment for age) (19, 20) and reflects mean TLs across all hematopoietic cells (21). Within every person, these cells, including the hematopoietic stem cells (HSCs) that top the hematopoietic hierarchy, show age-dependent telomere shortening (22, 23). Although telomerase, the reverse transcriptase that maintains telomeres (24), is active in subsets of hematopoietic cells, in most hematopoietic cells, this activity is insufficient to prevent telomere shortening that ultimately leads to cellular senescence, which culminates in cessation of replication.
Figure 1 depicts a model of hematopoiesis in the bone marrow under ‘steady state’ conditions. Atop the hierarchy are a few HSCs with high proliferative capacity; they replicate approximately once a year (22, 23). At the bottom of the hierarchy, multitudes of cells committed to specific lineages replicate approximately daily, producing ~ 350 billion cells (erythrocytes and leukocytes) in adults (25). Numerous mitotic divisions occur as cells are formed from top to bottom of the hierarchy; consequently, TL is shorter in unipotent cells at the bottom than cells at the top. Typically, about three months might elapse between the replication of HSCs and the release of their fully differentiated progenies into the circulation (25). This hierarchal configuration of hematopoiesis, with its numerous unipotent cells with shorter telomeres at the bottom and progressively fewer cells with longer telomeres at the top, works efficiently to maintain homeostasis of blood cells not only young persons with long LTL but also older persons with a short LTL (26).
Figure 1. Replicative potential, replicative rate and telomere shortening across the hematopoietic hierarchy.
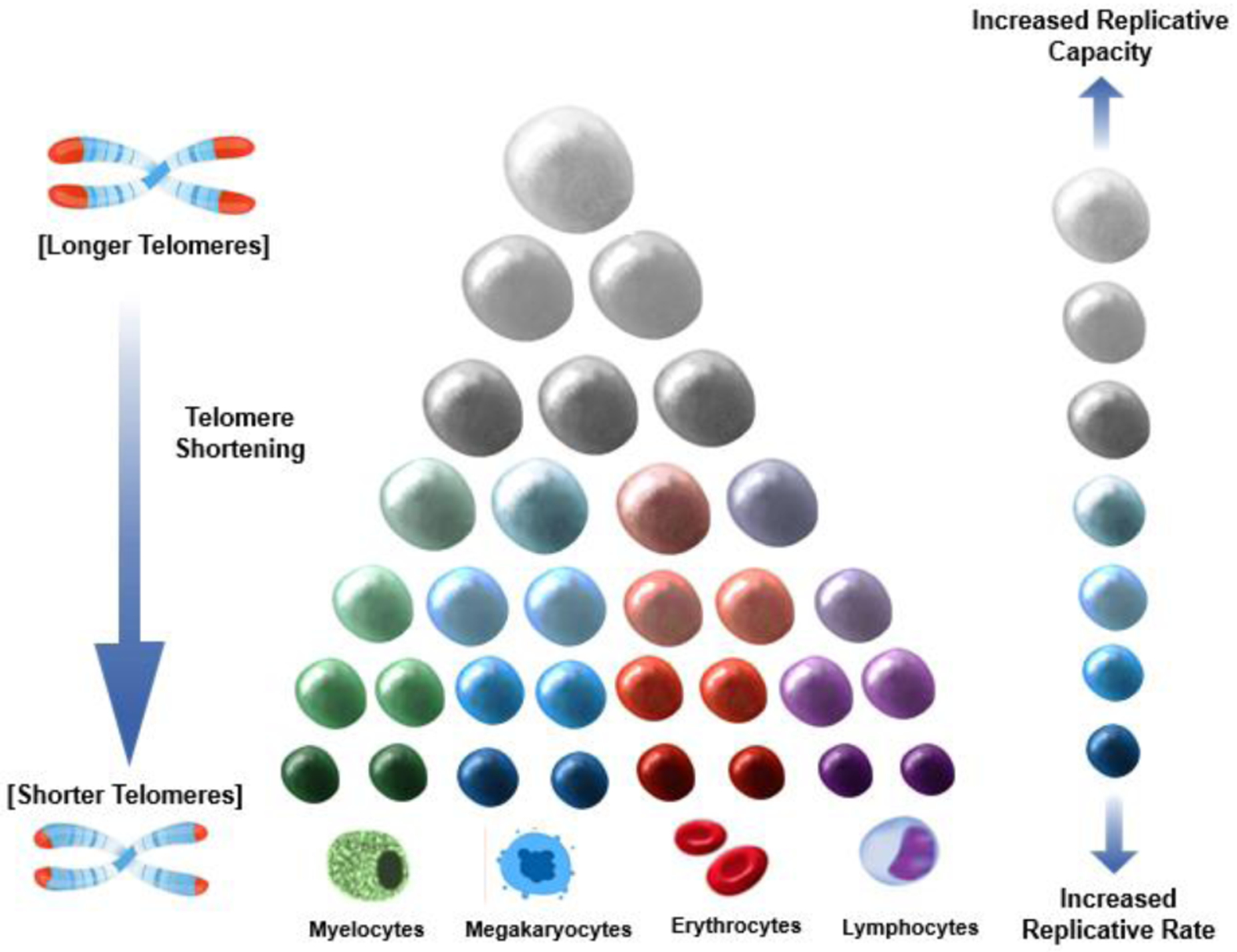
Larger cells denote more replicative capacity; darker cells denote faster replication. Cells atop the hierarchy replicate at a slow pace but have a high replicative capacity. Cells at the bottom replicate at a fast pace but have a lower replicative capacity. The length of telomeres (shown as the red caps at the end of the chromosomes) is progressively shorter towards the bottom due to the greater number of cell replications that occur moving down the hierarchy.
That said, hematopoiesis might stall in the face of a massive loss of circulating blood cells (non-steady state condition). When this happens, the fast-replicating unipotent cells at the bottom of the hierarchy (first responding cells) increase their replicative pace to offset the loss of circulating cells. Replication ‘waves’ propagated up the hierarchy likely occur in tandem with the increased demand for replication of the cells at the bottom. Replications at the top, albeit slow, ultimately serve to replete the ranks of more differentiated cells at the bottom. However, in response to massive loss of circulating cells, first responding cells will exhaust their TL-dependent replicative capacity and reach senescence more quickly in individuals with shorter LTL than those with longer LTL. This is because an individual’s LTL (short or long) reflects TL across all cells of her/his hematopoietic hierarchy. The slow ‘refilling’ of the stockpile of replicating cells at the bottom of the hierarchy would therefore stall hematopoiesis among individuals with a shorter LTL. Simply put, the recovery pace from a massive loss of circulating cells would be inversely related to LTL.
The kinetics of cell replication of the immune system are much more complex than the above model, which principally applies to cells, including lymphocytes, produced in the bone marrow. The development of lymphoid progenitors in the thymus during early life, maturation of lymphocytes and their production in secondary lymphoid organs add more layers of complexity to the model. We know little about the hierarchal configuration of human lymphopoiesis in lymphoid organs and in the circulation. But we do know that lymphopoiesis is tightly linked to TL and telomerase, whose activity varies in different lymphocyte lineages. Both B and T cells show telomerase activity, which elongates telomeres in memory B cells (27–29). In contrast, telomerase fails to maintain TL in T cells and consequently TL is shorter and activation-induced proliferation slower in memory T cells and in older persons (29–31). Therefore, inter-individual differences in TL and the propensity of their T cells to undergo senescence due to aging and in response of inherent factors (e.g. genetically determined TL) might play a critical role in severe lymphopenia- associated with COVID-19 and its often fatal outcome. Indeed, the lymphopenia associated with COVID-19 is marked by reduction in CD4/CD8 cells, but not B cells (16, 32) consistent with their different TL dynamics during development and activation, i.e., telomere elongation in B cells (27–29) and telomere shortening in T cells (29–31). Finally, in adults, TL is shorter by approximately one kb in lymphocytes than in granulocytes (33), potentially explaining with an exception (34), the absence of leukopenia (8–10, 32) in the majority of patients with COVID-19.
The advantage of having a longer LTL in the face of COVID-19 infection
The average LTL at birth (in the US) is ~9.5 kb (20). Thereafter, LTL shortens by ~2 kb by the 3rd decade (19, 20) and by ~3.5 kb by the 9th decade of life (20, 33). We know little about how much telomeres shorten per replication of hematopoietic cells in vivo. Estimates (~0.05–0.1 kb) rely on cultured cells (35, 36). Still, consider a child whose LTL is only one kb longer than that of an adult. Based on the loss of 0.1 kb per replication, all else being equal, the TL-dependent replicative capacity of the first responding cells is 210 larger for the child than the adult, meaning that the child has an enormous restorative advantage compared with the adult in the ability to respond to an acute and massive loss of circulating cells. Similarly, the average difference in LTL between adults with CVD vs. those without CVD is ~ 0.3 kb (37, 38). The TL-dependent replicative capacity of the first responding cells in adults with CVD would thus be 23 smaller than in those without CVD.
At present, we little knowledge of the etiology of lymphopenia in patients with COVID-19, but prompt recovery of the immune response requires massive lymphopoiesis, which is TL-dependent. The shorter telomeres of hematopoietic cells of the elderly, persons with cardio-metabolic disease, and men might impede their lymphopoiesis, particularly CD4/CD8 lymphopoiesis, in the face of COVID-19, increasing the risk of severe disease and a fatal outcome. In principle, all adults ranked in the lower part of the TL distribution, regardless of age, could be susceptible to severe COVID-19-associated drop in CD4/CD8 because their telomeres might be too short to sustain the speedy replicative response of these cells to acute and massive losses of lymphocytes.
Ramifications
The potential exists to diagnose clinically useful biomarkers of risk based on the presumed LTL-COVID connection. Such biomarkers might help identifying persons at greater risk of severe COVID-19 in whom intensive management should be initiated sooner. Further, the potential connection between LTL and the severity of COVID-19 raises a host of questions vital to public and global health. For instance, LTL in African Americans is ~ 0.2 kb longer than that of Americans of European ancestry (39, 40), and in sub-Saharan Africans it is ~ 0.3 kb longer than in African Americans (40). How can one reconcile the longer LTL of African Americans with their higher mortality rate from COVID-19 (41), assuming the finding holds after adjustment for age, sex, obesity and demographic settings? The incidence of essential hypertension is higher (42) and the activity of the renin-angiotensin-aldosterone (RAAS) lower (43) in African Americans than in Americans of European ancestry. As mortality from COVID-19 is presumably higher in patients with essential hypertension (1–3) and given that angiotensin converting enzyme-2, which facilitates the intracellular entry of SARS-CoV-2, is a component of RAAS (44, 45), different factors might influence the severity of COVID-19 in individuals of different ancestries. In this light, preliminary data, which require validation, suggest disproportionally less cases of COVID-19 in sub-Saharan regions (46). Falciparum malaria – a disease that still kills numerous individuals, the majority of whom are sub-Saharan children younger than five years of age (47) – causes massive hemolysis and often severe anemia in children (48, 49). Might co-infection of infants and children with COVID-19 and malaria have a synergistic effect that greatly increases children mortality because of heightened telomeric demands for both lymphopoiesis and erythropoiesis?
Evolutionary Context
Humans have been infected by zoonotic viruses, including betacoronaviruses, since the dawn of their evolution (50, 51). Ever since the development of agriculture and settlements (52, 53), they have also been exposed to Plasmodium falciparum, which causes the most lethal form of malaria. These infections unleashed powerful selective forces, explaining, for instance, the high prevalence of pleiotropic alleles that confer resistance to severe malaria among populations indigenous to malaria endemic regions. By increasing the turnover of lymphocytes and erythrocytes, betacoronaviruses, including SARS-CoV-2, Plasmodium falciparum, and other infectious and parasitic pathogens probably served to lengthen human telomeres through selection (Figure 2). Cancer, in contrast, has been a powerful evolutionary force to shorten telomeres in humans and other mammals (Figure 2) (7). Human migration, endemic infections, and other exposures likely caused TL to fluctuate above and below an optimal value that maintained the balance between these and other selective forces (54) that typically exert influence during the reproductive years. The majority of contemporary humans, however, largely experiences the lasting effects of such forces on TL in late adulthood and old age (55, 56). In this sense, the severe impact of COVID-19 on older individuals, persons with the cardio-metabolic syndrome, and men reaffirms the dictum: Nothing in biology makes sense except in the light of evolution (57).
Figure 2. Evolutionary forces that regulate optimal TL (Model).
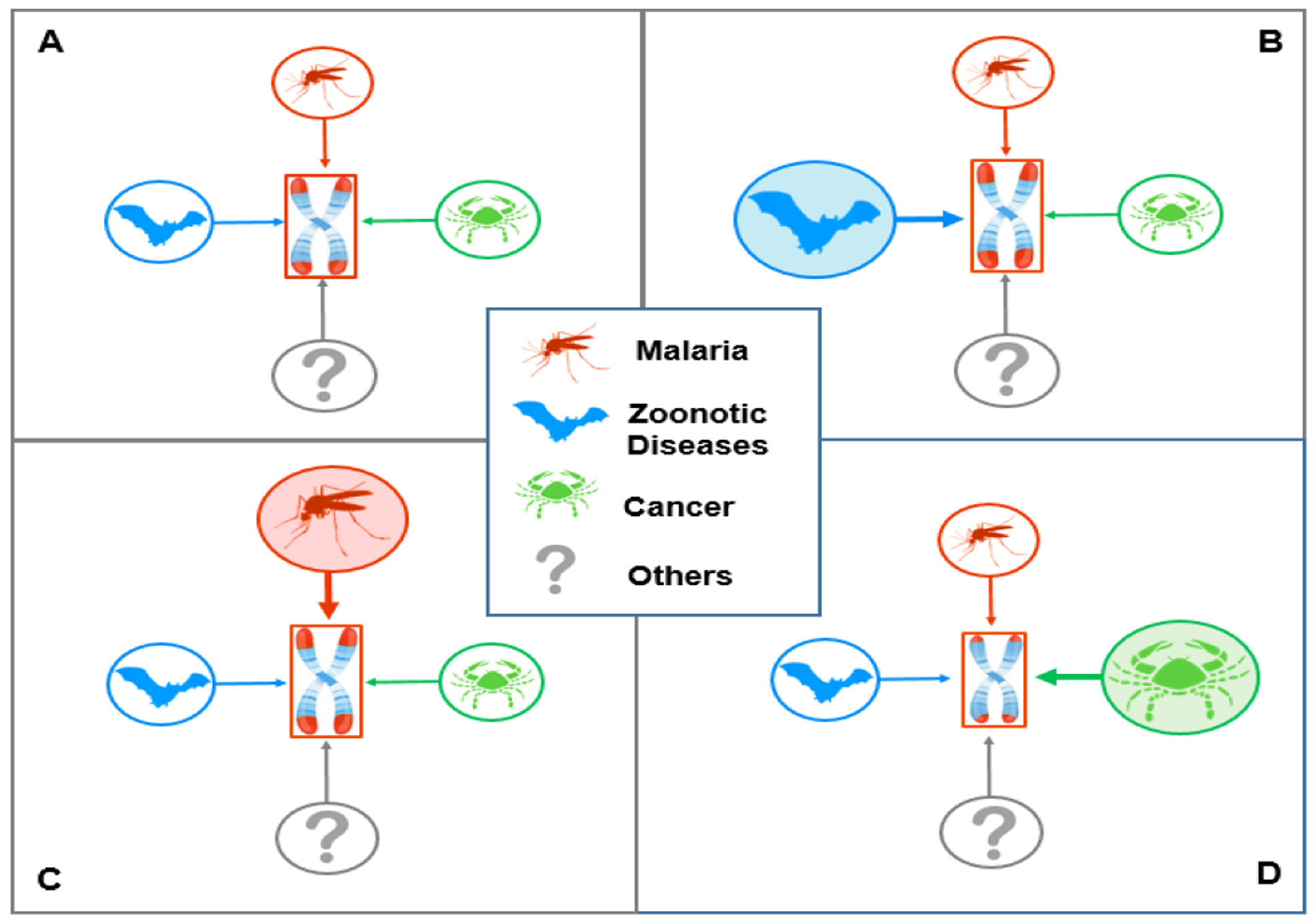
Optimal TL is set by opposing factors that lengthen or shorten telomeres through natural selection. Displayed for illustration, are (compared to A) the effect of zoonotic viral diseases, e.g., betacoronaviruses, which increases the demand for lymphpoiesis (B), and falciparum malaria, which increases the demand for erythropoiesis (C). Longer telomeres (red caps at the ends of the chromosomes) increase the chance of surviving these diseases. Therefore, repeated exposures to such diseases (B and C) in succeeding generations would lengthen telomeres. Cancer (D) might be an evolutionary force to shorten telomeres, because longer telomeres entail increased replicative potential and a higher cancer risk. Other factors that increase demand for somatic repair through cell replication might lengthen telomeres.
Acknowledgments:
I thank my colleagues Jennifer R Harris, Christophe G Lambert, Lawrence C. Kleinman, Ezra Susser, and Daniel Levy for valuable suggestions, and my student Anita Zhang for creating the figures.
Funding: A. Aviv current telomere research is funded by the NIH (R01 HL134840 and U01AG066529) and the Norwegian Research Council (NFR) ES562296.
Abbreviations:
- TL
telomere length
- LTL
Leukocyte telomere length
- HSCs
hematopoietic stem cells
- kb
kilobase
References
- 1.Wu Z, and McGoogan JM (2020) Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72314 Cases From the Chinese Center for Disease Control and Prevention. Jama [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19) [Pdf]. 2020. [cited 23 March 2020]. Available from https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-finalreport.pdf. [Google Scholar]
- 3.Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, Cereda D, Coluccello A, Foti G, Fumagalli R, Iotti G, Latronico N, Lorini L, Merler S, Natalini G, Piatti A, Ranieri MV, Scandroglio AM, Storti E, Cecconi M, and Pesenti A (2020) Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. Jama [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dong Y, Mo X, Hu Y, Qi X, Jiang F, Jiang Z, and Tong S (2020) Epidemiological Characteristics of 2143 Pediatric Patients With 2019 Coronavirus Disease in China. Pediatrics, e2020070232179660 [Google Scholar]
- 5.Coronavirus Disease 2019 in Children - United States, February 12-April 2, 2020. MMWR Morbidity and mortality weekly report. 2020;69(14):422–6. Epub 2020/04/10. doi: 10.15585/mmwr.mm6914e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu Q, and Shi Y (2020) Coronavirus disease (COVID-19) and neonate: What neonatologist need to know. Journal of medical virology [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aviv A, and Shay JW (2018) Reflections on telomere dynamics and ageing-related diseases in humans. Philosophical transactions of the Royal Society of London. Series B, Biological sciences 373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, and Peng Z (2020) Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. Jama [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, and Zhang L (2020) Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet (London, England) 395, 507–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang JJ, Dong X, Cao YY, Yuan YD, Yang YB, Yan YQ, Akdis CA, and Gao YD (2020) Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy [DOI] [PubMed] [Google Scholar]
- 11.Liu WJ, Zhao M, Liu K, Xu K, Wong G, Tan W, and Gao GF (2017) T-cell immunity of SARS-CoV: Implications for vaccine development against MERS-CoV. Antiviral research 137, 82–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee N, Hui D, Wu A, Chan P, Cameron P, Joynt GM, Ahuja A, Yung MY, Leung CB, To KF, Lui SF, Szeto CC, Chung S, and Sung JJ (2003) A major outbreak of severe acute respiratory syndrome in Hong Kong. The New England journal of medicine 348, 1986–1994 [DOI] [PubMed] [Google Scholar]
- 13.Booth CM, Matukas LM, Tomlinson GA, Rachlis AR, Rose DB, Dwosh HA, Walmsley SL, Mazzulli T, Avendano M, Derkach P, Ephtimios IE, Kitai I, Mederski BD, Shadowitz SB, Gold WL, Hawryluck LA, Rea E, Chenkin JS, Cescon DW, Poutanen SM, and Detsky AS (2003) Clinical features and short-term outcomes of 144 patients with SARS in the greater Toronto area. Jama 289, 2801–2809 [DOI] [PubMed] [Google Scholar]
- 14.Min CK, Cheon S, Ha NY, Sohn KM, Kim Y, Aigerim A, Shin HM, Choi JY, Inn KS, Kim JH, Moon JY, Choi MS, Cho NH, and Kim YS (2016) Comparative and kinetic analysis of viral shedding and immunological responses in MERS patients representing a broad spectrum of disease severity. Scientific reports 6, 25359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ko JH, Park GE, Lee JY, Lee JY, Cho SY, Ha YE, Kang CI, Kang JM, Kim YJ, Huh HJ, Ki CS, Jeong BH, Park J, Chung CR, Chung DR, Song JH, and Peck KR (2016) Predictive factors for pneumonia development and progression to respiratory failure in MERS-CoV infected patients. The Journal of infection 73, 468–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diao B, Wang C, Tan Y, Chen X, Liu Y, Ning L, Chen L, Li M, Liu Y, Wang G, Yuan Z, Feng Z, Wu Y, and Chen Y (2020) Reduction and Functional Exhaustion of T Cells in Patients with Coronavirus Disease 2019 (COVID-19). medRxiv, 2020.2002.2018.20024364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hjelmborg JB, Dalgard C, Moller S, Steenstrup T, Kimura M, Christensen K, Kyvik KO, and Aviv A (2015) The heritability of leucocyte telomere length dynamics. Journal of medical genetics 52, 297–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Slagboom PE, Droog S, and Boomsma DI (1994) Genetic determination of telomere size in humans: a twin study of three age groups. American journal of human genetics 55, 876–882 [PMC free article] [PubMed] [Google Scholar]
- 19.Factor-Litvak P, Susser E, Kezios K, McKeague I, Kark JD, Hoffman M, Kimura M, Wapner R, and Aviv A (2016) Leukocyte Telomere Length in Newborns: Implications for the Role of Telomeres in Human Disease. Pediatrics 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steenstrup T, Kark JD, Verhulst S, Thinggaard M, Hjelmborg JVB, Dalgard C, Kyvik KO, Christiansen L, Mangino M, Spector TD, Petersen I, Kimura M, Benetos A, Labat C, Sinnreich R, Hwang SJ, Levy D, Hunt SC, Fitzpatrick AL, Chen W, Berenson GS, Barbieri M, Paolisso G, Gadalla SM, Savage SA, Christensen K, Yashin AI, Arbeev KG, and Aviv A (2017) Telomeres and the natural lifespan limit in humans. Aging 9, 1130–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kimura M, Gazitt Y, Cao X, Zhao X, Lansdorp PM, and Aviv A (2010) Synchrony of telomere length among hematopoietic cells. Experimental hematology 38, 854–859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sidorov I, Kimura M, Yashin A, and Aviv A (2009) Leukocyte telomere dynamics and human hematopoietic stem cell kinetics during somatic growth. Experimental hematology 37, 514–524 [DOI] [PubMed] [Google Scholar]
- 23.Shepherd BE, Guttorp P, Lansdorp PM, and Abkowitz JL (2004) Estimating human hematopoietic stem cell kinetics using granulocyte telomere lengths. Experimental hematology 32, 1040–1050 [DOI] [PubMed] [Google Scholar]
- 24.Blackburn EH, and Collins K (2011) Telomerase: an RNP enzyme synthesizes DNA. Cold Spring Harbor perspectives in biology 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dingli D, Traulsen A, and Pacheco JM (2007) Compartmental architecture and dynamics of hematopoiesis. PloS one 2, e345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mollica L, Fleury I, Belisle C, Provost S, Roy DC, and Busque L (2009) No association between telomere length and blood cell counts in elderly individuals. The journals of gerontology. Series A, Biological sciences and medical sciences 64, 965–967 [DOI] [PubMed] [Google Scholar]
- 27.Hodes RJ, Hathcock KS, and Weng NP (2002) Telomeres in T and B cells. Nature reviews. Immunology 2, 699–706 [DOI] [PubMed] [Google Scholar]
- 28.Martens UM, Brass V, Sedlacek L, Pantic M, Exner C, Guo Y, Engelhardt M, Lansdorp PM, Waller CF, and Lange W (2002) Telomere maintenance in human B lymphocytes. British journal of haematology 119, 810–818 [DOI] [PubMed] [Google Scholar]
- 29.Weng NP, Palmer LD, Levine BL, Lane HC, June CH, and Hodes RJ (1997) Tales of tails: regulation of telomere length and telomerase activity during lymphocyte development, differentiation, activation, and aging. Immunological reviews 160, 43–54 [DOI] [PubMed] [Google Scholar]
- 30.Patrick M, and Weng NP (2019) Expression and regulation of telomerase in human T cell differentiation, activation, aging and diseases. Cellular immunology 345, 103989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patrick MS, Cheng NL, Kim J, An J, Dong F, Yang Q, Zou I, and Weng NP (2019) Human T Cell Differentiation Negatively Regulates Telomerase Expression Resulting in Reduced Activation-Induced Proliferation and Survival. Frontiers in immunology 10, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen G, Wu D, Guo W, Cao Y, Huang D, Wang H, Wang T, Zhang X, Chen H, Yu H, Zhang X, Zhang M, Wu S, Song J, Chen T, Han M, Li S, Luo X, Zhao J, and Ning Q (2020) Clinical and immunologic features in severe and moderate Coronavirus Disease 2019. The Journal of clinical investigation [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aubert G, Baerlocher GM, Vulto I, Poon SS, and Lansdorp PM (2012) Collapse of telomere homeostasis in hematopoietic cells caused by heterozygous mutations in telomerase genes. PLoS genetics 8, e1002696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zheng HY, Zhang M, Yang CX, Zhang N, Wang XC, Yang XP, Dong XQ, and Zheng YT (2020) Elevated exhaustion levels and reduced functional diversity of T cells in peripheral blood may predict severe progression in COVID-19 patients. Cellular & molecular immunology [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vaziri H, Dragowska W, Allsopp RC, Thomas TE, Harley CB, and Lansdorp PM (1994) Evidence for a mitotic clock in human hematopoietic stem cells: loss of telomeric DNA with age. Proceedings of the National Academy of Sciences of the United States of America 91, 9857–9860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Allsopp RC, Vaziri H, Patterson C, Goldstein S, Younglai EV, Futcher AB, Greider CW, and Harley CB (1992) Telomere length predicts replicative capacity of human fibroblasts. Proceedings of the National Academy of Sciences of the United States of America 89, 10114–10118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Benetos A, Toupance S, Gautier S, Labat C, Kimura M, Rossi PM, Settembre N, Hubert J, Frimat L, Bertrand B, Boufi M, Flecher X, Sadoul N, Eschwege P, Kessler M, Tzanetakou IP, Doulamis IP, Konstantopoulos P, Tzani A, Korou M, Gkogkos A, Perreas K, Menenakos E, Samanidis G, Vasiloglou-Gkanis M, Kark JD, Malikov S, Verhulst S, and Aviv A (2018) Short Leukocyte Telomere Length Precedes Clinical Expression of Atherosclerosis: The Blood-and-Muscle Model. Circulation research 122, 616–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brouilette S, Singh RK, Thompson JR, Goodall AH, and Samani NJ (2003) White cell telomere length and risk of premature myocardial infarction. Arteriosclerosis, thrombosis, and vascular biology 23, 842–846 [DOI] [PubMed] [Google Scholar]
- 39.Hunt SC, Chen W, Gardner JP, Kimura M, Srinivasan SR, Eckfeldt JH, Berenson GS, and Aviv A (2008) Leukocyte telomeres are longer in African Americans than in whites: the National Heart, Lung, and Blood Institute Family Heart Study and the Bogalusa Heart Study. Aging cell 7, 451–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hansen ME, Hunt SC, Stone RC, Horvath K, Herbig U, Ranciaro A, Hirbo J, Beggs W, Reiner AP, Wilson JG, Kimura M, De Vivo I, Chen MM, Kark JD, Levy D, Nyambo T, Tishkoff SA, and Aviv A (2016) Shorter telomere length in Europeans than in Africans due to polygenetic adaptation. Human molecular genetics 25, 2324–2330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yancy CW (2020) COVID-19 and African Americans. Jama [DOI] [PubMed] [Google Scholar]
- 42.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, and Turner MB (2015) Heart disease and stroke statistics−−2015 update: a report from the American Heart Association. Circulation 131, e29–322 [DOI] [PubMed] [Google Scholar]
- 43.He FJ, Markandu ND, Sagnella GA, and MacGregor GA (1998) Importance of the renin system in determining blood pressure fall with salt restriction in black and white hypertensives. Hypertension (Dallas, Tex. : 1979) 32, 820–824 [DOI] [PubMed] [Google Scholar]
- 44.Danser AHJ, Epstein M, and Batlle D (2020) Renin-Angiotensin System Blockers and the COVID-19 Pandemic: At Present There Is No Evidence to Abandon Renin-Angiotensin System Blockers. Hypertension (Dallas, Tex. : 1979), Hypertensionaha12015082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.AlGhatrif M, Cingolani O, and Lakatta EG (2020) The Dilemma of Coronavirus Disease 2019, Aging, and Cardiovascular Disease: Insights From Cardiovascular Aging Science. JAMA cardiology [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Napoli PE, and Nioi M (2020) Global Spread of Coronavirus Disease 2019 and Malaria: An Epidemiological Paradox in the Early Stage of A Pandemic. Journal of clinical medicine 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.World Health Organization. This year’s world malaria report at a glance. (2018, November 19). Available from https://www.who.int/malaria/media/world-malaria-report-2018/en/#Preventing%20malaria
- 48.White NJ (2018) Anaemia and malaria. Malaria journal 17, 371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Doolan DL, Dobano C, and Baird JK (2009) Acquired immunity to malaria. Clinical microbiology reviews 22, 13–36, Table of Contents [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Parrish CR, Holmes EC, Morens DM, Park EC, Burke DS, Calisher CH, Laughlin CA, Saif LJ, and Daszak P (2008) Cross-species virus transmission and the emergence of new epidemic diseases. Microbiology and molecular biology reviews : MMBR 72, 457–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Van Blerkom LM (2003) Role of viruses in human evolution. American journal of physical anthropology Suppl 37, 14–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Joy DA, Feng X, Mu J, Furuya T, Chotivanich K, Krettli AU, Ho M, Wang A, White NJ, Suh E, Beerli P, and Su XZ (2003) Early origin and recent expansion of Plasmodium falciparum. Science (New York, N.Y.) 300, 318–321 [DOI] [PubMed] [Google Scholar]
- 53.Carter R, and Mendis KN (2002) Evolutionary and historical aspects of the burden of malaria. Clinical microbiology reviews 15, 564–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Horvath K, Eisenberg D, Stone R, Anderson J, Kark J, and Aviv A (2019) Paternal Age and Transgenerational Telomere Length Maintenance: A Simulation Model. Scientific reports 9, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stone RC, Horvath K, Kark JD, Susser E, Tishkoff SA, and Aviv A (2016) Telomere Length and the Cancer-Atherosclerosis Trade-Off. PLoS genetics 12, e1006144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aviv A, Anderson JJ, and Shay JW (2017) Mutations, Cancer and the Telomere Length Paradox. Trends in cancer 3, 253–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dobzhansky T (1973) Nothing in Biology Makes Sense except in the Light of Evolution. The American Biology Teacher 35, 125–129 [Google Scholar]


